Abstract
Introduction
TRPM2 channels have been suggested to play a role in ischemic neuronal injury, specifically in males. A major hindrance to TRPM2 research has been the lack of specific TRPM2 inhibitors. The current study characterized the specificity and neuroprotective efficacy of a novel TRPM2 inhibitor.
Methods
Fluorescent calcium imaging (Fluo5F) was used to determine inhibitor efficacy of the TRPM2 peptide inhibitor (tat-M2NX) in HEK293 cells stably expressing hTRPM2. Adult (2–3months) and aged (18–20 months) mice were subjected to 60 min middle cerebral artery occlusion (MCAO) and injected with tat-M2NX, control scrambled peptide (tat-SCR) or clotrimazole (CTZ) either 20 min prior or 3 h after reperfusion. Infarct size was assessed using TTC staining.
Results
TRPM2 inhibition by tat-M2NX was observed by decreased Ca2+ influx following H2O2 exposure human TRPM2 expressing cells. Male mice pre-treated with tat-M2NX had smaller infarct volume compared to tat-SCR. No effect of tat-M2NX on infarct size was observed in female mice. Importantly, male TRPM2−/− mice were not further protected by tat-M2NX, demonstrating selectivity of tat-M2NX. Administration of tat-M2NX 3 h after reperfusion provided significant protection to males when analyzed at 24 h or 4 days after MCAO. Finally, we observed that tat-M2NX reduced ischemic injury in aged male mice.
Conclusions
These data demonstrate the development of a new peptide inhibitor of TRPM2 channels that provides protection from ischemic stroke in young adult and aged male animals with a clinically relevant therapeutic window.
Keywords: TRPM2 channel, Experimental stroke, Cerebral ischemia, Aging, Sex
1. Introduction
Stroke is the second leading cause of death worldwide with a significant financial burden and poor life quality among recovered patients due to cognitive disability (Mozaffarian et al., 2015). Unfortunately, the clinical pharmacological tools available to reduce brain injury and treat patients with stroke are extremely limited, possibly due to the vast majority of experimental stroke studies using young adult male animals (Herson and Traystman, 2014). In contrast, two of the most important and non-modifiable risk factors for stroke are age and gender (Romero et al., 2008). Clinical stroke is well known to affect the elderly (Mozaffarian et al., 2015), with the risk for stroke doubling every decade after age 55 years old (Wolf et al., 1992), independent of other risk factors (Romero et al., 2008). Aging has numerous effects on the brain, including numerous biochemical changes, neurochemical changes, alterations in blood flow and decreases in white matter, to name a few. Further, stroke is well known to affect men to a larger extent than women until later in life where the rate of stroke increases in elderly women (Reeves et al., 2008). This observation is likely due to effects of androgens and estrogen in cell death pathways (Herson et al., 2009). However, most neuroprotection investigations either have not used female animals or have not been powered to find differences between sexes in human trials. Thus, it is critical to more accurately model the human patient population in order to determine the therapeutic potential of any new compounds.
Transient receptor potentialM2(TRPM2) channels are non-selective cation channels activated by ADP ribose (ADPr) (Perraud et al., 2001). ADPr is generated by PARP-1 in response to oxidative stress and cerebral ischemia, which is particularly relevant in the setting of reperfusion injury after ischemia (Shimizu et al., 2013). Inhibition of TRPM2 ion channels with clotrimazole (CTZ) or genetic knockdown reduces ischemic injury in males, but not females (Jia et al., 2011; Verma et al., 2012; Shimizu et al., 2013; Quillinan et al., 2014; Shimizu et al., 2016). While we have previously shown that CTZ can be administered up to 2 h after onset of ischemia in stroke models (Shimizu et al., 2013), we have not previously investigated whether inhibiting TRPM2 at more extended time points provides neuroprotection. Even though TRPM2 appears to be a viable target for therapeutic interventions for stroke in males, preclinical studies have been limited by the lack of a specific inhibitor. Therefore, one goal of this study was to develop and test a membrane-permeable, selective inhibitor of TRPM2 ion channels that can be used to further the understanding of TRPM2 in neuronal injury following focal cerebral ischemia. Targeting the Nudix motif located on the C-terminal of the TRPM2 channel that directly binds ADPr, we designed a peptide that would inhibit TRPM2 activity and attached it to a tat domain of HIV to promote cell permeability (tat-M2NX). The current study uses both sexes and aged mice to show that tat-M2NX is a novel neuroprotective agent with a clinically relevant therapeutic window.
2. Methods
2.1. Animals
All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Colorado and adhered to the National Institute of Health guidelines for the care and use of animals in research. Young adult (2–3 month old) and aged (18–20 month old) C57Bl/6 mice were purchased from Charles River Laboratories (CRL). Male and female TRPM2 KO (TRPM2−/−) mice were provided by Dr. Perraud (Knowles et al., 2011) and corresponding WT mice were purchased from The Jackson Laboratory (JAX). All mice were permitted access to water and standard lab chow ad libitum with standard 12-h light/dark cycles. All experiments were performed in a blinded and randomized manner according to the ARRIVE guidelines (Kilkenny et al., 2010a; Kilkenny et al., 2010b), with a separate investigator generating the experimental code.
2.2. Novel inhibitor of TRPM2
We generated a cell permeable peptide-inhibitor of TRPM2 by fusing a portion of the C-terminus that corresponds > 90% with the Nudix domain (M2NX) of the C-terminus of TRPM2channelswith the tat inducer of HIV (tat-M2NX). In addition, a control peptide, which contained the same amino acids in a scrambled sequence (tat-SCR), was generated. Dose of tat-M2NX was chosen based upon previous literature using tat-fusion proteins for neuroprotection which utilized 8–9 mg/kg (Cao et al., 2002; Soriano et al., 2008).
2.3. In vitro inhibition of TRPM2 channels
HEK-293 cells stably expressing tetracycline-regulated FLAG-tagged human TRPM2 were used. TRPM2 expressing HEK-293 cells were grown in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal bovine serum, L-glutamine (2 mM), and penicillin/streptomycin (100 units/mL) at 37 °C in a 5% CO2 incubator. HEK cells were grown in media containing doxycycline (1 µg/mL) to drive TRPM2 expression 24 h prior to experiments. Cells were pre-incubated for 2 h with 50 µL of doxycycline-containing media with tat-M2NX (25, 50, and 100 µM), tat-SCR (25, 50, and 100 µM) or vehicle. Fluo-5F AM (Life Technologies, 10 µM), a membrane permeable Ca2+ indicator, was added to the media during the last 30 min of incubation. Cells were washed twice and placed in 50 µL of saline solution (135 mM NaCl, 5mMKCl, 1mMMgCl2, 1mMCaCl2, 10mMHEPES; pH 7.4). Fluorescence was measured using a microplate reader (BioTek Synergy 2) and Gen5 software to measure fluorescence (485/20, 528/20) every 20 s before and after exposure to H2O2 (200 µM final concentration) or saline (control).
2.4. Middle cerebral artery occlusion (MCAO)
Transient focal cerebral ischemia (60 min) was induced using reversible MCAO through the intra-luminal filament techniques described previously (Shimizu et al., 2013). Briefly, mice were anesthetized with isoflurane delivered through a face mask (5% induction and 1–2%maintenance). Cerebral ischemia was induced for 60 min of MCAO via intraluminal suture method (6-0 nylon monofilament coated with silicone to diameter of 0.22–0.24 mm). Adequacy of MCAO confirmed by laser Doppler flowmetry (Moor Instruments) over the right parietal cortex (> 80% drop required).
2.5. Infarct volume analysis
After the corresponding reperfusion period, the mice were anesthetized with 5% isoflurane and decapitated for brain collection. The mice were excluded from the study if subarachnoid hemorrhage was observed. Each cerebrum was sliced into four 2-mm-thick coronal sections. The sections were placed in 1.2% of 2,3,4-triphenyltetrazolium chloride (TTC, Sigma) for 30 min at 37 °C and fixed in 10% formalin for 24 h. Each coronal slice was stained and photographed on both sides using a digital camera, infarction was measured with ImageJ (NIH), and integrated across all five slices. In order to include the effect of edema, the infarct volume was estimated indirectly and plotted as percentage of the contralateral structure and presented as a corrected hemisphere infarct.
2.6. Testosterone and dihydrotestosterone enzyme-linked immunoassay
A cohort of naïve male mice was sacrificed by Avertin overdose (intraperitoneal) for blood sample collections from the right ventricle of the heart using heparinized syringes. The blood sample was centrifuged at 3300g for 10min at 4 °C to yield serum for hormone detection. Enzyme-linked immunoassay for testosterone (Calbiotech) and dihydrotestosterone (DHT, Alpha Diagnostic International, TX) were performed following the manufacturers protocol.
2.7. Statistical analysis
All data are presented as mean±SEM. Each n represents an individual culture for in vitro experiments and an individual animal for in vivo experiments. All experiments were performed in a randomized and blinded manner, with analysis and surgery performed by separate investigators. Statistical significance was determined using students t-test (unpaired, 2-tailed) for 2 groups and one-way analysis of variance (ANOVA) with Newman-Keuls post hoc analysis or studies with > 2 groups. Statistical significance was established at p < 0.05.
3. Results
3.1. tat-M2NX inhibits the human TRPM2 channel in vitro
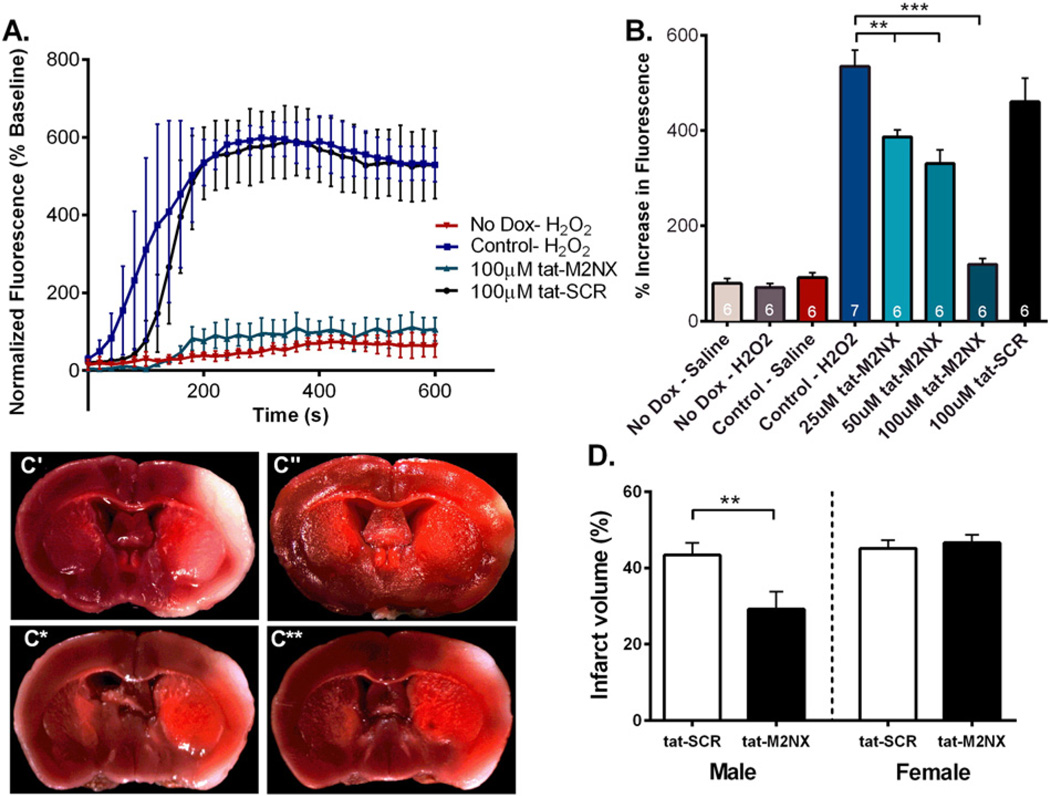
In order to determine the ability of tat-M2NX to inhibit TRPM2 channels, we used HEK-293 cells stably expressing tetracycline-induced human FLAG-tagged TRPM2 channel (hTRPM2). TRPM2 channel activity was measured using the Ca2+ indicator Fluo-5F to monitor changes in fluorescence following treatment with 200 µM H2O2. Exposure to H2O2 increased fluorescence in HEK-293 cells expressing hTRPM2, while no changes were observed in non-induced HEK-293 cells, indicating functionality of the channel in the in vitro system (Fig. 1A & B). HEK-293 expressing the hTRPM2 had reduced H2O2-induced Ca2+ influx after 2 h incubation with 100 µM tat-M2NX, while treatment with the tat-SCR peptide had no effect on H2O2-induced Ca2+ influx (Fig. 1A & B). Further experiments revealed a concentration-dependent decrease in Ca2+ influx following exposure to 25, 50, and 100 µM tat-M2NX in hTRPM2 expressing HEK-293 cells (Fig. 1A & B). These data suggest that tat-M2NX inhibits human TRPM2 channels.
Fig. 1.
tat-M2NX inhibits Ca2+ influx through TRPM2 channels and provides in vivo neuroprotection. (A) Average fluorescent changes in Fluo-5F fluorescence shown as percentage increase from baseline in HEK-293 cells expressing tetracycline-regulated cytomegalovirus-driven transcription of FLAG-tagged human TRPM2. Samples were treated with treated with 100 µM tat-M2NX (green) or tat-SCR (black) in the presence of 200 µMH2O2. (B) Quantification of percentage increase in Fluo-5F fluorescence in HEK-293 cells treated with 25, 50, 100 µM tat-M2NX or 100 µM tat-SCR. (C) TTC staining from male brains treated with (C') tat-SCR (n= 8) and (C") tat-M2NX (n= 8), and female brains treated with (C*) tat-SCR (n= 7) and (C**) tat-M2NX (n= 7) 20 min prior to MCAO. (E) Quantification of percentage of infarct volume in WT male and female mice treated with vehicle, 20 mg/kg tat-SCR, or 20 mg/kg tat-M2NX.
3.2. tat-M2NX reduces infarct volume in male brains, but not female brains
To investigate the effects of tat-M2NX on ischemic injury following experimental stroke, male and female WT mice were subjected to 60 min transient MCAO and total hemisphere infarct volume was analyzed. Either tat-M2NX or tat-SCR was injected 20 min prior to occlusion of the MCA and infarct volume was analyzed 24 h after reperfusion. MCA occlusion was similar in all groups tested, measured by laser Doppler flowmetry (Table 1) and importantly, no effect of tat-M2NX was observed on blood pressure (Supplemental Fig. 1). Males treated with tat-M2NX showed smaller infarct volume compared to tat-SCR (29.2 ± 4.6% [n = 8] vs. 43.4 ± 3.2% [n= 8; p < 0.01]), respectively (Fig. 1). In contrast, there was no difference in infarct size after stroke in female mice exposed to tat-SCR (46.6 ± 2.1% [n = 7]) or tat-M2NX (45.1 ± 2.1% [n = 7], Fig. 1). Together, these data demonstrate the ability of tat-M2NX to produce male-specific neuroprotection, as previously described using CTZ (Jia et al., 2011; Verma et al., 2012; Shimizu et al., 2013).
Table 1.
LDF and tympanic temperature.
| LDF (%) | Temperature (°C) | |||
|---|---|---|---|---|
| Just before reperfusion | Reperfusion 5 min | Rectal | Tympanic | |
| Male + tat-M2NX (n = 8) | 9 ± 1 | 76 ± 4 | 36.6 ± 0.2 | 36.8 ± 0.3 |
| Male + tat-SCR (n = 8) | 9 ± 1 | 72 ± 5 | 36.8 ± 0.1 | 36.6 ± 0.2 |
| Female + tat-M2NX (n = 7) | 7 ± 1 | 69 ± 3 | 36.7 ± 0.1 | 36.7 ± 0.2 |
| Female + tat-SCR (n = 7) | 6 ± 1 | 87 ± 10 | 36.8 ± 0.1 | 36.7 ± 0.1 |
| JAX WT male (n = 7) | 7 ± 1 | 70 ± 6 | 36.6 ± 0.1 | 36.8 ± 0.2 |
| JAX WT female (n = 7) | 10 ± 3 | 91 ± 10 | 36.4 ± 0.1 | 36.5 ± 0.1 |
| TRPM2 KO male (n = 7) | 12 ± 3 | 75 ± 5 | 36.6 ± 0.1 | 37.2 ± 0.2 |
| TRPM2 KO female (n = 6) | 8 ± 2 | 88 ± 9 | 36.3 ± 0.2 | 36.5 ± 0.3 |
| TRPM2 KO male + tat-M2NX (n = 5) | 12 ± 2 | 88 ± 14 | 36.7 ± 0.2 | 36.7 ± 0.1 |
| tat-M2NX 3 h 1 d (n = 7) | 8 ± 1 | 79 ± 4 | 36.6 ± 0.1 | 36.5 ± 0.2 |
| tat-SCR 3 h 1 d (n = 6) | 8 ± 2 | 82 ± 13 | 36.7 ± 0.2 | 36.7 ± 0.2 |
| tat-M2NX 3 h 4 d (n = 8) | 9 ± 1 | 91 ± 6 | 36.8 ± 0.1 | 36.8 ± 0.1 |
| tat-SCR 3 h 4 d (n = 8) | 9 ± 1 | 72 ± 3 | 36.9 ± 0.1 | 36.8 ± 0.1 |
Abbreviations: 1 d, 24 h reperfusion; 4 d, 96 h reperfusion; JAX; Jackson Laboratory, LDF, laser-Doppler flowmetry; MCAO, middle cerebral artery occlusion; TRPM2 KO, transient receptor potential M2 knockout mice; WT, wild type.
Values are mean ± SEM. No statistical differences were observed between groups using one-way ANOVA.
3.3. tat-M2NX provides no further protection compared to TRPM2 knockout
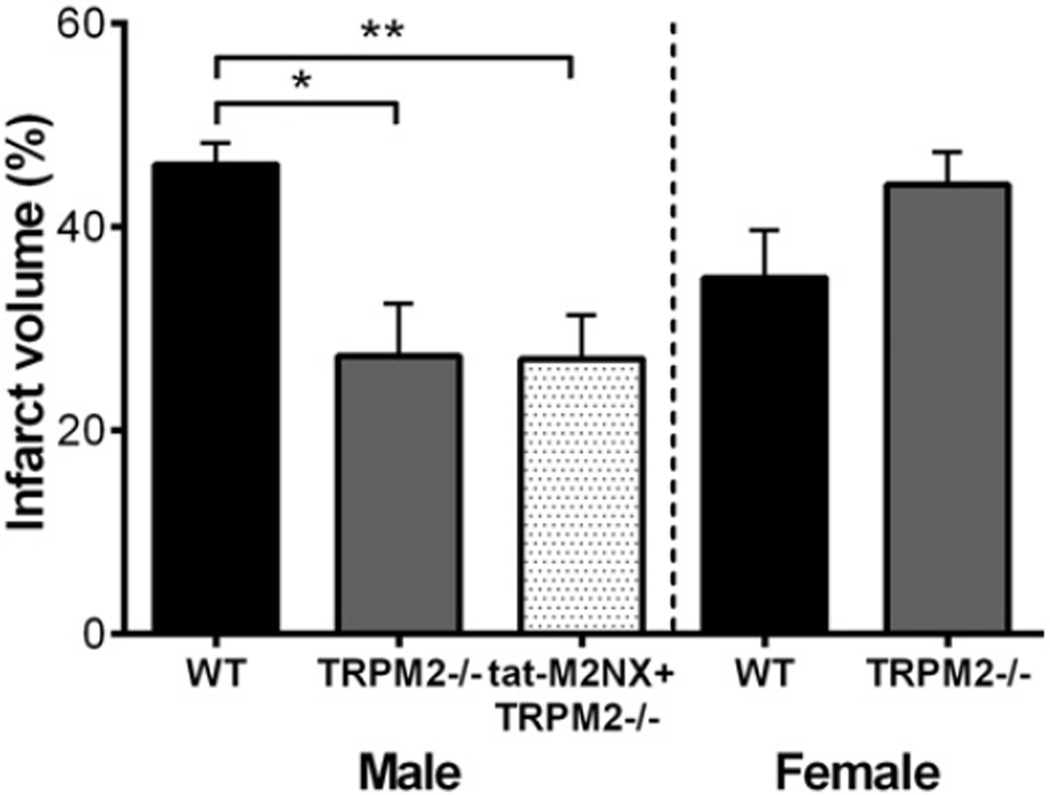
To further characterize the specificity of tat-M2NX for TRPM2 channels, we compared the neuroprotective efficacy of tat-M2NX inTRPM2 knock out mice (TRPM2−/−). Infarct volume was reduced in male TRPM2−/− compared to WT male mice, 27.3 ± 5.1% (n = 8) vs. 46.1±6.0% (n=7; p < 0.05), respectively (Fig. 2). There was no difference in infarct volume observed between WT and TRPM2−/− females, (35.0 ± 4.6% [n = 9] vs. 44.2 ± 3.2% [n = 6], Fig. 2). Administration of tat-M2NX did not further reduce infarct volume in male TRPM2−/−, providing further evidence of specificity for TRPM2 channels by tat-M2NX.
Fig. 2.
tat-M2NX does not protect TRPM2−/− mice following 60 min tMCAO. Quantification of percentage of infarct volume from WT and TRPM2−/− mice brains stained with TTC. TRPM2−/− male mice were treated with 20 mg/kg tat-M2NX peptide or vehicle.
3.4. tat-M2NX demonstrates a translation-relevant therapeutic window
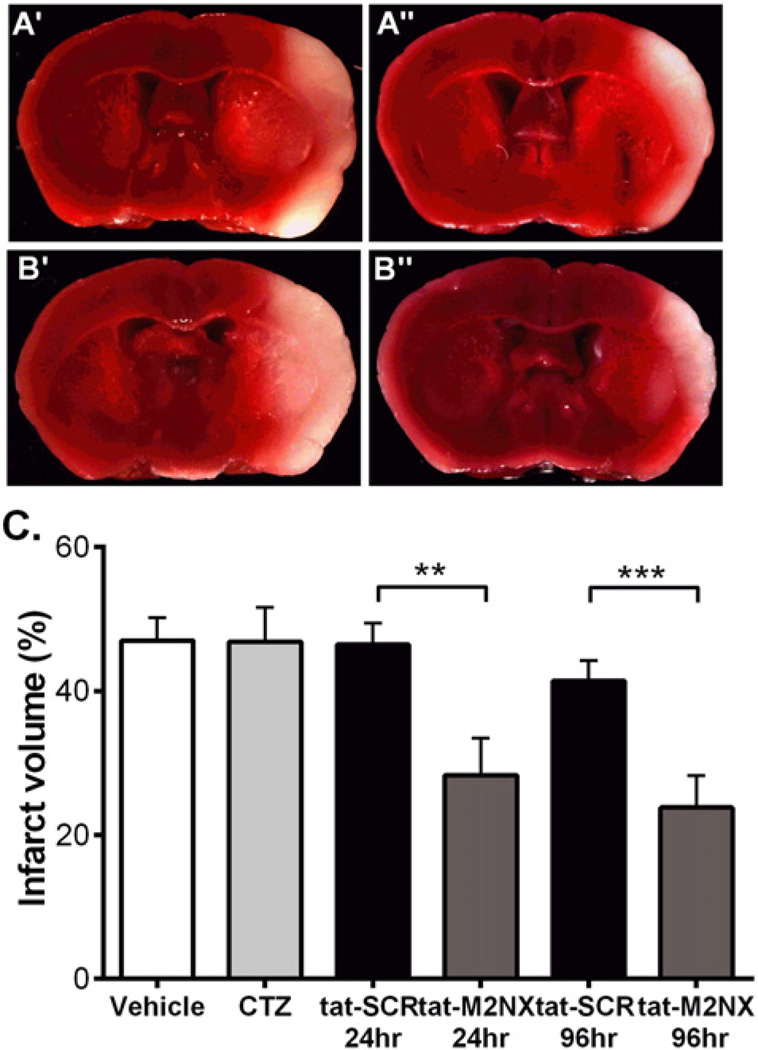
Clinical trials in stroke have failed for a variety of reasons, but many have failed due to a relevant therapeutic window (Neuhaus et al., 2014; Tymianski, 2014). Therefore, we tested whether tat-M2NX administered 3 h after reperfusion (4 h after onset of ischemia) in WT male mice can provide neuroprotection. tat-M2NX or tat-SCR was administered retro-orbitally 3 h post-reperfusion and the brain was collected at either 24 h or 96 h after injection to analyze the extent of infarct size. tat-M2NX significantly reduced infarct volume at 24 h and 96 h post-stroke compared to tat-SCR (Fig. 3, 24 h: tat-SCR 46.5 ± 7.3% (n = 6) vs. tat-M2NX 28.3 ± 13.5% (n = 7, p < 0.05); 96 h: tat-SCR 41.4 ± 8.0% (n = 8) vs. tat-M2NX 23.8 ± 12.5% (n = 8, p < 0.05)). In contrast, administration of the non-selective TRPM2 inhibitor clotrimazole (CTZ) 3 h after reperfusion had no effects on infarct volume (Fig. 3, vehicle 47.0 ± 9.9% vs. CTZ 46.8 ± 7.4%). This data suggests that not only is tat-M2NX neuroprotective, but also has a wider therapeutic window than CTZ and is similar to the clinical intervention of tPA.
Fig. 3.
tat-M2NX exhibits a clinically relevant therapeutic window. (A) Representative TTC staining of male mice treated with tat-SCR (A') or tat-M2NX (A") 3 h after reperfusion and stained 24 h after reperfusion and male mice treated with tat-SCR (B') or tat-M2NX (B") 3 h after reperfusion and stained 96 h after reperfusion. (C) Quantification of percentage of infarct volume in male and female brains stained with TTC 24 h and 96 h after receiving drug. Male and female mice were treated with vehicle, clotrimazole (CTZ), tat-SCR (20 mg/kg) or tat-M2NX (20 mg/kg).
3.5. tat-M2NX provides neuroprotection in aged male mice
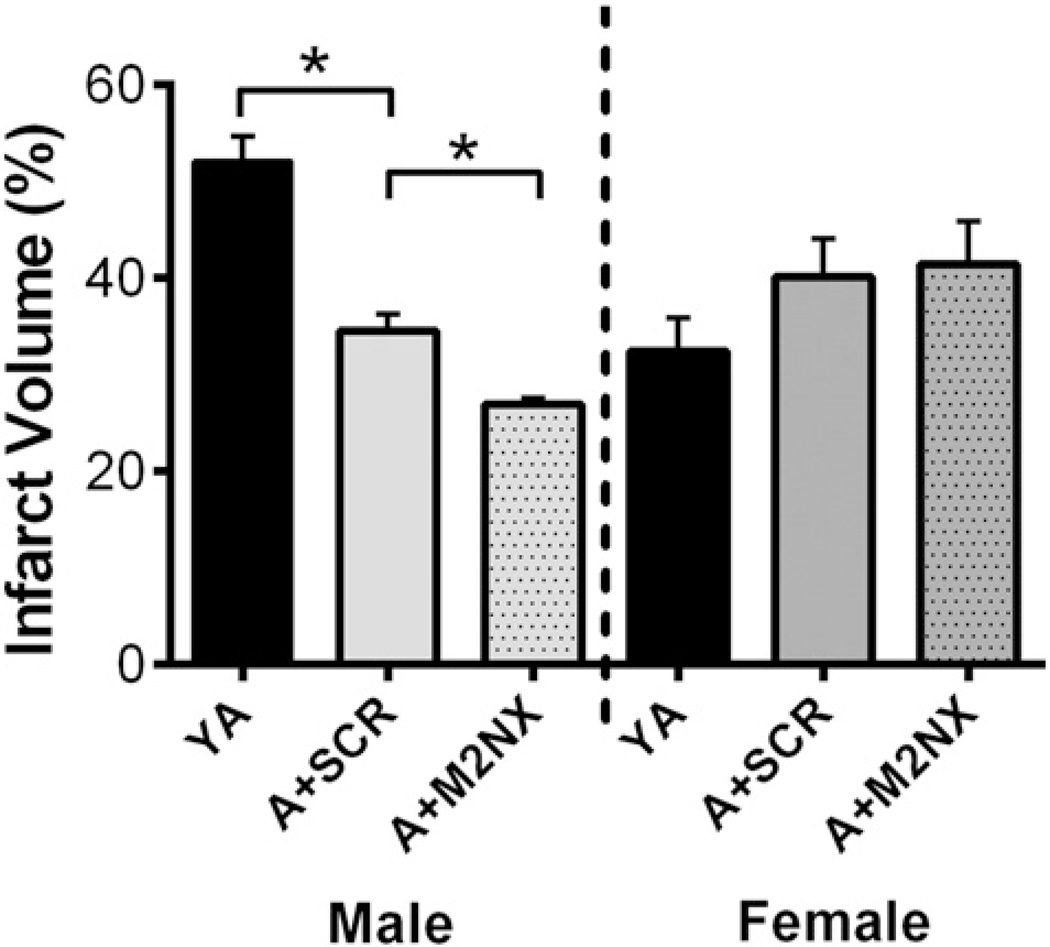
The incidence of stroke increases with age among all races and both sexes (Herson et al., 2009; Mozaffarian et al., 2015). Therefore, we tested whether tat-M2NX would provide protection in aged mice. 18–20 month-old male and female mice were subjected to 60 min MCAO and tat-M2NX or tat-SCR was administered 30min after reperfusion. Infarct volume was analyzed 24 h later. Consistent with the data from young adults, aged male mice given tat-M2NX had smaller infarct volumes compared to aged males given tat-SCR (27.0 ± 0.7% [n = 6] vs. 34.6±1.7% [n=6], respectively [Fig. 4]; p < 0.01). Interestingly, control aged male mice (tat-SCR treated) had smaller infarcts compared to young adult (YA) mice, consistent with previous reports (Liu et al., 2009a) (27.0 ± 0.7% [n = 6] vs. 52.1 ± 2.7% [n = 6], respectively [Fig. 4]). In contrast, tat-M2NX did not provide neuroprotection in aged females compared to tat-SCR (40.2 ± 4.0% [n = 6] vs. 41.5 ± 4.5% [n = 6], Fig. 4). Together, these data suggest that TRPM2 channels contribute to acute ischemic cell death following stroke in aged males, but not in females.
Fig. 4.
tat-M2NX provides neuroprotection in aged males. Quantification of percentage of infarct in 18–20 month old male and female mice receiving either tat-SCR (SCR) or tat-M2NX (M2NX). YA= young adult; A = aged. *p < 0.05.
4. Discussion
This study presents data showing that a novel inhibitor of TRPM2, tat-M2NX, can dramatically decrease infarct volume in males. Further, our data shows that the novel peptide has specificity for TRPM2 by way of blocking TRPM2-mediated Ca2+ entry in vitro and exhibiting a ceiling effect with TRPM2−/− mice. tat-M2NX appears to have a therapeutic window similar to that of tPA, giving hope that inhibiting TRPM2 channels with this novel peptide will have translational potential. To further the translational potential, our data indicate that tat-M2NX is effective in reducing infarct size in aged males, thereby targeting a population that has increased risk for focal ischemia.
TRPM2 channels have been implicated in ischemic neuronal damage for over a decade, yet the field has been plagued by lack of an inhibitor specific for the channel (Jiang et al., 2011). Here we present the characterization of a novel and specific peptide inhibitor of TRPM2 channels, tat-M2NX. The observation that tat-M2NX inhibits human TRPM2 channels presents translational intrigue and the possibility that this peptide could be used in clinical conditions. Currently, tat-bound peptides are being used in clinical trials (Hill and Fitch, 2012), representing a potential therapeutic strategy for human pathology. A distinguishing property of TRPM2 channels is that they are gated by adenosine-5′-diphosphoribose (ADPr), via binding to an ADPr hydrolase (term NUDT9-H) homology domain in the C-terminus (Perraud et al., 2001). The catalytic domain of NUDT9-H is the Nudix domain, which in coordination with several distant amino acids form the ADPr binding pocket (Shen et al., 2003; Kuhn and Luckhoff, 2004). We targeted the ADPr binding pocket as a strategy to inhibit channel activation, as mutations in this domain render the channel inactive (Kuhn and Luckhoff, 2004). Therefore, we generated a peptide (M2NX), fused to cell permeable tat sequence (tat 47–57) that inhibits TRPM2 channel activity via interaction with the ADPr binding pocket of the NUDT9-H domain of the channel. This rational design, coupled with our data showing a ceiling effect with TRPM2−/− mice, gives us great confidence in the ion channel specificity for TRPM2.
Current data using tat-M2NX is consistent with our previous studies using the less specific TRPM2 inhibitor CTZ or shRNA, demonstrating efficacy specifically in males. Interestingly, the current study uses a second TRPM2 KO mouse and observed the same male-specific protection observed previously (Shimizu et al., 2013), indicating the phenomena is independent of strain. Sex differences in outcomes following stroke are well described and emerging data suggests discrete cell death pathways triggered after cerebral ischemia in male or female animals (Herson et al., 2009; Siegel et al., 2010). Male mice demonstrate activation of cell death pathways predominantly mediated by excessive reactive oxygen species production and subsequent over-activation of poly(ADP-ribose) polymerase-1 (PARP-1) (Goto et al., 2002; Siegel et al., 2010). This pathway ultimately leads to the release of apoptosis-inducing factor following mitochondrial dysfunction, resulting in cell death. In contrast, cell death pathways in females involve caspase dependent apoptosis (Zhu et al., 2005; Zhu et al., 2006). The male-specific over-activation of PARP-1 in cell death following cerebral ischemia is particularly relevant to the data here, as PARP-1 generates adenosine-5′-diphosphoribose (ADPr), which goes on to activate TRPM2 channels (Perraud et al., 2001; Perraud et al., 2005; Hill et al., 2006; Eisfeld and Luckhoff, 2007; Buelow et al., 2008; Shimizu et al., 2013). We have taken advantage of this signaling pathway in designing tat-M2NX that targets the ADPr binding pocket of TRPM2, thereby inhibiting its activation. The possibility remains that the lack of effect in females could be due to inadequate dosing of tat-M2NX, though we have performed extensive studies using in vitro neuronal cultures and TRPM2 KO mice in combination with several known TRPM2 channel inhibitors and have never observed a protective effect in female cells or animals (Jia et al., 2011; Verma et al., 2012; Nakayama et al., 2013; Shimizu et al., 2013; Quillinan et al., 2014). Therefore, it is highly unlikely that the lack of protection observed in female mice in the current study using tat-M2NX is related to incorrect dosing. As such, the male-specific activity of tat-M2NX is consistent with previous data and entirely expected by the design of the peptide.
This study makes use of aged animals to demonstrate continued efficacy in older animals. Much has been written about the need for aged animal studies in preclinical studies (Herson and Traystman, 2014; Neuhaus et al., 2014), however relatively few studies report the interaction between age and treatment. This issue is particularly relevant given the changes in androgen levels at older ages (Gray et al., 1991) and the activation of TRPM2 channels by androgen related pathway (Shimizu et al., 2013). Therefore it may be speculated that low androgens in aged male mice would cause the loss of tat-M2NX neuroprotection. We assessed serum levels of testosterone and the higher potency dihydrotestosterone (DHT) across the mouse lifespan. Testosterone levels were decreased in older mice compared to young adults, however there was no change across ages in the higher potency dihydrotestosterone (DHT) (Supplemental Fig. 2), suggesting that DHT levels remain sufficiently high to engage TRPM2 channels in aged male mice. Despite this, our data, consistent with previous observations (Shapira et al., 2002; Liu et al., 2009b; Manwani et al., 2013), reveal that aged males have smaller infarcts than younger males. In contrast, aged female mice have larger infarcts than young females, as previously described (Liu et al., 2009b). Together, the data presented here represent an important pre-clinical study using a novel peptide to block TRPM2 channels and reduce infarct volume in males, but not females.
One of the major goals of ischemia research has been to develop neuroprotectants that could reduce ischemic damage. With the exception of recombinant tissue plasminogen activator, little preclinical research has translated into effective stroke therapies. It has been noted that many failures have been due to widespread use of young male animals and insufficient therapeutic windows (Neuhaus et al., 2014). Further, prior to recent advances in thrombectomy and revascularization strategies (Palaniswami and Yan, 2015), delivery of neuroprotectants after stroke was most likely non-efficacious. However, now that thrombectomy and revascularization are becoming standard in stroke treatment, reperfusion and oxidative stress targets such as TRPM2 are extremely attractive. Indeed, we report here in our transient focal ischemia model that tat-M2NX reduces injury after reperfusion. Importantly, we observed a relatively wide therapeutic window for tat-M2NX as a neuroprotectant (≥4 h). This is consistent with our understanding of the mechanism of TRPM2 channel regulation. Data clearly implicates NMDA receptor over-activation (excitotoxicity) as an early initiating event in ischemic injury, triggering a cascade of deleterious events, including oxidative stress and apoptosis. However, glutamate receptor blockade and antioxidant therapies have failed in clinical trials. Therefore, we and others have focused on downstream signaling pathways that may provide both improved specificity and wider therapeutic window of opportunity. Indeed, our data indicating a window of opportunity for our novel TRPM2 inhibitor (tat-M2NX) of at least 4 h is consistent with its role as an oxidative-stress activated signaling pathway downstream of NMDA receptor over-activation and consequent oxidative stress. This is equivalent to the guidelines for rTPA use published by the American Stroke Association (Powers et al., 2015). While there are some situations where rTPA can be given later (Powers et al., 2015), the data presented here are within the therapeutic window of the current standard of care, and likely relevant after endovascular thrombectomy. Interestingly, we report here that clotrimazole, a non-specific inhibitor of TRPM2, does not offer a similar therapeutic window, though we have shown previously that CTZ can provide protection when given up to 2 h after induction of MCAO (Shimizu et al., 2013). The mechanisms whereby CTZ has a shorter therapeutic window remain unknown. Nonetheless, the therapeutic window of tat-M2NX demonstrated here make tat-M2NX a potential therapeutic avenue for stroke.
5. Summary
The current study demonstrates that a specific inhibitor of TRPM2 channels can reduce stroke injury in a sexually dimorphic manner. The ability of tat-M2NX to inhibit a human homolog of TRPM2, combined with efficacy in aged animals and clinically relevant therapeutic window allows for optimism that this agent can someday be used in combination with other ischemia therapies to improve outcomes in people suffering from stroke. While this study does not address the important issues of comorbidities and behavioral outcomes that often complicate translation, our data provide important proof of concept for a novel strategy to combat stroke. As outlined above, we make use of both males and females to show that TRPM2 is an important contributor in male ischemic damage, but the same is not true for females. The majority of human clinical trials do not power to assess sex differences in efficacy and are therefore not investigated. Further, we show that tat-M2NX can effectively decrease infarct volume when given at least 4 h after onset of symptom. However, a potential weakness of the current study is the reliance on histological measurements to assess the protective efficacy of tat-M2NX. Future studies will need to assess the functional outcomes of such a therapeutic approach. As such, the current study provides important insights into the mechanisms of TRPM2-related cell death and begins to reveal strategies that may be targeted to reduce ischemic impairments.
Supplementary Material
Acknowledgments
We are grateful to Dr. Anne-Laure Perraud for graciously providing the TRPM2 expressing HEK cells. Project was supported by the following grants: NIH R01NS092645, NIHR01NS080851, AHA14GRNT18190012, NIH T32 HD 007186.
Footnotes
Conflict of interest
None.
Disclosures
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.expneurol.2016.06.015.
References
- Buelow B, Song Y, Scharenberg AM. The poly(ADP-ribose) polymerase PARP-1 is required for oxidative stress-induced TRPM2 activation in lymphocytes. J. Biol. Chem. 2008;283:24571–24583. doi: 10.1074/jbc.M802673200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, Lu A, Ran R, Graham SH, Chen J. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J. Neurosci. 2002;22:5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisfeld J, Luckhoff A. TRPM2. Handb. Exp. Pharmacol. 2007:237–252. doi: 10.1007/978-3-540-34891-7_14. [DOI] [PubMed] [Google Scholar]
- Goto S, Xue R, Sugo N, Sawada M, Blizzard KK, Poitras MF, Johns DC, Dawson TM, Dawson VL, Crain BJ, Traystman RJ, Mori S, Hurn PD. Poly(ADP-ribose) polymerase impairs early and long-term experimental stroke recovery. Stroke. 2002;33:1101–1106. doi: 10.1161/01.str.0000014203.65693.1e. [DOI] [PubMed] [Google Scholar]
- Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts male aging study. J. Clin. Endocrinol. Metab. 1991;73:1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- Herson PS, Traystman RJ. Animal models of stroke: translational potential at present and in 2050. Future Neurol. 2014;9:541–551. doi: 10.2217/fnl.14.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson PS, Koerner IP, Hurn PD. Sex, sex steroids, and brain injury. Semin. Reprod. Med. 2009;27:229–239. doi: 10.1055/s-0029-1216276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CA, Fitch RH. Sex differences in mechanisms and outcome of neonatal hypoxia-ischemia in rodentmodels: implications for sex-specific neuroprotection in clinical neonatal practice. Neurol. Res. Int. 2012;2012:867531. doi: 10.1155/2012/867531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Tigue NJ, Kelsell RE, Benham CD, McNulty S, Schaefer M, Randall AD. Characterisation of recombinant rat TRPM2 and a TRPM2-like conductance in cultured rat striatal neurones. Neuropharmacology. 2006;50:89–97. doi: 10.1016/j.neuropharm.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Jia J, Verma S, Nakayama S, Quillinan N, Grafe MR, Hurn PD, Herson PS. Sex differences in neuroprotection provided by inhibition of TRPM2 channels following experimental stroke. J. Cereb. Blood Flow Metab. 2011;31:2160–2168. doi: 10.1038/jcbfm.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LH, Gamper N, Beech DJ. Properties and therapeutic potential of transient receptor potential channels with putative roles in adversity: focus on TRPC5, TRPM2 and TRPA1. Curr. Drug Targets. 2011;12:724–736. doi: 10.2174/138945011795378568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. J. Gene Med. 2010a;12:561–563. doi: 10.1002/jgm.1473. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010b;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles H, Heizer JW, Li Y, Chapman K, Ogden CA, Andreasen K, Shapland E, Kucera G, Mogan J, Humann J, Lenz LL, Morrison AD, Perraud AL. Transient receptor potential melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11578–11583. doi: 10.1073/pnas.1010678108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn FJ, Luckhoff A. Sites of the NUDT9-H domain critical for ADP-ribose activation of the cation channel TRPM2. J. Biol. Chem. 2004;279:46431–46437. doi: 10.1074/jbc.M407263200. [DOI] [PubMed] [Google Scholar]
- Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke. 2009a;40:1842–1848. doi: 10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J. Cereb. Blood Flow Metab. 2009b;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp. Neurol. 2013;249:120–131. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics C., Stroke Statistics S. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Vest R, Traystman RJ, Herson PS. Sexually dimorphic response of TRPM2 inhibition following cardiac arrest-induced global cerebral ischemia in mice. J. Mol. Neurosci. 2013;51:92–98. doi: 10.1007/s12031-013-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus AA, Rabie T, Sutherland BA, Papadakis M, Hadley G, Cai R, Buchan AM. Importance of preclinical research in the development of neuroprotective strategies for ischemic stroke. JAMA Neurol. 2014;71:634–639. doi: 10.1001/jamaneurol.2013.6299. [DOI] [PubMed] [Google Scholar]
- Palaniswami M, Yan B. Mechanical thrombectomy is now the gold standard for acute ischemic stroke: implications for routine clinical practice. Interv. Neurol. 2015;4:18–29. doi: 10.1159/000438774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraud AL, Fleig A, Dunn CA, Bagley LA, Launay P, Schmitz C, Stokes AJ, Zhu Q, Bessman MJ, Penner R, Kinet JP, Scharenberg AM. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, Knowles HM, Ferraris D, Li W, Zhang J, Stoddard BL, Scharenberg AM. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J. Biol. Chem. 2005;280:6138–6148. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, Johnston KC, Johnston SC, Khalessi AA, Kidwell CS, Meschia JF, Ovbiagele B, Yavagal DR American Heart Association Stroke C. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–3035. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- Quillinan N, Grewal H, Klawitter J, Herson PS. Sex steroids do not modulate TRPM2-mediated injury in females following middle cerebral artery occlusion(1,2,3) eNeuro. 2014;1 doi: 10.1523/ENEURO.0022-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero JR, Morris J, Pikula A. Stroke prevention: modifying risk factors. Ther. Adv. Cardiovasc. Dis. 2008;2:287–303. doi: 10.1177/1753944708093847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira S, Sapir M, Wengier A, Grauer E, Kadar T. Aging has a complex effect on a rat model of ischemic stroke. Brain Res. 2002;925:148–158. doi: 10.1016/s0006-8993(01)03270-x. [DOI] [PubMed] [Google Scholar]
- Shen BW, Perraud AL, Scharenberg A, Stoddard BL. The crystal structure and mutational analysis of human NUDT9. J. Mol. Biol. 2003;332:385–398. doi: 10.1016/s0022-2836(03)00954-9. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Macey TA, Quillinan N, Klawitter J, Perraud AL, Traystman RJ, Herson PS. Androgen and PARP-1 regulation of TRPM2 channels after ischemic injury. J. Cereb. Blood Flow Metab. 2013;33:1549–1555. doi: 10.1038/jcbfm.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Quillinan N, Orfila JE, Herson PS. Sirtuin-2 mediates male specific neuronal injury following experimental cardiac arrest through activation of TRPM2 ion channels. Exp. Neurol. 2016;275(Pt 1):78–83. doi: 10.1016/j.expneurol.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel C, Turtzo C, McCullough LD. Sex differences in cerebral ischemia: possible molecular mechanisms. J. Neurosci. Res. 2010;88:2765–2774. doi: 10.1002/jnr.22406. [DOI] [PubMed] [Google Scholar]
- Soriano FX, Martel MA, Papadia S, Vaslin A, Baxter P, Rickman C, Forder J, Tymianski M, Duncan R, Aarts M, Clarke P, Wyllie DJ, Hardingham GE. Specific targeting of pro-death NMDA receptor signals with differing reliance on the NR2B PDZ ligand. J. Neurosci. 2008;28:10696–10710. doi: 10.1523/JNEUROSCI.1207-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymianski M. Stroke in 2013: disappointments and advances in acute stroke intervention. Nat. Rev. Neurol. 2014;10:66–68. doi: 10.1038/nrneurol.2013.271. [DOI] [PubMed] [Google Scholar]
- Verma S, Quillinan N, Yang YF, Nakayama S, Cheng J, Kelley MH, Herson PS. TRPM2 channel activation following in vitro ischemia contributes to male hippocampal cell death. Neurosci. Lett. 2012 doi: 10.1016/j.neulet.2012.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf PA, D'Agostino RB, O'Neal MA, Sytkowski P, Kase CS, Belanger AJ, Kannel WB. Secular trends in stroke incidence and mortality. The Framingham study. Stroke. 1992;23:1551–1555. doi: 10.1161/01.str.23.11.1551. [DOI] [PubMed] [Google Scholar]
- Zhu C, Wang X, Xu F, Bahr BA, Shibata M, Uchiyama Y, Hagberg H, Blomgren K. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 2005;12:162–176. doi: 10.1038/sj.cdd.4401545. [DOI] [PubMed] [Google Scholar]
- Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J. Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.