Abstract
Background
A common mechanism of resistance to beta-lactam antibiotics is the production of beta-lactamase by Gram-negative bacteria. Recently, nonderivative extended-spectrum beta-lactamases (ESBLs) from the TEM and SHV enzymes, such as CTX-M, that were related to different geographical regions have been recognized.
Objectives
The aim of this study was to determine the frequency of the CTX-M gene in ESBL-producing Enterobacteriaceae isolates in hospitalized patients in the teaching hospitals of Ahvaz, Iran.
Methods
Enterobacteriaceae isolates from clinical specimens (other than stool), such as wounds, blood, urine, trachea, discharge, and abscess, were collected and examined. All the isolates were identified using standard biochemical tests. The combination test was carried out based on CLSI criteria for the phenotypic detection of ESBL-producing isolates. After DNA extraction, the CTX-M and CTX-M-1 genes were amplified using PCR among phenotypically positive ESBL isolates.
Results
Among 240 Enterobacteriaceae isolates, Escherichia coli and Enterobacter were the most common isolates with 171 (71.3%) and 65 (27.1%), respectively. The combination test results also showed that 108 (45%) Enterobacteriaceae isolates were phenotypic ESBL producers, but 104 (96%) isolates were positive for the blaCTX-M gene and 99 (92%) were positive for the blaCTX-M-1 gene according to the PCR method.
Conclusions
The results of this study phenotypically and genotypically confirmed the high frequency of ESBL-producing strains, such as the CTX-M and CTX-M-1 genes, among Enterobacteriaceae isolates in our region. Therefore, use of antibiotic susceptibility testing for the detection of ESBL isolates prior to the prescription of beta-lactam antibiotics is recommended. This could help prevent the spread of bacteria strains that are resistant to beta-lactam antibiotics.
Keywords: Extended-Spectrum Beta-Lactamase, Polymerase Chain Reaction, CTX-M, Enterobacteriaceae
1. Introduction
Beta-lactam has been an efficient antibiotic that has been frequently used for the treatment of infections for many years. However, bacterial resistance to this antibiotic has increased gradually due to the transfer of beta-lactamase-encoding genes, especially through conjugation. This has recently caused the resistance of bacteria to beta-lactam antibiotics (1, 2). Extended-spectrum beta-lactamases (ESBLs) are a group of beta-lactamase enzymes that are able to hydrolyze oximinocephalosporins (3). Two of these enzymes are TEM and SHV. They are productions of point mutations in the main inactive extended-spectrum enzymes. In recent years, nonderivative beta-lactamases of TEM and SHV have been reported, and most of them have been CTX-M enzymes (4-6).
In 1989, the CTX-M family of ESBLs was reported in Germany for the first time, and later, these ESBLs spread to different parts of the world. The CTX-M enzyme has been found frequently in Escherichia coli and Klebsiella, but it has also been reported in other Enterobacteriaceae species (3). Generally, plasmids are responsible for the gene development of beta-lactamases among similar or different Enterobacteriaceae strains. Some strains of Enterobacteriaceae produce beta-lactamases that are encoded by chromosomes and that could cause antibiotic resistance (7, 8).
Studies have indicated that more than 60 types of CTX-M enzymes have been identified. These enzymes are categorized into five main groups based on amino acid variations (9). In comparison to TEM and SHV beta-lactamases, CTX-M has a more harmful effect on cefotaxime and ceftriaxone than ceftazidime. The CTX-M enzyme is inhibited by beta-lactamases, such as clavulanic acid, sulbactam, and tazobactam (10, 11).
2. Objectives
Since blaCTX-M and blaCTX-M-1 are common genes among ESBL producers (12), this study aimed to investigate the frequency of the beta-lactamase CTX-M gene in Enterobacteriaceae strains that have been isolated from hospitalized patients in the teaching hospitals of Ahvaz, Iran. The results of this study could help physicians choose more effective antibiotics for treating patients.
3. Methods
3.1. Bacteria Isolation from Specimens
In this study, Enterobacteriaceae isolates were collected from the Imam Khomeini and Golestan teaching hospitals in Ahvaz, Iran over the course of 11 months from April 2012 to February 2013. These bacteria were isolated from wounds (n = 5), blood (n = 10), urine (n = 210), trachea (n = 10), and fluids (n = 2) and were transported to the microbiology lab at the school of medicine. The isolates were identified using standard biochemical tests, such as triple sugar iron agar, lysine urea, SIM, MR-VP, citrate, and gas production testing (all the culture mediums were provided by Merck, Germany) (13).
3.2. Phenotypic ESBLs with The Combination Disk Method
The ESBL-producing Enterobacteriaceae were identified using the phenotypic combination disk method based on CLSI directions (13). With this method, combination disks of cefotaxime-clavulanic acid (CTX30-CA10) and ceftazidime-clavulanic acid (CAZ30-CA10) with single disks of cefotaxime and ceftazidime (Mast company, England) in Mueller-Hinton agar (Merck, Germany) were used. First, a microbe suspension equal with the McFarland 0.5 standard was prepared and was thoroughly spread in the culture medium. Then, paper disks of the antibiotics were placed on the plate in 2.5 cm apart from each other. The plates were incubated at 35°C for 18 - 24 hours. Then, the bacterial sensitivity or resistance to the antibiotics were examined. If the inhibition zone around the CAZ-CA and CTX-CA combination disks were at least 5 mm more than the single disks, a tested isolate was considered to be an ESBL-producing strain (Figure 1).
Figure 1. ESBL-Producing Enterobacteriaceae Isolates that Were Phenotypically, Combination Disk Method.
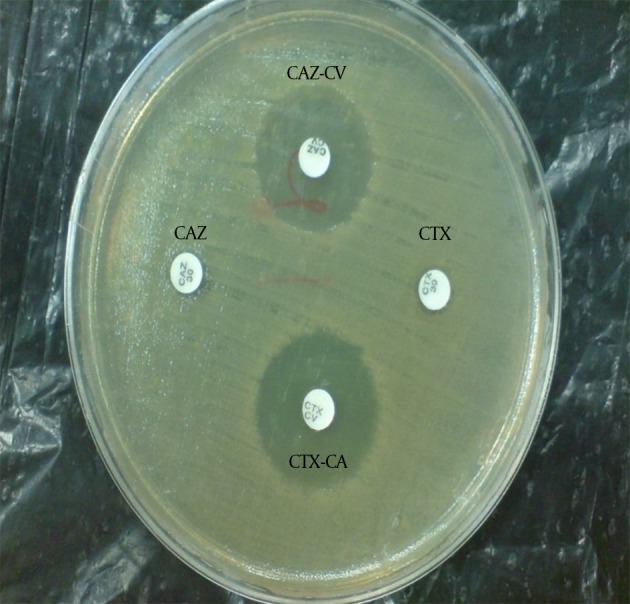
CAZ, ceftazidime; CAZ-CV, ceftazidime-clavulanic acid; CTX, cefotaxime; CTX-CV, cefotaxime-clavulanic acid; No growth inhibition zone around the single disk of ceftazidime and cefotaxime indicates bacterial resistance. The growth inhibition zone around the ceftazidime and cefotaxime disks in combination with clavulanic acid shows the sensitivity of the bacteria to the combination disks and confirms the production of ESBLs by the isolate.
3.3. DNA Extraction and PCR of the CTX-M and CTX-M-1 Genes
DNA extraction was performed using the boiling method (14). In this method, three to four colonies of positive phenotypic strains were suspended in microtubes with 500 µL of sterile distilled water. They were placed in a thermo black machine for ten minutes at 100°C. Then, the samples were centrifuged for 10 minutes at 4°C and 12000 rpm. In addition, 200 µL of the surface fluid was separated in a sterile condition and was stored in Eppendorf micro tubes at -20°C. The concentration of the extracted DNA was measured using a BioPhotometer (Eppendorf, Germany).
The PCR reactions were carried out using two pairs of primers: CTX-M and CTX-M-1 genes (microbiology biotechnology gene). The sequences of these primers are presented in Table 1. For each PCR reaction, a master mix of 25 µL that included 2.5 µL of PCR buffer 10X, 0.5 µL of 10 mM dNTP mix, 0.75 µL of 50 mM MgCl2, 0.2 µL of 5u/µL Taq DNA polymerase, 2 µL of template DNA, 2 µL of 10 µM F and R primers, and 17.05 µL of distilled water was prepared (15, 16).
Table 1. Primer Sequences of the ESBL Genes Amplified By PCR.
The best conditions for the PCR reaction were recognized in a pilot method by performing multiple repetitions of the test and concentration changes and the temperature gradient in a Master Cycler (Ependorff, Germany). The program of gene amplification in the Master Cycler consisted of an initial denaturation at 94°C for 5 minutes, annealing at 55°C for 45 seconds, 30 cycles, an extension at 72°C for 1 minute, 30 cycles, and a final extension for and 5 minute, one cycle.
3.4. PCR Production Electrophoresis
PCR production was evaluated using 1.2% gel agarose with the addition of 0.5 µg/mL ethidium bromide (CinaGen Co., Iran). The photography of the related gel was carried out using Gel documentation (Protein Simple Co., US). The standard strains of E. coli ATCC 25922 and an E. coli strain possessing the CTX-M gene, which was determined by sequencing, were used as negative and positive controls, respectively.
4. Results
The isolation results of the different types of Enterobacteriaceae isolates are summarized in Table 2. Based on these results, the most frequent isolates were E. coli (71.3%), Enterobacter (27%), and Klebsiella (1.2%). In addition, the phenotypic investigation of the ESBLs in the 240 Enterobacteriaceae isolates indicated that 108 isolates (45%) were producers of ESBLs and that most of these producers (79 isolates) were E. coli (Table 3). However, the PCR results confirmed that 104 strains of the ESBL-producing isolates (96%) had the CTX-M gene and 4 isolates did not have this gene.
Table 2. Frequency of Enterobacteriaceae Species Isolated From Examined Clinical Specimens.
| Bacterial Names | No. (%) of Isolates |
|---|---|
| E. coli | 171 (71.3) |
| K. pneumoniae | 2 (0.8) |
| K. oxytoca | 1 (0.4) |
| E. aerogenes | 19 (8) |
| E. cloacae | 5 (2) |
| E. taylore | 2 (0.8) |
| E. intermedium | 1 (0.4) |
| E. gergoviae | 38 (15.9) |
| C. freundii | 1 (0.4) |
| Total | 240 (100) |
Table 3. Frequency of Phenotypic ESBL-Positive and ESBL-Negative Enterobacteriaceae Isolated from Clinical Specimens.
| Name | ESBL+, No. (%) | ESBL-, No. (%) | Total Isolates, No. (%) |
|---|---|---|---|
| E. coli | 79 (46.1) | 92 (53.9) | 171 (100) |
| K. pneumoniae | 0 (0) | 1 (100) | 1 (100) |
| K. oxytoca | 2 (100) | 0 (0) | 2 (100) |
| E. aerogenes | 8 (42.1) | 11 (57.9) | 19 (100) |
| E. cloacae | 5 (100) | 0 (0) | 5 (100) |
| E. taylore | 0 (0) | 2 (100) | 2 (100) |
| E. intermedium | 0 (0) | 1 (100) | 1 (100) |
| E. gergoviae | 14 (36.9) | 24 (63.1) | 38 (100) |
| C. freundii | 0 (0) | 1 (100) | 1 (100) |
| Total | 108 (45) | 132 (55) | 240 (100) |
Our results also showed that among 108 positive phenotype ESBL isolates, 99 isolates (92%) had the CTX-M-1 gene and 9 isolates lacked this gene according to the results of the PCR method. The electrophoresis of the PCR products for the CTX-M and CTX-M-1 genes are presented in the Figures 2 and 3.
Figure 2. The PCR Products of Electrophoresis for CTX-M Genes from Enterobacteriaceae Isolates.
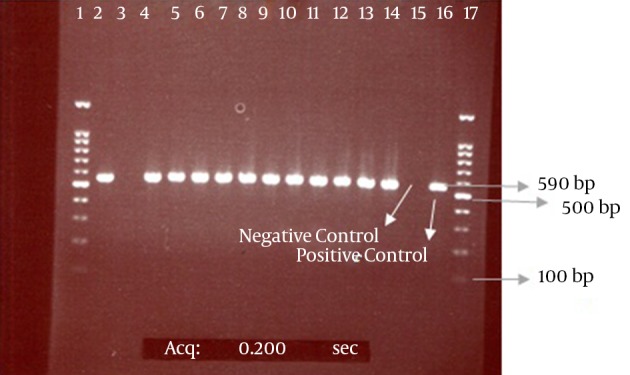
The bond 590 bp is related to the CTX-M beta-lactamase gene. Lanes 1 and 17 contain a 100-bp DNA size marker; Lane 3, CTX-M-negative isolate; Lanes 2 and 4 - 14, CTX-M-positive isolates; Lanes 15 and 16, negative (E. coli ATCC 25922) and positive (E. coli strain possessing the CTX-M gene, which was determined by sequencing) controls, respectively.
Figure 3. The PCR Products of Electrophoresis for CTX-M-1 Genes from Enterobacteriaceae Isolates.
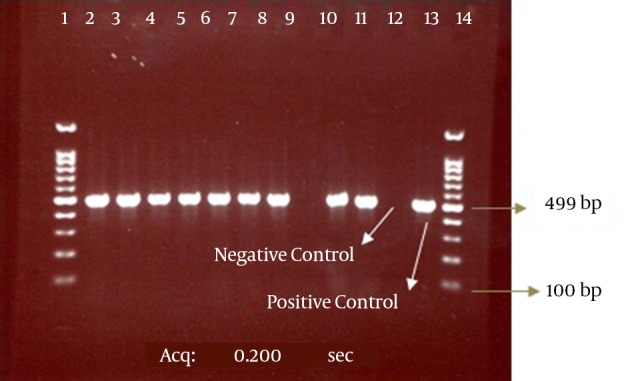
The bond 499 bp is related to the CTX-M-1 beta-lactamase gene. Lanes 1 and 14, DNA size marker; Line 9, CTX-M-1-negative isolate; Lanes 2 - 8, 10, and 11, CTX-M-1-positive isolates; Lanes 12 and 13, negative (E. coli ATCC 25922) and positive (E. coli strains possessing the CTX-M-1 gene, which was determined by sequencing) controls, respectively.
5. Discussion
Beta-lactam antibiotics are the first medicine option for the treatment of infections due to the Gram-negative bacilli of Enterobacteriaceae. However, in the past decade, bacterial resistance to these antibiotics has increased (17, 18). The main mechanism of resistance among Enterobacteriaceae members to beta-lactam antibiotics is the production of beta-lactamases enzymes (18). ESBL enzymes were separated from Klebsiella pneumoniae for the first time in Germany in 1983, but today, nasocomial infections due to infections by ESBL-producing organisms have developed all over the world (19, 20).
Among the ESBLs produced by Enterobacteriaceae isolates, the CTX-M family has had the most destructive impact on cefotaxime and ceftazidime. The appropriate phenotypic method for the detection of beta-lactamase-producing bacteria is the combination disk method according to CLSI directions. With this method, beta-lactamase activity is inhibited by clavulanic acid, so the growth inhibition zone around the combination disk is increased to a single antibiotic disk, which is a criteria for the detection of ESBL isolates (13). The results of our study on Enterobacteriaceae isolates showed that 108 isolates (45%) were phenotypic ESBL producers, and the most frequent producers belonged to the Klebsiella (66.6%), E. coli (46.1%), and Enterobacter (41.5%) isolates.
Some studies in different countries reported various frequencies of ESBLs among Enterobacteriaceae isolates. For instance, a study carried out on Enterobacteriaceae isolates by Luzzaro et al. (2006) in Italy showed that 7.4% of isolates were phenotypic ESBL producers and that the most frequent ESBL producers were E. coli strains (31.9%) (21). In addition, in a study on Enterobacteriaceae isolates in Pakistan, Riaz et al. (2011) showed that among 1018 isolates, 300 (29.5%) isolates were phenotypic ESBL producers and that the frequency of E. coli and Klebsiella among these ESBL producers was (39.3%) and (26.1%), respectively (22).
Studies have also been conducted on this subject in Iran. In a study in Sannandaj, Ramezanzadeh et al. (2010) revealed that 14.5% of Gram-negative bacilli were ESBL-producing isolates (23). In addition, in a study carried out by Moosavian et al. (2012) in Dezful, Iran, the ESBL rate in Enterobacteriaceae isolates was 30.5%, and the prevalence of K. pneumoniae and E. coli among the ESBL producers in this study was 45.4% and 28.8%, respectively (24). Moreover, in a study on Klebsiella isolates in Tehran, Nasehi et al. (2010) showed that the ESBL frequency was 38.5 %, which was lower than the present study’s result (25).
The results discrepancy between the present study and these other studies might be due to the consumption patterns of antibiotics, particularly cephalosporins; the number of antibiotic prescriptions; and the differences in the time periods during which the Enterobacteriaceae isolates were collected (4). The results of our study showed that among 108 phenotypically positive Enterobacteriaceae isolates, 104 isolates (96%) had the CTX-M gene and 4 isolates did not have this gene. The frequency of the CTX-M gene in Klebsiella was 66.6%, but in E. coli and Enterobacter, this frequency was 46.1% and 41.5%, respectively. In addition, among 108 phenotypically positive Enterobacteriaceae isolates, 99 (92%) isolates had the CTX-M-1 gene and 5 isolates lacked the gene. The nonexistence of the CTX-M and CTX-M-1 genes in strains that were phenotypically positive in the combination disk test revealed that these strains can have other ESBL enzymes, such as TEM and SHV (26).
Maina et al. (2012) reported that among 52 phenotypically positive Enterobacteriaceae isolates in Kenya, 46 (88.5%) had the CTX-M gene but 6 isolates lacked the gene (26). The reported frequencies of this gene in was 96% for E. coli and 79% for K. pneumoniae, which are consistent with the results of the present study in Ahvaz. Furthermore, the frequency of the CTX-M gene in Peirano et al.’s study on E. coli isolates in Turkey was reported to be 63.9%. (27). In Iran, there also have been studies that indicated the frequency of ESBL-producing Enterobacteriaceae isolates, such as CTX-M.
Soltan Dalal et al. (2011) studied 188 isolates of E. coli strains collected from Tabriz hospitals in Iran which indicated 84.1% of strains were positive for CTX-M-1 (28). The results of studies by Shahcheraghi et al. (2010) and Mirzaiee et al. (2009) concerning isolates from Tehran reported the frequencies of the CTX-M gene in E. coli isolates to be 20.8% and 35.7%, respectively (16, 29). Although cephalosporins are effective for treating Enterobacteriaceae infections, different studies have indicated the regionally high frequency of ESBLs, especially CTX-M. Since CTX-M genes are associated with the appended sequential of ISEcp1 and since these sequences are responsible for the movement and expression of the CTX-M gene, the transfer and movement of appended ISEcp1 sequences between different strains motivates the expression of the CTX-M gene and the subsequent epidemic development of ESBLs (30). The transferability of these genes from animal sources to humans is considered to be one of the important causes of the high prevalence of the CTX-M gene (31).
5.1. Conclusion
The results of this study phenotypically and genotypically confirmed the high frequency of ESBL-producing strains among Enterobacteriaceae isolates, such as the CTX-M and CTX-M-1 genes, in our region. Therefore, conducting antibiotic susceptibility tests for the detection of ESBL isolates prior to prescribe of beta-lactam antibiotics is recommended. Conducting these tests could help prevent the spread of strains that are resistant to beta-lactam antibiotics.
Acknowledgments
This study was part of an approved research plan (No. B-9101 by Arvand international branch, Jundishapur University of Ahvaz, Iran). We are grateful to the head and staff of the research center of Arvand branch and the infectious and tropical diseases research center, Jundishapur University of Ahvaz.
References
- 1.Ambler RP. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980;289(1036):321–31. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 2.Medeiros A, Mayer K, Opal SM. Plasmid Beta-lactamases. AntimicrobiolNewsl. 1988;5:61–5. [Google Scholar]
- 3.Bush K. Classification of beta-lactamases: groups 1, 2a, 2b, and 2b'. Antimicrob Agents Chemother. 1989;33(3):264–70. doi: 10.1128/aac.33.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Agamy MH, Shibl AM, Tawfik AF. Prevalence and molecular characterization of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in Riyadh, Saudi Arabia. Ann Saudi Med. 2009;29(4):253–7. doi: 10.4103/0256-4947.55306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamudha PR, Srinivas AN, Rahul D, Harish BN, Parija SC. Molecular epidemiology of Multidrug resistant Extended-Spectrum β-Lactamase Producing Klebsiella pneumoniae outbreak in a neonatal intensive care unit. IJCRIMPH. 2010;2(7):226–38. [Google Scholar]
- 6.Munier GK, Johnson CL, Snyder JW, Moland ES, Hanson ND, Thomson KS. Positive extended-spectrum-beta-lactamase (ESBL) screening results may be due to AmpC beta-lactamases more often than to ESBLs. J Clin Microbiol. 2010;48(2):673–4. doi: 10.1128/JCM.01544-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnet R. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother. 2004;48(1):1–14. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford PA. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14(4):933–51. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monstein HJ, Tarnberg M, Nilsson LE. Molecular identification of CTX-M and blaOXY/K1 beta-lactamase genes in Enterobacteriaceae by sequencing of universal M13-sequence tagged PCR-amplicons. BMC Infect Dis. 2009;9:7. doi: 10.1186/1471-2334-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelstein M, Pimkin M, Palagin I, Edelstein I, Stratchounski L. Prevalence and molecular epidemiology of CTX-M extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrob Agents Chemother. 2003;47(12):3724–32. doi: 10.1128/AAC.47.12.3724-3732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govinden U, Mocktar C, Moodley P, Stum AW, Essack SY. Geographical evolution of the CTX-M-lactamase an update. African J Biotech. 2007;6(7):831–9. [Google Scholar]
- 12.Valenzuela de Silva EM, Mantilla Anaya JR, Reguero Reza MT, Gonzalez Mejia EB, Pulido Manrique IY, Dario Llerena I, et al. Detection of CTX-M-1, CTX-M-15, and CTX-M-2 in clinical isolates of Enterobacteriaceae in Bogota, Colombia. J Clin Microbiol. 2006;44(5):1919–20. doi: 10.1128/JCM.44.5.1919-1920.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CLSI. Performance standards for antimicrobialsusebtibility testing:20th information supplement , document M100-S20. wayne: Clinical and Laboratory Standards Institue; 2010. [Google Scholar]
- 14.Sambrook MJ, Russel DW. Molecular colning Laboratory manual . 3 ed. Vol. 2. NewYork: CSHL Press; 2001. [Google Scholar]
- 15.Amaral SN, Peixe LV, Machado E. Characterization of ctx-m-type extended-spectrum beta-lactamases (esbls) among enterobacteriaceae from a portuguese hospital. 2009;6:254–63. [Google Scholar]
- 16.Mirzaee M, Pourmand MR, Chitsaz M, Mansouri S. Antibiotic resistance to third generation cephalosporins due to CTX-M-Type extended-spectrum β-lactamases in clinical isolates of Escherichia coli. I J Public Health. 2009;38(1):10–7. [Google Scholar]
- 17.Rossolini GM, D'Andrea MM, Mugnaioli C. The spread of CTX-M-type extended-spectrum beta-lactamases. Clin Microbiol Infect. 2008;14 Suppl 1:33–41. doi: 10.1111/j.1469-0691.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 18.Livermore DM. beta-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;8(4):557–84. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canton R, Novais A, Valverde A, Machado E, Peixe L, Baquero F, et al. Prevalence and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Europe. Clin Microbiol Infect. 2008;14 Suppl 1:144–53. doi: 10.1111/j.1469-0691.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 20.Philippon A, Arlet G, Lagrange PH. Origin and impact of plasmid-mediated extended-spectrum beta-lactamases. Eur J Clin Microbiol Infect Dis. 1994;13 Suppl 1:S17–29. doi: 10.1007/BF02390681. [DOI] [PubMed] [Google Scholar]
- 21.Luzzaro F, Mezzatesta M, Mugnaioli C, Perilli M, Stefani S, Amicosante G, et al. Trends in production of extended-spectrum beta-lactamases among enterobacteria of medical interest: report of the second Italian nationwide survey. J Clin Microbiol. 2006;44(5):1659–64. doi: 10.1128/JCM.44.5.1659-1664.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riaz S, Faisal M, Hasnain S. Prevalence and comparison of Beta-lactamase producing Escherichia coli and Klebsiella spp from clinical and environmental sources in Lahore, Pakistan. African J Microbiol Res. 2012;6(2):465–70. [Google Scholar]
- 23.Ramazanzadeh R. Etiologic agents and extended-spectrum beta-lactamase production in urinary tract infections in Sanandaj, Iran. Eastern J Med. 2010;15(2):57–62. [Google Scholar]
- 24.Moosavian M, Deiham B. Distribution of TEM, SHV and CTX-M Genes among ESBL-producing Enterobacteriaceae isolates in Iran. African J Microbiol Res. 2012;6(26):5433–9. [Google Scholar]
- 25.Nasehi L, Shahcheraghi F, Nikbin V, Nematzadeh S. PER, CTX-M, TEM and SHV Beta-lactamases in clinical isolates of Klebsiella pneumoniae isolated from Tehran, Iran. Iranian J Basic Med Sci. 2010;13(3):111–8. [Google Scholar]
- 26.Maina D, Revathi G, Kariuki S, Ozwara H. Genotypes and cephalosporin susceptibility in extended-spectrum beta-lactamase producing enterobacteriaceae in the community. J Infect Dev Ctries. 2012;6(6):470–7. doi: 10.3855/jidc.1456. [DOI] [PubMed] [Google Scholar]
- 27.Peirano G, Pitout JD. Molecular epidemiology of Escherichia coli producing CTX-M beta-lactamases: the worldwide emergence of clone ST131 O25:H4. Int J Antimicrob Agents. 2010;35(4):316–21. doi: 10.1016/j.ijantimicag.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Soltan Dallal MM, Shirazi MH. The prevalence of extended-spectrum beta-lactamases and CTX-M-1 producing Escherichia coli in urine samples collected at Tabriz city Hospitals. Tehran Univ Med J TUMS Publ. 2011;69(5):273–8. [Google Scholar]
- 29.Shahcheraghi F, Nasiri S, Noveiri H. The Survey of Genes Encoding Beta-Lactamases, in Escherichia Coli Resistant to Beta-Lactam and Non-Beta-Lactam Antibiotics. Iran J Basic Med Sci. 2010;13(1):230–7. [Google Scholar]
- 30.Bertrand S, Weill FX, Cloeckaert A, Vrints M, Mairiaux E, Praud K, et al. Clonal emergence of extended-spectrum beta-lactamase (CTX-M-2)-producing Salmonella enterica serovar Virchow isolates with reduced susceptibilities to ciprofloxacin among poultry and humans in Belgium and France (2000 to 2003). J Clin Microbiol. 2006;44(8):2897–903. doi: 10.1128/JCM.02549-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poirel L, Decousser JW, Nordmann P. Insertion sequence ISEcp1B is involved in expression and mobilization of a bla(CTX-M) beta-lactamase gene. Antimicrob Agents Chemother. 2003;47(9):2938–45. doi: 10.1128/AAC.47.9.2938-2945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


