Abstract
Sustained abstinence from cocaine use is frequently compromised by exposure to environmental stimuli that have previously been strongly associated with drug taking. Such cues trigger memories of the effects of the drug, leading to craving and potential relapse. Our work has demonstrated that manipulating cocaine-cue memories by destabilizing them through interfering with the reconsolidation process is one potential therapeutic tool by which to prolong abstinence. Here, we examine the use of the naturally occurring amnestic agent garcinol to manipulate an established cocaine-cue memory. Rats underwent 12 days of cocaine self-administration training during which time active lever presses resulted in an i.v. infusion of cocaine that was paired with a light/tone cue. Next rats underwent lever extinction for 8 days followed by light/tone reactivation and a test of cue-induced cocaine-seeking behavior. Systemic injection of garcinol 30 min after reactivation significantly impaired the reconsolidation of the cocaine-associated cue memory. Further testing revealed that garcinol had no effect on drug-induced cocaine-seeking, but was capable of blocking the initial conditioned reinforcing properties of the cue and prevents the acquisition of a new response. Additional experiments showed that the effects of garcinol are specific to reactivated memories only, temporally constrained, cue-specific, long-lasting, and persist following extended cocaine access. These data provide strong evidence that the naturally occurring compound, garcinol, may be a potentially useful tool to sustain abstinence from drug abuse.
INTRODUCTION
Drug addiction is commonly described as the process whereby repeated drug use results in physiological and behavioral alterations and ultimately dependence, resulting in craving and withdrawal upon cessation. Numerous factors contribute to craving and relapse to drug seeking, preventing sustained abstinence. One powerful component associated with relapse is exposure to drug-associated cues, which evoke memories of the pleasurable and rewarding effects of the drug. These strong associations lead to stimulus-induced craving and potential relapse. Thus, identifying clinically relevant mechanisms by which to weaken the strength of these drug-associated memories is critical to supporting sustained abstinence. There are two main methods by which to manipulate an already established or consolidated memory: exposure therapy/extinction and interference with the reconsolidation process. Attempts to permanently disrupt cocaine-associated memories using exposure therapy alone have proved unsuccessful (Weiss et al, 2001; Conklin and Tiffany, 2002; Taylor et al, 2009; Torregrossa and Taylor, 2013). In addition, because repeated exposure results in the formation of a new memory where the cue is no longer associated with drug taking, the effects of this form of therapy are not permanent. For example, the initial memory can return under stressful conditions (Shaham et al, 2000; Mantsch et al, 2016) and can also renew in different contexts (Nakajima et al, 2000; Allerweireldt et al, 2001; Crombag and Shaham, 2002), making exposure therapy a less than ideal form of treatment. However, manipulations targeted at interfering with the reconsolidation process are not subject to these constraints and appear to be persistent (Debiec and LeDoux, 2004; Duvarci and Nader, 2004; Kindt et al, 2009). Therefore, we believe manipulations of reconsolidation to be a more clinically useful approach. Once an established memory is retrieved or recalled, it enters a brief state of destabilization where it is labile and sensitive to disruption. If no disruption occurs during this period, the memory will once again undergo long-term storage during the process of restabilization or reconsolidation. Conversely, if the memory is disrupted during the destabilized state, it is possible to block its reconsolidation into permanent storage (Tronson and Taylor, 2007; Sorg, 2012). The amygdala, specifically the lateral nucleus (LA), is widely accepted as the brain region where this process occurs and where emotional memories are stored and, therefore, is the target of much research (Feltenstein and See, 2007; Robbins et al, 2008). Studies have demonstrated that disruption of the LA diminishes its ability to form associative memories and specifically cocaine-associated memories (Kantak et al, 2002; Fuchs et al, 2006; Feltenstein and See, 2007).
Previous work has shown that epigenetic mechanisms in the LA, such as histone acetylation (Ac), underlie the reconsolidation process (Maddox and Schafe, 2011; Maddox et al, 2013a, b). For example, studies have shown that memory retrieval induces levels of histone H3 protein in the LA and that the use of histone acetyltransferase (HAT) inhibitors, such as the amnestic agent garcinol, can interfere with the reconsolidation of an auditory fear memory (Maddox and Schafe, 2011; Maddox et al, 2013a, b). However, the specific epigenetic mechanisms underlying the reconsolidation of cocaine-cue memories remain unclear. It is for this reason that we chose to examine the use of garcinol, a naturally occurring HAT inhibitor, to block the reconsolidation of cocaine-associated cue memories.
Garcinol is a naturally derived HAT inhibitor obtained from the rind of the fruit of the Kokum tree (Garcinia indica). It has been widely studied for its anti-inflammatory, antioxidant, antibacterial, and chemoprotective properties (Yamaguchi et al, 2000; Koeberle et al, 2009; Padhye et al, 2009; Ahmad et al, 2010). Garcinol is a potent and selective inhibitor of the two HATs CBP/p300 and p300/CBP-associated factor (PCAF), and as such can alter gene expression (Balasubramanyam et al, 2004). In light of this it has been investigated as a treatment for cancer, arthritis, AIDS, and ulcers (Padhye et al, 2009). The following experiments examined the use of garcinol in impairing the reconsolidation of a cocaine-associated cue memory as assessed by a decrease in cocaine-seeking behavior measured in a ‘reinstatement test'. We additionally examined whether garcinol is capable of blocking drug-induced reinstatement and the ability of the cue to serve as a conditioned reinforcer in the acquisition of a new response. Further, we investigated whether the effects of garcinol on cocaine-cue memory reconsolidation are specific to reactivated memories, temporally constrained, persist following extended cocaine access, cue-specific, and long-lasting.
MATERIALS AND METHODS
Subjects
Adult male Sprague-Dawley rats (Charles River), weighing 275–300 g and aged 2–3 months, were housed individually in plastic cages and maintained on a 12 h light/dark cycle. Throughout the experiment food was restricted to maintain rats at 90–95% of their free-feeding body weight following recovery from surgery. Water was provided ad libitum.
Surgical Procedures
Rats were anesthetized with 75 mg/kg ketamine and 5 mg/kg xylazine i.p. They were provided with 5 mg/kg Rimadyl and 5 ml of lactated Ringer's solution s.c. Rats were implanted with indwelling catheters into the right jugular vein. Catheters were kept patent by infusions of heparinized saline every other day. Rats were allowed 1 week to recover from surgery at which time they were individually housed with ad libitum access to food and water. Rats were weighed throughout the recovery period and daily throughout behavioral training/testing.
Behavioral Procedures
Rats were trained on cocaine self-administration in sound-attenuated operant conditioning chambers (Med Associates). Each operant box contained two retractable levers (one to the far left and one to the far right on the same wall), a house light, a cue light, a tone generator, and a fan to provide background noise (65 dB). Rats were food restricted to 90–95% of their weight measured on the last day of recovery from surgery for the duration of the experiment.
Rats received 12 days (or 24 days for extended access experiment) of cocaine self-administration (SA) training. Each daily session lasted 1 h, during which time an active and inactive lever were extended in the chamber. Active lever presses resulted in a 1 mg/kg i.v. infusion of cocaine while concurrently a cue light and tone (75 dB) were presented for 10 s. Inactive lever presses had no outcome. Self-administration was on a fixed ratio 1 schedule where every active lever press resulted in one cocaine infusion and cue presentation. In experiments involving tests of cue-induced cocaine-seeking, rats underwent lever extinction for 8 days, where lever pressing did not result in cocaine infusion or cue presentation.
Rats that met the acquisition criteria (≥6 infusions for each of the last 3 days of SA) were then divided into two groups, ensuring an equivalent total number of infusions across the 12 days of SA and similar levels of extinction: to-be-vehicle or to-be-garcinol. The day following the last lever extinction session, rats underwent memory reactivation in a novel context (geometrically different chamber, different lighting, new odor). During the reactivation session the cues (tone and light) were presented three times for 10 s as in self-administration with an intertrial interval of 60 s and a total session duration of 5 min to reactivate the cocaine-cue memory. No levers were present during the reactivation session. No-reactivation controls were placed in the same novel chamber as in our reactivation experiments, but received no stimulus presentation. Rats were then placed in a quiet laboratory room until injections. Thirty minutes after reactivation (or 6 h after in the delayed reactivation control group) rats received either a 10 mg/kg i.p. injection of garcinol or vehicle and were returned to the animal colony. The 30 min post-reactivation time point was used based on initial studies using garcinol to impair fear memory reconsolidation. Twenty-four hours after the reactivation session, rats were placed back in the original chamber for a test of cue-induced cocaine-seeking where each active lever press resulted in a 10 s cue presentation but no cocaine infusion. In the experiment testing drug-induced cocaine-seeking, self-administration, extinction, reactivation, and injections were performed as before. Twenty-fours after the reactivation session, rats received a non-contingent 10 mg/kg i.p. injection of cocaine immediately prior to being put into the chambers for a drug-induced cocaine-seeking test. During this test lever responses did not result in cue presentation or cocaine infusion.
Conditioned Reinforcement
Rats underwent surgery, self-administration training, memory reactivation, and received injections as above. Here, rats did not receive lever extinction sessions, but were kept in the animal colony for the same length of time as other groups following SA (8 days). Twenty-four hours after the memory reactivation session and garcinol or vehicle injections, rats underwent a conditioned reinforcement test where they had to learn a new instrumental response solely reinforced by cue presentation. Here, rats were placed in a third context that was a different operant chamber with plastic floors and scented with peppermint. No levers were present; however, rats had access to two illuminated nose ports. Responses into the active nose port resulted in a 5 s cue presentation, while responses into the inactive nose port had no consequence. Total active and inactive nose port entries were recorded over a 30-min session.
Two Cue Behavioral Procedure
Here, during self-administration cocaine infusion was paired with either the light 50% of the time or the tone 50% of the time in response to active lever presses. The first cue presented on each daily session was altered every day as they did throughout the session (ie, 1st lever press=tone, 2nd=light, 3rd=tone, etc.). To assess whether lever extinction affects the results of our cue-induced cocaine-seeking tests, here rats did not undergo lever extinction. On memory reactivation day half the rats were reactivated to the light only and the other half to the tone only. Each of these groups then received injections of vehicle or garcinol as before. Twenty-four hours later, rats underwent a test of cue-induced cocaine-seeking where for 30 min all rats were responding for presentation of the light cue and for another 30 min they responded for presentation of the tone cue. Groups were counterbalanced so that half of the rats were first responding for the light and half were first responding for the tone. The total number of active and inactive lever responses was recorded.
Statistical Analysis
Cocaine self-administration data were analyzed using repeated measure analysis of variance (ANOVA) across all days for number of infusions, total active lever presses, and total inactive lever presses. Lever extinction was also analyzed using ANOVA across each day to measure total active and inactive lever responses. For all experiments involving lever extinction, the tests of cocaine-seeking were analyzed using ANOVAs measuring total active and inactive lever responses on the last day of extinction and test day. The conditioned reinforcement test data were analyzed using ANOVAs for active and inactive nose poke entries during the test. Bonferroni adjustment and post hoc tests were used where appropriate.
RESULTS
Systemic Garcinol Impairs Cue-, but not Drug-Induced Cocaine-Seeking Behavior Following Retrieval of a Cocaine-Associated Memory
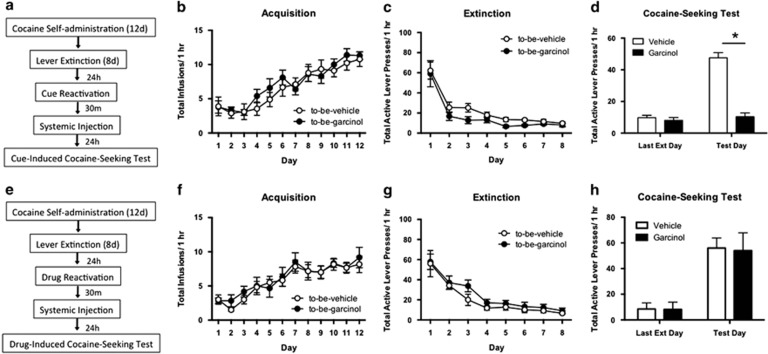
In our first experiment we asked whether post-reactivation systemic garcinol is capable of impairing the reconsolidation of a cocaine-associated memory (Figure 1). Acquisition of cocaine self-administration did not differ between to-be-vehicle (N=9) and to-be-garcinol (N=9) groups; there were no significant differences in the total number of cocaine infusions (p>0.05; Figure 1b). Likewise, there were no differences across lever extinction between groups for total active lever presses (p>0.05; Figure 1c). The ANOVA across the last day of extinction and the cue-induced cocaine-seeking test day revealed a significant main effect of day (F(1,34)=74.29, p<0.0001) and of drug (F(1,34)=69.75, p<0.0001) as well as a significant interaction in active lever presses between garcinol- and vehicle-injected rats (F(1,34)=58.21, p<0.0001; Figure 1d). Post-reactivation garcinol resulted in a subsequent decrease in active lever responses during the cocaine-seeking reinstatement test compared with vehicle controls (p<0.05), while there were no differences between groups on the last day of extinction, suggesting systemic garcinol injection can block the reconsolidation of a cocaine-cue memory and decrease drug-seeking behavior. Follow-up studies examining vehicle- vs garcinol-injected rats' locomotor activity 24 h after injection revealed no differences between groups (p>0.05). This suggests the differences observed in cue-induced cocaine-seeking are not due to garcinol impairing locomotor activity. Additional studies assessing post-reactivation short-term memory revealed no differences between vehicle (N=5) and garcinol (N=5) injected rats on cue-induced cocaine-seeking 2 h after memory retrieval (Supplementary Figure S1), indicating garcinol only interferes with the long-term storage (reconsolidation) of the cocaine-cue memory. In a separate experiment, we assessed whether garcinol would also impair drug-induced cocaine-seeking of a cocaine-cue memory. There were no differences in acquisition of cocaine self-administration between to-be-vehicle (N=6) or to-be-garcinol (N=6) injected rats (p>0.05; Figure 1f). Additionally, there were no differences across lever extinction between groups for total active lever presses (p>0.05; Figure 1g). Finally, the ANOVA across the last day of extinction and the drug-induced cocaine-seeking reinstatement test day revealed a significant main effect of day (F(1,10)=286.40, p<0.0001), but no significant effect of group and no significant interaction in active lever presses between garcinol- and vehicle-injected rats (p>0.05; Figure 1h). Thus, the amnestic effect of garcinol here is specific to the mnemonic processing of the cocaine-associated cues and does not interfere with drug-induced cocaine-seeking.
Figure 1.
Systemic garcinol impairs cue-, but not drug-induced cocaine-seeking behavior following retrieval of a cocaine-associated memory. (a) Schematic of the behavioral protocol. (b) Total infusions across a 1 h session for each day of cocaine self-administration for to-be-vehicle (N=9) and to-be-garcinol (N=9) injected rats. (c) Total active lever presses across each day of extinction. (d) Total active lever presses on the last day of extinction compared with during the cue-induced cocaine-seeking test. (e) Schematic of the behavioral protocol. (f) Total infusions across each day of cocaine self-administration for to-be-vehicle (N=6) and to-be-garcinol (N=6) injected rats. (g) Total active lever presses across each day of extinction. (h) Total active lever presses on the last day of extinction compared with the drug-induced cocaine-seeking test. Behavioral data are shown as mean±SEM number over a 1 h session. *p<0.05.
Effects of Garcinol on Cocaine-Cue Memory are Specific to Reactivated Memories Only
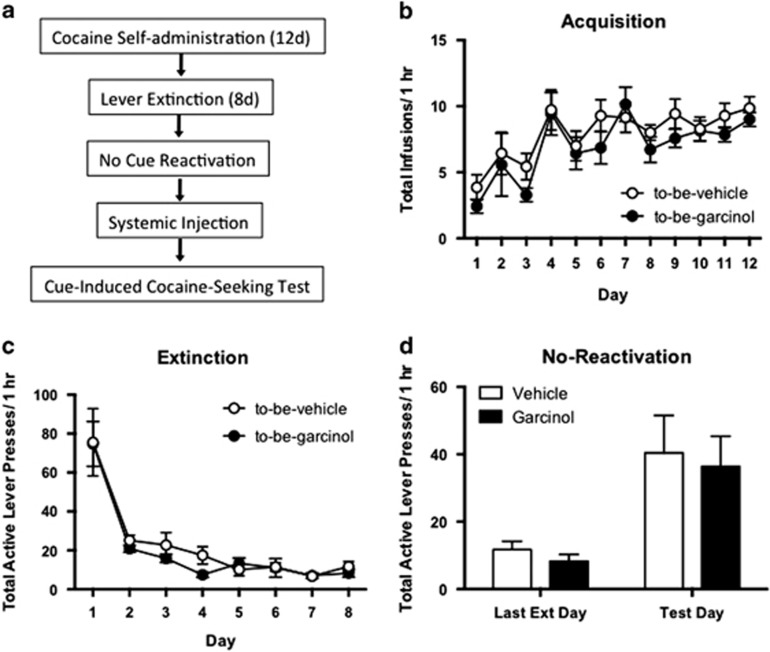
For garcinol to be clinically useful, it is necessary to ensure that the compound blocks only memories that have been reactivated and not those that have not. As a control for our reconsolidation experiments, we next examined the effect of garcinol on a cocaine-cue memory receiving ‘no-reactivation'. To test this, a separate group of rats received self-administration training and lever extinction as in our first experiment. However, the next day following the last extinction session, rats were placed in the reactivation chamber for the same duration as the reactivated groups, but did not receive cue presentations. Thirty minutes after being placed in the box they were injected with either vehicle or garcinol and were administered a cue-induced cocaine-seeking test 24 h later (Figure 2). Similar to our reactivation experiments, there were no significant differences in total infusions across the 12 days of cocaine self-administration between to-be-vehicle (N=7) and to-be-garcinol (N=7) groups (p>0.05; Figure 2b). Likewise, there were no differences in active lever presses during lever extinction (p>0.05; Figure 2c). Here, the ANOVA revealed a significant main effect of day (F(1,12)=15.60, p<0.01), but a nonsignificant effect of group and no significant interaction in active lever presses between garcinol- and vehicle-injected rats (p>0.05; Figure 1d). These findings suggest that the ability of systemic garcinol to effectively impair the reconsolidation of a cocaine-associated memory is predicated on active memory recall during the reactivation session; that is, in the absence of memory retrieval, garcinol has no effect on the retention of the memory.
Figure 2.
The effects of garcinol on cocaine-cue memory are specific to reactivated memories only. (a) Schematic of the behavioral protocol. (b) Total infusions across each day of cocaine self-administration for to-be-vehicle (N=7) and to-be-garcinol (N=7) injected rats. (c) Total active lever presses across each day of extinction. (d) Total active lever presses on the last day of extinction compared with the cue-induced cocaine-seeking test. Behavioral data are shown as mean±SEM number over a 1 h session.
Systemic Garcinol Reduces the Subsequent Conditioned Reinforcement of a New Instrumental Response
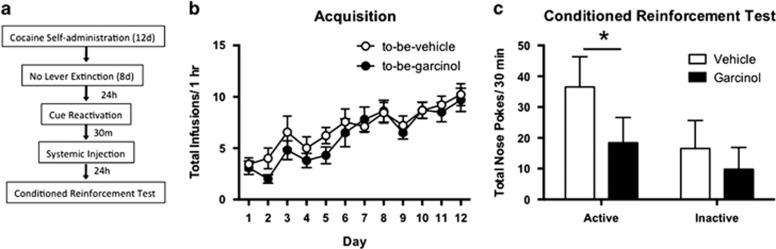
In this experiment we sought to assess garcinol's ability to block the learning of a new instrumental response reinforced by the previous cocaine-associated cue using a conditioned reinforcement test (Figure 3). Here, we were also able to assess whether the effects of garcinol are dependent on context by training rats in Context A, performing memory reactivation in Context B, and testing in Context C. No differences were seen across acquisition of cocaine self-administration in total infusions between to-be-vehicle (N=10) and to-be-garcinol (N=10) groups (p>0.05; Figure 3b). Rats did not undergo lever extinction prior to conditioned reinforcement testing. During this test rats were placed in a novel context and learned to acquire a new response (nose poke) solely reinforced by the cocaine-associated cue. This experiment assesses whether garcinol injection blocks the initial conditioned reinforcing effects of the cue and prevents the acquisition of this new response. The ANOVA revealed a significant main effect of operandum (F(1,34)=26.45, p<0.0001) and drug (F(1,34)=20.07, p<0.0001) and a significant interaction between active and inactive nose poke entries between vehicle- and garcinol-injected rats (F(1,34)=4.20, p<0.05), with the garcinol-injected group showing a significant decrease in number of active, but not inactive nose pokes when compared with the vehicle-injected group (p<0.05; Figure 3c). Thus, garcinol significantly decreases the ability of the cue to serve as a conditioned reinforcer. Additionally, this experiment suggests that the effects of garcinol are independent of context as training, reactivation, and conditioned reinforcement testing were all performed in distinct contexts.
Figure 3.
Systemic garcinol reduces the subsequent conditioned reinforcement of a new instrumental response. (a) Schematic of the behavioral protocol. (b) Total infusions across each day of cocaine self-administration for to-be-vehicle (N=10) and to-be-garcinol (N=10) injected rats. (c) Total active and inactive nose pokes during the conditioned reinforcement test. Behavioral data are shown as mean±SEM number over session length. *p<0.05.
Effects of Systemic Garcinol are Temporally Constrained and Persist Following Extended Access to Cocaine
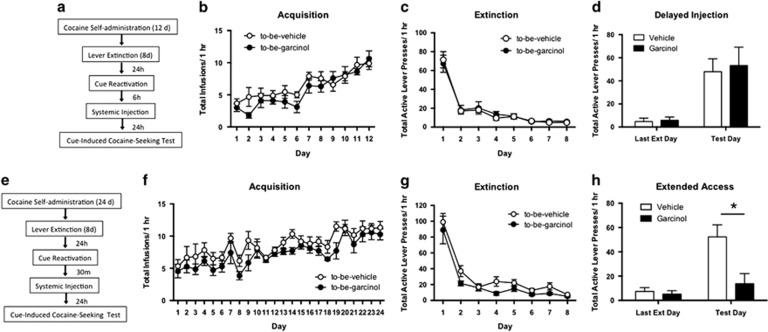
Our next series of experiments was aimed at examining the temporal specificity of garcinol and whether the drugs ability to interfere with memory reconsolidation persists following extended access to cocaine (Figure 4). Following retrieval, memories return to a labile state that is sensitive to disruption before the memory trace becomes reconsolidated and permanently stored once again. Previous studies have shown that behavioral and pharmacological manipulations are successful at blocking drug-cue memory reconsolidation when performed within 1 h following memory retrieval, but not when performed 3 or 6 h later (Sanchez et al, 2010; Xue et al, 2012). Therefore, if our drug manipulation is indeed interfering with the reconsolidation process the effects should be temporally specific to the post-retrieval lability period. For the first experiment, rats received a delayed injection of systemic garcinol 6 h following memory reactivation (ie, a time point when the memory should already be reconsolidated). Here, we observed no differences in total infusions across self-administration training between to-be-vehicle (N=9) and to-be-garcinol (N=10) groups (p>0.05; Figure 4b). Similarly, no differences were observed between groups in active lever responses during lever extinction (p>0.05; Figure 4c). The ANOVA revealed a significant main effect of day (F(1,17)=175.60, p<0.0001), but no significant effect of group and no significant interaction in active lever presses between garcinol- and vehicle-injected rats (p>0.05; Figure 4d). These data suggest that the effect of garcinol on cocaine-associated memory reconsolidation is temporally constrained; when rats are given systemic garcinol 6 h after reactivation there is no effect on cocaine-seeking behavior. Therefore, systemic garcinol within a narrow window following memory retrieval can significantly impair the reconsolidation of a cocaine-associated memory.
Figure 4.
Effects of systemic garcinol are temporally constrained and persist following extended access to cocaine. (a) Schematic of the behavioral protocol. (b) Total infusions across each day of cocaine self-administration for to-be-vehicle (N=9) and to-be-garcinol (N=10) injected rats. (c) Total active lever presses across each day of extinction. (d) Total active lever presses on the last day of extinction compared with the cocaine-seeking test between groups receiving delayed injections. (e) Schematic of the behavioral protocol. (f) Total infusions across 24 days of cocaine self-administration for to-be-vehicle (N=6) and to-be-garcinol (N=7) injected rats. (g) Total active lever presses across lever extinction. (h) Total active lever responses on the last day of extinction compared with the cocaine-seeking test. Behavioral data are shown as mean±SEM number over a 1 h session. *p<0.05.
Next we asked whether the effects of garcinol persist following extended access to cocaine self-administration, which may lead to the formation of a stronger cocaine-cue memory. To assess this, we doubled the number of days of the acquisition period and trained rats over 24 days of cocaine self-administration instead of 12 days as in other experiments. No significant differences were observed in the total number of infusions across self-administration between to-be-vehicle (N=6) and to-be-garcinol (N=7) groups (p>0.05; Figure 4f). Further, there were no differences in active lever responses made across the extinction period (p>0.05; Figure 4g). However, the ANOVA across the last day of extinction and the cocaine-seeking test day revealed a significant main effect of day (F(1,11)=159.40, p<0.0001) and drug (F(1,11)=42.57, p<0.0001) and a significant interaction in active lever presses between garcinol- and vehicle-injected rats (F(1,11)=73.72, p<0.0001; Figure 4h). Systemic garcinol 30 min following memory retrieval resulted in a significant decrease in active lever responses during the cue-induced cocaine-seeking test when compared with vehicle controls (p<0.05), while there were no differences between groups on the last day of extinction. These data suggest that this drug manipulation is additionally capable of impairing the reconsolidation of a cocaine-cue memory even following extended access.
Effects of Systemic Garcinol are Cue Specific, not Dependent on Extinction, and Long-lasting
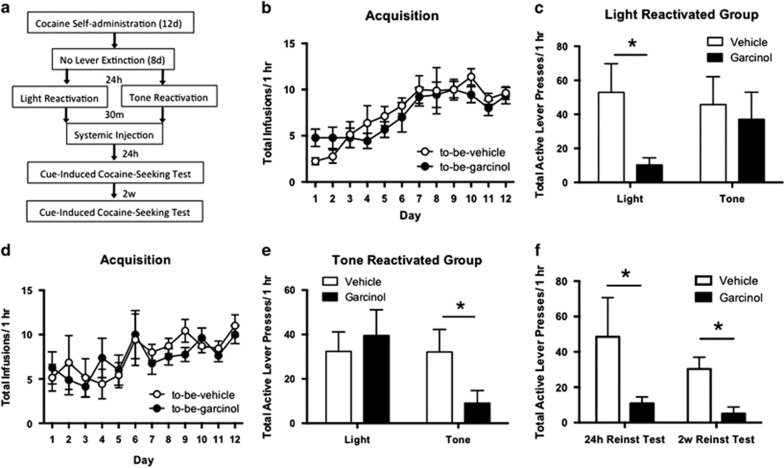
In our final set of behavioral experiments we wanted to assess whether the effects of garcinol on cocaine-cue memory reconsolidation are cue specific (Figure 5). Here, two different cues were separately paired with active lever responses and cocaine infusion during self-administration (ie, 50% of active lever presses resulted in light presentation and 50% of active lever presses resulted in tone presentation). For this experiment rats did not undergo lever extinction as here we additionally sought to examine whether extinction training has any impact on the outcome of our cocaine-seeking test. Nine days following acquisition rats were split into two groups and underwent reactivation to either light alone or tone alone. During acquisition, there were no significant differences in total number of infusions between to-be-vehicle (N=8) and to-be-garcinol (N=9) light reactivated groups or the to-be-vehicle (N=7) and to-be-garcinol (N=8) tone reactivated groups (p>0.05; Figures 5b and d). During the 1-h cocaine-seeking test, active lever presses resulted in light presentation for 30 min and in tone presentation for 30 min in a counterbalanced manner. The ANOVA for the light alone reactivated group showed a significant main effect of session (F(1,30)=4.01, p=0.05) and drug (F(1,30)=27.64, p<0.0001) and a significant interaction for active lever presses across light and tone cocaine-seeking tests (F(1,30)=12.00, p<0.01), with the garcinol-injected group pressing significantly less than the vehicle-injected group during the light cocaine-seeking test (p<0.05; Figure 5c), while there were no differences between groups during tone cocaine-seeking test. The ANOVA for the tone alone reactivated group showed a significant main effect of session (F(1,26)=20.32, p<0.0001) and drug (F(1,26)=5.49, p<0.05) and a significant interaction for active lever presses across light and tone cocaine-seeking behavior tests (F(1,26)=19.57, p=0.001), with the garcinol-injected group pressing significantly less than the vehicle-injected group during the tone cocaine-seeking behavior test (p<0.05; Figure 5e), while there were no differences between groups during the light cocaine-seeking behavior test.
Figure 5.
Effects of systemic garcinol are cue specific, not dependent on extinction, and long-lasting. (a) Schematic of the behavioral protocol. (b) Total infusions across each day of cocaine self-administration for to-be-vehicle (N=8) and to-be-garcinol (N=9) injected rats reactivated to light. (c) Total active lever responses in rats reactivated to the light cue during light or tone presentations in the cocaine-seeking test. (d) Total infusions per group across each day or self-administration for the to-be-vehicle (N=7) and to-be-garcinol (N=8) injected rats reactivated to tone. (e) Total active lever responses in rats reactivated to the tone cue during light or tone presentations in the cocaine-seeking behavior test. (f) Total active lever presses during a cocaine-seeking test 24 h after reactivation compared with 2 weeks after reactivation. Behavioral data are shown as mean±SEM number over session length. *p<0.05.
To test whether the effects of garcinol are long-lasting, a subset of rats was retested for cocaine-seeking 2 weeks after the initial test. The ANOVA revealed a significant main effect of group (F(1,9)=31.30, p<0.001; Figure 5f) with the garcinol group showing a decrease in active lever presses on the 2-week cocaine-seeking test when compared with the vehicle group. Thus, garcinol can impair the reconsolidation of a cocaine-associated memory in a cue-specific and long-lasting manner. Additionally, the effects of garcinol appear to be independent of extinction. In this experiment rats did not undergo lever press extinction; however, they show comparable levels of cue-induced cocaine-seeking behavior as groups that had previously received extinction.
DISCUSSION
In the present study, we demonstrate that a post-reactivation systemic injection of garcinol can significantly impair the reconsolidation of a cocaine-associated memory as evidenced by a reduction in cue-induced reinstatement and drug-seeking behavior in rats. We show that systemic garcinol given shortly after memory reactivation can impair cue-induced cocaine-seeking behavior. This effect appears to be specific to the mnemonic processing of the cocaine-associated cue as we did not find any effect on drug-induced cocaine-seeking behavior. These data also suggest that the effects of garcinol are due to interfering with the reconsolidation process itself and not due to an overall decrease in responding. Importantly, in separate experiments we showed that the effects of garcinol are specific to reactivated memories only, indicating that garcinol can only impair the reconsolidation of a memory if administered around the time of active memory recall. Further, we show that the effects of garcinol on cocaine-cue memory reconsolidation are temporally constrained, persist following extended access to cocaine, cue-specific, and long-lasting.
Systemic Garcinol as a Compound with Translational Utility
Sustained abstinence from drug use in humans is commonly jeopardized by exposure to environmental cues that have been strongly associated with drug taking, inducing stimulus-elicited cravings that often lead to relapse. Recent work in rodents has been aimed at targeting the reconsolidation process and interfering with drug-associated memories to reduce craving and prolong abstinence (cf., Taylor et al, 2009; Sorg 2012; Torregrossa and Taylor, 2013; Everitt, 2014; Taylor and Torregrossa, 2015; Torregrossa and Taylor, 2016). Indeed, it has been demonstrated that specifically manipulating cocaine-cue memories following memory retrieval using pharmacological agents can block the reconsolidation of these memories and reduce drug-seeking behavior in rats (Lee et al, 2005, 2006; Sanchez et al, 2010; Wan et al, 2014; Shi et al, 2015; Merlo et al, 2015). However, most of these studies to date have used intracranial drug infusions. For this method of manipulating established memories to be clinically useful, however, it is necessary to identify a compound that can be given systemically and it also would be beneficial to be natural with minimal side effects. Given our findings, the naturally occurring amnestic agent garcinol may be one compound with this translational potential. Several recent preliminary reports in humans have attempted to target drug-cue reconsolidation and garcinol could be investigated using a similar procedure (Zhao et al, 2009, 2011; Saladin et al, 2013, Lonergan et al, 2016).
Garcinol's Effects on Conditioned Reinforcement
Similar to our first experiment where garcinol diminished the cocaine-associated cue's ability to trigger drug-seeking, further testing showed that garcinol was also capable of blocking the initial conditioned reinforcing properties of the cue and prevented the acquisition of a new instrumental response. For this experiment we additionally assessed whether extinction training following self-administration altered the outcome of our behavioral paradigm. Here rats did not undergo lever extinction as other groups had in the past; however, the effect of garcinol persisted and blocked conditioned reinforcement. Moreover, this experiment also serves as a context shift control as many studies examining reconsolidation processes associated with cocaine-cue memories have failed to explore whether drug manipulations are context independent (Lee et al, 2005, 2006; Milton et al, 2008). In our experiments, conditioned reinforcement testing was performed in a novel environment distinct from both the self-administration context and the memory reactivation context. The ability of garcinol to decrease the cue's ability to serve as a conditioned reinforcer in this new environment provides evidence that the effects of this drug manipulation are context independent, which is critical for its therapeutic potential. Attempts to permanently disrupt such memories using exposure-based therapies have been ineffective as extinguished memories are capable of renewing in different contexts (Weiss et al, 2001; Conklin and Tiffany, 2002; Taylor et al, 2009; Torregrossa and Taylor, 2013). However, as these data suggest, memories that have been disrupted by interfering with the reconsolidation process are not subject to these constraints; they appear to be persistent and do not renew in other contexts.
The Use of Garcinol Following Extended Access to Cocaine
Additional experiments showed that the effect of garcinol on cocaine-cue memory reconsolidation persists following extended access to cocaine, which could lead to the formation of a stronger cocaine-cue memory. This is of great clinical importance as many individuals who seek treatment have been using a substance for a considerable amount of time, potentially enhancing the powerful reinforcing properties of drug-associated cues. An ideal compound to block reconsolidation in this context should have the capability of being given to those who have been dependent on a substance for months or years in order to maximize its treatment value. Previous studies using intracranial pharmacological manipulations have used cocaine self-administration procedures with 9–12 days of acquisition (Lee et al, 2005, 2006; Milton et al, 2008; Sanchez et al, 2010; Wan et al, 2014; Shi et al, 2015). To explore the effects of garcinol following extended cocaine access, we increased this self-administration acquisition period to 24 days. Interestingly, rats that received either 12 or 24 days of self-administration peaked to the same number of cocaine infusions throughout the acquisition period reaching an average of 11 infusions per day. Conversely, rats that received 12 days of self-administration in our first experiment responded on the first day of extinction with an average of 59 active lever presses, while those that had 24 days of acquisition responded with an average of 97 active lever presses on the first day of extinction. These data suggest that doubling the length of self-administration may have created a more robust cocaine-cue association, which garcinol was additionally capable of blocking. However, it is difficult to pinpoint whether doubling the number of self-administration days resulted in a change in the actual strength of the memory, or the animal's level of motivation, or both. Further studies are needed to assess these underlying factors. In light of these data, it would be of interest to examine whether garcinol would also be effective in other models of drug craving and relapse such as those using incubation of cocaine craving after withdrawal (Grimm et al, 2001; Pickens et al, 2011). Additionally, it would be important to assess garcinol's effectiveness following addiction-like models examining the more compulsive aspects of drug seeking or taking such as those using schedules of cocaine administration in the face of aversive outcomes (Belin et al, 2008; Jonkman et al, 2012; Everitt, 2014).
Garcinol, Cue-Specificity, and Persistence
Also of clinical importance is the cue specificity of our drug manipulation using garcinol. By separately pairing two different cues with cocaine infusion we were able to show that garcinol only impairs the reconsolidation of a directly reactivated cue and did not generalize to another cue that was also associated with cocaine infusion. This importantly allows for the targeting of specific memories to be impaired while others remain intact. To our knowledge, this is the first demonstration of a drug manipulation having cue-specific effects following memory reactivation. Others have used a second-order fear conditioning behavioral procedure to examine associative networks and assess whether reactivation of one memory generalizes to other associated memories leaving them labile in a manner that requires reconsolidation (Debiec et al, 2006). Consistent with our results they found that only directly reactivated memories became labile and sensitive to disruption while indirectly reactivated or associated memories did not (Debiec et al, 2006). However, this study did not address whether first-order associations behave in a similar manner. Using the amnestic agent garcinol, our data are the first to show that it is possible to produce cue-specific blockade of reconsolidation.
While this finding is exciting, one criticism of using reconsolidation manipulations to sustain abstinence and prevent relapse is the fact that drugs of abuse are most often strongly associated with many different cues. Therefore, it may be translationally problematic to block the reconsolidation of only one cocaine-associated cue. For this method to be useful, it may require many sessions in order to impair the reconsolidation of the numerous cues associated with drug use. However, recent unpublished data from our lab suggest that systemic garcinol administration following drug (instead of cue) reactivation may also impair the reconsolidation of multiple cocaine-associated cues. This method of interfering with cocaine-cue memories may lead to more promising clinical implications.
To examine the persistence of this cue-specific reconsolidation impairment, these rats were also tested 2 weeks after their initial cocaine-seeking test and the results indicated that garcinol can interfere with cocaine-cue memory reconsolidation in a long-lasting manner. In this experiment we additionally addressed the question of whether or not lever extinction has any impact on the outcome of our cocaine-seeking reinstatement test. In the past, our lab has included lever extinction training in the behavioral procedures as it produces a robust level of cocaine-seeking behavior and prevents a floor effect, thus allowing for clearer interpretation of the results of our drug manipulations. However, this model has been scrutinized in the past as it less accurately parallels what occurs in addicted individuals and many labs have adapted a model that excludes lever extinction training (Milton et al, 2008; Merlo et al, 2015; Zhang et al, 2015). For this reason, in this experiment rats did not undergo extinction as they did in our previous experiments; however, this had no impact on the ability of garcinol to impair reconsolidation.
Possible Mechanisms of Garcinol's Mnemonic Actions
The precise mechanism by which post-reactivation systemic garcinol is capable of impairing cocaine-cue memory reconsolidation remains unclear. Previous studies have demonstrated that HAT inhibitors, such as garcinol, are capable of impairing memory reconsolidation following an aversive fear learning paradigm (auditory fear conditioning) in rats (Maddox and Schafe, 2011; Maddox et al, 2013a, b; Monsey et al, 2015) and that epigenetic mechanisms in the LA, such as histone acetylation, underlie the reconsolidation process in these learning paradigms (Maddox and Schafe, 2011; Maddox et al, 2013a). For example, studies examining fear memory reconsolidation have demonstrated that histone H3 acetylation levels are significantly enhanced following memory reactivation and that the use of HAT inhibitors is capable of blunting these retrieval-induced increases and blocking reconsolidation (Maddox and Schafe, 2011; Maddox et al, 2013a, b). Others have shown that epigenetic mechanisms play a critical role in response to cocaine self-administration. For example, histone H3 acetylation in the nucleus accumbens and striatum has been shown to underlie motivation for drug reinforcement as well as cocaine-induced neuroplasticity (Kumar et al, 2005; Wang et al, 2010). Further testing is needed to more stringently dissect the underlying epigenetic pathway and to confirm a direct causal role between garcinol's epigenetic actions and its effects on reconsolidation. In addition, future studies should examine other brain regions implicated in the reconsolidation of drug memories. While the amygdala is an important site involved in this process, other studies have revealed that areas such as the hippocampus, nucleus accumbens, and prefrontal cortex, among others, are also critically involved (Miller and Marshall, 2005; Ramirez et al, 2009; Wells et al, 2011, 2016; Li et al, 2016). Future studies could utilize intracranial infusions of garcinol into such regions to examine the neural circuits involved in the effect of this compound on drug-cue memory reconsolidation. Further, it would be important to also assess whether garcinol alters levels of histone acetylation and changes in downstream gene transcription in these regions.
In summary, our findings provide compelling evidence that a systemically administered naturally occurring compound can significantly impair the reconsolidation of cocaine-associated memories in a widely studied animal model of cocaine addiction. Further, we show that this compound is specific to reactivated memories, capable of blocking conditioned reinforcement, temporally restricted to a brief window following memory retrieval, persist following extended cocaine access, cue-specific, and long-lasting. These data provide the first evidence that the HAT inhibitor garcinol may hold great promise as a therapeutic agent in the treatment of addiction disorders in efforts to prolong abstinence.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
This work was supported by DA015222 (to JRT), 5T32 MH14276, the Charles B.G. Murphy Fund, and the Connecticut Mental Health Center.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Ahmad A, Wang Z, Ali R, Miatah MY, Kong D, Banerjee S et al (2010). Apoptosis-inducing effect of garcinol is mediated by NF-kappaB signaling in breast cancer cells. J Cell Biochem 109: 1134–1141. [DOI] [PubMed] [Google Scholar]
- Allerweireldt AT, Weber SM, Neisewander JL (2001). Passive exposure to a contextual discriminative stimulus reinstates cocaine-seeking behavior in rats. Pharmacol Biochem Behav 69: 555–560. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam K, Altaf M, Varier RA, Swaminathan V, Ravindran A, Sadhale PP et al (2004). Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J Biol Chem 279: 33716–33726. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008). High impulsivity predicts the switch to compulsive cocaine-taking. Science 320: 1352–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST (2002). Applying extinction research and theory to cue-exposure addiction treatments. Addiction 97: 155–167. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y (2002). Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci 116: 169–173. [DOI] [PubMed] [Google Scholar]
- Debiec J, Doyere V, Nader K, LeDoux JE (2006). Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc Natl Acad Sci USA 103: 3428–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE (2004). Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neurosci 129: 267–272. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K (2004). Characterization of fear memory reconsolidation. J Neurosci 24: 9269–9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ (2014). Neural and psychological mechanisms underlying compulsive drug seeking habits and drug-memories – indications for novel treatments of addiction. Eur J Neurosci 40: 2163–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE (2007). NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol Learn Mem 88: 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Feltenstein MW, See RE (2006). The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci 23: 2809–2813. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y (2001). Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412: 141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman S, Pelloux Y, Everitt BJ (2012). Drug intake is sufficient, but conditioning is not necessary for the emergence of compulsive cocaine seeking after extended self-administration. Neuropsychopharm 37: 1612–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB (2002). Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci 22: 1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B (2009). Beyond extinction: erasing human fear responses and preventing return of fear. Nat Neurosci 12: 256–258. [DOI] [PubMed] [Google Scholar]
- Koeberle A, Northoff H, Werz O (2009). Identification of 5-lipoxygenase and microsomal prostaglandin E2 synthase-1 as functional targets of the anti-inflammatory and anti-carcinogenic garcinol. Biochem Pharmacol 77: 1513–1521. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT et al (2005). Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 48: 303–314. [DOI] [PubMed] [Google Scholar]
- Lee JL, DiCiano P, Thomas KL, Everitt BJ (2005). Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron 47: 795–801. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ (2006). Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci 26: 5881–5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ge S, Li N, Chen L, Zhang S et al (2016). NMDA and dopamine D1 receptors within NAc-shell regulate IEG proteins expression in reward circuit during cocaine memory reconsolidation. Neuroscience 315: 45–69. [DOI] [PubMed] [Google Scholar]
- Lonergan MH, Saumier D, Tremblay J, Kieffer B, Brown TG et al (2016). Reactivating addiction-related memories under propranolol to reduce craving: a pilot randomized controlled trial. J Behav Ther Exp Psychiatry 50: 245–249. [DOI] [PubMed] [Google Scholar]
- Maddox SA, Schafe GE (2011). Epigenetic alterations in the lateral amygdala are required for reconsolidation of a Pavlovian fear memory. Learn Mem 8: 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Watts CS, Doyere V, Schafe GE (2013. a). A naturally-occurring histone acetyltransferase inhibitor derived from Garcinia indica impairs newly acquired and reactivated fear memories. PLoS One 8: e54463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Watts CS, Schafe GE (2013. b). p300/CBP histone acetyltransferase activity is required for newly acquired and reactivated fear memories in the lateral amygdala. Learn Mem 20: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y (2016). Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharm 41: 335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo E, Ratano P, Ilioi EC, Robbins MA, Everitt BJ, Milton AL (2015). Amygdala dopamine receptors are required for the destabilization of a reconsolidating appetitive memory. eNeuro 2 ENEURO.0024-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF (2005). Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 47: 873–884. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ (2008). Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci 28: 8230–8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsey MS, Gerhard DM, Boyle LM, Briones MA, Seligsohn M, Schafe GE (2015). A diet enriched with curcumin impairs newly acquired and reactivated fear memories. Neuropsychopharmacology 40: 1278–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Tanaka S, Urushihara K, Imada H (2000). Renewal of extinguished lever-press responses upon return to the training context. Learn Motiv 31: 416–431. [Google Scholar]
- Padhye S, Ahmad A, Oswal N, Sarkar FH (2009). Emerging role of Garcinol, the antioxidant chalcone from Garcinia indica Choisy and its synthetic analogs. J Hematol Oncol 2: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y (2011). Neurobiology of the incubation of drug craving. Trends Neurosci 34: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DR, Bell GH, Lasseter HC, Xie X, Traina SA et al (2009). Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur J Neurosci 30: 901–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everett BJ (2008). Drug addiction and the memory systems of the brain. Ann NY Acad Sci 1141: 1–21. [DOI] [PubMed] [Google Scholar]
- Saladin ME, Gray KM, McRae-Clark AL, Larowe SD, Yeatts SD et al (2013). A double blind, placebo-controlled study of the effects of post-retrieval propranolol on reconsolidation of memory for craving and cue reactivity in cocaine dependent humans. Psychopharmacology (Berl) 226: 721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR (2010). Reconsolidation of a cocaine-associated stimulus requires amygdalar protein kinase A. J Neurosci 30: 4401–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J (2000). Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev 33: 13–33. [DOI] [PubMed] [Google Scholar]
- Shi HS, Luo YX, Yin X, Wu HH, Xue G, Geng XH et al (2015). Reconsolidation of a cocaine associated memory requires DNA methyltransferase activity in the basolateral amygdala. Science Rep 5: 13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA (2012). Reconsolidation of drug memories. Neurosci and Behav Rev 36: 1400–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quin JJ, Torregrossa MM (2009). Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharm 56: 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Torregrossa MM (2015). Pharmacological disruption of maladaptive memory. Handb Exp Pharmacol 228: 381–415. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Taylor JR (2013). Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology 226: 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Taylor JR (2016). Neuroscience of learning and memory for addiction medicine: from habit formation to memory reconsolidation. Prog Brain Res 223: 91–113. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR (2007). Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 8: 262–275. [DOI] [PubMed] [Google Scholar]
- Wan X, Torregrossa MM, Sanchez H, Nairn AC, Taylor JR (2014). Activation of exchange protein activated by cAMP in the rat basolateral amygdala impairs reconsolidation of a memory associated with self-administered cocaine. PLoS One 9: e107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lv Z, Hu Z, Sheng J, Hui B, Sun J et al (2010). Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIalpha in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology 35: 913–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL et al (2001). Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology 25: 361–372. [DOI] [PubMed] [Google Scholar]
- Wells AM, Lasseter HC, Xie X, Cowhey KE, Rittinger AM et al (2011). Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem 18: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Xie X, Higginbotham JA, Arguello AA, Healy KL et al (2016). Contribution of an SFK-mediated signaling pathway in the dorsal hippocampus to cocaine-memory reconsolidation in rats. Neuropsychopharmacology 41: 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C et al (2012). A memory retrieval-extinction procedure to prevent drug craving and relapse. Science 336: 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi F, Ariga T, Yoshimura Y, Nakazawa H (2000). Antioxidative and anti-glycation activity of garcinol from Garcinia indica fruit rind. J Agric Food Chem 48: 180–185. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xue Y, Meng S, Luo Y, Liang J, Li J et al (2015). Inhibition of lactate transport erases drug memory and prevents drug relapse. Biol Psychiatry 79: 928–939. [DOI] [PubMed] [Google Scholar]
- Zhao LY, Zhang XL, Shi J, Epstein DH, Lu L (2009). Psychosocial stress after reactivation of drug-related memory impairs later recall in abstinent heroin addicts. Psychopharmacology (Berl) 203: 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LY, Sun LL, Shi J, Li P, Zhang Y et al (2011). Effects of β-adrenergic receptor blockade on drug-related memory reconsolidation in abstinent heroin addicts. Drug Alcohol Depend 118: 224–229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.