Summary
Histone post-translational modifications (PTMs) play an essential role in chromatin biology, as they model chromatin structure and recruit enzymes involved in gene regulation, DNA repair and chromosome condensation. Such PTMs are mostly localized on histone N-terminal tails where, as single units or in a combinatorial manner, influence chromatin reader protein binding and fine-tune the abovementioned activities. Mass spectrometry (MS) is currently the most adopted strategy to characterize proteins and protein PTMs. We hereby describe the protocols to identify and quantify histone PTMs and their patterns using either bottom-up or middle-down proteomics. In the bottom-up strategy we obtain 5–20 aa peptides by derivatization with propionylation followed by trypsin digestion. The newly generated N-termini of histone peptides can be further derivatized with light or isotopically heavy propionyl groups to increase chromatographic retention and allow multiplexed analyses. Moreover, we describe how to perform derivatization and trypsin digestion of histones loaded into a gel, which is usually the final step of immunoprecipitation experiments. In the middle-down strategy we obtain intact histone tails of 50–60 aa by digestion with the enzyme GluC. This allows characterization of combinatorial histone PTMs on N-terminal tails.
Keywords: histones, mass spectrometry, proteomics, bottom-up, middle-down
1. Introduction
Epigenetics is defined as the study of inheritable changes in the phenotype of an organism caused by mechanisms other than changes in the underlying DNA sequence [1]. The phenotype of a complex organism changes dramatically during development, from the embryo to the adult form, even though its DNA remains mostly unaltered. The epigenetic machinery involves different cellular biomolecules, including histone post-translational modifications (PTMs), histone variants, non-coding RNAs, DNA methylation and DNA binding factors[2]. While DNA methylation is known as irreversible modification that inactivates chromatin regions from being translated [3], histone variants and histone PTMs are more dynamic units that influence chromatin-related functions. PTMs are mostly localized in the N-terminal tails, as it is the region of the histones most exposed and flexible. Even though histone marks have been extensively characterized in the last decade, many links between known histone marks and their function are still missing. This is mostly due to the peculiarity of histone PTMs to shuffle in a large variety of combinations, modifying dramatically the affinity with histone interacting proteins and thus their role in the chromatin. The presence of sequence variants also contributes to increase the complexity of histone analysis, as histone isotypes are generally highly similar in sequence, but they might have different roles in the chromatin; e.g. H2A.x has a C-terminal sequence which is more easily phosphorylated in case of DNA damage compared to canonical H2A [4] and it is required for inactivation of sex chromosomes in male mouse meiosis [5], while CENP-A substitutes canonical histone H3 in centromers [6].
Antibody-based techniques such as western blotting have been extensively adopted to characterize histones. However, this approach is limited for the following reasons: (i) antibodies only work as confirmation, they cannot identify unknown PTMs; (ii) they are biased through presence of co-existing marks, which might influence binding affinity; (iii) they cannot identify combinatorial marks, as only very few antibodies are available for such purpose and (iv) they happen to cross-react between highly similar histone variants or multiple PTMs (e.g., di- and trimethylation of lysine residues). Egelhofer et al. described that more than 25% of commercial antibodies fail specificity tests by dot blot or western blot, and among specific antibodies more than 20% fail in chromatin immunoprecipitation experiments [7]. Mass spectrometry (MS) is currently the most suitable analytical tool to study novel and/or combinatorial PTMs, and it has been extensively implemented for histone proteins (reviewed in [8]). This is mostly due to MS high sensitivity, high mass accuracy and the possibility to perform large-scale analyses. In this chapter, we describe the workflow to purify histones and prepare them for PTM analysis via bottom-up or middle-down proteomics (Fig. 1). Both strategies achieve quantification of single histone marks. While bottom-up is more sensitive and requires less advanced instrumentation, middle-down is more suitable to characterize distant co-existing marks and their respective histone variants. An overview of the major differences between the bottom-up and the middle-down proteomics strategies is illustrated in Table 1. We recommend consulting the table prior deciding which strategy to follow for histone PTM analysis, as it specifies both requirements and the different type of results that can be achieved.
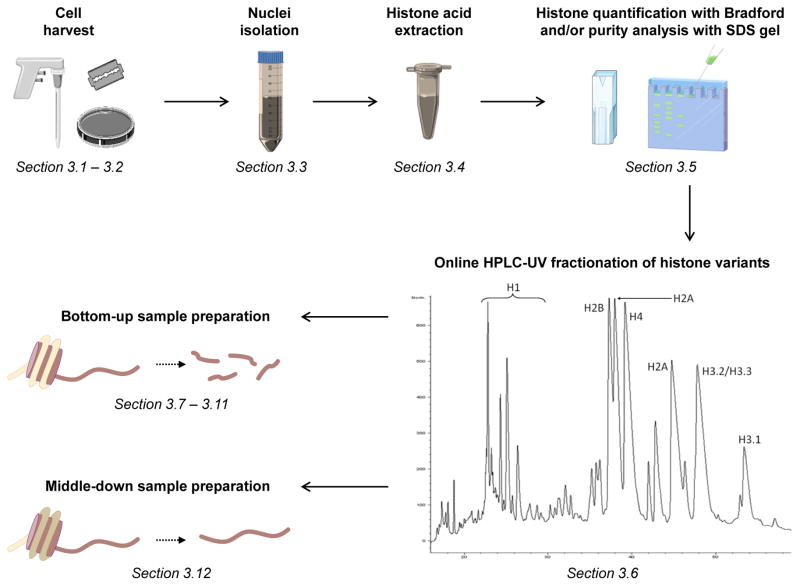
Figure 1. workflow for histone sample preparation.
After cell harvesting, nuclei are extracted with acid precipitation (TCA). The yield of histone extraction can be verified by using protein quantification methods such as Bradford, and the purity of the sample by SDS gel. When histone amount is sufficient it is possible to fractionate the different histone variants by reversed-phase HPLC. The different fractions (or the crude histone extract in case of low sample amounts) can then be digested for bottom-up or middle-down analysis.
Table 1. overview of bottom-up and middle-down strategies.
| Step of the workflow | Bottom-up | Middle-down |
|---|---|---|
| Sample preparation | medium-easy. Derivatization with propionic anhydride is required if trypsin is used | easy |
| Fractionation of histone isotypes and separate analysis | Does not provide significant gain in sensitivity as compared to the LC-MS/MS analysis of the crude histone mixture | Provides significant gain in sensitivity as compared to the LC-MS/MS analysis of the crude histone mixture (ref. Sidoli) |
| Enzyme for digestion | Trypsin (recommended), ArgC | GluC (recommended), AspN |
| Possibility of multiplexing | yes | not at the moment |
| Chromatography | C18 reversed-phase. C18-AQ material is recommended, as it can tolerate 100% H2O | Weak cation exchange – hydrophilic interaction chromatography (WCX-HILIC). Polycat A (PolyLC, USA) |
| is recommended | ||
| HPLC | two channels required; | three channels required; |
| -buffer A/loading buffer: 0.1% formic acid | -loading buffer: 0.1% formic acid | |
| -buffer B: 95% acetonitrile, 0.1% formic acid | -buffer A: 75% acetonitrile, 20 mM propionic acid, pH 6 | |
| -buffer B: 25% acetonitrile, 0.1% formic acid, pH 2.5 | ||
| Mass spectrometer | must provide at least high resolution full MS and collision induced dissociation (CID) as fragmentation method for MS/MS | must provide high resolution full MS and MS/MS, with electron transfer dissociation (ETD) as fragmentation method |
| MS acquisition method | Data dependent acquisition. Targeted MS/MS for isobaric peptides (identical precursor mass) required | Data dependent acquisition. No targeted MS/MS required. Dynamic exclusion disabled to allow selection of isobaric peptides |
| Database searching | Mascot (Matrix Science, UK) is recommended. Other tools can be used. However, some database searching engines do not properly deal with many dynamic modifications | Mascot is required, due to the optimization of the following bioinformatics steps |
| Quantification | Extracted ion chromatogram, which can be performed manually or with software | Total MS/MS ion intensity and fragment ion relative ratio calculated by isoScale (ref. [10]). Too demanding to be done manually |
| Results | ||
| Quantification of single PTMs | Yes, but not on arginines (target of digestion enzyme) | yes (only on histone tails) |
| Quantification of combinatorial PTMs | no, unless both sites of interested are on the same peptide | yes (only on histone tails) |
| Interplay evaluation between co-existing PTMs | no, unless both sites of interested are on the same peptide | yes (only on histone tails) |
| Discrimination of the histone isotype where the PTM resides | very limited for histones with highly homolog sequence | possible for several histone isotypes |
2. Materials
2.1. Reagents and abbreviations
Bradford protein assay reagent
Ammonium hydroxide (NH4OH), 28% NH3 in water
Trichloroacetic acid (TCA)
Trifluoroacetic acid (TFA)
Propionic anhydride (D0 and D10) and 2-propanol for propionylation mixture
Kasil®#1 (PQ corporation, Valley Forge, PA, USA) and formamide to prepare frits for nano-columns
2.2. Buffers
Phosphate-buffered saline (PBS): 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4
Nuclei isolation buffer (NIB-250): 15 mM Tris–HCl (pH 7.5), 15 mM NaCl, 60 mM KCl, 5 mM MgCl2, 1 mM CaCl2, 250 mM sucrose
Ammonium bicarbonate (NH4HCO3): 50 mM NH4HCO3, pH 8.0
In gel digestion buffer: 50 mM NH4HCO3, 12.5 ng/μL trypsin (sequencing grade)
HPLC-UV buffer A: 5% acetonitrile, 0.1% TFA in HPLC grade water
HPLC-UV HPLC buffer B: 95% acetonitrile, 0.1% TFA in HPLC grade water
Stage-tip loading and wash buffer: 0.1% TFA
Stage-tip elution buffer: 75% acetonitrile, 0.025% TFA
Bottom-up online HPLC loading buffer and buffer A: 0.1% formic acid in HPLC grade water
Bottom-up online HPLC buffer B: 0.1% formic acid, 95% HPLC grade acetonitrile, in HPLC grade water
Middle-down online HPLC loading buffer: 0.1% formic acid in HPLC grade water
Middle-down online HPLC buffer A: 75% HPLC grade acetonitrile, 20 mm propionic acid, adjusted to pH 6.0 with NH4OH in HPLC grade water
Middle-down online HPLC buffer B: 25% HPLC grade acetonitrile adjusted to pH 2.5 with formic acid in HPLC grade water
2.3. Solutions
Protease inhibitors (add fresh to buffers prior to use): 1 M dithiothreitol (DTT) in ddH2O (1000 ×); 200 mM AEBSF in ddH2O (400 ×)
Phosphatase inhibitor (add fresh to buffers prior to use): 2.5 μM microcystin in 100% ethanol (500 ×)
HDAC inhibitor (add fresh to buffers prior to use): 5 M sodium butyrate, made by titration of 5 M butyric acid using NaOH to pH 7.0 (500 ×)
10% (v/v) NP-40 Alternative in ddH2O
0.2 M H2SO4 in ddH2O
100% TCA (w/v) in ddH2O
2.4. Equipment
Tissue and cell homogenizers (optional)
Glass Pasteur pipettes
pH indicator strips (pH 0–14)
Liquid nitrogen
Razor blades
0.5 and 1.5 mL microcentrifuge tubes
15 and 50 mL conical tubes
Pipettes from P10 to P1000 range with respective tips
− 80 °C refrigerator
Heat blocks or water baths
HPLC-UV (~0.1–1 mL/min flow range), equipped with C18 5 μm particle commercial column (size 4.6 x 250 mm or 2.1 x 250 mm) (optional)
3 M Empore™ Solid Phase Extraction Disks C18
75 and 100 μm internal diameter fused silica tubings
Micro-stir magnets
C18-AQ 3 μm bulk resin with 200–300 Å pore sizefor trap column and analytical column for nanoLC (bottom-up strategy)
Polycat A bulk resin with 1500 Å pore size (PolyLC, Columbia, MD, USA) for analytical column for nanoLC (middle-down strategy)
Pressure cell for capillary column packing with respective compressed gas bomb (either helium, nitrogen or air)
Appropriate nanoLC-MS setup. Bottom-up analysis requires HPLC with at least two channels (one for buffer A/loading buffer and one for buffer B) and high resolution MS. High resolution MS/MS is optional. Middle-down analysis requires HPLC with at least three channels (one for loading buffer, one for buffer A and one for buffer B) and high resolution MS and MS/MS with electron transfer dissociation (ETD) as fragmentation technique.
3. Methods
Carry out all procedures at room temperature, unless otherwise specified.
3.1. Cell harvest from tissue culture
In case cells in suspension are grown, centrifuge cells at 300 rcf for 5–10 min. In case attached cells are grown, trypsinize cells, stop the trypsinization and centrifuge at 300 rcf for 5–10 min
Remove supernatant
Resuspend cells in PBS and transfer them in a 15 or 50 mL conical tube, depending on the volume of the suspension
Centrifuge cells at 300 rcf for 5–10 min and remove supernatant
Add PBS for a second wash and repeat Step 4
-
Estimate the volume of cell pellets
PAUSE – Sample can be frozen into liquid nitrogen and stored at −80 °C
Continue with cell nuclei isolation (section 3.3)
3.2 Cell harvest from tissue (alternative to 3.1)
Dissect out desired tissue and rinse with ice-cold PBS
Mince fresh or frozen tissue with a razor blade into small pieces to increase surface contact for nuclei isolation
-
Collect minced tissue in microcentrifuge tubes or 15 mL conical tubes and estimate the volume of tissue
PAUSE – Sample can be frozen into liquid nitrogen and stored at −80 °C
Continue with cell nuclei isolation (section 3.3)
3.3. Cell nuclei isolation
Add protease inhibitors and other inhibitors to NIB-250 buffer. For 1 mL of cell pellet, approximately 50 mL of NIB-250 buffer is prepared. Add to 50 mL NIB-250 buffer 50 μL of 1 M DTT, 125 μL of 200 mM AEBSF, 100 μL of 2.5 μM microcystin and 100 μL of 5 M sodium butyrate
Lyse the cell pellet with 10:1 (v/v) ratio of NIB-250 + inhibitors + 0.2% NP-40 Alternative
Homogenize with the appropriate instrument. For instance, liver samples can be homogenized using pestles or dounce homogenizers. Tissue culture cells can be homogenized by gentle pipetting
Incubate homogenized cells on ice for 5–10 min; the cells will lyse and release nuclei
Centrifuge at 1000 rcf for 5–10 min at 4 °C. The pellet contains mostly cell nuclei, while the supernatant contains mostly cytoplasmic components
Wash the nuclei pellet by gently resuspending with 10:1 (v/v) NIB-250 + inhibitors. Do not add NP-40 Alternative anymore, as detergents should be removed previous histone extraction
Centrifuge at 1000 rcf for 5 min at 4 °C and remove supernatant
-
Repeat Step 6–7 from two to four times to completely remove NP-40 Alternative. Removal of NP-40 Alternative is evident as gentle pipetting during the washing step does not form bubbles anymore
PAUSE – Optionally, sample can be resuspended in the minimum volume possible of NIB-250 + inhibitors + 5% glycerol, and stored at −80 °C
Continue to purification of histone proteins (section 3.4). Alternatively, nuclei can be used to perform immunoprecipitation of nucleosomes or other chromatin-binding proteins. For analysis of histone PTMs enriched from immunoprecipitation experiments skip directly to derivatization and proteolytic digestion of histones (section 3.9)
3.4. Purification of histone proteins
Histones are highly enriched in basic amino acid residues. This property highly facilitates their interaction with DNA, which has a backbone containing phosphoric acid. The described histone extraction protocol is based on their acid solubility (with 0.2 M H2SO4) followed by precipitation with highly concentrated TCA (33%) (see Note 1 for alternative protocol).
Resuspend cell nuclei with 0.2M H2SO4 with about 5 times the volume of the nuclei pellet by gentle pipetting
Incubate the sample with constant rotation or gentle shaking for 2–4 h at 4 °C. For samples with more than 500 μL cell pellet, a 2-h extraction is enough incubation time. Longer incubation is not recommended, as other basic proteins will be also extracted. For small sample size (<200 μL cell pellet), 4-h extraction gives a better yield
Centrifuge at 3400 rcf for 5 min
Transfer the supernatant to a new 1.5 or 15 mL tube, depending on the sample volume
Repeat Steps 3–4
Add 100% TCA to the sample solution with a ratio of 1:3 (v/v), in order to obtain a final TCA concentration of 33%. This step will precipitate histones
Let the mixture precipitate on ice for at least 1 h. Do not disturb the precipitation. For samples that start with small cell numbers, overnight precipitation is recommended
Centrifuge at 3400 rcf for 5 min. Remove the supernatant by aspiration without touching the precipitated proteins. In particular, do not touch the white layer condensed around the bottom of the tube, as these are the histones. The pellet in the very bottom of the tube contains mostly other proteins or other biomolecules
By using a glass Pasteur pipette rinse the tube with acetone + 0.1%HCl to cover the precipitated proteins
Centrifuge at 3400 rcf for 2 min and discard supernatant
Repeat Steps 9–10 using acetone without 0.1% HCl
Dry pellet with air flow or with a SpeedVac centrifuge, or just by leaving the tube open. Acetone evaporates quickly
Dissolve the histones with ddH2O in the minimum volume possible to dissolve completely the white layer. For pellets in a 1.5 mL microcentrifuge tube, 100 μL ddH2O is usually enough
-
Centrifuge at 3400 rcf for 2 min and transfer the supernatant to a new tube. Discard the pellet at the very bottom of the tube, as it contains mostly non-histone proteins and other biomolecules
PAUSE – Sample can be stored at −80 °C. Before freezing collect few μL for quality control of histone extraction (section 3.5)
3.5 Quality control of histone extraction
Measure protein concentration. BCA, Bradford protein assay or amino acid analysis (AAA) are recommended. Do not use techniques that adopt absorbance at 280 nm, as histones are poor in aromatic amino acid residues
Verify the purity of extracted histones with SDS gel and coomassie staining (optional)
If high purity of single histone variants is desired continue to HPLC-UV fractionation of histone variants (section 3.6). Alternatively, skip directly to sample preparation for either bottom-up or middle-down histone PTM analysis (sections 3.7 and 3.12, respectively)
3.6. Online HPLC-UV fractionation of histone variants (optional)
The crude histone mixture can be fractionated with reversed-phase HPLC coupled to a UV detector. This step allows for purification of histone variants and thus leads to more sensitive analyses for single histones as compared to the analysis of the crude histone mixture (see Note 2 for recommendations on when to perform this step).
Connect a C18 5 μm column to an HPLC. We recommend either a 4.6 x 250 mm or a 2.1 mm x 250 mm column. For the first, use a flow-rate of ~0.8 mL/min, for the second a flow-rate of ~0.2 mL/min. Use buffer A and buffer B as described in Buffers (section 2.2, number 5 and 6)
Connect the other extreme of the column to a UV detector, and set the absorbance to 210–220 nm
Acidify the histone sample dissolved in water with 100% TFA to achieve a final concentration of 0.1–1% TFA
Equilibrate the column with 100% buffer A for at least 15 minutes at the recommended flow-rate, which corresponds approximately to three column volumes. Use this signal to set the zero-absorbance level of the UV detector
Prepare 1.5 or 15 mL tubes to collect fractions or, if available, an automatic sample collector
Inject sample at the concentration of ~1 μg/μL or higher, if using 2.1 mm ID column, or at the concentration of ~0.75 μg/μL or higher, if using 4.6 mm ID column. Samples dissolved in larger volumes might alter the equilibration of the column during loading and lead to lower retention
Run the gradient, programmed as follows: from 0 to 30% B in 1 min, 30 to 60% B in 50 min, and 60 to 90% B in 1 min
Collect 1–2 min fractions between 10 and 50 min (see Note 3 for further instructions). Elution of histone variants is displayed in figure 1
-
Merge fractions containing the same chromatographic peak and dry down in a SpeedVac centrifuge
PAUSE – Sample can be stored at −80 °C as dry or reconstituted in ddH2O
Continue to sample preparation for bottom-up or middle-down histone PTM analysis (sections 3.7 and 3.12, respectively)
3.7. Propionic anhydride derivatization prior histone digestion for bottom-up analysis
The bottom-up strategy is the most commonly used MS-based proteomics strategy for histone characterization, as it is based on histone digestion into short peptides (5–20 aa), which facilitates both HPLC separation and MS detection (Fig. 2). Masses in the range of 600–2000 Da are commonly more easily ionized, and identified with higher mass accuracy and resolution than larger masses. Smaller masses are instead hard to retain by chromatography. MS/MS fragmentation is also facilitated, as short peptides are generally well-suited for collision induced dissociation (CID). However, histones are highly enriched in basic amino acid residues such as lysine and arginines. Therefore, trypsin digestion leads to the generation of too short peptides for HPLC retention and unambiguous localization of the PTMs. Our protocol includes a step of lysine and peptide N-terminal chemical derivatization [9]. We use propionic anhydride for this purpose, as we recently proved that it is the most suitable anhydride for the purpose [10]. Such derivatization blocks the ε-amino groups of unmodified and monomethyl lysine residues, allowing trypsin to perform proteolysis only at the C-terminal of arginine residues. Moreover, derivatized lysine residues, contrary to unmodified ones, cannot exchange protons with the solution and thus the peptides are generally only doubly or triply charged, facilitating MS and MS/MS detection. N-terminal derivatization increases peptide hydrophobicity and thus reversed-phase chromatographic retention (see Note 4 for alternative protocol).
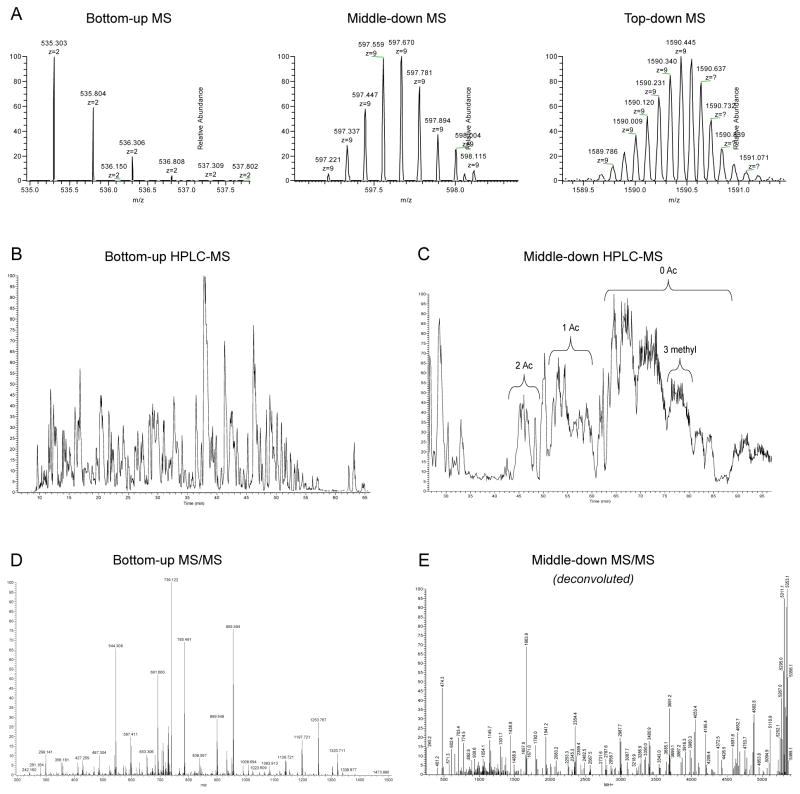
Figure 2. comparison of HPLC-MS performance for bottom-up and middle-down analysis.
A) Full MS scan performed at 60,000 resolution with an Orbitrap Fusion (Thermo Fisher Scientific) of a bottom-up-like peptide (1 kDa), a middle-down-like peptide (5 kDa) and a mass comparable to an intact histone (14–15 kDa). Higher masses lead to lower efficiency in resolving the isotopic distribution of the analyzed molecule. B) Reversed-phase HPLC separation of a histone mixture digested with trypsin after derivatization with propionic anhydride. Peptides are eluted in sharp, mostly baseline separated, peaks. C) WCX/HILIC separation of histone H3 N-terminal tails. The different peaks correspond to the same peptide sequence with different number of PTM equivalents. Heavily methylated and acetylated peptides elute first, while poorly modified tails elute in the final part of the chromatography. D) MS/MS spectrum of a bottom-up like peptide (1 kDa). E) Deconvoluted MS/MS spectrum of a middle-down-like peptide (5 kDa), where ETD fragmentation was used. On the right side of the spectrum the precursor mass and its respective neutral losses are the most abundant species in the spectrum, indicating that the fragmentation is not complete
Dissolve histone samples in 30 μL of 50 mM NH4HCO3, pH 8.0 (recommended amount: 50–100 μg). If samples were in pure ddH2O, add concentrated NH4HCO3 until reaching 50 mM
Dip a P10 pipette tip into the sample and touch with this a pH indicator strip to monitor the pH. It should be enough to have an idea of the current pH without having sample losses. NH4OH and glacial acetic acid can be used to adjust the pH (see Note 5 for safety instructions)
Prepare propionylation reagent by mixing propionic anhydride with 2-propanol in the ratio 1:3 (v/v); e.g. for three samples that have the volume of 20–30 μL, mix 15 μL of propionic anhydride and 45 μL of 2-propanol. This reagent must be made fresh every 3–4 samples (see Note 6 for details regarding reagents’ reactivity)
Add rapidly the propionylation reaction to the histone sample with a ratio of 1:2 (v/v); e.g. 15 μL propionylation reaction for 30 μL sample
-
Add rapidly NH4OH to re-establish pH 8.0 to the solution. Propionic anhydride reacting with the free amines of the peptides produces propionic acid that decreases pH. Usually, adding NH4OH to the sample with a ratio of 1:5 (v/v) is appropriate to re-establish pH 8.0; e.g. 6 μL of NH4OH to 30 μL of sample
WARNING – When pH is larger than 10.0, labeling of other amino acid residues with higher pKa is possible
Mix immediately by vortex
Check pH with the same procedure as Step 2
Briefly centrifuge and incubate samples at 37 °C on a heat block or in a water bath for 15 min
Repeat Steps 3–8, always taking care to not perform the reaction for more than 3–4 samples per batch of propionylation reagent
Dry samples down to 5–10 μL in a SpeedVac centrifuge. This evaporates unreacted propionic anhydride, 2-propanol, acetic acid and ammonia gas released from NH4OH. If samples dry out completely, no significant sample losses occur
Resuspend or dilute samples with ddH2O until achieving 30 μL of final volume
-
Repeat Steps 2–10. A double round of histone propionylation ensures >95% of reaction completion
PAUSE – Sample can be stored at −80 °C as dry or reconstituted in ddH2O
Continue with with proteolytic digestion with trypsin (section 3.8)
3.8. Proteolytic digestion with trypsin (in solution)
Resuspend histones in 50 mM NH4HCO3 to achieve a concentration of 1 μg/μL or higher. More diluted samples lead to lower trypsin efficiency
Verify that pH is about 8.0
Add trypsin to histone samples at a 1:20 ratio; e.g. 5 μg of trypsin for 100 μg of histones
Incubate at 37 °C for 6 h
Stop the digestion by adding 2–5 μL (or more) of glacial acetic acid to reach pH 3.0, or 1–2 μL of TFA
-
Dry down the sample to 5–10 μL in a SpeedVac centrifuge
PAUSE – Sample can be stored at −80 °C
Continue with propionylation of histone peptides at N-termini after trypsin digestion (section 3.10)
3.9 Derivatization and proteolytic digestion of histones (in gel – alternative to 3.7–8)
This part of the protocol should be used for histones loaded in gel. Protein separation using SDS-PAGE is an efficient technique to achieve both sample fractionation and removal of detergents (e.g. if sample is an elution from immunoprecipitation). This part is alternative to sections 3.7 and 3.8. Section 3.10 is in common for both in solution and in gel histone derivatization and digestion.
Excise the histone fraction from the polyacrylamide gel. Cut as close to the protein band as possible to reduce the amount of background
Cut the excised piece into ~1 mm3 cubes and transfer them to a clean 1.5 mL or 0.5 mL microcentrifuge tube
Wash the gel pieces with ddH2O corresponding to 5 times gel volume. For washing the tubes can be left for 15 min on a shaker or vortex
Remove water, and replace it with the same volume of 50% acetonitrile. Repeat the wash
Remove the solution. If gel bands are still heavily stained repeat Steps 3–4
Remove the solution and replace it with the same volume of 100% acetonitrile. Repeat the wash
Remove acetonitrile, taking care to not aspirate shrunk gel pieces
Add 50 μL (or sufficient volume to cover gel pieces) of 100 mM NH4HCO3, immediately followed by 100 μL of propionic anhydride. Quickly vortex and incubate for 20 min at room temperature
Spin down and remove supernatant
Wash with 500 μL of 100 mM NH4HCO3
Repeat Step 10 once or twice. Check pH of the second wash, if not ~8.0 repeat the wash
Remove supernatant and add 5 times gel volume of acetonitrile to shrink gel pieces
Remove acetonitrile
Repeat Steps 8–13 to assure completion of derivatization
On ice (4 °C) rehydrate gel particles with digestion buffer (50 mM NH4HCO3, and 12.5 ng/μL trypsin). Add enough digestion buffer to cover the gel pieces. If after 2 minutes all the initially added volume has been absorbed by the gel pieces add 20 μL more digestion buffer
Incubate at room temperature overnight
The next day transfer supernatant with peptides to a clean 1.5 mL tube. The supernatant contains the digested peptides eluted from gel bands. To increase peptide recovery (e.g. low sample amounts), follow the Steps 18–21
Add 20 μL of ddH2O and wash gel pieces for 15 min
Add the same volume of acetonitrile and wash gel pieces for 15 min
Transfer the supernatant to the same 1.5 mL tube of Step 17
Repeat Steps 18–20
-
Dry the sample in a SpeedVac centrifuge
PAUSE – Sample can be stored dry at −80 °C
Continue with propionylation of histone peptides at N-termini after trypsin digestion (section 3.10)
3.10. Propionylation of histone peptides at N-termini after trypsin digestion
This section describes the derivatization of peptide N-termini. Such procedure is not essential for most of histone peptides, but it facilitates the HPLC retention of the shortest ones (e.g. aa 3–8 histone H3), as the propionyl group increases peptide hydrophobicity (see Note 7 for modifications of the protocol that includes multiplexing).
Resuspend samples in 30 μL of 100 mM NH4HCO3
Repeat Steps 2–12 of section 3.7, also if in gel digestion was performed. In case light and heavy anhydride is used, perform propionylation with the light form in one sample, and with the heavy form in the other sample
-
Resuspend or dilute samples with 50–100 μL ddH2O + 0.1% TFA. If propionylation with light and heavy anhydride was performed, samples can now be mixed together
PAUSE – Sample can be stored at −80 °C
Continue to sample desalting with Stage-tips (section 3.11). If your HPLC-MS setup is equipped with a trap column skip directly to preparation of the nano HPLC setup for online HPLC-MS analysis (section 3.13)
3.11. Sample desalting with Stage-tips (this step can be omitted if using trap column in HPLC-MS)
The protocol we describe leads to presence of salts in the sample at this stage of the preparation. Salts are detrimental for HPLC-MS analysis. First, ionized salts are also injected into the mass spectrometer, contributing in suppressing the signal of the peptides and contaminating the instrument. Moreover, salts might form ionic adducts with peptides, reducing the signal intensity of the “clean” peptide, as a percentage of such peptide would be detected with a different molecular weight. This prevents efficient identification and quantification of the given peptide. Desalting can be performed offline with Stage-tips or online when the HPLC-MS setup consists of a two column system (Fig. 3). In this section we describe the offline protocol.
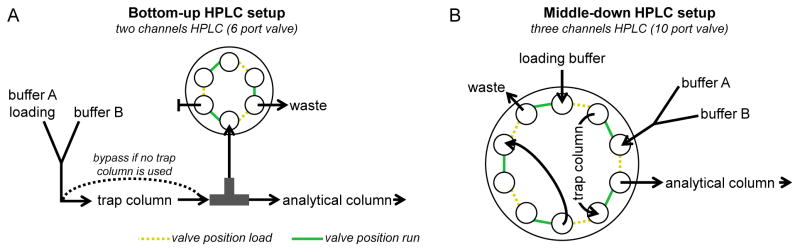
Figure 3. Column configuration for online HPLC-MS.
A) Connection of trap and analytical column for bottom-up analysis. The arrows represent the direction of the flow. During loading the loading buffer, which is also the buffer A, is pumped through the trap column and to the waste, as the valve leaves open the waste line. During the run the valve blocks the tee and the gradient flows through trap and analytical column. If trap column is omitted both load and run is performed with the valve in the position run, while the valve in position load is used only to rapidly change buffer composition without system backpressure. B) Connections for middle-down analysis. During loading the loading buffer is pumped through the trap column, connected to generate a loop within the valve, while the analytical column can be equilibrated with buffer A
By using a P1000 pipette tip cut a disk of C18 material from a 3 M Empore™ Solid Phase Extraction Disk C18, and deposit this minidisk to the bottom of a P100/200 pipette tip. You can push the minidisk out of the P1000 tip by using a fused silica capillary. Ensure that the disk is securely wedged in the bottom of the tip
Repeat Step 1 in the same P100/200 tip if you are desalting more than 25 μg of sample
Wash disc by flushing 100 μL of 75% acetonitrile and 0.025% TFA with air pressure, e.g. using a syringe (see Note 8 for alternative procedure)
Equilibrate disk by flushing 50 μL of 0.1% TFA by air pressure
Load sample onto the disk by applying air pressure
Wash sample by flushing 50 μL of 0.1% TFA by applying air pressure
Elute sample by flushing 50 μL 75% acetonitrile and 0.025% TFA by air pressure. Collect the sample in a 0.5 or 1.5 mL tube
-
Dry sample in a SpeedVac centrifuge to ~5 μL
PAUSE –Sample can be stored at −80 °C
Continue to preparation of the nano HPLC setup for online HPLC-MS analysis (section 3.13)
3.12. Sample preparation for middle-down histone PTM analysis (alternative to sections 3.7–11)
The middle-down strategy takes advantage of the fact that the N-terminal tail of the histones can be proteolytically digested by GluC, an enzyme that cleaves at the C-terminal of the glutamic acid residue. This generates a polypeptide of 40–50 aa residues (5–6 kDa) that contain the majority of histone PTMs. For instance, histone H3 isotypes in mammals and many model organisms contain the first glutamic acid in position 50. This strategy is an effective compromise between bottom-up and top-down (intact protein analysis), as it allows precise mapping and quantification of single PTMs, still technically challenging with top-down, and combinatorial PTMs, not possible with bottom-up.
Resuspend histones in 50 mM NH4HCO3 (pH 8.0) to achieve a concentration of 1 μg/μL or higher. More diluted samples lead to lower GluC efficiency. Alternatively, histones can be resuspended in 50 mM ammonium acetate (NH4C2H3O2, pH 4.0). At pH > 10 deamidation of glutamine has high kinetics; this is an issue as glutamine is present on all histone tails and, if deamidated, it is converted into glutamic acid
Add GluC to the sample at a 1:20 enzyme:sample ratio (w/w); e.g. 5 μg of GluC for 100 μg of histones
Incubate at room temperature for 6 h. Higher temperatures increase deamidation kinetics
-
Stop the digestion by adding 2–5 μL (or more) of glacial acetic acid to reach pH 3.0, or 1–2 μL of TFA
PAUSE – Freeze samples at −80 °C until analysis
Continue with preparation of the nano HPLC setup for online HPLC-MS analysis (section 3.13)
3.13. Preparation of the nano HPLC setup for online HPLC-MS analysis
Online HPLC-MS in proteomics is commonly performed using nano liquid chromatography. This is because nanoHPLC allows for loading of low amounts of material, and guarantees high sensitive analyses. However, particular attention must be used when preparing the HPLC setup, as small errors in column cuts or connections highly affect chromatographic performance. Here we describe how to prepare nanoHPLC columns (steps 1–10 can be omitted if using commercial columns) and how to configure the HPLC setup for bottom-up and middle-down analysis.
Cut ~30 cm of fused silica capillary in which you wish to make a frit. We recommend the use of 75 μm internal diameter (ID) capillaries for analytical columns and 100 μm ID for trap columns
Transfer 88 μL Kasil® to a 0.5 mL tube
Add 16 μL formamide to the tube and vortex quickly for 10–15 seconds. Formamide is toxic, and all the necessary safety precautions should be taken
Dip 1 cm of fused silica into the mixture and remove quickly. The mixture will enter into the capillary for about 1–2 cm by capillarity
Leave the fused silica overnight for polymerization. Alternatively, polymerization can be catalyzed by placing the capillary in a heater at ~ 110 °C for 3–4 hours
Cut the frit to leave no more than 3–4 mm at the top of the column. The frit should appear bright below illumination. The fused silica is now ready for packing (see Note 9 for alternative procedure)
Prepare in a clean HPLC glass vial the resin slurry for column packing in 100% methanol or any other organic solvent and add a micro-stir magnet. Use C18-AQ reversed-phase 3 μm particles for trap columns and bottom-up analytical column, and Polycat A resin 3 μm 1500 Å pore size for middle-down analytical column
Place the resin slurry in a pressure bomb and turn on magnetic stirring
Place the Kasil-fritted fused silica in the pressure bomb. Pressure is delivered by a gas bomb, containing helium, nitrogen or air. Traditional pressure bombs cannot stand pressures above 100–150 bars. Verify that value on the pressure limiting valve placed on the gas bomb
-
Turn on pressure and leave the column packing. The recommended lengths for columns are:
15–18 cm for C18 analytical column for bottom-up analysis. The column can be packed indefinitely, and then cut the desired length
10–12 cm for Polycat A analytical column for middle-down analysis. The column can be packed indefinitely, and then cut the desired length
1.5 cm for trap columns. Make sure that you turn off the pressure at the desired length, as it is not possible to cut a capillary so short and then make the HPLC connections. Leave at least 5 cm of empty capillary for bottom-up analysis, and 8 cm for middle-down
Connect the trap and the analytical column as indicated in figure 3. For the bottom-up analysis the trap column can be omitted if Stage-tips desalting was performed (section 3.11)
Continue to bottom-up or middle-down analysis of histone peptides (sections 3.14 and 3.15, respectively)
3.14. Bottom-up analysis of histone peptides
At this stage the histone sample and the HPLC setup are ready. It is possible now to proceed to the HPLC-MS/MS analysis. The method described is meant to be used for the columns we previously recommended (section 3.13).
Program the HPLC method as follows: from 0 to 30% buffer B in 30 min, from 30 to 100% B in 5 min and 8 min at isocratic 100% B. If the HPLC is not programmed for automated column equilibration before sample loading then include this part in the method: from 100 to 0% B in 1 min and isocratic flow at 0% B for 10 min. The flow rate of the analysis should be 250–300 nL/min
Program the MS acquisition method to perform MS/MS data dependent acquisition. With C18 chromatography the average baseline peak width is about 30 seconds for the gradient we described. Make sure that the MS duty cycle allows one full MS scan every ~2 seconds, in order to have enough data points to draw accurately the peak shape of the eluted peptides.
Include in the MS acquisition method targeted MS/MS for peptides that have isobaric species (displayed with an asterisk in Table 2). These peptides need to be selected for fragmentation through their entire elution, as the discrimination of the relative abundance of the isobaric species is performed by monitoring the elution profile of the fragment ions. All the other settings are common to other standard proteomics experiments
Load ~1 μg of sample onto the HPLC column
Run the HPLC-MS/MS method as programmed
Perform label-free quantification by extracting the area below the curve of the chromatographic peak for each peptide. This step can be performed manually or with dedicated software. The area of the chromatographic peak should be calculated for the [M + H]+, [M + 2H]2+, and [M + 3H]3+ ions of the same peptide, even though in most cases the [M + 2H]2+ is the prevalent form (see Note 10 for further instructions on how to discriminate the differently modified peptides)
Calculate the relative abundance of each PTM by calculating the sum of all different modified forms of a histone peptide (100%), and divide the area of the particular peptide by the total histone peptide. When isobaric species are present, e.g. K18ac and K23ac, MS/MS information is used to find the ratio between the two species (Fig. 4). This ratio is used to divide the area of the chromatographic peak between the two species
Table 2. peptides of most common interest in bottom-up histone analysis.
The table displays the histone variant and the peptide position in the protein sequence. Each peptide is then present in all most common modified states, and we calculated their respective m/z signal for singly, doubly, triply and, where possible, quadruply charged forms.
| Histone | Peptide position | Modified peptide | (+1) | (+2) | (+3) | (+4) | Histone | Peptide position | Modified peptide | (+1) | (+2) | (+3) | (+4) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone H3 | 1 8 | ARTKQTAR | 1043.596 | 522.302 | 348.537 | Histone H3 | 41 49 | YRPGTVALR | 1088.622 | 544.815 | 363.546 | ||
| ARme1TKQTAR | 1057.612 | 529.310 | 353.209 | ||||||||||
| 54 63 | YQKSTELLIR | 1362.763 | 681.886 | 454.926 | |||||||||
| 3 8 | TKQTAR | 816.458 | 408.733 | YQKacSTELLIR | 1348.747 | 674.877 | 450.254 | ||||||
| TKme1QTAR | 830.474 | 415.741 | |||||||||||
| TKme2QTAR | 788.463 | 394.735 | 73 83 | EIAQDFKTDLR | 1447.743 | 724.376 | 483.253 | ||||||
| TKme3QTAR | 802.478 | 401.743 | EIAQDFKme1TDLR | 1461.759 | 731.383 | 487.925 | |||||||
| TphosKQTAR | 896.424 | 448.716 | EIAQDFKme2TDLR | 1419.748 | 710.378 | 473.921 | |||||||
| EIAQDFKme3TDLR | 1433.763 | 717.386 | 478.593 | ||||||||||
| 9 17 | KSTGGKAPR | 1069.601 | 535.304 | 357.205 | |||||||||
| Kme1STGGKAPR | 1083.616 | 542.312 | 361.877 | Histone H4 | 1 17 | acSGRGKGGKGLGKGGAKR | 1837.040 | 919.024 | 613.019 | 460.016 | |||
| Kme2STGGKAPR | 1041.606 | 521.307 | 347.874 | acSGRme1GKGGKGLGKGGAKR | 1851.056 | 926.032 | 617.690 | 463.520 | |||||
| Kme3STGGKAPR | 1055.621 | 528.314 | 352.545 | ||||||||||
| *KacSTGGKAPR | 1055.584 | 528.296 | 352.533 | 4 17 | GKGGKGLGKGGAKR | 1550.902 | 775.955 | 517.639 | 388.481 | ||||
| *KSTGGKacAPR | 1055.584 | 528.296 | 352.533 | *GKGGKGLGKGGAKacR | 1536.886 | 768.947 | 512.967 | 384.977 | |||||
| Kme1STGGKacAPR | 1069.600 | 535.304 | 357.205 | *GKGGKGLGKacGGAKR | 1536.886 | 768.947 | 512.967 | 384.977 | |||||
| Kme2STGGKacAPR | 1027.589 | 514.299 | 343.202 | *GKGGKacGLGKGGAKR | 1536.886 | 768.947 | 512.967 | 384.977 | |||||
| Kme3STGGKacAPR | 1041.605 | 521.306 | 347.873 | *GKacGGKGLGKGGAKR | 1536.886 | 768.947 | 512.967 | 384.977 | |||||
| KacSTGGKacAPR | 1041.568 | 521.288 | 347.861 | *GKacGGKGLGKGGAKacR | 1522.869 | 761.939 | 508.295 | 381.473 | |||||
| KSphosTGGKAPR | 1149.566 | 575.287 | 383.861 | *GKGGKacGLGKGGAKacR | 1522.869 | 761.939 | 508.295 | 381.473 | |||||
| Kme1SphosTGGKAPR | 1163.582 | 582.295 | 388.533 | *GKGGKGLGKacGGAKacR | 1522.869 | 761.939 | 508.295 | 381.473 | |||||
| Kme2SphosTGGKAPR | 1121.571 | 561.290 | 374.529 | *GKacGGKGLGKacGGAKR | 1522.869 | 761.939 | 508.295 | 381.473 | |||||
| Kme3SphosTGGKAPR | 1135.587 | 568.297 | 379.201 | *GKGGKacGLGKacGGAKR | 1522.869 | 761.939 | 508.295 | 381.473 | |||||
| *GKacGGKacGLGKGGAKR | 1522.869 | 761.939 | 508.295 | 381.473 | |||||||||
| 18 26 | KQLATKAAR | 1154.690 | 577.849 | 385.568 | *GKGGKacGLGKacGGAKacR | 1508.853 | 754.930 | 503.623 | 377.969 | ||||
| *Kme1QLATKAAR | 1168.705 | 584.857 | 390.240 | *GKacGGKGLGKacGGAKacR | 1508.853 | 754.930 | 503.623 | 377.969 | |||||
| *KQLATKme1AAR | 1168.705 | 584.857 | 390.240 | *GKacGGKacGLGKGGAKacR | 1508.853 | 754.930 | 503.623 | 377.969 | |||||
| *KacQLATKAAR | 1140.674 | 570.841 | 380.896 | *GKacGGKacGLGKacGGAKR | 1508.853 | 754.930 | 503.623 | 377.969 | |||||
| *KQLATKacAAR | 1140.674 | 570.841 | 380.896 | GKacGGKacGLGKacGGAKacR | 1494.837 | 747.922 | 498.951 | 374.465 | |||||
| KacQLATKacAAR | 1126.657 | 563.833 | 376.224 | ||||||||||
| 20 23 | KVLR | 627.419 | 314.214 | ||||||||||
| 27 36 | KSAPATGGVKKPHR | 1657.939 | 829.473 | 553.318 | 415.241 | Kme1VLR | 641.435 | 321.221 | |||||
| *Kme1SAPATGGVKKPHR | 1671.955 | 836.481 | 557.990 | 418.745 | Kme2VLR | 599.424 | 300.216 | ||||||
| *Kme2SAPATGGVKKPHR | 1629.944 | 815.476 | 543.987 | 408.242 | Kme3VLR | 613.440 | 307.224 | ||||||
| *Kme3SAPATGGVKKPHR | 1643.959 | 822.483 | 548.658 | 411.746 | |||||||||
| KacSAPATGGVKKPHR | 1643.923 | 822.465 | 548.646 | 411.737 | 68 78 | DAVTYTEHAKR | 1402.697 | 701.852 | 468.237 | ||||
| *KSAPATGGVKme1KPHR | 1671.955 | 836.481 | 557.990 | 418.745 | |||||||||
| *KSAPATGGVKme2KPHR | 1629.944 | 815.476 | 543.987 | 408.242 | Histone H2A | 82 88 | HLQLAIR | 906.552 | 453.780 | ||||
| *KSAPATGGVKme3KPHR | 1643.959 | 822.483 | 548.658 | 411.746 | |||||||||
| Kme1SAPATGGVKme1KPHR | 1685.970 | 843.489 | 562.662 | 422.248 | 4 11 | GKQGGKAR | 969.548 | 485.278 | |||||
| *Kme1SAPATGGVKme2KPHR | 1643.959 | 822.483 | 548.658 | 411.746 | *GKQGGKacAR | 955.532 | 478.270 | ||||||
| *Kme1SAPATGGVKme3KPHR | 1657.975 | 829.491 | 553.330 | 415.250 | *GKacQGGKAR | 955.532 | 478.270 | ||||||
| *Kme2SAPATGGVKme1KPHR | 1643.959 | 822.483 | 548.658 | 411.746 | GKacQGGKacAR | 941.516 | 471.262 | ||||||
| Kme2SAPATGGVKme2KPHR | 1601.949 | 801.478 | 534.655 | 401.243 | |||||||||
| *Kme2SAPATGGVKme3KPHR | 1615.964 | 808.486 | 539.327 | 404.747 | H2A.Z | 1 19 | AGGKAGKDSGKAKTKAVSR | 2153.193 | 1077.100 | 718.403 | 539.054 | ||
| *Kme3SAPATGGVKme1KPHR | 1657.975 | 829.491 | 553.330 | 415.250 | *AGGKacAGKDSGKAKTKAVSR | 2139.177 | 1070.092 | 713.731 | 535.550 | ||||
| *Kme3SAPATGGVKme2KPHR | 1615.964 | 808.486 | 539.327 | 404.747 | *AGGKAGKacDSGKAKTKAVSR | 2139.177 | 1070.092 | 713.731 | 535.550 | ||||
| Kme3SAPATGGVKme3KPHR | 1629.979 | 815.494 | 543.998 | 408.251 | *AGGKAGKDSGKacAKTKAVSR | 2139.177 | 1070.092 | 713.731 | 535.550 | ||||
| KSphosAPATGGVKKPHR | 1737.905 | 869.456 | 579.973 | 435.232 | *AGGKAGKacDSGKacAKTKAVSR | 2125.160 | 1063.084 | 709.059 | 532.046 | ||||
| Kme1SphosAPATGGVKKPHR | 1751.920 | 876.464 | 584.645 | 438.736 | *AGGKacAGKDSGKacAKTKAVSR | 2125.160 | 1063.084 | 709.059 | 532.046 | ||||
| Kme2SphosAPATGGVKKPHR | 1709.910 | 855.459 | 570.642 | 428.233 | *AGGKacAGKacDSGKAKTKAVSR | 2125.160 | 1063.084 | 709.059 | 532.046 | ||||
| Kme3SphosAPATGGVKKPHR | 1723.925 | 862.466 | 575.314 | 431.737 | AGGKacAGKacDSGKacAKTKAVSR | 2111.144 | 1056.076 | 704.387 | 528.542 | ||||
| H2A.X | 4 11 | GKTGGKAR | 942.537 | 471.772 | macroH2A | 15 26 | SAKAGVIFPVGR | 1313.758 | 657.383 | 438.591 |
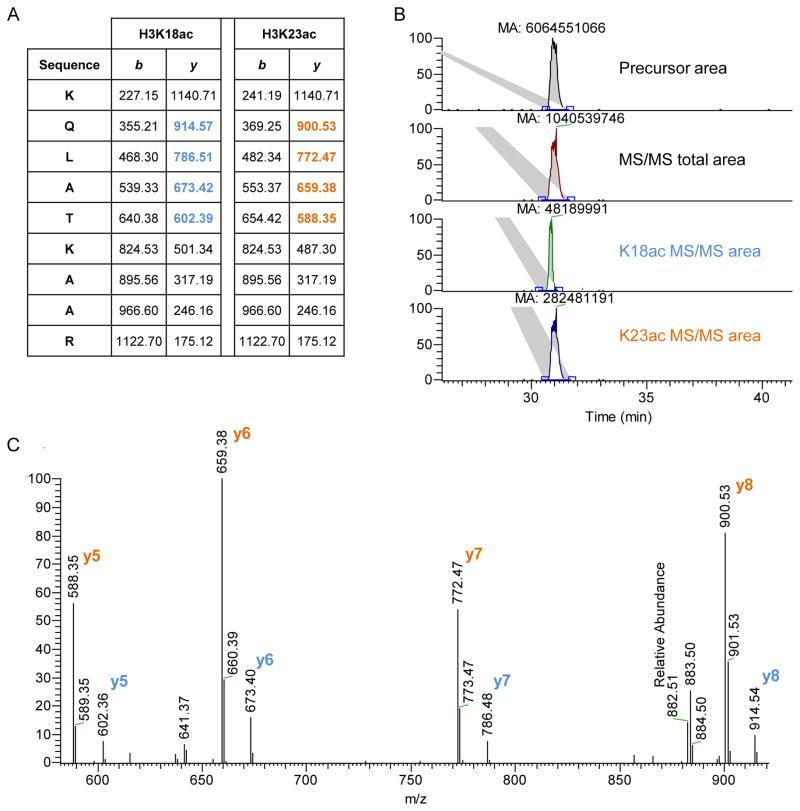
Figure 4. quantification of two co-eluted isobaric peptides.
A) The histone H3 peptide KQLATKAAR (aa 18–26) was found acetylated in both K18 and K23 residues. The two peptides generate different MS/MS fragments. We used the fragments y5–8 to calculate their relative abundance, as they were the most intense ones (highlighted). B) Extracted ion chromatogram of the precursor mass corresponding to the peptide sequence + one acetyl group (top) and the ion chromatography of the targeted MS/MS scans (top-middle). The extracted MS/MS ion chromatography generates a smaller area than the extracted precursor mass, as the fragmented peptide has a lower signal than the precursor mass. Below, extracted MS/MS ion chromatography of the fragments y5–8 of the K18ac (bottom-middle) and the K23ac peptide (bottom). The area of the K23ac chromatogram is about 6 times higher than K18ac. The total precursor area should then be divided by the two species according to their calculated ratio. C) MS/MS spectrum of co-fragmented K18ac and K23ac peptides. Also from the single MS/MS spectrum it is possible to calculate the ratio between K23ac and K18ac, which is about 6-folds.
3.15. Middle-down analysis of intact histone tails (alternative to section 3.14)
WCX/HILIC is currently the best suited column material to online separate histone tails. Large basic and hydrophilic polypeptides bind efficiently in high organic solvent (75%) and near-neutral pH (6.00), since the hydrophilic stationary phase contains glutamic acid that deprotonates and generates ionic bonds with positively charged polypeptides. Elution is performed with a gradient of water and decreasing pH, avoiding the use of salts that are potentially detrimental for MS. Detection is performed with high resolution MS/MS and ETD fragmentation. Afterwards, database searching is mandatory to follow our workflow, which is currently the only one publicly available to map and quantify precisely single and combinatorial histone PTMs with middle-down proteomics. The method described is meant to be used with the column type and configuration we described previously (section 3.13).
Program the HPLC method as follows: from 0 to 55% buffer B in 1 min, from 55 to 85% B in 160 min and from 85 to 100% in 5 min. If the HPLC is not programmed for automated column equilibration before sample loading then include this part in the method: switch the valve in position load (Fig. 3), from 100 to 0% B in 1 min and isocratic flow at 0% B for 10 min. The flow rate of the analysis should be 250–300 nL/min
Program the MS acquisition method to perform MS/MS data dependent acquisition of the 6–8 most abundant precursor masses without dynamic exclusion. The full MS scan range should be 450–750 m/z to avoid repetitive selection of the same peptides in multiple charge states. If only histone H3 is analyzed, the window can be narrowed to 660–720 m/z
Program the MS/MS acquisition to be performed with ETD at a resolution of ~30,000. The reaction time should be around 20 ms for polypeptides with 8–10 charges. Include 3 microscans to improve the quality of the MS/MS spectra acquired, as ETD spectra are overall less reproducible than CID. If using a trapping mass analyzer, i.e. orbitrap, please note that the automatic gain control should be increased of about one order of magnitude as compared to traditional peptide fragmentation. This is because the number of histone tails accumulated is underestimated by the ion counter, as each of them is heavily charged. All the other settings are common to other standard proteomics experiments
Load ~2 μg of sample onto the HPLC trap column
Run the HPLC-MS/MS method as programmed. The HPLC elution profile should look like in figure 2C if only histone H3 is analyzed
Continue to middle-down data processing (section 3.16)
3.16. Middle-down data processing
While bottom-up LC-MS runs do not necessarily need a proper database searching, in the case of middle-down it is mandatory with our developed workflow. In middle-down each precursor mass might easily correspond to more than 30 isobaric peptides (value estimated in [11]), which should be discriminated at the MS/MS level. Peptide-spectrum match validation and peptide quantification are performed with our in-house developed bioinformatics tools, freely available at http://middle-down.github.io/Software. However, they require the result file of Mascot (Matrix Science, UK) as input.
Collect all raw files and submit them to a deconvolution tool. MS/MS spectra ions should be all singly charged previous Mascot database searching. We recommend Xtract as deconvolution algorithm if Thermo Fisher Scientific instrument is used (e.g. LTQ-Orbitrap). Xtract can be directly used in Proteome Discoverer (Thermo Fisher Scientific, Bremen, Germany) as workflow node. Alternatively, any other deconvolution algorithm that generates Mascot Generic Format (.mgf) files can be used
Perform database searching using the following parameters: MS mass tolerance: 2.2 Da, to include possible errors of the deconvolution algorithm in isotopic recognition. MS/MS mass tolerance: 0.01 Da. Enzyme: GluC with no missed cleavages allowed. No static modifications. Variable modifications: mono- and dimethylation (KR), trimethylation (K), acetylation (K) and, optionally, phosphorylation (ST). The sequence database should contain only histones; large databases increase dramatically searching time
Export Mascot results in .csv file extension. In the export include the following information to the file: all Query level information, all the default information (already ticked when export page is opened)
Import the .csv file in isoScale slim, which you can find at http://middle-down.github.io/Software. Select the tolerance for the search (recommended: 30 ppm) and the type of fragmentation adopted. The result table is in the same folder where the software is located. This table contains the list of peptides that passed the site determining ions validation and their absolute and relative intensity. isoScale outputs the calculated relative abundance for each combinatorial PTM identified and validated (software principle described in [11])
-
The output table contains duplicates (peptides with the same sequence and PTM combination). Remove them by using the “Remove duplicates” option in Excel
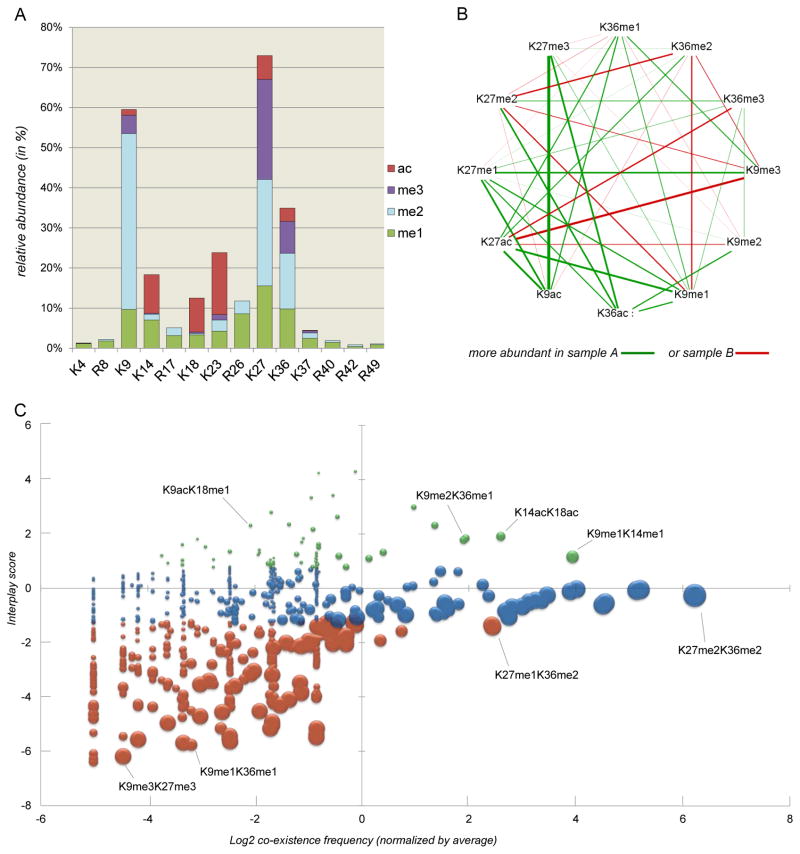
Middle-down is used to study PTM co-existence, but also to compare multiple conditions. However, all samples need to be run separately (multiplexing is still not possible). From the relative abundance of the combinatorial marks it is possible to extract the relative abundance of single marks simply by summing all relative abundances of peptides that contain the given PTM. In figure 5 we display some examples of how to represent middle-down data.
To estimate which histone marks tend to co-exist with each other with high or low frequency it is possible to calculate the interplay score [12,13]. This score is calculated as:
where Ixy is the interplay score between the marks X and Y, Fxy is the co-existence frequency (or relative abundance) of the two marks and Fx or Fy are the frequencies of the single marks in the dataset. In other words, Fxy is the observed co-existence frequency, while is the theoretical co-existence frequency, calculated based on the relative abundance of single PTMs. The interplay score provides how much two marks “like” to share the same histone tail. Positive values indicate tendency to co-exist higher than if the two marks were completely independent from each other, while negative values indicate the opposite. The interplay score calculated for binary PTMs quantified in middle-down experiments could be used as indicator to predict cross-talk between histone marks.
Figure 5. examples of middle-down data representation.
A) Bar plot of the relative abundance of single PTMs. Middle-down analysis allows for quantification of arginine methylations, while with bottom-up arginine is the cleavage site of the proteolytic enzyme. B) Comparison of co-existence frequency of binary marks in two conditions. The graph displays the relative abundance of co-existing marks in sample A (green) and sample B (red); e.g. the combination K9acK27me3 is the binary PTM with the highest A/B ratio. C) Bubble plot of binary PTMs. The graph displays three levels of information: the observed co-existence frequency of the binary marks (X axis), the interplay score of the two marks (Y axis) and the relative abundance of the single marks summed together (bubble size). The colors green, blue and red represent binary marks with interplay score higher than 1, between 1 and −1, and below −1, respectively. Single marks with high relative abundance (large bubble size) and with low co-existence frequency have intuitively low interplay score, as they are abundant marks that rarely share the same histone tail.
Footnotes
The high-salt extraction protocol can be used to purify histones [14] in alternative to TCA precipitation. Such protocol is intrinsically milder, as it does not use strong acid. This preserves acid-labile PTMs and increases the yield of extracted histones, as TCA precipitation co-precipitates many other chromatin binding proteins. However, high-salt extraction leads to samples containing too concentrated salt for HPLC-MS/MS. The Stage-tip step (section 3.11) is often not sufficient in this case. Salt removal might generate consistent sample losses, making high-salt extraction procedure not favorable for our workflow
Fractionation of intact histone variants ideally requires at least 100 μg of starting material (if 2.1 mm ID column is used), or 300 μg (if 4.6 mm ID column is used). In case the available sample is less than 25% of these references, we recommend avoiding HPLC-UV fractionation
The time and time windows of fraction collection might vary depending on your HPLC system and the collecting tubes in use. Perform a test run at first
Histone digestion for bottom-up proteomics analysis can be performed without propionylation, for instance by reducing trypsin incubation time and the enzyme/substrate ratio [15] or using ArgC as digestion enzyme [16–18]. However, we recommend our described protocol, as it leads to the generation of more hydrophobic peptides which are better retained during liquid chromatography
Propionic anhydride, NH4OH and acetic acid should be handled in the fumehood. After its use, the bottle of propionic anhydride should be filled with argon to prevent slow conversion to propionic acid due to water vapor from air
The propionylation mixture rapidly becomes inefficient, due to the conversion of propionic anhydride to propionic acid. For this reason, it is highly recommended to perform the reaction rapidly and for a limited number of samples for each batch
In bottom-up sample preparation it is possible to differentially derivatize peptide N-termini of two samples with light and heavy propionic anhydride. One sample can be modified with a D0 propionic anhydride (CH3CH2CO)2O, while the other with a D10 propionic anhydride (CD3CD2CO)2O at this step. This leads to a delta mass between the light and heavy labeled peptides of +5 Da and multiplexing analysis can be performed. The two samples should be mixed in equal amounts to obtain the least variation in ionization efficiency. The procedure to extract the area of heavy labeled peptides is equal to the light version
To desalt a large number of samples, centrifugation can be used instead of air pressure. Do not simply place the tips into 1.5 mL microcentrifuge tubes, as they might break. Use appropriate holders, or drill a hole on the top of the tube using a suitable size screw driver or a mini drill
Alternatively to preparing frit for in-house packed column, it is possible to pull a tip from one extreme of the capillary, if the lab is equipped with a laser tip puller. Such procedure can be performed only for the analytical column, as it can be directly placed at the front of the mass spectrometer
For the bottom-up analysis lysine acetylation (+42.011 Da) can be discriminated from the nearly isobaric trimethylation (+42.047 Da) by using high resolution MS acquisition (>30,000). Moreover, acetylation is more hydrophobic than trimethylation, leading to later elution of acetylated peptides as compared to the respective trimethylated ones. The unmodified form of the same peptide elutes even later, due to the fact that the lysine is propionylated. In summary, the order of hydrophobicity for a peptide with one modifiable site is di- and trimethylated < acetylated < unmodified (propionylated) < monomethylated (propionylated)
References
- 1.Waddington CH. Canalization of development and the inheritance of acquired characters. Nature. 1942;150:563–565. doi: 10.1038/150563a0. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293(5532):1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 4.van Attikum H, Gasser SM. Crosstalk between histone modifications during the DNA damage response. Trends Cell Biol. 2009;19(5):207–217. doi: 10.1016/j.tcb.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Capetillo O, Mahadevaiah SK, Celeste A, Romanienko PJ, Camerini-Otero RD, Bonner WM, Manova K, Burgoyne P, Nussenzweig A. H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev Cell. 2003;4(4):497–508. doi: 10.1016/S1534-5807(03)00093-5. [DOI] [PubMed] [Google Scholar]
- 6.Santaguida S, Musacchio A. The life and miracles of kinetochores. Embo Journal. 2009;28(17):2511–2531. doi: 10.1038/emboj.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egelhofer TA, Minoda A, Klugman S, Lee K, Kolasinska-Zwierz P, Alekseyenko AA, Cheung MS, Day DS, Gadel S, Gorchakov AA, Gu TT, Kharchenko PV, Kuan S, Latorre I, Linder-Basso D, Luu Y, Ngo Q, Perry M, Rechtsteiner A, Riddle NC, Schwartz YB, Shanower GA, Vielle A, Ahringer J, Elgin SCR, Kuroda MI, Pirrotta V, Ren B, Strome S, Park PJ, Karpen GH, Hawkins RD, Lieb JD. An assessment of histone-modification antibody quality. Nat Struct Mol Biol. 2011;18(1):91. doi: 10.1038/Nsmb.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidoli S, Cheng L, Jensen ON. Proteomics in chromatin biology and epigenetics: Elucidation of post-translational modifications of histone proteins by mass spectrometry. Journal of proteomics. 2012;75(12):3419–3433. doi: 10.1016/j.jprot.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Plazas-Mayorca MD, Zee BM, Young NL, Fingerman IM, LeRoy G, Briggs SD, Garcia BA. One-Pot Shotgun Quantitative Mass Spectrometry Characterization of Histones. Journal of proteome research. 2009;8(11):5367–5374. doi: 10.1021/Pr900777e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sidoli S, Yuan ZF, Lin S, Karch K, Wang X, Bhanu N, Arnaudo AM, Britton LM, Cao XJ, Gonzales-Cope M, Han Y, Liu S, Molden RC, Wein S, Afjehi-Sadat L, Garcia BA. Drawbacks in the use of unconventional hydrophobic anhydrides for histone derivatization in bottom-up proteomics PTM analysis. Proteomics. 2015 doi: 10.1002/pmic.201400483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidoli S, Schwammle V, Ruminowicz C, Hansen TA, Wu X, Helin K, Jensen ON. Middle-down hybrid chromatography/tandem mass spectrometry workflow for characterization of combinatorial post-translational modifications in histones. Proteomics. 2014 doi: 10.1002/pmic.201400084. [DOI] [PubMed] [Google Scholar]
- 12.Schwammle V, Aspalter CM, Sidoli S, Jensen ON. Large-scale analysis of co-existing post-translational modifications on histone tails reveals global fine-structure of crosstalk. Molecular & cellular proteomics : MCP. 2014 doi: 10.1074/mcp.O113.036335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung HR, Sidoli S, Haldbo S, Sprenger RR, Schwammle V, Pasini D, Helin K, Jensen ON. Precision mapping of coexisting modifications in histone H3 tails from embryonic stem cells by ETD-MS/MS. Analytical chemistry. 2013;85(17):8232–8239. doi: 10.1021/ac401299w. [DOI] [PubMed] [Google Scholar]
- 14.Vonholt C, Brandt WF, Greyling HJ, Lindsey GG, Retief JD, Rodrigues JD, Schwager S, Sewell BT. Isolation and Characterization of Histones. Method Enzymol. 1989;170:431–523. doi: 10.1016/0076-6879(89)70061-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhang KL, Tang H, Huang L, Blankenship JW, Jones PR, Xiang F, Yau PM, Burlingame AL. Identification of acetylation and methylation sites of histone H3 from chicken erythrocytes by high-accuracy matrix-assisted laser desorption ionization-time-of-flight, matrix-assisted laser desorption ionization-postsource decay, and nanoelectrospray ionization tandem mass spectrometry. Analytical biochemistry. 2002;306(2):259–269. doi: 10.1006/abio.2002.5719. [DOI] [PubMed] [Google Scholar]
- 16.Jufvas A, Stralfors P, Vener AV. Histone Variants and Their Post-Translational Modifications in Primary Human Fat Cells. PloS one. 2011;6(1) doi: 10.1371/journal.pone.0015960. ARTN e15960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonaldi T, Imhof A, Regula JT. A combination of different mass spectroscopic techniques for the analysis of dynamic changes of histone modifications. Proteomics. 2004;4(5):1382–1396. doi: 10.1002/pmic.200300743. [DOI] [PubMed] [Google Scholar]
- 18.Zhao XL, Sidoli S, Wang LL, Wang WJ, Guo L, Jensen ON, Zheng L. Comparative Proteomic Analysis of Histone Post-translational Modifications upon Ischemia/Reperfusion-Induced Retinal Injury. Journal of proteome research. 2014;13(4):2175–2186. doi: 10.1021/Pr500040a. [DOI] [PubMed] [Google Scholar]