Abstract
Biological control is the purposeful introduction of parasites, predators, and pathogens to reduce or suppress pest populations. Wolbachia are inherited bacteria of arthropods that have recently attracted attention for their potential as new biocontrol agents. Wolbachia manipulate host reproduction by using several strategies, one of which is cytoplasmic incompatibility (CI) [Stouthamer, R., Breeuwer, J. A. J. & Hurst, G. D. D. (1999) Annu. Rev. Microbiol. 53, 71–102]. We established Wolbachia-infected lines of the medfly Ceratitis capitata using the infected cherry fruit fly Rhagoletis cerasi as donor. Wolbachia induced complete CI in the novel host. Laboratory cage populations were completely suppressed by single releases of infected males, suggesting that Wolbachia-induced CI could be used as a novel environmentally friendly tool for the control of medfly populations. The results also encourage the introduction of Wolbachia into pest and vector species of economic and hygenic relevance to suppress or modify natural populations.
Cytoplasmic incompatibility (CI) is the most widespread and, perhaps, the most prominent feature that Wolbachia endosymbionts impose on their hosts (1, 2). CI results in embryonic mortality (EM) in matings between insects of the same species with different Wolbachia infection status (3, 4). It can be either unidirectional or bidirectional. Unidirectional CI is typically expressed when an infected male mates with an uninfected female. The reciprocal mating is fully compatible, as are matings between infected individuals. Bidirectional CI usually occurs in matings between infected individuals harboring different strains of Wolbachia. Although the mechanism of CI has not yet been elucidated on the molecular level, several lines of evidence suggest that Wolbachia somehow modifies the paternal chromosomes during spermatogenesis (mature sperm does not contain the bacteria). This modification influences their behavior during the first mitotic divisions and results in loss of mitotic synchrony (5, 6). Even before the etiological connection between Wolbachia and CI was revealed in mosquitoes (7), attempts were made to exploit CI as a method to suppress natural populations of arthropod pests in a way analogous to the sterile insect technique (S.I.T.) (8). These early attempts involved the mass production and release of incompatible male insects to control wild populations of disease vectors such as the mosquito Culex pipiens (9) and of agricultural pests such as the European cherry fruit fly, Rhagoletis cerasi (10) and, at a smaller scale, the almond moth Cadra (Ephestia) cautella (11).
The Mediterranean fruit fly (medfly) Ceratitis capitata is a worldwide pest that infests >250 fruit varieties of economic importance (12). Extensive screenings of both laboratory and natural populations of medfly have shown that this insect pest is not infected with Wolbachia (13). However, at the time of this writing, ongoing experimental work suggests that some Brazilian natural populations of medfly may be infected with Wolbachia (D. Selivon, personal communication). To determine whether the medfly can support Wolbachia infections and express CI, we used conventional embryonic cytoplasmic injections (14–17) for transfer of natural bacterial symbionts from a related species, R. cerasi (Diptera, Tephritidae) (18), to an uninfected laboratory strain of medf ly C. capitata (Diptera, Tephritidae), the Benakeion strain.
Previous studies have demonstrated high levels of incompatibility between natural populations of R. cerasi (10, 19), the basis of which was recently shown to be Wolbachia (18). Populations of R. cerasi are either infected by a single Wolbachia variant, wCer1, or coinfected by two variants, wCer1 and wCer2. Incompatibility occurs between males from doubly infected populations and females from singly infected populations, suggesting the wCer2 infection as the cause of CI (18). Additionally, transfer of wCer2 in Drosophila simulans also resulted to the induction of CI (17). An additional, yet uncharacterized, Wolbachia strain (wCer3) has been recently found in Sicilian populations of R. cerasi (M.R. and C. Stauffer, unpublished results).
The aim of our study was to establish Wolbachia-infected lines of the medfly by using the infected cherry fruit fly as donor. Previous attempts to transfer Wolbachia strains from species such as Drosophila melanogaster, D. simulans (infected with wRi), and Cadra cautella into medfly failed (K.B., unpublished data), due to either an unsupportive host background for Wolbachia, or the lack of adaptation of the transferred Wolbachia strains to the new host. A transfer of Wolbachia strains from the more closely related host species R. cerasi would have a greater potential. Furthermore, we were interested in whether transferred Wolbachia could induce CI in the novel host, also in an attempt to use the mechanism of Wolbachia-induced CI as a novel environmentally friendly tool for the control of medfly populations. The results presented here are very supportive and encourage the introduction of Wolbachia into pest and vector species of economic and health relevance to suppress or modify natural populations.
Materials and Methods
Insects. Ceratitis capitata. Benakeion is an uninfected laboratory strain. A71 is an uninfected white eye mutant laboratory strain. Both strains are kept in mass in population cages at 24°C on standard medfly diet (13).
Rhagoletis cerasi. Two natural populations of cherry fruit flies were collected from Austria and from Sicily (Italy). The first was doubly infected with wCer1 and wCer2 and the second infected with wCer1 (18) and wCer4 (this study). The Sicilian population had previously been found to carry additional Wolbachia strains (M.R. and C. Stauffer, unpublished results).
Embryonic Cytoplasm Transfer. Wolbachia was transferred from naturally infected cherry fruit fly populations into the Benakeion laboratory strain of C. capitata. Microinjections were carried out by using a microcapillary needle (Boehringer Femtotips). Medfly embryos were collected for 60 min, dechorionated, and slightly desiccated. The cytoplasm was taken from the posterior region of donor mature oocytes and injected at the posterior pole of the recipient preblastoderm embryos (16, 17).
Establishment of the Infected C. capitata Lines. Females deriving from injected embryos represent the generation G0 postinjection. G0 females were each crossed with two Benakeion males. The progeny of transinfected G0 females were kept in mass culture, establishing a line.
DNA Extraction, PCR, and Sequencing. Total DNA was extracted from single individuals following the STE boiling method (20). Wolbachia was detected by PCR using the 16S rDNA Wolbachia-specific primers 99F and 994R (20). At least 60 individuals from the WolMed 88.6 line and 40 individuals from the WolMed S10.3 line were screened for infections every generation. Distinction between different Wolbachia variants was done with strain-specific wsp primers (18). For sequencing, PCR products were amplified by using the wsp primers 81F and 691R (21) and were subjected to direct sequencing. At least three individuals from each established line were sequenced.
CI Assays and Statistical Analysis. CI levels were measured in two different ways, in single pair matings and in cage populations. All of the crosses were performed at 24°C. For single-pair matings, 2-day-old virgin females were individually mated with 1-day-old virgin males. The egg-laying plates were removed daily, and all eggs were scored for a period of 6–8 days. Cage populations consisted of 100 2-day-old virgin females mated with 100 1-day-old virgin males. A random sample of 500 eggs was taken every day. Hatching rates were scored 72 h after egg collection. EM was determined as the percentage of unhatched eggs. The standard error (SD) for EM, was determined according to Ott (22). EM in the progeny of infected males and uninfected females was complete, which let us assume that CI is complete.
Suppression of C. capitata Populations. The population suppression experiments were performed in six population cages at 24°C. Each cage contained equal numbers of 2-day-old virgin uninfected females and 1-day-old virgin uninfected males (1:1), plus 1-day-old virgin transinfected males at different ratios 1:1:0, 1:1:1, 1:1:10, 1:1:20, 1:1:30, and 1:1:50. The first five cages contained ≈300 flies and the last one (ratio 1:1:50) 520 flies. Egg-laying plates were removed every day for a period of 6–8 days. In the first two cages, a random sample of 500 eggs was kept daily, whereas, in the rest of them, all layed eggs were collected. Hatching rates were scored 72 h after egg collection. Survival was determined as percentage of hatched eggs.
Detection of Wolbachia by Immunofluorescence. Embryos were collected and dechorionated in 50% commercial bleach for 5 min. After a quick rinse with washing buffer (0.7% NaCl, 0.3% Triton X-100), they were transferred to 1:1 heptane-methanol solution and shaken vigorously for a couple of minutes. They were then briefly washed three times with methanol and three times with TBST (50 mM Tris·HCl/150 mM NaCl/0.1% Tween 20/0.05% NaN3, pH 7.5), 15 min each; were then blocked in 1% BSA in TBST; and incubated with the Wolbachia surface protein (WSP) antibody with a 1:500 dilution overnight at 4°C. After three washes with TBST, the eggs were incubated for 1 h at room temperature with 1:500 dilution of Alexa Fluor 488 goat anti-rabbit IgG-labeled antibody (Molecular Probes) and 2 mg/ml Rnase A (Sigma) in TBST. After several washes in TBST, eggs were stained with 5 μg/ml propidium iodide (Molecular Probes) for 20 min, rinsed, and mounted with ProLong Antifade kit (Molecular Probes).
Ovaries from 2- to 3-day-old females and testes from 1-day-old males were removed in TBST and further dissected on glass slides. Tissue samples were flattened under a cover glass and frozen in liquid nitrogen. Cover glasses were removed by using a razor blade, and the slides were placed in ice-cold ethanol for 3 min and fixed in 4% paraformaldehyde for 12 min. Slides were rehydrated in TBST, blocked, and incubated with antibodies and propidium iodide as described (23).
Optical sections were taken by using a confocal laser-scanning microscope (TCS-NT, Leica, Deerfield, IL), and they were projected onto single images. Images were further processed by using photoshop 6.0 (Adobe Systems, San Jose, CA).
Results and Discussion
Establishment of Wolbachia-Infected Medfly Lines. To determine whether the medfly can support Wolbachia infections and express CI, we used conventional embryonic cytoplasmic injections (14–17) to transfer natural bacterial symbionts from a related species, R. cerasi (Diptera, Tephritidae) (18), to an uninfected laboratory strain of medfly C. capitata (Diptera, Tephritidae), the Benakeion strain. Infected lines of R. cerasi from Sicily (Italy) and Austria were used as donors of Wolbachia-infected embryonic cytoplasm. Eighty-eight G0 isofemale lines were established and were monitored at each generation for the presence of Wolbachia by using a specific PCR assay (20). After three generations of monitoring, 2 of initially 11 positive transinfected isofemale lines remained positive for the presence of Wolbachia, namely WolMed 88.6 and WolMed S10.3. Analysis of PCR-amplified sequences of bacterial wsp gene showed that each of the 2 lines was infected by a different Wolbachia strain. Line WolMed 88.6 was found to be infected with the wCer2 strain, which originated from the R. cerasi Austrian population (accession no. AF418557), whereas line WolMed S10.3 was found to be infected with wCer4. This Wolbachia strain was previously undetected in its original host, but originated from an island population (Sicily) that has characteristic infection types (18), including previously nondescribed Wolbachia strains (M.R. and C. Stauffer, unpublished results). The wCer4 strain was found to be 100% identical with the wIrr-A1 strain (accession no. AF217714) based on a partial wsp gene sequence. At the time of this writing, 26 generations (≈23 months) postinjection, both transinfected lines are stably infected, with infection rates of 100%.
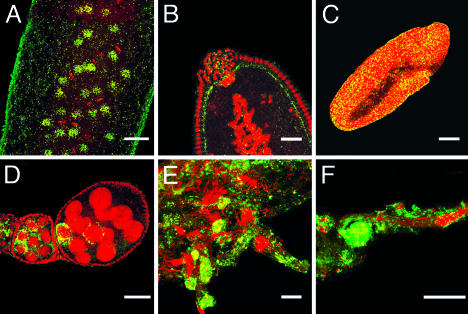
Confocal microscopy analysis was performed in embryos, ovaries, and testes of both transinfected medfly lines by using an anti-WSP (Wolbachia surface protein) antiserum (24) (Fig. 1). Both lines present high infection levels and distribution patterns as in characterized Drosophila–Wolbachia associations (23, 25–27). In early medfly embryos, Wolbachia show a clear association with the host cytoskeleton interacting with the mitotic spindle apparatus (Fig. 1 A). The bacteria then comigrate with the nuclei toward the periphery of the embryo and at the same time get incorporated into pole cells (Fig. 1B). It is notable that, in medfly preblastoderm embryos, the bacteria are concentrated at the basal side of the nuclei, in contrast with most Drosophila–Wolbachia symbioses where the bacterium is associated with the apical side of precellular nuclei (23). At later stages, Wolbachia are evenly distributed throughout the developing embryo, and this pattern remains constant until late gastrulation (Fig. 1C). In the transinfected medfly lines, Wolbachia were present in the ovaries, mostly within the egg chamber surrounding the nuclei of the nurse cells, probably infecting the oocyte at later stages of the oogenesis through the cytoplasmic dumping process (Fig. 1D). Wolbachia density is quite high throughout spermatogenesis, the bacteria being present at high levels within the sperm cysts until their removal to the waste bags during the individualization process of spermatozoa (Fig. 1 E and F).
Fig. 1.
Presence of Wolbachia in transinfected C. capitata embryos, ovaries, and testes. (A) Transinfected medfly embryo undergoing synchronous mitotic divisions showing Wolbachia localization at the mitotic spindles. (B) The posterior part of a transinfected medfly embryo, where pole cells (the precursors of gonads) are being formed, showing incorporation of Wolbachia in the pole plasm. (C) Uniform distribution of Wolbachia bacteria in a postgastrulation transinfected embryo. (D) Distribution of Wolbachia during oogenesis, when oocytes start to form. The bacteria are mostly concentrated in nurse cells, presumably infecting the oocyte at later stages of oogenesis. (E) Large numbers of Wolbachia are present in adult testes of transinfected medfly. (F) A sperm cyst is shown with the distal end toward the left and nuclei to the right. After elongation is complete, most cytoplasmic components, including Wolbachia, are stripped away from the sperm during individualization and sequestered in the waste bag (shown as a “green ball” in the left). Bacteria are visualized green-yellow and nuclei red. [Scale bars: 40 μm(A, B, and D–F) and 100 μm(C).]
Wolbachia-Infected Medfly C. capitata Lines and Expression of CI. To determine whether the two transinfected lines were capable of inducing CI, test crosses were performed between each transinfected line and the parental naturally uninfected Benakeion strain. Crossing experiments in cages with 100 uninfected females and equal numbers of infected males from each transinfected line (seventh generation post injection) resulted in 100% egg mortality; the reciprocal crosses resulted in <32% egg mortality under identical experimental conditions (Table 1, No tetracycline). Similar results were obtained in cage experiments between uninfected females from the medfly strain A71 (a white eye mutant C. capitata line) and infected males from each transinfected line (sixth generation postinjection, data not shown).
Table 1. Wolbachia-induced cytoplasmic incompatibility in two transinfected lines of the medfly C. capitata.
| Cross (females × males) | Embryos scored | Embryonic mortality, % |
|---|---|---|
| No tetracycline* | ||
| Uninfected × WolMed 88.6 (wCer2) | 3,000 | 100 ± 0 |
| Uninfected × WolMed S10.3 (wCer4) | 3,000 | 100 ± 0 |
| WolMed 88.6 (wCer2) × Uninfected | 3,000 | 16.73 ± 0.68 |
| WolMed S10.3 (wCer4) × Uninfected | 3,000 | 32.03 ± 0.85 |
| WolMed S10.3 (wCer4) × WolMed 88.6 (wCer2) | 3,000 | 100 ± 0 |
| WolMed 88.6 (wCer2) × WolMed S10.3 (wCer4) | 3,000 | 100 ± 0 |
| WolMed 88.6 (wCer2) × WolMed 88.6 (wCer2) | 3,000 | 64.77 ± 0.87 |
| WolMed S10.3 (wCer4) × WolMed S10.3 (wCer4) | 3,000 | 67.25 ± 0.87 |
| Uninfected × Uninfected | 3,000 | 12.17 ± 0.60 |
| Tetracycline† | ||
| WolMed S10.3 tet × WolMed S10.3 tet‡ | 1,890 | 23.44 ± 0.97 |
| WolMed S10.3 tet × WolMed S10.3 tet§ | 3,000 | 11.80 ± 0.59 |
| WolMed 88.6 tet × WolMed 88.6 tet‡ | 2,283 | 25.10 ± 0.91 |
Wolbachia-induced cytoplasmic incompatibility is expressed as percentage of unhatched eggs ± SE. Egg-laying plates were removed daily for a period of 6 days. Hatching rates were scored 72 h after egg collection.
Test crosses between each transinfected line and the parental naturally uninfected Benakeion strain as well as between the two transinfected lines. Crosses between 100 females (2-3 days old) and equal numbers of males (1 day old) were performed in cages.
Test crosses between tetracycline-treated individuals of the line WolMed S10.3. Crosses between 30 females and equal numbers of males were performed in cages, three generations (‡) and five generations (§) after the tetracycline treatment.
Persistence of Wolbachia-induced CI was also tested by single-pair matings. Twenty-six single-pair crosses between WolMed 88.6 (wCer2) transinfected males (fifth generation postinjection) and uninfected females resulted in 100% egg mortality (no hatched eggs out of 2164). Similarly, 17 single-pair crosses between WolMed S10.3 (wCer4) transinfected males (fifth generation postinjection) and uninfected females resulted again in 100% egg mortality (no hatched eggs out of 1,325). The same experiments were repeated again in the tenth generation postinjection, providing the same results, 100% egg mortality (data not shown). It is notable that complete CI has been observed only in very few Wolbachia-infected species such as C. pipiens (9). To our knowledge, there have been no previous reports that a novel transinfected host species presents high stability of the infection and, at the same time, expresses 100% CI.
The two transinfected medfly lines WolMed 88.6 (wCer2) and WolMed S10.3 (wCer4), each infected with a different Wolbachia strain, were 100% bidirectionally incompatible in appropriate genetic cross experiments performed in cages (Table 1).
Control crosses between infected males and females resulted in high EM, ≈65%, in both transinfected lines (Table 1, No tetracycline). This effect is caused by Wolbachia, as shown by eliminating the bacteria by treatment with antibiotics: Crosses between individuals of the tetracycline cured lines, three and five generations after curing, resulted in normal rates of EM (Table 1, Tetracycline). One explanation for these high EM could be inefficient transmission of both wCer2 and wCer4 in medfly. However, at least 60 individuals from the WolMed 88.6 line and 40 individuals from the WolMed S10.3 line were tested by PCR in every generation for 26 generations (≈23 months), and all individuals have been found positive for Wolbachia. A more likely cause for the high mortalities observed in the crosses between infected individuals could be additional fertility effects of wCer2 and wCer4 on medfly females, effects other than CI. Alternatively, it is possible that infected medfly females can only partially rescue the modification induced by the bacteria in infected medfly males. The last two hypotheses will require further investigation.
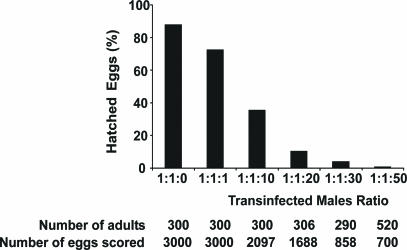
Wolbachia-Induced CI as a Potential Tool for Medfly Population Suppression. To determine whether cytoplasmic incompatibility expressed by the Wolbachia-infected medfly lines could be used for population suppression, we set up cage populations containing different ratios of uninfected females to uninfected males to transinfected males (1:1:0, 1:1:1, 1:1:10, 1:1:20, 1:1:30, 1:1:50). These experiments were performed by using transinfected WolMed 88.6 (wCer2) males from generations 8 to 11 postinjection. As shown in Fig. 2, the laboratory cage medfly populations were suppressed by these single “releases” of incompatible males in a ratio-dependent manner. Under these conditions, population suppression reached levels higher than 99% in releases of incompatible males at a ratio 1:1:50. Similar results were obtained in cage experiments by using as target population the medfly white eye mutant strain A71 (data not shown). These data clearly demonstrate that Wolbachia-induced cytoplasmic incompatibility could be used as a tool for the population control of this major agricultural pest.
Fig. 2.
Suppression of medfly populations using Wolbachia-induced CI. Population suppression is expressed as percentage of eggs that hatched. The numbers of the adults used and the number of eggs scored per cage are shown below the graph.
In recent years, there has been increasing interest in the biology of Wolbachia and in its application as an agent for control or modification of insect populations. It has been proposed that Wolbachia might be used (i) as a vector for the expression of genes of interest, (ii) as a tool to drive desirable genotypes into arthropod populations, and (iii) to directly suppress arthropod populations (28–31). The recent publication of the genome sequence of a CI-inducing strain (32) should accelerate progress in elucidating, at the molecular level, the interactions between Wolbachia and its hosts that lead to CI. Our present study clearly shows that Wolbachia endosymbionts can be experimentally transferred over genus barriers into a novel host, forming associations that express complete CI. The population cage experiments strongly suggest that Wolbachia-induced CI (unidirectional and, importantly, bidirectional) could be used as a means for control of natural medfly populations. The observed increased lethality in crosses between infected males and females will have some negative impact in mass rearing; however, Wolbachia transinfection experiments into other host insects have shown that deleterious fitness effects can attenuate rapidly in consecutive selection processes (33). Alternatively, the system may be improved by testing additional bacterial strains that may not be causing this lethality effect. For effective Wolbachia-based population suppression, an efficient genetic sexing system producing males only is necessary. Such systems are available in medfly (34). However, because accidental release of even one female can theoretically result in the replacement of the target population by a second, the sexing system has to be 100% effective. Altogether, our results should encourage efforts to transfer Wolbachia into other insect pests or disease vectors for suppression or modification of natural populations.
Acknowledgments
We thank S. L. O'Neill for comments on the manuscript. We particularly acknowledge Y. Livadaras for help with cytoplasmic injections. We also thank Z. Kovacs, S. Longo, M. Porto, and A. Pulvirenti, who helped with the collection of the cherry fruit flies. This research was supported by European Union (EU) Grant QLK3-CT-2000-01079 (to K.B.), an Austrian Science Foundation grant (to C. Stauffer), and an EU Cooperation in the Field of Scientific and Technological Research (COST) 850 travel grant (to M.R.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CI, cytoplasmic incompatibility; EM, embryonic mortality.
References
- 1.Stouthamer, R., Breeuwer, J. A. J. & Hurst, G. D. D. (1999) Annu. Rev. Microbiol. 53, 71–102. [DOI] [PubMed] [Google Scholar]
- 2.Werren, J. H. (1997) Annu. Rev. Entomol. 42, 587–609. [DOI] [PubMed] [Google Scholar]
- 3.Bourtzis, K., Dobson, S. L., Braig, H. R. & O'Neill, S. L. (1998) Nature 391, 852–853. [DOI] [PubMed] [Google Scholar]
- 4.Bourtzis, K., Braig, H. & Karr, T. L. (2003) in Insect Symbiosis, eds. Bourtzis, K. & Miller, T. A. (CRC, Boca Raton, FL), pp. 217–246.
- 5.O'Neill, S. L. & Karr, T. L. (1990) Nature 348, 178–180. [DOI] [PubMed] [Google Scholar]
- 6.Tram, U. & Sullivan, W. (2002) Science 296, 1124–1126. [DOI] [PubMed] [Google Scholar]
- 7.Yen, J. H. & Barr, A. R. (1971) Nature 232, 657–658. [DOI] [PubMed] [Google Scholar]
- 8.Krafsur, E. S. (1998) J. Agric. Entomol. 15, 303–317. [Google Scholar]
- 9.Laven, H. (1967) Nature 261, 383–384. [DOI] [PubMed] [Google Scholar]
- 10.Boller, E. F., Russ, K., Vallo, V. & Bush, G. L. (1976) Entomol. Exp. Appl. 20, 237–247. [Google Scholar]
- 11.Brower, J. H. (1980) J. Econ. Entomol. 73, 415–418. [Google Scholar]
- 12.Robinson, A. S. & Hooper, G. (1989) in Fruit Flies, Their Biology, Natural Enemies and Control (Elsevier, Amsterdam), Vol. 3B.
- 13.Bourtzis, K., Nirgianaki, A., Onyango, C. & Savakis, C. (1994) Insect Mol. Biol. 3, 131–142. [DOI] [PubMed] [Google Scholar]
- 14.Boyle, L., O'Neill, S. L., Robertson, H. M. & Karr, T. L. (1993) Science 260, 1796–1799. [DOI] [PubMed] [Google Scholar]
- 15.Braig, H. R., Guzman, H., Tesh, R. B. & O'Neill, S. L. (1994) Nature 367, 453–455. [DOI] [PubMed] [Google Scholar]
- 16.Poinsot, D., Bourtzis, K., Markakis, G., Savakis, C. & Merçot, H. (1998) Genetics 150, 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riegler, M., Charlat, S., Stauffer, C. & Merçot, H. (2004) Appl. Environ. Microbiol. 70, 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riegler, M. & Stauffer, C. (2002) Mol. Ecol. 11, 2425–2434. [DOI] [PubMed] [Google Scholar]
- 19.Boller, E. F. & Bush, G. L. (1974) Entomol. Exp. Appl. 17, 279–293. [Google Scholar]
- 20.O'Neill, S. L., Giordano, R., Colbert, A. M. E., Karr, T. L. & Robertson, H. M. (1992) Proc. Natl. Acad. Sci. USA 89, 2699–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braig, H. R., Zhou, W. G., Dobson, S. L. & O'Neill, S. L. (1998) J. Bacteriol. 180, 2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ott, L. (1988) in An Introduction to Statistical Methods and Data Analysis (PWS-Kent, Boston), 3rd Ed., pp. 227.
- 23.Veneti, Z., Clark, M. E., Karr, T. L., Savakis, C. & Bourtzis, K. (2004). Appl. Environ. Microbiol. 70, 5366–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobson, S., Bourtzis, K., Braig, H. R. Jones, B. F. Zhou, W. Rousset, F. & O'Neill, S. L. (1999) Insect Biochem. Mol. Biol. 29, 153–160. [DOI] [PubMed] [Google Scholar]
- 25.Clark, M. E., Veneti, Z., Bourtzis, K. & Karr, T. L. (2002) Mech. Dev. 111, 3–15. [DOI] [PubMed] [Google Scholar]
- 26.Clark, M. E., Veneti, Z., Bourtzis, K. & Karr, T. L. (2003) Mech. Dev. 120, 185–198. [DOI] [PubMed] [Google Scholar]
- 27.Veneti, Z., Clark, M. E., Zabalou, S., Karr, T. L., Savakis, C. & Bourtzis, K. (2003) Genetics 164, 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beard, C. B., O'Neill, S. L., Mason, P., Mandelco, L., Woese, C. R., Tesh, R. B., Richards, F. F. & Aksoy, S. (1993) Insect Mol. Biol. 1, 123–131. [DOI] [PubMed] [Google Scholar]
- 29.Sinkins, S. P., Curtis, C. F. & O'Neill, S. L. (1997) in Influential Passengers: Inherited Microorganisms and Arthropod Reproduction, eds. O'Neill, S. L., Hoffmann, A. A. & Werren, J. H. (Oxford Univ. Press, Oxford), pp. 155–175.
- 30.Bourtzis, K. & Braig, H. (1999) in Rickettsiae and Rickettsial Diseases at the Turn of the Third Millenium, eds. Raoult, D. & Brouqui, P. (Elsevier, Amsterdam), pp. 199–221.
- 31.Sinkins, S. P. & O'Neill, S. L. (2000) in Insect Transgenesis: Methods and Applications, eds. Handler, A. M. & James, A. C. (CRC, Boca Raton, FL), pp. 271–287.
- 32.Wu, M., Sun, L. V., Vamathevan, J., Riegler, M., Deboy, R., Brownlie, J. C., McGraw, E. A., Martin, W., Esser, C., Ahmadinejad, N., et al. (2004) PLoS Biol. 2, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGraw, E. A., Merritt, D. J., Droller, J. N. & O'Neill, S. L. (2002) Proc. Natl. Acad. Sci. USA 99, 2918–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson, A. S. (2002) Genetica 116, 5–13. [DOI] [PubMed] [Google Scholar]