Abstract
Presynaptic N-type Ca2+ channels (Cav2.2, α1B) are thought to bind to SNARE (SNAP-25 receptor) complex proteins through a synaptic protein interaction (synprint) site on the intracellular loop between domains II and III of the α1B subunit. Whether binding of syntaxin to the N-type Ca2+ channels is required for coupling Ca2+ ion influx to rapid exocytosis has been the subject of considerable investigation. In this study, we deleted the synprint site from a recombinant α1B Ca2+ channel subunit and transiently transfected either the wild-type α1B or the synprint deletion mutant into mouse pheochromocytoma (MPC) cell line 9/3L, a cell line that has the machinery required for rapid stimulated exocytosis but lacks endogenous voltage-dependent Ca2+ channels. Secretion was elicited by activation of exogenously transfected Ca2+ channel subunits. The current-voltage relationship was similar for the wild-type and mutant α1B-containing Ca2+ channels. Although total Ca2+ entry was slightly larger for the synprint deletion channel, compared with the wild-type channel, when Ca2+ entry was normalized to cell size and limited to cells with similar Ca2+ entry (≈150 × 106 Ca2+ ions/pF cell size), total secretion and the rate of secretion, determined by capacitance measurements, were significantly reduced in cells expressing the synprint deletion mutant channels, compared with wild-type channels. Furthermore, the amount of endocytosis was significantly reduced in cells with the α1B synprint deletion mutant, compared with the wild-type subunit. These results suggest that the synprint site is necessary for efficient coupling of Ca2+ influx through α1B-containing Ca2+ channels to exocytosis.
Release of neurotransmitter from presynaptic vesicles is triggered by Ca2+ influx through voltage-dependent Ca2+ channels (1-3). Presynaptic vesicles are docked at release sites by binding of the vesicular protein synaptobrevin (also called VAMP) to two plasma membrane proteins, syntaxin and SNAP-25 (synaptosome-associated protein of 25 kDa) (4-6). Collectively, these three proteins form the SNAP-25 receptor (SNARE) complex, which is an essential component of the exocytotic machinery (7). In addition to participating in SNARE complex formation, syntaxin and SNAP-25 bind to and modulate voltage-dependent Ca2+ channels (8-10).
The association of Ca2+ channels with proteins of the SNARE complex was first demonstrated by coimmunoprecipitation of the N-type Ca2+ channel subunits with syntaxin and synaptotagmin (11-13). Like other voltage-dependent Ca2+ channels, the N-type channel is composed of at least three subunits: α1, the pore-forming subunit, which is distinctive for each Ca2+ channel subtype (α1B for the N-type Ca2+ channel), and two auxiliary subunits, α2δ and β, which alter the voltage-dependent properties of the channels as well as surface expression of the channel complex (14, 15). Subsequent in vitro-binding assays with recombinant fusion proteins identified the minimal site of interaction between the N-type Ca2+ channel and syntaxin as amino acids 773-859 of the intracellular loop between transmembrane domains II and III of the α1B subunit (16); this region was named the synaptic protein interaction (synprint) site (17). Subsequently, a longer region of the intracellular loop between domains II and III (amino acids 718-963) of the Ca2+ channel α1B subunit was shown to bind syntaxin with higher affinity than the originally defined synprint region (16, 17). A similar synprint site was identified in the α1A subunit of the P/Q-type Ca2+ channel (17). Short and long synprint peptides bind to syntaxin and SNAP-25 in a biphasic, Ca2+-dependent manner with maximal binding at 20 μM free Ca2+ and reduced affinity at higher Ca2+ concentrations (18). Functional effects on neurotransmitter release also have been attributed to the synprint site. Injection of peptides corresponding to the synprint region into presynaptic neurons resulted in a 24-42% reduction in synaptic transmission in rat sympathetic neurons (19) or ≈25% reduction in Xenopus spinal neurons (20). From these combined data, it was hypothesized that the synprint site docks vesicles near Ca2+ channels such that Ca2+ influx is efficiently coupled to secretion (9, 10). Consistent with this hypothesis, two recent papers by Mochida et al. (21, 22) suggested that the synprint site is necessary for both the localization of Ca2+ channels at sites of neurotransmitter release and for functional synaptic transmission.
To further examine the importance of the synprint site for exocytosis, we deleted the synprint site from the α1B subunit (Cav2.2) and expressed this mutant as well as wild-type α1B in mouse pheochromocytoma (MPC) 9/3L cells. We have shown in a previous study (23) that MPC 9/3L cells contain vesicles and many of the proteins involved in vesicle fusion, including SNAP-25 and syntaxin 1A. MPC 9/3L cells express little or no endogenous Ca2+ current and so exhibit no exocytosis when stimulated by trains of depolarizations. In contrast, when MPC 9/3L cells are transfected with exogenous Ca2+ channel subunits, they rapidly release vesicles in a Ca2+-dependent manner similar to PC12 cells and adrenal chromaffin cells (23). To assess the functional consequences of disrupting the synaptic protein interaction site between the N-type Ca2+ channel and the SNARE complex, we transiently transfected MPC 9/3L cells with both wild-type and mutant α1B Ca2+ channel subunits that had the short synprint site (amino acids 772-856) deleted. No currents were detected in cells transfected with the α1B long synprint site deletion mutants (amino acids 718-963). An earlier study by Mochida et al. (21) showed that P/Q-type Ca2+ channels with a similar long synprint deletion also did not express functional Ca2+ channels. For both wild-type and mutant Ca2+ channel expression, cells were chosen that had a similar Ca2+-influx. Measurements of release showed that the cells transfected with the short synprint deletion mutant had significantly reduced secretion, compared with wild-type cells. Furthermore, both the maximal rate of release and the amount of endocytosis 40 s after stimulation were significantly reduced in cells expressing the short synprint deletion mutant, compared with wild-type cells. Our results suggest that the synprint site of the N-type Ca2+ channel is necessary for efficient coupling of the channel to the release machinery.
Methods
Ca2+ Channel cDNAs. Cloning of the bovine chromaffin cell α1B (GenBank accession no. AF173882) and β2a (GenBank accession no. AF174417) subunits of the N-type Ca2+ channel has been described (24). Regions of the α1B cDNA coding for portions of the synprint site (see Fig. 1) were deleted by using the “splicing-by-overlap extension” method (25). The cDNA flanking each deletion was sequenced on both strands to assure that the desired deletions had been accomplished without introducing other unintended mutations. A rat α2δ Ca2+ channel subunit cDNA (GenBank accession no. M86621) was a kind gift from T. Snutch (University of British Columbia, Vancouver). All of these Ca2+ channel subunits were subcloned into pcDNA3.1(+) (Invitrogen) for expression in the MPC 9/3L cells.
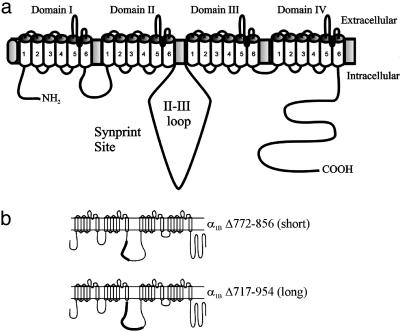
Fig. 1.
Domain structure of the α1B subunit of the N-type (Cav2.2) Ca2+ channel. (a) The II-III loop between domains II and III contains the synprint site. (b) Two mutant α1B Ca2+ channel subunits were constructed by deleting portions of the intracellular loop between domains II and III (schematized as the thicker, darkened areas). The short synprint deletion (α1B Δ772-856) and long synprint deletion (α1B Δ717-954) in the bovine sequence correspond to the originally described short and extended synprint sites, respectively, for the rat α1B Ca2+ channel subunit (16, 17).
Cell Culture and Transfection. MPC cell line 9/3L was grown in culture medium that consisted of RPMI medium 1640, 10% heat-inactivated horse serum, 5% FBS (HyClone), 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen) in a humidified 5% CO2 incubator at 37°C. The day before transfection, the cells were replated to collagen-coated coverslips. Cells were transfected with Qiagen-purified plasmids in a 5:5:5:1 μg ratio of the α1:β2a:α2δ:enhanced GFP plasmids by using Lipofectamine 2000 (Invitrogen). Recordings were done 48-72 h after transfection.
Electrophysiology. A coverslip of cells was set into a recording chamber and perfused with an external Na+ solution that contained 135 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM CaCl2, 12 mM Hepes, and 10 mM glucose (pH 7.3; ≈295 mOsm). A single transfected cell was selected based on its fluorescence due to enhanced GFP expression and voltage-clamped in the whole-cell configuration (26) with an Axopatch-1C amplifier (Axon Instruments, Union City, CA) modified for capacitance measurements. Gigaohm seals were obtained with sylgard-coated electrodes filled with an internal solution that contained 120 mM CsAsp, 5 mM MgCl2, 0.1 mM EGTA, 40 mM Hepes, 2 mM ATP, and 0.3 mM GTP (pH 7.3; ≈310 mOsm). Ca2+ currents were isolated with an Na+-free external solution that contained 140 mM tetraethylammonium-Cl, 0.0001 mM tetrodotoxin, 5 mM CaCl2, 10 mM Hepes, and 10 mM glucose (pH 7.3; ≈300 mOsm).
Capacitance measurements were made with the phase-tracking technique in which a 60-mV peak-to-peak sine wave was superimposed on a holding potential of -80 mV (27, 28). Details of the technique have been described (29). The data were collected at a 500-μs sampling rate and filtered at 2 kHz. All current records were compensated for series resistance and whole-cell capacitance. Single-pulse current data were leak-subtracted by an average of 10 hyperpolarizing sweeps. Experiments were carried out at room temperature (22-24°C).
Stimulation Protocol. Five minutes after the whole-cell configuration was obtained, each cell was stimulated with a train of five-step depolarizations. The membrane was depolarized to 20 mV for 200 ms with a 50-ms interpulse duration. The current-voltage (I-V) relationships were obtained by applying 50-ms step depolarizations to various test potentials every 15 s. Each current was leak- and capacitance-subtracted.
Data Analysis. Statistical analysis of the data are expressed as mean ± SEM, and an independent Student t test (origin, Microcal, Amherst, MA) was performed to test statistical significance (P < 0.05). For I-V curve calculations, the peak current from each depolarization was recorded and normalized to the whole-cell capacitance, and cells were pooled to calculate the mean and SEM. The maximal rate of secretion was calculated as the maximal release that occurred for a single depolarization divided by the duration of the depolarization. Ca2+ current inactivation was fit with a single exponential function by using origin.
Results
The Synprint Deletion Mutant of the α1B Ca2+ Channel Subunit Produces Ca2+ Currents That Are Similar to Wild-Type α1B Ca2+ Currents. N-type Ca2+ channels contain at least three subunits (α1B, β, and α2δ). The pore-forming α1B subunit is composed of four homologous domains, each of which contains six membrane-spanning domains (15, 30) (Fig. 1a). Between domains II and III is a large cytoplasmic loop that is thought to interact with SNARE proteins (15). Previously, two overlapping synprint sites have been defined for the Ca2+ channel α1B subunit, a short region and a long region of the II-III intracellular loop (16, 17). In our current study, each region was independently deleted from the bovine chromaffin cell α1B subunit. One synprint deletion mutant was made that removed the short synprint site of bovine α1B (Δ772-856) that correlated to the originally defined synprint site of rat α1B (773-859; Fig. 1b) (16), whereas a second bovine α1B mutant was constructed that deleted a longer synprint site (bovine α1B Δ717-954) that correlated to the long synprint site of rat α1B (718-963; Fig. 1b) (17). Each of these mutant α1B subunits was transiently transfected into MPC 9/3L cells along with the auxiliary Ca2+ channel subunits, β2a and α2δ, and an enhanced GFP vector to allow visual selection of cells that had been successfully transfected. The external Na+ solution was exchanged with a Na+-free solution (5 mM Ca2+) designed to isolate the Ca2+ currents (ICa).
As we have reported (23), nontransfected MPC 9/3L cells express little if any endogenous ICa. In our earlier study, 94% of cells tested had an endogenous Na+ current (INa) that was blocked by bath application of tetrodotoxin, and 18% of cells exhibited a small endogenous ICa that averaged 70 ± 8 pA (n = 23). After transfection with either the wild-type α1B Ca2+ channel subunit or the short synprint deletion mutant (and β2A and α2δ subunits), ≈75% of the GFP-positive cells expressed exogenous N-type Ca2+ currents (Fig. 2 a and b). No difference in current kinetics was observed for the two α1B subunits. The time to peak was 6.42 ± 0.39 ms for wild-type channels and 5.03 ± 0.51 ms for synprint deletion channels, whereas the time constants for the decay rate were 184.4 ± 28.3 ms for wild-type channels and 199.0 ± 45.2 ms for synprint deletion channels. These currents were sensitive to the specific N-type Ca2+ channel blocker ω-CgTx GVIA (data not shown, but see ref. 23). Step depolarizations were applied to different test potentials from a holding potential of -80 mV. The currents elicited were normalized by cell size (whole-cell capacitance). Normalized current-voltage plots for wild-type and synprint deletion mutant channels are shown in Fig. 2 c and d.
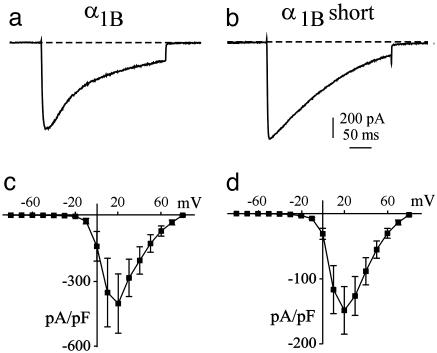
Fig. 2.
Similar N-type Ca2+ currents were observed in cells expressing either wild-type or short synprint deletion α1B subunits. Cells were transiently transfected with the α1B (a) or α1B short synprint deletion mutant (b) subunits (α1B Δ772-856) in addition to β2a and α2δ subunits. ICa was recorded from individual cells in response to a voltage step stimulation to 20 mV and duration of 200 ms. The current vs. voltage plots show the peak current normalized by the whole-cell capacitance (pF) for α1B (c) and α1B short synprint deletion (d) Ca2+ channel subunits. Each plot shows data averaged from seven cells. The data are expressed as mean ± SEM. Please note that current decay was not completely consistent between cells. In some cells, two exponentials were required to fit the data, whereas in others a single exponential fit well.
Cells transfected with either the wild-type α1B subunit or the short synprint deletion α1B subunit (and accessory subunits) exhibited large Ca2+ currents that were similar in amplitude to each other. Average peak Ca2+ currents were measured from the first of five stimulations and averaged 1984 ± 319 pA (n = 44) for the wild-type α1B subunit and 1812 ± 359 pA (n = 16; P = 0.76, compared with wild-type) for the short synprint deletion mutant subunit. These peak amplitudes were >25-fold greater than endogenous peak Ca2+ currents measured in untransfected cells. Cells transfected with the long synprint deletion α1B subunit exhibited little if any Ca2+ current, which suggests that the long deletion mutant subunit did not form functional Ca2+ channels [see also Mochida et al. (21)].
Fig. 3 compares the average total Ca2+ influx in response to five-step depolarizations to +20 mV (200-ms duration) from cells transfected with either wild-type α1B subunits or the short synprint deletion mutant. Fig. 3a plots representative current traces from two cells expressing either wild-type (Upper) or short synprint deletion mutant channels (Lower). Fig. 3b shows that, on average, the cells expressing wild-type α1B subunits had a Ca2+ influx of 858 ± 100 × 106 ions (n = 44), whereas cells expressing the short synprint deletion mutant subunit were not significantly different, with a Ca2+ influx of 1,067 ± 130 × 106 ions (n = 16).
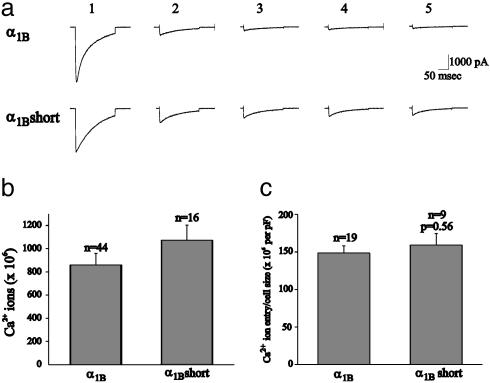
Fig. 3.
Similar Ca2+ influx was observed in cells expressing either wild-type or short synprint deletion α1B subunits. (a) Current records from two cells expressing either wild-type (Upper) or short synprint deletion mutant channels (Lower). Five depolarizations were applied, separated by 50 ms. The numbers on top of the traces correspond to the order in which the currents were obtained. (b) The total number of Ca2+ ions to enter each cell in response to stimulation is shown for cells that were transfected with either wild-type α1B or the short synprint deletion mutant subunit. (c) Ca2+ entry was normalized by the whole-cell capacitance (pF) for currents recorded in cells transfected with either the wild-type α1B or the short synprint deletion mutant. A reduced pool of cells was selected from the total number of cells shown in b that had similar normalized Ca2+ influx to ensure that Ca2+ entry was the same between the two sets of cells (P = 0.56).
Neurotransmitter release is thought to be a nonlinear function of Ca2+-influx. Small differences in Ca2+-influx produce large changes in secretion. To compare secretion between MPC 9/3L cells expressing wild-type and short synprint deletion mutant α1B subunits, the Ca2+ influx was normalized by cell size and limited to cells with similar Ca2+ influx per unit area (measured as pF; Fig. 3c). On average, normalized Ca2+ influx for the wild-type α1B subunit was 148.6 ± 9.4 pA/pF (n = 19), and the short synprint deletion α1B subunit was not significantly different (P = 0.56) at 158.9 ± 15.4 pA/pF (n = 9). This group of cells was subsequently used to determine the amount of secretion supported by each of the α1B Ca2+ channel subunits.
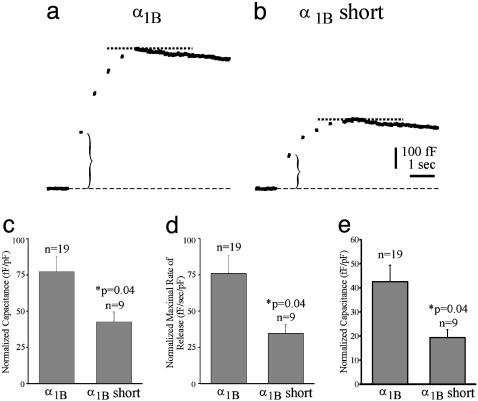
Secretion Is Significantly Reduced in Cells That Express the Short Synprint Mutant α1B Ca2+ Channel. To determine whether the synprint deletion mutant could support vesicle fusion, membrane capacitance was measured in response to stimulation with a train of five depolarizations to +20 mV (current was maximal at +20 mV, see Fig. 2 c and d) for 200 ms with a 50-ms interpulse duration. Representative capacitance traces for MPC 9/3L cells expressing either wild-type α1B or the α1B short synprint deletion mutant are shown in Fig. 4 a and b, respectively. The gaps in the capacitance trace represent the times the cell was stimulated with five-step depolarizations. In the capacitance traces, the dashed lines indicate the peak capacitance changes. It is interesting to note that exocytosis could be significant for all five depolarizations, even though the later Ca2+ currents were small (see Fig. 3a). This result may be due to residual Ca2+ summating with a relatively small Ca2+-influx, or, alternatively, intracellular Ca2+ stores may help support secretion. The bar graphs in Fig. 4 c and d plot averaged data for cells transfected with either the wild-type or the synprint deletion α1B subunits. Peak membrane capacitance and the maximal rate of release for each cell was normalized by the cell size (pF), and the normalized values were then averaged. Secretion was significantly reduced in the synprint deletion mutant, compared with the wild-type α1B subunit. The average peak change in membrane capacitance was 77 ± 10 fF/pF (n = 19) for the wild-type α1B subunit and 43 ± 7 fF/pF (n = 9; P = 0.04, compared with wild type) for the α1B synprint deletion mutant. Similarly, the rate of release was reduced in the α1B synprint deletion mutant, with an average of 35 ± 6 fF/s per pF (n = 19), compared with wild-type α1B, which averaged 76 ± 12 fF/s per pF (n = 9; P = 0.04). To ensure minimal interference by endocytosis in these measurements, Fig. 4e plots normalized capacitance changes produced by the first voltage depolarization. The average change in membrane capacitance was 42.5 ± 6.9 fF/pF (n = 19) for the wild-type α1B subunit and 19.3 ± 3.4 fF/pF (n = 9; P = 0.04, compared with wild type) for the α1B synprint deletion mutant. Therefore, deletion of the synprint site functionally alters release. Furthermore, this reduced secretion was not caused by a difference in Ca2+ influx during stimulation.
Fig. 4.
Cells transfected with the synprint deletion mutant exhibited reduced secretion, compared with cells expressing wild-type α1B subunits. Representative capacitance traces are plotted as a function of time for individual cells transfected with either wild-type α1B (a) or the short synprint deletion mutant (b). Each cell was stimulated with trains of five depolarizing voltage steps to +20 mV (duration 200 ms, interpulse 50 ms). The traces are shown on an expanded time scale (5 s). The bar graphs depict the averaged data for the cells transfected with either wild-type α1B (n = 19) or the short synprint deletion mutant (n = 9). The graphs plot total amount of secretion (fF) normalized by cell size (pF) in response to stimulation (c), the maximal rate of secretion (fF/s) normalized by cell size (pF) (d), and secretion produced by the first depolarization (e) (indicated by the curly brackets in a and b).
Secretion of catecholamines from cells closely related to MPC cells (e.g., chromaffin cells and PC12 cells) can be measured by amperometry. To date, we have had no successful amperometric recordings from the MPC cells, possibly because these cells do not store sufficient quantities of oxidizable neurotransmitter. We attempted to load the cells with false neurotransmitters, a commonly used technique (31). However, even after loading, there were no amperometric events.
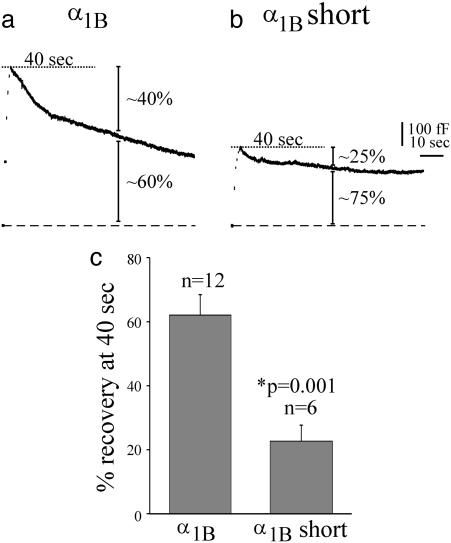
Membrane Retrieval Is Significantly Reduced in Cells That Express the Synprint Deletion Mutant α1B. Fig. 5 a and b shows traces from the same cells as those shown in Fig. 4 a and b, except plotted as ≈80 s of capacitance data. Membrane retrieval was defined as the percentage recovery of the peak capacitance change (indicated by the dashed line in the figure). Forty seconds after stimulation, ≈40% of the membrane had been retrieved in the cell expressing the wild-type α1B (Fig. 5a), but only ≈25% of the membrane had been retrieved in the cell expressing the α1B synprint deletion mutant (Fig. 5b). The bar graph in Fig. 5c plots the average membrane retrieval values for both wild-type α1B and the synprint deletion mutant. On average, the cells expressing wild-type α1B retrieved 62 ± 6% (n = 12) of their membrane after 40 s compared with 23 ± 5% (n = 6; P = 0.001) for the synprint deletion mutant. These data indicate not only that the synprint deletion mutant channel is less effective at coupling the channel to neurotransmitter release, but also that membrane retrieval was less efficient in cells expressing the mutant channel.
Fig. 5.
Endocytosis was significantly reduced in cells expressing the short synprint deletion mutant. Capacitance traces are shown from cells transfected with wild-type α1B (a) and short synprint deletion α1B subunits (b). These are the same traces shown in Fig. 4 a and b, except on a compressed time scale. (c) The bar graph depicts the percentage of membrane recovered at 40 s post-stimulation for cells transfected with either the α1B (n = 12) or α1B short synprint deletion (n = 6) subunits. Forty seconds was chosen arbitrarily to include several cells for which only 40 s of data were available.
The capacitance technique used in this study measures the sum of exocytosis and endocytosis and does not distinguish between the two processes. It might be argued that an alternative explanation for the diminished exocytosis observed in cells expressing the short synprint deletion Ca2+ channel mutant is that the channels somehow augment endocytosis, thereby masking the true exocytosis. It also could be argued that the diminished endocytosis is due to delayed exocytosis masking endocytosis. Amperometry measures exocytosis exclusively. Haller et al. (32) made detailed comparisons between exocytosis measured with capacitance and amperometry and concluded that the data from both techniques were in agreement for measurements of exocytosis. Thus, our data, taken immediately after stimulation ends, should provide a reliable estimate of exocytosis. In addition, exocytosis in neuroendocrine cells is thought to end tens of milliseconds after Ca2+ influx has stopped (32). Therefore, our measurement of endocytosis tens of seconds after stimulation also should be free of overlap.
Discussion
Cav2 family members (N-, P/Q-, and R-type Ca2+ channels) are known to regulate neurotransmitter release (33-38). N- and P/Q-type Ca2+ channels are abundant in nerve terminals, where they colocalize with synaptic vesicles. The channels are thought to be in close physical proximity to neurotransmitter release proteins, including the SNARE proteins and the associated synaptic vesicles containing neurotransmitters (39-42). N- and P/Q-type Ca2+ channels bind to the SNARE proteins syntaxin 1A and SNAP-25 at their synprint sites (11, 16, 43). There has been considerable interest in understanding the functional consequences of this link between the synprint site on N- and P/Q-type Ca2+ channels and the SNARE complex.
We recently showed that a mouse pheochromocytoma cell line (MPC 9/3L), established by Powers et al. (44), is a useful model for studying Ca2+-dependent neurotransmitter release (23). MPC 9/3L cells express many of the proteins involved in Ca2+-dependent release but do not express endogenous voltage-dependent Ca2+ channels. They exhibit no exocytosis when stimulated by trains of depolarizations. MPC 9/3L cells therefore represent an excellent single-cell system in which to study the roles of specific Ca2+-channel subunits in secretion. Subtypes of known subunit composition can be expressed in isolation or in assorted combinations. Introducing exogenous N-type Ca2+ channels initiated vesicle fusion in these cells (23) in a manner similar to that found in neurons and neurosecretory cells (37, 38, 45). In an earlier study, we expressed L- and T-type Ca2+ channels (which do not possess synprint sites) in addition to N-type channels into MPC cells. All three types of Ca2+ channel were shown to elicit robust vesicle fusion (23), but no direct comparison of secretion elicited by each type of channel was attempted, in part due to the different gating and permeation properties of the channels. Furthermore, L-type Ca2+ channels seem to interact with SNARE proteins by means of a mechanism that does not involve the synprint site (46). In the present study, the importance of the synprint site was tested. N-type Ca2+ channels (α1B, β2A, and α2δ), with and without the synprint site, were transfected into MPC cells to better understand the effect that this region of the α1B subunit has on release. We found that the N-type channels are coupled less efficiently to secretion when the synprint site was deleted. Secretion was smaller and slower, even though Ca2+ current amplitude was not affected. In addition, endocytosis was slowed in cells expressing N-type channels that lacked the synprint site. The slowing of endocytosis may be a secondary consequence of the reduction in exocytosis because there would be less synaptic vesicle membrane available in the plasma membrane for retrieval.
After showing that N- and P/Q-type Ca2+ channels bound to SNARE proteins, different studies investigated the functional consequences of this interaction. The authors of these studies hypothesized that interaction was required for the efficient release of neurotransmitter (19, 20). If the release machinery and Ca2+ channels are tethered at the synprint site, then disrupting that interaction should interfere with release. Injection of fusion proteins containing the synprint site may dissociate N-type channels from the SNARE core. Mochida et al. (19) showed that introduction of the synprint peptides into presynaptic superior cervical ganglion neurons inhibited synaptic transmission. In this case, fast excitatory postsynaptic potentials due to synchronous transmitter release were partially inhibited, but late asynchronous release was increased. The injected peptides did not affect the Ca2+ current. Rettig and colleagues (20) showed that injection of the synprint peptide into embryonic Xenopus spinal neurons altered the Ca2+-dependence of release even though Ca2+ influx was not altered. In that study, the authors observed a 25% reduction in relative release when the cells were stimulated in an extracellular solution containing 1.8 mM Ca2+. The authors modeled their results and proposed that ≈70% of the previously linked synaptic vesicles became uncoupled by the addition of the synprint peptide (20). These earlier studies with the synprint peptide did not assay the peptide concentration at individual synapses. Thus, they could not assess the number of functional Ca2+ channel/SNARE interactions remaining in the presence of the synprint peptide. Our model system, MPC cells, allowed us to completely eliminate Ca2+ channel/SNARE interactions. Our data, which are consistent with these earlier studies, show that eliminating Ca2+ channel/SNARE interactions results in ≈50% reduction in release. This reduction in exocytotic amplitude may represent the fact that the Ca2+ channels are no longer optimally located for efficient release. Our results also indicate that the synprint site is not absolutely required for release.
Not only does the synprint site position Ca2+ channels appropriately for vesicle fusion, but it may be involved in targeting the N- and P/Q-type Ca2+ channels to synapses. In a recent study, Mochida et al. (21) deleted the synprint site in the α1A subunit of P/Q-type channels and then expressed this construct in superior cervical ganglion neurons. They showed that these channels had a reduced effectiveness in synaptic transmission corresponding with a reduced localization to presynaptic terminals. Wild-type P/Q-type Ca2+ channels localized correctly. L-type channels are not typically localized at synapses, but, after Mochida et al. (21) spliced in a synprint site, the L-type channels localized correctly to the presynaptic nerve terminals and were able to establish synaptic transmission. Studies from other laboratories suggest that additional Ca2+ channel regions are used for targeting to synapses. Maximov and Bezprozvanny (47) showed that the C-terminal of the N-type Ca2+ channel contained a sequence that targeted the channel to synapses and that the modular adaptor proteins Mint1 and CASK may play a role in this targeting. More recently, Missler et al. (48) generated knockout mice in which one, two, or three of the α-neurexin genes were disrupted. In the triple-knockout animals, neurotransmitter release was greatly diminished due to loss of Ca2+ channel activity (48). Missler et al. (48) proposed that neurexins are required to couple the presynaptic N-type Ca2+ channels to the secretory machinery. If true, this finding would imply that there are at least two or more separate pathways for localizing Ca2+ channels to presynaptic nerve terminals and the secretory machinery. An alternate interpretation of the data presented in Missler et al. (48) is that the Ca2+ channels are correctly localized but that they are not functional in the absence of neurexin.
Not only do Ca2+ channels initiate neurotransmitter release, but also SNARE proteins, in turn, modify Ca2+ channel function, perhaps as a form of feedback modulation. For example, coexpressing syntaxin 1A with N-, P/Q-, and, in some cases, L-type Ca2+ channels results in decreased current amplitude, increased voltage-dependent inactivation, and/or enhanced Gβγ inhibition (8, 46, 49-55) presumably through interactions with the synprint site. The modulation of Ca2+ channel function by syntaxin 1A has also been demonstrated in nerve terminals. Stanley and Mirotznik (56) demonstrated that botulinum toxin, which cleaves syntaxin 1A, prevents G protein modulation of presynaptic Ca2+ channels. Although the mechanism(s) by which syntaxin 1A exerts its effects on Ca2+ channels are not well understood, it is clear that these types of interactions may have significant consequences for neurotransmitter release. Interestingly, other laboratories have shown that coexpression of other secretory proteins, like synaptotagmin, can relieve the syntaxin 1A-mediated inhibition of Ca2+ channels (46, 49, 57), suggesting that this kind of inhibition is dynamic. Our results showed that Ca2+ currents in MPC 9/3L cells were very similar regardless of whether or not the expressed Ca2+ channels lack a synprint site. Ca2+ channel availability was unchanged in the synprint deletion mutants. Our results therefore suggest that the N-type Ca2+ channels were in close proximity to mature SNARE complexes exclusively.
Although some aspects of the functional interactions between the synprint site and SNARE proteins remain to be elucidated, a wealth of information suggests that the site is important for both neurotransmitter release and Ca2+ channel function in both neurons and secretory cells. Further studies with MPC 9/3L cells and related cell lines will allow us to explore the functional links between a variety of different Ca2+ channels and secretion in greater detail.
Acknowledgments
We thank Dr. Terry Snutch for his kind gift of cDNA. This work was supported by National Institutes of Health Grants NS26189 (to A.P.F.), NS37685 (to A.S.T.), and NS42681 (to A.B.H.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SNAP-25, synaptosome-associated protein of 25 kDa; SNARE, SNAP-25 receptor; synprint, synaptic protein interaction; MPC, mouse pheochromocytoma.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. AF173882 (α1B), AF174417 (β2a), and M86621 (α2δ)].
References
- 1.Fatt, P. & Katz, B. (1951) J. Physiol. (London) 115, 320-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llinas, R., Steinberg, I. Z. & Walton, K. (1976) Proc. Natl. Acad. Sci. USA 73, 2913-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunlap, K., Luebke, J. I. & Turner, T. J. (1995) Trends Neurosci. 18, 89-98. [PubMed] [Google Scholar]
- 4.Bajjalieh, S. M. & Scheller, R. H. (1995) J. Biol. Chem. 270, 1971-1974. [DOI] [PubMed] [Google Scholar]
- 5.Sudhof, T. C. (1995) Nature 375, 645-653. [DOI] [PubMed] [Google Scholar]
- 6.Bajjalieh, S. (2001) Trends Neurosci. 24, 678-680. [DOI] [PubMed] [Google Scholar]
- 7.Gerst, J. E. (1999) Cell. Mol. Life Sci. 55, 707-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bezprozvanny, I., Scheller, R. H. & Tsien, R. W. (1995) Nature 378, 623-626. [DOI] [PubMed] [Google Scholar]
- 9.Catterall, W. A. (1998) Cell Calcium 24, 307-323. [DOI] [PubMed] [Google Scholar]
- 10.Atlas, D. (2001) J. Neurochem. 77, 972-985. [DOI] [PubMed] [Google Scholar]
- 11.Bennett, M. K., Calakos, N. & Scheller, R. H. (1992) Science 257, 255-259. [DOI] [PubMed] [Google Scholar]
- 12.Leveque, C., Hoshino, T., David, P., Shoji-Kasai, Y., Leys, K., Omori, A., Lang, B., el Far, O., Sato, K., Martin-Moutot, N., et al. (1992) Proc. Natl. Acad. Sci. USA 89, 3625-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida, A., Oho, C., Omori, A., Kuwahara, R., Ito, T. & Takahashi, M. (1992) J. Biol. Chem. 267, 24925-24928. [PubMed] [Google Scholar]
- 14.Walker, D. & De Waard, M. (1998) Trends Neurosci. 21, 148-154. [DOI] [PubMed] [Google Scholar]
- 15.Catterall, W. A. (2000) Annu. Rev. Cell Dev. Biol. 16, 521-555. [DOI] [PubMed] [Google Scholar]
- 16.Sheng, Z. H., Rettig, J., Takahashi, M. & Catterall, W. A. (1994) Neuron 13, 1303-1313. [DOI] [PubMed] [Google Scholar]
- 17.Rettig, J., Sheng, Z. H., Kim, D. K., Hodson, C. D., Snutch, T. P. & Catterall, W. A. (1996) Proc. Natl. Acad. Sci. USA 93, 7363-7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheng, Z. H., Rettig, J., Cook, T. & Catterall, W. A. (1996) Nature 379, 451-454. [DOI] [PubMed] [Google Scholar]
- 19.Mochida, S., Sheng, Z. H., Baker, C., Kobayashi, H. & Catterall, W. A. (1996) Neuron 17, 781-788. [DOI] [PubMed] [Google Scholar]
- 20.Rettig, J., Heinemann, C., Ashery, U., Sheng, Z. H., Yokoyama, C. T., Catterall, W. A. & Neher, E. (1997) J. Neurosci. 17, 6647-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mochida, S., Westenbroek, R. E., Yokoyama, C. T., Zhong, H., Myers, S. J., Scheuer, T., Itoh, K. & Catterall, W. A. (2003) Proc. Natl. Acad. Sci. USA 100, 2819-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mochida, S., Westenbroek, R. E., Yokoyama, C. T., Itoh, K. & Catterall, W. A. (2003) Proc. Natl. Acad. Sci. USA 100, 2813-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harkins, A. B., Cahill, A. L., Powers, J. F., Tischler, A. S. & Fox, A. P. (2003) J. Neurophysiol. 90, 2325-2333. [DOI] [PubMed] [Google Scholar]
- 24.Cahill, A. L., Hurley, J. H. & Fox, A. P. (2000) J. Neurosci. 20, 1685-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horton, R. M., Hunt, H. D., Ho, S. N., Pullen, J. K. & Pease, L. R. (1989) Gene 77, 61-68. [DOI] [PubMed] [Google Scholar]
- 26.Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. (1981) Pflügers Arch. 391, 85-100. [DOI] [PubMed] [Google Scholar]
- 27.Neher, E. & Marty, A. (1982) Proc. Natl. Acad. Sci. USA 79, 6712-6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi, C. & Fernandez, J. M. (1988) Biophys. J. 53, 885-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harkins, A. B. & Fox, A. P. (1998) J. Gen. Physiol. 111, 257-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Waard, M., Liu, H., Walker, D., Scott, V. E., Gurnett, C. A. & Campbell, K. P. (1997) Nature 385, 446-450. [DOI] [PubMed] [Google Scholar]
- 31.Kim, K. T., Koh, D. S. & Hille, B. (2000) J. Neurosci. 20, RC101: 1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haller, M., Heinemann, C., Chow, R. H., Heidelberger, R. & Neher, E. (1998) Biophys. J. 74, 2100-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirning, L. D., Fox, A. P., McCleskey, E. W., Olivera, B. M., Thayer, S. A., Miller, R. J. & Tsien, R. W. (1988) Science 239, 57-61. [DOI] [PubMed] [Google Scholar]
- 34.Horne, A. L. & Kemp, J. A. (1991) Br. J. Pharmacol. 103, 1733-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luebke, J. I., Dunlap, K. & Turner, T. J. (1993) Neuron 11, 895-902. [DOI] [PubMed] [Google Scholar]
- 36.Turner, T. J., Adams, M. E. & Dunlap, K. (1993) Proc. Natl. Acad. Sci. USA 90, 9518-9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheeler, D. B., Randall, A. & Tsien, R. W. (1994) Science 264, 107-111. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi, T. & Momiyama, A. (1993) Nature 366, 156-158. [DOI] [PubMed] [Google Scholar]
- 39.Stanley, E. F. (1993) Neuron 11, 1007-1011. [DOI] [PubMed] [Google Scholar]
- 40.Westenbroek, R. E., Sakurai, T., Elliott, E. M., Hell, J. W., Starr, T. V., Snutch, T. P. & Catterall, W. A. (1995) J. Neurosci. 15, 6403-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheng, Z. H., Westenbroek, R. E. & Catterall, W. A. (1998) J. Bioenerg. Biomembr. 30, 335-345. [DOI] [PubMed] [Google Scholar]
- 42.Catterall, W. A. (1999) Ann. N.Y. Acad. Sci. 868, 144-159. [DOI] [PubMed] [Google Scholar]
- 43.Leveque, C., el Far, O., Martin-Moutot, N., Sato, K., Kato, R., Takahashi, M. & Seagar, M. J. (1994) J. Biol. Chem. 269, 6306-6312. [PubMed] [Google Scholar]
- 44.Powers, J. F., Evinger, M. J., Tsokas, P., Bedri, S., Alroy, J., Shahsavari, M. & Tischler, A. S. (2000) Cell Tissue Res. 302, 309-320. [DOI] [PubMed] [Google Scholar]
- 45.Artalejo, C. R., Adams, M. E. & Fox, A. P. (1994) Nature 367, 72-76. [DOI] [PubMed] [Google Scholar]
- 46.Wiser, O., Trus, M., Hernandez, A., Renstrom, E., Barg, S., Rorsman, P. & Atlas, D. (1999) Proc. Natl. Acad. Sci. USA 96, 248-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maximov, A. & Bezprozvanny, I. (2002) J. Neurosci. 22, 6939-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Missler, M., Zhang, W., Rohlmann, A., Kattenstroth, G., Hammer, R. E., Gottmann, K. & Sudhof, T. C. (2003) Nature 424, 939-948. [DOI] [PubMed] [Google Scholar]
- 49.Wiser, O., Bennett, M. K. & Atlas, D. (1996) EMBO J. 15, 4100-4110. [PMC free article] [PubMed] [Google Scholar]
- 50.Bezprozvanny, I., Zhong, P., Scheller, R. H. & Tsien, R. W. (2000) Proc. Natl. Acad. Sci. USA 97, 13943-13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jarvis, S. E., Magga, J. M., Beedle, A. M., Braun, J. E. & Zamponi, G. W. (2000) J. Biol. Chem. 275, 6388-6394. [DOI] [PubMed] [Google Scholar]
- 52.Jarvis, S. E. & Zamponi, G. W. (2001) Trends Pharmacol. Sci. 22, 519-525. [DOI] [PubMed] [Google Scholar]
- 53.Jarvis, S. E. & Zamponi, G. W. (2001) J. Neurosci. 21, 2939-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trus, M., Wiser, O., Goodnough, M. C. & Atlas, D. (2001) Neuroscience 104, 599-607. [DOI] [PubMed] [Google Scholar]
- 55.Jarvis, S. E., Barr, W., Feng, Z. P., Hamid, J. & Zamponi, G. W. (2002) J. Biol. Chem. 277, 44399-44407. [DOI] [PubMed] [Google Scholar]
- 56.Stanley, E. F. & Mirotznik, R. R. (1997) Nature 385, 340-343. [DOI] [PubMed] [Google Scholar]
- 57.Zhong, H., Yokoyama, C. T., Scheuer, T. & Catterall, W. A. (1999) Nat. Neurosci. 2, 939-941. [DOI] [PubMed] [Google Scholar]