Abstract
GABAA receptor subunit composition is a critical determinant of receptor localization and physiology, with synaptic receptors generating phasic inhibition and extrasynaptic receptors producing tonic inhibition. Extrasynaptically localized α5 GABAA receptors are largely responsible for tonic inhibition in hippocampal neurons. However, we show here that inhibitory synapses also contain a constant level of α5 GABAA receptors throughout neuronal development, as measured by its colocalization with gephyrin, the inhibitory postsynaptic scaffolding protein. Immunoprecipitation of the α5 subunit from both cultured neurons and adult rat brain coimmunoprecipitated gephyrin, confirming this interaction in vivo. Furthermore, the α5 subunit can interact with gephyrin independent of other synaptically localized alpha subunits, as shown by immunoprecipitation experiments in HEK cells. By replacing the α5 predicted gephyrin binding domain (Residues 370–385) with either the high affinity gephyrin binding domain of the α2 subunit or homologous residues from the extrasynaptic α4 subunit that does not interact with gephyrin, α5 GABAA receptor localization shifted into or out of the synapse, respectively. These shifts in the ratio of synaptic/extrasynaptic α5 localization disrupted dendritic outgrowth and spine maturation. In contrast to the predominant view of α5 GABAA receptors being extrasynaptic and modulating tonic inhibition, we identify an intimate association of the α5 subunit with gephyrin, resulting in constant synaptic levels of α5 GABAAR throughout circuit formation that regulates neu ronal development.
Keywords: alpha 5 GABA(A)R, gephyrin, spine morphology, dendritic outgrowth
INTRODUCTION
Gamma-aminobutyric acid type A receptors (GABAARs) are pentameric chloride permeable ligand gated ion channels mediating GABAergic inhibition in the brain. Synaptic release of GABA activates postsynaptically localized GABAARs to trigger fast inhibitory currents, while ambient GABA results in activation of extrasynaptic receptors and generation of tonic inhibition. The subunit composition of the receptor, typically two α subunits, two β subunits, and either a γ or a δ subunit, determines receptor cell surface localization as well as physiological and pharmacological properties. Receptors containing α1, α2, and/or α3 subunits are primarily located at GABAergic synapses via direct association with gephyrin, the key inhibitory postsynaptic scaffolding protein responsible for GABAAR and glycine receptor localization (Tyagarajan and Fritschy, 2014). GABAARs containing the α4 and α6 subunits do not interact with gephyrin, are extrasynaptically localized, and are responsible for tonic GABA currents (Glykys and Mody, 2007). The α5 subunit assembles with β and γ subunits, is reported to be primarily localized extrasynaptically (Brunig et al., 2002; Christie and de Blas, 2002), and is primarily responsible for generating tonic inhibition in the hippocampus (Glykys et al., 2008). Extrasynaptically, the α5 subunit colocalizes with radixin, a cytoskeletal protein linking actin to the plasma membrane (Loebrich et al., 2006).
The α5 subunit is suggested to play an important role in hippocampal pyramidal neuron development (Marchionni et al., 2007; Giusi et al., 2009). However, the contribution of α5-containing GABAAR localization to neuronal development is unknown, and the mechanism for synaptic accumulation of the α5 subunit has not been established. We show here that surface α5 GABAARs significantly colocalize with gephyrin, with α5 synaptic content being maintained at a constant ratio throughout development. We then demonstrate that the α5 subunit directly interacts with gephyrin, and that disruption of Residues 370–385 of the α5 subunit alters both α5 association with gephyrin and receptor synaptic/extrasynaptic localization, but is not required for cluster formation or maintenance of surface receptor number. Finally, we show that balanced distribution of the α5 subunit at synaptic and extrasynaptic sites is important for proper dendritic outgrowth and spine maturation. Together these results support a synaptic role in neuronal development for the α5 GABAAR subtype.
METHODS
DNA Constructs and Antibodies
The α5WT, α5α4GBD, α5α2GBD, and RFP (red monomeric fluorescent protein mCherry) tagged gephyrin construct were generated by PCR cloning (Li et al., 2011) from CDNAs (kindly provided by Stephen J. Moss). The pH-sensitive GFP tagged α2 GABAAR subunit (α2WT) has been described previously (Tretter et al, 2008). All constructs were fully sequenced. The following antibodies were used: rabbit anti-α5 (Cat # 224503), mouse anti-gephyrin (RRID: AB_887719), rabbit anti-VGAT (RRID: AB_887871), mouse anti-α1 (RRID: AB_10597955), and rabbit anti-α2 (RRID: AB_2108839; Synaptic Systems, Gottingen, Germany); rabbit anti-radixin (Cat # R3653, Sigma, St. Louis, MO); chicken anti-GFP (RRID: AB_10000240, Aves Labs, Tigard, OR); rabbit anti-GFP (RRID: AB_221570, Life Technologies, Carlsbad, CA); mouse anti-RFP (RRID: AB_1141717, Abcam, Cambridge, MA) and secondary antibodies for immunofluorescence (goat anti-rabbit Alexa Fluor 568 RRID: AB_143011; goat anti-rabbit Alexa Fluor 488 RRID: AB_10562715; Goat anti-mouse Alexa Fluor 647 RRID: AB_141725; goat anti-chicken Alexa Fluor 488 Cat # 11309, Life Technologies, Carlsbad, CA). Rabbit IgG (RRID: AB_737197), mouse IgG (RRID: AB_737182), and goat anti-α5 (RRID: AB_2109314) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Culture and Transfection
All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Hippocampal neurons were prepared from embryonic day 18 rats and nucleofected (Lonza) at plating (Jacob et al., 2005). HEK-293 cells were also transfected using nucleofection.
Immunofluorescence, Imaging, and Analysis of Cells
Surface GABAAR α5 or anti-GFP staining was done under nonpermeabilized conditions, followed by permeabilization and staining for α5, GFP, and/or gephyrin as previously described (Jacob et al., 2005). Images were taken on a Nikon A1 Confocal microscope using a 60× oil immersion objective (NA 1.49) at a 2× zoom. Samples for confocal imaging were sequentially scanned with individual lasers (488, 561, and 640 nm) and an appropriate emission band pass filter (500–550 and 575–625 nm) or long pass filter (650LP) to avoid any spectral bleed-through between channels. Data were analyzed using NIS Elements software (Nikon, NY), with colocalization determined as previously described (Tretter et al., 2008). In the gephyrin and α5 GABAAR colocalization studies, 20-μm length regions (ROIs) from two to three proximal dendrites per neuron were used for analysis. For comparison between α5WT, α5α4GBD, and α5α2GBD, the researcher was blinded to the experimental conditions. For cluster analysis, thresholding was kept constant between cultures. For total α5 surface fluorescence measurements (both clustered and diffuse receptors), fluorescence values in the same ROIs were measured without thresholding. 3D reconstructions of confocal z series acquired with a 40× oil immersion objective, 1× zoom, were used for Sholl analysis, and analyzed using ImageJ software. Spine morphology studies were performed as previously described (Jacob et al., 2009). Briefly, images were analyzed from 3D reconstructions of confocal z series acquired with a 60× oil immersion objective using a 4× zoom. Spine length was determined using NIS Elements by measuring the length from the spine head to where the spine intersected the dendritic shaft. We classified spines as either mushroom or filopodia, with a mushroom spine having a spine head twice the size of the spine neck, and the remaining spines classified as filopodia.
Immunoprecipitation and Western Blots
For immunoprecipitation from hippocampal neuronal cultures, neurons were nucleofected with α5WT at plating. At DIV 14, cells were lysed in Buffer A containing 50 mM Tris-HCl (pH 7.6), 50 mM NaCl, 1 mM EDTA, 0.25% Igepal, 10 mM NaF, 2 mM sodium orthovanadate, and protease inhibitor cocktail (Sigma, St. Louis, MO), solubilized for 15 min at 4°C, and spun at 1,000g for 10 min. Extracts were then immunoprecipitated with either rabbit anti-GFP (5 μg), or rabbit IgG as a negative control. For hippocampal and cortical rat tissue, adult female rats were killed using CO2 followed by cervical dislocation. The hippocampus and cortex were dissected and homogenized as previously described (Brady et al., 2013), diluted approximately fivefold in Buffer A, and immunoprecipitated with rabbit anti-α5 antibody crosslinked to Protein A Sepharose beads with BS3 (Thermo Scientific, Rockford, IL). The beads were eluted by incubating beads twice with 50 mM glycine for 5 min, as previously described (Sousa et al., 2011), and SDS-PAGE loading buffer was added. For immunoprecipitation from HEK cells, HEK cells were transfected with RFP-gephyrin, an untagged β3 subunit and either a α2WT or α5WT construct, and lysates were immunoprecipitated with rabbit anti-GFP as described above.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 5 (San Diego, CA) on 3–4 independent cultures, 12–18 neurons per culture, using two way ANOVA with Tukey post hoc test for dendritic morphology analysis or one-way ANOVA with paired t-test post-hoc tests for all other studies. All values are given as mean ± standard error of the mean.
RESULTS
Inhibitory Synapses Contain a Constant Level of α5-Containing GABAARs Throughout Development
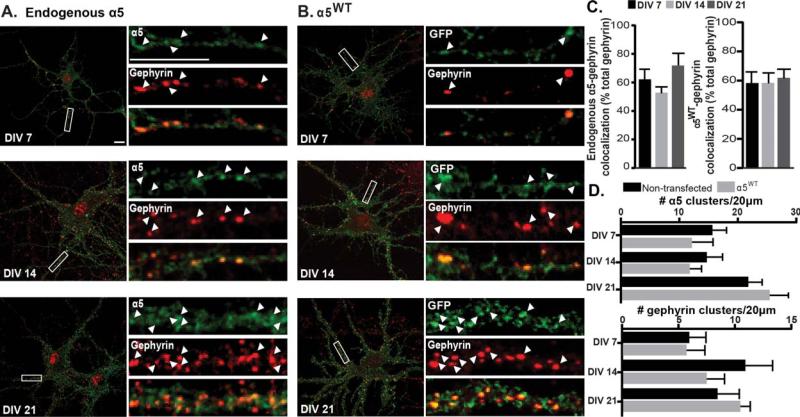
GABAARs containing the α5 subunit are generally considered to be extrasynaptic; however, previous studies have found some evidence for synaptic localization (Christie and de Blas, 2002; Serwanski et al., 2006). We first examined whether a pool of endogenous α5 GABAARs was synaptically located and if receptor localization changes throughout development. Hippocampal neurons were fixed and stained for surface α5 and gephyrin, the postsynaptic inhibitory scaffold protein (Jacob et al., 2005; Flores and Mendez, 2014; Tyagarajan and Fritschy, 2014). Analysis of the number of gephyrin clusters colocalized with α5 found a high level of colocalization, with approximately a 60% association maintained at a uniform level throughout development [DIV 7 62.67 ± 7.38, DIV 14 53.34 ± 4.62, DIV 21 72.34 ± 9.06; Fig. 1(A,C)]. These data support a role for synaptically located α5 GABAARs contributing to GABAergic inhibition, in addition to the established role of extrasynaptic α5 GABAARs.
Figure 1.
GABAAR α5 association with gephyrin throughout development in hipppocampal neurons. (A) DIV 7, 14, and 21 neurons were fixed and immunostained for surface α5 GABAAR (anti-α5, green, non permeabilized conditions) and gephyrin (red, permeabilized conditions). Colocalized clusters are indicated by arrowheads. Scale bar = 10 μm. (B) Neurons expressing α5WT were fixed and stained for surface tagged GABAAR (anti-GFP, green, nonpermeabilized conditions) and gephyrin (red). (C) The percentage of gephyrin clusters colocalized with surface clusters of endogenous α5 or tagged α5 GABAAR remained constant throughout development. (D) The number of surface α5 GABAAR and gephyrin clusters in 20 μm dendritic segments in nontransfected and transfected cells was compared. No significant difference was found between groups for either α5 or gephyrin.
To further investigate mechanisms of α5 GABAAR synaptic localization, we then introduced a myc tag and a pH-sensitive GFP variant at the N-terminus of the α5 subunit (α5WT). It has been shown that insertion of these tags into other GABAAR subunits are functionally silent (Jacob et al., 2005; Tretter et al., 2008, 2011; Mukherjee et al., 2011). To confirm that these tags do not induce major alterations, we examined the total number of surface α5 containing GABAAR clusters and the total number of gephyrin clusters between nontransfected and transfected neurons at all developmental stages [Fig. 1(A,B,D)]. We found no significant difference between groups, indicating that GABAARs containing tagged α5 subunits do not induce changes in the development of GABAergic synapses. We then examined synaptic localization of the α5WT construct. Similar to nontransfected neurons, the number of gephyrin clusters associated with α5WT remains constant throughout development [DIV 7 59.24 ± 7.95, DIV 14 59.25 ± 7.25, DIV 21 62.76 ± 6.45; Fig. 1(B,C)]. As the α5 subunit has been shown to associate with radixin in the extrasynaptic membrane at DIV 13–14 (Loebrich et al., 2006), surface α5WT colocalization with radixin was also assessed: there was a significant decrease in association of radixin and α5 between DIV 7 and DIV 21 (DIV 7 70.45 ± 10.12%, DIV 21 26.60 ±9.05%, p = 0.0270; Supporting Information Fig. S1). These data suggest that during this developmental period, while α5-radixin association decreases over time, the number of synapses (as represented by gephyrin) with α5-containing GABAARs remains constant. In summary, these data show that a major portion of α5 GABAARs is observed at synaptic locations throughout the key period of synaptogenesis and in an established hippocampal neuron culture circuit.
α5-Containing GABAAR and Gephyrin Are Intimately Associated in Cultured Neurons and In Vivo
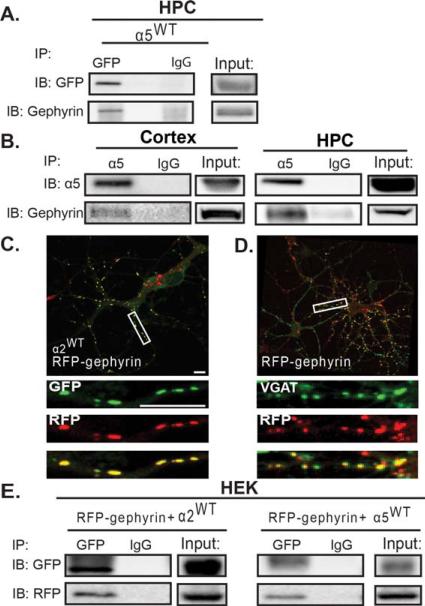
To further demonstrate a close association of α5 with gephyrin in neurons, we performed immunoprecipitations on hippocampal neuron cultures transfected with the α5WT construct. Immunprecipitation of the tagged α5 subunit with an anti-GFP antibody also coimmunprecipitated gephyrin [Fig. 2(A)]. To confirm that α5 and gephyrin interact in vivo, endogenous α5 was immunoprecipitated from adult rat hippocampal and cortical tissue, and the association of gephyrin was determined. As shown in Figure 2(B), the α5 subunit coimmunoprecipitated gephyrin, confirming that these two proteins are associated in vivo.
Figure 2.
The GABAAR α5 subunit is able to directly associate with gephyrin. (A) Cultured hippocampal neurons were transfected with α5WT and lysates were immunoprecipitated with anti-GFP or anti-IgG. Precipitates were immunoblotted for GFP and gephyrin. Immunoprecipitation of GFP resulted in coimmunoprecipitation of gephyrin. (B) Cortical and hippocampal (HPC) lysates from adult rat brain were immunoprecipitated with either anti-α5 or anti-IgG and immunoblotted for α5 and gephyrin. In both cortex and HPC, gephyrin coimmunoprecipitates with α5 (n = 3 animals). (C) Neurons were transfected with RFP-gephyrin and α2WT, then fixed, stained under nonpermeabilized conditions with anti-GFP, then permeabilized and stained with anti-RFP. (D) Neurons were transfected with RFP-gephyrin, fixed, permeabilized, and stained for RFP and VGAT. (E) HEK cells were transfected with α5WT, β3, and RFP-gephyrin. Lysates were then immunoprecipitated for GFP and immunoblotted for RFP and GFP. GFP immunoprecipitation resulted in coimmunoprecipitation of RFP-gephyrin. HEK cells transfected with α2WT, β3, and RFP-gephyrin were used as a positive control.
The α5 Subunit Intracellular Domain Is Sufficient for Gephyrin Binding
Our data indicate that the α5 subunit is associating with gephyrin, although this could occur solely via a direct interaction of the α5 subunit with gephyrin (via the α5 intracellular domain), or via α5 interactions with other proteins capable of directly interacting with gephyrin, such as another α subunit. A potential weak direct interaction between the α5 intracellular loop and gephyrin was suggested by minimal binding in a GST overlay assay using an α5 intracellular loop GST-fusion protein overlaid with 35S-methionine labeled gephyrin (Mukherjee et al., 2011). To further examine the mechanism for α5 GABAAR synaptic localization with a complete α5 subunit, we cotransfected the α5WT and an RFP-gephyrin construct into HEK cells, along with an untagged β3 construct (surface expression of functional GABAARs requires a β subunit; Connolly et al., 1996). As a positive control, a pH-sensitive GFP tagged α2WT subunit was cotransfected with RFP-gephyrin and the β3 subunit in a separate set of HEK cell experiments. In neurons the RFP-gephyrin exhibits equivalent subcellular localization as endogenous gephyrin, being highly colocalized with synaptic α2 GABAAR and closely apposed to the presynaptic inhibitory terminal maker VGAT [vesicular GABA/glycine transporter; Fig. 2(C,D)], indicating its ability to interact with GABAARs. Immunoprecipitation of HEK lysates with anti-GFP resulted in coimmunoprecipitation of RFP-gephyrin by both α2WT and α5WT [Fig. 2(E)], indicating that these proteins are associated in a heterologous system, supporting a direct interaction between the α5 and gephyrin rather than via α1 or α2 subunits.
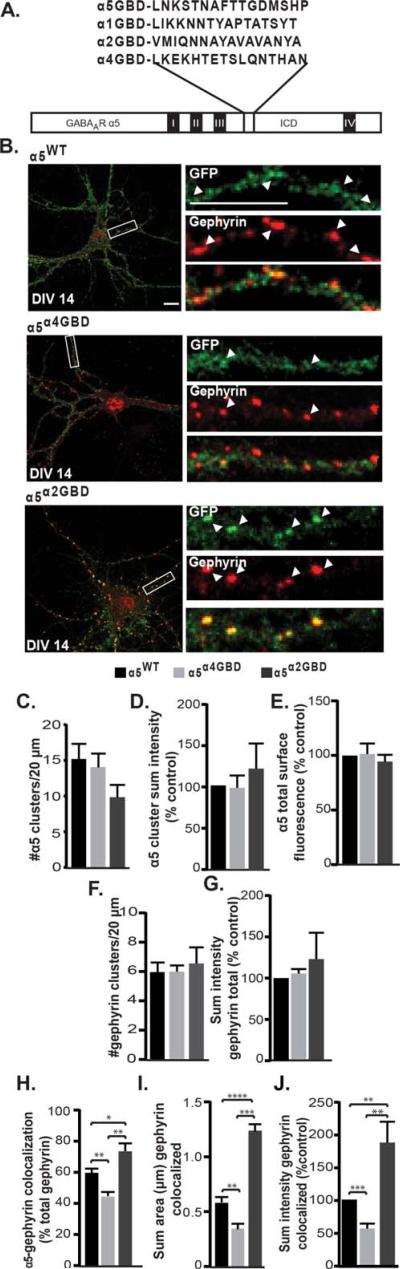
The α5 Gephyrin Binding Domain Contributes to Synaptic Localization of α5 GABAAR
GABAAR α1, α2, and α3 subunits localize to the synapse via interaction with gephyrin through specific domains in the subunit intracellular loops between transmembrane Domains 3 and 4 (Tretter et al., 2008, 2011; Mukherjee et al., 2011), whereas extrasynaptic α4 or α6 GABAARs do not colocalize or interact with gephyrin (Sun et al., 2004; Kralic et al., 2006). To determine if the α5 sequence (Residues 370–385) homologous to the gephyrin binding domains in other alpha subunits enables α5 interaction with gephyrin, we created two different α5 chimeras; one in which the predicted gephyrin binding domain (GBD; Mukherjee et al., 2011) of α5 was replaced with the homologous region from α4 (α5α4GBD) and another in which the α5 GBD was replaced with the α2 GBD [α5α2GBD; Fig. 3(A)]. Chimeric GABAAR expression levels, assembly and surface trafficking were equivalent to α5WT in HEK cells (Supporting Information Fig. S2). We then transfected neurons with the α5WT, α5α4GBD, or α5α2GBD constructs and analyzed surface GABAAR α5 subunit clustering and synaptic localization at DIV 14. In neurons expressing the chimeric receptors, both surface tagged α5 GABAAR clusters and gephyrin overall cluster attributes were unchanged, indicating normal synaptogenesis: the total number and sum intensity of clusters were not significantly different [Fig. 3(C,D,F,G)]. In addition, the total surface fluorescence of α5 (both clustered and diffuse), was unchanged between groups, indicating no apparent change in receptor trafficking [Fig. 3(E)]. We found a significant decrease in surface receptor colocalization with gephyrin in neurons transfected with α5α4GBD compared to α5WT and α5α2GBD, and a significant increase in colocalization in neurons transfected with α5α2GBD compared to α5WT [α5WT 60.80 ± 3.16%; α5α4GBD 45.29 ± 2.64%; α5α2GBD 74.68 ± 5.06%; α5WT vs. α5α4GBD p = 0.0055, α5WT vs. α5α2GBD p = 0.048, α5α4GBD vs. α5α2GBD p = 0.0012, Fig. 3(B,H)]. Gephyrin clusters colocalized with α5 also exhibited corresponding changes: cluster sum area [α5WT 0.54 ± 0.045; α5α4GBD 0.32 ± 0.046; α5α2GBD 1.15 ± 0.063; α5WT vs. α5α4GBD p = 0.0096, α5WT vs. α5α2GBD p = 0.0002, α5α4GBD vs. α5α2GBD p < 0.0001, Fig. 3(B,I)] and sum intensity normalized to control [α5WT 100; α5α4GBD 56.89 ± 7.52; α5α2GBD 189.10 ± 31.81; α5WT vs. α5α4GBD p = 0.0004, α5WT vs. α5α2GBD p = 0.0086, α5α4GBD vs. α5α2GBD p = 0.002, Fig. 3(B,J)]. The colocalization data indicate that replacement of the putative gephyrin binding domain in α5 with α2GBD, representing a high affinity gephyrin binding site, increased α5 synaptic levels, while disruption with the α4GBD decreased, but did not eliminate, α5 synaptic levels. The remaining association of α5 and gephyrin seen here may be due to a gephyrin interaction with an endogenous α1 or α2 subunit, as previous studies have shown mixed subunit populations occurring in vivo (Araujo et al., 1999; del Rio et al., 2001). In summary, these data show that the α5 gephyrin binding domain significantly contributes to α5 GABAAR synaptic localization.
Figure 3.
Disruption of the gephyrin binding domain in the α5 subunit decreases its association with gephyrin. (A) Alignment of homologous regions of the gephyrin binding domain (GBD) of different alpha subunits, and construction of the α5α4GBD and α5α2GBD chimeras. (B) DIV 14 neurons expressing the α5WT, α5α4GBD, or α5α2GBD constructs were fixed and stained for surface GABAAR (anti-GFP, green) and gephyrin (red). (C–E) In transfected neurons, there was no significant difference in the total number of α5 clusters per 20 μm, the total sum intensity of α5 clusters, or the total α5 surface fluorescence. (F and G) There was no significant difference in the total number of gephyrin clusters per 20 μm or the overall sum intensity of gephyrin between groups. (H) Swapping the α5 GBD for the α4 GBD significantly decreased colocalization of the α5 subunit with gephyrin, while exchanging the α5 GBD for the α2 GBD significantly increased gephyrin colocalization (*p < 0.05, **p < 0.01). (I and J) The sum area and sum intensity of gephyrin clusters colocalized with tagged GABAAR was significantly decreased in neurons transfected with the α5α4GBD compared to control, while neurons transfected with α5α2GBD showed a significant increase in sum area and sum intensity of colocalized gephyrin clusters (**p < 0.01, ***p < 0.001, and ****p < 0.0001).
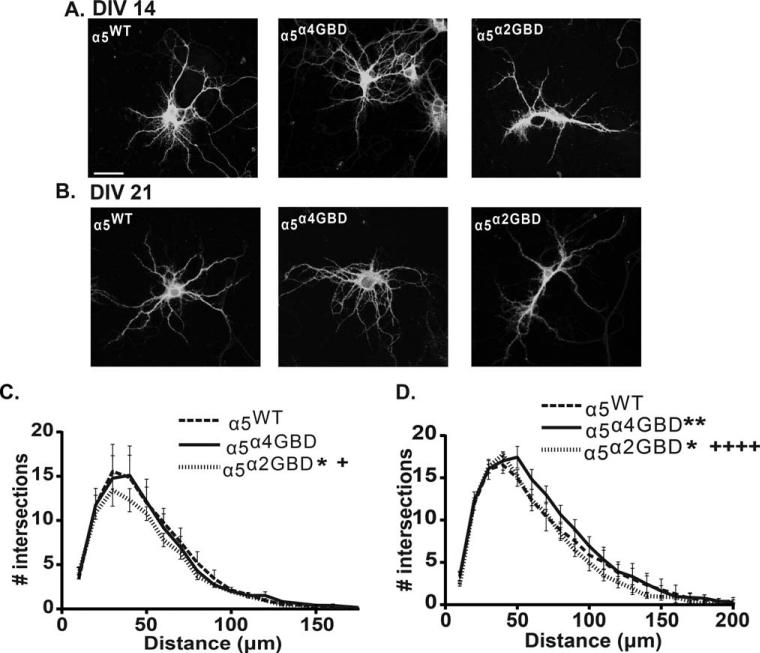
Proper Localization of α5-Containing GABAARs Is Important for Neuronal Development
As previous genetic and pharmacological studies have implicated a role of α5-containing GABAARs in proper neuronal development (Curia et al., 2009; Giusi et al., 2009; Fatemi et al., 2010) we examined the effect of α5 redistribution on neuronal morphology at DIV 14 and 21. Neurons were transfected with the α5WT, α5α4GBD, or α5α2GBD constructs at plating, then fixed, permeabilized, and stained with anti-GFP and anti-MAP-2. 3-D reconstructions of confocal z stacks were analyzed by Sholl analysis using ImageJ. At DIV 14, α5α2GBD neurons exhibited a significant decrease in the number of intersections compared to α5WT and α5α4GBD [two-way ANOVA significant effect of transfection p = 0.0116; Tukey post hoc test α5α2GBD vs. α5WT p < 0.05; α5α2GBD vs. α5α4GBD p < 0.05; Fig. 4(A,C)]. At DIV 21, we observed a significant decrease in the number of intersections between α5α2GBD neurons compared to α5WT and α5α4GBD [two-way ANOVA significant effect of transfection p < 0.0001; Tukey post hoc test α5α2GBD vs. α5WT p < 0.05; α5α2GBD vs. α5α4GBD p < 0.0001 Fig. 4(B,D)], and a significant increase in the number of intersections between α5α4GBD and α5WT [p < 0.05 Fig. 4(B,D)]. Further analysis found no significant difference in number of primary, secondary, or tertiary dendrites, or total dendritic length, either at DIV 14 or 21 (Supporting Information Fig. S3). Together, these data indicate that shifting α5 localization into the synapse via the α2 GBD impairs dendritic outgrowth, while moving the α5 subunit out of the synapse via the α4 GBD increases dendritic outgrowth.
Figure 4.
α5 localization is important in controlling dendritic outgrowth. Sholl analysis was performed on hippocampal cultures transfected with the α5WT, α5α4GBD, or α5α2GBD, then fixed and stained under permeabilized conditions with anti-GFP and anti-MAP-2 antibodies. Confocal z-series were acquired with a 40× objective, and 3D constructions were used to analyze dendritic morphology. (A and C) At DIV 14, neurons transfected with α5α2GBD exhibited impaired dendritic growth compared to those transfected with α5WT or α5α4GBD (*p < 0.05 compared to α5WT, +p < 0.05 compared to α5α4GBD; number of dendrites examined: α5WT 1054, α5α4GBD 1076, α5α2GBD 1020). (B and D) At DIV 21, neurons transfected with α5α4GBD showed more dendritic intersections than either α5WT or α5α2GBD, while neurons expressing α5α2GBD showed fewer dendritic intersections (*p < 0.05 compared to α5WT, ** p < 0.01 compared to α5WT, ++++p < 0.0001 compared to α5α4GBD; number of dendrites examined: α5WT 1126, α5α4GBD 1213, α5α2GBD 1269).
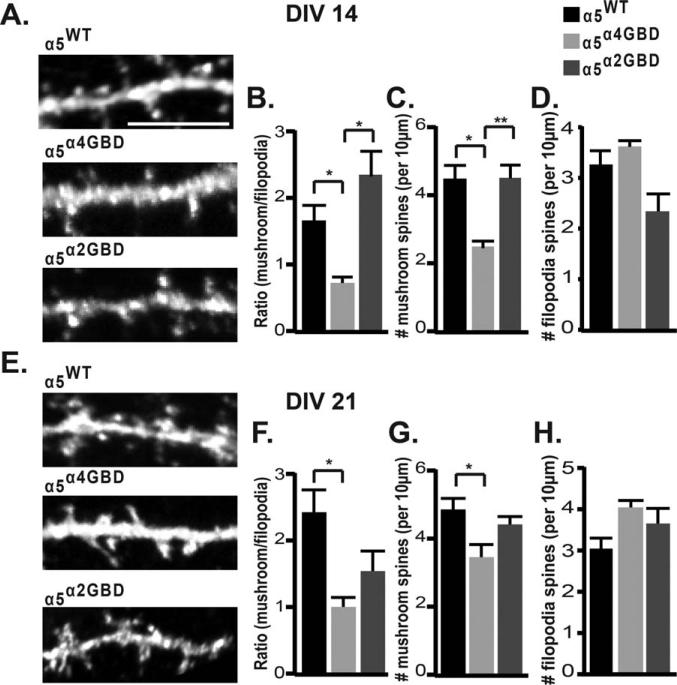
We also examined the effect of α5 mislocalization on dendritic spine morphology, since alterations in GABAAR surface levels have been shown to alter spine maturation (Jacob et al., 2009). At DIV 14, while overall spine length and spine density were unchanged (Supporting Information Fig. S3), there was a significant decrease in the number of mushroom spines in α5α4GBD neurons compared to α5WT and α5α2GBD [α5α4GBD 2.502 ± 0.16 spines/10 μm, α5WT 4.48 ± 0.46 spines/10 μm, α5α2GBD 4.50 ± 0.38 spines/10 μm; α5α4GBD vs. α5WT p = 0.016; α5α4GBD vs. α5α2GBD p = 0.0083, Fig. 5(A,C)], which led to a shift in mushroom/filopodia ratio in α5α4GBD neurons compared to α5WT and α5α2GBD [α5α4GBD 0.73 ± 0.033, α5WT 1.66 ± 0.24, α5α2GBD 2.34 ± 0.38; α5α4GBD vs. α5WT p = 0.019; α5α4GBD vs. α5α2GBD p = 0.0131, Fig. 5(A,B)]. At DIV 21, the number of mushroom spines in α5α4GBD neurons were still reduced compared to α5WT [α5α4GBD 3.50 ± 0.37, α5WT 4.90 ± 0.32, α5α2GBD 4.46 ± 0.23; α5α4GBD vs. α5WT p = 0.0461, Fig. 5(E,G)], again leading to a decrease in the mushroom/filopodia ratio [α5α4GBD 1.02 ± 0.14, α5WT 2.45 ± 0.34, α5α2GBD 1.56 ± 0.30; α5α4GBD vs. α5WT p = 0.018, Fig. 5(E,F)]. Neither spine density nor lengths were altered at DIV 21 (Supporting Information Fig. S4). The decrease in the mushroom/filopodia ratio suggests that a shift of α5-containing GABAARs out of the GABAergic synapse results in a less mature spine, and that this immature phenotype is maintained throughout development.
Figure 5.
Altered ratio of synaptic/extrasynaptic α5 GABAAR disrupts spine maturation. Neurons were transfected with α5WT, α5α4GBD, or α5α2GBD, then fixed and stained under permeabilized conditions with anti-GFP and anti-MAP-2 antibodies. High magnification confocal z-series through dendritic regions were obtained, and 3D reconstructions were used to analyze spine length, density, and morphology. 3D reconstructions of confocal images from neurons expressing α5WT, α5α4GBD, or α5α2GBD at DIV 14 (A) and DIV 21 (E). (B) At DIV 14, neurons expressing α5α4GBD exhibited a significantly lower mushroom/filopodia spine ratio compared to either α5WT or α5α2GBD (for B–H, *p < 0.05, **p < 0.01; number of spines examined: α5WT 394, α5α4GBD 311, α5α2GBD 358). (C) Neurons expressing α5α4GBD exhibited significantly fewer mushroom spines per 10 μm compared to either α5WT or α5α2GBD. (F) At DIV 21, neurons expressing α5α4GBD exhibited a significantly lower mushroom/filopodia spine ratio compared to α5WT (number of spines examined: α5WT 474, α5α4GBD 453, α5α2GBD 455). (G) Neurons expressing α5α4GBD exhibited significantly fewer mushroom spines per 10 μm compared to α5WT. There was no significant difference between the number of filopodia spines per 10 μm between constructs at DIV 14 (D) or at DIV 21 (H).
DISCUSSION
α5 GABAARs are emerging as key contributors to learning and memory processes and potential targets for pharmacological modulation in treating cognitive and neurodevelopmental disorders, with clinical trials ongoing in Down syndrome treatment (Rudolph and Mohler, 2014). Extrasynaptically localized α5 GABAARs generate tonic inhibition in the hippocampus. However, the function and contribution of synaptically localized α5 GABAAR to inhibition has largely been overlooked. To resolve this issue, we investigated the localization of the α5 GABAAR sub-type during development, the mechanism of α5 restriction at synapses, and how its regulated distribution at synaptic versus extrasynaptic sites contributes to the function of α5 in neuronal development. We found that a significant portion of GABAergic post-synaptic compartments contain the α5 GABAAR subunit, both in vitro and in vivo. Surface α5 GABAARs are significantly colocalized with gephyrin in hippocampal neurons, with α5 synaptic content being maintained at a constant ratio from 7 to 21 DIV. The association between gephyrin and the α5 subunit occurs independent of other α subunits that bind gephyrin and requires Residues 370–385 of the α5 subunit. By exchanging these residues with the corresponding residues of either the high affinity gephyrin binding domain of the α2 subunit or homologous residues from the extrasynaptic α4 subunit that does not interact with gephyrin, we created chimeras that shifted the localization of the α5 subunit without changing α5 total surface levels, thus altering the synaptic/extrasynaptic ratio of α5. Redistribution of the α5 subunit had significant functional consequences, as these chimeras disrupted proper neuronal dendritic morphology and spine maturation.
A role for α5 GABAAR in dendritic development is supported from in vitro pharmacological and genetic studies. α5-inverse agonist treatment of hippocampal neurons decreased dendritic arborization and reduced glutamate receptor expression (Giusi et al., 2009). Furthermore, neurodevelopmental disorders, such as autism and Fragile X syndrome, have demonstrated alterations in α5 subunit levels (Curia et al., 2009; Mendez et al., 2013). However, the precise function of α5-containing GABAAR signaling, particularly of the prevalent synaptic α5 GABAAR population identified here, in dendritic outgrowth is not known. The α5α4GBD and α5α2GBD chimeras provided the opportunity to assess the contributions of α5 synaptic and extrasynaptic signaling to neuronal development. Replacing the α5 GBD with the α2 GBD increased α5-containing GABAAR synaptic localization similar to levels previously reported for the endogenous α2 subunit (Tretter et al., 2008). We found that shifting the α5 subunit into the synapse reduced the number of dendritic intersections in Sholl analysis compared to wild type at DIV 14 and 21, indicating impaired dendritic growth. Conversely, moving the α5 subunit out of the synapse, by way of the α5α4GBD chimera, increased the number of intersections at DIV 21. Previous studies have shown that both tonic and phasic GABAergic inhibition can control Ca2+ transients in dendrites (Pan and Lipton, 1995; Kanemoto et al., 2011; Hayama et al., 2013), making this a possible mechanism by which altering the synaptic and extrasynaptic α5 levels may influence dendritic outgrowth. While the increased dendritic outgrowth observed at DIV 21 in neurons transfected with the α5α4GBD chimera implies enhanced neuronal maturation, it is also possible that decreasing the ratio of synaptic/extrasynaptic α5 GABAAR signaling reduced dendritic pruning. Few studies have examined the role of GABAAR in dendrite pruning, although one study found that propofol, an anesthetic agent that binds to GABAAR β subunits and potentiates GABAAR, results in GABAAR-dependent neurite retraction in primary cortical cultures (Turina et al., 2008). It is also known that local Ca2+ transients, both through ion channels and intracellular Ca2+ stores, can help to stabilize dendrites, and alterations in these transients may result in dendrite retraction (Wong and Ghosh, 2002; Kanamori et al., 2013). A recent study found that glycogen synthase kinase 3β (GSK3β) can regulate dendrite retraction in hippocampal cultures between DIV 14–18 by regulating γ2 surface expression (Rui et al., 2013). In neurons expressing α5α4GBD, the shift of the α5 subunit from the synapse to the extrasynaptic membrane may alter the overall excitatory/inhibitory balance, which in turn alters local Ca2+ signaling and affects dendritic pruning.
We also found that altering the ratio of synaptic/extrasynaptic α5 GABAAR affected spine maturation. Shifting the α5 subunit out of the synapse resulted in a less mature dendritic spine phenotype. This suggests a specific role for α5 synaptic signaling in spine development, as removing extrasynaptic α5 via α5α2GBD had no significant effect on spine maturity. As with dendritic morphology, the role of synaptic and extrasynaptic GABAergic signaling has not been fully examined in spine development. GABAergic inhibition, primarily through GABAergic synaptic signaling, can focally suppress Ca2+ transients on individual spines (Chiu et al., 2013), and induce spine shrinkage or elimination (Hayama et al., 2013). Furthermore, alterations to other GABAAR subunits, such as the α1 and β3 subunits, disrupted spine maturation in neurons (Heinen et al., 2003; Jacob et al., 2009). Less mature spines show enhanced Ca2+ diffusion between the spine head and dendrite resulting in lower spine calcium accumulation (Zito et al., 2009), which could also play a role in the changes in dendritic morphology seen here.
A large body of evidence indicates that alpha subunit type plays a direct role in controlling GABAAR localization via gephyrin interactions (Tretter et al., 2008, 2011; Saiepour et al., 2010; Mukherjee et al., 2011; Wu et al., 2012). It has been hypothesized that gephyrin trimers provide multiple binding sites (Sola et al., 2004; Fritschy et al., 2008; Tretter et al., 2012; Specht et al., 2013), such that two subunits within a GABAAR may bind the gephyrin scaffold. Our study supports a direct interaction between the α5 subunit and gephyrin, which would alter receptor avidity for gephyrin and diffusion within and out of the synapse. This has implications for both α5/α5 and mixed alpha subunit GABAAR, which exist in vivo (Araujo et al., 1999; del Rio et al., 2001). To assess alpha subunit synaptic localization independent of receptor oligomers, a recent study compared α5 or α2 subunit intracellular domains fused to a single pass transmembrane domain, revealing reduced colocalization of the α5 chimera with gephyrin and a higher diffusion rate (Gerrow and Triller, 2014). This has significant implications for the receptor composition of inhibitory synapses, as a mixed α2/α5 receptor would diffuse out of the synapse faster than a homogenous α2/α2 receptor, but slower than an α5/α5 receptor. This subtle tuning of synaptic GABAergic signaling could contribute to neuronal development by modulating local spine and dendritic Ca2+ influx.
In conclusion, we found that a significant portion of GABAergic postsynaptic compartments contain the α5 GABAAR subunit, both in vitro and in vivo. Shifting the synaptic/extrasynaptic localization of α5 GABAAR through substitution of the α5 gephyrin binding domain disrupts proper neuronal development, indicating that an appropriate balance of phasic and tonic α5 GABAergic inhibition regulates dendritic outgrowth and spine maturation. Further research is needed to elucidate the precise roles of synaptic and extrasynaptic α5 GABAAR at early stages of neuronal circuit development and later in learning and memory.
Supplementary Material
Acknowledgments
The authors thank Nick Graff and Charles Moon for technical help. MLB designed the experiments, performed the research, analyzed the data, and wrote the article. TCJ designed the experiments, analyzed the data, and wrote the article.
Contract grant sponsor: Pharmacology and Chemical Biology Department Startup funds.
Contract grant sponsor: MLB is in part supported by the Whitehall Foundation; contract grant number: 2012-12-36.
Contract grant sponsor: NINDS; contract grant number: T32 NS086749.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Araujo F, Ruano D, Vitorica J. Native gamma-aminobutyric acid type A receptors from rat hippocampus, containing both alpha 1 and alpha 5 subunits, exhibit a single benzodiazepine binding site with alpha 5 pharmacological properties. J Pharmacol Exp Ther. 1999;290:989–997. [PubMed] [Google Scholar]
- Brady ML, Diaz MR, Iuso A, Everett JC, Valenzuela CF, Caldwell KK. Moderate prenatal alcohol exposure reduces plasticity and alters NMDA receptor subunit composition in the dentate gyrus. J Neurosci. 2013;33:1062–1067. doi: 10.1523/JNEUROSCI.1217-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GC, Higley MJ. Compartmentalization of GABAergic inhibition by dendritic spines. Science. 2013;340:759–762. doi: 10.1126/science.1234274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, de Blas AL. alpha5 Subunit-containing GABA(A) receptors form clusters at GABAergic synapses in hippocampal cultures. Neuroreport. 2002;13:2355–2358. doi: 10.1097/00001756-200212030-00037. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem. 1996;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- Curia G, Papouin T, Seguela P, Avoli M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb Cortex. 2009;19:1515–1520. doi: 10.1093/cercor/bhn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio JC, Araujo F, Ramos B, Ruano D, Vitorica J. Prevalence between different alpha subunits performing the benzodiazepine binding sites in native heterologous GABA(A) receptors containing the alpha2 subunit. J Neurochem. 2001;79:183–191. doi: 10.1046/j.1471-4159.2001.00551.x. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Rooney RJ, Patel DH, Thuras PD. mRNA and protein levels for GABAAalpha4, alpha5, beta1 and GABABR1 receptors are altered in brains from subjects with autism. J Autism Dev Disord. 2010;40:743–750. doi: 10.1007/s10803-009-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CE, Mendez P. Shaping inhibition: activity dependent structural plasticity of GABAergic synapses. Front Cell Neurosci. 2014;8:327. doi: 10.3389/fncel.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 2008;31:257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Gerrow K, Triller A. GABAA receptor subunit composition and competition at synapses are tuned by GABAB receptor activity. Mol Cell Neurosci. 2014;60:97–107. doi: 10.1016/j.mcn.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Giusi G, Facciolo RM, Rende M, Alo R, Di Vito A, Salerno S, Morelli S, De Bartolo L, Drioli E, Canonaco M. Distinct alpha subunits of the GABAA receptor are responsible for early hippocampal silent neuron-related activities. Hippocampus. 2009;19:1103–1114. doi: 10.1002/hipo.20584. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Hayama T, Noguchi J, Watanabe S, Takahashi N, Hayashi-Takagi A, Ellis-Davies GC, Matsuzaki M, Kasai H. GABA promotes the competitive selection of dendritic spines by controlling local Ca21 signaling. Nat Neurosci. 2013;16:1409–1416. doi: 10.1038/nn.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen K, Baker RE, Spijker S, Rosahl T, van Pelt J, Brussaard AB. Impaired dendritic spine maturation in GABAA receptor alpha1 subunit knock out mice. Neuroscience. 2003;122:699–705. doi: 10.1016/s0306-4522(03)00477-9. [DOI] [PubMed] [Google Scholar]
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci. 2005;25:10469–10478. doi: 10.1523/JNEUROSCI.2267-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Wan Q, Vithlani M, Saliba RS, Succol F, Pangalos MN, Moss SJ. GABA(A) receptor membrane trafficking regulates spine maturity. Proc Natl Acad Sci USA. 2009;106:12500–12505. doi: 10.1073/pnas.0903943106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori T, Kanai MI, Dairyo Y, Yasunaga K, Morikawa RK, Emoto K. Compartmentalized calcium transients trigger dendrite pruning in Drosophila sensory neurons. Science. 2013;340:1475–1478. doi: 10.1126/science.1234879. [DOI] [PubMed] [Google Scholar]
- Kanemoto Y, Matsuzaki M, Morita S, Hayama T, Noguchi J, Senda N, Momotake A, Arai T, Kasai H. Spatial distributions of GABA receptors and local inhibition of Ca21 transients studied with GABA uncaging in the dendrites of CA1 pyramidal neurons. PLoS One. 2011;6:e22652. doi: 10.1371/journal.pone.0022652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralic JE, Sidler C, Parpan F, Homanics GE, Morrow AL, Fritschy JM. Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of gamma-aminobutyric acid type A receptor alpha1 subunit knockout mice. J Comp Neurol. 2006;495:408–421. doi: 10.1002/cne.20866. [DOI] [PubMed] [Google Scholar]
- Li C, Wen A, Shen B, Lu J, Huang Y, Chang Y. Fast-Cloning: a highly simplified, purification-free, sequence-and ligation-independent PCR cloning method. BMC Biotechnol. 2011;11:92. doi: 10.1186/1472-6750-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebrich S, Bahring R, Katsuno T, Tsukita S, Kneussel M. Activated radixin is essential for GABAA receptor alpha5 subunit anchoring at the actin cytoskeleton. EMBO J. 2006;25:987–999. doi: 10.1038/sj.emboj.7600995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni I, Omrani A, Cherubini E. In the developing rat hippocampus a tonic GABAA-mediated conductance selectively enhances the glutamatergic drive of principal cells. J Physiol. 2007;581:515–528. doi: 10.1113/jphysiol.2006.125609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MA, Horder J, Myers J, Coghlan S, Stokes P, Erritzoe D, Howes O, Lingford-Hughes A, Murphy D, Nutt D. The brain GABA-benzodiazepine receptor alpha-5 subtype in autism spectrum disorder: a pilot [(11)C]Ro15-4513 positron emission tomography study. Neuropharmacology. 2013;68:195–201. doi: 10.1016/j.neuropharm.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J, Kretschmannova K, Gouzer G, Maric HM, Ramsden S, Tretter V, Harvey K, Davies PA, Triller A, Schindelin H, Moss SJ. The residence time of GABA(A)Rs at inhibitory synapses is determined by direct binding of the receptor alpha1 subunit to gephyrin. J Neurosci. 2011;31:14677–14687. doi: 10.1523/JNEUROSCI.2001-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZH, Lipton SA. Multiple GABA receptor sub-types mediate inhibition of calcium influx at rat retinal bipolar cell terminals. J Neurosci. 1995;15:2668–2679. doi: 10.1523/JNEUROSCI.15-04-02668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. GABAA receptor subtypes: Therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu Rev Pharmacol Toxicol. 2014;54:483–507. doi: 10.1146/annurev-pharmtox-011613-135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui Y, Myers KR, Yu K, Wise A, De Blas AL, Hartzell HC, Zheng JQ. Activity-dependent regulation of dendritic growth and maintenance by glycogen synthase kinase 3beta. Nat Commun. 2013;4:2628. doi: 10.1038/ncomms3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiepour L, Fuchs C, Patrizi A, Sassoe-Pognetto M, Harvey RJ, Harvey K. Complex role of collybistin and gephyrin in GABAA receptor clustering. J Biol Chem. 2010;285:29623–29631. doi: 10.1074/jbc.M110.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL. Synaptic and nonsynaptic localization of GABAA receptors containing the alpha5 subunit in the rat brain. J Comp Neurol. 2006;499:458–470. doi: 10.1002/cne.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sola M, Bavro VN, Timmins J, Franz T, Ricard-Blum S, Schoehn G, Ruigrok RW, Paarmann I, Saiyed T, O'Sullivan GA, Schmitt B, Betz H, Weissenhorn W. Structural basis of dynamic glycine receptor clustering by gephyrin. EMBO J. 2004;23:2510–2519. doi: 10.1038/sj.emboj.7600256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa MM, Steen KW, Hagen L, Slupphaug G. Antibody cross-linking and target elution protocols used for immunoprecipitation significantly modulate signal-to noise ratio in downstream 2D-PAGE analysis. Proteome Sci. 2011;9:45. doi: 10.1186/1477-5956-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht CG, Izeddin I, Rodriguez PC, El Beheiry M, Rostaing P, Darzacq X, Dahan M, Triller A. Quantitative nanoscopy of inhibitory synapses: counting gephyrin molecules and receptor binding sites. Neuron. 2013;79:308–321. doi: 10.1016/j.neuron.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Sun C, Sieghart W, Kapur J. Distribution of alpha1, alpha4, gamma2, and delta subunits of GABAA receptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. J Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Kerschner B, Milenkovic I, Ramsden SL, Ramerstorfer J, Saiepour L, Maric HM, Moss SJ, Schindelin H, Harvey RJ, Sieghart W, Harvey K. Molecular basis of the gamma-aminobutyric acid A receptor alpha3 subunit interaction with the clustering protein gephyrin. J Biol Chem. 2011;286:37702–37711. doi: 10.1074/jbc.M111.291336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Mukherjee J, Maric HM, Schindelin H, Sieghart W, Moss SJ. Gephyrin, the enigmatic organizer at GABAergic synapses. Front Cell Neurosci. 2012;6:23. doi: 10.3389/fncel.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turina D, Loitto VM, Bjornstrom K, Sundqvist T, Eintrei C. Propofol causes neurite retraction in neurones. Br J Anaesth. 2008;101:374–379. doi: 10.1093/bja/aen185. [DOI] [PubMed] [Google Scholar]
- Tyagarajan SK, Fritschy JM. Gephyrin: a master regulator of neuronal function? Nat Rev Neurosci. 2014;15:141–156. doi: 10.1038/nrn3670. [DOI] [PubMed] [Google Scholar]
- Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Wu X, Wu Z, Ning G, Guo Y, Ali R, Macdonald RL, De Blas AL, Luscher B, Chen G. gamma-Aminobutyric acid type A (GABAA) receptor alpha subunits play a direct role in synaptic versus extrasynaptic targeting. J Biol Chem. 2012;287:27417–27430. doi: 10.1074/jbc.M112.360461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito K, Scheuss V, Knott G, Hill T, Svoboda K. Rapid functional maturation of nascent dendritic spines. Neuron. 2009;61:247–258. doi: 10.1016/j.neuron.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.