Abstract
Ascidians, belonging to the subphylum Urochordata, the earliest branch from the lineage to the vertebrates, exhibit a prototypical morphogenesis of chordates in the larval development, although they subsequently metamorphose into adults with a unique body structure. Recent draft genome analysis of the ascidian Ciona intestinalis has identified 9 Hox genes, which, however, have been located on five scaffolds. Similarly, expression patterns of Ciona Hox genes are largely unknown, although some data have been available for a few Hox member genes. Thus, the cluster structure and colinearity of Hox genes are still an enigma in C. intestinalis. To address these issues, we used fluorescence in situ hybridization and whole-mount in situ hybridization techniques and examined the genomic organization and spatiotemporal expression of all Hox as well as extended Hox member genes (Evx and Mox) of C. intestinalis. We found that seven of nine Ciona Hox genes are located on a single chromosome with some ordering exceptions, and the other genes, including Evx and Mox, are located on three other chromosomes. Some Ciona Hox genes, if not all, exhibited spatially coordinated expression within the larval central nervous system and the gut of the juvenile. In light of these observations, we suggest that the cluster organization and colinearity of the Hox genes are under dispersing and disintegrating conditions in C. intestinalis.
Hox genes have been noted to play a central role in the anterior–posterior patterning throughout animal phylogeny (1, 2). They are characterized by the clustering organization on a chromosome and the colinear expression during development. These observations have led to the hypothesis that the physical organization of the Hox genes on the chromosome is closely related to their role in animal development. Variations in Hox gene numbers among species reflect an evolutionary history characterized by gain or loss of Hox gene members or duplications of a Hox gene cluster (3). These events could have brought about changes in expression or functions of Hox genes. Therefore, despite their remarkable conservation, Hox genes have been regarded as responsible for the dramatic developmental differences in body plans within and between phyla.
In view of this situation, it is important to understand the cluster organization and expression pattern of Hox genes in different species, especially in animals occupying unique evolutionary positions. Ascidians belong to the subphylum Urochordata, the earliest branch in the monophyletic phylum Chordata, and their larvae share the prototypical morphogenesis and body plan of chordates as characterized by the presence of a dorsal hollow neural tube, a notochord, paraxial mesoderm, and so on. In the ascidian Ciona intestinalis, previous studies have identified 9 Hox genes of distinct subgroups, suggesting the presence of a single Hox gene cluster (4, 5). However, these Hox genes have been located by the whole draft genome analysis (6) on five scaffolds containing Ci-Hox1; Ci-Hox2, -3, and -4 (Ci-Hox2–4); Ci-Hox5 and -6 (Ci-Hox5–6); Ci-Hox10; and Ci-Hox12 and -13 (Ci-Hox12–13), respectively. Thus, the organization of the Ciona Hox gene cluster has been unclear. Likewise, information about developmental expression of member genes has been incomplete. Expression patterns have been described previously for four ascidian Hox genes: Ci-Hox1, Ci-Hox3, and Ci-Hox5 of C. intestinalis (7–9) and HrHox-1 of Halocynthia roretzi (10). From these studies, it has been expected that ascidian Hox1, -3, and -5 may retain coordinated expression in the neural tube (11). However, expression patterns of the other genes have been largely unknown. Moreover, these studies were focused on embryogenesis and not extended to postlarval stages.
In this work, we examined the organization of the Hox gene cluster and the expression pattern, spanning from the egg to juvenile of all of the known Hox genes in the ascidian C. intestinalis by using fluorescence in situ hybridization (FISH) and whole-mount in situ hybridization (WISH). We also paid attention to Evx and Mox genes. These genes are classified as the extended Hox gene group because of the similarity of their nucleotide sequences to those of Hox genes and close location to the Hox gene cluster (12). Evx genes have been known to be involved in gastrulation and neurogenesis (13), and Mox genes have a function in myogenesis in a variety of phyla (14). In the previous C. intestinalis genome analysis, two Evx genes, Ci-EvxA and Ci-EvxB, and a single Mox gene, Ci-Mox, were identified (6). However, no information has been available about their linkage to Hox genes or developmental expression.
We have shown that seven of nine Hox genes as well as one Evx gene are on a single chromosome. However, the organization of the member genes was quite unusual in the spacing and order of the genes. Developmental expression patterns of these genes also exhibit some unusual features. However, some of the Hox genes, if not all, exhibited spatially coordinated expression patterns before and after metamorphosis. In light of the present findings, we propose that the cluster structure of Hox genes is under dispersing conditions, and, accordingly, the colinearity is under disintegrating conditions in C. intestinalis.
Materials and Methods
Ascidians. C. intestinalis were cultivated at the Maizuru Fisheries Research Station of Kyoto University or at the Misaki Marine Biological Station of the University of Tokyo. Eggs and sperm were obtained surgically from the gonoducts. After insemination, embryos were raised in filtered seawater at 15–18°C. Larvae were allowed to undergo metamorphosis naturally in culture dishes. Juveniles were cultured for ≈2 weeks and fed with the diatom Chaetoceros gracillis. For FISH, 64-cell embryos were treated with 0.05% colchicine in seawater for 20 min, and then transferred into methanol/glacial acetic acid (3:1) fixative. For WISH, eggs, embryos, larvae, and the juveniles were collected and fixed (15, 16).
FISH. Two-color FISH was carried out as described in ref. 17 with the following modifications. For preparation of metaphase spreads, increased volume (1 ml) of 60% acetic acid was added to the microtube containing 50–100 embryos. Three minutes later, the embryos were agitated by gentle pipetting ≈50 times and allowed to stand at room temperature for 5 min. The burst embryonic cells were collected by centrifugation at 800 × g for 2 min and resuspended in 75 μl of 60% acetic acid. These modifications reduced the contamination of cytoplasmic debris and contributed to lower background and efficient probe accessibility. Hybridization was carried out for 16 h, which we found to be sufficient. Images were taken with an Olympus BX60 microscope equipped with an Olympus DP70 camera and processed by using photoshop (Version 6.0, Adobe Systems, San Jose, CA).
FISH Probes. Probes for FISH were derived from clones out of the C. intestinalis genomic bacterial artificial chromosome (BAC) library (18). To select the clones, we referred extensively to data for the C. intestinalis genome sequence (http://genome.jgipsf.org/ciona4) and the BAC end sequence (http://ghost.zool.kyoto-u.ac.jp/indexr1.html). The BAC clones GECi36A15, GECi36A 24, GECi48L19, GECi12G21, GECi45K08, GECi07I24, GECi46E03, and GECi27M09 were used for preparation of probes for Ci-Hox1, Ci-Hox2–4, Ci-Hox5–6, Ci-Hox10, Ci-Hox12–13, Ci-EvxA, Ci-EvxB, and Ci-Mox, respectively. All of the BAC clones except for GECi48L19 cover the Hox gene(s). As regards the clone GECi48L19 for the probe of Ci-Hox5-6, we selected the BAC clone that covers the scaffold (Scaffold 659) adjacent to that including Ci-Hox5–6 (Scaffold 765) because BAC clones that cover Ci-Hox5 and Ci-Hox6 gave numerous signals, likely because of the presence of repetitive sequences surrounding the genes (data not shown). The localization of each BAC clone in a given scaffold was confirmed by nucleotide sequence determination of the BAC clone ends. BAC clone DNA was isolated and labeled with biotin or digoxigenin by using a nick translation kit (Roche).
WISH. WISH was carried out by using digoxigenin-labeled RNA probes essentially as described in ref. 19. Briefly, fixed embryos or juveniles were treated with 2 μg/ml proteinase K in PBS containing 0.1% Tween 20 (PBST) at 37°C for 30–40 min or 10 μg/ml proteinase K in PBST at room temperature for 30 min, respectively. The tunic of the juveniles was removed by repeated flash vortexing ≈50 times. The specimens were postfixed with 4% paraformaldehyde in PBST at room temperature for 1 h. After prehybridization at 42°C for 1 h, the specimens were allowed to hybridize with digoxigenin-labeled probes at the concentration of 0.5–1.0 μg/ml at 42°C for at least 16 h. After the coloring procedure, the specimens were cleared, when necessary, by stepwise transfer into 30%, 60%, and 80% glycerol in PBST. The stages examined for the gene expression were the egg, 32-cell, 64-cell, 110-cell, gastrula, neurula, early tailbud, mid-tailbud, late tailbud, larva, and juvenile.
WISH Probes. RNA probes for Ci-Hox3, -4, -10, and -13 were synthesized by using EST clones as templates, which were from Ciona intestinalis Gene Collection release 1 (http://ghost.zool.kyoto-u.ac.jp/indexr1.html). DNA fragments for probe synthesis of Ci-Hox5 and -12 and Ci-Mox were obtained by PCR using C. intestinalis genomic DNA as a template, and those for Ci-Hox1, -2, and -6, and Ci-EvxA and -B probe synthesis were obtained through RT-PCR and 5′ and/or 3′ RACE using total RNA prepared from tailbud-stage embryos. The DNA fragments were cloned into the pBluescript KS vector (Stratagene). Details are available upon request. The RNA probes were between ≈500 and 1,500 nucleotides long.
Results
Chromosomal Mapping of Extended Hox Genes by FISH. To study the cluster organization of Ciona Hox, Evx, and Mox genes, we performed two-color FISH to determine the relative locations on chromosomes. C. intestinalis has the diploid karyotype of 28 chromosomes, although these chromosomes have not been distinguished yet (17). Nevertheless, we consistently observed a pair of chromosomes with a single signal per metaphase plate for each probe.
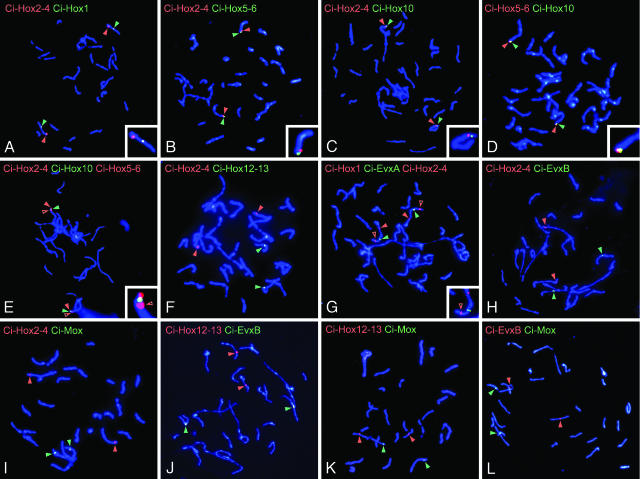
As shown in Fig. 1A, signals for Ci-Hox1 and for Ci-Hox2-4 were located on the same chromosome, but they are at distant positions. The signal of Ci-Hox1 was located in the center of the chromosome, whereas that of Ci-Hox2–4 was located close to the terminal. A signal for Ci-Hox5–6 or Ci-Hox10 was detected close to, but on the terminal side of, the signal for Ci-Hox2–4, as shown in Fig. 1 B and C. When we examined the relationships among Ci-Hox2–4, Ci-Hox5–6, and Ci-Hox10, we found that the signal for Ci-Hox5–6 was the most terminally located (Fig. 1 D and E). Thus, Ci-Hox1, Ci-Hox2–4, Ci-Hox10, and Ci-Hox5–6 are likely to be aligned on the same chromosome in this order, although we could not determine the alignment within each scaffold because of the resolution of FISH analysis. Ci-Hox12 and -13, however, were located on another chromosome (Fig. 1F). Next, we examined the locations of Evx and Mox genes in relation to those of Hox genes. As shown in Fig. 1G, Ci-EvxA was mapped between Ci-Hox1 and Ci-Hox2–4, whereas Ci-EvxB and Ci-Mox were located on different chromosomes, on which signals of neither Ci-Hox2–4 nor Ci-Hox12–13 were detected (Fig. 1 H–L).
Fig. 1.
The mapping of C. intestinalis extended Hox genes onto metaphase chromosomes by using FISH. Metaphase chromosome spreads prepared from 64-cell stage embryos were hybridized with two or three probes labeled with digoxigenin (red) or biotin (green) for the genes indicated at the top in A–L. Red and green arrowheads indicate the signals for the gene of the same color code. In E and G, red arrowheads with a black dot indicate the signals for Ci-Hox2–4.In A–E and G, enlargement of the chromosome with signals is indicated in Insets. (Bars, 5 μm.)
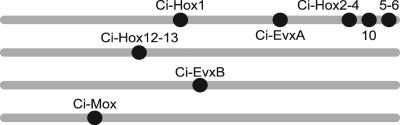
These mapping results are summarized in Fig. 2, which suggests an occurrence of multiple rearrangement and translocation of the member genes. We conclude that the genomic organization of the extended Hox genes is under dispersing conditions in C. intestinalis.
Fig. 2.
A summary of the chromosomal mapping of the extended Hox genes by using FISH. The result of the chromosomal mapping analysis is schematically presented. Gray bars and black spots indicate chromosomes and positions of the mapped genes. Because the chromosomes of C. intestinalis have not been distinguished by morphology, the chromosomes are drawn the same size.
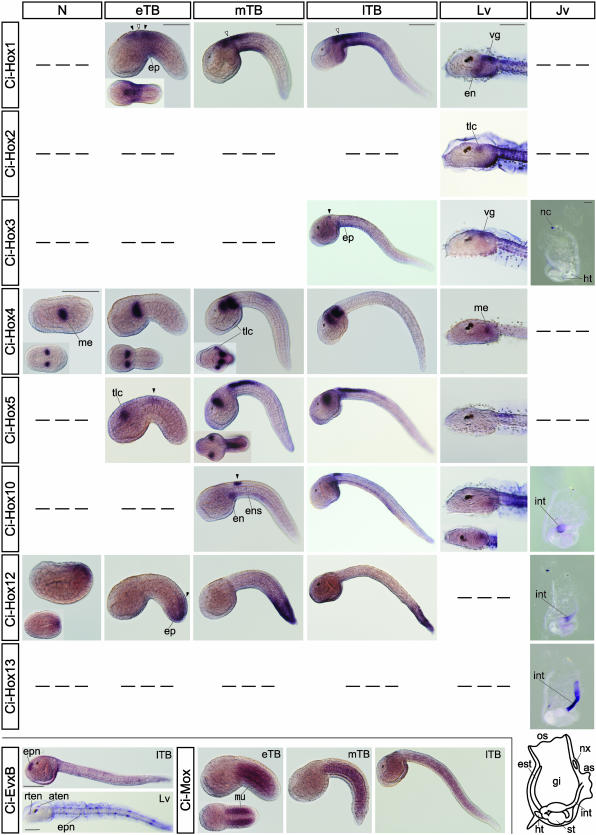
Expression Patterns of the Hox Genes in Larval and Juvenile Development. We investigated expression patterns of the Hox genes as well as Evx and Mox by using WISH to see whether the colinear expression of Hox genes might be retained in the development of C. intestinalis. The expression patterns examined with the specimens from the unfertilized egg to the juvenile are described below (Fig. 3).
Fig. 3.
Expression patterns of C. intestinalis extended Hox genes examined by WISH. WISH was carried out with nine Hox, two Evx, and one Mox genes by using specimens from fertilized eggs to juveniles, but only the genes that exhibited positive signals are shown. Thus, Ci-Hox6 and Ci-EvxA have been omitted. Also excluded are the stages, from the egg to gastrula, in which no signals for expression of any of the 12 genes were detected. Gene names are indicated on the left and stages at the top. For Evx and Mox genes, stages are indicated at the top. For all specimens, lateral views are shown, except for those of Insets, and the anterior is to the left. For Insets, the dorsal view is shown except for the Ci-Hox10 specimen at the larva stage, for which the ventral view is shown. For juveniles, anterior is to the top and dorsal to the right, except in the Ci-Hox3 specimen, where the dorsal is to the left. Filled arrowheads indicate the expression in the CNS. Open arrowheads indicate the gap between two expression domains of Ci-Hox1 in the CNS. Also shown are names of the tissues in which the expression was detected earlier in development. A dash signifies that signals were not detected at that stage. A schematic drawing of the juvenile is shown at the bottom right. as, atrial siphon; aten, apical trunk epidermal neuron; ep, epidermis; en, endoderm; ens, endodermal strand; epn, epidermal neuron; est, endostyle; gi, gill; ht, heart; int, intestine; me, mesenchyme; mu, muscle; nx, neural complex; os, oral siphon; rten, rostral trunk epidermal neuron; st, stomach; tlc, trunk lateral cells; vg, visceral ganglion. Stages shown are N, neurula; eTB, early tailbud; mTB, mid-tailbud; lTB, late tailbud; Lv, larva; Jv, juvenile. (Bars, 100 μm, which is, if not specifically indicated, applicable to all specimens below.)
Ci-Hox1. Expression of Ci-Hox1 was first detected at the early tailbud stage in development in the epidermis and the central nervous system (CNS) around the junction of the trunk (head of the ascidian tadpole) and the tail without clear anterior and posterior expression boundaries. The expression domain in the CNS consisted of two subdomains aligned along the anterior–posterior axis as described in ref. 9. The anterior expression eventually localized to the visceral ganglion in the larva. The posterior expression extended to the posterior in the mid- to late tailbud stage and gradually disappeared by the larva stage. This expression pattern is very similar to that of HrHox-1 of Halocynthia roretzi, another ascidian species (10). In C. intestinalis larvae, weak expression of Ci-Hox1 also was detected in the endodermal cells. In juveniles, no signal was detected.
Ci-Hox2. Expression of Ci-Hox2 was detected only at the larva stage in the trunk lateral cells.
Ci-Hox3. Expression of Ci-Hox3 was evident from the late tailbud stage onward in a small region just behind the sensory vesicle and also in the anterior tail epidermis. At the larva stage, Ci-Hox3 expression was observed in the visceral ganglion, as described in ref. 7. In the juvenile, although the visceral ganglion degenerates during metamorphosis, expression of Ci-Hox3 was maintained in the neural complex, the adult CNS. Weak expression of Ci-Hox3 also was detected in a part of the heart in juveniles.
Ci-Hox4. Expression of Ci-Hox4 was detected from the neurula stage. Expression was observed in the lateral of the embryo, which appears to correspond to the mesenchyme. At the mid-tailbud stage, expression in the trunk lateral cells was also evident anterior to the mesenchyme, but the expression disappeared by the larva stage. In juveniles, we could not detect any signal of Ci-Hox4, although the EST database (http://ghost.zool.kyoto-u.ac.jp/indexr1.html) suggests that this gene is transcribed in the blood cells.
Ci-Hox5. Expression of Ci-Hox5 was first detectable at the early tailbud stage in the two distinct sites, as described in ref. 8. One was the trunk lateral cell, in which the expression was observed during tailbud stages. The other was the anterior nerve cord, in which Ci-Hox5 was expressed with sharp anterior and decreasing-to-the-posterior expression boundaries. The latter expression continued until the larva stage, but no signals were detected in juveniles.
Ci-Hox6. Expression of Ci-Hox6 was not detectable by WISH in the present study, although weak expression was observed upon RT-PCR (data not shown).
Ci-Hox10. Expression of Ci-Hox10 was first detectable at the mid-tailbud stage in two regions. One was a small region of the anterior nerve cord, and the other was a small area of the posterior ventral endoderm and the adjacent tissue, the endodermal strand. As the tail elongated, the latter expression became intense. In larvae, expression was observed only in the left ventral endoderm. In juveniles, expression of Ci-Hox10 was detected in the anterior bulged part of the intestine. Upon transverse sectioning of the specimen, the staining was observed solely in the endodermal epithelial layer (data not shown).
Ci-Hox12. Expression of Ci-Hox12 was observed from the neurula to larva stages in the dorsal ectodermal cells at the posterior end of the embryo. During tailbud stages, the expression extended to the ventral side of the embryo and became evident in the posterior-most nerve cord and epidermis. Unlike other Ciona Hox genes, Ci-Hox12 was expressed with the anteriorly decreasing expression boundary. In juveniles, Ci-Hox12 was expressed in a small region around the junction of the anterior bulged part and the posterior slender part of the intestine without clear expression boundaries. Transcripts were restricted to the endodermal epithelial layer (data not shown).
Ci-Hox13. Expression of Ci-Hox13 was detectable only in the juvenile. The expression was observed over the posterior slender part of the intestine, which had a clear anterior expression boundary and posteriorly decreasing gradient. This signal also was restricted to the endodermal epithelial layer (data not shown). According to the EST database, Ci-Hox13 should be expressed maternally. In our study, however, no signals were detected by WISH in the egg or in larval development.
Other extended Hox member genes. Expression of Ci-EvxB was detectable from the late tailbud to the larva stage in a relatively small number of cells on the surface of embryos. Judging from the locations, these cells correspond to the epidermal neurons (20). Expression of Ci-EvxA was not detected by WISH, although weak expression could be detected by RT-PCR (data not shown). Expression of Ci-Mox started in the embryonic muscle around the onset of tail elongation, continued during the tailbud stage, and thereafter disappeared. Expression patterns of Ci-EvxB and Ci-Mox are very reminiscent of those reported in other organisms (21, 22).
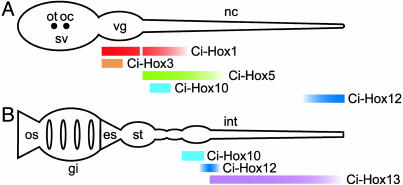
Summarizing the analysis of these expression patterns, we point out the presence of two sets of spatially coordinated expressions with several members of Hox genes (shown schematically in Fig. 4), in the CNS during larval development and in the gut endoderm of the juvenile after metamorphosis. Thus, despite the dispersed cluster organization, the spatially coordinated expression still seems to be retained by the Hox genes to a certain extent in the development of C. intestinalis.
Fig. 4.
Schematic representation of coordinated expression of Ciona Hox genes in the developing larval CNS and the juvenile gut. Expression domains of the Hox genes in the CNS in the tailbud stages (A) and in the gut of the juvenile (B) are shown schematically. The gut is drawn straightened to clearly indicate the anterior–posterior axis. gi, gill; es, esophagus; sv, sensory vesicle; oc, ocellus; os, oral siphon; ot, otolith; vg, visceral ganglion; nc, nerve cord; st, stomach; int, intestine.
Discussion
The present FISH analysis has shown unusual features of the Hox gene cluster organization of C. intestinalis. First, the organization of the C. intestinalis Hox gene cluster has dispersed and disintegrated to a significant extent. The member genes are separated onto two chromosomes. Although Ci-Hox1 to -10 are located on the same chromosome, Ci-Hox1 is situated far from the more posterior group genes, and Ci-Hox10 is between Ci-Hox2–4 and Ci-Hox5–6. In Drosophila melanogaster, the Hox gene cluster is split into two complexes, Antennapedia and Bithorax. In Caenorhabditis elegans, the cluster is also split into three parts with the reversed order of the two 3′-most genes (23, 24). Compared with these instances, the situation observed in C. intestinalis is much more complicated, likely including the rearrangement of the member gene positions at least three times, assuming that the ancestral organism had a typical Hox gene cluster like that of amphioxus. Second, unlike other chordate Hox gene clusters examined so far, the distances between member genes are very long, inserted with many non-Hox genes in C. intestinalis. Seven Hox genes, Ci-Hox1 to -10, are spanning about half the length of a chromosome, which could be calculated as 5 megabases, extraordinarily longer than an ordinary vertebrate Hox gene cluster (100–120 kb) (25). Because sea urchin and amphioxus have a relatively compact Hox gene cluster with almost all or all members of 13 paralogous subgroup genes arranged in linear order, the dispersion and breakage of a Hox gene cluster, including the loss of 4 member genes, might have occurred in the Urochordata lineage. The observation that central Hox genes seem to be lost in the larvacean genome (26) supports this notion. Third, linkage of the other extended Hox member genes to the Hox genes is lost in C. intestinalis. It is known that Evx genes are located adjacent to the 5′ end of the Hox gene cluster in vertebrates and cnidaria. Likewise, Mox genes are mapped near the opposite end of the HoxA or HoxB cluster in the human genome (27). In C. intestinalis, however, Ci-EvxA is in between Ci-Hox1 and Ci-Hox2–4, and neither Ci-EvxB nor Ci-Mox coexists with any of the Hox genes on the chromosomes. Furthermore, many non-Hox genes situated between Hox member genes in the C. intestinalis genome have no apparent “synteny” relationship. In other words, none of their vertebrate orthologues are located in the vicinity of Hox genes in vertebrate genomes (M. Nonaka, personal communication). From these observations, it is suggested that extensive shuffling of the genome has occurred during the evolution of ascidians.
Nevertheless, the present WISH analysis has demonstrated the presence of the two temporally separated sets of coordinated expressions of the Hox genes in the development of C. intestinalis. Ci-Hox1 and -3 are expressed in the visceral ganglion, anterior to the nerve cord in the CNS, and more posteriorly Ci-Hox5, -10, and -12 are expressed in the distinct parts within the nerve cord. Thus, Ci-Hox1, -3, -5, -10, and -12 exhibit spatially coordinated expression in the CNS. The temporal coordination, however, seems to be lost, because Ci-Hox1, -5, and -12 are transcribed in the neural ectoderm earlier than Ci-Hox3 and -10. Ferrier and Holland (28) have suggested that the temporal colinearity as well as constraint on cluster organization might have been removed from animals that undergo rapid embryogenesis with a low cell number and predominantly mosaic development. Our data offer further support to their suggestion. Furthermore, some of the Ciona Hox genes do not seem to be expressed in the CNS. Expression of Ci-Hox2, -4, and -13 was not detected in the ectoderm, and the expression of Ci-Hox6, if any, seems to be at quite a low level throughout the larval development or in the juvenile. It is noted here that in the middle of the tail, none of the Hox gene expressions were observed (Fig. 4). This region, without any apparent morphological regional distinction, might have expressed central/posterior member genes. In any case, the central/posterior Hox member genes appear to have lost their roles in the formation of the embryonic body plan, and probably the adult-type body plan too, in the development of ascidians.
C. intestinalis Hox genes also are expressed coordinately in the gut endoderm during postlarval development. This finding is reminiscent of the situation in the developing hindgut of chicken embryo, in which Hoxa-10, -11, and -13 are expressed in the endoderm in a coordinated manner (29). In C. intestinalis, endodermal expression of Ci-Hox10 starts at the mid-tailbud stage and continues to the larva stage, although the expression becomes restricted to the ventral posterior left endoderm. Because the posterior ventral endoderm and the endodermal strand have been reported to contribute to the adult intestine (30), the observations suggest that regionalization of the gut endoderm may be initiated at the mid-tailbud stage, continuing to the postlarval stage.
In the mesoderm, C. intestinalis Hox genes do not achieve the colinear expression. Alternatively, there are points to be noted with mesodermal expression of C. intestinalis Hox and extended member genes. Ci-Hox3 is expressed in a part of the heart of the juvenile. This finding is reminiscent of the observation that Hoxd-3 is expressed in the heart-forming region in the avian embryo (31).
In conclusion, we suggest that the cluster structure of the Hox genes is under a dispersed condition and the colinearity is disintegrating in C. intestinalis. Thus, C. intestinalis Hox genes retain residual colinearity. However, this situation does not necessarily mean the decline of whole roles of Hox genes in axial patterning. In C. intestinalis, some Hox genes, if not all, seem to be deployed in the patterning along the anterior–posterior axis of the developing larval CNS, and the posterior Hox genes seem to contribute to axial structure of the gut endoderm in the juvenile. To understand in depth the current state of Hox genes in C. intestinalis, it is essential to clarify functions of the Hox genes in ascidian development.
Acknowledgments
We thank Dr. M. Nonaka (University of Tokyo) for communicating unpublished results and Ms. K. Hirayama (Kyoto University) and Dr. Y. Kimura (University of Tokyo) for their kind help in collecting Ciona embryos and adult animals. T.I was supported by the Sasakawa Scientific Grant from The Japan Science Society. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) (to H.S.).
Author contributions: T.I. and H.S. designed research; T.I., N.Y., and H.S. performed research; N.S. contributed new reagents/analytical tools; T.I. and N.S. analyzed data; and T.I. and H.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: BAC, bacterial artificial chromosome; FISH, fluorescence in situ hybridization; WISH, whole-mount in situ hybridization.
References
- 1.Carroll, S. B. (1995) Nature 376, 479–485. [DOI] [PubMed] [Google Scholar]
- 2.de Rosa, R., Grenier, J. K., Andreeva, T., Cook, C. E., Adoutte, A., Akam, M., Carroll, S. B. & Balavoine, G. (1999) Nature 399, 772–776. [DOI] [PubMed] [Google Scholar]
- 3.Balavoine, G., de Rosa, R. & Adoutte, A. (2002) Mol. Phylogenet. Evol. 24, 366–373. [DOI] [PubMed] [Google Scholar]
- 4.Dehal, P., Satou, Y., Campbell, R. K., Chapman, J., Degnan, B., De Tomaso, A., Davidson, B., Di Gregorio, A., Gelpke, M., Goodstein, D. M., et al. (2002) Science 298, 2157–2167. [DOI] [PubMed] [Google Scholar]
- 5.Spagnuolo, A., Ristoratore, F., Di Gregorio, A., Aniello, F., Branno, M. & Di Lauro, R. (2003) Gene 309, 71–79. [DOI] [PubMed] [Google Scholar]
- 6.Wada, S., Tokuoka, M., Shoguchi, E., Kobayashi, K., Di Gregorio, A., Spagnuolo, A., Branno, M., Kohara, Y., Rokhsar, D., Levine, M., et al. (2003) Dev. Genes Evol. 213, 222–234. [DOI] [PubMed] [Google Scholar]
- 7.Locascio, A., Aniello, F., Amoroso, A., Manzanares, M., Krumlauf, R. & Branno, M. (1999) Development (Cambridge, U.K.) 126, 4737–4748. [DOI] [PubMed] [Google Scholar]
- 8.Gionti, M., Ristoratore, F., Di Gregorio, A., Aniello, F., Branno, M. & Di Lauro, R. (1998) Dev. Genes Evol. 207, 515–523. [DOI] [PubMed] [Google Scholar]
- 9.Nagatomo, K. & Fujiwara, S. (2003) Gene Expression Patterns 3, 273–277. [DOI] [PubMed] [Google Scholar]
- 10.Katsuyama, Y., Wada, S., Yasugi, S. & Saiga, H. (1995) Development (Cambridge, U.K.) 121, 3197–3205. [DOI] [PubMed] [Google Scholar]
- 11.Lemaire, P., Bertrand, V. & Hudson, C. (2002) Dev. Biol. 252, 151–169. [DOI] [PubMed] [Google Scholar]
- 12.Pollard, S. L. & Holland, P. W. (2000) Curr. Biol. 10, 1059–1062. [DOI] [PubMed] [Google Scholar]
- 13.Ferrier, D. E., Minguillon, C., Cebrian, C. & Garcia-Fernandez, J. (2001) Dev. Biol. 237, 270–281. [DOI] [PubMed] [Google Scholar]
- 14.Minguillon, C. & Garcia-Fernandez, J. (2002) Dev. Biol. 246, 455–465. [DOI] [PubMed] [Google Scholar]
- 15.Satou, Y., Takatori, N., Yamada, L., Mochizuki, Y., Hamaguchi, M., Ishikawa, H., Chiba, S., Imai, K., Kano, S., Murakami, S. D., et al. (2001) Development (Cambridge, U.K.) 128, 2893–2904. [DOI] [PubMed] [Google Scholar]
- 16.Ogasawara, M., Sasaki, A., Metoki, H., Shin-i, T., Kohara, Y., Satoh, N. & Satou, Y. (2002) Dev. Genes Evol. 212, 173–185. [DOI] [PubMed] [Google Scholar]
- 17.Shoguchi, E., Ikuta, T., Yoshizaki, F., Satou, Y., Satoh, N., Asano, K., Saiga, H. & Nishikata, T. (2004) Zool. Sci. 21, 153–157. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi, M., Matsuda, M., Asakawa, S., Shimizu, N., Nagahama, Y., Satou, Y. & Satoh, N. (2002) Genes Genet. Syst. 77, 283–285. [DOI] [PubMed] [Google Scholar]
- 19.Wada, S., Katsuyama, Y., Yasugi, S. & Saiga, H. (1995) Mech. Dev. 51, 115–126. [DOI] [PubMed] [Google Scholar]
- 20.Takamura, K. (1998) Dev. Genes Evol. 208, 1–8. [DOI] [PubMed] [Google Scholar]
- 21.Bastian, H. & Gruss, P. (1990) EMBO J. 9, 1839–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Candia, A. F., Hu, J., Crosby, J., Lalley, P. A., Noden, D., Nadeau, J. H. & Wright, C. V. (1992) Development (Cambridge, U.K.) 116, 1123–1136. [DOI] [PubMed] [Google Scholar]
- 23.Ferrier, D. E. & Akam, M. (1996) Proc. Natl. Acad. Sci. USA 93, 13024–13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Auken, K., Weaver, D. C., Edgar, L. G. & Wood, W. B. (2000) Proc. Natl. Acad. Sci. USA 97, 4499–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGinnis, W. & Krumlauf, R. (1992) Cell 68, 283–302. [DOI] [PubMed] [Google Scholar]
- 26.Seo, H. C., Edvardsen, R. B., Maeland, A. D., Bjordal, M., Jensen, M. F., Hamsen, A., Flaat, M., Weissenbach, J., Lehrach, H., Wincker, P., et al. (2004) Nature 431, 67–71. [DOI] [PubMed] [Google Scholar]
- 27.Minguillon, C. & Garcia-Fernandez, J. (2003) Genome Biol. 4, R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrier, D. E. & Holland, P. W. (2002) Mol. Phylogenet. Evol. 24, 412–417. [DOI] [PubMed] [Google Scholar]
- 29.Yokouchi, Y., Sakiyama, J. & Kuroiwa, A. (1995) Dev. Biol. 169, 76–89. [DOI] [PubMed] [Google Scholar]
- 30.Hirano, T. & Nishida, H. (2000) Dev. Genes Evol. 210, 55–63. [DOI] [PubMed] [Google Scholar]
- 31.Searcy, R. D. & Yutzey, K. E. (1998) Dev. Dyn. 213, 82–91. [DOI] [PubMed] [Google Scholar]