Abstract
Over the past five years immune-checkpoint inhibitors have dramatically changed the therapeutic landscape of advanced solid and hematologic malignancies. The currently approved immune-checkpoint inhibitors include antibodies to cytotoxic T-lymphocyte antigen-4, programmed cell death (PD-1), and programmed cell death ligand (PD-L1 and PD-L2). Response to immune-checkpoint inhibitors is evaluated on imaging using the immune-related response criteria. Activation of immune system results in a unique toxicity profile termed immune-related adverse events. This article will review the molecular mechanism, clinical applications, imaging of immune-related response patterns and adverse events associated with immune-checkpoint inhibitors.
Keywords: Immune-checkpoint inhibitor, Toxicity, Response, Immune-related Response Criteria
INTRODUCTION
The management of cancer patients has been revolutionized in the last few decades by advances in targeted therapies, which, unlike conventional chemotherapy, enable the precise targeting of cancer cells giving rise to the concept of precision medicine. The term targeted therapy encompasses a wide spectrum of drug classes including inhibitors of signal transduction (receptor tyrosine kinases), hormonal agents, inhibitors of angiogenesis, DNA damage modulators and modulators of immune system, to name a few. Of these targeted therapies, immune modulators or immune-checkpoint inhibitors (ICIs) have garnered significant attention in the last 5 years due to their increasing spectrum of activity in oncology and have, as such, radically changed the therapeutic landscape of advanced solid and hematologic malignancies. To date, four different ICIs have been approved by the Food and Drug Administration (FDA) for various malignancies since 2011 (1). The use of ICIs is expected to increase significantly in the near future with several new single and combination therapies under active research in phase I and II trials.
Owing to their unique anti-cancer mechanism, ICIs are associated with unusual response patterns and adverse event profiles on imaging, which are currently under investigation. As ICIs become the standard of care for an increasing number of cancers and prolong life expectancy of patients with metastatic disease, radiologists, as an integral part of the multidisciplinary oncologic patient care, need be familiar with the mechanistic background of these anticancer agents, their immune-related tumor response patterns, immune-related adverse event (irAE) profiles, to contribute to clinical decision making. In this article, we will review the molecular bases of anti-cancer immunotherapeutic agents, their clinical application in solid and hematologic malignancies, the immune-related patterns of response observed on imaging studies and the imaging features of irAEs.
Immunotherapy
Using the immune system to treat diseases dates back to 1796, when Edward Jenner produced the first vaccine involving immunization with cowpox to prevent smallpox. It was only in the late 1980s that immunotherapy has been used to treat cancer, when Rosenberg (2) and colleagues reported a low tumor regression rate (2.6–3.3%) in 1205 patients with metastatic cancer who underwent different types of immunotherapy. Since then, cancer immunotherapy, which utilizes the immune system to kill cancer cells, has become an established modality for cancer care. Several agents have been used over the years, targeting the immune systems at different levels and in different ways. Cytokine IL-2 has shown objective response in patients with melanoma and renal cell carcinoma (RCC) (3). Vaccine sipuleucel-T has been FDA approved in April 2010 for treatment of metastatic prostate cancer (4). Patient’s own T-cells have been engineered with chimeric antigen receptors to recognize and fight leukemias and lymphomas (5). More recently, ICIs, which act “unleashing” the immune system to fight the cancer, have gained FDA approval for treatment of different types of solid and hematologic malignancies (6).
Immune-Checkpoint Inhibitors: Mechanism of Action
Immune-checkpoint inhibitors act by inhibiting crucial regulatory steps in the immune system, promoting the activation and proliferation of T-cells to induce tumor infiltration and regression. Based on the checkpoint target, three main types of ICIs have been studied so far and gained FDA approval: Cytotoxic T-lymphocyte antigen-4 (CTLA-4) antibodies and programmed cell death (PD-1) and programmed cell death ligand (PD-L1 and PD-L2) antibodies. CTLA-4, discovered in 1987, is a negative regulator of T cell activation, inhibiting CD4+ and CD8+ cell activation, triggered by the antigen presenting cells (7,8,9). Ipilimumab (Yervoy; Bristol-Myers Squibb, New York, NY, USA), a CTLA4 inhibitor, was the first ICI, to gain approval by the U.S. FDA in 2011, based on two pivotal randomized trials (1), which have shown improved overall survival in patients with advanced melanoma. It has also been approved as adjuvant therapy for high-risk melanoma, as an alternative to interferon. Ipilimumab is administered intravenously every 3 weeks for a total of 4 doses, called the induction regimen. Additional CTLA-4 inhibiting monoclonal antibodies have been investigated in other solid malignancies, such as Tremelimumab for malignant mesothelioma (10,11,12).
PD-1, PD-L1 and PD-L2 are transmembrane inhibitory proteins expressed on T cells, B cells and natural killer (NK) cells, binding to PD-ligand 1 and PD-ligand 2 on multiple tissue types and hematopoietic cells, respectively (13). Nivolumab, Pemrolizumab and Atezolizumab have been FDA approved and are increasingly used in multiple cancer types (14,15,16). Nivolumab, IgG4 subclass PD-1 inhibitor, is FDA approved for metastatic melanoma, advanced non-small cell lung cancer (NSCLC) (17), RCC (18) and Hodgkin’s disease (19,20,21,22,23,24,25,26). Pembrolizumab is FDA approved for metastatic melanoma and advanced NSCLC and Atezolizumab has been recently FDA approved for the treatment of urothelial bladder cancer (27). In addition, these agents and other antibodies targeting PD-1 or PD-L1 are under investigation in a broad spectrum of malignancies, such as mismatch-repair deficient colorectal carcinoma, non-Hodgkin’s lymphoma and cancers of the head and neck. Other potential targets for checkpoint inhibition have been studied, including T-cell immunoglobulin and mucin domain 3, lymphocyte activation gene 3, B and T-cell lymphocyte attenuator, and V-domain Ig suppressor of T-cell activation. In addition, multiple clinical trials investigating combination regimen of checkpoint inhibitors are underway. Blocking the CTLA-4 and the PD-1/PD-L1 pathways, in fact, has been proved to have synergistic effect (28,29). The combination Ipilimumab and Nivolumab has demonstrated a significant higher response rate than Ipilumamb alone in metastatic melanoma and has already been FDA approved for the treatment of unresectable or metastatic melanoma across BRAF status (28,29). Clinical trials combining Ipilimumab and Nivolumab are underway in NSCLC, epithelial ovarian/fallopian tube carcinoma, Hodgkin’s lymphoma, RCC and soft tissue sarcoma (28,29).
Immune-Related Imaging Features
Immune-Related Response and Immune Response Criteria
Cancer immunotherapy is associated with a variety of new imaging features, including unconventional response patterns and new spectrum of drug-related toxicities. The patterns of response seen with ICIs differ in many ways from the ones seen with conventional cytotoxic agents and molecular targeted therapies. Treatment response to immune-modulatory agents, in fact, can be seen long after the start of treatment; furthermore, increased and new lesions soon after start of treatment do not necessarily represent worsening of disease, as they do with conventional therapies. The immuno-modulation elicits an inflammatory reaction, which can cause initial increase in tumor size, giving the appearance of pseudoprogression, before leading to response or stability of disease (30,31,32,33). New lesions can also be seen in the context of the initial inflammatory reaction, likely representing undetected lesions at baseline, increasing in size during the initial immune reaction. The pseudoprogression pattern of response is supported by a case study of an Ipilimumab-treated patient which showed increased tumor burden on imaging after 12 weeks of therapy, while histologic exam demonstrated that the increased in size was due to inflammatory response caused by T-cell infiltration rather than tumor cell proliferation (30). In this setting of unconventional response, the application of conventional tumor response criteria, such as Response Evaluation Criteria for Solid Tumors (RECIST) and World Health Organiziation (WHO) criterion, where new lesions and increase tumor size suggest progression of disease, would underestimate the benefit of therapy and lead to premature discontinuation of treatment. Hence, to assess this atypical pattern of response, in 2009, a committee of 200 oncologists, immunotherapists, and regulatory experts proposed a new immune-related Response Criteria (irRC) (34,35,36,37).
Immune-related Response Criteria revisits the definition of progression of disease, preventing new measurable or non-measurable lesions from defining progression and incorporates new measurable lesions into the tumor burden. Radiologic aspects of immune-related tumor response criteria and patterns of irAEs in patients undergoing Ipilimumab therapy (31,33). Maintaining the concept of measurable and non-measurable lesions and the bidimensional measurement according to WHO criterion, irRC proposes a new immune-related (ir) response assessment, as follow:
1) Immune-related complete response: complete response is defined as disappearance of all measureable and non-measurable lesions, with no new lesions. Complete response must be confirmed by a subsequent, consecutive assessment at least four weeks apart.
2) Immune-related partial response: partial response is defined as decrease in the total tumor burden of 50 percent or more from the baseline, confirmed by a subsequent scan at least four weeks later. Increase in size of some of the lesions and/or appearance of new lesions, as long as the total tumor burden meets the response criterion of 50 percent decrease, are allowed.
3) Immune-related stable disease: stability of the tumor burden, not meeting the criteria for partial, complete response or for progressive disease.
4) Immune-related progressive disease: progression of disease is defined as 25 percent increase in the tumor burden from the baseline or nadir study. A short-term follow-up imaging study, at least four weeks apart, should rule out true progression.
While irRC accurately revisits the parameter defining the response assessment, it presents some limitations and scope for areas for improvement. For example, a major pitfall of the original irRC is the use of bidimensional measurements according to WHO criterion, defined as the product of the longest diameter and the longest perpendicular diameter (LD × LPD). Prior studies have in fact demonstrated that bidimensional measurements are not suitable for capturing small changes of tumor burden, as they are subject to a higher variability than unidimensional measurements (38,39,40,41). Furthermore, since multiple prior trials have used RECIST, which is based on unidimensional measurements, the use of bidimensional measurements in irRC makes it difficult to directly compare results (42,43). Tumor density, volume, metabolic activities and other functional information, should also be incorporated in the criterion to evaluate immune-related response and are under investigation (36,44). The field of atypical response with ICIs, as single agents or in combination therapy, has to be further explored (45,46,47,48).
A recent study by Hodi et al. (49) evaluated atypical response patterns using irRC in comparison with RECIST1.1 in 327 patients with melanoma during treatment with Pembrolizumab, the largest retrospective study to date, evaluating immune-related responses in patients on PD-1 inhibitor therapy. The study found that, based on survival analysis, conventional RECIST underestimates the benefit of Pembrolizumab in approximately 15% of patients in comparison with irRC. The article provides important insights for future directions toward an irRECIST 1.1, which, maintaining the modifications introduced by irRC, will propose a common denominator with conventional RECIST, for allowing accurate comparison of the immune-related response (39).
Immune-Related Response in the Radiology Reading Room
In the clinical practice of a radiology reading room of a tertiary cancer center, the non-conventional patterns of response elicited by immunomodulatory agents, translate into three main categories, which radiologists should be aware of:
1) Initial Progression followed by response or stability: increase in tumor burden and/or presence of new lesions at the first follow up imaging study after start of immunomodulatory agent should not be reported as progression of disease. In fact, this could represent initial transient flare up of tumor burden, followed by improvement or stabilization of disease. Patients with this type of response pattern are usually asymptomatic during the flare; conversely, patients with true progression will show clinical symptoms. In the absence of clear symptomatic progression, a short-term follow up scan (at least 4 weeks) should be suggested in the radiology report, to avoid considering failure of immunotherapy.
2) Prolonged stable disease followed by response: stability of the tumor burden can be seen for a long period of time after initiation of ICIs, before seeing response, which could become apparent only after the induction period of 4 doses adminsistered 3 weeks apart. Practically, after the induction period of 12 weeks, is the time a radiologist should carefully look for changes in the tumor burden.
3) Long period of stability: although there is no visible decrease in size of the lesions, even long period of stability can be clinically significant. In the clinical practice, reporting stability of an otherwise incurable and aggressive disease after multiple imaging studies can be clinically relevant. Hence, the time of initiation of treatment should be known to the radiologist, and the stability over a long period of time should be emphasized in the radiology report.
Immune-Related Adverse Events
Immune-checkpoint inhibitors can cause a variety of irAEs, either symptomatic or clinically silent, most of them associated with radiology manifestations. While both clinical and radiographic characterization of irAEs is still ongoing, several have been described and are well characterized. These include enterocolitis, hepatitis, thyroid disorders, hypophysitis, pancreatitis, pneumonitis and dermatitis (50). Additional imaging findings, such as retroperitoneal stranding, sarcoid-like distributed lymphadenopathy, myositis and arthritis have also been attributed to the proinflammatory state elicited by ICIs. irAEs are quite frequent as the body’s innate control against autoimmunity is down-regulated by ICIs (50), more so with CTLA-4 inhibitors, which involves the early activation of T-cells, less with PD-1 and PD-L1 antibodies, involved in the later phases of the activation. With early detection, irAEs can be successfully managed delaying or discontinuing the therapy, based on the severity of the event, and high dose corticosteroids. Hence, prompt recognition of drug-related toxicity by the radiologist is crucial for clinical decisionmaking.
Pneumonitis: pneumonitis is a serious, potentially life-threatening irAEs, which resulted in three deaths in a phase I trial. Pneumonitis as new consolidative or ground-glass opacities has been reported in up to 5% of patients receiving immune checkpoint inhibition (Fig. 1; please also see Fig. 4M, P regarding PET findings) (50). Although imaging features have yet to be described, initial reports of ICI-related pneumonitis indicate a spectrum of radiographic manifestations of the entity. Three cases of pneumonitis associated with the use of anti-PD-1 antibodies in patients with melanoma have been reported with two main radiologic patterns: an acute respiratory distress syndrome-like pattern, with diffuse ground-glass and reticular opacities, consolidation associated with traction bronchiectasis, decreased lung volumes and pleural effusion and a non-specific interstitial pneumonia (NSIP)-pattern, with peripheral ground-glass and reticular opacities, predominantly at the lung bases (51,52). A case study of two patients reports anti-PD-1 pneumonitis in advanced NSCLC patients treated with Nivolumab (51). CT of both patients demonstrated ground-glass, reticular opacities and rapidly evolving consolidations in a peripheral distribution, in a radiographic pattern of cryptogenic organizing pneumonia. Nivolumab was stopped and treatment with high dose corticosteroids was initiated, with improvement of respiratory symptoms and radiographic findings. After steroid taper, one patient, not on any active treatment, developed another episode of pneumonitis (“pneumonitis flare”) and had to be restarted on corticosteroids with subsequent clinical and radiologic improvement, further indicating the complicated nature of this irAE (Fig. 2).
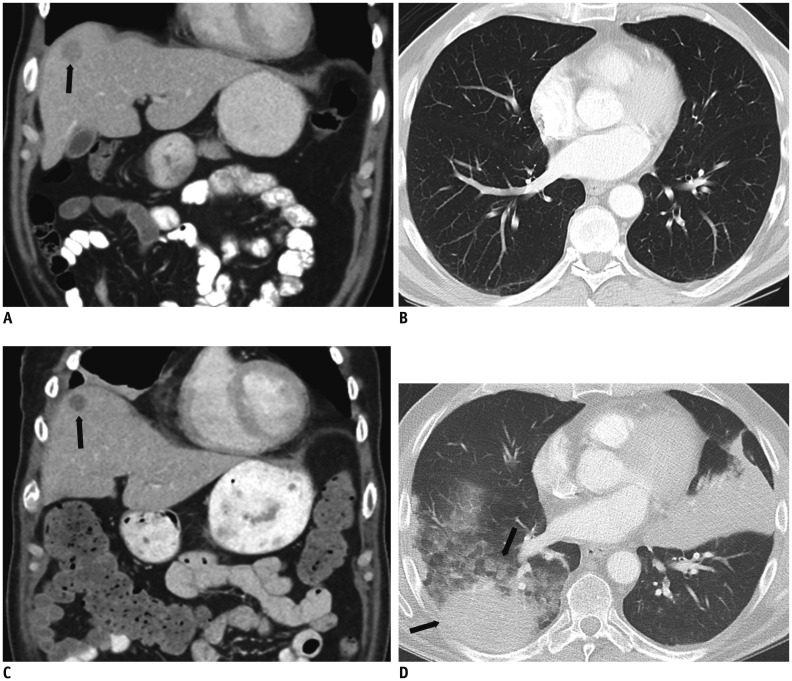
Fig. 1. 62-year-old male with history of ocular melanoma metastatic to liver.
A, B. Contrast enhanced coronal abdominal CT image shows single solid hepatic metastasis (black arrow, A); lung parenchyma is normal. C, D. After 8 weeks of treatment with combination therapy Ipilimumab/Nivolumab, patient presents to emergency department complaining of shortness of breath. While contrast enhanced coronal abdominal CT image shows decreased size and density of hepatic metastasis (black arrow, C), suggesting response to treatment, new lung consolidative opacities are concerning for drug pneumonitis (black arrows, D).
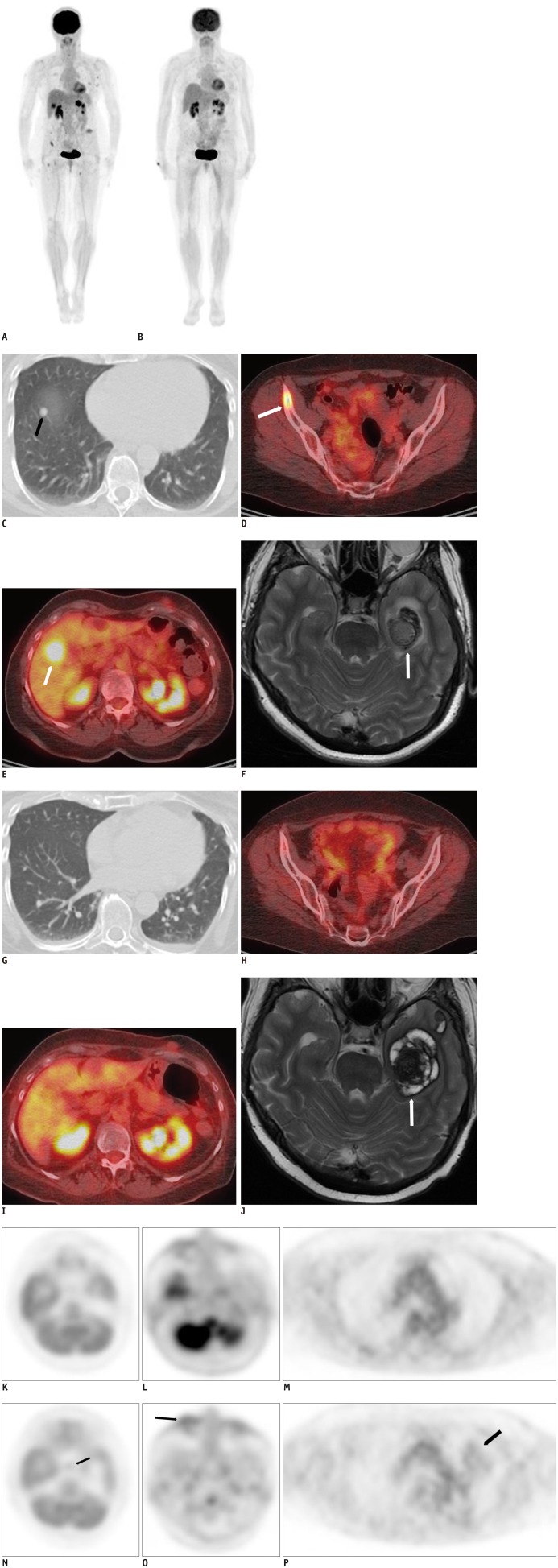
Fig. 4. 59-year-old female with history of left arm melanoma, metastatic to lung, bones, liver brain and subcutaneous tissue, before and after 12 weeks of treatment with Ipilimumab.
A. Whole body MIP F-FDG PET/CT image at baseline demonstrates tracer uptake within lung, bones, liver and subcutaneous tissue, suggesting widespread metastatic disease. B. Whole body MIP F-FDG PET/CT image after 12 weeks of treatment with Ipilimumab demonstrates interval resolution of tracer avidity at sites of metastases. F-FDG = fludeoxyglucose, MIP = maximum intensity projection, PET = positron emission tomography
C-F. Axial CT image in lung window, fused PET/CT images of pelvis and liver, contrast enhanced T2 weighted MRI image of brain demonstrate right lower lobe nodule (black arrow, C), right iliac bone (white arrow, D), right liver (white arrow, E) and left temporal lobe metastases (white arrow, F). F-FDG = fludeoxyglucose, MIP = maximum intensity projection, PET = positron emission tomography
G-J. Restaging imaging at 12 weeks after induction treatment with Ipilimumab show significant decrease of lung nodule, significant decrease uptake of right iliac bone lesion, of liver metastasis and decreased enhancement of brain metastasis (white arrow), suggesting response to treatment.
K-M. Axial PET image of brain at level of hypophysis, mid face and mid thorax before treatment show physiologic tracer uptake. N-P. Restaging PET images at 12 weeks after induction treatment with Ipilimumab show interval increased uptake within hypophysis (black arrow, N), in region of palpebrae (black arrow, O) and left lung parenchyma (black arrow, P), which suggest drug associated hypophysitis, blepharoconjunctivitis and pneumonitis. F-FDG = fludeoxyglucose, MIP = maximum intensity projection, PET = positron emission tomography
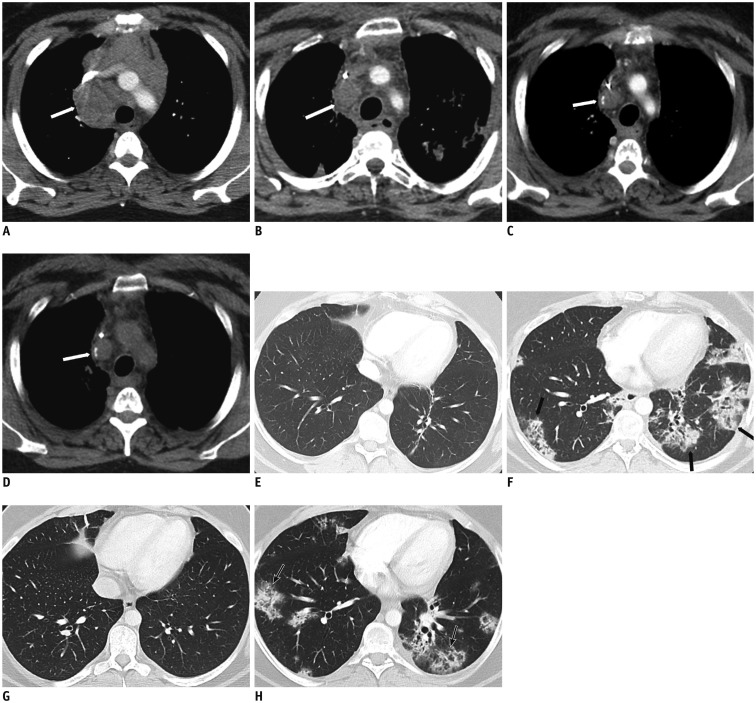
Fig. 2. 33-year-old woman with history of Hodgkin’s lymphoma.
A, E. Axial contrast enhanced CT image of mediastinum shows bulky mediastinal adenopathy (white arrow, A); lung parenchyma is normal. B, F. After 12 weeks of treatment with Nivolumab, while mediastinal lymph nodes have significantly decreased (white arrow, B), new parenchymal patchy bilateral opacities suggest drug pneumonitis (black arrows, F). C, G. Nivolumab was stopped, high dose therapy with corticosteroid initiated. After 4 weeks of steroid, axial CT image of lung parenchyma demonstrates resolution of pneumonitis, while mediastinal disease continues to decrease (white arrow). D, H. After steroid taper, while mediastinal adenopathy has not significantly changed (white arrow), axial chest CT in lung window shows recurrent pneumonitis (black arrows).
Immune-related colitis is the most commonly reported irAE, and can be observed even in asymptomatic patients. It is most commonly associated with Ipilimumab and usually occurs between 5–10 weeks of initiation of therapy (Fig. 3) (53). Two distinct CT appearances have been described: diffuse bowel wall thickening, with or without fluid filled distended colon, with mesenteric vessel engorgement, or segmental bowel wall thickening and pericolonic stranding, often superimposed to diverticulitis, so called ‘segmental colitis associated with diverticulosis’ (54). It is important to differentiate between the two patterns as their management differs: after stopping the drug, diffuse colitis is treated with high dose steroids, segmental colitis is treated with steroids and antibiotics.
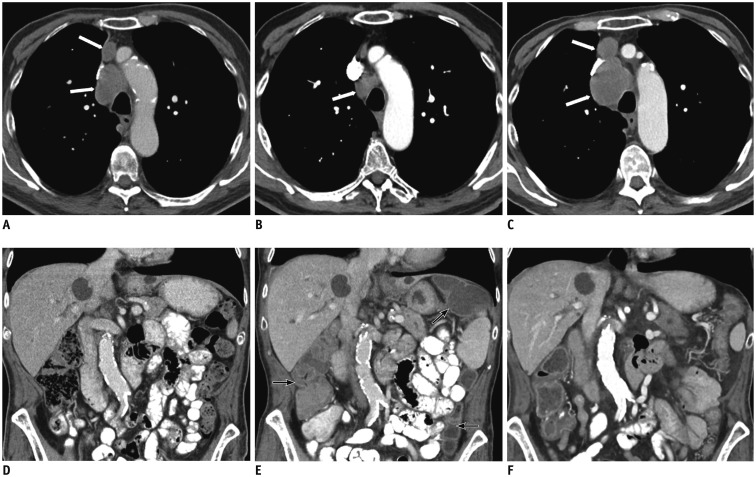
Fig. 3. 73-year-old gentleman with history of recurrent squamous cell lung cancer.
A, D. Axial contrast-enhanced CT image of mediastinum shows mediastinal adenopathy (white arrows, A); coronal abdominal image is unremarkable. B, E. After 4 weeks of treatment with Nivolumab, while mediastinal lymph nodes have significantly decreased (white arrow, B), visualized colon is hyperemic and fluid filled, suggesting colitis (black arrows, E). C, F. Nivolumab was stopped, high dose therapy with corticosteroid initiated. After 8 weeks, restaging contrast-enhanced abdominal CT shows resolution of colitis, but there has been significant increased in size of mediastinal adenopathy (white arrows, C).
Hepatitis: immune-related hepatitis usually appears between 6–14 weeks of initiation of therapy. Imaging findings can be subtle and a high index of suspicion should be maintained in patients with systemic symptoms and altered liver function tests (LFTs). Classic imaging appearance of acute hepatitis is seen in such patients, as reported in a study of 6 melanoma patients with Ipilimumab-associated hepatitis (55). Periportal edema, mild hepatomegaly, and periportal lymphadenopathy are the most common findings. Conversely, imaging of asymptomatic patients, with mildly increased LFTs can be normal (55,56). Histologically, Ipilimumab-associated hepatitis has been described as hepatocyte injury, with acute hepatitis pattern or as bile duct injury (56).
Endocrine irAEs are quite common and usually appear between 7–20 weeks of initiation of therapy. They might cause serious and life-changing symptoms due to permanent damage of the hypophysis or the thyroid glands. They have to be promptly recognized by the radiologist, as symptoms can resolve with adequate hormone replacement, allowing the patients to continue a therapy they are benefiting from. Thyroid disorders are the most common clinically reported endocrine irAEs, particularly in women on anti-PD-1 inhibitors and can clinically manifests with hypothyroidism, most commonly, followed by hyperthyroidisim, whereas thyroiditis has not yet been reported (50,57). Imaging features have been described as new enlargement of the thyroid gland, enhancement and diffuse fludeoxyglucose (FDG) uptake on PET/CT, as reported by a study on Ipilimumab related adverse event (50).
Hypophysitis is more commonly associated with Ipilimumab, is rare with PD-1 and PD-L1 inhibitor monotherapies, and has a median appearance at 11 weeks, but can be seen as early as 4 weeks (Fig. 4N) (58). Clinical picture and imaging findings resemble those seen with primary lymphocytic hypophysitis, with avid enhancement of the hypophysis on contrast enhanced CT, T1 precontrast and T2 hypointensity and homogeneous enhancement on MRI (57). Additional rare immune-related endocrinopathies have been described and include Ipilimumab Graves’ ophthalmopathy and anti PD-1 Type I diabetes mellitus, which has been reported in only 4 patients to date, but imaging features have yet to be described (Fig. 4O).
Clinically silent new imaging findings have been observed. Awareness that these findings might reflect inflammatory response rather than new sites of disease is crucial for the radiologist. Examples include new sarcoid-like distributed lymph nodes (bilateral hilar and mediastinal), which have been reported in up to 5% of patients on ICIs treatment (50,59,60,61,62). Although the imaging appearance of these nodes can be indistinguishable from nodal metastases, the distribution resembling typical sarcoidosis, in absence of infection and occurring in the setting of response at other sites, favor sarcoid-like reaction and ACE-level and tissue diagnosis can support the diagnosis of irAE (50). Treatment should not be discontinued as these nodes are likely a flare phenomenon at initiation of therapy and might regress spontaneously. New abdominal lymphadenopathy, stranding in perirenal or retroperitoneal fat and focal muscle abnormalities have also been observed and are also likely a marker of lymphocytic infiltration, indicating treatment effect, rather than new sites of disease. New focal intramuscular enhancement seen on contrast-enhanced CT or new intramuscular tracer uptake on PET/CT has been described as drug-related myositis and should be reported.
An interesting relationship between tumor response and irAEs has been described: radiologic manifestations of irAEs have been associated with improved tumor response and disease control (53). A study of 119 patients with metastatic melanoma on Ipilimumab therapy showed irAEs in 17% of patients, who were more likely to show response and disease control (55%) (63). This relationship is more apparent in combination therapies. For example, Ipilimumab and Nivolumab therapy for the treatment of metastatic melanoma, as it demonstrates increase antitumor activity, shows increased incidence of drug-associated adverse events. The combination of Pembrolizumab and Ipilimumab has also been associated with increased adverse events, the thyroid gland being most affected organ, manifesting as hypothyroidism. This interesting relationship between response and toxicities, however, need to be further investigated with a rigorous study design controlling for important factors such as time of therapy duration.
CONCLUSION
Immune-checkpoint inhibitors consist of a new paradigm of anti-cancer drugs approved for treatment of a variety of solid and hematologic malignancies. With the increasing use of ICIs in a growing number of tumor types, awareness of the immune-related patterns of response, the clinical and imaging manifestations of irAEs is key for radiologists, as they become increasingly involved in and key contributors of cancer patient care.
Footnotes
Conflict of interest Disclosures: Dr. Nishino’s disclosures:
Consulting or Advisory Role: Bristol-Myers Squibb, Toshiba Medical Systems, Worldcare Clinical
Research Funding: Merck (Inst), Canon (Inst)
Dr. Hodi’s disclosures:
Consulting or Advisory Role: Merck Sharp & Dohme, Novartis, Genentech, Amgen
Research Funding: Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Genentech (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending as per institutional policy; patent pending royalties received on MICA related disorders application to institution per institutional intellectual property policy
Travel, Accommodations, Expenses: Novartis, Bristol-Myers Squibb
Other Relationship: Bristol-Myers Squibb, Genentech
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA. Adoptive immunotherapy of cancer: accomplishments and prospects. Cancer Treat Rep. 1984;68:233–255. [PubMed] [Google Scholar]
- 3.Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology. 2016;5:e1163462. doi: 10.1080/2162402X.2016.1163462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoo S, Choi SY, You D, Kim CS. New drugs in prostate cancer. Prostate Int. 2016;4:37–42. doi: 10.1016/j.prnil.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Liu D. Chimeric antigen receptor (CAR)-directed adoptive immunotherapy: a new era in targeted cancer therapy. Stem Cell Investig. 2014;1:2. doi: 10.3978/j.issn.2306-9759.2013.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 8.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 9.Freeman GJ, Gribben JG, Boussiotis VA, Ng JW, Restivo VA, Jr, Lombard LA, et al. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993;262:909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 10.Calabrò L, Morra A, Fonsatti E, Cutaia O, Fazio C, Annesi D, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med. 2015;3:301–309. doi: 10.1016/S2213-2600(15)00092-2. [DOI] [PubMed] [Google Scholar]
- 11.Hodi FS, Fisher DE. Adoptive transfer of antigen-specific CD4+ T cells in the treatment of metastatic melanoma. Nat Clin Pract Oncol. 2008;5:696–697. doi: 10.1038/ncponc1259. [DOI] [PubMed] [Google Scholar]
- 12.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 13.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 16.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choueiri TK, Fishman MN, Escudier B, McDermott DF, Drake CG, Kluger H, et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res. 2016 May 11; doi: 10.1158/1078-0432.CCR-15-2839. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34:2980–2987. doi: 10.1200/JCO.2016.66.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armand P. Immune checkpoint blockade in hematologic malignancies. Blood. 2015;125:3393–3400. doi: 10.1182/blood-2015-02-567453. [DOI] [PubMed] [Google Scholar]
- 20.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perales MA, Sauter CS, Armand P. Fast cars and no brakes: autologous stem cell transplantation as a platform for novel immunotherapies. Biol Blood Marrow Transplant. 2016;22:17–22. doi: 10.1016/j.bbmt.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34:2690–2697. doi: 10.1200/JCO.2016.66.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib Study. J Clin Oncol. 2016;34:2698–2704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armand P. Checkpoint blockade in lymphoma. Hematology Am Soc Hematol Educ Program. 2015;2015:69–73. doi: 10.1182/asheducation-2015.1.69. [DOI] [PubMed] [Google Scholar]
- 25.Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med. 2016;375:143–153. doi: 10.1056/NEJMoa1601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. 2016 Jun 27; doi: 10.1200/JCO.2016.67.3467. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 29.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 31.O’Regan KN, Jagannathan JP, Ramaiya N, Hodi FS. Radiologic aspects of immune-related tumor response criteria and patterns of immune-related adverse events in patients undergoing ipilimumab therapy. AJR Am J Roentgenol. 2011;197:W241–W246. doi: 10.2214/AJR.10.6032. [DOI] [PubMed] [Google Scholar]
- 32.Kwak JJ, Tirumani SH, Van den Abbeele AD, Koo PJ, Jacene HA. Cancer immunotherapy: imaging assessment of novel treatment response patterns and immune-related adverse events. Radiographics. 2015;35:424–437. doi: 10.1148/rg.352140121. [DOI] [PubMed] [Google Scholar]
- 33.Nishino M, Tirumani SH, Ramaiya NH, Hodi FS. Cancer immunotherapy and immune-related response assessment: the role of radiologists in the new arena of cancer treatment. Eur J Radiol. 2015;84:1259–1268. doi: 10.1016/j.ejrad.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishino M, Giobbie-Hurder A, Ramaiya NH, Hodi FS. Response assessment in metastatic melanoma treated with ipilimumab and bevacizumab: CT tumor size and density as markers for response and outcome. J Immunother Cancer. 2014;2:40. doi: 10.1186/s40425-014-0040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishino M, Gargano M, Suda M, Ramaiya NH, Hodi FS. Optimizing immune-related tumor response assessment: does reducing the number of lesions impact response assessment in melanoma patients treated with ipilimumab? J Immunother Cancer. 2014;2:17. doi: 10.1186/2051-1426-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19:3936–3943. doi: 10.1158/1078-0432.CCR-13-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishino M. Immune-related response evaluations during immune-checkpoint inhibitor therapy: establishing a “common language” for the new arena of cancer treatment. J Immunother Cancer. 2016;4:30. doi: 10.1186/s40425-016-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erasmus JJ, Gladish GW, Broemeling L, Sabloff BS, Truong MT, Herbst RS, et al. Interobserver and intraobserver variability in measurement of non-small-cell carcinoma lung lesions: implications for assessment of tumor response. J Clin Oncol. 2003;21:2574–2582. doi: 10.1200/JCO.2003.01.144. [DOI] [PubMed] [Google Scholar]
- 39.Nishino M, Guo M, Jackman DM, DiPiro PJ, Yap JT, Ho TK, et al. CT tumor volume measurement in advanced non-small-cell lung cancer: performance characteristics of an emerging clinical tool. Acad Radiol. 2011;18:54–62. doi: 10.1016/j.acra.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao B, James LP, Moskowitz CS, Guo P, Ginsberg MS, Lefkowitz RA, et al. Evaluating variability in tumor measurements from same-day repeat CT scans of patients with non-small cell lung cancer. Radiology. 2009;252:263–272. doi: 10.1148/radiol.2522081593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Zhao B, Tan Y, Bell DJ, Marley SE, Guo P, Mann H, et al. Exploring intra- and inter-reader variability in uni-dimensional, bi-dimensional, and volumetric measurements of solid tumors on CT scans reconstructed at different slice intervals. Eur J Radiol. 2013;82:959–968. doi: 10.1016/j.ejrad.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishino M, Hatabu H, Johnson BE, McLoud TC. State of the art: response assessment in lung cancer in the era of genomic medicine. Radiology. 2014;271:6–27. doi: 10.1148/radiol.14122524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bohnsack O, Ludajic K, Hoos A. Adaptation of the immune-related response criteria: irRECIST. Massachusetts: ESMO; 2014. p. Abstract 4958. [Google Scholar]
- 45.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 46.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 47.Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. AJR Am J Roentgenol. 2010;195:281–289. doi: 10.2214/AJR.09.4110. [DOI] [PubMed] [Google Scholar]
- 48.Nishino M, Jagannathan JP, Krajewski KM, O’Regan K, Hatabu H, Shapiro G, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012;198:737–745. doi: 10.2214/AJR.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510–1517. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tirumani SH, Ramaiya NH, Keraliya A, Bailey ND, Ott PA, Hodi FS, et al. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res. 2015;3:1185–1192. doi: 10.1158/2326-6066.CIR-15-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishino M, Chambers ES, Chong CR, Ramaiya NH, Gray SW, Marcoux JP, et al. Anti-PD-1 inhibitor-related pneumonitis in non-small cell lung cancer. Cancer Immunol Res. 2016;4:289–293. doi: 10.1158/2326-6066.CIR-15-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med. 2015;373:288–290. doi: 10.1056/NEJMc1505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol. 2011;197:W992–W1000. doi: 10.2214/AJR.10.6198. [DOI] [PubMed] [Google Scholar]
- 54.Kim KW, Ramaiya NH, Krajewski KM, Shinagare AB, Howard SA, Jagannathan JP, et al. Ipilimumab-associated colitis: CT findings. AJR Am J Roentgenol. 2013;200:W468–W474. doi: 10.2214/AJR.12.9751. [DOI] [PubMed] [Google Scholar]
- 55.Min JH, Lee HY, Lim H, Ahn MJ, Park K, Chung MP, et al. Drug-induced interstitial lung disease in tyrosine kinase inhibitor therapy for non-small cell lung cancer: a review on current insight. Cancer Chemother Pharmacol. 2011;68:1099–1109. doi: 10.1007/s00280-011-1737-2. [DOI] [PubMed] [Google Scholar]
- 56.Kim KW, Ramaiya NH, Krajewski KM, Jagannathan JP, Tirumani SH, Srivastava A, et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs. 2013;31:1071–1077. doi: 10.1007/s10637-013-9939-6. [DOI] [PubMed] [Google Scholar]
- 57.González-Rodríguez E, Rodríguez-Abreu D Spanish Group for Cancer Immuno-Biotherapy (GETICA) Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. Oncologist. 2016;21:804–816. doi: 10.1634/theoncologist.2015-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carpenter KJ, Murtagh RD, Lilienfeld H, Weber J, Murtagh FR. Ipilimumab-induced hypophysitis: MR imaging findings. AJNR Am J Neuroradiol. 2009;30:1751–1753. doi: 10.3174/ajnr.A1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eckert A, Schoeffler A, Dalle S, Phan A, Kiakouama L, Thomas L. Anti-CTLA4 monoclonal antibody induced sarcoidosis in a metastatic melanoma patient. Dermatology. 2009;218:69–70. doi: 10.1159/000161122. [DOI] [PubMed] [Google Scholar]
- 60.Berthod G, Lazor R, Letovanec I, Romano E, Noirez L, Mazza Stalder J, et al. Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J Clin Oncol. 2012;30:e156–e159. doi: 10.1200/JCO.2011.39.3298. [DOI] [PubMed] [Google Scholar]
- 61.Hunter G, Voll C, Robinson CA. Autoimmune inflammatory myopathy after treatment with ipilimumab. Can J Neurol Sci. 2009;36:518–520. doi: 10.1017/s0317167100007939. [DOI] [PubMed] [Google Scholar]
- 62.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 63.Howard SA, Krajewski KM, Jagannathan JP, Braschi-Amirfarzan M, Tirumani SH, Shinagare AB, et al. A new look at toxicity in the era of precision oncology: imaging findings, their relationship with tumor response, and effect on metastasectomy. AJR Am J Roentgenol. 2016;207:4–14. doi: 10.2214/AJR.15.15480. [DOI] [PubMed] [Google Scholar]