Abstract
High-resolution rectal MRI plays a crucial role in evaluating rectal cancer by providing multiple prognostic findings and imaging features that guide proper patient management. Quality reporting is critical for accurate effective communication of the information among multiple disciplines, for which a systematic structured approach is beneficial. Existing guides on reporting of rectal MRI are divergent on some issues, largely reflecting the differences in overall management of rectal cancer patients between the United States and Europe. The Korean Society of Abdominal Radiology (KSAR) study group for rectal cancer has developed an expert consensus recommendation regarding essential items for structured reporting of rectal cancer MRI using a modified Delphi method. This recommendation aims at presenting an up-to-date, evidence-based, practical, structured reporting template that can be readily adopted in daily clinical practice. In addition, a thorough explanation of the clinical and scientific rationale underlying the reporting items and their formats is provided. This KSAR recommendation may serve as a useful tool to help achieve more standardized optimal care for rectal cancer patients using rectal MRI.
Keywords: Rectum, Rectal cancer, Staging, Magnetic resonance imaging, Structured, Report, Consensus, Recommendation, Guideline
INTRODUCTION
High-resolution rectal MRI plays a crucial role in evaluating rectal cancer patients to make management decisions, in particular for the selection of patients who would benefit from neoadjuvant therapy primarily using radiation prior to surgery. This is because the paradigm for supplementary treatment to surgery for rectal cancer has shifted from adjuvant to neoadjuvant therapy (1,2). Neoadjuvant radiation therapy decreases the risk of local tumor recurrence and may increase patient survival following rectal cancer surgery (1,2,3,4). In order to help achieve a systematic assessment and reporting of findings on rectal MRI in rectal cancer patients, several recommendations and guides for structured reporting of rectal MRI have been proposed (5,6,7,8,9,10). These guides generally follow similar principles but do not concur completely. In fact, they are quite divergent on some issues, creating some confusion. The discrepancies between the existing guides largely reflect the differences between the United States and Europe in the overall management strategies for rectal cancer patients (11,12). To this end, the Korean Society of Abdominal Radiology (KSAR) study group for rectal cancer has developed an expert consensus recommendation regarding essential items for structured reporting of rectal cancer MRI. This consensus recommendation represents the official position of KSAR. It aims at presenting an up-to-date, evidence-based, practical reporting template that can be readily adopted in daily clinical practice, avoiding redundancy. We have tried to reconcile the confusions and regional differences between the existing guides. This recommendation primarily focuses on the initial staging evaluation of rectal cancer and is not customized for reporting the response to neoadjuvant therapy. We also do not intend to provide guidance regarding MRI scan techniques.
Methods of Development
The KSAR study group for rectal cancer comprised 27 board-certified abdominal radiologists from 20 different hospitals in South Korea. All of them were members of KSAR and were experienced with rectal cancer MRI. We used a modified Delphi method to develop the proposed consensus recommendation. We started with a comprehensive summary of the relevant data and evidences in the literature using 1) thorough PubMed MEDLINE search of original research articles; 2) review of existing recommendations/guides on structural reporting of rectal MRI, including the User's guide for the synoptic MRI report for rectal cancer supported by Cancer Care Ontario and the Canadian Cancer Society (5,6), Proforma-based reporting by a British group (8), Radiology reporting template of the Radiological Society of North America (7), recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology consensus meeting (9), and radiology experts review of the European Registration of Cancer Care (EURECCA) consensus (10); 3) review of general management or staging guidelines for rectal cancer, including the National Comprehensive Cancer Network (NCCN) 2016 guidelines of the United States (11), EURECCA consensus of Europe (12), and the Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) staging manuals (13,14,15); and 4) review of relevant articles included in the bibliography of the aforementioned recommendations and guides. We made a list of candidate issues, which were deemed potentially essential for the reporting of rectal cancer MRI. Multiple teams, each comprising two study group members, were organized and each team was assigned one candidate issue. The teams consolidated relevant evidences regarding their assigned issue and prepared a draft of specific reporting items and their formats, along with a summary of clinical and scientific rationale in support of their suggestions. All study group members discussed these materials at two face-to-face meetings and one online discussion. We tried to develop a single reporting format for a particular item, which was then subjected to a modified Delphi voting among the study group members. In case a single reporting format could not be designed, we used multiple choice question formats for the voting. The modified Delphi method, where the participant could express their agreement for a particular single reporting format, was based on a six point scale; strongly agree, agree with minor reservation, agree with major reservation, disagree with minor reservation, disagree with major reservation, and strongly disagree. Consensus level was predefined as ≥ 80% of the sum of the votes in favor of strongly agree or agree with minor reservation. A pre-planned second-round modified Delphi voting was carried out on items for which the agreement did not meet the 80% threshold, but was close during the first round of voting and if any of the choices from the multiple choice voting questions had been selected by close to 80% of the participants. The second round of voting was conducted after a thorough discussion on the pros and cons of the items to be re-voted upon. Initially, there were 16 modified Delphi-type and 3 multiple choice question-type voting questions. Of these, 1 item, the maximum extramural depth of tumor invasion, initially a multiple-choice question, was voted again in the second round as a modified Delphi question. The final draft of the consensus recommendation was presented to the KSAR members outside the study group for feedback through a half-day satellite conference (128 board-certified radiologists specializing in abdominal radiology were in attendance) during the 2016 Scientific Assembly and Annual Meeting of KSAR, which consisted of didactic lectures, a case workshop, and a panel discussion.
Essential Items for Structured Reporting of Rectal Cancer MRI
The KSAR consensus recommendation on the essential items for structured reporting of rectal cancer MRI is shown in the Table 1 along with the level of agreement on each item among the 27 study group members. We included the use of rectal filling as an essential reporting item (85.2% agreement, 23 of 27) because it may affect certain key findings, such as longitudinal tumor distance from the anal verge or the anorectal junction and the tumor distance from the mesorectal fascia, although this consensus recommendation does not intend to address scan techniques.
Table 1. KSAR Recommendation of Essential Items and Formats for Structured Reporting of Rectal Cancer MRI.
| Reporting Item and Format | Degree of Agreement | |
|---|---|---|
| Technique | ||
| Use of rectal filling (using such as ultrasound gel) | 85.2% (23 of 27) | |
| □ Used | ||
| □ Unused | ||
| Findings | ||
| 1. Distance of lowest tumor margin from anal verge | 96.3% (26 of 27) | |
| □ | ||
| □ Cannot be measured: please specify | ||
| 2. Distance of lowest tumor margin from anorectal junction | 81.5% (22 of 27) | |
| □ | ||
| □ Cannot be measured: please specify | ||
| 3. Tumor relationship to anterior peritoneal reflection | 92.6% (25 of 27) | |
| □ Entirely above anterior peritoneal reflection | ||
| □ Straddling anterior peritoneal reflection | ||
| □ Entirely below anterior peritoneal reflection | ||
| □ Other: please specify | ||
| 4. Circumferential tumor location | 92.6% (25 of 27) | |
| □ Completely encircling lumen | ||
| □ Partially encircling: to o’clock in clockwise manner | ||
| □ Other: please specify | ||
| Most ventral point of rectum is designated as 12 o’clock | ||
| 5. Longitudinal tumor size | 88.9% (24 of 27) | |
| □ | ||
| □ Cannot be measured: please specify | ||
| 6. T stage: | 92.6% (25 of 27) | |
| 7. For ≥ T3 lesion, maximum extramural depth of tumor invasion | 85.2% (23 of 27) | |
| □ ≤ 5 mm | ||
| □ > 5 mm | ||
| □ Other: please specify | ||
| 8. For T4b lesion, involved structures | 100% (27 of 27) | |
| □ Small bowel | □ Bladder | □ Right ureter | □ Left ureter | □ Right seminal vesicle | □ Left seminal vesicle | □ Prostate | □ Uterus | □ Right ovary and adnexa | □ Left ovary and adnexa | □ Vagina | □ Right obturator internus | □ Left obturator internus | □ Right piriformis | □ Left piriformis | □ Right levator ani | □ Left levator ani | □ Right internal iliac vessels | □ Left internal iliac vessels | □ Right external iliac vessels | □ Left external iliac vessels | □ Sacrum/coccyx | □ Other (please specify) | ||
| 9. Shortest tumor distance* from mesorectal fascia or levator (for low tumors close to levator), i.e., risk of CRM tumor involvement | 96.3% (26 of 27) | |
| 10. Anal canal involvement | 88.9% (24 of 27) | |
| □ Absent | ||
| □ Partial thickness of internal sphincter | ||
| □ Full thickness of internal sphincter | ||
| □ Into intersphincteric fat plane | ||
| □ Into external sphincter | ||
| □ Beyond external sphincter and into ischiorectal tissue | ||
| □ Other: please specify | ||
| 11. Mesorectal lymph node spread | 92.6% (25 of 27) | |
| □ Absent | ||
| □ Present | ||
| □ Other: please specify | ||
| 12. Extramesorectal lymph node spread | 96.3% (26 of 27) | |
| □ Absent | ||
| □ Present: □ Right internal iliac | □ Right external iliac | □ Right obturator | □ Right common iliac | □ Right inguinal | □ Left internal iliac | □ Left external iliac | □ Left obturator | □ Left common iliac | □ Left inguinal | ||
| □ Other: please specify | ||
| 13. Extramural venous invasion | 88.9% (24 of 27) | |
| □ Absent | ||
| □ Present | ||
| □ Other: please specify | ||
*Consensus regarding any specific reporting format could not be made (please see “Shortest Tumor Distance from the Mesorectal Fascia or the Levator (Item 9)” section in main text for further explanations). CRM = circumferential resection margin, KSAR = Korean Society of Abdominal Radiology
Elaboration on the Reporting Items, Formats, and Their Clinical and Scientific Rationale
Longitudinal Tumor Location (Items 1,2)
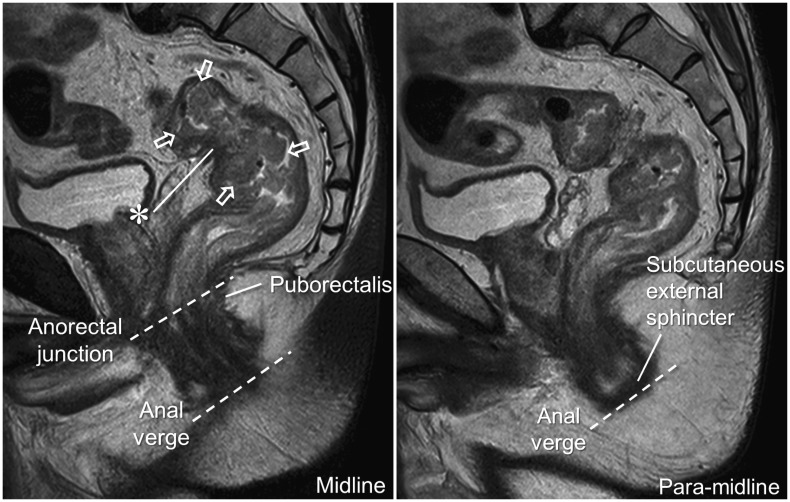
Upper, mid, and lower are commonly used terms to describe the height (i.e., longitudinal location) of a rectal cancer. These terms are useful in providing a quick grasp of the lesion location, for example, approximately upper 1/3, mid 1/3, and lower 1/3, respectively, of the rectum. However, using these terms alone in official radiology reports is not recommended because there are no cohesive definitions for these terms. The definitions of these terms have been inconsistent in published literature (5,7,16,17,18,19). These terms should be accompanied by objective measurements of the tumor distances from fixed reference anatomical structures for clarity, if they are to be included in the report. Both the anal verge and the anorectal junction, which are generally clearly visible on MRI, can serve as reference structures (Fig. 1). The anal verge refers to the mucocutaneous junction of the anus and is located at the lower margin of the subcutaneous external sphincter muscle (20), which is clearly visualized on MRI in a para-midline sagittal plane (Fig. 1). Different definitions exist with respect to the anorectal junction. Anatomists consider the dentate line as the border between the anus and the rectum, which is not clinically relevant. The surgical definition, the top border of the puborectalis muscle (16,21,22), is more clinically relevant and should be used for clinical reporting of rectal MRI (Fig. 1). Almost all the study group members (96.3%, 26 of 27) consider that the distance of the lowest tumor margin from the anal verge is essential for reporting. This is largely because this distance is measured in clinical practice using other techniques such as digital rectal exam and rigid or flexible sigmoidoscope. Nevertheless, it is important to recognize that the distance from the anal verge, measured on MRI, may not necessarily coincide with other corresponding clinical measurements, as the latter typically straightens the anal/rectal lumen, particularly when measured with a rigid sigmoidoscope. Some investigators recommend measuring the tumor height of a low rectal cancer on MRI in a straight line from the anal verge to simulate the distance measured with a rigid sigmoidoscope (23). However, this method cannot be applied to rectal tumors located higher due to the curvature of the rectum. A slightly smaller number of the members (81.5%, 22 of 27) considered the distance from the anorectal junction to be essential. Apart from MRI, this distance is difficult to measure by a digital rectal exam or with an endoscope and, therefore, is rarely used in clinical practice. However, from the viewpoint of treatment planning, the distance from the anorectal junction is practically more useful.
Fig. 1. Longitudinal tumor location.
Rectal cancer mass (arrows) is identified by raised rolled-up edges. Anal verge and anorectal junction (dashed lines) are clearly seen on these sagittal planes. Distances from anal verge and anorectal junction to lowest tumor margin, measured on MRI along approximate luminal center of rectum and anus, were 11.5 and 8 cm, respectively. Tumor distance from anal verge measured with flexible sigmoidoscope was 9.5 cm. Mass (arrows) is seen straddling anterior peritoneal reflection, as apex of anterior peritoneal reflection (*) is seen at mid-portion of mass (please see “Tumor Relationship with the Anterior Peritoneal Reflection [Item 3]” section and Figure 2 for further explanations).
As mentioned earlier, simulating a rigid sigmoidoscopic measurement using a straight line on MRI is only applicable to those tumors located close to the anus. Moreover, measurements using a straight line are subject to observer variation depending on how the line is drawn. For these reasons, a vast majority of the study members (92.6%, 25 of 27) concurred that the measurement of the distances from the anal verge and the anorectal junction on MRI should be made approximately along the luminal center of the rectum and the anus, for example, by performing a curvilinear measurement on the midline sagittal plane.
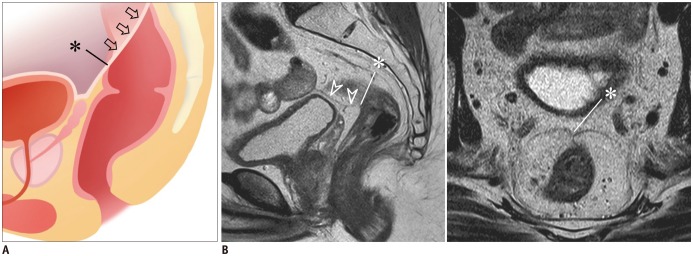
Tumor Relationship with the Anterior Peritoneal Reflection (Item 3)
The anterior peritoneal reflection refers to the transition between the peritonealized and non-peritonealized portions of the rectum (Fig. 2). The apex (i.e., the lowermost point) of the anterior peritoneal reflection attaches to the anterior rectal wall approximately at the midline and is visible on MRI in most (81.6–88.5%) patients (Fig. 2) (24). The anterior peritoneal reflection gradually extends from the apex cranially and posterolaterally on both sides of the rectal wall creating a V-shaped configuration (Fig. 2). Describing the tumor location in relation to the anterior peritoneal reflection is important (92.6% agreement, 25 of 27) because the relative location is closely related to treatment decisions (21). The tumor region that is located above the anterior peritoneal reflection in the peritonealized anterior or anterolateral rectal wall does not require direct surgical resection, unless the tumor has directly invaded adjacent organs/structures. Therefore, above the anterior peritoneal reflection, tumor involvement in the circumferential resection margin (CRM; i.e., the lateral surgical resection margin of rectal cancer surgery) does not apply to a tumor located in the peritonealized area (5,6), unlike a tumor located posteriorly in the sacral side of the rectum (25). The risk of tumor involvement in the CRM generally applies to a tumor (or a portion of a tumor) that is located below the anterior peritoneal reflection (5,6) (please refer to “Shortest Tumor Distance from the Mesorectal Fascia or the Levator [Item 9]” section).
Fig. 2. Anterior peritoneal reflection.
A. Schematic shows anterior peritoneal reflection (arrows) and its apex (*), i.e., lowermost point of anterior peritoneal reflection.
B. Apex of anterior peritoneal reflection (*) can be identified on sagittal MRI (left) in this male patient by following peritoneal line (arrowheads) over urinary bladder and seminal vesicle and by noting its termination in anterior rectal wall (*). On axial view (right), peritoneal attachment makes “seagull”-like appearance.
Circumferential Tumor Location (Item 4)
It is important to clearly recognize and describe the circumferential location of a rectal tumor because rectal tumors are frequently not circumferential. In instances of partially encircling tumors, the circumferential extent of involvement should be described as clock face in clockwise manner (92.6% agreement, 25 of 27) (Fig. 3). Extramural tumor invasion occurs at the site of the tumor-involved wall and the deepest invading tumor edge typically exists near the central tumoral ulcer.
Fig. 3. Circumferential tumor location.
Ulceroinfiltrative cancer mass is observed in rectal wall from 1 o'clock to 8 o'clock position in clockwise direction (arrowheads). Metastatic lymph node abutting left posterolateral side of mesorectal fascia (arrow) is also noted, which predicts tumor involvement of CRM. CRM = circumferential resection margin
Tumor Size (Item 5)
According to UICC/AJCC staging system, tumor size per se is not recognized as a separate prognostic factor for rectal cancer and, therefore, is not considered for staging (13,15). Therefore, when it comes to the staging evaluation of rectal cancer, typical oncologic measurements, such as the largest tumor diameter or the cross product of the long and short diameters, do not seem to offer a particular benefit. On the contrary, we believe that the longitudinal tumor size, i.e., craniocaudal tumor extent along the long axis of the colorectum, should be reported (88.9% agreement, 24 of 27), as it influences the treatment planning in terms of the length of the colorectum to be resected or the area to be irradiated.
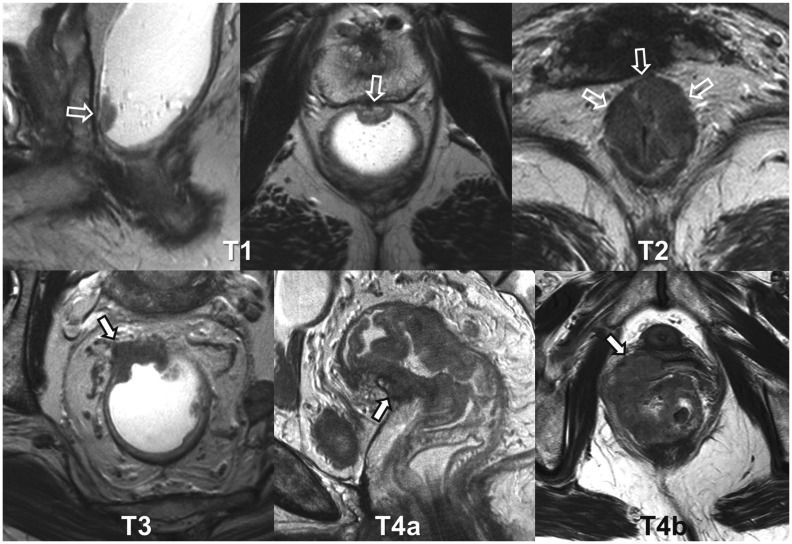
T Stage (Items 6,8)
Depth of tumor invasion is a clear prognostic factor for rectal cancer. The current standard method of describing the depth of tumor invasion is based on the UICC/AJCC T staging system. According to the seventh edition of the AJCC staging system (13), T0, Tis, T1, T2, T3, T4a, and T4b tumors are defined as no evidence of primary tumor, intraepithelial or invasion of lamina propria, invasion of submucosa, invasion of muscularis propria, invasion through the muscularis propria into perirectal tissue, penetration to the surface of the visceral peritoneum, and direct invasion of other organs or structures, respectively (Fig. 4). Description of T stages on MRI, by directly borrowing the AJCC staging system, has several challenges and issues. First, a reliable distinction between T1 and T2 is difficult on MRI (9). Secondly, the prognosis of T3 tumors is actually quite heterogeneous according to the exact depth of tumor invasion (26). T3 with minimal extramural invasion of less than 1 mm (i.e., T3a according to the UICC sub-categorization (15)) may not have a different prognosis from T2 (26), not to mention the distinction between them on MRI is also not straightforward (27). Considering these factors, some previous guidelines for structured reporting of rectal cancer include a few specific intermediate T stages, such as T1/2, T2/early T3, and T3/possible T4, as their T stage choices in addition to the standard AJCC stages (5,6,7). The majority of our study group members (77.8%, 21 of 27) agreed that the plain AJCC T staging style is not flexible enough for reporting MRI findings in clinical practice. Although we agreed that reporting T stage is essential (92.6%, 25 of 27), we could not reach a clear agreement for any particular multiple choice T staging formats: for example, 51.9% (14 of 27) agreed on combining T1 and T2 and 25.9% (7 of 27) agreed on including T1/2, T2/early T3, and T3/possible T4. Given the small intrinsic inaccuracy of MRI T staging, some subjectivity of the MRI findings, and varied appearance of rectal cancers, indeterminate MRI interpretations that cannot fit in the standard T staging descriptions may inevitably occur at times. In such cases, it would be better to explain the indeterminate nature in the report rather than brute force assigning them to any of the fixed T stage categories.
Fig. 4. T stages.
Examples of rectal cancers of various T stages (arrows), including T1 (sagittal and axial), T2 (axial), T3 (axial), T4a (sagittal), and T4b with vaginal invasion (axial).
Some reporting guidelines give specific instructions regarding the assignment of findings to a corresponding T stage, for example, “spiculation of the perirectal fat should be reported as a T2/early T3 tumor” (5,6). Although such an approach may help reduce observer variability, more than half (51.9%, 14 of 27) of our study group members think that such an approach is not yet supported by strong evidence.
For T4 tumors, it is important to distinguish between T4a and T4b tumors because they are managed quite differently. As T4a indicates perforation of visceral peritoneum by tumor, it only applies when at least a portion of a rectal cancer involves the peritonealized rectal wall above the anterior peritoneal reflection. The peritoneal involvement was reported to be an independent risk factor for intraperitoneal tumor recurrence (28) and should thus be included when reporting rectal MRI, although its management and the role of any preoperative therapy are currently undetermined (10). Unlike T4a tumors, T4b tumors require a combined resection of the involved adjacent organs/structures for a curative-intent surgery and, therefore, the involved organs/structures should be clearly specified. A structured checklist of “T4b organs/structures” (Table 1) would help accomplish complete evaluation and reporting (100% agreement, 27 of 27). We did not include the presacral fascia, the mesorectal fascia, and the anal sphincters in our T4b checklist because the current AJCC staging system is a bit ambiguous about T staging of the direct involvement of these structures, although the TNM supplement considers an involvement of the presacral fascia as T4b and favors categorizing an involvement of the mesorectal fascia or the anal sphincter as T3 (14). Tumor involvement in (vs. a lack thereof) the mesorectal fascia and the anal sphincter is particularly important for patient management and, therefore, should be reported clearly, regardless of their classification as T3 or T4. Therefore, these structures are addressed as separate reporting items (items 9,10).
Extramural Depth of Tumor Invasion (Item 7)
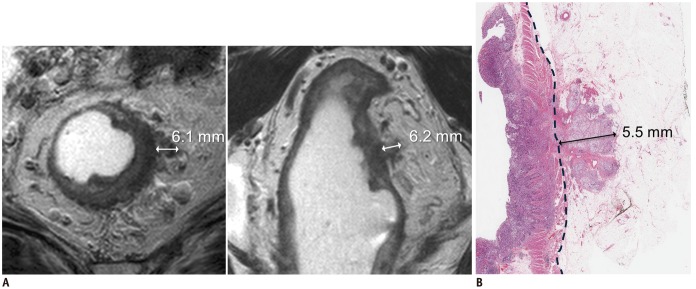
The NCCN guidelines basically consider all T3 rectal cancers (without distant metastasis) as a single group and recommend a preoperative radiation therapy with or without combined chemotherapy (11). However, as briefly mentioned earlier, the prognosis of T3 rectal cancers is actually quite heterogeneous according to the depth of extramural tumor invasion (26,29,30). Accordingly in Europe, contrary to the NCCN guidelines, the extramural depth of tumor invasion is used as a parameter to distinguish between patients who need and do not need preoperative neoadjuvant radiation therapy (10). Extramural depth of tumor invasion is measured from the outer border of the longitudinal muscle layer to the deepest part of the tumor invasion perpendicular to the rectal wall (Fig. 5) (31). When the outer border of the muscular layer is not clearly identified due to tumor replacement, a tentative line is drawn to connect the outer muscular borders at both ends of the tumor and the distance is measured from this line (31). The measurement should include all types of tumor tissues as long as they are contiguous from the primary tumor mass, including a direct extension from the main tumor, nodal spread, and extramural venous invasion (EMVI). The measurement can be quantitatively made on MRI in millimeters or in categories, i.e., higher vs. lower than a threshold value. We recommend measuring the depth in a binary manner, ≤ 5 mm vs. > 5 mm, for clinical practice (85.2% agreement, 23 of 27) considering the following factors. First, although the quantitative measurement of the absolute depth of tumor invasion may be valuable from a research viewpoint, its clinical value compared with the binary categorization has not been demonstrated yet. According to available data, the rate of local recurrence and patient survival change substantially across an extramural tumor invasion depth of 5 mm and patients who have the depth of invasion greater than 5 mm present a significantly worse prognosis than those who did not (26,29). Collapsing the quantitative measurements into the binary categories still provides enough useful information for clinical practice, despite some information loss, and makes the interpretation more straightforward and convenient. Secondly, while the quantitative measurement is shown to be free from any remarkable systematic bias compared with pathological measurements (i.e., no substantial tendency of over- or under-estimation), it can have a measurement error up to approximately ± 7–8 mm in each patient (32,33,34). The measurement errors are supposedly related to selection and angulation of the imaging plane, definition of outer muscular border and the outermost tumor boundary, and exact measurement direction rather than technical errors intrinsic to MRI. The binary categorical interpretation would likely be less sensitive to these factors compared with absolute quantitative measurements. The binary interpretation using the 5 mm threshold showed an interobserver agreement of 84–94% (0.65–0.87 in kappa) (35) and an accuracy of 78% (34). Furthermore, the 5 mm threshold matches the UICC T3 sub-categorization, i.e., T3a, T3b, T3c, and T3d for extramural invasion of < 1, 1–5, 5–15, and > 15 mm, respectively (15).
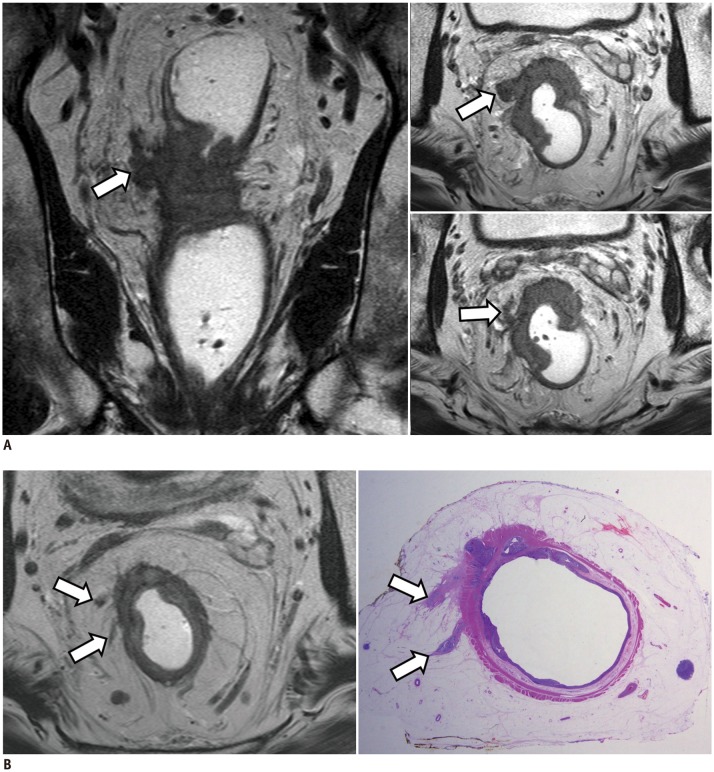
Fig. 5. Measurement of extramural depth of tumor invasion in T3 rectal cancer.
A. MRI measurements on axial (left) and coronal (right) views are 6.1 and 6.2 mm, respectively.
B. Pathologic measurement using surgical specimen is also 5.5 mm (hematoxylin and eosin stain). All measurements are coherently > 5 mm.
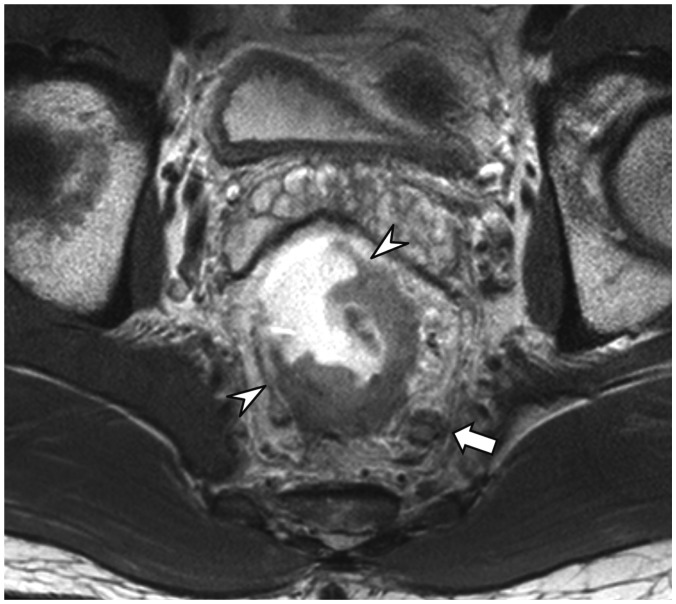
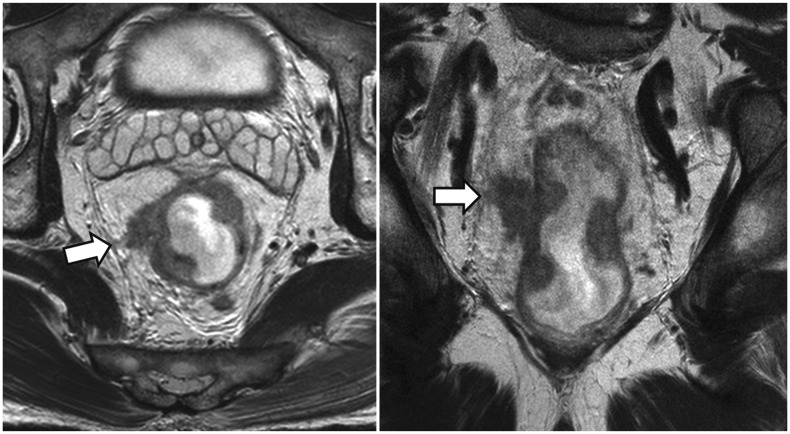
Shortest Tumor Distance from the Mesorectal Fascia or the Levator (Item 9)
The mesorectal fascia, also referred to as the fascia propria of the rectum, is identified as a thin, low-signal-intensity structure on MRI that envelops the rectum and the surrounding perirectal fat (colloquially referred to as the mesorectum, albeit anatomically incorrect). It attaches anteriorly to Denonvilliers' fascia and is separated from the presacral fascia posteriorly by the potential retrorectal space (21). The mesorectal fascia forms the boundary of the surgical excision plane during total mesorectal excision (TME) and the surgical dissection follows a plane between Denonvilliers' fascia and the mesorectal fascia, a plane between the mesorectal fascia and surrounding lateral parietal tissue, and through the retrorectal space (21).
Tumor involvement of the surgical resection margin is an apparent risk factor for postsurgical tumor recurrence and metastasis. Therefore, patients who have CRM threatened by tumor involvement are recommended to undergo preoperative neoadjuvant radiation therapy. As the mesorectal fascia is the boundary of standard TME surgery, the risk of tumor involvement of CRM is evaluated on MRI by the shortest tumor distance from the mesorectal fascia (or the levator, for low tumors close to the levator, as explained later in this section) and the distance should be reported (96.3% agreement, 26 of 27) (Fig. 6). Pathologically, tumor involvement of CRM is defined as presence of tumor within 1 mm of the surgical resection margin (36,37). Unlike the clear pathological criterion of tumor-involved CRM, there are differing opinions regarding the MRI criteria to predict CRM tumor involvement. The MERCURY study group (38), a European multidisciplinary study group, proposes ≤ 1 mm from the mesorectal fascia as the MRI criterion to determine tumor-involved CRM (39,40), which is basically similar to the pathologic definition. This criterion is based upon their prospective trial, which showed that the negative predictive value of the MRI criterion (i.e., the probability for a patient with the shortest tumor distance from the mesorectal fascia > 1 mm on MRI not to have CRM tumor involvement) was 94% (327 of 349 patients) (38). In comparison, another landmark study by a group of Dutch investigators (27) showed a result which at a glance appears to be contradictory to the MERCURY result. The Dutch study proposed that the shortest tumor distance from the mesorectal fascia should be ≥ 5 mm on MRI to opt out of neoadjuvant radiotherapy (27). Although the two threshold values, 1 mm vs. 5 mm, appear to be different, the two studies actually report similar results from different viewpoints. In the study by MERCURY study group (38), the high negative predictive value was in part due to the low prevalence of CRM-positive patients (13%) and, in fact, the “≤ 1 mm” criteria on MRI correctly identified only 59% (32 of 54) of patients who had CRM tumor involvement, i.e., 59% sensitivity. In other words, the result clearly shows that a substantial number of patients who have CRM tumor involvement would show the shortest tumor distance of > 1 mm from the mesorectal fascia on MRI. The Dutch study (27) directly analyzed the correlation between MRI and pathologic measurements of the shortest tumor distance to the CRM and found that a histological distance of at least 1 mm (i.e., lack of CRM involvement) could be confidently predicted when the distance measured on MRI was at least 5 mm. In other words, there is little chance of CRM tumor involvement if the MRI-measured distance is ≥ 5 mm. The MERCURY and the Dutch results are coherent in that both studies demonstrate that CRM tumor involvement is possible when the shortest tumor distance from the mesorectal fascia on MRI is 1–5 mm. The Dutch study should not be misinterpreted as suggesting that the shortest tumor distance from the mesorectal fascia < 5 mm is an indication of CRM tumor involvement. Many patients with the shortest tumor distance of 1–5 mm would be devoid of CRM involvement. In fact, the Dutch investigators later adopted the shortest tumor distance of < 2 mm from the mesorectal fascia on MRI as a criterion to predict CRM involvement and recommend preoperative radiation therapy, considering the widely recognized histological threshold of 1 mm and an additional 1-mm safety margin to compensate for small MRI measurement errors (41). Several studies specifically investigated the possibility of defining a line between 1 and 5 mm as the threshold to diagnose CRM involvement on MRI. According to a recent meta-analysis (42), of three different threshold values of 1, 2, and 5 mm for the shortest tumor distance from the mesorectal fascia on MRI, the highest overall accuracy for diagnosing CRM involvement, demonstrating 76% pooled sensitivity, 88% pooled specificity, and diagnostic odd ratio of 22.4, was observed for a distance of 1 mm. Another study analyzed the risk of local recurrence according to the shortest tumor distance from the mesorectal fascia on MRI and showed that, relative to the distance > 5 mm, the risk of local recurrence was not significantly different in 1–2 mm and 2–5 mm groups, but was significantly higher in ≤ 1 mm group (hazard ratio of 3.72) (39). These results collectively indicate that 1 mm on MRI might be a reasonable threshold to predict CRM tumor involvement. However, there may still be some uncertainties and reservations, as the threshold would neglect a sizeable number of patients with CRM tumor involvement, even though the incorrect categorization may probably not affect the ultimate oncologic outcome. Our voting results reflect all the issues discussed above accurately. The largest fraction of the members, still not an absolute majority (44.4%, 12 of 27), chose 1 mm as the criterion to predict tumor-involved CRM on MRI, 29.6% (8 of 27) selected 2 mm as the threshold, and 25.9% (7 of 27) believed that we could not choose a single threshold value yet.
Fig. 6. Shortest tumor distance from mesorectal fascia.
Direct tumor involvement of right lateral side of mesorectal fascia (arrows), i.e., shortest tumor distance of 0 mm, is noted on axial (left) and coronal (right) views, which predicts tumor involvement of CRM. CRM = circumferential resection margin
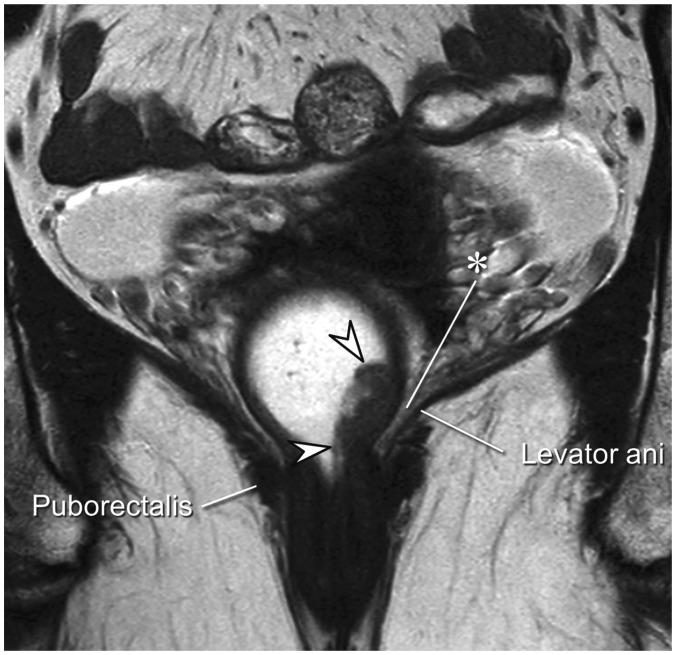
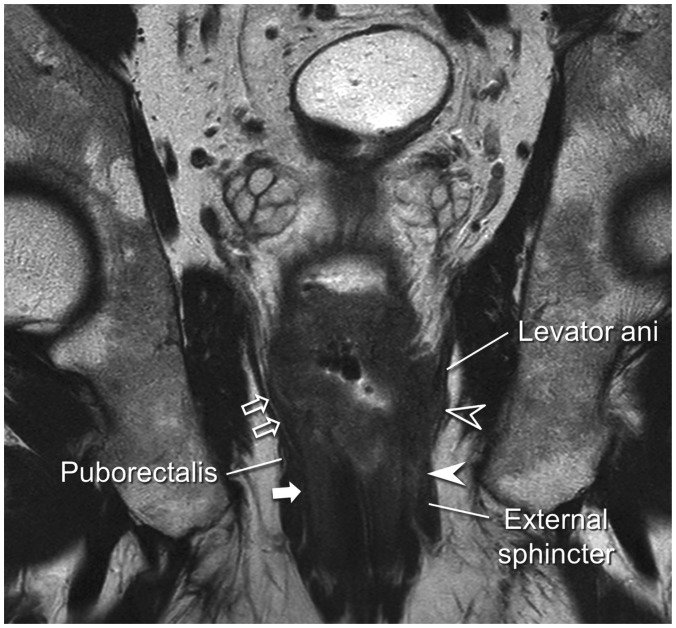
The mesorectal fascia tapers below the origin of the levator muscles and is lost in the far distal rectal area (43,44). Therefore, for low rectal tumors located close to the anus, the aforementioned principle can similarly be applied to the distance from the levator muscle in lieu of the mesorectal fascia (Figs. 7,8). The fatty tissue surrounding the far distal rectum is much thinner than the fairly thick mesorectum and the threshold distance from the tumor to the levator to diagnose CRM tumor involvement on MRI has not been thoroughly investigated. Therefore, there is some ambiguity regarding the optimal threshold. Some investigators adopted presence of the tumor within 1 mm of the levator muscle on MRI as the criterion for tumor-involved CRM (23,40,44).
Fig. 7. Far distal rectal cancer without perirectal extension.
Ulcerofungating mass (arrowheads) is noted just above anorectal junction on this coronal image. It is confined within rectal wall and perirectal fat (*) in between lesion and left levator muscle is intact.
Fig. 8. Distal rectal cancer with extension to levator ani and anal canal.
Large mass with central ulcer is noted in distal rectum on this coronal image. Mass involves both internal and external (white arrowhead) sphincters. Unlike patient's right side, where thin perirectal fatty tissue (open arrows) between far distal rectum and right levator and intersphincteric plane (white arrow) remain intact, tumor has replaced perirectal fat and has extended to levator (open arrowhead) on patient's left side.
The shortest tumor distances to the mesorectal fascia or the levator should be measured from any tumor tissue that is closest to the structures. If distinguishable, the specific nature of CRM involvement, i.e., direct extension of the primary tumor, metastatic lymph node, EMVI, or other tumor deposits, should also be described (Figs. 3,6) because, unlike direct tumor involvement, CRM involvement solely via a lymph node harboring metastatic tumor deposits is reported to be uncommon (45) and may not increase the rate of local recurrence (46).
Anal Sphincter Involvement (Item 10)
When the tumor is located in the far distal rectal area, it is important to evaluate if there is a tumor extension to the anal canal and identify the specific mural layers of the anal canal involved (88.9% agreement, 24 of 27) (Fig. 8). This information and the shortest tumor distance from the levator, as explained in the previous section, are crucial in determining the appropriate surgical procedure to ensure complete tumor removal such as intersphincteric resection and conventional or extralevator abdominoperineal resections (23,47). For example, intersphincteric resection would be inappropriate if the tumor has involved the full thickness of the internal sphincter or has extended into the intersphincteric plane because it would cause tumor involvement of the resection margin (23). Recently, the MERCURY investigators proposed a new descriptive anatomic term, “MRI low rectal plane,” in order to specifically describe the extension of a far distal rectal cancer located near the anus (48). It is a combination of the intersphincteric plane and the mesorectal fascia/levator plane (48). We did not include this new term in our recommendation because its impact/usefulness requires further scientific evaluation.
Mesorectal and Extramesorectal Lymph Node Spread (Items 11,12)
Cancer spread to pelvic lymph nodes has traditionally been regarded as a risk factor for local recurrence and distant metastasis after surgical resection of a rectal tumor. Likewise, the current NCCN guidelines recommend that patients who have a rectal cancer associated with cN1–2 stages of nodal spread (“c” prefix refers to clinical staging such as with CT or MRI, whereas “p” and “y” prefixes are given by pathologic examination of a surgical specimen and after neoadjuvant chemotherapy and/or radiation therapy, respectively) should undergo preoperative neoadjuvant radiation therapy, with or without combined chemotherapy, regardless of their T stages (11). By contrast, some recent studies question the appropriateness of such an approach. According to an analysis of published literature and a German rectal cancer registry (49), while nodal involvement was considered a predictive factor for local recurrence regardless of the number of involved nodes in the pre- TME era, after the adoption of TME as a standard surgical procedure for rectal cancer, the local recurrence risk was found to increase when there were at least four metastatic regional nodes (i.e., pN2) (49). In addition, another study by the MERCURY group (50) reported that the local recurrence rate was maintained at a very low level (3.3%) regardless of MRI cN stage as long as the MRI-predicted tumor T stage was T3b or lower (i.e., less than 5 mm of extramural depth of tumor invasion), the MRI-predicted CRM was safe, and there was no demonstrable EMVI on MRI. Furthermore, the accuracy of cN staging using MRI is low. According to meta-analyses (51,52,53), the sensitivity of MRI was only 66–77% and specificity was 71–76% for diagnosing nodal metastasis in rectal cancer. Combining all of these findings, we believe that there is evidence indicating that MRI cN staging is not as strong a prognostic factor as pN staging, especially in the TME era. We also believe that distinguishing N sub stages such as N1 vs. N2 on MRI would be neither very meaningful nor accurate. However, we are not convinced if it is acceptable to disregard MRI cN stages at all; and we essentially unanimously agree that lymphadenopathies that are suspicious of metastasis with a reasonable confidence should be reported. Locations of suspicious lymphadenopathy should distinguish the mesorectum (92.6% agreement, 25 of 27) and the extra-mesorectal area (96.3% agreement, 26 of 27) because extra-mesorectal lymph nodes require particular attention as they are not included within the boundary of standard TME surgery and the routine field of preoperative radiation therapy. When extra-mesorectal nodal metastasis is suspected, the specific locations should also be reported (Figs. 9,10).
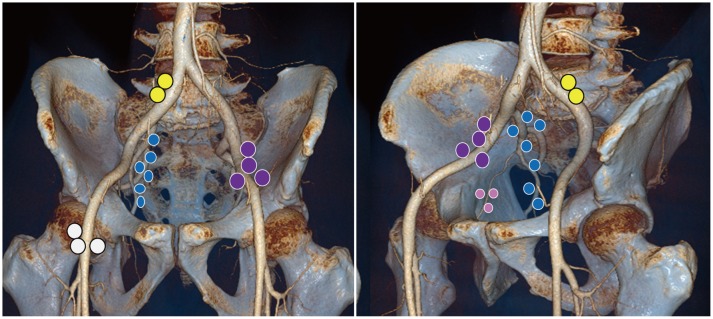
Fig. 9. Extramesorectal lymph node stations associated with rectal cancer spread.
Locations of inguinal nodes (white), external iliac nodes (purple), internal iliac nodes (blue), obturator nodes (pink), and common iliac nodes (yellow) are marked by different colors.
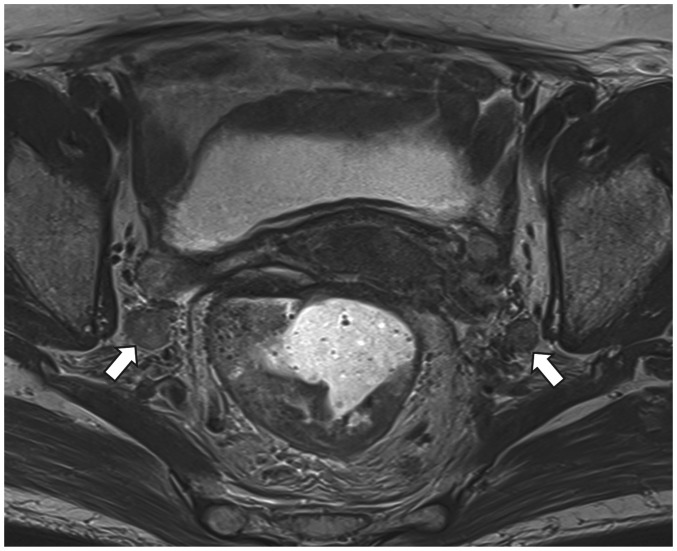
Fig. 10. Rectal cancer patient with bilateral internal iliac nodal metastasis (arrows).
It is difficult to frame criteria to diagnose metastatic lymph nodes from a rectal cancer on MRI. The use of size criteria alone is limited because metastatic lymph nodes distribute across a very wide range of node sizes and, therefore, small metastatic nodes (Fig. 11) are understaged, while large-sized reactive nodes are overstaged. Indeed, 15–17% of lymph nodes ≤ 5 mm in rectal cancer, were reported to be involved with metastatic disease (54,55), suggesting that a threshold for ruling out nodal metastasis does not exist (Fig. 11). On the other hand, large lymph nodes such as ≥ 8 mm in short-axis diameter (56), ≥ 10 mm in short-axis diameter (57) or ≥ 10 mm in maximal diameter (54) were reported to be highly specific for nodal metastasis, albeit not sensitive. The Canadian guide for reporting of rectal cancer MRI (5,6) provides specific instructions regarding lymph node size that should be reported to be suspicious of metastasis, i.e., ≥ 8 mm and ≥ 10 mm in short-axis diameter for mesorectal and extramesorectal nodes, respectively. These imaging criteria to provide high specificity seems reasonable considering somewhat subdued prognostic implications of N stages in the TME era (49,50) and low accuracy of MRI cN staging (51,52,53) as explained earlier. Nevertheless, there was a reservation among our study group members about recommending any particular size criteria and we do not propose any single size threshold to diagnose nodal metastasis on MRI in this consensus recommendation. In addition to the size, other MRI morphological features such as margin irregularity, heterogeneity of nodal texture, and shape are beneficial for differentiating benign and malignant nodes (Figs. 12,13) (54,56). Lymph nodes showing uniform and homogenous signal intensity are not considered to be suspicious of metastasis (Fig. 12), while nodes are judged suspicious if they have irregular margin or mixed signal intensity or both (Fig. 13) (54,56).
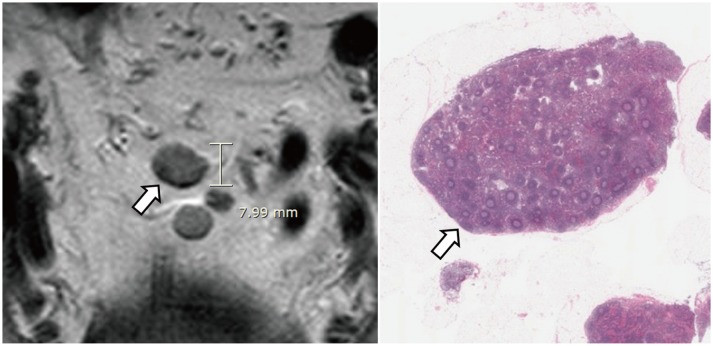
Fig. 11. Microscopic view of 1-mm lymph node (arrows) that is harboring metastatic foci (arrowheads) (hematoxylin and eosin stain, magnification × 30).
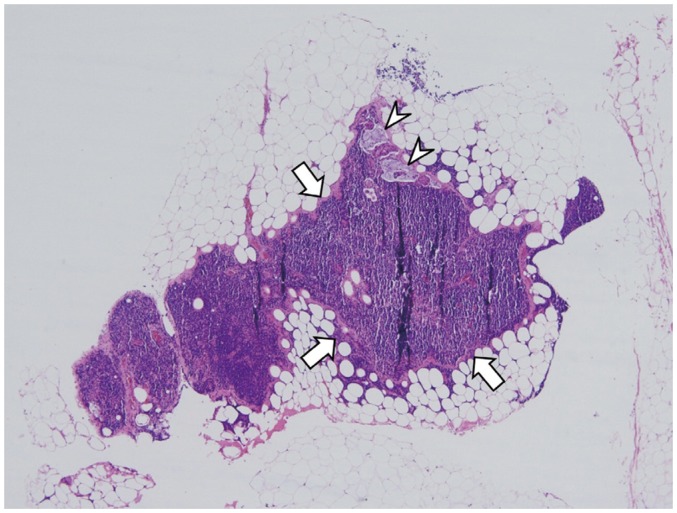
Fig. 12. 8-mm in short axis, non-metastatic, mesorectal lymph node.
Node (arrow) is not considered suspicious if it has regular border and homogeneous signal intensity on MRI (left). Lower rim of lymph node shows low signal intensity due to chemical shift artifact. Microscopic image (right) shows normal lymph node (arrow) (hematoxylin and eosin stain, magnification × 5).
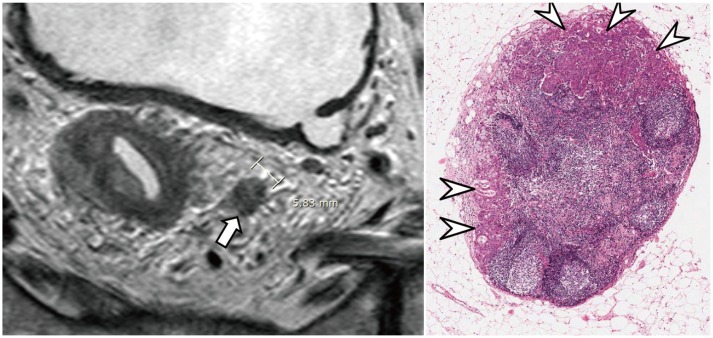
Fig. 13. 6-mm in short axis, metastatic, mesorectal lymph node.
Lymph node (arrow) with irregular border and heterogeneous signal intensity on MRI (left) suggests metastasis. Microscopic image (right) shows metastatic foci (arrowheads) in lymph node (hematoxylin and eosin stain, magnification × 30).
EMVI (Item 13)
Extramural venous invasion is a term that has evolved relatively recently, although this entity was reported in rectal cancer fairly early (58). EMVI refers to the presence of cancer cells/tissue within the veins beyond the muscularis propria of the rectal wall. EMVI is regarded as a bad prognostic factor in terms of local recurrence, distant metastasis, as well as overall patient survival. According to a meta-analysis (59), the pooled overall 5-year survival was 39.5% in patients with rectal cancer accompanied by positive EMVI at histology. Postsurgical recurrence of rectal cancer was also shown to differ according to MRI-predicted EMVI status in one study: 3-year recurrence-free survival of 35% in patients with EMVI-positive findings on MRI compared with 74% in EMVI-negative patients (60). Tumor extension into the extramural veins may provide a pathway of hematogenous dissemination of the tumor cells and would explain the reason why EMVI is a risk factor for synchronous or metachronous metastasis in patients with rectal cancer (61,62). EMVI is currently not considered for TNM staging of a rectal cancer, one reason for which is presumably the highly variable reported prevalence (9–61% (59)), which may indicate inconsistent recognition and reporting of the findings. Nonetheless, evidences are accumulating to support a therapeutic paradigm shift to neoadjuvant or adjuvant therapy for patients who have a locally advanced rectal cancer associated with EMVI (63). Therefore, we believe that describing presence vs. absence of EMVI in the report is timely and we include EMVI status as an essential reporting item (88.9% agreement, 24 of 27).
Extramural venous invasion is typically seen on MRI as intermediate tumor signal intensity within extramural vessels contiguous to the primary tumor (Fig. 14) (60,64). The contour and caliber of the involved vessels can only be slightly expanded or remarkably dilated with obvious irregular nodular margins according to the size and extent of intravascular tumor extension (Fig. 14) (60,64). MRI is reported to be highly specific for diagnosing EMVI associated with rectal cancer, but is not sensitive (61,65). One study reported a 54% sensitivity for EMVI in veins 3 mm or greater in diameter and the sensitivity was even lower when smaller vessels were also considered (61,65). Unlike MRI-detected EMVI in large vessels (≥ 3 mm in diameter), the prognostic implications of EMVI in smaller vessels (< 3 mm in diameter) seems somewhat unclear (61). Given the effect of the vessel size, an existing MRI reporting guide has included a specific mention of the involved vessel size (small, medium, and large) in addition to the presence vs. absence of EMVI (8). However, we do not recommend reporting the vessel size, considering the low sensitivity of MRI for EMVI in small vessels and a lack of enough evidence and reader expertise to ensure a consistent use of such an approach in daily clinical practice. Another uncommon challenge is the presentation of EMVI as a lesion discontinuous from the primary tumor. In such a case, there is often no good means to distinguish a discontinuous EMVI from a completely replaced metastatic lymph node or an isolated discontinuous tumor extension on MRI.
Fig. 14. Rectal cancer associated with EMVI.
A. At initial presentation. EMVI is seen on MRI (coronal image in left and two axial images at slightly different levels in right) as intermediate tumor signal intensity within extramural vessels contiguous to primary tumor (arrows). One involved vessel is markedly expanded with irregular nodular margins. B. After preoperative neoadjuvant chemoradiation therapy, EMVI has markedly decreased (arrows) on MRI (left). Whole mounted surgical specimen (right) shows residual tumor within vessels (arrows) (hematoxylin and eosin stain). EMVI = extramural venous invasion
SUMMARY
Management of rectal cancer is increasingly reliant upon MRI. A lot of evidence has been accumulated indicating that high-resolution MRI can provide multiple prognostic findings and imaging features to guide proper management of rectal cancer patients. These include longitudinal (especially in relation to the anterior peritoneal reflection) and circumferential tumor locations, the maximum extramural depth of tumor invasion and the shortest tumor distance from the mesorectal fascia or the levator in addition to the standard T staging, anatomical details of anal canal involvement, mesorectal and extramesorectal nodal spread, and EMVI. There are still regional differences regarding how these various imaging factors are specifically used to determine the patient management, especially regarding the selection of patients who need preoperative neoadjuvant radiation therapy (10,11,41,50). Quality reporting of rectal MRI using a systematic structured approach would facilitate accurate effective communication among multiple disciplines and this KSAR consensus recommendation can serve as a useful tool to help achieve a more standardized optimal care of rectal cancer patients using rectal MRI.
Full Author List (in Alphabetical Order)
References
- 1.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 4.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy ED, Milot L, Fruitman M, Al-Sukhni E, Heine G, Schmocker S, et al. Development and implementation of a synoptic MRI report for preoperative staging of rectal cancer on a population-based level. Dis Colon Rectum. 2014;57:700–708. doi: 10.1097/DCR.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 6.Al-Sukhni E, Milot L, Fruitman M, Brown G, Schmocker S, Kennedy E. User's guide for the synoptic MRI report for rectal cancer. Web site. [Accessed June 25, 2016]. https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=133269.
- 7.Al-Sukhni E, Milot L, Fruitman M, Brown G, Schmocker S, Kennedy E. MR Rectal Tumour. Web site. [Accessed June 25, 2016]. http://www.radreport.org/template/0000240.
- 8.Taylor F, Mangat N, Swift IR, Brown G. Proforma-based reporting in rectal cancer. Cancer Imaging. 2010;10 Spec no A:S142–S150. doi: 10.1102/1470-7330.2010.9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2013;23:2522–2531. doi: 10.1007/s00330-013-2864-4. [DOI] [PubMed] [Google Scholar]
- 10.Tudyka V, Blomqvist L, Beets-Tan RG, Boelens PG, Valentini V, van de Velde CJ, et al. EURECCA consensus conference highlights about colon & rectal cancer multidisciplinary management: the radiology experts review. Eur J Surg Oncol. 2014;40:469–475. doi: 10.1016/j.ejso.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer version 2. Web site. [Accessed June 25, 2016]. https://www.nccn.org/professionals/physician_gls/PDF/rectal.pdf.
- 12.van de Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer. 2014;50:1.e1–1.e34. doi: 10.1016/j.ejca.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 13.American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. Chicago, IL: Springer; 2010. [Google Scholar]
- 14.Wittekind C, Compton CC, Brierley JR, Sobin L. TNM supplement: a commentary on uniform use. 4th ed. Oxford: Wiley-Blackwell; 2012. [Google Scholar]
- 15.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th ed. Oxford, UK: Wiley-Blackwell; 2009. [Google Scholar]
- 16.Salerno G, Sinnatamby C, Branagan G, Daniels IR, Heald RJ, Moran BJ. Defining the rectum: surgically, radiologically and anatomically. Colorectal Dis. 2006;8(Suppl 3):5–9. doi: 10.1111/j.1463-1318.2006.01062.x. [DOI] [PubMed] [Google Scholar]
- 17.Schoellhammer HF, Gregorian AC, Sarkisyan GG, Petrie BA. How important is rigid proctosigmoidoscopy in localizing rectal cancer? Am J Surg. 2008;196:904–908. doi: 10.1016/j.amjsurg.2008.08.005. discussion 908. [DOI] [PubMed] [Google Scholar]
- 18.Taylor FG, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol. 2008;191:1827–1835. doi: 10.2214/AJR.08.1004. [DOI] [PubMed] [Google Scholar]
- 19.Gowdra Halappa V, Corona Villalobos CP, Bonekamp S, Gearhart SL, Efron J, Herman J, et al. Rectal imaging: part 1, high-resolution MRI of carcinoma of the rectum at 3 T. AJR Am J Roentgenol. 2012;199:W35–W42. doi: 10.2214/AJR.11.8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torkzad MR, Påhlman L, Glimelius B. Magnetic resonance imaging (MRI) in rectal cancer: a comprehensive review. Insights Imaging. 2010;1:245–267. doi: 10.1007/s13244-010-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corman ML, Bergamaschi RCM, Nicholls RJ, Fazio VW. Corman's colon and rectal surgery. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 22.Jorge JM, Wexner SD. Anatomy and physiology of the rectum and anus. Eur J Surg. 1997;163:723–731. [PubMed] [Google Scholar]
- 23.Shihab OC, Moran BJ, Heald RJ, Quirke P, Brown G. MRI staging of low rectal cancer. Eur Radiol. 2009;19:643–650. doi: 10.1007/s00330-008-1184-6. [DOI] [PubMed] [Google Scholar]
- 24.Gollub MJ, Maas M, Weiser M, Beets GL, Goodman K, Berkers L, et al. Recognition of the anterior peritoneal reflection at rectal MRI. AJR Am J Roentgenol. 2013;200:97–101. doi: 10.2214/AJR.11.7602. [DOI] [PubMed] [Google Scholar]
- 25.Chang JS, Lee Y, Lim JS, Kim NK, Baik SH, Min BS, et al. The magnetic resonance imaging-based approach for identification of high-risk patients with upper rectal cancer. Ann Surg. 2014;260:293–298. doi: 10.1097/SLA.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 26.Merkel S, Mansmann U, Siassi M, Papadopoulos T, Hohenberger W, Hermanek P. The prognostic inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis. 2001;16:298–304. doi: 10.1007/s003840100309. [DOI] [PubMed] [Google Scholar]
- 27.Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De Bruine A, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001;357:497–504. doi: 10.1016/s0140-6736(00)04040-x. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd NA, Baxter KJ, Love SB. Influence of local peritoneal involvement on pelvic recurrence and prognosis in rectal cancer. J Clin Pathol. 1995;48:849–855. doi: 10.1136/jcp.48.9.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin R, Jeong SY, Yoo HY, Park KJ, Heo SC, Kang GH, et al. Depth of mesorectal extension has prognostic significance in patients with T3 rectal cancer. Dis Colon Rectum. 2012;55:1220–1228. doi: 10.1097/DCR.0b013e31826fea6a. [DOI] [PubMed] [Google Scholar]
- 30.Willett CG, Badizadegan K, Ancukiewicz M, Shellito PC. Prognostic factors in stage T3N0 rectal cancer: do all patients require postoperative pelvic irradiation and chemotherapy? Dis Colon Rectum. 1999;42:167–173. doi: 10.1007/BF02237122. [DOI] [PubMed] [Google Scholar]
- 31.Shirouzu K, Akagi Y, Fujita S, Ueno H, Takii Y, Komori K, et al. Clinical significance of the mesorectal extension of rectal cancer: a Japanese multi-institutional study. Ann Surg. 2011;253:704–710. doi: 10.1097/SLA.0b013e3182119331. [DOI] [PubMed] [Google Scholar]
- 32.MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243:132–139. doi: 10.1148/radiol.2431051825. [DOI] [PubMed] [Google Scholar]
- 33.Park SH. Degree of error of thin-section MR in measuring extramural depth of tumor invasion in patients with rectal cancer. Radiology. 2008;246:647. doi: 10.1148/radiol.2462070843. author reply 647-648. [DOI] [PubMed] [Google Scholar]
- 34.Cho SH, Kim SH, Bae JH, Jang YJ, Kim HJ, Lee D, et al. Prognostic stratification by extramural depth of tumor invasion of primary rectal cancer based on the Radiological Society of North America proposal. AJR Am J Roentgenol. 2014;202:1238–1244. doi: 10.2214/AJR.13.11311. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen BG, Moran B, Brown G, Blomqvist L, Fenger-Grøn M, Laurberg S. Reproducibility of depth of extramural tumor spread and distance to circumferential resection margin at rectal MRI: enhancement of clinical guidelines for neoadjuvant therapy. AJR Am J Roentgenol. 2011;197:1360–1366. doi: 10.2214/AJR.11.6508. [DOI] [PubMed] [Google Scholar]
- 36.Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 37.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996–999. doi: 10.1016/s0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 38.MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, et al. One millimetre is the safe cut-off for magnetic resonance imaging prediction of surgical margin status in rectal cancer. Br J Surg. 2011;98:872–879. doi: 10.1002/bjs.7458. [DOI] [PubMed] [Google Scholar]
- 40.Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32:34–43. doi: 10.1200/JCO.2012.45.3258. [DOI] [PubMed] [Google Scholar]
- 41.Engelen SM, Maas M, Lahaye MJ, Leijtens JW, van Berlo CL, Jansen RL, et al. Modern multidisciplinary treatment of rectal cancer based on staging with magnetic resonance imaging leads to excellent local control, but distant control remains a challenge. Eur J Cancer. 2013;49:2311–2320. doi: 10.1016/j.ejca.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Xie H, Zhou X, Zhuo Z, Che S, Xie L, Fu W. Effectiveness of MRI for the assessment of mesorectal fascia involvement in patients with rectal cancer: a systematic review and meta-analysis. Dig Surg. 2014;31:123–134. doi: 10.1159/000363075. [DOI] [PubMed] [Google Scholar]
- 43.Shihab OC, Heald RJ, Rullier E, Brown G, Holm T, Quirke P, et al. Defining the surgical planes on MRI improves surgery for cancer of the low rectum. Lancet Oncol. 2009;10:1207–1211. doi: 10.1016/S1470-2045(09)70084-1. [DOI] [PubMed] [Google Scholar]
- 44.Salerno GV, Daniels IR, Moran BJ, Heald RJ, Thomas K, Brown G. Magnetic resonance imaging prediction of an involved surgical resection margin in low rectal cancer. Dis Colon Rectum. 2009;52:632–639. doi: 10.1007/DCR.0b013e3181a0a37e. [DOI] [PubMed] [Google Scholar]
- 45.Shihab OC, Quirke P, Heald RJ, Moran BJ, Brown G. Magnetic resonance imaging-detected lymph nodes close to the mesorectal fascia are rarely a cause of margin involvement after total mesorectal excision. Br J Surg. 2010;97:1431–1436. doi: 10.1002/bjs.7116. [DOI] [PubMed] [Google Scholar]
- 46.Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH Pathology Review Committee; Cooperative Clinical Investigators. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26:350–357. doi: 10.1097/00000478-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 47.van Loenhout R, Zijta F, Lahaye M, Beets-Tan R, Smithuis R. Radiology assistant: rectal cancer-MR staging 2.0. Web site. 2016. [Accessed June 25]. http://www.radiologyassistant.nl/en/p56195b237699d/rectal-cancer-mr-staging-20.html.
- 48.Battersby NJ, How P, Moran B, Stelzner S, West NP, Branagan G, et al. Prospective validation of a low rectal cancer magnetic resonance imaging staging system and development of a local recurrence risk stratification model: the mercury II study. Ann Surg. 2016;263:751–760. doi: 10.1097/SLA.0000000000001193. [DOI] [PubMed] [Google Scholar]
- 49.Hermanek P, Merkel S, Fietkau R, Rödel C, Hohenberger W. Regional lymph node metastasis and locoregional recurrence of rectal carcinoma in the era of TME [corrected] surgery. Implications for treatment decisions. Int J Colorectal Dis. 2010;25:359–368. doi: 10.1007/s00384-009-0864-2. [DOI] [PubMed] [Google Scholar]
- 50.Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711–719. doi: 10.1097/SLA.0b013e31820b8d52. [DOI] [PubMed] [Google Scholar]
- 51.Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773–783. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 52.Lahaye MJ, Engelen SM, Nelemans PJ, Beets GL, van de Velde CJ, van Engelshoven JM, et al. Imaging for predicting the risk factors--the circumferential resection margin and nodal disease--of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR. 2005;26:259–268. doi: 10.1053/j.sult.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, Schmocker S, et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:2212–2223. doi: 10.1245/s10434-011-2210-5. [DOI] [PubMed] [Google Scholar]
- 54.Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227:371–377. doi: 10.1148/radiol.2272011747. [DOI] [PubMed] [Google Scholar]
- 55.Wang C, Zhou Z, Wang Z, Zheng Y, Zhao G, Yu Y, et al. Patterns of neoplastic foci and lymph node micrometastasis within the mesorectum. Langenbecks Arch Surg. 2005;390:312–318. doi: 10.1007/s00423-005-0562-7. [DOI] [PubMed] [Google Scholar]
- 56.Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol. 2004;52:78–83. doi: 10.1016/j.ejrad.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Ogawa S, Hida J, Ike H, Kinugasa T, Ota M, Shinto E, et al. Selection of lymph node-positive cases based on perirectal and lateral pelvic lymph nodes using magnetic resonance imaging: study of the japanese society for cancer of the colon and rectum. Ann Surg Oncol. 2016;23:1187–1194. doi: 10.1245/s10434-015-5021-2. [DOI] [PubMed] [Google Scholar]
- 58.Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980;67:439–442. doi: 10.1002/bjs.1800670619. [DOI] [PubMed] [Google Scholar]
- 59.Chand M, Siddiqui MR, Swift I, Brown G. Systematic review of prognostic importance of extramural venous invasion in rectal cancer. World J Gastroenterol. 2016;22:1721–1726. doi: 10.3748/wjg.v22.i4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229–236. doi: 10.1002/bjs.5917. [DOI] [PubMed] [Google Scholar]
- 61.Sohn B, Lim JS, Kim H, Myoung S, Choi J, Kim NK, et al. MRI-detected extramural vascular invasion is an independent prognostic factor for synchronous metastasis in patients with rectal cancer. Eur Radiol. 2015;25:1347–1355. doi: 10.1007/s00330-014-3527-9. [DOI] [PubMed] [Google Scholar]
- 62.Bugg WG, Andreou AK, Biswas D, Toms AP, Williams SM. The prognostic significance of MRI-detected extramural venous invasion in rectal carcinoma. Clin Radiol. 2014;69:619–623. doi: 10.1016/j.crad.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 63.Chand M, Bhangu A, Wotherspoon A, Stamp GW, Swift RI, Chau I, et al. EMVI-positive stage II rectal cancer has similar clinical outcomes as stage III disease following pre-operative chemoradiotherapy. Ann Oncol. 2014;25:858–863. doi: 10.1093/annonc/mdu029. [DOI] [PubMed] [Google Scholar]
- 64.Smith NJ, Shihab O, Arnaout A, Swift RI, Brown G. MRI for detection of extramural vascular invasion in rectal cancer. AJR Am J Roentgenol. 2008;191:1517–1522. doi: 10.2214/AJR.08.1298. [DOI] [PubMed] [Google Scholar]
- 65.Jhaveri KS, Hosseini-Nik H, Thipphavong S, Assarzadegan N, Menezes RJ, Kennedy ED, et al. MRI detection of extramural venous invasion in rectal cancer: correlation with histopathology using elastin stain. AJR Am J Roentgenol. 2016;206:747–755. doi: 10.2214/AJR.15.15568. [DOI] [PubMed] [Google Scholar]