Abstract
Almost 30% of hip fractures occur in men; the mortality, morbidity, and loss of independence after hip fractures are greater in men than in women. To comprehensively evaluate risk factors for hip fracture in older men, we performed a prospective study of 5994 men, primarily white, age 65+ years recruited at six US clinical centers. During a mean of 8.6 years of 97% complete follow-up, 178 men experienced incident hip fractures. Information on risk factors including femoral neck bone mineral density (FNBMD) was obtained at the baseline visit. Cox proportional hazards models were used to calculate the hazard ratio (HR) with 95% confidence intervals; Fine and Gray models adjusted for competing mortality risk. Older age (≥75 years), low FNBMD, currently smoking, greater height and height loss since age 25 years, history of fracture, use of tricyclic antidepressants, history of myocardial infarction or angina, hyperthyroidism or Parkinson’s disease, lower protein intake, and lower executive function were all associated with an increased hip fracture risk. Further adjustment for competing mortality attenuated HR for smoking, hyperthyroidism, and Parkinson’s disease. The incidence rate of hip fracture per 1000 person-years (PY) was greatest in men with FNBMD T-scores <−2.5 (white women reference database) who also had 4+ risk factors, 33.4. Men age ≥80 years with 3+ major comorbidities experienced hip fracture at rates of 14.52 versus 0.88 per 1000 PY in men age <70 years with zero comorbidities. Older men with low FNBMD, multiple risk factors, and multimorbidity have a high risk of hip fracture. Many of these assessments can easily be incorporated into routine clinical practice and may lead to improved risk stratification.
Introduction
Hip fractures are the most devastating consequence of osteoporosis.(1) Almost 30% of all hip fractures occur in men(2) and the morbidity, mortality, and loss of independence after a hip fracture are greater in men than women.(3,4) Although hip fracture rates declined in US women (2000 to 2009), rates were stable in US men.(4) Given the significant increase in life expectancy in men and current demographic trends, there will be an increasing number of hip fractures in men. Many potential risk factors for hip fractures have been identified, but most previous studies have focused on women,(5) the association with bone mineral density (BMD),(6–8) a single set of risk factors,(9–11) or relied on a case-control design.(12–14) The Dubbo Osteoporosis Epidemiology Study showed that BMD, postural instability and/or quadriceps weakness, a history of falls, and prior fracture were risk factors for hip fracture in men.(15) For the most part, the number of hip fractures in these studies was small (20 to 77),(6,8–11,15) except for large database studies that focused on a single risk factor and did not evaluate important covariates, particularly, BMD.(16–20)
The goal of this report is to expand knowledge of important risk factors for hip fracture in men. This information is important to improve our understanding of the pathophysiology of fracture and contribute to improvements in prediction of risk and prevention of fractures in older men. We recruited a large cohort of older men, measured many potential risk factors, including BMD, and followed the men for incident hip fractures up to 10 years.
Materials and Methods
Participants
The Osteoporotic Fractures in Men Study (MrOS) is a multicenter prospective study of risk factors for fractures. The design, measures, and recruitment have been previously described.(21,22) One of the primary specific aims of MrOS was to identify risk factors for hip fracture in older men. In brief, 5994 men aged >65 years were recruited from 2000 to 2002 in Birmingham, AL; Minneapolis, MN; the Monongahela Valley near Pittsburgh, PA; Palo Alto, CA; Portland, OR; and San Diego, CA. Approval of the conduct of MrOS was obtained from the institutional review boards of the participating institutions, and written informed consent was obtained from all participants before data collection. Men were excluded from the study if they could not walk without the assistance of another or had bilateral hip replacements. Of the 5994 men enrolled, we excluded 118 men who reported taking osteoporosis medications at baseline. This analysis includes 5876 men (98.0%) who were followed for an average of 8.6 years (up to March 2012).
Risk factors
Information on demographic, lifestyle, personal and family medical history, functional status, anthropometric, and cognitive, visual, and neuromuscular function data were obtained at the baseline visit by self-report, interview, or examination.(21) General health status was self-rated as excellent/good versus fair/poor/very poor. We defined comorbidity as the sum of self-report of 10 common chronic conditions. Functional status was assessed by summing the amount of difficulty (on a 0 to 3 scale) with five instrumental activities of daily living (difficulty walking two to three blocks, climbing 10 steps, preparing meals, heavy housework, and shopping). Modified physical and mental health summary scales from the 12-item Medical Outcomes Study Short Form 12 (SF-12)(23) were also reported.
Lifestyle factors included alcohol consumption (average number of drinks per week), smoking, and dietary intake. The Block 98 semiquantitative food-frequency questionnaire (Block Dietary Data Systems, Berkeley, CA, USA) was administered.(24,25) Diet quality was calculated using the validated Quality Index Revised (DQI-R).(26) Physical activity was assessed with the Physical Activity Scale for the Elderly(27) (PASE) and additional questions on daily walking for exercise and sedentary activity (sometimes/often sit >4 h/d). Participants were asked to bring all current (any use within the past 30 days) prescription medications with them to the clinic. All prescription medications were recorded in an electronic medication inventory database and matched to its ingredients(s) based on the Iowa Drug information Service drug vocabulary (College Pharmacy, University of Iowa, Iowa City, IA, USA).(28)
We measured body weight (kg) and height (cm). Radial pulse was palpated for 30 seconds after a 5-minute rest. The Modified Minimental State (3MS) examination was used to assess global cognitive function (scored 0 to 100)(29) and the Trail-making Test B, a test of executive function; higher scores mean lower executive function. BMD of the femoral neck (FN) was measured by DXA (model QDR 4500; Hologic, Waltham, MA, USA) with extensive quality-assurance protocols used throughout.(30) T-scores were calculated using the National Health and Nutrition Examination Survey (NHANES) female reference database.(31) Tests of neuromuscular function included the ability and time required to complete five chair stands and gait speed at usual pace over a 6-m course.(9)
Hip fractures
Men were followed for incident hip fracture with triannual questionnaires administered by mail or telephone; >97% complete follow-up. Hip fractures were verified by physician adjudication of medical records. Pathologic fractures were excluded. Because exclusion of fractures resulting from excess trauma underestimates the contribution of osteoporosis to fractures,(32) we included fractures regardless of trauma level.
Statistical analysis
All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). We compared the baseline characteristics of men by incident hip fracture status using chi-square tests, t tests, Fisher’s exact test, and Wilcoxon rank sum tests, as appropriate.
Cox proportional hazards analysis was used to estimate the hazard ratio (HR) and 95% confidence intervals (CI). Our model-building strategy followed the guidelines of Shtatland and colleagues.(33) To develop a parsimonious, multivariable (MV) prediction model from a large number of variables, we generated a list of potential risk factors based on the literature. We first tested for association with hip fractures in age-, race-, and site-adjusted models (base models). We then adjusted for FNBMD to test if the association could be explained by BMD. Among the variables associated with fracture in the univariate analyses, some were highly correlated with each other, eg, body weight and body mass index (BMI). We selected those that performed best in a stepwise regression models and the variable with the least missing data. The Akaike Information Criteria (AIC) was used to determine the quality of the models. Models with the lowest AIC are shown as the final model. Potential risk factors from base plus FNBMD models (associations of p <0.10) were entered into stepwise proportional hazards models (using p <0.10 to enter and p <0.20 to stop) to identify risk factors associated with incident hip fractures. Finally, Fine and Gray competing risk regression models were carried out using the same risk factors to account for competing risk of mortality.(34) For continuous variables, we calculated the HR per one standard deviation increase or decrease, depending on the hypothesized direction of an effect. Men were censored at the time of event (first hip fracture), death, or end of the follow-up period.
We next created a score for each participant based on his number of independent clinical risk factors. We examined the incidence of hip fracture according to the number of risk factors within strata of the World Health Organization’s classification of FN T-scores: T-score <−2.5; T-score −2.5 to −1.0; T-score >1.0. Finally, we calculated the incidence of hip fracture by age and number of comorbidities.
Results
Over the 10-year follow-up period, 178 (3%) men experienced an incident hip fracture, age-adjusted incidence rate, 3.5 per 1000 person-years (PY). Men who sustained a hip fracture differed from men who did not on a number of characteristics (Table 1). Men with a hip fracture were older, less likely to be married, had a lower body weight and BMD, and experienced greater height loss since age 25 years. They were also more likely to report smoking, a previous fracture, falls, hyperthyroid disease, stroke, Parkinson’s disease, myocardial infarction, and cataracts. Several medications were more commonly reported among men with a hip fracture. Physical performance, physical activity, and cognitive function tended to be lower at baseline in men who experienced a hip fracture.
Table 1.
Descriptive Baseline Characteristics of Men by Incident Hip Fracture Status
| Variable | No hip fracture (n =5698) | Hip fracture (n =178) | p Value |
|---|---|---|---|
| Demographics | |||
| Age at enrollment (years) | 73.48 ± 5.81 | 77.81 ± 6.08 | |
| Age ≥75 years | 2318 (40.68) | 131 (73.6) | <0.001 |
| Education | |||
| ≥High school | 4337 (76.11) | 129 (72.47) | 0.26 |
| Health status (good/excellent) | 4903 (86.08) | 149 (83.71) | 0.37 |
| Height (cm) | 174.22 ± 6.8 | 173.43 ± 6.33 | 0.13 |
| Height change since age 25 years (cm) | 3.64 ± 2.94 | 5.09 ± 3.09 | <0.001 |
| Weight (kg) | 83.4 ± 13.3 | 79.25 ± 11.99 | <0.001 |
| Weight change since age 25 years (kg) | 10.51 ± 11.36 | 7.59 ± 11.14 | 0.001 |
| Radial pulse average (beats/min) | 64.39 ± 9.88 | 64.59 ± 10.33 | 0.83 |
| ≥80 beats/min | 405 (7.13) | 18 (10.11) | 0.13 |
| Femoral neck BMD (g/cm2) | 0.79 ± 0.13 | 0.67 ± 0.11 | <0.001 |
| Fracture/fall history | |||
| Any fracture after age 50 years | 1332 (23.42) | 73 (41.01) | <0.001 |
| Fall 1 time in past 12 months | 672 (11.79) | 29 (16.29) | 0.012 |
| Fall 2+ times previous 12 months | 505 (8.86) | 24 (13.48) | 0.012 |
| Hip fracture after age 50 years | 60 (1.05) | 8 (4.49) | <0.001 |
| Maternal history of any fracture | 1251 (21.96) | 39 (21.91) | 0.99 |
| No falls in past 12 months | 4521 (79.34) | 125 (70.22) | 0.012 |
| Paternal history of any fracture | 860 (15.09) | 29 (16.29) | 0.66 |
| Medical history | |||
| Cataracts | 1939 (34.03) | 81 (45.5) | 0.002 |
| Chronic obstructive pulmonary | 594 (10.42) | 24 (13.48) | 0.19 |
| Comorbidity indexa | 0.83 ± 1.04 | 1.2 ± 1.13 | <0.001 |
| Congestive heart failure | 298 (5.23) | 14 (7.87) | 0.12 |
| Diabetes | 839 (15.79) | 24 (14.37) | 0.62 |
| Hypertension | 2453 (43.05) | 88 (49.44) | 0.09 |
| Hyperthyroid disease | 89 (1.56) | 7 (3.93) | 0.01 |
| Hypothyroid disease | 387 (6.79) | 17 (9.55) | 0.15 |
| Myocardial infarction or angina | 778 (13.65) | 42 (23.6) | <0.001 |
| Parkinson’s disease | 43 (0.75) | 5 (2.81) | 0.003 |
| Rheumatoid arthritis | 289 (5.07) | 13 (7.3) | 0.18 |
| Stroke | 311 (5.46) | 24 (13.48) | <0.001 |
| Medications | |||
| Alpha adrenergic blocker | 864 (15.81) | 39 (22.81) | 0.01 |
| Anticonvulsants | 121 (2.21) | 5 (2.92) | 0.54 |
| Benzodiazepine (long acting) | 76 (1.39) | 4 (2.34) | 0.30 |
| Benzodiazepine (short acting) | 113 (2.07) | 4 (2.34) | 0.80 |
| Beta blockers, non-opthamalic | 1002 (18.33) | 38 (22.22) | 0.20 |
| Corticosteroid (oral or inhaled) | 458 (8.38) | 16 (9.36) | 0.65 |
| Hypoglycemic agent | 452 (8.27) | 15 (8.77) | 0.81 |
| Loop diuretics | 277 (5.07) | 14 (8.19) | 0.07 |
| Nitrates | 227 (4.15) | 13 (7.6) | 0.03 |
| SSRI | 150 (2.74) | 6 (3.51) | 0.55 |
| Thiazide diuretics | 695 (12.71) | 22 (12.87) | 0.95 |
| Thyroid medications | 361 (6.6) | 16 (9.36) | 0.15 |
| Tricyclic antidepressants | 99 (1.81) | 7 (4.09) | 0.03 |
| Physical performance | |||
| PASE score | 147.58 ± 68.16 | 131.89 ± 67.45 | 0.003 |
| Seconds to complete 5 stands | 11.03 ± 3.25 | 12.39 ± 3.98 | <0.001 |
| Sitting (>4 hours) | 1647 (28.9) | 61 (34.27) | 0.12 |
| Take walks for exercise daily? | 2835 (49.75) | 79 (44.38) | 0.16 |
| Chair stands (Highest quartile or unable) | 1495 (26.3) | 78 (44.1) | <0.001 |
| Walking speed ≤ than 0.8 m/s | 185 (3.25) | 22 (12.5) | <0.001 |
| Cigarette smoking status | |||
| No | 2125 (37.3) | 81 (45.51) | 0.005 |
| Past | 3379 (59.03) | 86 (48.3) | 0.005 |
| Current | 193 (3.39) | 11 (6.18) | 0.005 |
| Alcohol (number of drinks/wk) | |||
| Average | 4.35 ± 6.88 | 3.03 ± 5.29 | <0.001 |
| Non use | 1985 (34.89) | 86 (48.31) | 0.001 |
| <14 | 3030 (53.25) | 77 (43.26) | 0.001 |
| ≥14 | 675 (11.86) | 15 (8.43) | 0.001 |
| IADL (no. of difficulties) | 0.52 ± 1.43 | 1.04 ± 2.03 | <0.001 |
| SF-12 Modified Mental Summary Scale | 55.63 ± 6.87 | 54.17 ± 9.45 | 0.29 |
| SF-12 Modified Physical Summary Scale | 49.01 ± 10.14 | 46.78 ± 11.79 | 0.05 |
| Cognitive function | |||
| Teng 3MS (0 to 100) | 93.3 ± 5.94 | 92.39 ± 5.18 | 0.002 |
| Trails B: total time (0 to 300 seconds) | 133.51 ± 58.33 | 162.43 ± 68.21 | <0.001 |
| Visual acuity 20/40 or worse (calc vear) | 273 (4.8) | 13 (7.43) | 0.113 |
| Diet | |||
| Caffeine (daily intake, mg) | 215.18 ± 233.34 | 195.7 ± 226.34 | 0.62 |
| Calcium (dietary and supplemental, mg) | 1130 ± 585.59 | 1080.74 ± 590.07 | 0.28 |
| Diet Quality Score | 62.71 ± 12.99 | 61.53 ± 13.72 | 0.24 |
| Protein (percent kcal) | 16.13 ± 2.91 | 15.3 ± 2.55 | <0.001 |
| Vitamin D (dietary and supplemental, IU) | 389.84 ± 244.62 | 356.5 ± 236.72 | 0.07 |
SSRI =selective serotonin reuptake inhibitors; PASE =Physical Activity Scale for the Elderly; IADL =instrumental activities of daily living.
Summary of the number of self-report of the following conditions: stroke, myocardial infarction, angina, chronic obstructive pulmonary disease, congestive heart failure, Parkinson’s disease, osteoarthritis, diabetes, thyroid disease, and osteoporosis.
Mean ±standard deviation or n (%).
Multivariable models
In models adjusting for age, race, site, and FNBMD, we identified 32 variables (p <0.10) for entry into the MV model. The multivariable model with the minimum AIC included all the variables listed in Table 2. Older age (≥75 years), lower FNBMD, currently smoking, protein %, fractre after age 50 greater height and height loss since age 25 years, use of tricyclic antidepressants (TCAs), self-reported history of physician diagnosed myocardial infarction or angina, Parkinson’s disease and hyperthyroidism, and poorer executive function were all related to an increased risk of hip fracture (MV model) (Table 2). Each standard deviation increase in total energy from protein was related to an 18% lower risk of hip fracture. Men with a history of previous fracture had a 48% increased risk of hip fracture.
Table 2.
Hazard Ratio (95% CI) of Hip Fracture: Base Model (Age, Race, Clinic), Base Model +Femoral Neck Bone Mineral Density (FNBMD), Multivariate Model (MV), and Competing Risk MV Model
| Variable | Referent/unit for HRa | Base | Base +FNBMD | MV model HR (95% CI) | MV model (competing risk) |
|---|---|---|---|---|---|
| Age (≥75 years) | <75 years | 4.56 (3.25, 6.39) | 3.20 (2.27, 4.50) | 2.30 (1.57, 3.36) | 2.01 (1.35, 2.99) |
| Femoral neck BMD | −0.128 g/cm2 | — | 3.26 (2.70, 3.93) | 3.04 (2.47, 3.73) | 3.01 (2.40, 3.76) |
| Chair stands (highest quartile or unable) | Quartiles 1, 2, 3 | 2.06 (1.52, 2.79) | 1.87 (1.38, 2.54) | 1.31 (0.94, 1.82) | 1.17 (0.84, 1.65) |
| Current smoker | No | 3.09 (1.66, 5.76) | 2.27 (1.22, 4.25) | 2.05 (1.05, 3.98) | 1.78 (0.91, 3.48) |
| Protein (% total energy) | 2.9% | 0.76 (0.64, 0.89) | 0.82 (0.70, 0.97) | 0.81 (0.69, 0.96) | 0.82 (0.69, 0.97) |
| Any fracture (≥50 years) | No | 2.15 (1.59, 2.90) | 1.59 (1.17, 2.16) | 1.48 (1.07, 2.04) | 1.41 (1.02, 1.95) |
| Height (average) | 6.89 cm | 0.98 (0.84, 1.14) | 1.12 (0.96, 1.30) | 1.24 (1.05, 1.47) | 1.24 (1.05, 1.45) |
| Height loss (since age 25 years) | −2.95 cm | 1.38 (1.22, 1.55) | 1.34 (1.17,1.53) | 1.34 (1.16, 1.56) | 1.26 (1.09, 1.46) |
| Medications | |||||
| Hypoglycemic use | No | 1.20 (0.71, 2.06) | 1.69 (0.99, 2.88) | 1.59 (0.92, 2.74) | 1.31 (0.73, 2.33) |
| Long-acting benzodiazepine | No | 2.03 (0.75, 5.50) | 2.96 (1.09, 8.06) | 2.50 (0.90, 6.98) | 2.60 (1.00, 6.76) |
| Tricyclic antidepressant | No | 2.56 (1.20, 5.48) | 2.93 (1.37, 6.27) | 2.77 (1.27, 6.04) | 2.29 (1.04, 5.08) |
| Medical history | |||||
| Hyperthyroidism | No | 2.83 (1.33, 6.02) | 2.86 (1.34, 6.10)) | 2.86 (1.32, 6.20) | 2.27 (0.93, 5.52) |
| Parkinson’s disease | No | 4.95 (2.03, 12.06) | 3.77 (1.55, 9.21) | 3.32 (1.21, 9.07) | 2.29 (0.81, 6.52) |
| Stroke | No | 2.41 (1.56, 3.73) | 1.97 (1.26, 3.06) | 1.56 (0.96, 2.56) | 1.40 (0.82, 2.38) |
| Myocardial infarction or Angina | No | 1.71 (1.25, 2.33) | 1.90 (1.38, 2.60) | 1.59 (1.13, 2.23) | 1.45 (1.02, 2.08) |
| Executive function (Trails B/s) | 58.8 seconds | 1.46 (1.24, 1.68) | 1.41 (1.23, 1.62) | 1.33 (1.15, 1.53) | 1.21 (1.04, 1.41) |
Unit for HR for continuous variable: 1 SD.
Bold =statistically significant.
Current smoking was associated with a twofold increased risk of hip fracture. Both a history of stroke and chair stands were associated with about a twofold increased risk of hip fracture in minimally adjusted models, but this was attenuated in the MV models. We found no association between use of hypoglycemic medications to hip fracture.
Competing risks of mortality models
Consideration of the competing risk of mortality did not substantially alter most of the associations. However, the association between smoking, hyperthyroidism, and Parkinson’s disease was attenuated (Table 2). In contrast, there was a 2.6-fold increased risk of hip fracture in men reporting current use of long-acting benzodiazepines in these competing mortality risk models.
Number of risk factors, BMD, and prediction of hip fracture
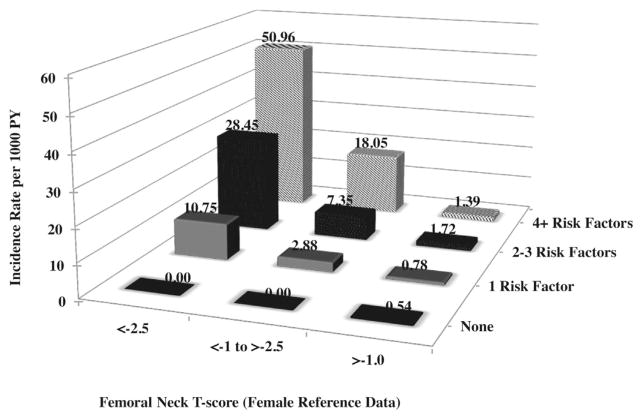
Overall, the incidence of hip fracture increased with increasing number of risk factors and decreasing BMD. A total of 128 (2.18%) men had BMD T-scores <−2.5. Among these men, the incidence of hip fracture increased with increasing numbers of risk factors (Fig. 1). For example, the incidence of hip fracture (per 1000 PY) was 33.4 (95% CI −32.1–98.8) among osteoporotic men with 4 or more risk factors compared with 4.09 (95% CI −4.7–14.5) among men with T-scores ≥ −1 and 4 or more risk factors. Similarly, among the 1923 (35.3%) men with T-scores of −1.0 to −2.5, the incidence of hip fracture (per 1000 PY) increased from 1.41 (95% CI 0.4–2.5) among men with 0 risk factors to 27.7 (95% CI 0.6–54.8) among men with 4 or more.
Fig. 1.
Incidence (per 1000 person-years) of hip fracture by femoral neck T-score and number of risk factors. Risk factors include age ≥75 years, being in the lowest quartile of % kcal from protein, any fracture after age 50 years, being divorced, using tricyclic antidepressants, using any hypoglycemic agent, highest quartile of height at age 25 years, hyperthyroidism, Parkinson’s disease, highest quartile or unable to complete chair stands, lowest quartile of Trails B, and current smoking.
Risk factors not significantly associated with the risk of hip fracture
Several risk factors were related to hip fractures in minimally adjusted models but not in multivariable analyses (Table 3, footnote c). These risk factors included history of previous hip fracture, congestive heart failure, diabetes, hypertension, falls, use of alpha adrenergic blockers, beta blockers, loop diuretics or nitrates, number of instrumental activities of daily living, sitting >4 hours, body weight, total vitamin D intake, and SF-12 mental score.
Table 3.
Risk Factors Not Significantly Associated With Hip Fractures
| Variable | Referent/unita | Age, race, clinic HR (95% CI) | Age, race, clinic, BMD HR (95% CI) |
|---|---|---|---|
| Demographics | |||
| High school education | ≤High school | 0.97 (0.68, 1.40) | 1.10 (0.76, 1.59) |
| Self-reported health status | Fair/poor | 0.70 (0.47, 1.04) | 0.80 (0.54, 1.20) |
| Fracture/fall history | |||
| History of previous hip fractureb | No | 3.12 (1.53, 6.38) | 1.87 (0.90, 3.86) |
| Maternal history of any fracture | No | 1.11 (0.77, 1.58) | 1.01 (0.71, 1.45) |
| Maternal history of hip fracture | No | 1.04 (0.63, 1.71) | 0.93 (0.56, 1.53) |
| Paternal history of hip fracture | No | 0.71 (0.26, 1.92) | 0.51 (0.19, 1.38) |
| Parental history of any fracture | No | 1.06 (0.77, 1.45) | 0.97 (0.71, 1.33) |
| Paternal history of any fracture | No | 1.06 (0.71, 1.57) | 1.00 (0.67, 1.48) |
| Spine fracture after age 50 years | No | 1.86 (0.87, 3.99) | 1.37 (0.63, 2.95) |
| Wrist fracture after age 50 years | No | 1.79 (1.03, 3.09) | 1.26 (0.73, 2.18) |
| Parental history of hip fracture | No | 0.93 (0.58, 1.48) | 0.77 (0.48, 1.24) |
| Medical history | |||
| Cataracts | No | 1.08 (0.79, 1.46) | 1.03 (0.75, 1.40) |
| Chronic obstructive pulmonary disease | No | 1.31 (0.85, 2.02) | 1.17 (0.76, 1.80) |
| Congestive heart failurec | No | 1.63 (0.94, 2.82) | 1.96 (1.10, 3.29) |
| Diabetesc | No | 1.10 (0.67, 1.80) | 1.50 (0.92, 2.45) |
| Gastrectomy | No | 1.12 (0.67, 1.88) | 1.08 (0.64, 1.81) |
| Hypertensionc | No | 1.30 (0.97, 1.75) | 1.48 (1.10, 1.99) |
| Hypothyroidism | No | 1.34 (0.81, 2.21) | 1.43 (0.86, 2.36) |
| Kidney stones | No | 1.18 (0.79, 1.77) | 1.15 (0.77, 1.72) |
| Rheumatoid arthritis | No | 1.39 (0.79, 2.45) | 1.48 (0.84, 2.62) |
| Falls in past 12 monthsc | |||
| 1 fall | 0 falls | 1.32 (0.88, 1.98) | 1.44 (0.96, 2.16) |
| >2 falls | 0 falls | 1.57 (1.01, 2.43) | 1.76 (1.13, 2.73) |
| Medications | |||
| Alpha adrenergic blockerc | No | 1.46 (1.02, 2.09) | 1.56 (1.09, 2.24) |
| Anticonvulsants | No | 1.19 (0.49, 2.92) | 1.10 (0.45, 2.68) |
| Benzodiazepine (short acting) | No | 1.03 (0.38, 2.80) | 0.73 (0.27, 1.98) |
| Beta blockerc | No | 1.14 (0.79, 1.63) | 1.31 (0.91, 1.89) |
| Corticosteroid (oral or inhaled) | No | 1.16 (0.69, 1.95) | 1.03 (0.61, 1.72) |
| Insulin | No | 0.97 (0.24, 3.95) | 1.20 (0.30, 4.88) |
| Loop diureticc | No | 1.58 (0.91, 2.77) | 1.73 (0.99, 3.02) |
| Nitratesc | No | 1.56 (0.88, 2.77) | 1.80 (1.01, 3.19) |
| NSAIDs | No | 0.98 (0.64, 1.50) | 1.23 (0.80, 1.87) |
| Proton pump inhibitors | No | 1.00 (0.60, 1.68) | 0.95 (0.57, 1.60) |
| SSRI | No | 1.76 (0.82, 3.75) | 1.30 (0.60, 2.79) |
| Statins | No | 0.76 (0.53, 1.10) | 0.85 (0.59, 1.23) |
| Thiazide diuretics | No | 0.91 (0.58, 1.42) | 1.09 (0.69, 1.71) |
| Thiazolidinediones | No | 1.09 (0.27, 4.41) | 1.29 (0.32, 5.24) |
| Thyroid medication | No | 1.37 (0.82, 2.30) | 1.37 (0.82, 2.31) |
| Visual acuity (20/40 or worse)b | No | 1.02 (0.90, 1.15) | 1.01 (0.89, 1.15) |
| Alcohol | |||
| >14 drinks/wk | 0 drinks/wk | 0.54 (0.31, 0.94) | 0.72 (0.41, 1.27) |
| Physical measures | |||
| IADLS (no. of difficulties)c | 1.5 | 1.28 (1.16, 1.42) | 1.27 (1.02, 1.44) |
| Physical activity (PASE) | 68.2 | 0.84 (0.71, 0.99) | 0.89 (0.75, 1.05) |
| Pulse ≥80 bpm | <80 bpm | 1.53 (0.94, 2.49) | 1.46 (0.89, 2.38) |
| Sitting (>4 h/d)c | No | 1.35 (0.99, 1.85) | 1.36 (0.99, 1.86) |
| Walk for exercise | No | 0.78 (0.58, 1.05) | 0.79 (0.58, 1.06) |
| Weightb | 13.3 kg | 0.81 (0.68, 0.96) | 1.21 (1.02, 1.44) |
| Dietary | |||
| Caffeine intake | 233 mg | 0.95 (0.81, 1.12) | 0.98 (0.84, 1.15) |
| Calcium (dietary and supplemental) | 586 mg/d | 0.90 (0.77, 1.06) | 0.91 (0.78, 1.07) |
| Diet Quality Score | 13.0 | 0.84 (0.72, 0.99) | 0.87 (0.74, 1.01) |
| Vitamin D (diet and supplemental)c | 244 IU/d | 0.88 (0.75, 1.02) | 0.86 (0.74, 1.01) |
| Past smoker | Never | 0.76 (0.56, 1.03) | 0.81 (0.60, 1.10) |
| Cognition | |||
| Global cognition (Teng 3 MS score) | 5.9 | 0.90 (0.79, 1.04) | 0.93 (0.80, 1.09) |
| SF-12 mental scorec | 7.0 | 0.83 (0.73, 0.94) | 0.88 (0.78, 0.99) |
SSRI =selective serotonin reuptake inhibitors; PASE =Physical Activity Scale for the Elderly.
Referent unit for continuous variables is 1 SD.
There was also no relationship with contrast sensitivity or depth perception.
p <0.10 in the Base +FNBMD models but not significant in the MV models.
Several risk factors previously identified in women were unrelated to hip fracture in men (Table 3). We found no association between previous self-reported history of wrist or spine fracture (after adjusting for FNBMD), parental history of fracture and several medical conditions, eg, rheumatoid arthritis. We found no association between corticosteroid use, anticonvulsants, SSRIs, alcohol consumption, calcium intake, visual function, and hip fracture. Greater physical activity was associated with a lower risk of hip fracture, but this was attenuated after adjusting for FNBMD.
Comorbidity, age, and prediction of hip fractures
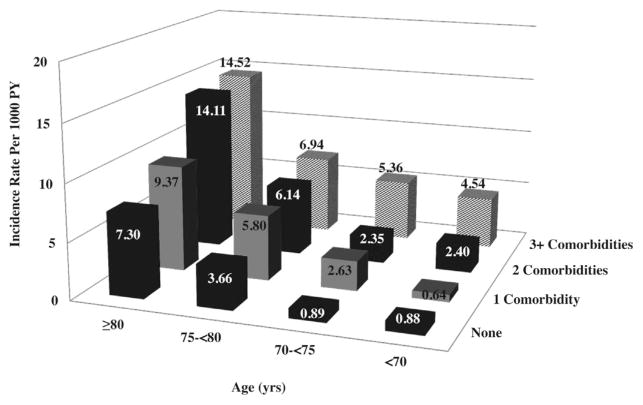
Hip fracture incidence increased with age (Fig. 2). However, within each age group, the incidence of hip fracture increased with increasing number of comorbidities. The highest incidence of hip fracture was observed in men age ≥80 years with 3 or more comorbidities.
Fig. 2.
Incidence of hip fracture (per 1000 person-years) by age and number of comorbidities. Comorbidities include self-report of stroke, myocardial infarction or angina, chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus, Parkinson’s disease, osteoarthritis, hyperthyroidism, and osteoporosis.
Discussion
Our large prospective study of almost 6000 older men followed for incident hip fractures for up to 10 years identified a number of important risk factors for incident hip fracture that were independent of FNBMD: older age, prior fracture history, greater height and height loss since age 25 years, use of TCAs, history of myocardial infarction or angina, Parkinson’s disease and hyperthyroidism, smoking, lower dietary protein, and worse executive function. Men with BMD in the osteoporotic range and 4 or more of these risk factors had about a 30-fold higher incidence of hip fracture compared with men with normal BMD and no risk factors. Thirty-five percent of men had a T-score in the low bone mass range; in them, the gradient of risk was 27 to 1 with increasing number of risk factors. Men age ≥80 years with multiple comorbidities also had an increased risk of hip fracture.
Consistent with current fracture risk assessment tools,(35,36) we found associations with increasing age, low BMD, smoking, and positive fracture history with an increased risk of hip fractures. Our results are also consistent with the Dubbo Study, which showed increased hip fracture incidence with low BMD and increasing number of risk factors, although this finding included men and women collectively.(15) Depression has been linked to an increased risk of fracture,(37) and it’s possible that the associations we observed with TCAs reflects underlying depression.(38) However, a recent meta-analysis suggested that TCA use was associated with an increased risk of fracture independent of depression status or symptoms.(38) TCA use was also found to be associated with a 2.4-fold increased risk of non-spine fractures in MrOS(39) but was unrelated to BMD.(40) This may suggest that TCAs may increase the risk of hip fracture by an increased risk of falls attributable to sedation, orthostatic hypotension, and/or confusion.(41) Other more common antidepressants, eg, SSRIs were unrelated to hip fracture risk. Of note, TCAs are listed on the updated BEERS criteria for inappropriate medications in older adults.(42)
Parkinson’s disease is the second most common neurodegenerative disease among those age >65 years(43) and carried a threefold increased risk of hip fracture, although CIs were wide. The point estimate of the HR was similar to a recent meta-analysis of Parkinson’s disease and any fracture.(44) The risk of fracture associated with Parkinson’s disease has been shown to be stronger in men than in women.(45) In addition, mortality after a hip fracture was also greater in men with Parkinson’s disease compared with women with Parkinson’s disease.(46) Men with Parkinson’s disease should be targeted for fracture prevention regardless of their BMD. The efficacy of bisphosphonates against hip fracture has been shown for Parkinson’s disease patients.(47)
Our findings regarding hyperthyroidism are consistent with results in women(5) and suggest that the hyperthyroidism may have lasting skeletal consequences. Hyperthyroidism may also impair muscle strength and quality, but our associations were independent of several physical performance tests. We previously examined subclinical hypothyroid and hyperthyroid-ism in MrOS and found no association with fractures.(48) We also found no association between use of thyroid medications and hip fracture, suggesting that the disease itself may increase risk.
Greater height may lead to a greater impact of a fall on the hip or it may reflect a longer hip axis length, which has been linked to hip fracture.(49) The association between height loss and hip fracture is likely explained by vertebral fractures, a major risk factor for future fractures.(50)
Cardiovascular disease (CVD) and osteoporosis are common age-related conditions, but biological and epidemiological evidence supports a link between the two diseases.(51–53) A recent study of more than 30,000 Swedish twins showed an increased risk of hip fracture after a CVD diagnosis.(54) Of importance, an increased risk of hip fracture was observed in co-twins that did not have a CVD event, suggesting that genetic factors play a role. Thus, the association between CVD and hip fracture may reflect shared etiology.
Each 3% (1 SD) increase in the percentage of dietary kcals from protein was associated with about a 20% lower risk of hip fracture. Previous literature has been mixed on the association between protein intake and bone health. A systematic review reported a positive association between protein intake and BMD, but there was no association with fracture risk.(53) A recent article from the Women’s Health Initiative reported that higher protein intake was associated with a 7% decreased risk of forearm fracture and improved BMD but not hip fracture.(55) Dietary protein is a major anabolic stimulus for muscle protein synthesis. Muscle protein synthesis declines with age at a rate of 3.5% per decade from ages 20 to 90 years. Low dietary protein may accelerate the loss of lean tissue mass.(56) Current dietary guidelines recommend that protein should account for 10% to 35% of kilocalories.(57) On average in MrOS, protein accounted for only about 15% of kilocalories.
Men with lower executive function had a higher risk of hip fracture, but we found no association with global cognitive function. The latter finding may reflect a ceiling effect whereby at study entry most men had good cognitive function. Executive function represents a domain of cognitive processes that regulate, control, and manage other cognitive processes. It is unlikely that executive function is directly linked to hip fracture, but rather it is linked through motor control, function, and speed.
In our long-term studies of older populations, the competing risk of death is very high.(58) Failure to account for competing risk of death could lead to spurious results. In our competing mortality risk models, the effects of smoking, Parkinson’s disease, and hypothyroid disease were attenuated. Premature mortality from smoking is well established. A recent meta-analysis showed that survival for Parkinson’s disease was reduced by approximately 5% for every year of follow-up, but major heterogeneity was observed across studies.(59) Both overt and subclinical hyperthyroid disease have been linked with increased mortality.(60) Despite their attenuation in these competing risk models, these variables are well-established risk factors for fractures. Outcomes after fracture may be worse in these patients, especially in people with Parkinson’s disease.(61) Hence, men with these characteristics may still benefit from primary prevention measures to lower hip fracture risk.
Several risk factors previously identified as risk factors in women were not related to hip fracture in our study of men. Lack of statistical power likely accounts for a number of these null results, eg, corticosteroids, long-acting benzodiazepines, anticonvulsants, and rheumatoid arthritis. The lack of association with wrist and spine fracture history may reflect the smaller number of men with a positive history. A history of falls was not associated with hip fracture in the multivariable model perhaps because of confounding by other risk factors for falls, eg, physical performance, and medications, eg, psychotropic agents, TCA, and hypoglycemic agents. Longer time and inability to complete the chair stand was a strong risk factor for hip fracture in women.(5) We previously showed in MrOS that repeated chair stands were strongly linked to hip fractures, but these models were minimally adjusted.(9) In our current analysis, chair stands were also associated with hip fracture in the minimally adjusted model but not in the multivariate model, suggesting that confounding by other factors in our multivariable model accounted for this association. The commonly used fracture risk assessment tool FRAX(36) includes >3 drinks of alcohol per day as a risk factor for fracture, but we found that drinking alcohol an average of 2 drinks per day was associated with a decreased risk of hip fracture in minimally adjusted models. We found no evidence that maternal or paternal history of any fracture or specifically hip fracture was associated with hip fracture in men. However, to our knowledge, there is no evidence that the heritability of BMD and fractures differs by sex. A large meta-analysis of 34,928 men and women reported an association between maternal or parental history of any fracture or hip fracture and future fractures that was independent of BMD.(62) However, effects were relatively modest, especially for a parental history of fracture, and we may have had limited power to detect these modest associations.
Self-reported physical activity was associated with hip fracture in minimally adjusted models. Adjustment for BMD attenuated these findings, suggesting that BMD may mediate the association between physical activity and hip fracture. Self-report is subject to recall bias and typically overestimates physical activity, especially lower-intensity activities. Higher objectively measured energy expenditure was associated with lower all non-spine fractures in MrOS.(63) Future analyses should examine the association between objective measures of physical activity and hip fracture.
Performance of fracture risk assessment tools in studies that compared the prediction in men and women have observed that the performance of these tools is poorer in men than in women.(64–66) This suggests the need for studies examining potential risk factors in men. We identified several risk factors that are not currently included in any current fracture risk assessment tool. If our results are confirmed, future studies pooling cohort data could evaluate whether the performance of fracture assessment tools is improved with the addition of these characteristics.
Our study has a number of strengths. We studied a large population of community-dwelling men and followed them for up to 10 years. The participants were well characterized for many risk factors for hip fracture, and follow-up rates were excellent. However, some limitations should be noted. All participants in MrOS were able to walk without assistance of another person and were generally in good health. Most were white. Hence, generalizability to less-mobile, institutionalized, non-white populations and to women may be limited. Nevertheless, the incidence rate of hip fracture in our study was only slightly less than the incidence rate using Medicare data, 3.75 per 1000 PY.(67) We examined many factors as potential risk factors for hip fracture but did not adjust for multiple comparisons. Some of these associations could have occurred by chance alone. We studied baseline risk factors and did not study prospective changes in risk factors.
In conclusion, men with low FNBMD, multiple risk factors, and multimorbidity have a high risk of hip fractures. In older men, hip fractures have immense personal and public health impact.(1) Improved methods for identifying those at risk are essential. Indeed, we identified a set of 11 risk factors plus BMD that were associated with hip fractures in older men. Many of these assessments can easily be incorporated into routine clinical practice and may lead to improved risk stratification.
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The funding source had no role in this manuscript.
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
Authors’ roles: Conception and design: JAC, SRC, KEE, and ESO. Analysis and interpretation of the data: JAC, KEP, KEE, DCB, BCT, and DMK. Drafting of the article: JAC. Critical revision of the article for important intellectual content: PMC, KEP, SRC, KEE, DCB, BCT, JMS, ARH, NEL, DMK, MLS, and ESO. Final approval of the article: JAC, PMC, KEP, SRC, KEE, DCB, BCT, JMS, ARH, NEL, DMK, MLS, and ESO. Statistical expertise: PMC, KEP. Obtaining funding: JAC, SRC, KEE, MLS, and ESO. Administrative, technical, or logistic support: JAC, PMC, and ESO. Collection and assembly of data: JAC, PMC, KEE, MLS, and ESO.
References
- 1.Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68(10):1243–51. doi: 10.1093/gerona/glt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29(4):441–64. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fransen M, Woodward M, Norton R, Robinson E, Butler M, Campbell AJ. Excess mortality or institutionalization after hip fracture: men are at greater risk than women. J Am Geriatr Soc. 2002;50(4):685–90. doi: 10.1046/j.1532-5415.2002.50163.x. [DOI] [PubMed] [Google Scholar]
- 4.Wright NC, Saag KG, Curtis JR, et al. Recent trends in hip fracture rates by race/ethnicity among older US adults. J Bone Miner Res. 2012;27(11):2325–32. doi: 10.1002/jbmr.1684. [DOI] [PubMed] [Google Scholar]
- 5.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332(12):767–73. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 6.De Laet CE, Van Hout BA, Burger H, Weel AE, Hofman A, Pols HA. Hip fracture prediction in elderly men and women: validation in the Rotterdam study. J Bone Miner Res. 1998;13(10):1587–93. doi: 10.1359/jbmr.1998.13.10.1587. [DOI] [PubMed] [Google Scholar]
- 7.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185–94. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 8.Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34(1):195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Cawthon PM, Fullman RL, Marshall L, et al. Physical performance and risk of hip fractures in older men. J Bone Miner Res. 2008;23(7):1037–44. doi: 10.1359/JBMR.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kauppi M, Stenholm S, Impivaara O, Maki J, Heliovaara M, Jula A. Fall-related risk factors and heel quantitative ultrasound in the assessment of hip fracture risk: a 10-year follow-up of a nationally representative adult population sample. Osteoporos Int. 2014;25(6):1685–95. doi: 10.1007/s00198-014-2674-9. [DOI] [PubMed] [Google Scholar]
- 11.Mussolino ME, Looker AC, Madans JH, Langlois JA, Orwoll ES. Risk factors for hip fracture in white men: the NHANES I Epidemiologic Follow-up Study. J Bone Miner Res. 1998;13(6):918–24. doi: 10.1359/jbmr.1998.13.6.918. [DOI] [PubMed] [Google Scholar]
- 12.Kanis J, Johnell O, Gullberg B, et al. Risk factors for hip fracture in men from southern Europe: the MEDOS study. Mediterranean Osteoporosis Study. Osteoporos Int. 1999;9(1):45–54. doi: 10.1007/s001980050115. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz AV, Kelsey JL, Sidney S, Grisso JA. Characteristics of falls and risk of hip fracture in elderly men. Osteoporos Int. 1998;8(3):240–6. doi: 10.1007/s001980050060. [DOI] [PubMed] [Google Scholar]
- 14.Grisso JA, Kelsey JL, O’Brien LA, et al. Risk factors for hip fracture in men. Hip Fracture Study Group. Am J Epidemiol. 1997;145(9):786–93. doi: 10.1093/oxfordjournals.aje.a009171. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen ND, Pongchaiyakul C, Center JR, Eisman JA, Nguyen TV. Identification of high-risk individuals for hip fracture: a 14-year prospective study. J Bone Miner Res. 2005;20(11):1921–8. doi: 10.1359/JBMR.050520. [DOI] [PubMed] [Google Scholar]
- 16.Michaelsson K, Wolk A, Langenskiold S, et al. Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ. 2014;349:g6015. doi: 10.1136/bmj.g6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feskanich D, Flint AJ, Willett WC. Physical activity and inactivity and risk of hip fractures in men. Am J Public Health. 2014;104(4):e75–81. doi: 10.2105/AJPH.2013.301667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feskanich D, Owusu W, Hunter DJ, et al. Use of toenail fluoride levels as an indicator for the risk of hip and forearm fractures in women. Epidemiology. 1998;9(4):412–6. [PubMed] [Google Scholar]
- 19.Benetou V, Orfanos P, Feskanich D, et al. Education, marital status, and risk of hip fractures in older men and women: the CHANCES project. Osteoporos Int. 2015;26(6):1733–46. doi: 10.1007/s00198-015-3054-9. [DOI] [PubMed] [Google Scholar]
- 20.Feskanich D, Bischoff-Ferrari HA, Frazier AL, Willett WC. Milk consumption during teenage years and risk of hip fractures in older adults. JAMA Pediatr. 2014;168(1):54–60. doi: 10.1001/jamapediatrics.2013.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006;9(1):84–93. doi: 10.1079/phn2005763. [DOI] [PubMed] [Google Scholar]
- 25.Johnson BA, Herring AH, Ibrahim JG, Siega-Riz AM. Structured measurement error in nutritional epidemiology: applications in the Pregnancy, Infection, and Nutrition (PIN) Study. J Am Stat Assoc. 2007;102(479):856–66. doi: 10.1198/016214506000000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haines PS, Siega-Riz AM, Popkin BM. The Diet Quality Index revised: a measurement instrument for populations. J Am Diet Assoc. 1999;99(6):697–704. doi: 10.1016/S0002-8223(99)00168-6. [DOI] [PubMed] [Google Scholar]
- 27.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 28.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10(4):405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 29.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–8. [PubMed] [Google Scholar]
- 30.Cauley JA, Blackwell T, Zmuda JM, et al. Correlates of trabecular and cortical volumetric bone mineral density at the femoral neck and lumbar spine: the osteoporotic fractures in men study (MrOS) J Bone Miner Res. 2010;25(9):1958–71. doi: 10.1002/jbmr.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Looker AC, Melton LJ, 3rd, Borrud LG, Shepherd JA. Changes in femur neck bone density in US adults between 1988–1994 and 2005–2008: demographic patterns and possible determinants. Osteoporos Int. 2012;23(2):771–80. doi: 10.1007/s00198-011-1623-0. [DOI] [PubMed] [Google Scholar]
- 32.Sanders KM, Pasco JA, Ugoni AM, et al. The exclusion of high trauma fractures may underestimate the prevalence of bone fragility fractures in the community: the Geelong Osteoporosis Study. J Bone Miner Res. 1998;13(8):1337–42. doi: 10.1359/jbmr.1998.13.8.1337. [DOI] [PubMed] [Google Scholar]
- 33.Shtatland ES, Kleinman K, Cain EM. Model building in PROC PHREG with automatic variable selection and information criteria. Proceedings of the 30th Annual SAS Users Group International Conference; 2005 Apr 10–13; pp. 1–10. Paper 206-30. http://www2.sas.com/proceedings/sugi30/toc.html. [Google Scholar]
- 34.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 35.Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV. Development of a nomogram for individualizing hip fracture risk in men and women. Osteoporos Int. 2007;18(8):1109–17. doi: 10.1007/s00198-007-0362-8. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. FRAX1 WHO Fracture Risk Assessment Tool. http://wwwshefacuk/FRAX/tooljsp.
- 37.Wu Q, Liu J, Gallegos-Orozco JF, Hentz JG. Depression, fracture risk, and bone loss: a meta-analysis of cohort studies. Osteoporos Int. 2010;21(10):1627–35. doi: 10.1007/s00198-010-1181-x. [DOI] [PubMed] [Google Scholar]
- 38.Wu Q, Qu W, Crowell MD, Hentz JG, Frey KA. Tricyclic antidepressant use and risk of fractures: a meta-analysis of cohort and case-control studies. J Bone Miner Res. 2013;28(4):753–63. doi: 10.1002/jbmr.1813. [DOI] [PubMed] [Google Scholar]
- 39.Lewis CE, Ewing SK, Taylor BC, et al. Predictors of non-spine fracture in elderly men: the MrOS study. J Bone Miner Res. 2007;22(2):211–9. doi: 10.1359/jbmr.061017. [DOI] [PubMed] [Google Scholar]
- 40.Cauley JA, Fullman RL, Stone KL, et al. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos Int. 2005;16(12):1525–37. doi: 10.1007/s00198-005-1866-8. [DOI] [PubMed] [Google Scholar]
- 41.Rabenda V, Nicolet D, Beaudart C, Bruyere O, Reginster JY. Relationship between use of antidepressants and risk of fractures: a meta-analysis. Osteoporos Int. 2013;24(1):121–37. doi: 10.1007/s00198-012-2015-9. [DOI] [PubMed] [Google Scholar]
- 42.American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–31. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Rijk MC, Launer LJ, Berger K, et al. Prevalence of Parkinson’s disease in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S21–3. [PubMed] [Google Scholar]
- 44.Tan L, Wang Y, Zhou L, et al. Parkinson’s disease and risk of fracture: a meta-analysis of prospective cohort studies. PLoS One. 2014;9(4):e94379. doi: 10.1371/journal.pone.0094379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benzinger P, Rapp K, Maetzler W, et al. Risk for femoral fractures in Parkinson’s disease patients with and without severe functional impairment. PLoS One. 2014;9(5):e97073. doi: 10.1371/journal.pone.0097073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris-Hayes M, Willis AW, Klein SE, Czuppon S, Crowner B, Racette BA. Relative mortality in U.S. Medicare beneficiaries with Parkinson disease and hip and pelvic fractures. J Bone Joint Surg Am. 2014;96(4):e27. doi: 10.2106/JBJS.L.01317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang YF, Cherng YG, Hsu SP, et al. Risk and adverse outcomes of fractures in patients with Parkinson’s disease: two nationwide studies. Osteoporos Int. 2015;26(6):1723–32. doi: 10.1007/s00198-015-3052-y. [DOI] [PubMed] [Google Scholar]
- 48.Waring AC, Harrison S, Fink HA, et al. A prospective study of thyroid function, bone loss, and fractures in older men: the MrOS study. J Bone Miner Res. 2013;28(3):472–9. doi: 10.1002/jbmr.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leslie WD, Pahlavan PS, Tsang JF, Lix LM Manitoba Bone Density Program. Prediction of hip and other osteoporotic fractures from hip geometry in a large clinical cohort. Osteoporos Int. 2009;20(10):1767–74. doi: 10.1007/s00198-009-0874-5. [DOI] [PubMed] [Google Scholar]
- 50.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15(4):721–39. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 51.Thompson B, Towler DA. Arterial calcification and bone physiology: role of the bone-vascular axis. Nat Rev Endocrinol. 2012;8(9):529–43. doi: 10.1038/nrendo.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302(15):1666–73. doi: 10.1001/jama.2009.1463. [DOI] [PubMed] [Google Scholar]
- 53.Darling AL, Millward DJ, Torgerson DJ, Hewitt CE, Lanham-New SA. Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr. 2009;90(6):1674–92. doi: 10.3945/ajcn.2009.27799. [DOI] [PubMed] [Google Scholar]
- 54.Sennerby U, Farahmand B, Ahlbom A, Ljunghall S, Michaelsson K. Cardiovascular diseases and future risk of hip fracture in women. Osteoporos Int. 2007;18(10):1355–62. doi: 10.1007/s00198-007-0386-0. [DOI] [PubMed] [Google Scholar]
- 55.Beasley JM, LaCroix AZ, Larson JC, et al. Biomarker-calibrated protein intake and bone health in the Women’s Health Initiative clinical trials and observational study. Am J Clin Nutr. 2014;99(4):934–40. doi: 10.3945/ajcn.113.076786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286(1):E92–101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- 57.US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans. 7. Washington, DC: US Government Printing Office; 2010. Dec, http://health.gov/dietaryguidelines/dga2010/dietaryguidelines2010.pdf. [Google Scholar]
- 58.Berry SD, Ngo L, Samelson EJ, Kiel DP. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58(4):783–7. doi: 10.1111/j.1532-5415.2010.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Macleod AD, Taylor KS, Counsell CE. Mortality in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2014;29(13):1615–22. doi: 10.1002/mds.25898. [DOI] [PubMed] [Google Scholar]
- 60.Laulund AS, Nybo M, Brix TH, Abrahamsen B, Jorgensen HL, Hegedus L. Duration of thyroid dysfunction correlates with all-cause mortality. The OPENTHYRO Register Cohort. PLoS One. 2014;9(10):e110437. doi: 10.1371/journal.pone.0110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Critchley R, Khan SK, Yarnall AJ, Parker MJ, Deehan DJ. Occurrence, management and outcomes of hip fractures in patients with Parkinson’s disease. Br Med Bull. 2015;115:135–42. doi: 10.1093/bmb/ldv029. [DOI] [PubMed] [Google Scholar]
- 62.Kanis JA, Johansson H, Oden A, et al. A family history of fracture and fracture risk: a meta-analysis. Bone. 2004;35(5):1029–37. doi: 10.1016/j.bone.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 63.Cauley JA, Harrison SL, Cawthon PM, et al. Objective measures of physical activity, fractures and falls: the Osteoporotic Fractures in Men study. J Am Geriatr Soc. 2013;61(7):1080–8. doi: 10.1111/jgs.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sandhu SK, Nguyen ND, Center JR, Pocock NA, Eisman JA, Nguyen TV. Prognosis of fracture: evaluation of predictive accuracy of the FRAX algorithm and Garvan nomogram. Osteoporos Int. 2010;21(5):863–71. doi: 10.1007/s00198-009-1026-7. [DOI] [PubMed] [Google Scholar]
- 65.Leslie WD, Lix LM, Johansson H, et al. Independent clinical validation of a Canadian FRAX tool: fracture prediction and model calibration. J Bone Miner Res. 2010;25(11):2350–8. doi: 10.1002/jbmr.123. [DOI] [PubMed] [Google Scholar]
- 66.Collins GS, Mallett S, Altman DG. Predicting risk of osteoporotic and hip fracture in the United Kingdom: prospective independent and external validation of QFractureScores. BMJ. 2011;342:d3651. doi: 10.1136/bmj.d3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–9. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]