Abstract
Most natural processes occur far from equilibrium and cannot be treated within the framework of classical thermodynamics. In 1998, Oono and Paniconi [Oono, Y. & Paniconi, M. (1998) Prog. Theor. Phys. Suppl. 130, 29–44] proposed a general phenomenological framework, steady-state thermodynamics, encompassing nonequilibrium steady states and transitions between such states. In 2001, Hatano and Sasa [Hatano, T. & Sasa, S. (2001) Phys. Rev. Lett. 86, 3463–3466] derived a testable prediction of this theory. Specifically, they were able to show that the exponential average of Y, a quantity similar to a dissipated work, should be equal to zero for arbitrary transitions between nonequilibrium steady states, –ln〈e–Y〉 = 0. We have tested this strong prediction by measuring the dissipation and fluctuations of microspheres optically driven through water. We have found that –ln〈e–Y〉 ≈ 0 for three different nonequilibrium systems, supporting Hatano and Sasa's proposed extension of thermodynamics to arbitrary steady states and irreversible transitions.
Classical thermodynamics and statistical mechanics are organized around the concept of “equilibrium states.” However, most processes of interest, such as fast switching between phonon distributions in optical cavities (1), oscillations of pumped mesoscopic chemical reaction systems (2, 3), and biological reactions by molecular machines (4), occur far from equilibrium. Molecular pumps, for example, maintain ions at nonequilibrium concentrations by active transport across membranes, and intermediate metabolism in cells involves transitions between nonequilibrium steady states for which there is no thermodynamic description.
Part of the difficulty in formulating a general theory of nonequilibrium thermodynamics is the diversity of dissipation regimes and mechanisms in systems away from thermal equilibrium. With this in mind, the study of “nonequilibrium steady states” occupies an interesting middle ground between the familiar territory of equilibrium thermodynamics and a nonequilibrium “thermodynamics of everything.” In fact, several theories have been proposed in which the state space includes such nonequilibrium steady states, which are characterized by the flow of constant currents (of energy, mass, or charge), as well as equilibrium states, which are defined by the absence of currents (5–8).
Any extension of thermodynamics should include a generalization of the second law. In one of its several classic formulations, this law places a constraint on transitions between equilibrium states, expressed by the Clausius inequality (9),
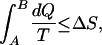 |
[1] |
where the left side pertains to a transition from state A to state B, and the right side is the entropy difference between these states. Does a similarly universal law govern transitions between nonequilibrium steady states?
One can address this question at the level of macroscopic phenomenology (see ref. 8), or one can seek the answer in a microscopic, statistical analysis. Taking the latter approach, Keizer (5, 10) derived a generalized Clausius inequality by considering the steady-state fluctuations of a set of extensive variables n = (n1, n2,...), which provide a coarse-grained snapshot of the system. This result describes linear-response behavior in the near-steady-state regime.
More recently, Hatano and Sasa (11), motivated by the phenomenological framework of Oono and Paniconi (8), have obtained predictions for transitions between steady states of a system expressed in terms of the evolution of its microscopic degrees of freedom. Their results remain valid even if the system is driven away from steady-state behavior, suggesting that a strong Clausius-like inequality for transitions between nonequilibrium steady states may exist. Specifically, they predict that a Boltzmann-weighted average of the transition dissipation is equal to zero, such that –ln〈e–Y〉 = 0. Here, we report experiments in which optically dragged microspheres are used to test the predictions of Hatano and Sasa (11), which we now introduce.
Theory and Background
Let x denote the microstate of some system of interest, let α denote an externally controlled parameter, and let us suppose that, when this parameter is held fixed, the system relaxes to a stationary state described by a probability distribution ρss(x;α). Here, we use the term “stationary state” quite generally, referring to either an equilibrium state or a nonequilibrium steady state. In the former case, ρss is the familiar Boltzmann–Gibbs distribution; in the latter case, ρss describes the microscopic fluctuations of the system in the steady state. When we carry the system from one stationary state to another, its microscopic evolution is specified by a trajectory x(t).
Imagine an idealized process by which we drive the system from one stationary state to another by holding the parameter fixed at α1 and then varying it over a finite time τ to a new constant value α2. If we change the parameter slowly and gently, the system moves through a continuous sequence of stationary states and the dissipated work is at a minimum. If we instead vary the parameter rapidly and violently, then the system cannot relax to the state consistent with the current parameter value because that value is changing too quickly. For transitions between equilibrium states, the Clausius inequality provides a quantitative measure of this lag: the more irreversible the process, the greater the difference between the two sides of Eq. 1. For transitions between nonequilibrium steady states, Hatano and Sasa (11) identified a new property, the Y value, which measures this lag. They construct the following quantity:
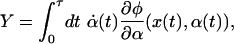 |
[2] |
where φ(x, α) = –ln ρss(x; α) and 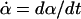 . The value of Y depends on both what we do to the system, as specified by the imposed time-dependence of the parameter α(t), and on how the system responds, which is represented by the phase-space trajectory x(t). Because our system is subject to thermal noise, each repetition of the process yields a different Y value. Now, consider a statistical ensemble of trajectories, obtained by repeatedly varying the control parameter according to the same schedule α(t). Under very general conditions, Hatano and Sasa have shown that:
. The value of Y depends on both what we do to the system, as specified by the imposed time-dependence of the parameter α(t), and on how the system responds, which is represented by the phase-space trajectory x(t). Because our system is subject to thermal noise, each repetition of the process yields a different Y value. Now, consider a statistical ensemble of trajectories, obtained by repeatedly varying the control parameter according to the same schedule α(t). Under very general conditions, Hatano and Sasa have shown that:
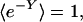 |
[3] |
where the angular brackets denote an average over our ensemble of repetitions of the process. By Jensen's inequality (12), Eq. 3 implies that:
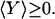 |
[4] |
Although the analysis of Hatano and Sasa (11) was carried out in the specific context of a trapped Brownian particle subject to a nonconservative force, it is evident from their derivation of Eqs. 3 and 4 that they are more general and do not depend on particular assumptions about the dynamics of the system.
Connecting their results to earlier work by Oono and Paniconi (8), Hatano and Sasa (11) interpret Eq. 4 as a generalized second law of thermodynamics that is applicable to transitions between (equilibrium or nonequilibrium) stationary states. As explained in greater detail in ref. 11, several observations support this interpretation. First, Y = 0 when the process is carried out reversibly, suggesting that in the more general case the nonnegative value 〈Y〉 provides a measure of the irreversibility of the process. Second, for the system studied in ref. 11, Eq. 4 is equivalent to a generalized Clausius inequality proposed within Oono and Paniconi's phenomenological steady-state thermodynamic framework (8). Finally, Eqs. 3 and 4 reduce to known results for transitions between equilibrium states in the appropriate limit (see Results and Discussion).
To test Hatano and Sasa's predictions, we dragged a microscopic bead through water by using a steerable harmonic optical trap. Our protocol created nonequilibrium steady states that are both experimentally tractable and theoretically understood. On the experimental side, laser tweezers and similar optical devices have proven to be ideal laboratory tools for probing nonequilibrum statistical physics at microscopic length scales (13–17). In the present work, this technology provided the means to pull the bead and also to observe its motion at the level of thermal fluctuations. On the theoretical side, treating the bead as a Brownian particle and the optical trap as a harmonic potential, we easily obtain an expression for the steady-state distribution, ρss, which enters into the definition of Y in Eq. 2. We subsequently confirmed the validity of the theoretical predictions for ρss of Mazonka and Jarzynski (18) by comparing them with the experimentally measured distributions, and we found excellent agreement (data not shown). We chose a system in which ρss is known a priori. In fact, Hatano and Sasa's relation for transitions between steady states can be applied to any physical system in which the stationary distribution ρss is known or can be extracted from experiments. The first step in applying Hatano and Sasa's relation to more complicated systems (for example, in turbulence or granular media) in which the steady-state distributions ρss are not known, will be to obtain them experimentally.
Methods
For each experiment, micron-sized polystyrene beads (diameter, 10.06 μm; Bangs Laboratories, Carmel, IN) were dispensed and diluted inside a microfluidics chamber, which was then sealed. A single bead was then trapped in a harmonic potential created by superposing the foci of two counterpropagating 834-nm laser beams (19), and an ultra-fast steerable mirror (Nano MTA-2, Mad City Labs, Madison, WI) was used to translate the trap. The optical force f(t) exerted by the trap is equal in magnitude and opposite in direction to the rate of change of the momentum of light (19), which we measured directly by using position-sensitive photodetectors (DL-10, United Detector Technology Sensors). The position of the trap was calculated from the angular rotation of the steerable mirror. Data were sampled at 10 kHz, with the average over 10 data points saved to a disk. The following further parameters characterized the experiments: the trap constant κ[pN/μm]; the number of switching repetitions N; the trap velocities v1 and v2 (μm/s), corresponding to the initial and final steady states, respectively; the switching time τ (s); and the value of q = κ/βγ[pN (μm/s)]. Values for the first experiment were as follows: κ = 4.25, n = 3,924, v1 = 8.12, v2 = 12.15, τ = 0.06, and q = 0.20. Values for the second experiment were as follows: κ = 4.51, n = 3,163, v1 = 9.93, v2 = 13.56, τ = 0.06, and q = 0.21. Values for the third experiment were as follows: κ = 4.9, n = 3,603, v1 = 7.53, v2 = 10.20, τ = 0.08, and q = 0.23.
Results and Discussion
Nonequilibrium steady states were created by translating the optical trap at constant speed (Fig. 1). After a relaxation time tR ∼ γ/κ, where γ is the friction coefficient of the bead in solution and κ is the spring constant of the trap, the bead settled into a steady state in which its position fluctuated around an average displacement γv/κ behind the minimum of the trap potential, so that the average force exerted by the trap balanced the average frictional force felt by the bead. This nonequilibrium steady state was maintained by a continual transfer of energy: the trap performed work on the bead at an average rate P̄ = γv2, which was dissipated as heat into the surrounding buffer.
Fig. 1.
Schematic representation of the experiment. Optically trapped bead lags a distance x behind the center of the trap translating at velocity v.
We began our experimental trajectories in such a steady state, with the trap moving at a speed v1, and we then changed the speed from v1 to v2 over a time interval of duration τ, after which we continued to move the trap at speed v2 (Fig. 2a). Thus, the trap speed v played the role of the parameter α in our earlier discussion. Modeling the bead as an overdamped Brownian particle (18), the steady-state distribution is ρss(x; v) ∝ exp[–β(κx + γv)2/2κ], where x is the bead displacement relative to the minimum of the trap and β is the inverse temperature of the solution. Eq. 2 then gives us 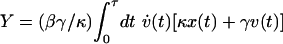 , where x(t) specifies the motion of the bead during a given realization of the process, and v(t) is the (externally imposed) trap speed.
, where x(t) specifies the motion of the bead during a given realization of the process, and v(t) is the (externally imposed) trap speed.
Fig. 2.
Trap-velocity profiles (a) and steady-state transitions producing low (b), medium (c) and high (d) dissipation. Each Y value distribution, shown in blue, is plotted with its ordinary average 〈Y〉 (black bar) as well as the Hatano and Sasa exponentiated average, –ln 〈e–Y〉= Ye (green bar). The bar heights are arbitrary, but their finite widths represent statistical errors, estimated with the bootstrap method, such that each green and black bar extends one standard deviation on either side of the computed value of Ye and 〈Y〉, respectively.
We used three distinct nonlinear transitions, and for each type, we repeated the experiment N times. Our three experiments differed by the switching protocol and the initial and final steady states (Fig. 2a). In the first experiment, a quarter-sine wave protocol was used to vary the trap speed: v(t) = v1 + (v2 – v1)sin(π t/2τ). For the second and third experiments, we used an inverted three-quarters sine wave: v(t) = v1 – (v2 – v1)sin(3π t/2τ). During each repetition, we recorded both the trap position and the time-dependent optical force on the sphere, and we used these signals to compute the Y value for each of N transitions. From these signals, the value Yn was evaluated for each realization. To compare with predictions, let 〈Y〉 and Ye = –ln 〈e–Y〉 denote the ordinary and exponential averages of the observed Y values. In Fig. 2 b–d, we show for each experiment the distribution of the N observed values Yn (blue histogram), as well as the averages Ye and 〈Y〉 computed from these values (green and black bars, respectively).
Two features are immediately apparent in Fig. 2 and Table 1. The first feature is the good agreement with the theoretical predictions (Eqs. 3 and 4). As shown in Fig. 2 b–d, the exponential averages of all three experiments are equal to zero, within estimated statistical error, confirming the strongest prediction of Hatano and Sasa (11). Second, in each of the three experiments, the error bars reveal greater statistical uncertainty in Ye than in 〈Y〉, although these quantities were computed from the same data. This discrepancy is typical of averages of highly nonlinear functions: if the distribution of Y values is significantly wider than unity, then Y values that are several standard deviations below the mean contribute disproportionately to the average of e–Y, resulting in poor convergence (20–24).
Table 1. Ordinary and exponential averages of the observed Y values for the three experiments.
| κtrap [pN/μm] | τ, ms | q = κ/βγ, pN μm/s | N | 〈Y〉 ± SE* | -In 〈e-Y〉 ± SE* |
|---|---|---|---|---|---|
| 4.25 | 60 | 0.20 | 3,924 | 2.184 ± 0.032 | -0.173 ± 0.162 |
| 4.90 | 80 | 0.23 | 3,603 | 3.444 ± 0.042 | -0.094 ± 0.267 |
| 4.51 | 60 | 0.21 | 3,163 | 7.377 ± 0.062 | +0.318 ± 0.838 |
The statistical errors of both averages were estimated with the bootstrap method (29) because a distribution of Boltzmann-weighted averages is not Gaussian.
Having shown that, for our system, the Y values satisfy Eqs. 3 and 4, we will now clarify their physical meaning and their correspondence to predictions for isothermal transitions between equilibrium states. When the stationary states corresponding to fixed values of α are equilibrium states (at a common temperature β–1), represented by Boltzmann–Gibbs distributions ρss ∝ e–βH(x,α), then a direct evaluation of Eq. 2 gives Y = β(W –ΔF), where W is the work performed on the system during the process and ΔF is the free energy difference between the initial and final equilibrium states. In this limit, Hatano and Sasa's results reduce to the nonequilibrium work relation (25–27) and the Clausius inequality (Eq. 1):
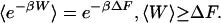 |
[5] |
We can develop corresponding predictions for transitions between nonequilibrium steady states by recasting Hatano and Sasa's result in terms of more familiar quantities. After incorporating the expression for the Gaussian steady-state distribution ρss into Eq. 2 and performing some simple algebra, we obtain the following:
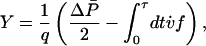 |
[6] |
where 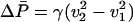 is the difference between the initial and final steady-state average dissipation rates, and q = κ/βγ is a constant with the dimensions of power, constructed from parameters characterizing the bead, trap, and surrounding water. During a transition, the instantaneous power that the moving trap delivers to the bead is the fluctuating quantity P(t) = v(t)f(t). The net change in the power delivered to the bead is ΔP = ΔvP + Δf P, where ΔvP = ∫dtv̇f is the contribution to ΔP from increments of the trap speed, and Δf P =∫dtv̇f is the contribution from fluctuations in the force f acting on the bead. By using Eq. 6 to rewrite Eqs. 3 and 4 as follows:
is the difference between the initial and final steady-state average dissipation rates, and q = κ/βγ is a constant with the dimensions of power, constructed from parameters characterizing the bead, trap, and surrounding water. During a transition, the instantaneous power that the moving trap delivers to the bead is the fluctuating quantity P(t) = v(t)f(t). The net change in the power delivered to the bead is ΔP = ΔvP + Δf P, where ΔvP = ∫dtv̇f is the contribution to ΔP from increments of the trap speed, and Δf P =∫dtv̇f is the contribution from fluctuations in the force f acting on the bead. By using Eq. 6 to rewrite Eqs. 3 and 4 as follows:
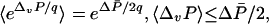 |
[7] |
we see a strong resemblance to the nonequilibrium work relation and the Clausius inequality (Eqs. 5). First, P̄ /2 = γv2/2 can be viewed as a “state function,” roughly analogous to the free energy F for equilibrium states. Next, the constant q = κ/βγ specifies a unit of power relevant to our dragged particle, just as β–1 specifies the relevant unit of energy in the equilibrium case. Last, in Eqs. 5, the work W is the contribution to the net change in the internal energy of the system, E, arising from increments in the value of α (25–27). Thus, by drawing parallels between P̄ and the free energy (P̄ /2 ∼ F), q and temperature (q ∼ β–1), and ΔvP and mechanical work (–ΔvP ∼ W), the analogy between equilibrium laws of Eqs. 5 and the nonequilibrium laws of Eqs. 7 is complete.
Our experiments support the suggestion that transitions between nonequilibrium steady states are subject to laws similar to those governing transitions between equilibrium states (Eqs. 5). These findings motivate several questions. First, does a steady-state thermodynamic formalism support a zeroth law? Such a law would define an effective temperature of nonequilibrium steady states (28). Second, can Eq. 4 also be understood in terms of the monotonic growth of some global quantity, just as the equilibrium second law states that the combined entropy of a system and its thermal surroundings never decreases? Last, at the microscopic level, can nonequilibrium steady states be described by a universal theory for ρss, analogous to the Boltzmann–Gibbs distribution for equilibrium states? If so, this result would greatly increase the predictive power of Eq. 4 as a “generalized second law” because the microscopic nature of the steady state enters into the definition of the quantity Y by means of the function φ = –ln ρss. For now, we can apply Eqs. 3 and 4 only to systems for which ρss can be deduced from a simple model or from direct experimental measurement of the distribution. Theoretical developments along these lines should be readily testable with the use of microscopic systems such as those described here, whose fluctuations are directly measurable.
Acknowledgments
We thank Yoshitsugu Oono, Ignacio Tinoco, Jr., Hari Shroff, Nathan Clack, and Merek Siu for valuable discussions and suggestions. J.L. is supported by the Director's Fellowship of Lawrence Berkeley National Laboratory. This work was supported by National Institutes of Health Grant GM-32543; U.S. Department of Energy Grants DE-AC03-76SF00098 and W-7405-ENG-36; National Science Foundation Grants MBC-9118482 and DBI-9732140; a David and Lucile Packard Foundation grant (to C.J.B.); and the Spanish Minesterio de Ciencia y Technoloía Grant BFM2001-3525.
Author contributions: E.H.T., C.J., and J.L. designed research; E.H.T. performed research; E.H.T., C.J., F.R., and G.E.C. analyzed data; E.H.T., C.J., C.J.B., and J.L. wrote the paper; and C.J. and F.R. performed computer simulations.
References
- 1.Chow, W. W., Scheider, H. C., Koch, S. W., Chang, C., Chrostowski, L. & Chang-Hasnian, C. J. (2002) IEEE J. Quantum Electron. 38, 402–409. [Google Scholar]
- 2.Qian, H., Saffarian, S. & Elson, E. L. (2002) Proc. Natl. Acad. Sci. USA 99, 10376–10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldbeter, A. (1997) in Biochemical Oscillations and Cellular Rhythms: The Molecular Bases of Periodic and Chaotic Behaviour (Cambridge Univ. Press, London).
- 4.Wang, H. & Oster, G. (1998) Nature 396, 279–282. [DOI] [PubMed] [Google Scholar]
- 5.Keizer, J. (1987) in Statistical Thermodynamics of Nonequilibrium Processes (Springer, Berlin).
- 6.Jou, D., Casas-Vázquez, J., and Lebon, G. (1988) Rep. Prog. Phys. 51, 1105–1179. [Google Scholar]
- 7.Eu, B. C. (1998) in Nonequilibrium Statistical Mechanics (Kluwer Academic, Dordrecht, The Netherlands).
- 8.Oono, Y. & Paniconi, M. (1998) Prog. Theor. Phys. Suppl. 130, 29–44. [Google Scholar]
- 9.Finn, C. B. P. (1993) in Thermal Physics (Chapman and Hall, London), 2nd Ed..
- 10.Keizer, J. (1985) J. Chem. Phys. 82, 2751–2771. [Google Scholar]
- 11.Hatano, T. & Sasa, S. (2001) Phys. Rev. Lett. 86, 3463–3466. [DOI] [PubMed] [Google Scholar]
- 12.Chandler, D. (1987) in Introduction to Modern Statistical Mechanics (Oxford Univ. Press, New York), Sect. 5.5.
- 13.Faucheux, L. P., Bourdieu, L. S., Kaplan, P. D. & Libchaber, A. J. (1995) Phys. Rev. Lett. 74, 1504–1507. [DOI] [PubMed] [Google Scholar]
- 14.Hales, J., Zhukov, A., Roy, R. & Dykman, M. I. (2000) Phys. Rev. Lett. 85, 78–81. [DOI] [PubMed] [Google Scholar]
- 15.Wang, G. M., Sevick, E. M., Mittag, E., Searls, D. J. & Evans, D .J. (2002) Phys. Rev. Lett. 89, 50601–50604. [DOI] [PubMed] [Google Scholar]
- 16.Hummer, G. & Szabo, A. (2001) Proc. Natl. Acad. Sci. USA 98, 3658–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liphardt, J., Dumont, S., Smith, S. B., Tinoco, I., Jr., & Bustamante, C. (2002) Science 296, 1832–1835. [DOI] [PubMed] [Google Scholar]
- 18.Mazonka, O. & Jarzynski, C. (Dec. 7, 1999) arXiv.org:cond-mat 9912121.
- 19.Smith, S.B., Cui, Y. & Bustamante, C. (2003) Methods Enzymol. 361, 134–162. [DOI] [PubMed] [Google Scholar]
- 20.Gore, J., Ritort, F. & Bustamante, C. (2003) Proc. Natl. Acad. Sci. USA 100, 12564–12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuckerman, D. M. & Woolf, T. B. (2002) Chem. Phys. Lett. 351, 445–453. [Google Scholar]
- 22.Zuckerman, D. M. & Woolf, T. B. (2002) Phys. Rev. Lett. 89, 180602–180604. [DOI] [PubMed] [Google Scholar]
- 23.Park, S., Khalili-Araghi, F., Tajkhorshid, E. & Schulten, K. (2003) J. Chem. Phys. 119, 3559–3566. [Google Scholar]
- 24.Ritort, F., Bustamante, C., Tinoco, I. Jr. (2002) Proc. Natl. Acad. Sci. USA 99, 13544–13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarzynski, C. (1997) Phys. Rev. Lett. 78, 2690–2693. [Google Scholar]
- 26.Jarzynski, C. (1997) Phys. Rev. E 56, 5018–5035. [Google Scholar]
- 27.Crooks, G. E. (1998) J. Stat. Phys. 90, 1481–1487. [Google Scholar]
- 28.Casas-Vazquez, J. & Jou, D. (2003) Rep. Prog. Phys. 66, 1937–2023. [Google Scholar]
- 29.Efron, B. & Tibshirani, R. J. (1993) in An Introduction to the Bootstrap (CRC, New York).




