Abstract
The fragile X mental retardation protein (FMRP) is a selective RNA-binding protein implicated in regulating translation of its mRNA ligands. The absence of FMRP results in fragile X syndrome, one of the leading causes of inherited mental retardation. Delayed dendritic spine maturation was found in fragile X mental retardation patients as well as in Fmr1 knockout (KO) mice, indicating the functional requirement of FMRP in synaptic development. However, the biochemical link between FMRP deficiency and the neuronal impairment during brain development has not been defined. How FMRP governs normal synapse development in the brain remains elusive. We report here that the developmentally programmed FMRP expression represses the translation of microtubule associated protein 1B (MAP1B) and is required for the accelerated decline of MAP1B during active synaptogenesis in neonatal brain development. The lack of FMRP results in misregulated MAP1B translation and delayed MAP1B decline in the Fmr1 KO brain. Furthermore, the aberrantly elevated MAP1B protein expression leads to abnormally increased microtubule stability in Fmr1 KO neurons. Together, these results indicate that FMRP plays critical roles in controlling cytoskeleton organization during neuronal development, and the abnormal microtubule dynamics is a conceivable underlying factor for the pathogenesis of fragile X mental retardation.
The absence of fragile X mental retardation protein (FMRP), a messenger ribonucleoprotein (mRNP) associated with a subclass of brain mRNAs, results in fragile X mental retardation, affecting 1 in 4,000 males and 1 in 8,000 females (1-3). The lack of FMRP results in delayed dendritic spine maturation in fragile X patients as well as in Fmr1 knockout (KO) mice (4-8), indicating the essential role of FMRP in synapse development. Furthermore, the association of FMRP with translating polyribosomes (9-12) and micro-RNA machinery (13) suggests that FMRP governs translation efficiency of its mRNA targets, which in turn modulates synaptic development and plasticity.
FMRP has been shown to act as a translation repressor for its associated mRNAs in vitro as well as in cultured cell lines (14-17). To date, >400 mRNAs have been identified to associate with FMRP in vivo (18-21). In fragile X patient cells and synaptoneurosomes isolated from the Fmr1 KO brain, the lack of FMRP causes abnormal polyribosome-association of several mRNAs that normally bind to FMRP (18, 21). Consistent with the proposed function of FMRP in regulating translation in synaptic plasticity, activity-dependent production of the synaptic protein PSD95 is abrogated in the Fmr1 KO primary neuronal cultures (22). In addition, the mRNA of the microtubule-associated protein 1B (MAP1B), a neuronal MAP playing principle roles in neurite and synapse development (23), is a predicted target of FMRP (18, 19, 21). Interestingly, deficiency of Drosophila Fmr1 (dFmr1) results in abnormally elevated expression of the microtubule associated protein Futsch and synaptic over growth at the neuromuscular junction (24), suggesting an evolutionarily conserved function of FMRP in synapse development, although the function of Futsch at neuromuscular junction may not be completely analogous to that of MAP1B in the mammalian brain. During brain development, MAP1B is the first MAP to be expressed, which controls neurite extension and growth cone motility via modulating microtubule dynamics (23). Therefore, FMRP-dependent translation regulation of MAP1B may serve as an important mechanism in controlling neuronal network formation. However, to date, no comprehensive model has been raised regarding how FMRP may regulate MAP1B production during normal neuronal development. Moreover, the biochemical consequence of misregulated MAP1B translation in fragile X neurons that leads to abnormal synapse development remains elusive.
Here we show the developmentally programmed FMRP expression governs the translation of MAP1B during active synaptogenesis in the neonatal brain. FMRP expression is elevated in the hippocampus, whereas MAP1B is rapidly down-regulated, when major neuritogenesis is completed and synaptogenesis begins. The lack of FMRP leads to misregulation of MAP1B translation, which delays the decline of MAP1B. Consequently, the aberrant MAP1B expression in Fmr1 KO neurons leads to abnormally increased microtubule stability. These results suggest that FMRP controls MAP1B translation to modulate the dynamic organization of neuronal cytoskeleton, and the abnormal microtubule stability caused by FMRP deficiency is a conceivable factor contributing to the impaired synaptic maturation in fragile X mental retardation.
Methods
Animals and Tissue Collection. WT and Fmr1 KO mice were raised at the Emory University animal facility and treated in accordance with National Institutes of Health regulations and under approval of the Emory University Institutional Animal Care and Use Committee. WT and Fmr1 KO littermates were produced by breeding heterozygous females with Fmr1 KO males in congenic background of C57BL/6. The genotype of each animal was initially mapped by PCR (25) and confirmed by immunoblot analysis of FMRP. For tissue collection, hippocampi were dissected on ice, followed by total RNA isolation using TRIzol extraction (Invitrogen), or subjected to preparation of whole tissue lysate (17). For immunostaining of neonatal hippocampus, postnatal day 7 (P7) brains were fixed, cryostat sectioned, and subjected to immunofluorescent staining (17). Primary cultures of cortical neurons were raised by using embryonic day 16 brain (26). Nocodazole and LiCl treatment were performed with the concentration and duration indicated in the corresponding figure legends.
Immunoprecipitation and RT-PCR. Cytoplasmic extracts derived from WT and Fmr1 KO P7 brain were subjected to immunoprecipitation using the monoclonal antibody 7G1-1 (18). RNA was extracted followed by RT-PCR analysis (Invitrogen). The sequence of primers used to detect MAP1B, MAP1A, and hypoxanthine phosphoribosyltransferase are available upon request.
Linear Sucrose Gradient Fractionation. Primary cultures of cortical neurons were incubated with cycloheximide (100 μg/ml) for 15 min to arrest polyribosome migration. Cytoplasmic extracts loaded on 15-45% (wt/vol) sucrose gradient were centrifuged at 39,000 rpm in a SW41 rotor for 60 min at 4°C and fractionated as described (9). EDTA-lysate was centrifuged on a parallel gradient lacking MgCl2 but containing 1 mM EDTA to dissociate polyribosomes into subunits. Total RNA was extracted from each fraction by phenol-chloroform extraction.
Antibodies and Immunodetection. For immunoblot analysis, the protein quantity of each sample was estimated by Bradford assay (Bio-Rad) before subjected to SDS/PAGE. Blots were stained by Ponceau S (Sigma) to confirm equal protein loading before immunoblot analysis. The primary antibodies were diluted as follows: 1C3, 1:1,000; translation initiation factor 5α (eIF5α), 1:5,000 (Santa Cruz Biotechnology); acetylated α-tubulin, 1:2,000 (Sigma); MAP1B, 1: 100,000 (a gift from I. Fischer, Drexel University, Philadelphia); and SMI-31, 1: 5,000 (Steinberg Monoclonals).
For indirect immunofluorescent staining, cells raised on coverslips were fixed in 3.7% paraformaldehyde, 0.05% glutaraldehyde, and 0.5% Triton X-100 in PHEMO buffer (0.068 M Pipes/0.025 M Hepes acid/0.015 M EGTA Na2/0.003 M MgCl2/10% DMSO) at room temperature, and incubated with the antibody against α-tubulin (Chemicon) and conjugated secondary antibody. Fluorescence was detected at room temperature by a Zeiss LMS510 confocal microscopic imaging system.
RNA Extraction and Analysis. Total RNA was extracted by using TRIzol (Invitrogen). The quantity of RNA was determined by OD260 reading and further confirmed by ethidium bromide-stained agarose gel electrophoresis. RNase protection analysis (RPA) was performed following standard protocols. The templates for RPA probes of MAP1B and GAP-43 were generated by RT-PCR of mouse brain RNA. Each RT-PCR fragment was cloned into pDrive (Qiagen, Valencia, CA), and sequence was confirmed to be 100% identical to those published previously. The GAPDH template was purchased from Ambion. Riboprobes were generated by in vitro transcription (Stratagene) in the presence of 32P-UTP (Amersham Pharmacia).
Results
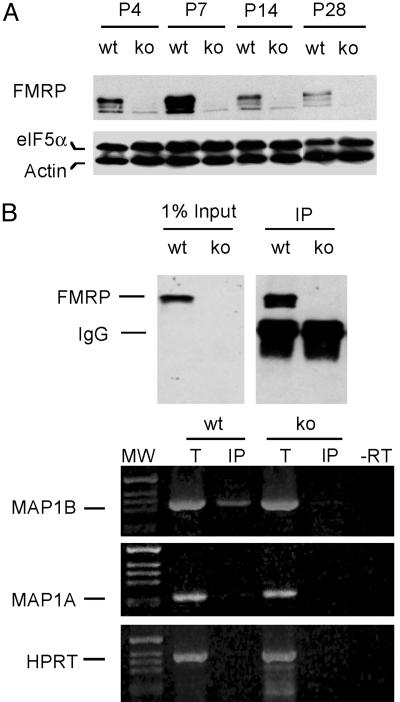
FMRP Is Up-Regulated in the Neonatal Brain, Selectively Associated with the MAP1B mRNA. To understand the role of FMRP in brain development, we first examined the expression profile of FMRP in the developing hippocampus. As shown in Fig. 1A, the level of FMRP reached a peak at the end of the first postnatal week, gradually declined thereafter, and was maintained at a moderate level through later development. As expected, FMRP was completely absent in the Fmr1 KO littermates at all ages examined. A similar expression profile of FMRP was also observed in the cerebellum during neonatal development (data not shown). The peak of FMRP expression coincides with the maximal polyribosome localization beneath the dendritic spine in the normal brain (27) and with the most severe dendritic spine abnormality in the Fmr1 KO brain (6), suggesting a functional requirement for high levels of FMRP in translation-dependent synapse maturation.
Fig. 1.
FMRP is vigorously up-regulated in the neonatal hippocampus and is selectively associated with the MAP1B mRNA. (A) Immunoblot analysis of FMRP in hippocampus derived from WT and Fmr1 KO littermates. A band is detectable in the Fmr1 KO lysates at the position of FXR1P on a prolonged exposure, likely caused by cross-reaction of the antibody to FXR1P. The blot was reprobed with antibodies against actin and eIF5α as loading controls. (B) Association of MAP1B mRNA with FMRP in the neonatal brain. (Upper) The input and immunoprecipitation of FMRP on immunoblot using WT and Fmr1 KO P7 brain. (Lower) RT-PCR analysis of mRNAs in the total input lysate (T) and in the immunoprecipitated FMRP-mRNP complexes (IP). A reaction without reverse transcriptase (-RT) was carried out as a negative control. The signals derived from MAP1B, MAP1A, and hypoxanthine phosphoribosyltransferase (HPRT) mRNA are marked on the left.
Among the proteins encoded by FMRP-associated brain mRNAs, MAP1B undergoes vigorous regulation when FMRP expression reaches the peak (28), therefore is a candidate for FMRP-dependent translation regulation during active synaptogenesis. As shown in Fig. 1B, abundant MAP1B mRNA was detected in the immunoprecipitated FMRP-mRNP complexes isolated from WT P7 brain. In contrast, no MAP1B mRNA was detected in the immunoprecipitates derived from age-matched Fmr1 KO brain in parallel experiments. Interestingly, the mRNA encoding MAP1A, a neuronal MAP harboring high levels of sequence and functional similarity to MAP1B (29), was negligible in the FMRP-mRNP complexes. In addition, as a negative control, the mRNA encoding the house keeping protein hypoxanthine phosphoribosyltransferase was absent in the FMRP-mRNP complexes. These results indicate that FMRP selectively interacts with MAP1B mRNA in the neonatal brain, consistent with the idea that MAP1B is a target of FMRP during synapse development.
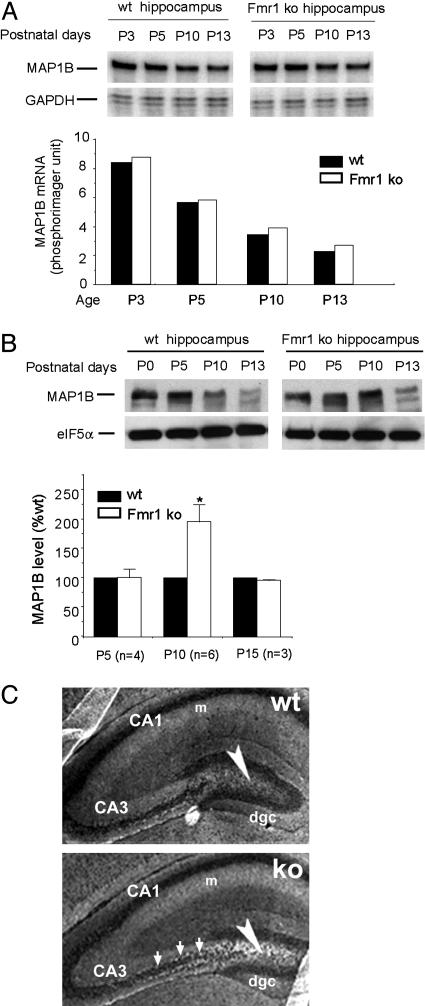
The Absence of FMRP Results in Delayed MAP1B Decline in the Developing Hippocampus. To determine how FMRP may influence the developmentally programmed MAP1B expression, we compared the level of MAP1B gene expression in the hippocampus derived from WT and Fmr1 KO mice. By using an RPA, we detected a gradual decline in the steady-state levels of MAP1B mRNA in WT hippocampus within the first two postnatal weeks (Fig. 2A). The quantity of hippocampal MAP1B mRNA was nearly identical in Fmr1 KO mice as that in the age-matched WT controls, demonstrating that the lack of FMRP did not affect the steady-state level of the MAP1B mRNA. On immunoblot, MAP1B protein started to decline after P5 in the WT hippocampus (Fig. 2B), coinciding with the up-regulated expression of FMRP (Fig. 1 A). However, the decline of MAP1B protein was delayed in the Fmr1 KO hippocampus, resulting in higher levels of MAP1B protein, with the most obvious difference detected at P10 (Fig. 2B). Immunofluorescent staining further revealed that the abnormally elevated MAP1B expression in the Fmr1 KO hippocampus was more obviously visualized in the hilus area and the mossy fiber terminals (Fig. 2C) that undergo vigorous neuronal network construction at this stage of development. These data support the hypothesis that FMRP functions to suppress MAP1B translation, and the absence of FMRP leads to misregulated MAP1B translation when MAP1B is subjected to the developmentally programmed down-regulation.
Fig. 2.
Misregulation of the developmentally programmed expression of MAP1B protein in Fmr1 KO hippocampus. (A) Comparable levels of MAP1B mRNA detected in the developing WT and Fmr1 KO hippocampus by RPA. MAP1B mRNA was quantified by a phosphorimager, normalized to the GAPDH mRNA, and illustrated in Lower. (B) Immunoblot analysis reveals a delayed decline of MAP1B protein in the Fmr1 KO hippocampus, most obvious at P10. The signal of MAP1B was quantified by the NIH image software and normalized to that of eIF5α. The average of MAP1B level in each WT litter was defined as 100%. The average of MAP1B level in each litter of the Fmr1 KO mice on the same immunoblot was normalized to that of the WT age-matched control. Results derived from multiple litters were subjected to standard t test. *, P < 0.05. (C) Immunofluorescent staining of MAP1B in WT and Fmr1 KO P7 hippocampus. MBP1B is mainly detected in the molecular layer (m) of CA1 and CA3 pyramidal cells and the hilus (arrowhead). An increase of MAP1B staining is more obviously detected in the hilus (arrowhead) and mossy fiber terminals (arrows) in the Fmr1 KO hippocampus. dgc, dentate gyrus granular cell layer.
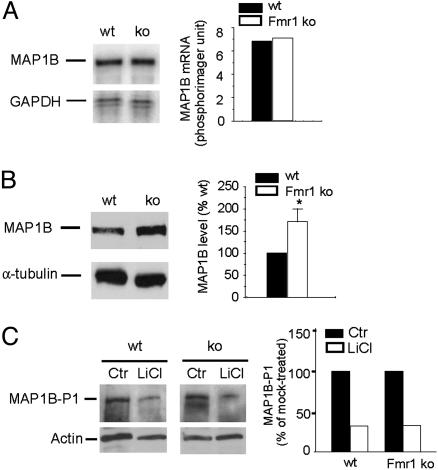
MAP1B Translation Is Abnormally Elevated in Primary Cultured Fmr1 KO Neurons. An important issue is to determine whether MAP1B is indeed misregulated in Fmr1 KO neurons, because expression of MAP1B is also found in the developing glia (30). To address this question, we compared MAP1B expression in primary cultured cortical neurons derived from embryonic day 16 WT and Fmr1 KO brain in parallel experiments. We found that, despite the normal quantity of MAP1B mRNA expression in the Fmr1 KO neurons (Fig. 3A), the level of MAP1B protein was significantly elevated (Fig. 3B).
Fig. 3.
Abnormally elevated MAP1B protein expression in primary cultures of Fmr1 KO cortical neurons. Parallel cultures were raised from embryonic day 16 brain of WT and Fmr1 KO mice, maintained in culture for 3 days before analysis for MAP1B expression. (A) Comparable MAP1B mRNA expression in WT and Fmr1 KO neurons. (Left) A representative image by RPA. (Right) The quantitative analysis by phosphorimager analysis normalizing MAP1B mRNA signal to that of GAPDH. (B) Immunoblot analysis reveals elevated MAP1B protein expression in Fmr1 KO neurons. The signal of MAP1B was normalized to that of house keeping genes in seven independent parallel cultures and statistically analyzed as shown in Left. *, P < 0.05 by standard t test. (C) Regulation of MAP1B-P1 by GSK3β kinase. Primary cortical neurons were subjected to mock or 10 mM LiCl treatment for 4 h. MAP1B-P1 was detected by the monoclonal antibody SMI-31 on immunoblot and quantified as described in B.
Because the activity for MAP1B to modulate microtubule stability is regulated by both the quantity and the phosphorylation of MAP1B (31, 32), we next examined the GSK-3β kinase-dependent regulation of mode-1 phosphorylation of MAP1B (MAP1B-P1), which modulates the microtubule binding activity of MAP1B, in WT and Fmr1 KO neurons. When treated with lithium under concentrations that inhibit GSK-3β kinase activity (32), a comparable decrease of MAP1B-P1 was detected in both WT and Fmr1 KO neurons (Fig. 3C). Therefore, the regulation of MAP1B phosphorylation by GSK-3β appears unaffected by the absence of FMRP.
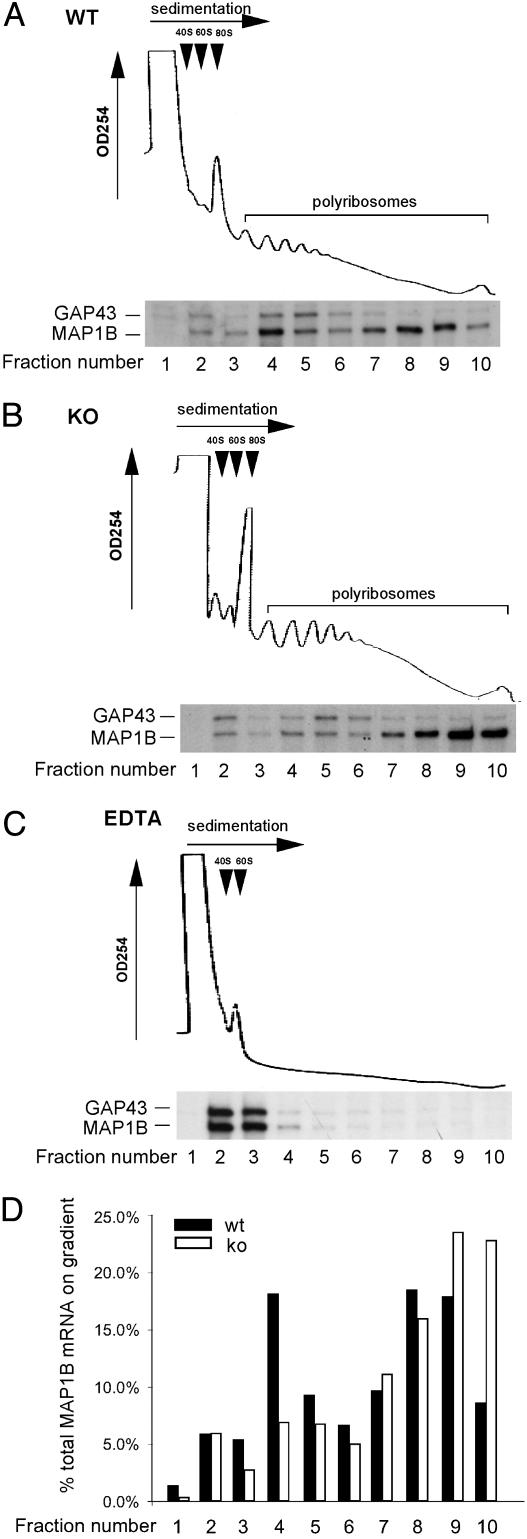
To test whether the abnormally increased MAP1B protein levels in Fmr1 KO neurons is indeed caused by misregulated translation, we analyzed the polyribosome profile of MAP1B mRNA in WT and Fmr1 KO primary cultured cortical neurons. As shown in Fig. 4 A and B, the majority of MAP1B mRNA was associated with translating polyribosomes (fraction 4-10) in both WT and Fmr1 KO neurons. EDTA-treatment dissociated polyribosomes into ribosomal subunits and released MAP1B mRNA into the mRNP fractions (Fig. 4C), supporting this interpretation. Noticeably, there was a clear shift of the MAP1B mRNA to the most actively translating polyribosomes in the Fmr1 KO neurons (fraction 9 and 10, Fig. 4 B and D). In contrast to MAP1B mRNA, the GAP43 mRNA that does not associate with FMRP (17) displayed nearly identical patterns of polyribosome association in WT and Fmr1 KO neurons (Fig. 4 A and B). These data are consistent with enhanced MAP1B translation caused by the absence of FMRP.
Fig. 4.
Polyribosome association of MAP1B mRNA in primary cultures of WT and Fmr1 KO neurons indicates misregulated translation. Cytoplasmic extracts were prepared in the presence of MgCl2 (A and B) or EDTA (C) for linear sucrose gradient fractionation. The sedimentation of translation components, including ribosome subunits (40S and 60S) and the 80S monoribosome and polyribosomes, were monitored by OD254 absorption as marked in each panel. MAP1B mRNA and GAP43 mRNA in each fraction were analyzed by RPA, and the corresponding signals are shown in correlation to fractions. (A) WT lysate fractionated on MgCl2 gradient. (B) Fmr1 KO lysate fractionated on MgCl2 gradient. (C) WT lysate fractionated on EDTA gradient. (D) Phosphorimager analysis of MAP1B mRNA distribution in A and B.
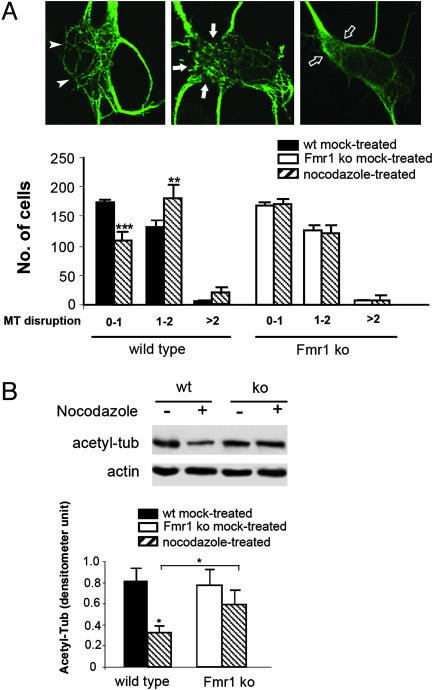
Fmr1 KO Neurons Are More Resistant to Microtubule Depolymerization Caused by Nocodazole. The primary function of MAP1B in neuronal development is to modulate microtubule dynamics (23, 33). Increased MAP1B expression is associated with microtubule stabilization (29, 34). Therefore, we next questioned whether the elevated MAP1B expression in Fmr1 KO neurons may result in aberrantly increased microtubule stability, which could limit microtubule dynamics in synaptic formation and remodeling. MAP1B has been shown to protect microtubule polymers from disassembly against the microtubule destabilization drug nocodazole (29), which can be used as an experimental index to assess the biological consequence of elevated MAP1B expression in Fmr1 KO neurons. We treated parallel cultures of WT and Fmr1 KO cortical neurons with low concentrations of nocodazole for 2 h, and evaluated the nocodazole-mediated microtubule disruption by indirect immunofluorescence confocal microscopy. As shown in Fig. 5A, nocodazole-treatment resulted in a significant increase in the number of WT neurons that harbor disrupted microtubule network, marked by either broken or dissolved microtubules. In contrast, such treatment did not have any effect on Fmr1 KO neurons. The increased resistance to nocodazole in Fmr1 KO neurons suggests an increase in microtubule stability, a predicted consequence of elevated MAP1B expression. High concentrations of nocodazole resulted in a severe disruption of microtubule networks and a blockade of neurite extension in both WT and Fmr1 KO neurons, indicating that the alterations in microtubule stability in Fmr1 KO neurons did not completely abrogate microtubule dynamics.
Fig. 5.
Abnormally increased microtubule stability in Fmr1 KO neurons. (A) Immunofluorescent staining of α-tubulin to visualize the microtubule networks. Cells were treated with 120 nM nocodazole for 2 h or mock-treated before being subjected to immunostaining. The disruption of microtubule polymers in the neuronal soma was scored as described below. Score 0 indicates no disruption of microtubule polymer, as indicated by arrowheads in Upper Left. Scores between 0 and 1 indicate negligible disruption of microtubule with majority of microtubule polymers remain intact. Scores between 1 and 2 indicate significantly disrupted microtubule polymers with obvious broken patches and dots, represented by the solid arrows in Upper Center. Scores larger than 2 indicate severely disrupted and dissolved microtubule networks, represented by open arrows in Upper Right. (Lower) The distribution of WT and Fmr1 KO neurons based on the above scale in mock- and nocodazole-treated cultures. Three hundred randomly selected neurons were subjected to the analysis in each parallel culture. Results from three independent experiments were subjected to statistical analysis. P < 0.05 by one-way ANOVA; **, P < 0.01; ***, P < 0.001 by standard t test when comparing mock- and nocodazole-treated cultures. (B) Immunoblot analysis of acetylated α-tubulin in WT and Fmr1 KO neurons with or without 120 nM nocodazole. The signal of acetylated α-tubulin was quantified by NIH image software and normalized to that of actin. Results from three independent experiments were subjected to statistical analysis. *, P < 0.05 by standard t test when comparing mock- and nocodazole-treated WT cells as well as by paired t test when comparing WT and Fmr1 KO cells after nocodazole treatment.
To more quantitatively evaluate the abnormal alterations of microtubule stability caused by the lack of FMRP, we measured the nocodazole-induced reduction of stable microtubule polymers in WT and Fmr1 KO neurons by immunoblot. In living cells, stable microtubule polymers are enriched with acetylated α-tubulin, whereas unstable microtubule polymers and soluble tubulin dimers are devoid of acetylated α-tubulin (35). Nocodazole inhibits microtubule polymerization, consequentially leading to microtubule depolymerization and reduced acetylated α-tubulin. As shown in Fig. 5B, exposing WT cortical neurons to nocodazole overnight resulted in a significant reduction of acetylated α-tubulin. However, such treatment resulted in only a minimal reduction of acetylated α-tubulin with no statistical significance in Fmr1 KO neurons. The increased resistance to nocodazole-mediated microtubule depolymerization in Fmr1 KO neurons leads us to conclude that microtubule stability is abnormally increased in fragile X neurons, and the elevated MAP1B translation caused by the loss of FMRP-dependent translation suppression is an obvious underlying mechanism for the aberrant microtubule dynamics.
Discussion
Our study demonstrates that FMRP binds to the MAP1B mRNA and acts to control the translation efficiency of MAP1B in brain neurons during neonatal development. The lack of FMRP-dependent translation suppression results in overproduction of MAP1B and aberrantly increased microtubule stability in Fmr1 KO neurons. This evidence suggests that FMRP is involved in regulating microtubule dynamics during brain neuron development. The activity-regulated localization of FMRP into dendrites and spines (36) further implicates the possibility for FMRP to regulate translation of MAP1B and microtubule dynamics within the individual dendrite and/or synapse, perhaps in response to neurotransmitter release. Considering the critical roles of microtubule dynamics in the development of neuronal networks, particularly in growth cone dynamics and synapse formation (32, 37-40), abnormal microtubule dynamics caused by FMRP deficiency provides a conceivable mechanism underlying the abnormalities in structural synaptic plasticity in fragile X mental retardation, represented by the delayed dendritic spine maturation in vivo (6) and the deficits in synapse formation in primary cultured neurons (41).
The essential role of MAP1B in supporting neurite extension has been well documented (34, 42). On the other hand, down-regulation of MAP1B in the neonatal brain coincides with the completion of the vigorous extension of neuronal processes and the initiation of active synaptogenesis (28). It is not understood whether the developmentally programmed down-regulation of MAP1B is physiologically important or simply represents a disused phenomenon. A gradual reduction of hippocampal MAP1B mRNA was observed during the first two postnatal weeks (Fig. 2 A), most likely caused by reduced MAP1B transcription (43), which was unaffected by the absence of FMRP. Interestingly, a sharp decrease of MAP1B protein levels was observed after the elevated expression of FMRP (Fig. 1 A), suggesting a role of FMRP in accelerating the decline of MAP1B by suppressing MAP1B translation. This hypothesis is further supported by the delayed decline of MAP1B protein in the Fmr1 KO hippocampus (Fig. 2B). The elevated MAP1B expression in the hilus of dentate gyrus and mossy fiber pathway (Fig. 2C) is consistent with the previously reported abnormal mossy fiber distribution in adult Fmr1 KO brain (44), suggesting a possibility for abnormal neuronal network formation that may contribute to the increased susceptibility to seizures in the Fmr1 KO mice and fragile X patients (45, 46). The fact that the delayed decline of MAP1B temporally associates with the most severe dendritic spine abnormality in the Fmr1 KO neurons in vivo also indicates the functional importance of MAP1B down-regulation in synapse development. At the adult stage, low levels of MAP1B expression is limited to brain neurons that are prone to drug and/or lesion induced plasticity (47, 48). In this regard, although misregulated MAP1B expression was more obviously detected in Fmr1 hippocampus in neonates (Fig. 2B), FMRP-dependent MAP1B regulation is likely used to govern the dynamic reconstruction of neuronal network throughout life. The fact that the Drosophila homologue of Fmr1 (dFmr1) regulates the microtubule-associated protein Futsch to control synapse development at the neuromuscular junction (24) further suggests that such regulation is evolutionarily conserved.
The enhanced polyribosome association of MAP1B mRNA in Fmr1 KO neurons (Fig. 4) indicates a loss of translation suppression by FMRP. The normal translation profile of mRNAs that are incapable for FMRP-association, represented by GAP43 mRNA (17), suggests that translation suppression of MAP1B mRNA depends on its biochemical association with FMRP, as demonstrated in the neonatal (Fig. 1B) and adult mouse brain (18). In addition, MAP1B mRNA harbors a G-quartet structure in the 5′ UTR, which has been shown to interact with FMRP directly in vitro (19). More recent studies raised the possibility that association of FMRP with its mRNA ligands may also involve micro-RNAs and other small noncoding RNAs (13, 21). Identification of sequence elements in MAP1B mRNA that mediate FMRP-dependent translation regulation in neuronal development is the next challenge for future studies.
An increasing number of recent reports indicate the critical role of MAP1B in modulating microtubule dynamics in the growing neurites, especially in the developing growth cones (23, 37, 38, 49) essential for neural network development. The elevated expression of MAP1B in Fmr1 KO neurons leads to aberrant microtubule dynamics (Fig. 5), which is expected to affect structural synaptic development. Abnormalities in dendritic spine maturation and synapse formation in the Fmr1 KO neurons (4-6, 41) are consistent with this view. Besides synaptic development, microtubule dynamics also controls the trafficking of neurotransmitter receptors to the synaptic junction (50). Thus, altered microtubule dynamics in Fmr1 KO neurons could affect neurotransmitter-dependent synaptic communication, as indicated by the abnormalities in glutamate receptor-mediated long-term plasticity (51). Moreover, interaction of MAP1B with actin (52) raises an intriguing possibility that MAP1B may be a link for the coordinated organization of microtubules and microfilaments to modulate growth cone dynamics and synaptic plasticity (23, 53).
In summary, our study demonstrates the functional requirement of FMRP in regulating the production of MAP1B in neonatal brain neurons, which governs the cytoskeleton dynamics for normal neuronal development and function. The absence of FMRP in fragile X neurons leads to elevated MAP1B expression due to the loss of translation suppression of the MAP1B mRNA. This, in turn, may result in more rigid cytoskeleton, which could account for the deficits of synaptic plasticity in fragile X mental retardation.
Acknowledgments
We thank Dr. I. Fischer for the MAP1B antibody and Dr. P. Giannakakou for advice on microtubule microscopy. This work is supported by National Institutes of Health Grant 5 PO1 HD35576 (to Y.F. and S.T.W.).
Author contributions: Y.F. designed research; R.L., H.W., Z.L., L.K., W.T.O., W.L., and Y.F. performed research; S.T.W. contributed new reagents/analytic tools; R.L., H.W., and Z.L. analyzed data; and S.T.W. and Y.F. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: FMRP, fragile X mental retardation protein; mRNP, messenger ribonucleoprotein; KO, knockout; MAP1B, microtubule-associated protein 1B; Pn, postnatal day n; eIF5α, translation initiation factor 5α; RPA, RNase protection analysis.
References
- 1.Jin, P. & Warren, S. T. (2003) Trends Biochem. Sci. 28, 152-158. [DOI] [PubMed] [Google Scholar]
- 2.O'Donnell, W. T. & Warren, S. T. (2002) Annu. Rev. Neurosci. 25, 315-338. [DOI] [PubMed] [Google Scholar]
- 3.Bardoni, B. & Mandel, J. L. (2002) Curr. Opin. Genet. Dev. 12, 284-293. [DOI] [PubMed] [Google Scholar]
- 4.Reiss, A. L., Aylward, E., Freund, L. S., Joshi, P. K. & Bryan, R. N. (1991) Ann. Neurol. 29, 26-32. [DOI] [PubMed] [Google Scholar]
- 5.Irwin, S. A., Galvez, R. & Greenough, W. T. (2000) Cereb. Cortex 10, 1038-1044. [DOI] [PubMed] [Google Scholar]
- 6.Nimchinsky, E. A., Oberlander, A. M. & Svoboda, K. (2001) J. Neurosci. 21, 5139-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwin, S. A., Patel, B., Idupulapati, M., Harris, J. B., Crisostomo, R. A., Larsen, B. P., Kooy, F., Willems, P. J., Cras, P., Kozlowski, P. B., et al. (2001) Am. J. Med. Genet. 98, 161-167. [DOI] [PubMed] [Google Scholar]
- 8.Comery, T. A., Harris, J. B., Willems, P. J., Oostra, B. A., Irwin, S. A., Weiler, I. J. & Greenough, W. T. (1997) Proc. Natl. Acad. Sci. USA 94, 5401-5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng, Y., Absher, D., Eberhart, D. E., Brown, V., Malter, H. E. & Warren, S. T. (1997) Mol. Cell 1, 109-118. [DOI] [PubMed] [Google Scholar]
- 10.Corbin, F., Bouillon, M., Fortin, A., Morin, S., Rousseau, F. & Khandjian, E. W. (1997) Hum. Mol. Genet. 6, 1465-1472. [DOI] [PubMed] [Google Scholar]
- 11.Khandjian, E. W., Huot, M. E., Tremblay, S., Davidovic, L., Mazroui, R. & Bardoni, B. (2004) Proc. Natl. Acad. Sci. USA 101, 13357-13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefani, G., Fraser, C. E., Darnell, J. C. & Darnell, R. B. (2004) J. Neurosci. 24, 9272-9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin, P., Zarnescu, D. C., Ceman, S., Nakamoto, M., Mowrey, J., Jongens, T. A., Nelson, D. L., Moses, K. & Warren, S. T. (2004) Nat. Neurosci. 7, 113-117. [DOI] [PubMed] [Google Scholar]
- 14.Laggerbauer, B., Ostareck, D., Keidel, E. M., Ostareck-Lederer, A. & Fischer, U. (2001) Hum. Mol. Genet. 10, 329-338. [DOI] [PubMed] [Google Scholar]
- 15.Li, Z., Zhang, Y., Ku, L., Wilkinson, K. D., Warren, S. T. & Feng, Y. (2001) Nucleic Acids Res. 29, 2276-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazroui, R., Huot, M. E., Tremblay, S., Filion, C., Labelle, Y. & Khandjian, E. W. (2002) Hum. Mol. Genet. 11, 3007-3017. [DOI] [PubMed] [Google Scholar]
- 17.Wang, H., Ku, L., Osterhout, D. J., Li, W., Ahmadian, A., Liang, Z. & Feng, Y. (2004) Hum. Mol. Genet. 13, 79-89. [DOI] [PubMed] [Google Scholar]
- 18.Brown, V., Jin, P., Ceman, S., Darnell, J. C., O'Donnell, W. T., Tenenbaum, S. A., Jin, X., Feng, Y., Wilkinson, K. D., Keene, J. D., et al. (2001) Cell 107, 477-487. [DOI] [PubMed] [Google Scholar]
- 19.Darnell, J. C., Jensen, K. B., Jin, P., Brown, V., Warren, S. T. & Darnell, R. B. (2001) Cell 107, 489-499. [DOI] [PubMed] [Google Scholar]
- 20.Miyashiro, K. Y., Beckel-Mitchener, A., Purk, T. P., Becker, K. G., Barret, T., Liu, L., Carbonetto, S., Weiler, I. J., Greenough, W. T. & Eberwine, J. (2003) Neuron 37, 417-431. [DOI] [PubMed] [Google Scholar]
- 21.Zalfa, F., Giorgi, M., Primerano, B., Moro, A., Di Penta, A., Reis, S., Oostra, B. & Bagni, C. (2003) Cell 112, 317-327. [DOI] [PubMed] [Google Scholar]
- 22.Todd, P. K., Mack, K. J. & Malter, J. S. (2003) Proc. Natl. Acad. Sci. USA 100, 14374-14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Billault, C., Jimenez-Mateos, E. M., Caceres, A., Diaz-Nido, J., Wandosell, F. & Avila, J. (2004) J. Neurobiol. 58, 48-59. [DOI] [PubMed] [Google Scholar]
- 24.Zhang, Y. Q., Bailey, A. M., Matthies, H. J., Renden, R. B., Smith, M. A., Speese, S. D., Rubin, G. M. & Broadie, K. (2001) Cell 107, 591-603. [DOI] [PubMed] [Google Scholar]
- 25.The Dutch-Belgian Fragile X Consortium (1994) Cell 78, 23-33. [PubMed] [Google Scholar]
- 26.Torre, E. R. & Steward, O. (1996) J. Neurosci. 16, 5967-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steward, O. & Falk, P. M. (1991) J. Comp. Neurol. 314, 545-557. [DOI] [PubMed] [Google Scholar]
- 28.Ma, D., Nothias, F., Boyne, L. J. & Fischer, I. (1997) J. Neurosci. Res. 49, 319-332. [DOI] [PubMed] [Google Scholar]
- 29.Noiges, R., Eichinger, R., Kutschera, W., Fischer, I., Nemeth, Z., Wiche, G. & Propst, F. (2002) J. Neurosci. 22, 2106-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulloa, L., Ibarrola, N., Avila, J. & Diez-Guerra, F. J. (1994) Glia 10, 266-275. [DOI] [PubMed] [Google Scholar]
- 31.Goold, R. G. & Gordon-Weeks, P. R. (2001) J. Cell Sci. 114, 4273-4284. [DOI] [PubMed] [Google Scholar]
- 32.Lucas, F. R., Goold, R. G., Gordon-Weeks, P. R. & Salinas, P. C. (1998) J. Cell Sci. 111, 1351-1361. [DOI] [PubMed] [Google Scholar]
- 33.Gordon-Weeks, P. R. & Fischer, I. (2000) Microsc. Res. Tech. 48, 63-74. [DOI] [PubMed] [Google Scholar]
- 34.Brugg, B., Reddy, D. & Matus, A. (1993) Neuroscience 52, 489-496. [DOI] [PubMed] [Google Scholar]
- 35.Baas, P. W. & Black, M. M. (1990) J. Cell Biol. 111, 495-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antar, L. N., Afroz, R., Dictenberg, J. B., Carroll, R. C. & Bassell, G. J. (2004) J. Neurosci. 24, 2648-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon-Weeks, P. R. (2004) J. Neurobiol. 58, 70-83. [DOI] [PubMed] [Google Scholar]
- 38.Mack, T. G., Koester, M. P. & Pollerberg, G. E. (2000) Mol. Cell. Neurosci. 15, 51-65. [DOI] [PubMed] [Google Scholar]
- 39.Buck, K. B. & Zheng, J. Q. (2002) J. Neurosci. 22, 9358-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz-Canada, C., Ashley, J., Moeckel-Cole, S., Drier, E., Yin, J. & Budnik, V. (2004) Neuron 42, 567-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braun, K. & Segal, M. (2000) Cereb. Cortex 10, 1045-1052. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Billault, C., Avila, J. & Caceres, A. (2001) Mol. Biol. Cell 12, 2087-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montesinos, M. L., Foucher, I., Conradt, M., Mainguy, G., Robel, L., Prochiantz, A. & Volovitch, M. (2001) J. Neurosci. 21, 3350-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ivanco, T. L. & Greenough, W. T. (2002) Hippocampus 12, 47-54. [DOI] [PubMed] [Google Scholar]
- 45.Berry-Kravis, E. (2002) Dev. Med. Child Neurol. 44, 724-728. [DOI] [PubMed] [Google Scholar]
- 46.Chen, L. & Toth, M. (2001) Neuroscience 103, 1043-1050. [DOI] [PubMed] [Google Scholar]
- 47.Popa-Wagner, A., Fischer, B., Schmoll, H., Platt, D. & Kessler, C. (1997) Exp. Neurol. 148, 73-82. [DOI] [PubMed] [Google Scholar]
- 48.Ma, D., Chow, S., Obrocka, M., Connors, T. & Fischer, I. (1999) Brain Res. 823, 141-153. [DOI] [PubMed] [Google Scholar]
- 49.Goold, R. G., Owen, R. & Gordon-Weeks, P. R. (1999) J. Cell Sci. 112, 3373-3384. [DOI] [PubMed] [Google Scholar]
- 50.Serge, A., Fourgeaud, L., Hemar, A. & Choquet, D. (2003) J. Cell Sci. 116, 5015-5022. [DOI] [PubMed] [Google Scholar]
- 51.Huber, K. M., Gallagher, S. M., Warren, S. T. & Bear, M. F. (2002) Proc. Natl. Acad. Sci. USA 99, 7746-7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Togel, M., Wiche, G. & Propst, F. (1998) J. Cell Biol. 143, 695-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dehmelt, L. & Halpain, S. (2004) J. Neurobiol. 58, 18-33. [DOI] [PubMed] [Google Scholar]