Abstract
Objective
Delirium is a highly prevalent syndrome of acute brain dysfunction among critically ill patients that has been linked to multiple risk factors such as age, pre-existing cognitive impairment, and use of sedatives; but to date the relationship between race and delirium is unclear. We conducted this study to identify whether African-American race is a risk factor for developing ICU delirium.
Design
A prospective cohort study.
Setting
Medical and Surgical ICUs of a university affiliated, safety-net hospital in Indianapolis, Indiana.
Patients
2087 consecutive admissions with 1008 African-Americans admitted to the ICU services from May 2009 to August 2012.
Interventions
None
Measurements and Main Results
Incident delirium defined as first positive Confusion Assessment Method for the ICU (CAM-ICU) result after an initial negative CAM-ICU; and prevalent delirium defined as positive CAM-ICU on first CAM-ICU assessment. The overall incident delirium rate in African-Americans was 8.7% compared to 10.4% in Caucasians (P: 0.26). The prevalent delirium rate was 14% in both African-Americans and Caucasians (P: 0.95). Significant age and race interactions were detected for incident delirium (P: 0.02), but not for prevalent delirium (P: 0.3). The hazard ratio for incident delirium for African-Americans in the 18–49 years age group compared to Caucasians of similar age was 0.4 (0.1– 0.9). The hazard and odds ratios for incident and prevalent delirium in other groups were not different.
Conclusions
African-American race does not confer any additional risk for developing incident or prevalent delirium in the ICU. Instead younger African-Americans tend to have lower rates of incident delirium compared to similar age Caucasians.
Introduction
Delirium is a syndrome of acute brain dysfunction characterized by altered consciousness with a reduced ability to focus, sustain, or shift attention that develops quickly and fluctuates over the course of the day.[1] Delirium is highly prevalent in critically ill patients;[2–6] and is associated with greater lengths of intensive care unit (ICU) and hospital stay, mortality, and cost of care.[3–5] Multiple risk factors such as age, pre-existing cognitive impairment, and use of sedatives have been implicated in delirium development in the ICU,[7–10] but thus far any relationship between race and delirium has not been systematically evaluated.
Race, either as a biological construct or a social construct has shown to be a contributing factor to various chronic disease conditions such as inflammatory bowel disease,[11] congestive heart failure[12] and cognitive impairment.[13–15] Recently, racial differences have been elucidated in critical illness with African-Americans having a higher prevalence of sepsis compared to whites,[16] higher rates of cardiac arrest,[17, 18] noncardiogenic acute respiratory failure,[19] and venous thromboembolism.[20] Race not only affects disease prevalence but also clinical manifestations, severity, and mortality.[12–14, 21] Although access to care has been the presumed default factor responsible for such disparities, recent evidence has shown that the relationship between race and disease is complicated with race modifying the disease through multiple mechanisms including genetic susceptibility, presence of comorbid conditions, socioeconomic status, and access to care.[12, 16, 22, 23] The attributable effect of race on chronic cognitive impairment has been mentioned in prior studies,[13, 14] but its contribution to acute brain dysfunction in the form of delirium in the ICU is unknown. We conducted a prospective, observational study with the objective to identify whether African-American race is independently associated with ICU delirium.
Materials and Methods
Indiana University Center for Aging Research (IU-CAR) is a research entity at Indiana University School of Medicine that has conducted multiple clinical studies on cognitive impairment over the last two decades.[24–28] A characteristic feature of all the studies is a significant representation of the African-American community with up to 50% of study subjects representing the minority race. In 2009, Indiana University Delirium working group consisting of IUCAR and the Pulmonary/ Critical Care division of Indiana University started a randomized, clinical trial known as the “Pharmacological Management of Delirium (PMD)” study.[29] As part of the enrollment process for the trial, patients admitted to the ICUs are screened daily for delirium. The presence of a large screening cohort with a significant number of African-American participants in the PMD trial allowed us to examine the relationship between African American race and delirium. This study was approved by the Research Compliance Administration of Indiana University-Purdue University at Indianapolis.
Study Setting
Consecutive patients admitted to ICU services of Wishard Memorial Hospital (WMH), now known as Eskenazi Health from May 2009 to August 2012 were included in this observational study. WMH is a 457-bed; university-affiliated, safety-net public hospital staffed by Indiana University School of Medicine faculty and house-staff. It has an 8-bed surgical ICU (SICU), and a 14-bed medical/coronary ICU (MICU).
Inclusion and Exclusion Criteria
Inclusion criteria: 1) admitted to the WMH ICUs and 2) age ≥ 18 years. Exclusion criteria: 1) not English speaking; 2) hearing impaired; 3) legally blind; 4) admitted with alcohol intoxication; 5) prisoners; 6) having an Axis 1 Psychiatric disorder; 7) persistently comatose as defined by Richmond Agitation Sedation Scale (RASS)[30] of −4 or −5 throughout the ICU stay; or 8) pregnant/nursing.
Screening and Outcome Measures
Richmond Agitation Sedation Scale (RASS)[30] and the Confusion Assessment Method for the ICU (CAM-ICU)[31] were used to assess patients’ sedation status and delirium respectively. Trained research assistants performed twice daily RASS and CAM-ICU assessments after patients’ ICU admission until the patients became delirious, died or were discharged from the ICU. A cut off of RASS ≥ −3 (i.e., any response to verbal stimulation) was used to identify CAM-ICU eligible patients. Patients were considered delirious if they had a positive CAM-ICU result, achieved by showing signs of acute change in mental status or fluctuating course, displaying features of inattention, and either disorganized thinking or altered level of consciousness.[31] Patients were deemed comatose if their RASS scores were −4 (responsive to physical but not to verbal stimulus) or −5 (unresponsive to verbal and physical stimulus).[30] Prevalent delirium was defined as a positive CAM-ICU result on the first delirium assessment during the ICU stay. Incident delirium was defined as first positive CAM-ICU assessment after an initial negative CAM-ICU. Delirium subtypes were classified based on the RASS assessment at the time of the first positive CAM-ICU screen. Hyperactive delirium was defined as a positive CAM-ICU plus a positive RASS (+1 to +4). Hypoactive delirium was defined as a positive CAM-ICU plus a neutral or negative RASS (0 to −3).
Data collection
Local electronic medical records, the Regenstrief Medical Record System (RMRS),32 was used to determine patients’ age, gender, race, insurance status, smoking and alcohol use, length of hospital stay and mortality. Race was documented within RMRS as reported by the patient or caregiver. Chronic Comorbidities and admission diagnoses were collected by reviewing ICD-9 codes in the RMRS. Drug exposure was assessed by active orders identified through the RMRS. Drugs with anticholinergic properties were classified using the Anticholinergic Cognitive Burden Scale.[32–34] Severity of illness was calculated through the Acute Physiology Score (APS) derived from the Acute Physiology and Chronic Health Evaluation (APACHE) III score.[35] Medicaid status and Wishard Advantage, a local health plan provided to patients without insurance were utilized as a socioeconomic adjustor variable.
Statistical Analysis
Variables are presented as mean and standard deviations for continuous variables and proportions for categorical variables. We used Chi-square tests to evaluate for differences in categorical variables and two sample t-tests for continuous variables across race for each of the outcomes. For continuous variables with skewed distributions such as length of stay and APS, we used the Wilcoxon-rank sum test in place of the two sample t-test. Logistic regression models were used to assess the relationship between race and prevalent delirium while adjusting for other demographic and socioeconomic factors, disease severity, and comorbidity. Cox proportional hazards regression was used to model the time to incident delirium. Time dependent variables were assessed differently for the two sets of models. For the prevalence analysis, medications and coma were only marked as yes if they occurred prior to the first CAM-ICU assessment. Ventilation status was marked as yes if patient was on mechanical ventilation prior to or during the CAM-ICU assessment. For the incidence analysis, medications and coma status was entered as time-dependent variables. Medications and coma would be counted as not present until after the day which they were prescribed or occurred. All data analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC).
Results
Study Population
2087 consecutive admissions from the MICU and SICU were included in the study after application of the exclusion criteria from May 2009 to August 2012. The prevalent cohort included all 2087 patients with an initial CAM-ICU evaluation. Only those with an initial negative CAM-ICU result and one or more subsequent CAM-ICU evaluations (1569 admissions) were included in the incident cohort. The mean age of patients in the incident cohort was 59.7 years with 48% African-Americans. The mean age of the study subjects in the prevalent cohort was 58.6 years with 48% African-Americans. 9794 RASS and CAM-ICU screens were performed during this time-period. Table 1 describes the baseline characteristics of both the incident and prevalent cohorts as stratified by race. There were more trauma patients in the Caucasian group as compared to African-Americans. Higher number of opioids, benzodiazepines, and haloperidol drugs were ordered in the Caucasian group. Both groups had comparable severity of illness and similar rates of mechanical ventilation and dementia diagnoses. Depression diagnosis was higher among Caucasians.
Table 1.
Patients’ characteristics of incident and prevalent delirium cohorts stratified by race
| Incident Cohort (n=1569) | Prevalent Cohort (n=2087) | |||||
|---|---|---|---|---|---|---|
| African- American (n=759) |
Caucasian (n=810) |
p- value |
African- American (n=1008) |
Caucasian (n=1079) |
p- value |
|
| Baseline demographics | ||||||
| Age mean (SD) | 59.3 (16.9) | 60.1 (14.4) | 0.80 | 58.3 (16.9) | 58.9 (14.6) | 0.77 |
| 18–49 n (%) | 188 (24.8) | 161 (19.9) | 0.03 | 275 (27.3) | 241 (22.4) | 0.01 |
| 50–64 n (%) | 273 (36.0) | 332 (41.0) | 363 (36.0) | 447 (41.4) | ||
| 65+ n (%) | 298 (39.3) | 317 (39.1) | 370 (36.7) | 391 (36.2) | ||
| Female n (%) | 397 (52.3) | 408 (50.4) | 0.44 | 521 (51.7) | 525 (48.7) | 0.16 |
| Insurance status | ||||||
| Medicaid/Wishard Advantage n (%) |
471 (62.5) | 449 (56.0) | 0.01 | 613 (61.3) | 597 (56.0) | 0.01 |
| Inpatient characteristics | ||||||
| APSa mean (SD) | 7.6 (4.5) | 7.5 (4.5) | 0.61 | 7.8 (4.7) | 7.7 (4.7) | 0.54 |
| Mechanical Ventilation n (%) |
187 (24.6) | 218 (26.9) | 0.30 | 275 (27.3) | 307 (28.4) | 0.55 |
| Sepsis n (%) | 161 (21.2) | 193 (23.8) | 0.21 | 236 (23.4) | 290 (26.9) | 0.07 |
| Acute Respiratory Failure n (%) |
191 (25.2) | 237 (29.3) | 0.07 | 310 (30.8) | 364 (33.7) | 0.14 |
| Trauma n (%) | 78 (10.3) | 110 (13.6) | 0.04 | 115 (11.4) | 154 (14.3) | 0.05 |
| Unit | ||||||
| MICUb n (%) | 535 (70.5) | 523 (64.6) | 0.01 | 713 (70.7) | 709 (65.7) | 0.01 |
| SICUc n (%) | 224 (29.5) | 287 (35.4) | 295 (29.3) | 370 (34.3) | ||
| Active Medications | ||||||
| Opioid Orderd n (%) | 468 (61.7) | 565 (69.8) | 0.001 | 592 (58.7) | 717 (66.4) | <0.001 |
| Benzodiazepine Ordere n (%) |
85 (11.2) | 124 (15.3) | 0.01 | 65 (6.4) | 76 (7.0) | 0.59 |
| Anticholinergic drug Order n (%) |
132 (17.4) | 131 (16.2) | 0.51 | 45 (4.5) | 61 (5.6) | 0.21 |
| Haloperidol Order n (%) |
54 (7.1) | 82 (10.1) | 0.03 | 52 (5.2) | 68 (6.3) | 0.26 |
| Chronic Comorbidities | ||||||
| Depression n (%) | 68 (9.0) | 105 (13.0) | 0.01 | 92 (9.1) | 130 (12.1) | 0.03 |
| Hypertension n (%) | 362 (47.7) | 405 (50.0) | 0.36 | 475 (47.1) | 508 (47.1) | 0.98 |
| Dementia n (%) | 163 (21.5) | 177 (21.8) | 0.85 | 214 (21.2) | 257 (23.8) | 0.15 |
| Smoking n (%) | 200 (26.4) | 245 (30.2) | 0.08 | 257 (25.5) | 325 (30.1) | 0.02 |
| History of alcohol abuse n (%) |
58 (7.6) | 84 (10.4) | 0.06 | 78 (7.7) | 104 (9.6) | 0.12 |
APS: Acute Physiology Score;
MICU: Medical intensive care unit;
SICU: Surgical intensive care unit:
Opioids includes morphine, fentanyl;
Benzodiazepines include lorazepam, midazolam
Incidence and Prevalence of Delirium in African Americans compared to Caucasians
150 patients develop incident delirium with an overall incidence rate of 9.5% (150/1569), whereas 293 patients in the prevalent cohort developed delirium (prevalent rate: 14%; 293/2087). The incidence in African-Americans was 8.7% (66/759) versus 10.4% in Caucasians (84/810) (P=0.26). The prevalence was 14.1% (142/1008) in African-Americans versus14% (151/1079) among Caucasians (P=0.95). The results did not change after adjusting for coma and other variables from Table 1. The median time to develop incident delirium was 4 (IQR 1–8) days. In both cohorts, there were higher numbers of hypoactive delirium cases as compared to hyperactive ones. Table 2 shows the incidence and prevalence results along with length of hospital stay and in-hospital mortality.
Table 2.
Clinical outcomes among African-Americans and Caucasians
| Outcomes | Incident Cohort (n=1569) | Prevalent Cohort (n=2087) | ||||
|---|---|---|---|---|---|---|
| African- American |
Caucasian |
p- value |
African- American |
Caucasian |
p- value |
|
| Delirium n (%) |
66 (8.7) | 84 (10.4) | 0.26 | 142 (14.1) | 151 (14.0) | 0.95 |
| Hyperactive (RASSa +1 to +4)n(%) |
7 (10.6) | 14 (16.6) | 0.16 | 22 (15.5) | 35 (23.2) | 0.13 |
| Hypoactive (RASS 0 to −3) n(%) |
59 (89.4) | 70 (83.4) | 0.53 | 120 (84.5) | 116 (76.8) | 0.40 |
| Coma n (%) |
139 (18.3) | 150 (18.5) | 0.91 | 189 (18.8) | 197 (18.3) | 0.77 |
| Length of hospital stay in days mean (SD) |
10.8 (11.1) |
10.9 (15.5) | 0.48 | 11.6 (12.3) |
11.9 (15.7) | 0.41 |
| In hospital mortality n (%) |
29 (3.9) | 33 (4.1) | 0.79 | 54 (5.4) | 55 (5.1) | 0.78 |
RASS; Richmond Agitation Sedation Scale.
As age has been associated with delirium, the cohorts were further divided into three groups based on age (18–49 years, 50–64 years, and ≥ 65 years). The incident delirium rate was 5.3% in African-Americans compared to 13% in Caucasians in the 18–49 years age-group (P=0.01); 9.9% vs 8.7% in 50–64 years age-group (P=0.65), and 9.7% vs 10.7% in the ≥ 65 years group (P=0.68). In the 18–49 years sub-group, delirium prevalence in African-Americans versus Caucasians was 10.9% vs 11.6% (P=0.79); 50–64 years (14.6% vs 16.3 %; P=0.49) and ≥ 65 years (16% vs 12.8%, P=0.21). Figure 1 shows the breakup stratified by race and age.
Figure 1.
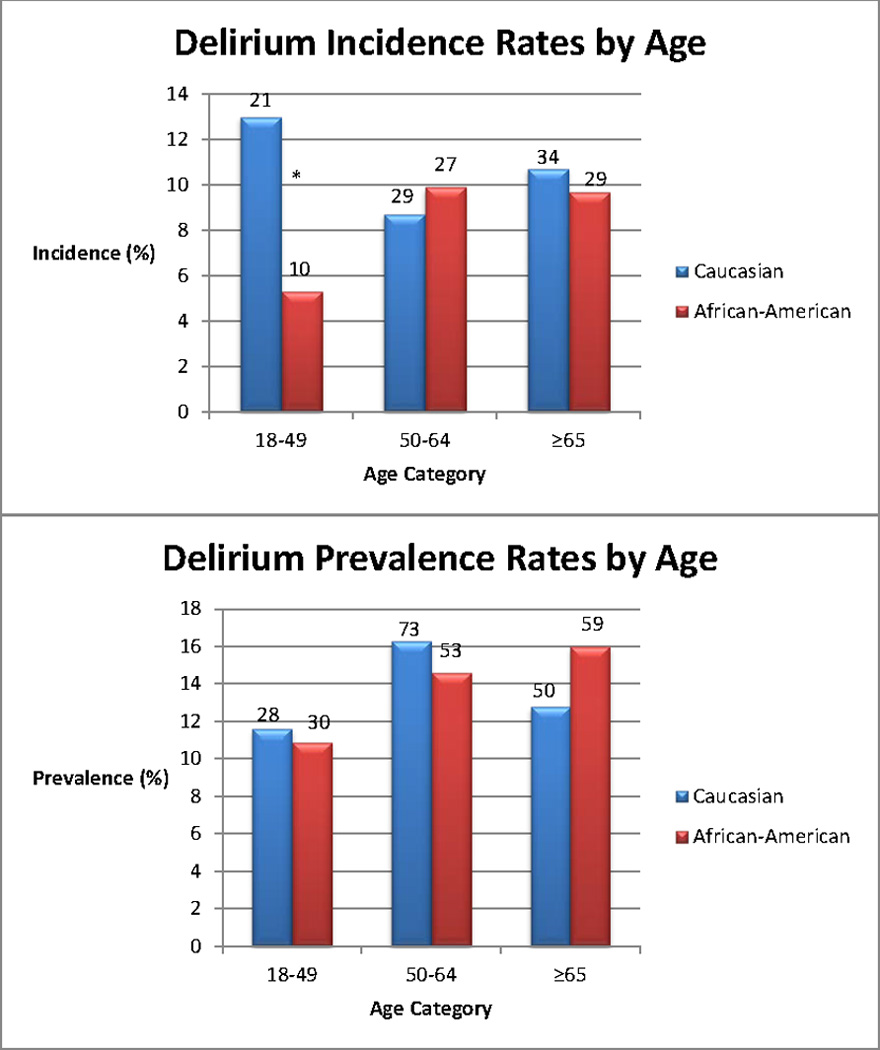
Incident and prevalent delirium rates in African-Americans and Caucasians
*P=0.01
Numbers on top of bars represent the number of delirious patients
Multivariate Analysis
The hazards ratio (HR) for incident delirium for African-Americans compared to Caucasians among all ages was 1.1 (CI: 0.8–1.6), whereas the odds ratio (OR) for prevalent delirium for African-Americans compared to Caucasians was 1.2 (CI: 0.8–1.7). After adjusting for baseline variables, an interaction term between race and delirium was tested for both incident and prevalent cohorts and was found to be significant for incident cohort, (P=0.02), but not for prevalent cohort (P=0.30). Table 3 shows the HR and OR for delirium associated with model variables after including the interaction term. Severity of illness, dementia and sepsis diagnoses, and haloperidol orders were associated with higher odds for prevalent delirium. Opioid orders and trauma diagnosis were associated with higher odds for incident delirium. The HR for African-Americans in the 18–49 years age group compared to similar age Caucasians was 0.4 (CI: 0.1–0.9) for incident delirium. The HR/ORs for incident or prevalent delirium in the other groups were not significant (Table 4). The interaction stayed significant with similar HR even with medications excluded from the final model.
Table 3.
Hazards Ratios (HR) and Odds Ratios (OR) for delirium in association with model variables
| Variables | Incident Cohort# HR (95% CI) |
Prevalent Cohort# OR (95% CI) |
|---|---|---|
| Female | 1.1 (0.8–1.6) | 1.0 (0.7–1.4) |
| Insurancea | 0.7 (0.5–1.0) | 1.0 (0.7–1.5) |
| APSb | 1.0 (1.0–1.05) | 1.0 (1.0–1.09) |
| Mechanical Ventilation | 3.0 (1.7–5.4) | 8.5 (5.0–14.3) |
| MICU* vs SICU# | 0.9 (0.6–1.4) | 0.6 (0.4–1.0) |
| Opioidc | 2.2 (1.1–4.3) | 1.5 (0.9–2.5) |
| Benzodiazepined | 0.7 (0.4–3.3) | 1.2 (0.7–2.0) |
| Anticholinergic | 1.3 (0.7–2.1) | 0.8 (0.4–1.7) |
| Haloperidol | 1.1 (0.7–1.7) | 2.7 (1.6–4.7) |
| Depression | 0.8 (0.4–1.6) | 1.4 (0.8–2.5) |
| Hypertension | 0.8 (0.5–1.2) | 0.9 (0.6–1.2) |
| Dementia | 0.7 (0.4–1.1) | 1.5 (1.0–2.2) |
| Sepsis | 1.2 (0.8–1.7) | 1.7 (1.1–2.4) |
| Acute Respiratory Failure |
0.9 (0.6–1.4) | 1.3 (0.8–1.9) |
| Trauma | 0.5 (0.3–0.9) | 1.0 (0.5–2.0) |
| Smoking | 0.9 (0.6–1.4) | 1.0 (0.7–1.5) |
| Alcohol | 1.4 (0.7–2.6) | 0.6 (0.3–1.3) |
| % RASSe 0 scores | 0.9 (0.97–0.99) | 0.06 (0.03–0.12) |
| Comaf | 1.2 (0.8–2.0) | 1.3 (0.8–1.9) |
MICU: Medical intensive care unit;
SICU: Surgical intensive care unit;
Medicaid/Wishard advantage;
APS: Acute Physiology Score;
Opioids includes morphine, fentanyl;
Benzodiazepines include lorazepam, midazolam;
RASS; Richmond Agitation Sedation Scale.
Race*Age interaction=0.02 for incident cohort; 0.30 for prevalent cohort
Table 4.
Multivariate analysis showing hazard (HR) and odds ratios (OR)* for delirium for African Americans compared to Caucasians
| Age (years) | Incident Cohort | Prevalent Cohort |
|---|---|---|
| HR (95% CI) | OR (95% CI) | |
| 18–49 | 0.4 (0.1 – 0.9) | 1.1 (0.5 – 2.1) |
| 50–64 | 1.7 (0.9 – 2.7) | 1.0 (0.6 – 1.7) |
| ≥ 65 | 1.3 (0.8 – 2.2) | 1.3 (0.9 – 1.8) |
Hazard and Odds Ratios, 95% confidence intervals, and p-values were calculated from the logistic regression model with the race by age interaction using appropriate contrasts
Discussion
ICU acquired delirium has been associated with multiple factors,[7–10, 36] but the relationship between race and development of delirium among critically ill adults has not been well defined. We sought to determine if African-American race is independently associated with ICU delirium compared to Caucasians. Our study findings do not indicate that African-American race is associated with a higher rate of delirium development in the ICU. On the contrary, incident delirium rates were found to be lower in younger African-Americans in the 18–49 years group as compared to Caucasians of similar age. No such differences were observed in the other age groups (50–64 years; ≥ 65 years). Given our strict definition of incident delirium requiring a negative CAM-ICU depicting a clear “brain-dysfunction free” period prior to a positive CAM-ICU, it seems that African-American race in younger patients may be a factor protecting this group from development of ICU acquired/incident delirium. This finding though did not translate into the prevalent cohort for younger African-Americans. This raises the question of an age-dependent interaction of race with ICU acquired risk factors. The very nature of this interaction given our limited understanding of the biological and social effects of race on delirium and other disease processes is not yet clear.
African-Americans tend to be disproportionately affected by disease conditions necessitating admission to the ICU such as sepsis,[16] cardiac arrests,[18] and respiratory failure[19] compared to whites. This coupled with the findings that they have a higher prevalence of cognitive impairment in the form of vascular dementia,[37] it seems to reason that they may have a higher delirium burden. This was not shown by our results as mentioned above. It could be because of comparable severity of illness scores and similar rates of mechanical ventilation between African-Americans and Caucasians in our study cohort along with equivalent number of sepsis, respiratory failure, and dementia diagnoses. We adjusted for all other relevant clinical factors that could contribute to delirium along with socioeconomic indicators through insurance status.
Further exploration of the 18–49 years age-group showed differences in the clinical characteristics between African-Americans and Caucasians with Caucasians more likely to have depression and be smokers. Caucasians were also in receipt of a higher number of benzodiazepine and opioid orders compared to African-Americans but the difference was not statistically significant (data shown in supplemental content Table). The differential rates of incident delirium in 18–49 age group persisted even after adjusting for the above factors and by analyzing the results without medication orders. The reasons behind lower rates of incident delirium among African-Americans are unclear and will need further exploration in future studies. The clinical implications of higher delirium incidence among younger Caucasians include raising awareness among healthcare personnel with implementation of personalized risk models along with targeted interventions to decrease delirium burden. Based on the vascular risk factors among African-Americans,[18, 37, 38] we were expecting a higher delirium burden in the ≥ 65 years age-group, but that was not shown by our results.
Similar to other studies evaluating the subtypes of delirium,[39, 40] we found a considerably higher number of hypoactive as opposed to hyperactive delirious cases on our first positive CAM-ICU screen. The rates of hyperactive and hypoactive delirium did not differ between African-Americans and Caucasians in both incident and prevalent cohorts. In contrast to other studies demonstrating a higher mortality among African-Americans,[22, 38, 41, 42] our in-hospital mortality rates were similar among African-Americans and Caucasians. A possible explanation may have been a similar case mix along with ready access to similar health care services and delivery at our local county hospital with majority of patients belonging to the urban greater Indianapolis area.
Evaluating the impact of race on disease prevalence, presentation, and clinical outcomes is complicated. Race could be classified purely as a genetic factor modifying the disease but this is an oversimplification as the physiologic changes resulting from gene disorders could never completely explain the disease burden discrepancy that currently exists between African-Americans and Caucasians. A better depiction of race could be organized taking into consideration the certain social factors including socioeconomic status, access to healthcare, and health habits. An omission of socioeconomic status and considering only the biologic race variable has the potential of introducing residual confounding with a bias assessment of association between race and particular disease of interest.[43] We tried to reduce residual confounding by adjusting for Indiana Medicaid and Wishard Advantage insurance status, an indigent care program unique to our hospital as a socioeconomic indicator in the final model.
Our study has several limitations. a) The study was conducted at a single site, a county hospital providing care to an urban, indigent population of Indianapolis metro area and hence applicability to other settings is limited. This on the other hand provides us with a large minority population allowing us to conduct our analysis with confidence to control for multiple variables. In addition, patients belong to a particular geographic area providing us with a homogenous group with similar access to health care. b) We did not have education or income status as proxy variables for socioeconomic status available for our patients. We instead utilized the insurance status as a socioeconomic indicator. Also the RASS and CAM-ICU assessments are not shown to be influenced by education. c) We used drug orders to adjust for drug exposure rather than drug dispensing, which may not accurately represent exposure to known delirium risk factors; however, we do not expect differences in medication administration due to race.
Our study also has several strengths. a) We had a diverse patient population with almost half African-Americans and half females in both African-Americans and Caucasian cohorts. b) We included all the relevant variables associated with delirium in the final analysis. c) As the study was conducted at a safety net hospital, our population irrespective of race had similar socio-demographic and socioeconomic characteristics. d) Valid and reliable sedation (RASS) and delirium (CAM-ICU) screening tools were administered. Unlike prior work determining the influence of race on cognitive impairment,[15] our measure of delirium was captured by trained research staff and not dependent on clinical documentation. e) Both RASS and CAM-ICU assessments were performed twice daily.
Conclusions
African-American race was not a risk factor for developing incident or prevalent delirium in the ICU. In fact younger African-Americans tend to have lower rates of incident delirium compared to Caucasians of similar age. This finding needs further confirmation in other studies.
Supplementary Material
Acknowledgments
The study was supported by a grant from the National Institute on Aging (R01AG034205). Dr. Khan’s work on the project was supported through a Career Development Award from the National Institute on Aging (NIA K23-AG043476).
Dr. Khan received support for article research from the National Institutes of Health (NIH). His institution received funding from NIA (RO1 AG 034205) and from NIA Career Development Award (NIA K23-AG 043476). Dr. Perkins received support for article research from the NIH. His institution received funding from the NIA, the NIH, and AHRQ. Dr. Hui received support for article research from the NIH. His institution received grant support from the NIH. Dr. Gao received support for article research from the NIH. His institution received grant support from the NIH. Dr. Campbell received support for article research from the NIH. His institution received grant support from the National Institute on Aging, Merck and Co., and Astellas Pharma US.
Footnotes
Work performed at: Indiana University School of Medicine, Indianapolis, IN
Conflicts of Interest:
The authors declare no relevant financial interests related to this manuscript.
Copyright form disclosures: The remaining authors have disclosed that they do not have any potential conflicts of interest.
Author Contributions:
NC and MF contributed to the conception and design and drafting the intellectual content of the manuscript. BK and MB contributed to the conception and design, analysis/interpretation of data, and drafting the intellectual content of the manuscript. AP, SH, and SJ contributed to conception and design and analysis/ interpretation of data.
References
- 1.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 2.Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32(11):2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 3.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 4.Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 6.Khan BA, Guzman O, Campbell NL, Walroth T, Tricker J, Hui SL, et al. Comparison and agreement between the Richmond Agitation-Sedation Scale and the Riker Sedation-Agitation Scale in evaluating patients' eligibility for delirium assessment in the ICU. Chest. 2012;142(1):48–54. doi: 10.1378/chest.11-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Rompaey B, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for intensive care delirium: a systematic review. Intensive Crit Care Nurs. 2008;24(2):98–107. doi: 10.1016/j.iccn.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JA, Jr, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 10.Zaal IJ, Devlin JW, Peelen LM, Slooter AJ. A systematic review of risk factors for delirium in the ICU. Crit Care Med. 2015;43(1):40–47. doi: 10.1097/CCM.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 11.Basu D, Lopez I, Kulkarni A, Sellin JH. Impact of race and ethnicity on inflammatory bowel disease. Am J Gastroenterol. 2005;100(10):2254–2261. doi: 10.1111/j.1572-0241.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 12.Deswal A, Petersen NJ, Souchek J, Ashton CM, Wray NP. Impact of race on health care utilization and outcomes in veterans with congestive heart failure. Am J Cardiol. 2004;43(5):778–784. doi: 10.1016/j.jacc.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 13.Cooper C, Tandy AR, Balamurali T, Livingston G. A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry. 2010;18(3):193–203. doi: 10.1097/JGP.0b013e3181bf9caf. [DOI] [PubMed] [Google Scholar]
- 14.Van Ryn M. Research on the provider contribution to race/ethnicity disparities in medical care. Med Care. 2002;40(1):I-140–I-151. doi: 10.1097/00005650-200201001-00015. [DOI] [PubMed] [Google Scholar]
- 15.Campbell NL, Cantor BB, Hui SL, Perkins A, Khan BA, Farber MO, et al. Race and Documentation of Cognitive Impairment in Hospitalized Older Adults. J Amer Geriatr Soc. 2014;62(3):506–511. doi: 10.1111/jgs.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. 2008;177(3):279–284. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker LB, Han BH, Meyer PM, Wright FA, Rhodes KV, Smith DW, et al. Racial differences in the incidence of cardiac arrest and subsequent survival. N Engl J Med. 1993;329(9):600–606. doi: 10.1056/NEJM199308263290902. [DOI] [PubMed] [Google Scholar]
- 18.Ehlenbach WJ, Barnato AE, Curtis JR, Kreuter W, Koepsell TD, Deyo RA, et al. Epidemiologic study of in-hospital cardiopulmonary resuscitation in the elderly. N Engl J Med. 2009;361(1):22–31. doi: 10.1056/NEJMoa0810245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooke CR, Erickson SE, Eisner MD, Martin GS. Trends in the incidence of noncardiogenic acute respiratory failure: the role of race. Crit Care Med. 2012;40(5):1532. doi: 10.1097/CCM.0b013e31824518f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heit JA, Beckman MG, Bockenstedt PL, Grant AM, Key NS, Kulkarni R, et al. Comparison of characteristics from White-and Black-Americans with venous thromboembolism: A cross-sectional study. Am J Hematol. 2010;85(7):467–471. doi: 10.1002/ajh.21735. [DOI] [PubMed] [Google Scholar]
- 21.Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: An analysis of multiple-cause mortality data (1979–1996)*. Crit Care Med. 2002;30(8):1679–1685. doi: 10.1097/00003246-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Soto GJ, Martin GS, Gong MN. Healthcare disparities in critical illness. Crit Care Med. 2013;41(12):2784–2793. doi: 10.1097/CCM.0b013e3182a84a43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosking JE, Ameratunga SN, Bramley DM, Crengle SM. Reducing ethnic disparities in the quality of trauma care: an important research gap. Ann Surg. 2011;253(2):233–237. doi: 10.1097/SLA.0b013e3182075553. [DOI] [PubMed] [Google Scholar]
- 24.Unützer J, Katon W, Callahan CM, Williams JW, Jr, Hunkeler E, Harpole L, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 25.Callahan CM, Boustani MA, Unverzagt FW, Austrom MG, Damush TM, Perkins AJ, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 295(18):2148–2157. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- 26.Counsell SR, Callahan CM, Clark DO, Tu W, Buttar AB, Stump TE, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 298(22):2623–2633. doi: 10.1001/jama.298.22.2623. [DOI] [PubMed] [Google Scholar]
- 27.Boustani MA, Campbell NL, Khan BA, Abernathy G, Zawahiri M, Campbell T, et al. Enhancing care for hospitalized older adults with cognitive impairment: a randomized controlled trial. JGIM. 2012;27(5):561–567. doi: 10.1007/s11606-012-1994-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan BA, Calvo-Ayala E, Campbell N, Perkins A, Ionescu R, Tricker J, et al. Clinical decision support system and incidence of delirium in cognitively impaired older adults transferred to intensive care. A J Crit Care. 2013;22(3):257–262. doi: 10.4037/ajcc2013447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell NL, Khan BA, Farber M, Campbell T, Perkins AJ, Hui SL, et al. Improving delirium care in the intensive care unit: The design of a pragmatic study. Trials. 2011;12(1):139. doi: 10.1186/1745-6215-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 31.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 32.Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311–320. [Google Scholar]
- 33.Campbell N, Boustani M, Lane K, Gao S, Hendrie H, Khan B, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology. 2010;75(2):152–159. doi: 10.1212/WNL.0b013e3181e7f2ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai X, Campbell N, Khan B, Callahan C, Boustani M. Long-term anticholinergic use and the aging brain. Alzheimers Dement. 2013;9:377–385. doi: 10.1016/j.jalz.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 36.Khan BA, Zawahiri M, Campbell NL, Fox GC, Weinstein EJ, Nazir A, et al. Delirium in hospitalized patients: implications of current evidence on clinical practice and future avenues for research—a systematic evidence review. J Hosp Med. 2012;7(7):580–589. doi: 10.1002/jhm.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miles TP, Froehlich TE, Bogardus ST, Inouye SK. Dementia and race: are there differences between African Americans and Caucasians? J Am Geriatr Soc. 2001;49(4):477–484. doi: 10.1046/j.1532-5415.2001.49096.x. [DOI] [PubMed] [Google Scholar]
- 38.Holmes JS, Arispe IE, Moy E. Heart disease and prevention: race and age differences in heart disease prevention, treatment, and mortality. Med Care. 2005;43(3):I-33–I-41. [PubMed] [Google Scholar]
- 39.Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, Shintani AK, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54(3):479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 40.Pandharipande P, Cotton BA, Shintani A, Thompson J, Costabile S, Pun BT, et al. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med. 2007;33(10):1726–1731. doi: 10.1007/s00134-007-0687-y. [DOI] [PubMed] [Google Scholar]
- 41.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 42.Erickson S, Shlipak M, Martin G, Wheeler A, Ancukiewicz M, Matthay M, et al. National Institutes of Health National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Racial and ethnic disparities in mortality from acute lung injury. Crit Care Med. 2009;37(1):1–6. doi: 10.1097/CCM.0b013e31819292ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufman JS, Cooper RS, McGee DL. Socioeconomic status and health in blacks and whites: the problem of residual confounding and the resiliency of race. Epidemiology. 1997:621–628. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


