CONSPECTUS
Light-responsive agents offer the promise of targeted therapy, whose benefits include (i) prolonged action at the target site, (ii) overall reduced systemic dosage, (iii) reduced adverse effects, and (iv) localized delivery of multiple agents. Although photoactivated prodrugs have been reported, these species generally require short wavelengths (<450 nm) for activation. However, maximal tissue penetrance by light occurs within the “optical window of tissue” (600–900 nm), well beyond the wavelength range of most existing photocleavable functional groups. Furthermore, since multidrug therapy holds promise for the treatment of complex diseases, from cancer to neurological disorders, controlling the action of multiple drugs via wavelength modulation would take advantage of a property that is unique to light. However, discrimination between existing photoresponsive moieties has thus far proven to be limited.
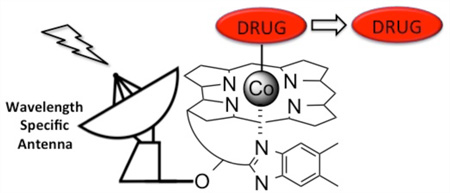
We have developed a vitamin B12/light-facilitated strategy for controlling drug action using red, far-red, and NIR light. The technology is based on a light-triggered reaction displayed by a subset of B12 derivatives: alkyl-cob(III)alamins suffer photohomolysis of the C–Co(III) bond. The C–Co(III) bond is weak (<30 kcal/mol), and therefore all wavelengths absorbed by the corrin ring (330–580 nm) induce photocleavage. In addition, by appending fluorophores to the corrin ring, long wavelength light (>600 nm) is readily captured and used to separate the Co-appended ligand (e.g., a drug) from B12. Consequently, it is now feasible to preassign the wavelength of homolysis by simply installing a fluorescent antenna with the desired photophysical properties. The wavelength malleability inherent within this strategy has been used to construct photoresponsive compounds that launch different drugs by simply modulating the wavelength of illumination. In addition, these phototherapeutics have been installed on the surface and interior of cells, such as erythrocytes or neural stem cells, and released upon expoure to the appropriate wavelength. We have shown that cytotoxic agents, such as doxorubicin, anti-inflammatories, such as dexamethasone, and anti- and pro-vascular agents are readily released from cellular vehicles as biologically active agents. We have also demonstrated that the concept of “optical window of tissue” phototherapeutics is not just limited to prodrugs. For example, stem cells have received considerable attention in the area of regenerative medicine. Hydrogels serve as scaffolds for stem cell growth and differentiation. We have shown that the formation of hydrogels can be triggered, in the presence of cells, using appropriately designed alkyl-cob(III)alamins and long wavelength light. The potential applications of phototherapeutics are broad and include drug delivery for a variety of indications, tissue engineering, and surgery.
Graphical abstract
1. INTRODUCTION
Light has been used in conjunction with photoresponsive agents1–4 to control the biochemistry of cells, manipulate the behavior of organisms, and treat a variety of pathological conditions. The applications of “phototherapeutics” are broad, including cancer therapy, tissue engineering, and surgery. Indeed, the opportunities associated with phototherapeutics have received considerable attention in the scientific literature as well as the popular media5 and culture.6 For example, an externally applied beam of light to perform surgery, as opposed to a scalpel, enjoys an array of potential advantages including (1) reduced exposure to infectious disease, (2) reduced reliance on anesthesia, (3) quicker recovery time, and (4) pinpoint accuracy along with the intriguing possibility of performing different procedures with different wavelengths of light (Figure 1). In this, and many other examples, light serves to trigger the action of dormant photoresponsive species at the site of illumination.
Figure 1.
Photosurgery in the 24th century? Wavelength-modulated illumination at the base of the brain triggers a series of surgical procedures to repair a traumatic brain injury. Photo Still from Star Trek: Voyager—Courtesy of CBS Television Studios.
A wide variety of light-responsive bioagents have been described, including biological/biochemical sensors, nucleic acids, enzyme substrates and inhibitors, proteins (optogenetics), and triggers for the generation of biomaterials.1–4 Most light-responsive species are prepared by covalently modifying an essential-for-activity functional group on the compound of interest.3 The modifying agent (often, but misleadingly, referred to as a “caging” agent) is a photocleavable moiety designed to furnish a biologically inactive species that can be restored to full activity upon photolysis. Although the simplicity of this idea is compelling, its application to the fields of biology and biomedicine faces a number of daunting hurdles. The most widely recognized challenge is that short wavelengths (<600 nm) do not readily penetrate the epidermis due to the presence of common biological chromophores, specifically melanin and hemoglobin. Furthermore, wavelengths beyond 950 nm are strongly absorbed by water. The sweet spot of tissue penetrance, namely, between 650–900 nm, is often referred to as the optical window of tissue. Unfortunately, the overwhelming majority of light-cleavable moieties suffer photolysis with UV or short visible light (<450 nm). One strategy to overcome this limitation is the construction red-shifted versions of photocleavable groups. However, this results in dramatically reduced photolytic quantum yields.7 The latter highlights another significant challenge, namely, “red and NIR photons have relatively low energy, limiting the range of processes they can initiate”.2 Nonetheless, red, far-red, and NIR wavelengths are tissue penetrating (up to 10 cm),8 are less subject to light scattering than shorter wavelengths, and offer a broad spectral range that could potentially be used to orthogonally control multiple photoagents.9 Consequently, a call has been put forth to extend “the wavelength coverage for activation… with visible and infrared light and [improve] the absorption properties for wavelength-selective, orthogonal activation”.2 A similar appeal has been made in the field of optogenetics.10
What kind of “processes” are triggered by red and near-IR photons? Two photon technology and upconverting nanoparticles transform the low energies of long wavelength photons into a form that drives high energy-requiring processes. Unfortunately, these approaches have their own unique set of limitations.11,12 Are there relatively weak covalent bonds that might be directly subject to photolysis by long wavelengths? One possibility is water-stable organometallic species. Indeed, a rich array of inorganic and organometallic photoactivatable systems has been described, including photodynamic agents (1O2 generators)13,14 and carriers of small gas molecules (CO,15 NO16). In addition, a few examples of the light-mediated release of larger bioactive compounds have also been reported, such as γ-amino butyric acid and glutamic acid.14,17 However, like their completely organic light-responsive brethren, these organometallic species generally require wavelengths shorter than the optical window of tissue to trigger photorelease (longer wavelength photolysis has been achieved via two-photon excitation18).
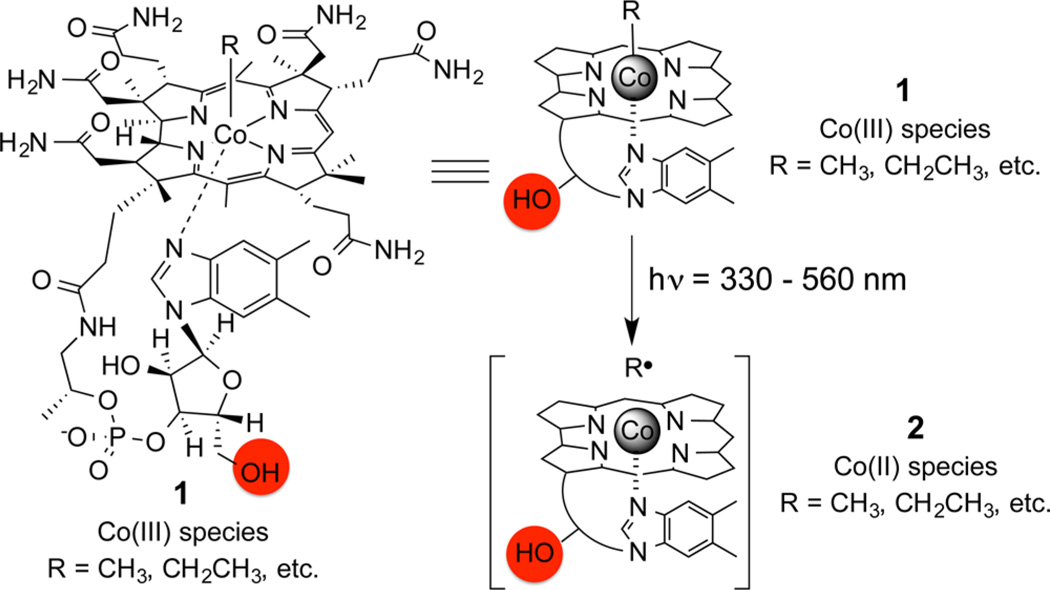
The photohomolysis of the C–Co bond in alkyl-cob(III)-alamins, 1 (alkyl-Cbls; R = CN, CH3, and adenosyl for vitamin B12), was noted a half-century ago (Scheme 1).19–22 The alkyl radical and Co(II) photoproducts (2) are identical to the intermediates formed in the enzyme-catalyzed reactions and thus offers a relatively simple means to model B12-dependent transformations. In addition, the C–Co bond is weak (<30 kcal/mol), and the energies supplied by any of the wavelengths absorbed by the corrin ring system (330–560 nm) are sufficient to induce cleavage with high quantum yields.23–26 We viewed multiwavelength-induced bond cleavage of alkyl-Cbls as a unique opportunity, one that we thought could be commandeered for biological and biomedical applications.
Scheme 1.
Structure of Vitamin B12 (R = CN, CH3, Adenosyl), 1, Shown in Full (left) and Schematic Forms (right)a
aPhotohomolysis of alkyl-Co(III)Cbl derivatives (R = alkyl) at wavelengths absorbed (330–560 nm) by the corrin ring furnish the Co(II)Cbl, 2, and Alkyl Radical Intermediates (R•).
2. EXPANSION OF THE PHOTOCHEMICAL TOOLKIT USING ALKYL-Cbls
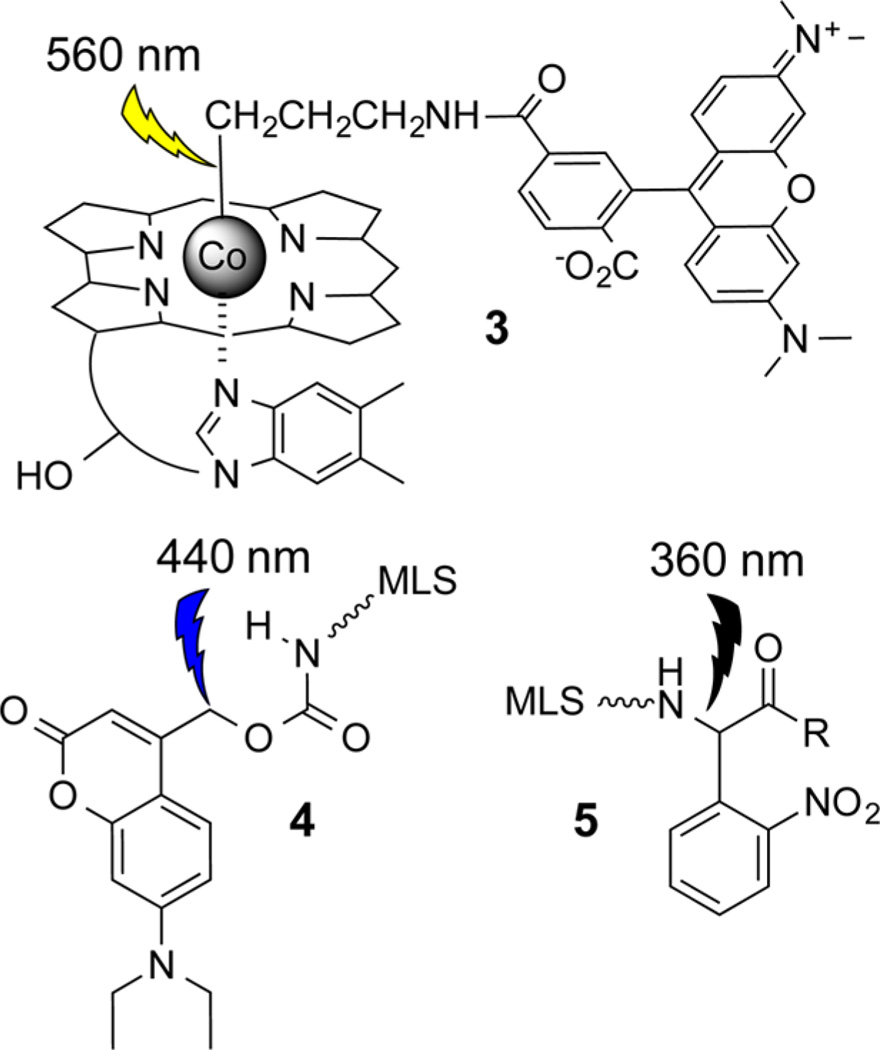
The o-nitrobenzyl group, which is sensitive to 330–360 nm, is the work horse of photomodulated bioreagents.27 However, there exists intense interest in expanding the toolkit of photoresponsive compounds to allow for the differential activation of more than one compound. For example, coumarin 4-ylmethyl modified bioagents2,28–33 are sensitive to longer wavelengths of light (400–450 nm) than o-nitrobenzyl substituted derivatives. We examined the potential utility of alkyl-Cbls as photoresponsive compounds by first preparing the derivative TAM-Cbl, 3 (Chart 1), which contains an appended TAM fluorophore moiety.34 Cbl quenches the fluorescence of a variety of fluorophores.35–38 We reasoned that this property could be useful since an increase in fluorescence would serve as a barometer of successful C–Co bond photohomolysis if the fluorophore were positioned on the bond undergoing photolysis.34,39–43 The ability to photolyze 3 in a wavelength selective fashion was assessed in the presence of UV-responsive 4 and blue-responsive 5 (Chart 1). These agents contain a mitochondrial localization sequence, which promotes mitochondrial association and was useful in visualizing the photocleavage event under biologically relevant conditions.34 As anticipated, the Cbl conjugate 3 undergoes photohomolysis at 560 nm, a wavelength that has no effect on 4 or 5, whereas 4 experiences photolysis at 440 nm, a wavelength that has no impact on 3. Finally, the latter is only susceptible to photolysis at 360 nm. Using this relatively simple set of compounds, we demonstrated the feasibility of issuing “commands” to individual entities via wavelength modulation, thereby demonstrating the potential of manipulating multiple, yet separate, biological processes using light alone.34 However, reliance upon the nitrobenzyl and coumarin moieties limits application to simple systems, such as cell culture. Furthermore, although 3 represents a profound 100 nm+ step in the right direction (560 nm versus 440 nm for 4 and 360 nm for 5), it still falls far short of breaching the optical window of tissue (650–900 nm).
Chart 1.
Wavelength-Distinguishable Photoresponsive Moieties 3–534
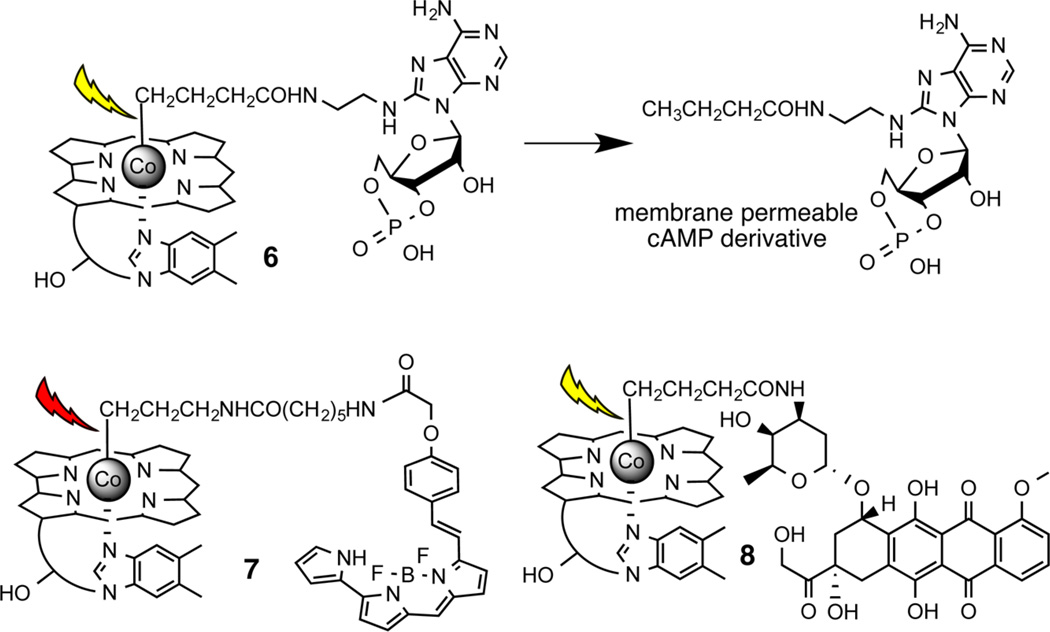
Light-responsive biological agents are commonly prepared via covalent modification of a functional group essential for biological activity.2,3 The substituted functionality is revealed upon illumination, thereby restoring activity to the molecule in question. Alcohols, amines, and carboxylates are common sites for substitution by photocleavable moieties. By contrast, we found that photohomolysis of RCH2-Co(III)Cbl primarily generates the alkyl, RCH3, product under cell culture conditions.44 It struck us as unlikely that attaching Cbl to “essential” methyl groups in biologically active compounds would serve as a general method for preparing light-activatable species. Initially, we wondered whether the steric bulk of the corrin ring might be sufficient to provide biological camouflage, precluding the interaction of inhibitors, activators, or sensors with their biological targets. For example, we prepared the Cbl modified cAMP derivative 6 (Chart 2) and examined its ability to activate the PKA protein kinase in the dark and following illumination at 530 nm.43 However, we found that the presence of the corrin ring fails to completely block biological activity. Although the “steric-bulk” strategy may ultimately find application in some systems, we sought a more reliable strategy for creating light-responsive Cbl-based agents.
Chart 2.
Cbl-Linked cAMP (6), BODIPY (7), and Dox (8)43a
aPhotolysis generates a membrane permeable bio-active agent (exemplified by the conversion of 6 to a cAMP derivative).
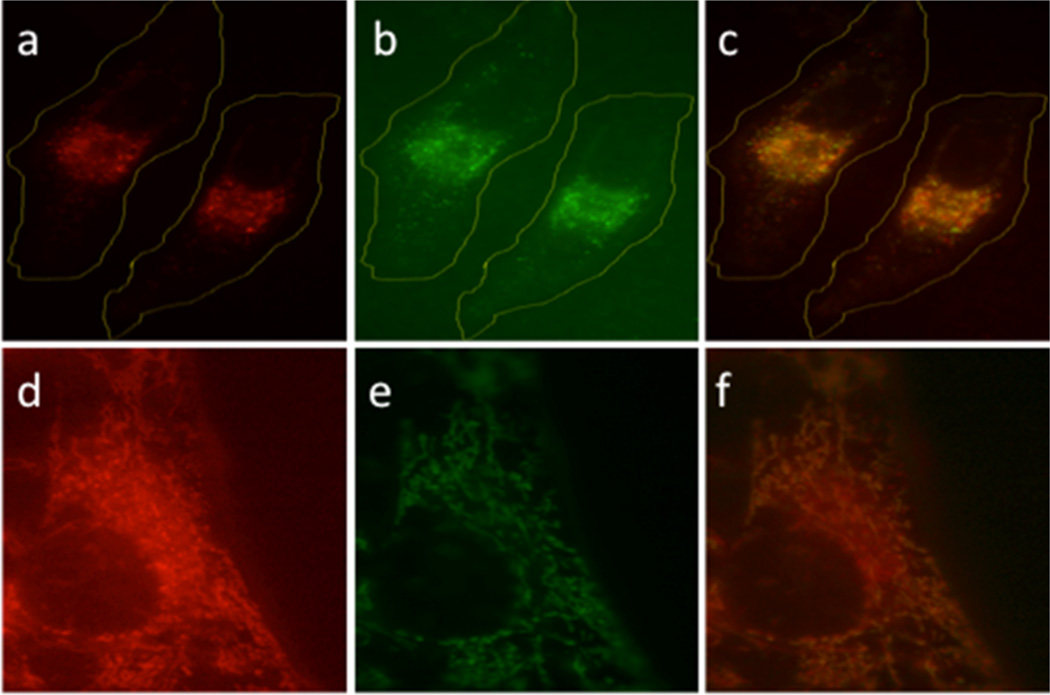
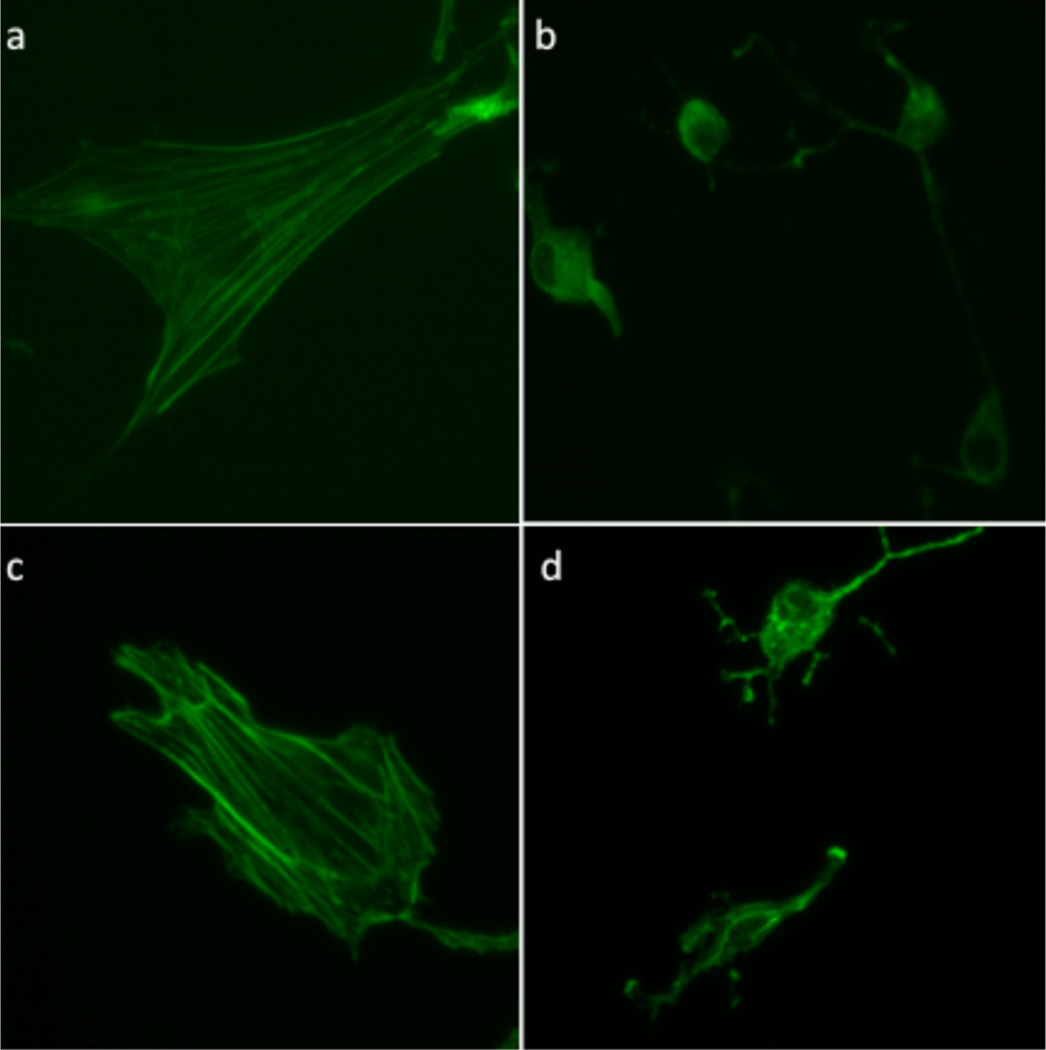
We have previously demonstrated that molecules can be biologically camouflaged by controlling their cellular location.40 Based on earlier reports, we anticipated that Cbl derivatives would be taken up and retained by endosomes,45,46 thereby offering an attractive means of keeping the reagent away from its intracellular biological target. Subsequent illumination should detach the cell-permeable cargo from the Cbl moiety, enabling escape from the endosomal depot. Our initial studies employed the BODIPY–Cbl conjugate 7 (Chart 2). The unattached BODIPY moiety freely enters cells and associates with mitochondria.47,48 By contrast, the conjugate 7 enters cells by receptor-mediated endocytosis and is retained in endosomes. Subsequent photolysis at 650 nm induces migration of the BODIPY fluorophore from endosomes to mitochondria (Figure 2).43 The use of Cbl as a membrane impermeant agent is not just limited to BODIPY. We also examined the effect of the cAMP derivative 6 on cell morphology prior to and following illumination. cAMP promotes significant morphological changes in cells, including loss of stress fibers and cell rounding. cAMP–Cbl 6 has no effect on cellular morphology in the absence of light. By contrast, illumination (530 nm) induces the expected loss in cellular stress fibers as well as cell shrinking and rounding (Figure 3). In an analogous vein, we synthesized Dox–Cbl 8 (Chart 2) and examined its cytotoxicity in HeLa cells.43 Dox is a powerful anticancer agent that, upon entering the nucleus, intercalates in DNA and thereby inhibits the progression of topoisomerase II. However, Dox’s severe cardiotoxicity is responsible for the intense interest in developing tumor-selective delivery strategies.49,50 As expected, cells simply incubated with 8 display no loss in viability.43 However, a light-dose-dependent increase in cell death is observed upon illumination (530 nm) in the presence of 8. The photorelease of cAMP from 6 and Dox from 8 occurs at a wavelength (530 nm) absorbed Cbl. By contrast, 650 nm triggers BODIPY–Cbl 7 photolysis, which is well beyond the wavelength range of the corrin ring.
Figure 2.
Light-induced (650 nm) translocation of BODIPY 650 in HeLa cells.43 (a) BODIPY–Cbl, 7, before photolysis. (b) Endosome visualization as revealed by an endosomal marker. (c) Overlay of panels a and b. (d) BODIPY–Cbl after photolysis. (e) Mitochondrial visualization as revealed by a mitochondria marker. (f) Overlay of panels d and e. Reproduced with permission from ref 43. Copyright 2014 John Wiley & Sons, Inc.
Figure 3.
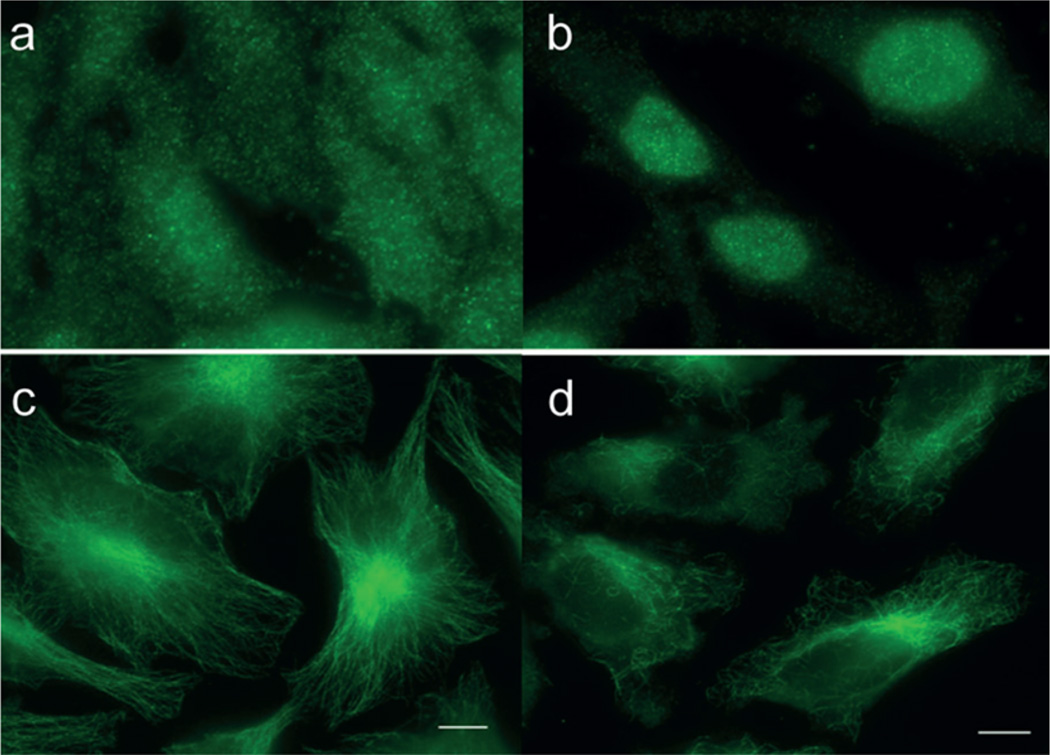
Light-triggered cAMP-Cbl (6)-mediated rearrangement of actin cytoskeleton in REF52 cells.43 Cells were exposed to 6, illuminated at 530 nm, and stained to visualize the actin stress fibers: (a) no treatment, media alone; (b) cell-permeable cAMP derivative as a positive control; (c) 6, no illumination; (d) 6 with illumination. Reproduced with permission from ref 43. Copyright 2014 John Wiley & Sons, Inc.
3. EXPANSION OF THE PHOTOCHEMICAL TOOLKIT INTO THE OPTICAL WINDOW OF TISSUE
The C–Co bond in alkyl-Cbl derivatives is weak (<30 kcal mol−1),20,25 and a back-of-the-envelope calculation suggests that wavelengths as long as 1000 nm might be sufficient to induce photohomolysis. Unfortunately, since Cbl only absorbs out to 560 nm, access to biomedically relevant wavelengths in the red, far-red, and near IR is, on first glance, simply not feasible. However, since the BODIPY derivative 7 undergoes photohomolysis at a wavelength (650 nm) beyond Cbl’s reach, we wondered whether fluorophores could act as antennas, capturing wavelengths that are otherwise invisible to the corrin ring system.
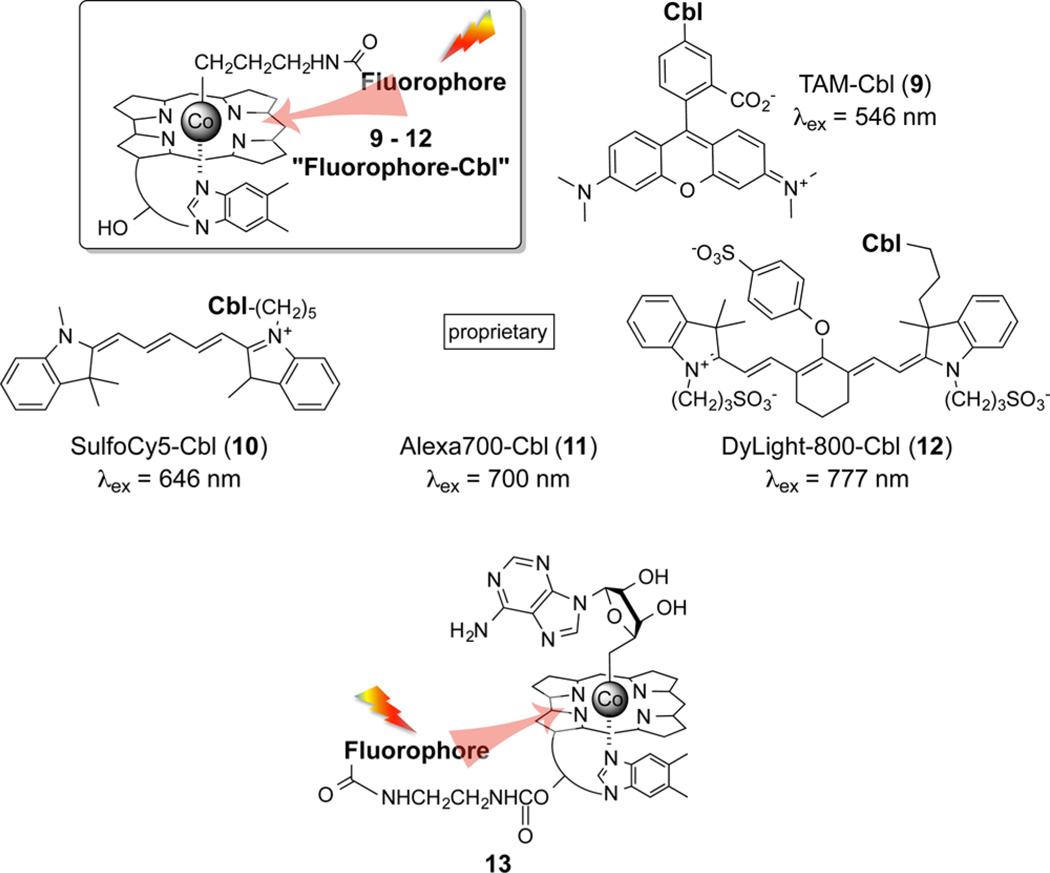
A series of alkyl-Cbl derivatives containing appended long wavelength fluorophores were prepared including SulfoCy5 (λex 647 nm, 9), AlexaFluor700 (λex 702 nm, 10), Atto725 (λex 730 nm, 11), and DyLight800 (λex 777 nm, 12) (Chart 3).43 In all cases, the fluorophore–Cbl conjugates undergo photohomolysis at wavelengths uniquely absorbed by the fluorophore. For example, we subjected a mixture of four photoresponsive compounds, each with distinct wavelength excitation profiles, to sequential illumination at 777, 700, 646, and 546 nm to obtain exquisitely controlled sequential homolysis of 12, 11, 10, and 9. Furthermore, since the excitation spectra of 12 and 10 are nonoverlapping beyond 600 nm, these compounds can be selectively photolyzed without resorting to a specific illumination sequence.43 However, the position occupied by the antennas in 9–12 is the photocleavable site, a position that we intended to reserve for drugs. Fortunately, the ribose ring hydroxyl (highlighted in red in 1) is readily modified, and this functional group serves as an ideal location to position the antenna. In a fashion analogous to 9–12, we prepared a family of long wavelength photoresponsive analogs of adenosyl-Cbl-fluorophore 13.43 Once again, the desired photocleavable wavelength (560–800 nm) is easily preassigned by simply installing the proper red, far-red, or NIR fluorescent antenna. As a side note, despite the structural complexity of these Cbl derivatives (as well as those described below), Cbl–fluorophore/drug conjugates are easily accessible in a few straightforward steps from readily available starting material and are stable at room temperature. We do take care to work under muted (indirect) lighting conditions that are nonetheless visually safe for detailed laboratory work.
Chart 3.
Photohomolysis of Fluorophore-Cbl Conjugates Where the Fluorophores Serve as Antennas To Capture 546 nm (9), 646 nm (10), 700 nm (11), and 777 nm (12) Wavelengths43a
aFluorophores appended to the ribose hydroxyl of Cbl likewise serve as antennas for wavelengths in the 650–800 nm realm, which trigger the release of Co-linked ligands (confirmed by LC-MS) as exemplified by release of the adenosyl moiety in 13.
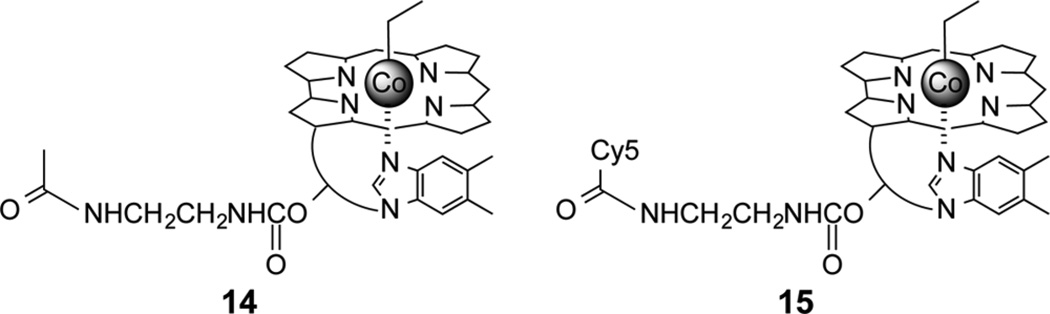
Do long wavelength responsive Cbls recognize deep tissue penetrating light under conditions that are otherwise challenging for shorter wavelengths? A dermal model, which mimics both various skin tones and the light scattering effects of tissue, was used to address this question.51 The concentration of melanin in this model is easily varied to simulate a variety of skin types from very pale (Fitzpatrick types I–II) to very dark (Fitzpatrick types V–VI). We illuminated, using the dermal tissue models as filters, unlabeled (14) and Cy5-labeled (15) ethyl-Cbl derivatives (Chart 4). As anticipated, in the absence of any skin type filter, both agents are rapidly photolyzed (confirmed by absorbance spectrometry and LC/MS) upon illumination at the appropriate wavelengths. However, introduction of even the palest Fitzpatrick level I–II model as a filter completely prevents the 520-nm-mediated photolysis of 14. By contrast, 660 nm illumination through either the Fitzpatrick level I–II filter or its darker III–IV counterpart photolyzes 15. Even in the presence of the darkest skin type (Fitzpatrick type V–VI), red light photolysis proceeds at only a slightly diminished pace. These results confirm the advantages of long wavelength responsive species as agents for mediating biochemical processes in tissue.
Chart 4.
Photohomolysis (520 nm) of 14 Is Compromised by Filters Mimicking Skin Types That Vary from Light to Dark;51 By Contrast, 15 Undergoes Photohomolysis at 660 nm in the Presence of All Skin Types
4. LONG WAVELENGTH TRIGGERED DRUG RELEASE FROM ALKYL-Cbls
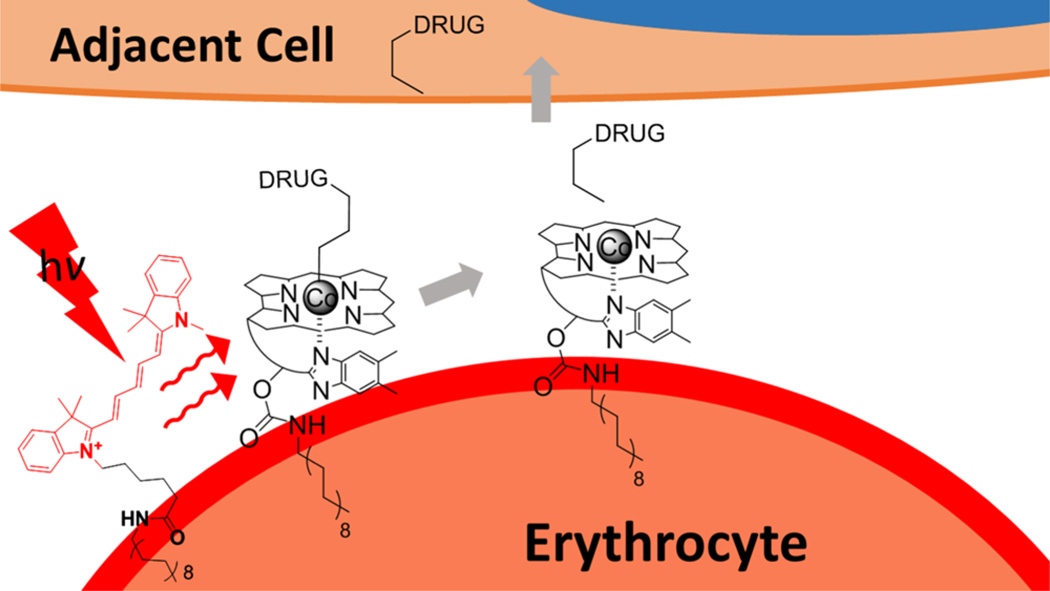
Photodynamic therapy (PDT) is used to treat a wide variety of conditions, including cancer.52 PDT employs photosensitizers that are selectively retained by the target tissue. Following sensitizer uptake, spatially focused illumination generates cytotoxic reactive oxygen species at the site of application.8 Despite the decades long effort in this field, PDT primarily remains a cytotoxic therapy, albeit one with undeniable clinical success. Is it possible to take full advantage of light’s extraordinary attributes to dramatically expand its biomedical applications? Can anti-inflammatories be selectively delivered to an arthritic knee, clotting agents to a hemorrhaging artery, or protein kinase inhibitors to a tumor? Can multiple drugs be applied to a diseased site in a specific sequence via wavelength modulation? We felt that affirmative answers to these questions would require a more robust delivery system than simple endosomal-mediated uptake as depicted in Figures 2 and 3. One obvious possibility is the use of nanoparticles as carriers of phototherapeutics. However, we decided, for our initial studies, to take advantage of the biological longevity and biocompatibility inherent with cell-based vehicles. For example, erythrocytes have a four-month life span, have a comparatively large size and thus potentially large loading capacity, and are easy to retrieve from and reintroduce into the patient. Finally, although erythrocytes have been described as the “the champions of drug delivery”, the use of red blood cells as therapeutic carriers has been limited by the inability to control drug release on command (a limitation potentially addressable with light).53
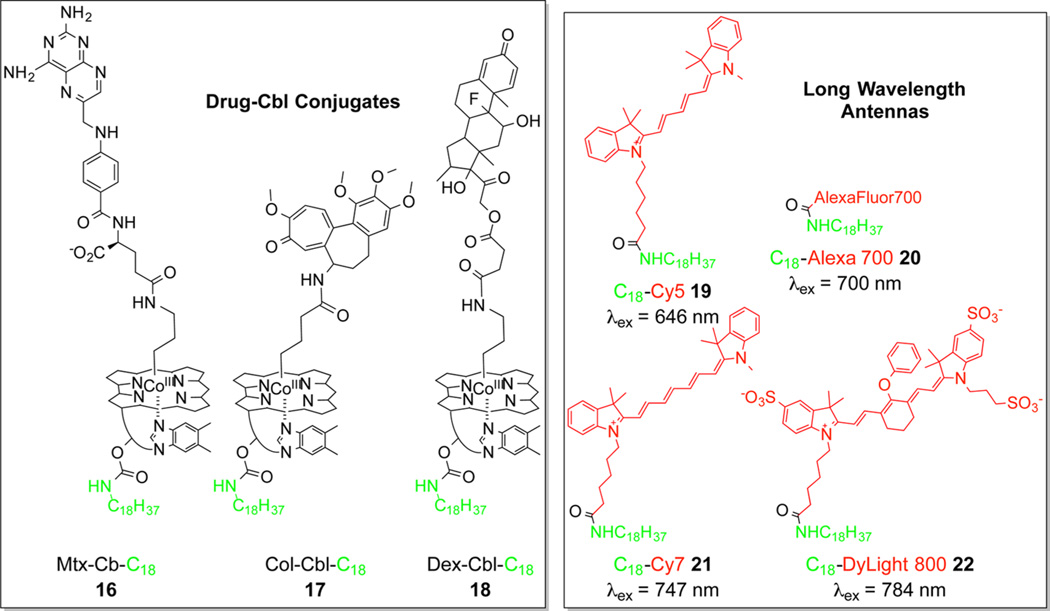
An assortment of lipidated (C18) conjugates of Cbl–drugs and fluorophores were prepared, with the expectation that the surface of erythrocytes would mediate the assembly of functional antenna/drug–Cbl arrays (Figure 4).44 We decided to focus on anti-inflammatory drugs based on their role in treating arthritis. Long-term use of anti-inflammatory agents can and does result in moderate to severe side effects. Not surprisingly, there is intense interest in targeting antiinflammatories to the site of inflammation in order to improve their therapeutic windows.54,55 The anti-inflammatory drugs methotrexate (Mtx), colchicine (Col), and dexamethasone (Dex) were conjugated to lipidated Cbls (16, 17, and 18, respectively; Chart 5). These conjugates were paired with different lipidated fluorophores (19–22; Chart 5) for assembly on the surface of blood cells. In all cases, phototherapeuticloaded erythrocytes were subsequently incubated with target adherent cell lines in the dark. Drug release from erythrocytes is only observed upon illumination at the proper wavelength. For example, Dex is released from RBCs loaded with 18/19 upon illumination at the wavelength (660 nm) recognized by Cy5. The released Dex induces the anticipated response in the target cells (Figure 5a,b). We found that it is quite straightforward to mix and match drug and antenna to create drug release systems with preassigned wavelength triggers. For example, 780 nm illumination of erythrocytes containing the DyLight800 antenna (22) and the surface anchored drug 17 liberates Col, which rapidly enters co-incubated cells leading to the loss of microtubule integrity (Figure 5c,d). Additionally, the presence of the Cy7 antenna (21) triggers the release of Mtx from surface-moored 16 at 725 nm. The liberated Mtx rapidly engages the dihydrofolate receptor in co-incubated cell lines. These studies establish that phototherapeutics assemble on the surface of carrier cells, tissue-penetrating wavelengths trigger drug release, and the wavelength of release is easily assigned. Furthermore, drug loading is not limited to cell surfaces on carrier cells but can be introduced into and photochemically released from both endosomes and the cytoplasm of cell carriers.56 For example, we have also successfully released drugs from neural stem cells,57 which display a pronounced ability to migrate to glioblastoma cells in vitro and tumors in vivo.58
Figure 4.
Self-assembly of a functional light-responsive drug release array on the surface of erythrocytes.44 Lipidated fluorophores serve as antennas, capturing the desired wavelength, which is then used to mediate drug release. The mechanism of “energy transfer” from antenna to drug–Cbl is currently under investigation. Reproduced with permission from ref 44. Copyright 2014 John Wiley & Sons, Inc.
Chart 5.
Drug–Cbl–Lipid Conjugates (16–18) and Lipidated Fluorophores (19–22) Self-Assemble on the Surface of Erythrocytes to Create Functional, Photoresponsive, Drug-Releasable Arrays44
Figure 5.
Impact of the light-triggered release of drugs from erythrocyte carriers on co-incubated cells.44 (a) HeLa cells incubated (in the dark) in the presence of erythrocytes containing the Dex conjugate 18 and the Cy5 antenna 19. HeLa cells are stained for the Dex receptor, GRα, which is cytoplasmic in the absence of Dex. (b) Illumination at 650 nm releases Dex from erythrocytes, which enters HeLa cells, binds to GRα, and induces nuclear localization. (c) HeLa cells incubated (in the dark) in the presence of erythrocytes containing the Col conjugate 17 and the DyLight antenna 22. HeLa cells are stained for microtubules. (d) Illumination at 780 nm releases Col from erythrocytes, which enters HeLa cells and binds to and depolymerizes microtubules. Reproduced with permission from ref 44. Copyright 2014 John Wiley & Sons, Inc.
5. ALKYL-Cbl-MEDIATED PHOTOPOLYMERIZATION OF HYDROGELS
Phototherapeutics, as broadly defined, are not just limited to the light-triggered release of drugs from cells or nanoparticles. A case in point are hydrogels, which have generated considerable biomedical interest in drug delivery and tissue engineering.4 These networks of cross-linked hydrophilic polymers mimic the softness and water content of natural tissue and serve in a number of capacities, including as scaffolds for implanted cells for use in tissue regeneration. The least invasive method of introduction is via injection of a viscous solution of pre-hydrogel and cargo (drug, cells, etc.) followed by gelation to create a spatially well-defined depot at the treatment site. This approach has the advantages that the (1) cargo is well dispersed throughout the hydrogel, (2) introduction of the hydrogel/cargo into the patient is simple, and (3) the low viscosity solution fills the diseased site and thus easily assumes an appropriate shape upon gelation. Transition from a viscous solution to a spatially discrete hydrogel is triggered by a number of methods, including photopolymerization. Photocross-linking of acrylated biopolymers with photoinitiators is typically conducted with α-hydroxyphenyl ketones, such as Irgacure 2959 (λmax < 350 nm). However, these short wavelengths limit curing depth and efficacy.59
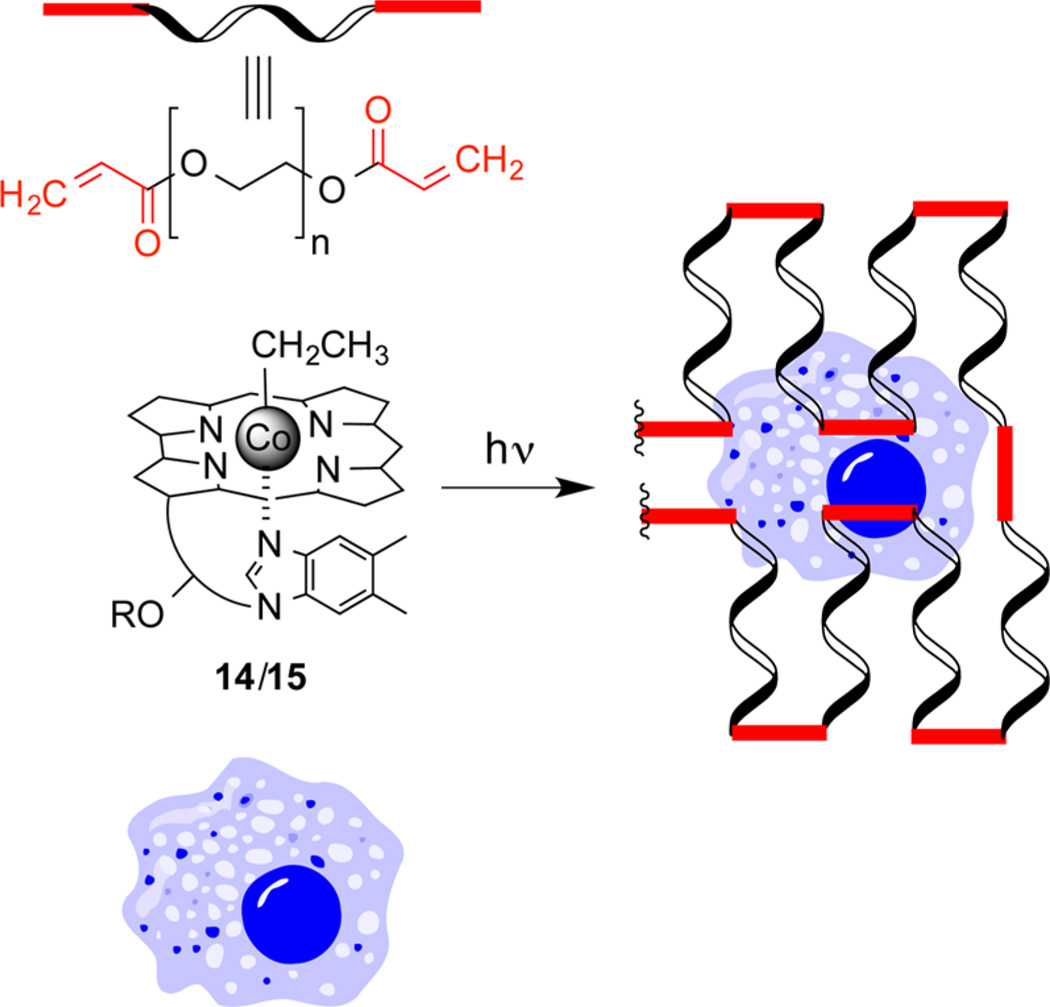
We developed a long wavelength photopolymerization hydrogel strategy (Scheme 2) that bears resemblance to the C–Co homolysis drug delivery approach outlined in Scheme 1.51 Both approaches use antennas to capture long wavelength light to induce C–Co homolysis. However, the drug-linked radical intermediate in Scheme 1 is rapidly quenched, generating a freely diffusible cell-permeable drug. By contrast, the ethyl radical in Scheme 2 is formed in the presence of high concentrations of acrylated monomers or biopolymers, inducing polymerization and gelation.
Scheme 2.
Photohomolysis of 14 or 15 at Wavelengths 520 or 660 nm, respectively, Generates an Ethyl Radical, Which Triggers Hydrogel Formation in the Presence of Cells
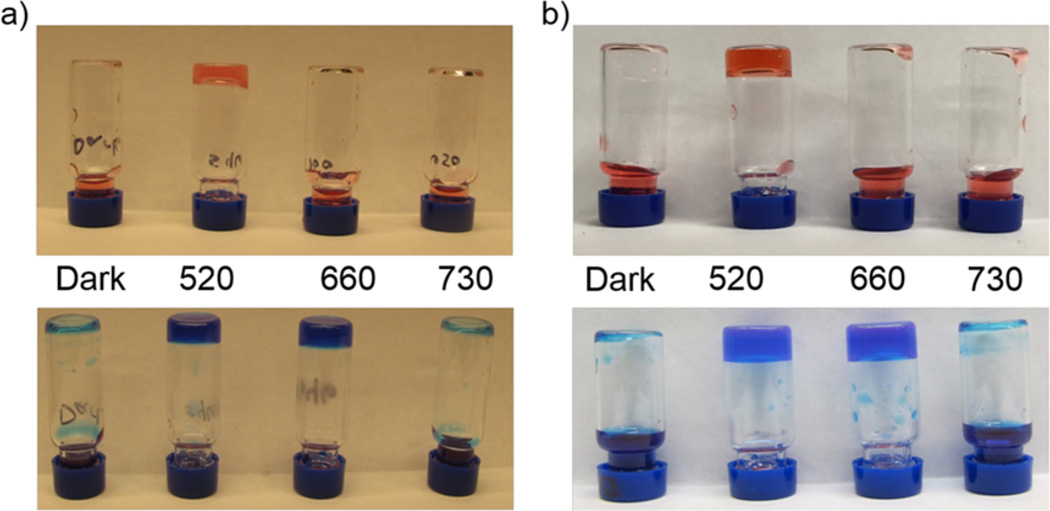
The green light absorbing (520 nm) ethyl-Cbl 14 and its red light absorbing (660 nm) counterpart 15 undergo photolysis at the anticipated wavelengths with high quantum yields, forming identical ethyl radicals.51 Both 14 and 15 promote polymerization of acrylic acid, acrylamide, N-isopropylacrylamide, hydroxyethyl acrylate, and PEG–monoacrylate upon illumination at 520 nm (Figure 6). However, only 15 supports photopolymerization at 660 nm. In addition, live cells are easily encapsulated in a phototriggered hydrogel derived from PEG-diacrylate and the photoinitiator (i.e., 14 or 15). The photophysical properties associated with wavelength-assigned alkyl-Cbls could prove useful for cell implantation, in conditions ranging from the replacement of damaged cartilage to the treatment of glioblastoma.
Figure 6.
Light-triggered gelation of (a) acrylamide and bis-(acrylamide) or (b) PEG-monoacrylate and PEG-diacrylate in the presence of 14 (top) or 15 (bottom).51 Following treatment (dark, 520, 660, or 730 nm), samples were inverted to visually assess gelation. Reproduced from ref 51. Copyright 2015 American Chemical Society.
6. CONCLUSIONS AND PERSPECTIVES
We have found that vitamin B12 can be used to create phototherapeutics that respond to wavelengths throughout the visible spectrum, from the edge of the UV to the NIR. Furthermore, the ability to preassign activation wavelengths to phototherapeutic-B12 conjugates, using commercially available fluorophores, confers extraordinary and unprecedented flexibility relative to other photoactivatable constructs. The consequences of investigator-designated wavelength triggers range from creating phototherapeutics with desired photorelease characteristics to enabling wavelength-specific communication with individual prodrugs in a phototherapeutic mixture. The latter potentially permits sequential delivery of multiple drugs with unmatched temporal precision. Furthermore, the advantages of the fluorophore-as-an-antenna technology are not limited to the important function of receiving wavelengths throughout the optical window of tissue. The extinction coefficient of fluorophores (e.g., Cy5 250 000 M−1 cm−1 at 650 nm) are commonly more than an order magnitude greater than the molecule undergoing photocleavage (e.g., CH3CH2-Cbl 8275 M−1 cm−1 at 520 nm),51 thereby dramatically enhancing the photon capturing ability of the molecular construct. The latter, in combination with recent extraordinary advances in the development of light-emitting devices,60,61 could prove pivotal in the challenging opaque environment of deep tissue. In addition, the quantum yields of photorelease are good relative to other photochemical processes (>5%).51 We do note that the mechanism by which fluorophore-mediated long wavelength capture leads to C–Co bond homolysis (energy transfer versus electron transfer?) remains unresolved. More significantly, in vivo studies will ultimately determine whether the biomedical implications of B12-dependent phototherapeutics, which range from high-resolution spatiotemporal drug delivery to tissue remodeling, are fully realized. These studies are in progress.
Acknowledgments
We thank our co-workers whose names are cited in the reference section for their valuable contributions. We are pleased to acknowledge funding from the National Institutes of Health to T.A.S. (IDeA Program, Grant P20GM103506) and D.S.L. (Grant R01 CA79954).
ABBREVIATIONS
- Cbl
cobalamin
- Col
colchicine
- Dex
dexamethasone
- Dox
doxorubicin
- GRα
glucocorticoid receptor α
- Mtx
methotrexate
- NIR
near-infrared
- PDT
photodynamic therapy
- PEG
poly(ethylene glycol)
- PKA
cAMP-dependent protein kinase
- TAM
5-carboxytetramethylrhodamine
Biographies
Thomas Shell was born and grew up in central Pennsylvania. He earned B.S. degrees in chemistry and biology from the University Richmond, where he performed undergraduate research on the synthesis of pyrroles using vinylogous iminium salts with Stuart Clough and John Gupton. As a graduate student at Emory University with Debra Mohler, he synthesized and studied light-responsive organometallic complexes that cleave DNA. He was a postdoctoral associate with David Lawrence at UNC, where he studied cobalamins as light-responsive compounds for the manipulation of biological systems. He is an assistant professor at Saint Anselm College.
David Lawrence was born in New York City and grew up in southern California. He earned his B.S. degree in the Biological Sciences from UCI, where he performed undergraduate research on the synthesis of quinone methides with Hal Moore. As a graduate student with the late Bob Stevens at UCLA, he was engaged in the total synthesis of steroids and vitamin D analogs. He also witnessed, up close, the application of the extraordinarily elegant strategy developed by Stevens’ for the total synthesis of vitamin B12. He was an NIH postdoctoral fellow with the late Tom Kaiser at the Rockefeller University, where he explored the intricacies of semisynthetic enzymes in the laboratory of one of the founders of the field of chemical biology. He is the Fred Eshelman Distinguished Professor in the Department of Chemistry, in the Division of Chemical Biology and Medicinal Chemistry (School of Pharmacy), and in the Department of Pharmacology (School of Medicine).
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Brieke C, Rohrbach F, Gottschalk A, Mayer G, Heckel A. Light-controlled tools. Angew. Chem., Int. Ed. 2012;51:8446–8476. doi: 10.1002/anie.201202134. [DOI] [PubMed] [Google Scholar]
- 2.Klán P, šolomek T, Bochet CG, Blanc A, Givens R, Rubina M, Popik V, Kostikov A, Wirz J. Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy. Chem. Rev. 2013;113:119–191. doi: 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H-M, Larson DR, Lawrence DS. Illuminating the Chemistry of Life: Design, Synthesis, and Applications of “Caged” and Related Photoresponsive Compounds. ACS Chem. Biol. 2009;4:409–427. doi: 10.1021/cb900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–4314. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 5.Lasers are Building the Future of Medical Science. [accessed October 16, 2015]; http://www.medicaldaily.com/lasers-are-building-future-medical-science-one-bald-head-and-brain-surgery-time-300626.
- 6.Star Trek: Subdermal Scalpel. [accessed October 16, 2015]; http://en.memory-alpha.org/wiki/Subdermal_scalpel.
- 7.Aujard I, Benbrahim C, Gouget M, Ruel O, Baudin JB, Neveu P, Jullien L. o-nitrobenzyl photolabile protecting groups with red-shifted absorption: syntheses and uncaging cross-sections for one-and two-photon excitation. Chem. Eur. J. 2006;12:6865–6879. doi: 10.1002/chem.200501393. [DOI] [PubMed] [Google Scholar]
- 8.Rai P, Mallidi S, Zheng X, Rahmanzadeh R, Mir Y, Elrington S, Khurshid A, Hasan T. Development and applications of photo-triggered theranostic agents. Adv. Drug Delivery Rev. 2010;62:1094–1124. doi: 10.1016/j.addr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tromberg BJ, Shah N, Lanning R, Cerussi A, Espinoza J, Pham T, Svaasand L, Butler J. Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia. 2000;2:26–40. doi: 10.1038/sj.neo.7900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BRAIN 2025: A Scientific Vision. [accessed October 16, 2015]; http://www.braininitiative.nih.gov/2025/index.htm.
- 11.Chen G, Qiu H, Prasad PN, Chen X. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem. Rev. 2014;114:5161–5214. doi: 10.1021/cr400425h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bort G, Gallavardin T, Ogden D, Dalko PI. From one-photon to two-photon probes: ″caged″ compounds, actuators, and photoswitches. Angew. Chem., Int. Ed. 2013;52:4526–4537. doi: 10.1002/anie.201204203. [DOI] [PubMed] [Google Scholar]
- 13.Knoll JD, Turro C. Control and utilization of ruthenium and rhodium metal complex excited states for photoactivated cancer therapy. Coord. Chem. Rev. 2015;282–283:110–126. doi: 10.1016/j.ccr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith NA, Sadler PJ. Photoactivatable metal complexes: from theory to applications in biotechnology and medicine. Philos. Trans. R. Soc., A. 2013;371:20120519. doi: 10.1098/rsta.2012.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakraborty I, Carrington SJ, Mascharak PK. Design strategies to improve the sensitivity of photoactive metal carbonyl complexes (photoCORMs) to visible light and their potential as CO-donors to biological targets. Acc. Chem. Res. 2014;47:2603–2611. doi: 10.1021/ar500172f. [DOI] [PubMed] [Google Scholar]
- 16.Ford PC. Photochemical delivery of nitric oxide. Nitric Oxide. 2013;34:56–64. doi: 10.1016/j.niox.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Zayat L, Filevich O, Baraldo LM, Etchenique R. Ruthenium polypyridyl phototriggers: from beginnings to perspectives. Philos. Trans. R. Soc., A. 2013;371:20120330. doi: 10.1098/rsta.2012.0330. [DOI] [PubMed] [Google Scholar]
- 18.Garcia JV, Zhang F, Ford PC. Multi-photon excitation in uncaging the small molecule bioregulator nitric oxide. Philos. Trans. R. Soc., A. 2013;371:20120129. doi: 10.1098/rsta.2012.0129. [DOI] [PubMed] [Google Scholar]
- 19.Pratt JM. Chemistry of Vitamin B12 0.2. Photochemical Reactions. J. Chem. Soc. 1964:5154–5160. [Google Scholar]
- 20.Hogenkamp HP. The photolysis of methylcobalamin. Biochemistry. 1966;5:417–422. doi: 10.1021/bi00866a005. [DOI] [PubMed] [Google Scholar]
- 21.Hogenkamp HP. Structure and Chemical Reactions of the Cobamide Coenzymes. Ann. N. Y. Acad. Sci. 1964;112:552–564. doi: 10.1111/j.1749-6632.1964.tb45031.x. [DOI] [PubMed] [Google Scholar]
- 22.Bernhauer K, Mueller O, Wagner F. New Chemical and Biochemical Developments in the Vitamin B12 Field. Angew. Chem., Int. Ed. Engl. 1964;3:200–211. doi: 10.1002/anie.196402001. [DOI] [PubMed] [Google Scholar]
- 23.Halpern J, Kim SH, Leung TW. Cobalt-carbon bond dissociation energy of coenzyme B12. J. Am. Chem. Soc. 1984;106:8317–8319. [Google Scholar]
- 24.Kozlowski PM, Kumar M, Piecuch P, Li W, Bauman NP, Hansen JA, Lodowski P, Jaworska M. The Cobalt-Methyl Bond Dissociation in Methylcobalamin: New Benchmark Analysis Based on Density Functional Theory and Completely Renormalized Coupled-Cluster Calculations. J. Chem. Theory Comput. 2012;8:1870–1894. doi: 10.1021/ct300170y. [DOI] [PubMed] [Google Scholar]
- 25.Schrauzer GN, Lee LP, Sibert JW. Alkylcobalamins and alkylcobaloximes Electronic structure, spectra, and mechanism of photodealkylation. J. Am. Chem. Soc. 1970;92:2997–3005. doi: 10.1021/ja00713a012. [DOI] [PubMed] [Google Scholar]
- 26.Taylor RT, Smucker L, Hanna ML, Gill J. Aerobic photolysis of alkylcobalamins: Quantum yields and light-action spectra. Arch. Biochem. Biophys. 1973;156:521–533. doi: 10.1016/0003-9861(73)90301-9. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan JH, Forbush B, Hoffman JF. Rapid photolytic release of adenosine 5′-triphosphate from a protected analog: utilization by the sodium:potassium pump of human red blood cell ghosts. Biochemistry. 1978;17:1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- 28.Fournier L, Gauron C, Xu L, Aujard I, Le Saux T, Gagey-Eilstein N, Maurin S, Dubruille S, Baudin J-B, Bensimon D, Volovitch M, Vriz S, Jullien L. A Blue-Absorbing Photolabile Protecting Group for in Vivo Chromatically Orthogonal Photo-activation. ACS Chem. Biol. 2013;8:1528–1536. doi: 10.1021/cb400178m. [DOI] [PubMed] [Google Scholar]
- 29.Goguen BN, Aemissegger A, Imperiali B. Sequential Activation and Deactivation of Protein Function Using Spectrally Differentiated Caged Phosphoamino Acids. J. Am. Chem. Soc. 2011;133:11038–11041. doi: 10.1021/ja2028074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotzur N, Briand B, Beyermann M, Hagen V. Wavelength-Selective Photoactivatable Protecting Groups for Thiols. J. Am. Chem. Soc. 2009;131:16927–16931. doi: 10.1021/ja907287n. [DOI] [PubMed] [Google Scholar]
- 31.Menge C, Heckel A. Coumarin-Caged dG for Improved Wavelength-Selective Uncaging of DNA. Org. Lett. 2011;13:4620–4623. doi: 10.1021/ol201842x. [DOI] [PubMed] [Google Scholar]
- 32.Priestman MA, Sun L, Lawrence DS. Dual Wavelength Photoactivation of cAMP- and cGMP-Dependent Protein Kinase Signaling Pathways. ACS Chem. Biol. 2011;6:377–384. doi: 10.1021/cb100398e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velema WA, van der Berg JP, Szymanski W, Driessen AJM, Feringa BL. Orthogonal Control of Antibacterial Activity with Light. ACS Chem. Biol. 2014;9:1969–1974. doi: 10.1021/cb500313f. [DOI] [PubMed] [Google Scholar]
- 34.Priestman MA, Shell TA, Sun L, Lee H-M, Lawrence DS. Merging of Confocal and Caging Technologies: Selective Three-Color Communication with Profluorescent Reporters. Angew. Chem., Int. Ed. 2012;51:7684–7687. doi: 10.1002/anie.201202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobsen DW. Preparation of cryptofluorescent analogs of cobalamin coenzymes. Methods Enzymol. 1980;67:12–19. doi: 10.1016/s0076-6879(80)67005-0. [DOI] [PubMed] [Google Scholar]
- 36.Lee M, Grissom CB. Design, synthesis, and characterization of fluorescent cobalamin analogues with high quantum efficiencies. Org. Lett. 2009;11:2499–2502. doi: 10.1021/ol900401z. [DOI] [PubMed] [Google Scholar]
- 37.Rosendahl MS, Omann GM, Leonard NJ. Synthesis and biological activity of a profluorescent analogue of coenzyme B12. Proc. Natl. Acad. Sci. U. S. A. 1982;79:3480–3484. doi: 10.1073/pnas.79.11.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smeltzer CC, Cannon MJ, Pinson PR, Munger JD, Jr, West FG, Grissom CB. Synthesis and characterization of fluorescent cobalamin (CobalaFluor) derivatives for imaging. Org. Lett. 2001;3:799–801. doi: 10.1021/ol006825v. [DOI] [PubMed] [Google Scholar]
- 39.Lee H-M, Xu W, Lawrence DS. Construction of a photoactivatable profluorescent enzyme via propinquity labeling. J. Am. Chem. Soc. 2011;133:2331–2333. doi: 10.1021/ja108950q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee H-M, Priestman MA, Lawrence DS. Light-mediated spatial control via photolabile fluorescently quenched peptide cassettes. J. Am. Chem. Soc. 2010;132:1446–1447. doi: 10.1021/ja907427p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majjigapu JR, Kurchan AN, Kottani R, Gustafson TP, Kutateladze AG. Release and report: a new photolabile caging system with a two-photon fluorescence reporting function. J. Am. Chem. Soc. 2005;127:12458–12459. doi: 10.1021/ja053654m. [DOI] [PubMed] [Google Scholar]
- 42.Pellois JP, Hahn ME, Muir TW. Simultaneous triggering of protein activity and fluorescence. J. Am. Chem. Soc. 2004;126:7170–7171. doi: 10.1021/ja0499142. [DOI] [PubMed] [Google Scholar]
- 43.Shell TA, Shell JR, Rodgers ZL, Lawrence DS. Tunable visible and near-IR photoactivation of light-responsive compounds by using fluorophores as light-capturing antennas. Angew. Chem., Int. Ed. 2014;53:875–878. doi: 10.1002/anie.201308816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith WJ, Oien NP, Hughes RM, Marvin CM, Rodgers ZL, Lee J, Lawrence DS. Cell-mediated assembly of phototherapeutics. Angew. Chem., Int. Ed. 2014;53:10945–10948. doi: 10.1002/anie.201406216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bagnato JD, Eilers AL, Horton RA, Grissom CB. Synthesis and characterization of a cobalamin-colchicine conjugate as a novel tumor-targeted cytotoxin. J. Org. Chem. 2004;69:8987–8996. doi: 10.1021/jo049953w. [DOI] [PubMed] [Google Scholar]
- 46.Gupta Y, Kohli DV, Jain SK. Vitamin B12-mediated transport: a potential tool for tumor targeting of antineoplastic drugs and imaging agents. Crit. Rev. Ther. Drug Carrier Syst. 2008;25:347–379. doi: 10.1615/critrevtherdrugcarriersyst.v25.i4.20. [DOI] [PubMed] [Google Scholar]
- 47.Awuah SG, Polreis J, Biradar V, You Y. Singlet oxygen generation by novel NIR BODIPY dyes. Org. Lett. 2011;13:3884–3887. doi: 10.1021/ol2014076. [DOI] [PubMed] [Google Scholar]
- 48.Kamkaew A, Lim SH, Lee HB, Kiew LV, Chung LY, Burgess K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013;42:77–88. doi: 10.1039/c2cs35216h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patil RR, Guhagarkar SA, Devarajan PV. Engineered nanocarriers of doxorubicin: a current update. Crit. Rev. Ther. Drug Carrier Syst. 2008;25:1–61. doi: 10.1615/critrevtherdrugcarriersyst.v25.i1.10. [DOI] [PubMed] [Google Scholar]
- 50.Volkova M, Russell R., 3rd Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr. Cardiol. Rev. 2012;7:214–220. doi: 10.2174/157340311799960645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodgers ZL, Hughes RM, Doherty LM, Shell JR, Molesky BP, Brugh AM, Forbes MDE, Moran AM, Lawrence DS. B12-Mediated, Long Wavelength Photopolymerization of Hydrogels. J. Am. Chem. Soc. 2015;137:3372–3378. doi: 10.1021/jacs.5b00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem. Rev. 2010;110:2795–2838. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muzykantov VR. Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert Opin. Drug Delivery. 2010;7:403–427. doi: 10.1517/17425241003610633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baschant U, Lane NE, Tuckermann J. The multiple facets of glucocorticoid action in rheumatoid arthritis. Nat. Rev. Rheumatol. 2012;8:645–655. doi: 10.1038/nrrheum.2012.166. [DOI] [PubMed] [Google Scholar]
- 55.Huscher D, Thiele K, Gromnica-Ihle E, Hein G, Demary W, Dreher R, Zink A, Buttgereit F. Dose-related patterns of glucocorticoid-induced side effects. Ann. Rheum. Dis. 2009;68:1119–1124. doi: 10.1136/ard.2008.092163. [DOI] [PubMed] [Google Scholar]
- 56.Hughes RM, Rodgers ZL, Smith WJ, Marvin CM, Shell TA, Lawrence DS. Manuscript in preparation. [Google Scholar]
- 57.O’Banion CP, Hingtgen SD, Lawrence DS. Unpublished results. [Google Scholar]
- 58.Stuckey DW, Hingtgen SD, Karakas N, Rich BE, Shah K. Engineering toxin-resistant therapeutic stem cells to treat brain tumors. Stem Cells. 2015;33:589–600. doi: 10.1002/stem.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith AM, Mancini MC, Nie S. Bioimaging: second window for in vivo imaging. Nat. Nanotechnol. 2009;4:710–711. doi: 10.1038/nnano.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Canales A, Jia X, Froriep UP, Koppes RA, Tringides CM, Selvidge J, Lu C, Hou C, Wei L, Fink Y, Anikeeva P. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 2015;33:277–284. doi: 10.1038/nbt.3093. [DOI] [PubMed] [Google Scholar]
- 61.Jeong JW, McCall JG, Shin G, Zhang Y, Al-Hasani R, Kim M, Li S, Sim JY, Jang KI, Shi Y, Hong DY, Liu Y, Schmitz GP, Xia L, He Z, Gamble P, Ray WZ, Huang Y, Bruchas MR, Rogers JA. Wireless Optofluidic Systems for Programmable In Vivo Pharmacology and Optogenetics. Cell. 2015;162:662–674. doi: 10.1016/j.cell.2015.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]