Abstract
Altered energy balance and insulin resistance are important characteristics of aging. Skeletal muscle is a major site of glucose disposal, and the role of aging-associated inflammation in skeletal muscle insulin resistance remains unclear. To investigate, we examined glucose metabolism in 18-mo-old transgenic mice with muscle-specific overexpression of IL-10 (MIL10) and in wild-type mice during hyperinsulinemic–euglycemic clamping. Despite similar fat mass and energy balance, MIL10 mice were protected from aging-associated insulin resistance with significant increases in glucose infusion rates, whole-body glucose turnover, and skeletal muscle glucose uptake (∼60%; P < 0.05), as compared to age-matched WT mice. This protective effect was associated with decreased muscle inflammation, but no changes in adipose tissue inflammation in aging MIL10 mice. These results demonstrate the importance of skeletal muscle inflammation in aging-mediated insulin resistance, and our findings further implicate a potential therapeutic role of anti-inflammatory cytokine in the treatment of aging-mediated insulin resistance.—Dagdeviren, S., Jung, D. Y., Friedline, R. H., Noh, H. L., Kim, J. H., Patel, P. R., Tsitsilianos, N., Inashima, K., Tran, D. A., Hu, X., Loubato, M. M., Craige, S. M., Kwon, J. Y., Lee, K. W., Kim, J. K. IL-10 prevents aging-associated inflammation and insulin resistance in skeletal muscle.
Keywords: cytokines, interleukins, aging, type 2 diabetes, glucose metabolism
Aging is characterized by alterations in metabolism that resemble a physiological state of obesity and insulin resistance (1). Molecular events underlying obesity-mediated insulin resistance have been extensively studied, but the mechanism of aging-associated insulin resistance remains poorly understood.
Adipose tissue inflammation develops in obesity, and macrophages and inflammatory cytokines are causally associated with insulin resistance (2). Aging is also associated with a low-grade chronic inflammation state referred to as “inflamm-aging” that involves a multitude of inflammation-related events contributing to morbidity and mortality (3–5). In that regard, circulating levels of inflammatory cytokines are shown to be elevated in elderly human subjects, and these aging-associated events are observed independent of obesity (6). Our recent study has also found that aging promotes moderate obesity, adipose tissue inflammation, and insulin resistance in mice (5), consistent with findings from human subjects.
Aging-associated inflammation and increased cytokine responses may be caused by a declining level of IL-10 during aging (7, 8). IL-10 is an essential cytokine, mainly produced by macrophages, and is responsible for suppressing proinflammatory response in various tissues, including skeletal muscle (9). IL-10 prevents inflammation by suppressing the activation of macrophages and blocking the antigen presentation, as well as the release and activity of inflammatory cytokines, such as IL-6, TNF-α, and IL-1β (7, 10). Consistent with this, IL-10-knockout (KO) mice showed elevated levels of inflammatory cytokines after LPS stimulation and aging further exacerbated the inflammatory response in these mice (8). Furthermore, IL-10 has a major beneficial effect on common inflammatory diseases such as chronic inflammatory bowel, rheumatoid arthritis, systemic lupus erythematosus, and multiple sclerosis (9). Therefore, IL-10 can be a suitable immunomodulatory candidate for anti-inflammatory therapy against inflammation-mediated conditions (9). Hacham et al. (11) have shown that IL-10 expression in skeletal muscle is reduced in aging mice. In that regard, we have shown that mice with muscle-specific overexpression of IL-10 (MIL10) are protected from diet-induced insulin resistance in skeletal muscle (12, 13). Thus, in the current study, we examined the effects of aging on energy balance, inflammation, and insulin resistance in MIL10 mice.
MATERIALS AND METHODS
Animals
Male muscle creatine kinase (MCK)-IL-10 (MIL10) mice (13) and wild-type (WT) littermates (C57BL/6J background) were studied from young (4 mo of age) to old (18 mo of age) age (n = 8/group). All mice were housed in controlled temperature and a light/dark cycle with free access to a standard chow diet (Prolab Isopro RMH 3000 5P75; Labdiet, St. Louis, MO, USA) and water. All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Body composition and in vivo assessment of energy balance
Whole-body fat and lean mass were noninvasively measured with 1H-magnetic resonance spectroscopy (MRS) (Echo-MRI; Echo Medical Systems, Houston, TX, USA). Food intake, physical activity, Vo2, Vco2, energy expenditure rates, and respiratory exchange ratios were measured via indirect calorimetry in specialized single-housed metabolic cages (TSE Systems Inc., Bad Homburg, Germany). For these metabolic cage measurements, acclimatized WT and MIL10 mice at 12, 14, 16, and 18 mo of age were fed a standard chow diet ad libitum at ambient temperature (20–23°C) in a 12 h light/dark cycle for 72 h. Data were collected via equipment-associated software.
Hyperinsulinemic–euglycemic clamp to assess insulin sensitivity
At 18 mo of age, we conducted a 2-h hyperinsulinemic–euglycemic clamping to measure insulin action and glucose metabolism in awake male MIL10 and WT mice (14). In brief, the clamping began with a primed and continuous infusion of human insulin (150 mU/kg bolus followed by 2.5 mU/kg per minute), and 20% glucose was infused at variable rates to maintain euglycemia. During the clamping, [3-3H]glucose was infused to assess whole-body glucose turnover, and 2-[14C]deoxyglucose (DG) was bolus injected after 75 min of clamping, to measure glucose uptake in individual organs (14).
Biochemical analysis of glucose metabolism and molecular analysis of insulin signaling
Plasma glucose levels during clamping were measured with a glucose analyzer (Analox Sensor Technology, Middlesrough, United Kingdom). Plasma concentrations of [3-3H]glucose, 2-[14C]DG, and 3H2O were measured as described previously (14). Tissue and serum triglyceride concentrations were measured with a triglyceride assay kit (Sigma-Aldrich, St. Louis, MO, USA) using plasma and tissue homogenates. Plasma insulin levels were determined with an ultrasensitive ELISA kit (Alpco Diagnostics, Salem, NH, USA).
For insulin signaling analysis, skeletal muscle samples (gastrocnemius) were collected at the end of clamping, and protein lysates were prepared for Western blot analysis with rabbit monoclonal p-Akt-Ser473 and Akt-Ser473 antibodies (Cell Signaling Technology, Danvers, MA, USA), with β-actin as a loading control. Gastrocnemius lysates were also used to measure local muscle levels of IL-1β, monocyte chemoattractant protein (MCP)-1, IL-10, and IFN-γ with multiplexed-ELISA assay with Luminex 200 Multiplex Bio-Plex 200 System (EMD Millipore, Billerica, MA, USA) using Milliplex Map kits (EMD Millipore). Plasma leptin, resistin, and adiponectin levels were also measured with Luminex.
For quantitative RT-PCR (qRT-PCR), RNA isolation was performed with homogenized skeletal muscle samples (gastrocnemius) and visceral white adipose tissue (WAT) with Trizol (Thermo Fisher Scientific, Carlsbad, CA, USA) used according to the manufacturer’s protocols. cDNA was synthesized from 2 µg of total RNA by the use of the Omniscript cDNA synthesis kit (Qiagen, Venlo, The Netherlands). cDNA and SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) was run on a CFX96 (Bio-Rad) real-time system, with the following primers: CD68, forward (F)–5′-TGTCTGATCTTGCTAGGACCG–3′ and reverse (R)-5′–GAGAGTAACGGCCTTTTTGTG–3′; IL-10, F-5′–GCTCTTACTGACTGGCATGAG–3′ and R-5′–CGCAGCTCTAGGAGCATGTG–3′; TNF-α F-5′–CAGGCGGTGCCTATGTCTC–3′ and R-5′–CGATCACCCCGAAGTTCAGTAG–3′; IL-6 F-5′–AGTTGCCTTCTTGGGACTGA–3′ and R-5′–TCCACGATTTCCCAGAGAAC-3′; F4/80 F-5′–TGACTCACCTTGTGGTCCTAA–3′ and R-5′–CTTCCCAGAATCCAGTCTTTCC–3′; citrate synthase F-5′–GGGAAGGCTAAGAACCCTTG–3′ and R-5′–TTCATCTCCGTCATGCCATA–3′; SDHA F-5′–GCTGGTGTGGATGTCACTAAG–3′ and R-5′–CCCACCCATGTTGTAATGCA–3′; UCP3 F-5′–TGCTGAGATGGTGACCTACG–3′ and R-5′–AGCTCCAAAGGCAGAGACAA–3′. Relative gene expression was calculated by using Bio-Rad CFX manager software normalized to the RPL32 housekeeping gene.
Histologic analysis
Gastrocnemius muscles were freshly extracted, fixed in 10% formalin overnight, and embedded in paraffin blocks. Sections (5 µm) were cut and stained with H&E to be observed under ×20 magnification.
Exercise study
Male MIL10 and WT mice at 24 mo of age were used for exercise study with treadmills (n = 4–5/group). After a 30 min acclimatization period, treadmill speed gradually increased every 3 min between 7.5 and 15.9 m/min until the mouse was exhausted and stopped running. The stopping time and total distance were recorded for each mouse.
Statistical analysis
Data are expressed as means ± se, and differences between groups were examined for statistical significance with ANCOVA and 2- or 1-tailed Student’s t test. A value of P < 0.05 was the criterion for statistical significance.
RESULTS
Body composition profile in aging mice
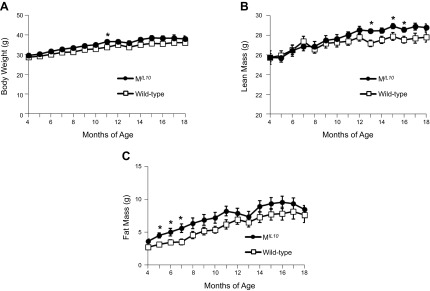
Longitudinal changes in body composition were recorded in male MIL10 and WT mice fed a standard chow diet from 4 to 18 mo of age. Body weights of MIL10 and WT mice increased gradually over the first 12 mo of age and plateaued with further aging (Fig. 1A). Whole-body lean mass also increased gradually with aging in both groups of mice, but between 13 and 16 mo of age, the MIL10 mice showed significantly higher lean mass than the WT mice (Fig. 1B). Whole-body fat mass increased more rapidly in MIL10 mice than in WT mice during the first 8 mo of age, but the groups were not significantly different with further aging (Fig. 1C).
Figure 1.
Body weight and composition of MIL-10 and WT mice between 4 and 18 mo of age (n = 10–12 per group). A) Body weight. B, C) Whole-body lean (B) and fat (C) masses measured with 1H-MRS. *P < 0.05 vs. WT mice.
Altered energy balance in aging mice
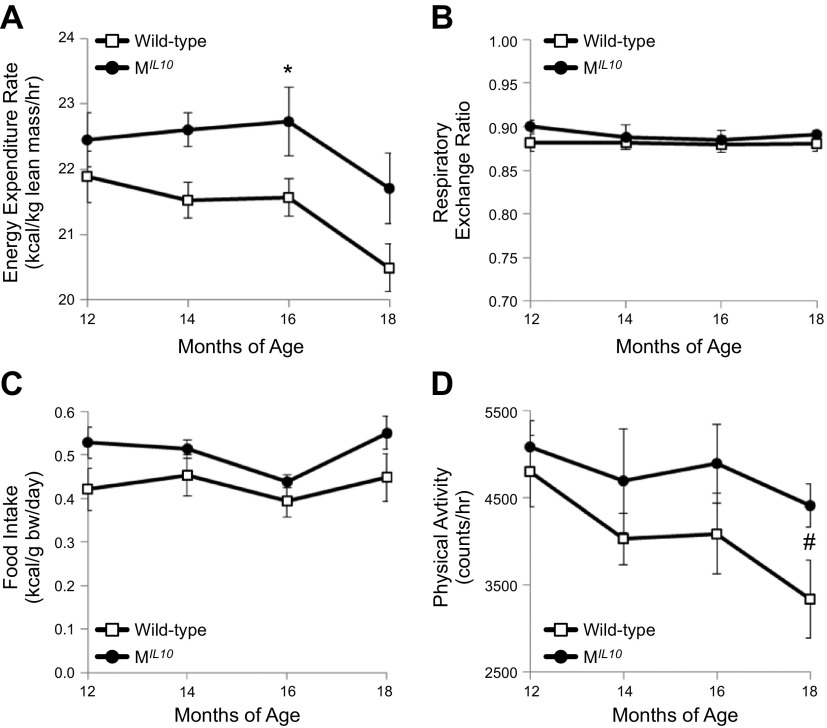
Aging-associated changes in energy balance were noninvasively assessed with metabolic cages in male MIL10 and WT mice at 12, 14, 16, and 18 mo of age. Energy expenditure and Vo2 rates were significantly elevated in MIL10 mice as compared to WT mice at 16 mo of age (Fig. 2A and Supplemental Fig. 1A). Vco2 rate also tended to be increased in MIL10 mice vs. WT mice at 16 mo of age (P = 0.07; Supplemental Fig. 1B). Respiratory exchange ratio did not differ between aging MIL10 and WT mice and reflected consumption of chow diet (Fig. 2B). Daily caloric intake was not significantly different between aging MIL10 and WT mice (Fig. 2C). We measured circulating levels of leptin, a major hormone regulating feeding behavior, and found that serum leptin levels were significantly reduced in aging MIL10 mice vs. aging WT mice (Table 1).
Figure 2.
Longitudinal assessment of energy balance using metabolic cages in aging MIL-10 and WT mice (n = 6 per group). Data are averaged from a 3-d continuous measurement in mice at 12, 14, 16, and 18 mo of age. A) Energy expenditure rate. B) Respiratory exchange ratio. C) Daily food intake (caloric intake normalized to body weight). D) Hourly physical activity. *P < 0.05 vs. WT mice. #P < 0.05 vs. physical activity in WT mice at 12 mo of age.
TABLE 1.
Metabolic profile in WT vs. aging MIL10 mice
| Group | Glucose (mg/dl) | Insulin (ng/ml) | Leptin (µg/ml) | Resistin (µg/ml) | Adiponectin (µg/ml) | Triglyceride (mg/dl) |
|---|---|---|---|---|---|---|
| WT | 151 ± 4 | 0.61 ± 0.16 | 14 ± 2 | 12 ± 1 | 13 ± 1 | 66 ± 3 |
| MIL10 | 135 ± 10 | 0.56 ± 0.15 | 5 ± 1* | 7 ± 1* | 10 ± 1 | 52 ± 3* |
Plasma levels in 18-mo-old WT and MIL10 mice left unfed overnight (n = 3–5/group). Values are means ± se.
P < 0.05 vs. WT mice.
We performed qRT-PCR analysis in muscle samples obtained from young (4 mo of age) and aging (18 mo of age) mice to assess mitochondrial metabolic genes. Skeletal muscle mRNA levels of UCP3 and succinate dehydrogenase were significantly decreased in both aging groups of WT and MIL10 mice, when compared with young cohorts. Muscle expression of citrate synthase also tended to decline in both groups of aging mice vs. young mice (P = 0.06; Supplemental Fig. 2). There were no significant differences in any of the mitochondrial gene expressions between aging WT and aging MIL10 mice.
Physical activity declined with aging and was significantly reduced in WT mice at 18 mo of age, compared with the same group of WT mice at 12 mo of age (Fig 2D). In contrast, this aging-associated decline in physical activity was not observed in MIL10 mice. As a result, MIL10 mice showed a tendency for higher activity than WT mice at 18 mo of age (P = 0.06). To determine whether this affects the exercise capacity, we performed a treadmill exercise test and found no significant difference between aging WT and MIL10 mice in total distance running on a treadmill until exhaustion (Supplemental Fig. 3).
MIL10 mice are protected from aging-associated insulin resistance
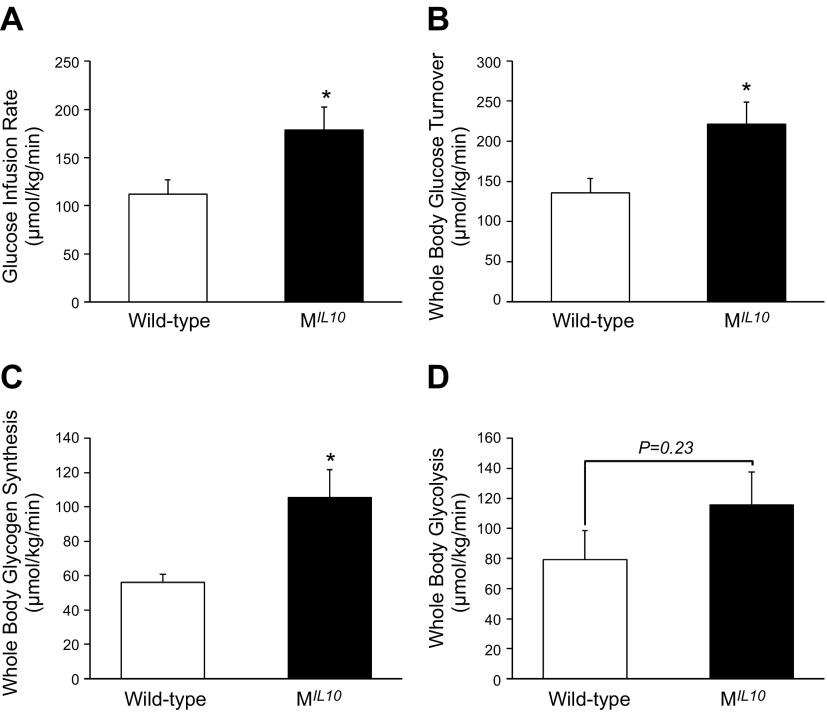
Basal glucose and insulin levels after remaining unfed overnight did not differ between MIL10 and WT mice at 18 mo of age (Table 1). A 2-h hyperinsulinemic–euglycemic clamping was performed to measure insulin action and glucose metabolism in awake age-matched MIL10 and WT mice at 18 mo of age. Steady-state rates of glucose infusion to maintain euglycemia during clamping were significantly increased (60%) in aging MIL10 mice vs. aging WT mice (Fig. 3A). Tracer analysis showed that whole-body glucose turnover was increased by ∼60%, and whole-body glycogen synthesis was increased by 2-fold in aging MIL10 mice (Fig. 3B, C). Whole-body glycolysis was not significantly different between the groups (Fig. 3D). These data indicate that aging MIL10 mice are more insulin sensitive than aging WT mice.
Figure 3.
A 2-h hyperinsulinemic–euglycemic clamping to assess insulin action and glucose metabolism in awake MIL-10 and WT mice at 18 mo of age (n = 9–10 per group). A) Steady-state glucose infusion rates during clamping. B–D) Whole-body glucose turnover (B), glycogen synthesis (C), and glycolysis (D). *P < 0.05 vs. WT mice.
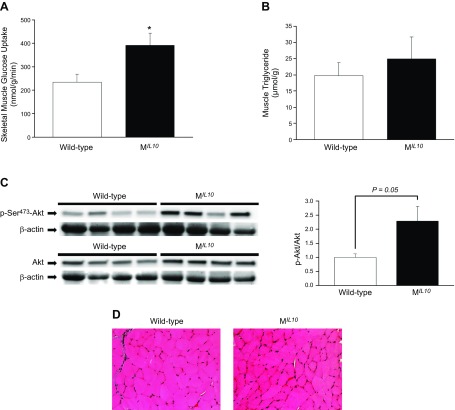
Increased insulin sensitivity in aging MIL10 mice was largely caused by a 70% increase in insulin-stimulated glucose uptake in skeletal muscle (gastrocnemius) (Fig. 4A). Because intramuscular lipid content is a major determinant of insulin action, we measured muscle triglyceride levels that did not differ between aging MIL10 and WT mice (Fig. 4B). Muscle insulin signaling was determined with Western blots with antibodies against Akt and phospho-Akt. Total Akt protein levels in skeletal muscle did not differ between groups, but phospho-Akt levels were increased by more than 2-fold in aging MIL10 mice vs. aging WT mice (P = 0.05; Fig. 4C). H&E staining showed that there were no obvious changes in skeletal muscle morphology between groups (Fig. 4D). We measured circulating hormones and metabolites known to affect insulin action using multiplexed Luminex and Cobas Analyzer (Roche Diagnostics, Indianapolis, IN, USA), and found significant decreases in plasma leptin, resistin and triglyceride levels in aging MIL10 mice vs. aging WT mice (Table 1). Plasma adiponectin levels did not differ between groups.
Figure 4.
Insulin signaling and glucose/lipid metabolism in skeletal muscle of MIL-10 and WT mice at 18 mo of age (n = 7–10 per group). A) Insulin-stimulated glucose uptake in skeletal muscle (gastrocnemius). B) Intramuscular (quadriceps) triglyceride levels. C) Total Akt and insulin-stimulated Akt phosphorylation at Ser473 in skeletal muscle (gastrocnemius). D) H&E staining of gastrocnemius muscle. *P < 0.05 vs. WT mice.
Aging-associated inflammation in skeletal muscle is reduced in MIL10 mice
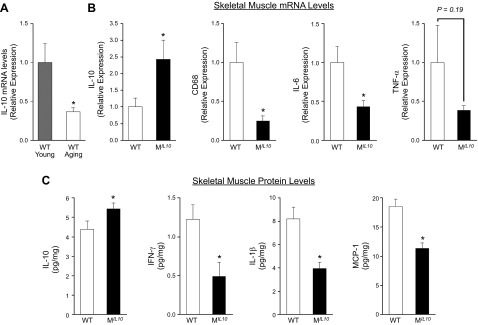
We have shown that local inflammation that develops in skeletal muscle in obesity is causally associated with insulin resistance (13). The qRT-PCR analysis of skeletal muscle samples showed that IL-10 expression was significantly reduced (>70%) with aging in WT mice (Fig. 5A), and this is consistent with previous observations in aging humans (15). Muscle IL-10 mRNA level in aging MIL10 mice was significantly increased (2.5-fold) compared with that in aging WT mice (Fig. 5B). This finding was associated with significant decreases in CD68 (macrophage marker) and IL-6 mRNA levels in aging MIL10 mice vs. aging WT mice. Muscle expression of TNF-α also tended to be lower in aging MIL10 mice. These important changes in inflammatory gene expressions were further confirmed at the protein levels. Skeletal muscle IL-10 levels were significantly higher in aging MIL10 mice than in aging WT mice (Fig. 5C). Local levels of inflammatory cytokines (IFN-γ and IL-1β) and chemokine (MCP-1) in skeletal muscle were all significantly reduced in aging MIL10 mice vs. aging WT mice (Fig. 5C).
Figure 5.
Skeletal muscle mRNA and protein levels of inflammatory cytokines, macrophages, and chemokines in MIL-10 and WT mice (n = 3–8 per group). A) Muscle IL-10 mRNA levels in WT mice at 4 mo (young) and 18 mo (aging) of age. B) Muscle mRNA levels of IL-10, CD68, IL-6, and TNF-α in aging MIL-10 and WT mice at 18 mo of age. C) Muscle protein levels of IL-10, IFN-γ, IL-1β, and MCP-1 in aging MIL-10 and WT mice at 18 mo of age. *P < 0.05 vs. young WT mice or WT mice.
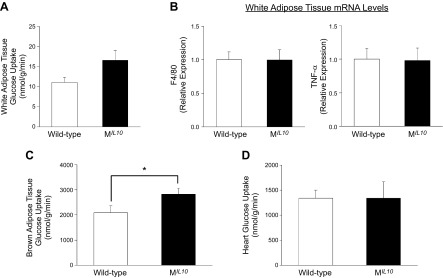
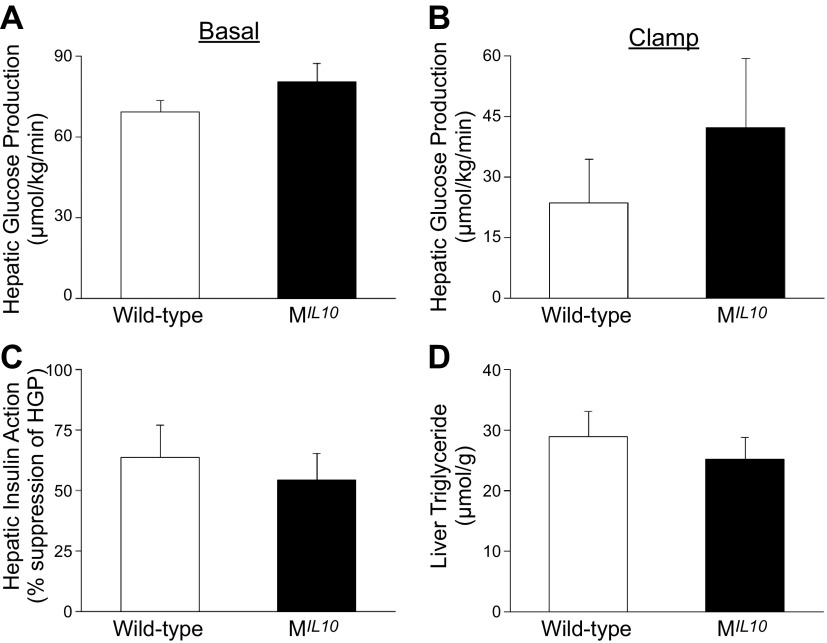
In contrast to the profound alterations in glucose metabolism and inflammation in skeletal muscle, insulin-stimulated glucose uptake in WAT and adipose expression of F4/80 and TNF-α did not differ between aging MIL10 and WT mice (Fig. 6A, B). Glucose metabolism in brown adipose tissue (BAT) was significantly increased in aging MIL10 mice vs. aging WT mice (Fig. 6C). Insulin-stimulated glucose uptake in heart was not altered in aging MIL10 mice (Fig. 6D). Furthermore, basal and clamp hepatic glucose production (HGP), hepatic insulin action, and intrahepatic triglyceride levels were not significantly altered in aging MIL10 mice (Fig. 7).These data indicate that aging-associated inflammation and insulin resistance were selectively attenuated in skeletal muscle of MIL10 mice.
Figure 6.
Glucose metabolism and inflammation in adipose tissue and heart of aging MIL-10 and WT mice at 18 mo of age. A) Insulin-stimulated glucose uptake in WAT (epididymal) (n = 9–10 per group). B) WAT (epididymal) mRNA levels of F4/80 and TNF-α (n = 4–9 per group). C) Insulin-stimulated glucose uptake in BAT (intrascapular) (n = 9–10/group). D) Insulin-stimulated glucose uptake in heart (n = 4–5 per group). *P < 0.05 vs. WT mice.
Figure 7.
HGP and hepatic insulin action during hyperinsulinemic–euglycemic clamp in awake MIL-10 and WT mice at 18 mo of age (n = 9–10 per group). A) Basal HGP. B) Insulin-stimulated HGP during clamping. C) Hepatic insulin action expressed as insulin-mediated percentage of suppression of basal HGP. D) Intrahepatic triglyceride levels. *P < 0.05 vs. WT mice.
DISCUSSION
Metabolic changes during aging are closely related to several prevalent medical conditions, such as obesity, insulin resistance, type 2 diabetes, cardiovascular diseases, and neurodegenerative disorders. We have shown that aging promotes modest obesity, adipose tissue inflammation, and insulin resistance and that these aging-associated disorders are ameliorated in mice with global deletion of protein tyrosine phosphatase 1B, a negative regulator of insulin signaling (5). Because obesity is an aging-related phenomenon that is causally associated with insulin resistance, it is difficult to delineate the contribution of aging vs. obesity effects on insulin resistance. In that regard, our current results show that aging mice with muscle-specific overexpression of IL-10 are more insulin sensitive than aging WT mice with matched adiposity and demonstrate that aging-associated insulin resistance can be rescued independent of obesity. Our findings also indicate that beneficial effects of IL-10 were caused by reduced local inflammation and improved insulin signaling and glucose metabolism in skeletal muscle.
A preponderance of recent evidence has highlighted the role of adipose tissue in obesity-mediated inflammation and insulin resistance and contributed to an adipocentric view of type 2 diabetes (2, 16, 17). However, aging-associated inflammation and insulin resistance may develop independent of obesity, suggesting that adipose tissue is not the primary organ responsible for dysregulated glucose metabolism in aging (6). Likewise, in our current study, the aging WT mice gained body weight and fat mass minimally from 4 to 18 mo of age and did not reach a pronounced obese state despite developing insulin resistance in multiple organs. Energy expenditure tended to decline slightly, whereas physical activity was significantly reduced with aging in WT mice, consistent with previous findings (18–20). However, both energy expenditure and physical activity were markedly elevated in aging MIL10 mice as compared to aging WT mice, suggesting that MIL10 mice were more metabolically active. Although we did not find significant differences in total distance running on treadmill until exhaustion, other exercise capacities may be altered in aging MIL10 mice.
We have shown that IL-10 overexpression in skeletal muscle prevents diet-induced insulin resistance in mice (13). Our current study demonstrates that muscle expression of IL-10 also protects against aging-associated insulin resistance, given that aging MIL10 mice showed marked increases in whole-body glucose turnover and muscle glucose metabolism vs. aging WT mice. This effect was in part caused by increased muscle Akt phosphorylation in aging MIL10 mice, supporting the previous notion that defects in muscle insulin signaling may underlie aging-associated insulin resistance (5). Although ectopic lipid accumulation is causally related to obesity-mediated defects in insulin signaling (21), our findings of comparable intramuscular triglyceride levels in MIL10 and WT mice suggest that other aging-associated factors are responsible for affecting muscle insulin signaling in aging mice.
Adipocyte-derived hormones, such as resistin, adiponectin, and leptin, are known to modulate insulin action, and their serum levels have been shown to increase with aging (22–25). Resistin is a negative regulator of insulin sensitivity and has been shown to increase HGP and induce hyperglycemia (22, 23). Aging MIL10 mice showed significantly lower plasma resistin levels vs. aging WT mice. However, neither basal HGP nor hepatic insulin action was affected in aging MIL10 mice (i.e., did not improve), suggesting minimal effects of resistin in this model. In addition, aging MIL10 mice showed a significantly lower leptin level than aging WT mice, although both groups had similar fat mass. In that regard, leptin levels usually correlate with adiposity, but this relationship has been shown to be affected by aging (26). Last, serum triglyceride levels also increase with aging in both humans and animals (27, 28), and aging MIL10 mice had a lower plasma triglyceride level than aging WT mice. Thus, aging MIL10 mice had an overall improved metabolic profile vs. aging WT mice.
Previous studies have reported that IL-10 levels and signaling in skeletal muscle decrease with aging, and those findings are consistent with our current data showing lower IL-10 levels in aging WT mice (11, 29–31). In contrast, MIL10 mice were protected from this aging-associated effect as muscle IL-10 levels remained consistently higher and within a physiological range. This increase in IL-10 expression was able to suppress local inflammation in skeletal muscle during aging, because macrophage marker (CD68), chemokine (MCP-1), and inflammatory cytokines (IL-6, IL-1β, IFN-γ, and TNF-α) were all reduced in aging MIL10 mice. The physiological role of IL-10 during aging is largely unknown. IL-10 polymorphisms were found to be associated with longevity in men (32), and global IL-10-KO mice were shown to develop frailty and altered muscle energy metabolism (33).
It is important to point out that improved insulin sensitivity in aging MIL10 mice is a result of selective increase in muscle glucose metabolism without affecting inflammation and metabolism in WAT, which is generally a major focus of the obesity–inflammation–diabetes paradigm. However, there was an increase in BAT glucose uptake in aging MIL10 mice. This increase may be caused by similarities in the metabolic machinery and developmental origin between skeletal muscle and brown fat (34) and secondary effects of improved muscle insulin action. To that end, myocardial glucose uptake was not affected in aging MIL10 mice. Furthermore, liver is an important organ in maintaining glucose homeostasis (35), and there were no significant effects on HGP or intrahepatic lipid levels in aging MIL10 mice. Although the main source of IL-10 is macrophages (7), several studies have shown that IL-10 is expressed in mouse skeletal muscle and myoblasts (11, 36). Thus, the profound effects of IL-10 in skeletal muscle may imply a physiological function of IL-10 signaling on muscle inflammation and glucose metabolism.
As aging-related diseases often involve mitochondrial dysfunction (20, 37–39), we examined muscle mitochondrial oxidative enzymes in aging mice. Both WT and MIL10 mice manifested significant decreases in skeletal muscle expression of mitochondrial oxidative enzymes with aging, and this effect was comparable between WT and MIL10 mice. Thus, aging-associated alterations in mitochondrial metabolic genes did not presumably play a major role in improved metabolic activity in MIL10 mice. In addition, suppression of muscle inflammation was shown to correlate with increased muscle function (40), but the aging MIL10 mice did not exhibit obvious improvement in muscle function based on the treadmill exhaustion test. However, future studies examining different aspects of muscle function and exercise capacity are clearly warranted.
In summary, our results demonstrate that IL-10, as an anti-inflammatory immune-modulator, attenuates aging-associated inflammation and improves insulin signaling and glucose metabolism in skeletal muscle. Also, our findings imply an important role of skeletal muscle inflammation in aging-associated insulin resistance and further suggest a therapeutic potential of anti-inflammatory modulators in treating insulin resistance and metabolic abnormalities during aging. To that end, application of recombinant IL-10 in human has turned out to be safe in clinical trials for the treatment of autoimmune diseases, neurodegenerative disorders, and several other conditions (9, 41, 42). Thus, IL-10 may be a promising therapeutic agent for the prevention of aging-associated decline in muscle glucose metabolism.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by U.S. National Institutes of Health, Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK080756, R01-DK079999, R24-DK090963, and UC2-DK093000 (to J.K.K.); American Diabetes Association Research Award 707-RA-80 (to J.K.K.); South Korean Midcareer Researcher Program Grant 2015R1A2A1A10053567 from the National Research Foundation, Ministry of Science, Information and Communications Technology, and Future Planning (to K.W.L.); High Value-Added Food Technology Development Program Grant 116030-3 through the Korean Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries, Ministry of Agriculture, Food and Rural Affairs (to K.W.L.).
Glossary
- BAT
brown adipose tissue
- DG
deoxyglucose
- H&E
hematoxylin and eosin
- HGP
hepatic glucose production
- KO
knockout
- MCP
monocyte chemoattractant protein
- MRS
magnetic resonance spectroscopy
- qRT-PCR
quantitative RT-PCR
- UCP
uncoupling protein
- WAT
white adipose tissue
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Dagdeviren, D. Y. Jung, R. H. Friedline, and J. K. Kim, contributed to the conception and design of the research; S. Dagdeviren, D. Y. Jung, R. H. Friedline, H. L. Noh, J. H. Kim, P. R. Patel, N. Tsitsilianos, K. Inashima, D. A.Tran, X. Hu, M. M. Loubato, and S. M. Craige performed the experiments; S. Dagdeviren, D. Y. Jung, R. H. Friedline, K. W. Lee, and J. K. Kim analyzed the data; S. Dagdeviren, D. Y. Jung, R. H. Friedline K. W. Lee, and J. K. Kim interpreted the results of the experiments; S. Dagdeviren and J. K. Kim prepared the figures; S. Dagdeviren and J. K. Kim drafted the manuscript; S. Dagdeviren, J. Y. Kwon, and J. K. Kim edited and revised the manuscript; and S. Dagdeviren, J. Y. Kwon, K. W. Lee, and J. K. Kim approved the final version of the manuscript.
REFERENCES
- 1.Fink R. I., Kolterman O. G., Griffin J., Olefsky J. M. (1983) Mechanisms of insulin resistance in aging. J. Clin. Invest. 71, 1523–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wellen K. E., Hotamisligil G. S. (2003) Obesity-induced inflammatory changes in adipose tissue. J. Clin. Invest. 112, 1785–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brüünsgaard H., Pedersen B. K. (2003) Age-related inflammatory cytokines and disease. Immunol. Allergy Clin. North Am. 23, 15–39 [DOI] [PubMed] [Google Scholar]
- 4.Franceschi C., Campisi J. (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69, S4–S9 [DOI] [PubMed] [Google Scholar]
- 5.González-Rodríguez A., Más-Gutierrez J. A., Mirasierra M., Fernandez-Pérez A., Lee Y. J., Ko H. J., Kim J. K., Romanos E., Carrascosa J. M., Ros M., Vallejo M., Rondinone C. M., Valverde Á. M. (2012) Essential role of protein tyrosine phosphatase 1B in obesity-induced inflammation and peripheral insulin resistance during aging. Aging Cell 11, 284–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karakelides H., Irving B. A., Short K. R., O’Brien P., Nair K. S. (2010) Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes 59, 89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore K. W., de Waal Malefyt R., Coffman R. L., O’Garra A. (2001) Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 [DOI] [PubMed] [Google Scholar]
- 8.Meador B. M., Krzyszton C. P., Johnson R. W., Huey K. A. (2008) Effects of IL-10 and age on IL-6, IL-1beta, and TNF-alpha responses in mouse skeletal and cardiac muscle to an acute inflammatory insult. J. Appl. Physiol. 104, 991–997 [DOI] [PubMed] [Google Scholar]
- 9.Asadullah K., Sterry W., Volk H. D. (2003) Interleukin-10 therapy: review of a new approach. Pharmacol. Rev. 55, 241–269 [DOI] [PubMed] [Google Scholar]
- 10.Mittal S. K., Roche P. A. (2015) Suppression of antigen presentation by IL-10. Curr. Opin. Immunol. 34, 22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacham M., White R. M., Argov S., Segal S., Apte R. N. (2004) Interleukin-6 and interleukin-10 are expressed in organs of normal young and old mice. Eur. Cytokine Netw. 15, 37–46 [PubMed] [Google Scholar]
- 12.Kim H. J., Higashimori T., Park S. Y., Choi H., Dong J., Kim Y. J., Noh H. L., Cho Y. R., Cline G., Kim Y. B., Kim J. K. (2004) Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes 53, 1060–1067 [DOI] [PubMed] [Google Scholar]
- 13.Hong E. G., Ko H. J., Cho Y. R., Kim H. J., Ma Z., Yu T. Y., Friedline R. H., Kurt-Jones E., Finberg R., Fischer M. A., Granger E. L., Norbury C. C., Hauschka S. D., Philbrick W. M., Lee C. G., Elias J. A., Kim J. K. (2009) Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes 58, 2525–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J. K. (2009) Hyperinsulinemic-euglycemic clamp to assess insulin sensitivity in vivo. Methods Mol. Biol. 560, 221–238 [DOI] [PubMed] [Google Scholar]
- 15.Van der Geest K. S., Lorencetti P. G., Abdulahad W. H., Horst G., Huitema M., Roozendaal C., Kroesen B. J., Brouwer E., Boots A. M. (2016) Aging-dependent decline of IL-10 producing B cells coincides with production of antinuclear antibodies but not rheumatoid factors. Exp. Gerontol. 75, 24–29 [DOI] [PubMed] [Google Scholar]
- 16.Wellen K. E., Hotamisligil G. S. (2005) Inflammation, stress, and diabetes. J. Clin. Invest. 115, 1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W. Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkins S., Wiswell R. (2003) Rate and mechanism of maximal oxygen consumption decline with aging: implications for exercise training. Sports Med. 33, 877–888 [DOI] [PubMed] [Google Scholar]
- 19.Conley K. E., Esselman P. C., Jubrias S. A., Cress M. E., Inglin B., Mogadam C., Schoene R. B. (2000) Ageing, muscle properties and maximal O(2) uptake rate in humans. J. Physiol. 526, 211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houtkooper R. H., Argmann C., Houten S. M., Cantó C., Jeninga E. H., Andreux P. A., Thomas C., Doenlen R., Schoonjans K., Auwerx J. (2011) The metabolic footprint of aging in mice. Sci. Rep. 1, 134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J. K., Fillmore J. J., Chen Y., Yu C., Moore I. K., Pypaert M., Lutz E. P., Kako Y., Velez-Carrasco W., Goldberg I. J., Breslow J. L., Shulman G. I. (2001) Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc. Natl. Acad. Sci. USA 98, 7522–7527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steppan C. M., Bailey S. T., Bhat S., Brown E. J., Banerjee R. R., Wright C. M., Patel H. R., Ahima R. S., Lazar M. A. (2001) The hormone resistin links obesity to diabetes. Nature 409, 307–312 [DOI] [PubMed] [Google Scholar]
- 23.Rajala M. W., Obici S., Scherer P. E., Rossetti L. (2003) Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J. Clin. Invest. 111, 225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamauchi T., Kamon J., Waki H., Terauchi Y., Kubota N., Hara K., Mori Y., Ide T., Murakami K., Tsuboyama-Kasaoka N., Ezaki O., Akanuma Y., Gavrilova O., Vinson C., Reitman M. L., Kagechika H., Shudo K., Yoda M., Nakano Y., Tobe K., Nagai R., Kimura S., Tomita M., Froguel P., Kadowaki T. (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7, 941–946 [DOI] [PubMed] [Google Scholar]
- 25.Wang J., Obici S., Morgan K., Barzilai N., Feng Z., Rossetti L. (2001) Overfeeding rapidly induces leptin and insulin resistance. Diabetes 50, 2786–2791 [DOI] [PubMed] [Google Scholar]
- 26.Moller N., O’Brien P., Nair K. S. (1998) Disruption of the relationship between fat content and leptin levels with aging in humans. J. Clin. Endocrinol. Metab. 83, 931–934 [DOI] [PubMed] [Google Scholar]
- 27.Zhou X., Hansson G. K. (2004) Effect of sex and age on serum biochemical reference ranges in C57BL/6J mice. Comp. Med. 54, 176–178 [PubMed] [Google Scholar]
- 28.Kreisberg R. A., Kasim S. (1987) Cholesterol metabolism and aging. Am. J. Med. 82, 54–60 [DOI] [PubMed] [Google Scholar]
- 29.Bernstein E. D., Gardner E. M., Abrutyn E., Gross P., Murasko D. M. (1998) Cytokine production after influenza vaccination in a healthy elderly population. Vaccine 16, 1722–1731 [DOI] [PubMed] [Google Scholar]
- 30.Llorente L., Richaud-Patin Y., Alvarado C., Vidaller A., Jakez-Ocampo J. (1997) Autoantibody production in healthy elderly people is not promoted by interleukin-10 although this cytokine is expressed in them by a peculiar CD8+CD3+ large granular cell subpopulation. Scand. J. Immunol. 45, 401–407 [DOI] [PubMed] [Google Scholar]
- 31.Bruunsgaard H., Pedersen A. N., Schroll M., Skinhøj P., Pedersen B. K. (2000) Proliferative responses of blood mononuclear cells (BMNC) in a cohort of elderly humans: role of lymphocyte phenotype and cytokine production. Clin. Exp. Immunol. 119, 433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khabour O. F., Barnawi J. M. (2010) Association of longevity with IL-10 -1082 G/A and TNF-alpha-308 G/A polymorphisms. Int. J. Immunogenet. 37, 293–298 [DOI] [PubMed] [Google Scholar]
- 33.Akki A., Yang H., Gupta A., Chacko V. P., Yano T., Leppo M. K., Steenbergen C., Walston J., Weiss R. G. (2014) Skeletal muscle ATP kinetics are impaired in frail mice. Age (Dordr.) 36, 21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scimè A., Devarakonda S., Conroe H.M., Erdjument-Bromage H., Tempst P., Rudnicki M.A., Beier D.R., Spiegelman B.M. (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 21, 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leclercq I. A., Da Silva Morais A., Schroyen B., Van Hul N., Geerts A. (2007) Insulin resistance in hepatocytes and sinusoidal liver cells: mechanisms and consequences. J. Hepatol. 47, 142–156 [DOI] [PubMed] [Google Scholar]
- 36.Alvarez B., Quinn L. S., Busquets S., López-Soriano F. J., Argilés J. M. (2002) TNF-α modulates cytokine and cytokine receptors in C2C12 myotubes. Cancer Lett. 175, 181–185 [DOI] [PubMed] [Google Scholar]
- 37.Brett J. O., Rando T. A. (2014) Alive and well? Exploring disease by studying lifespan. Curr. Opin. Genet. Dev. 26, 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin M. T., Beal M. F. (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 [DOI] [PubMed] [Google Scholar]
- 39.Houtkooper R. H., Williams R. W., Auwerx J. (2010) Metabolic networks of longevity. Cell 9, 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dagdeviren S., Kandilci H. B., Uysal B., Zeybek N. D., Korkusuz P., Gümüsel B., Korkusuz F. (2011) Tumor necrosis factor-alpha antagonist administration recovers skeletal muscle dysfunction in ovariectomized rats. J. Orthop. Res. 29, 275–280 [DOI] [PubMed] [Google Scholar]
- 41.Sziksz E., Pap D., Lippai R., Béres N. J., Fekete A., Szabó A. J., Vannay Á. (2015) Fibrosis related inflammatory mediators: role of the IL-10 cytokine family. Mediators Inflamm. 2015, 764641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levast B., Li Z., Madrenas J., (2015) The role of IL-10 in microbiome-associated immune modulation and disease tolerance. Cytokine 75, 291–301 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.