Abstract
Clinical trials have shown that administration of the nematode Trichuris suis can be beneficial in treating various immune disorders. To provide insight into the mechanisms by which this worm suppresses inflammatory responses, an active component was purified from T. suis soluble products (TsSPs) that suppress TNF and IL-12 secretion from LPS-activated human dendritic cells (DCs). Analysis by liquid chromatography tandem mass spectrometry identified this compound as prostaglandin (PG)E2. The purified compound showed similar properties compared with TsSPs and commercial PGE2 in modulating LPS-induced expression of many cytokines and chemokines and in modulating Rab7B and P2RX7 expression in human DCs. Furthermore, the TsSP-induced reduction of TNF secretion from DCs is reversed by receptor antagonists for EP2 and EP4, indicating PGE2 action. T. suis secretes extremely high amounts of PGE2 (45–90 ng/mg protein) within their excretory/secretory products but few related lipid mediators as established by metabololipidomic analysis. Culture of T. suis with several cyclooxygenase (COX) inhibitors that inhibit mammalian prostaglandin synthesis affected the worm’s motility but did not inhibit PGE2 secretion, suggesting that the worms can synthesize PGE2 via a COX-independent pathway. We conclude that T. suis secretes PGE2 to suppress proinflammatory responses in human DCs, thereby modulating the host’s immune response.—Laan, L. C., Williams, A. R., Stavenhagen, K., Giera, M., Kooij, G., Vlasakov, I., Kalay, H., Kringel, H., Nejsum, P., Thamsborg, S. M., Wuhrer, M., Dijkstra, C. D., Cummings, R. D., van Die, I. The whipworm (Trichuris suis) secretes prostaglandin E2 to suppress proinflammatory properties in human dendritic cells.
Keywords: multiple sclerosis, inflammatory bowel disease, helminth therapy, immune regulation, lipids
Parasitic helminths are a burden to humanity and animal welfare due to their ability to cause chronic infection by modulating host immune responses in favor of their survival (1). Intriguingly, this ability causes positive bystander effects such as the protection to several immune disorders, including multiple sclerosis, inflammatory bowel disease, and allergies (1, 2). Many animal studies and human in vitro studies indicate that helminths and their products have strong immune-modulating capacities (2, 3) that might be exploited to improve inflammatory conditions in autoimmune diseases. Over 25 clinical trials have been performed to evaluate the therapeutic potential of helminth treatment (4). Most of these trials were carried out using the pig whipworm Trichuris suis, a parasite that cannot multiply in humans and therefore is considered a safe choice. Whereas earlier helminth therapy trials have given promising results, the outcomes of recent larger trials have been disappointing (4, 5). Our aim in the present study was to elucidate the structural identity of the immune modulatory compounds of the worms to facilitate the design of optimized treatment regimens for inflammatory diseases, possibly without the use of live parasites and thus avoiding infection.
Extracted T. suis soluble products (TsSPs) significantly reduce disease severity in a murine model for multiple sclerosis (6), and excretory/secretory (E/S) products of this worm have been reported to reduce hyperreactivity in a model for allergic disease (7). These data indicate that infection with live worms is not essential for the immunoregulatory action of T. suis. In previous studies, we showed that secretion of proinflammatory mediators from human activated dendritic cells (DCs) and macrophages is strongly suppressed by TsSPs, and we have unraveled several mechanistic aspects underlying the potential of TsSPs and T. suis E/S products to suppress inflammatory responses (6, 8, 9). In line with these findings, TsSPs suppress the polarization of DCs into a T helper (Th)1/17-inducing phenotype and instead induce a Th2-inducing DC subtype (6). TsSP treatment of monocytes increases a patrolling monocyte subtype with enhanced CD16 and reduced CCR2 expression, which shows decreased migration through a human brain endothelial cell line compared with untreated monocytes (10). Such a property might restrict the influx of monocytes into the brain in multiple sclerosis. During human monocyte-to-macrophage differentiation, TsSPs induce epigenetic changes in the cells, resulting in prolonged TNF reduction in activated macrophages (type M1), and strongly induce IL-10 production and a macrophage subtype that resembles that of an alternatively activated macrophage (type M2) (11). In summary, these data provide insight into the anti-inflammatory properties of T. suis, which contributes to understanding of its protective potential in inflammatory diseases.
Here, we report the purification and identification of a major component present within TsSPs and T. suis E/S products, which modulates DC phenotype and function in a manner similar to crude TsSPs. Remarkably, characterization of this component by liquid chromatography tandem mass spectrometry (LC-MS/MS) revealed that this component is prostaglandin (PG)E2, which acts at least partly via the PGE2 receptors EP2 and EP4 on DCs.
MATERIALS AND METHODS
Preparation of soluble worm products and isolation of E/S products
Adult T. suis worms were isolated from pigs experimentally infected with 5000–7000 T. suis eggs for 50 d. Immature stages were obtained at d 18 after inoculation (larval stage 3) and d 28 after inoculation (larval stage 4) using 15,000 and 10,000 infective eggs, respectively. Infection was approved and carried out according to the guidelines of the Danish Animal Experimentation Inspectorate (2015-15-0201-00760 and 2010-561-1914). Pig intestines were washed with 0.9% NaCl, and the adult worms were manually harvested from the large intestine in 0.9% NaCl in a 37°C room. The larvae were isolated using the protocol described by Kringel et al. (12), except that the incubation of the intestines was reduced to 2 h at 37°C. The worms were transferred to large Petri dishes (100–200 worms/dish) with HBSS (Thermo Fisher Scientific, Breda, The Netherlands) and washed 5 times with HBSS at 37°C, with 15–30 min in between washes. Then the worms were transferred to Petri dishes with serum-free RPMI 1640 with 2 mM L-glutamine, 100 µg/ml streptomycin, and 100 U/ml penicillin (culture medium) and incubated for 2 h at 37°C to let the worms empty their guts. The medium was refreshed every 15–30 min. Finally, the worms were collected, washed twice with HBSS, and either frozen (for preparation of TsSPs) or transferred to fresh culture medium to collect E/S products. To collect E/S products, the worms were incubated for 24 h at 37°C in culture medium, and the supernatants containing the E/S products filtered (0.2-µm syringe) and frozen at −80°C until use. Ascaris suum fourth-stage larvae were isolated from pigs 14 d after experimental infection with 10,000 infective A. suum eggs (13). After 48 h of in vitro culture, the E/S-containing medium was collected as previously described. TsSPs were prepared as described previously (9). Endotoxin levels were below 0.2 EU/ml, as determined with a Limulus Amebocyte Lysate assay (Lonza, Basel, Switzerland). When indicated, compounds were treated with 10 mM sodium periodate (PI) as previously described (8). Crude Schistosoma mansoni soluble egg antigen was prepared as previously described (14).
DC assays
Human immature DCs were generated as described previously (6). In all assays, the DCs were reseeded (1 × 106 cells/ml) after 4 d differentiation and stimulated with TsSPs (40 µg protein/ml), isolated TsSP fractions, or PGE2 (at concentrations indicated; Sigma-Aldrich, St. Louis, MO, USA) for 15 min before addition of 10 ng/ml LPS (Escherichia coli strain 0111:B4, Sigma-Aldrich). When indicated, the following PGE2 receptor antagonists were used: for EP1/2, AH6809 (Abcam), for EP3, L-798,106 (Santa Cruz Biotechnology), and for EP4, GW 627368X (both from Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Purification of T. suis compounds
TsSPs (∼70 mg dry weight in MilliQ water; Millipore, Billerica, MA, USA) and 150 ml of T. suis culture medium containing E/S products were preseparated using Sep-Pak C18 chromatography (6 ml Vac cartridge, 1 g sorbent per cartridge, 55–105 µm; Waters, Etten-Leur, The Netherlands). Sep-Pak columns were activated with 2-propanol and washed with 30 ml MilliQ before application of the T. suis compounds. Elution was performed stepwise using 20–100% propanol. Collected fractions were lyophilized and dissolved in MilliQ. Reverse-phase HPLC was carried out on an Ultimate 3000 Prep system (Dionex, Sunnyvale, CA, USA) using a semipreparative RP C18 column (218MS RP 10 × 250 mm) (Grace–Alltech, Breda, The Netherlands); fractionation size was 1 min at a flow rate of 4 ml/min. All solvents were purchased from Biosolve (Valkenswaard, The Netherlands). UV was measured at 215 nm. Buffer A consisted of MilliQ acidified with 0.01 TFA. Gradient used was isocratic elution at 3% buffer B (100% MeCN) for 5 min, 3–60% B in 30 min, and 60–95% B in 5 min and kept isocratic for 5 min before returning to starting conditions. All fractions were lyophilized and resuspended in 200 µl MilliQ. Subsequent ion exchange HPLC was performed using a polymeric weak anion exchange column (Prosphere P-WAX, 7.5 × 75 mm; Grace–Alltech) on an Ultimate 3000 Ultra High Performance Liquid Chromatography (UHPLC) system (Dionex). UV was measured at 215 nm, and fractionation size was 1 min at a flow rate of 1 ml/min. Buffer A consisted of MilliQ. Gradient used was isocratic elution at 0% buffer B (0.5 M NaCl in MilliQ) for 5 min, 0–100% B in 18 min, and kept isocratic for 2 min before returning to starting conditions. Fractions were collected and desalted using 50 mg Sep-Pak C18 Solid Phase Extraction (SPE) columns. After desalting, the eluates were lyophilized and dissolved in 200 µl MilliQ.
MS of TsSP and T. suis E/S products
LC-MS/MS in positive electrospray ionization (ESI+) mode was performed by C18 RP-LC-ESI+ MS/MS as described (15) on a maXis HD Quadrupole Time-of-Flight (QTOF) mass spectrometer equipped with a CaptiveSpray nanoBooster source (both Bruker Daltonics, Bremen, Germany) coupled to an Ultimate 3000 nanoUPLC system (Dionex). The mass spectrometer and the LC were controlled by Hystar 3.2 (Bruker Daltonics), and data analysis was performed using DataAnalysis 4.2 (Bruker Daltonics). Two microliters of the purified TsSP fractions (P2–4) were loaded onto a C18 microprecolumn (C18 PepMap 100, 300 µm × 5 mm, 5 µm, 100 Å; Dionex) with 10 μl/min of loading solvent (99% water/1% ACN/0.05% TFA) for 4 min. The analytes were separated on a C18 analytical column (Acclaim PepMap RSLC, 75 μm × 15 cm, 2 µm, 100 Å; Dionex) at 45°C column oven temperature. Elution was performed at a flow rate of 0.5 μl/min with solvent A (water containing 0.1% FA, v/v) and solvent B (80% acetonitrile/20% water containing 0.1% FA, v/v). A linear gradient of 3–54% solvent B for 31.5 min was applied followed by column washing and reconditioning.
For targeted LC-MS/MS in negative ESI mode, 1 µl P3 sample was diluted 1:200 with 40% methanol, 4 µl internal standard solution (containing PGE4-d4, 50 ng/ml in methanol) was added, and the sample analyzed as described (16). Briefly, a QTrap 6500 mass spectrometer operated in negative ESI mode (Sciex, Nieuwerkerk aan den Ijssel, The Netherlands) was coupled to a LC system using 2 LC-30AD pumps, a SIL-30AC autosampler, and a CTO-20AC column oven (Shimadzu, Hertogenbosch, The Netherlands). A Kinetex C18 (50 × 2.1 mm, 1.7 µm) protected with a C8 precolumn (Phenomenex, Utrecht, The Netherlands) was used. The column was kept at 50°C. A binary gradient of water (solvent A) and methanol (solvent B) containing 0.01% acetic acid was generated: 0 min 30% B, held for 1 min, then ramped to 45% at 1.1 min, to 53.5% at 2 min, to 55.5% at 4 min, to 90% at 7 min, and to 100% B at 7.1 min, held for 1.9 min. The injection volume was 40 µl, and the flow rate 400 µl/min. The mass spectrometer was operated as described in Schlegel, et al. (16). For analyte identification, the characteristic mass transition combined with the compounds relative retention time were used. For analyzing the effect of PI, 4-µl PI-treated TsSP and P3 samples were diluted with 96 µl methanol and centrifuged for 3 min at 16,100 g. To 50 µl of the supernatant, 2 µl internal standard (containing d4-PGE4, 50 ng/ml in methanol) and 48 µl water was added. The samples were analyzed as previously described.
A 24 h, T. suis culture medium, containing T. suis E/S products, was subjected to solid-phase extraction and lipid mediator (LM) metabololipidomics as previously described (17). Before sample extraction, d8-5-HETE, d5-RvD2, d5-LXA4, d4-LTB4, and d4-PGE2 internal standards (500 pg each) were added to facilitate quantification. Extracted samples were analyzed by a LC-ultraviolet MS/MS system consisting of a QTrap 5500 (Sciex) equipped with a Shimadzu LC-20AD HPLC (Tokyo, Japan). A Poroshell 120 EC-18 column (100 mm × 4.6 mm × 2.7 μm) (Agilent Technologies, Santa Clara, CA, USA) was kept in a column oven maintained at 50°C, and LMs were eluted with a gradient of methanol/water/acetic acid from 55:45:0.01 (v/v/v) to 100:0:0.01 at a flow rate of 0.5 ml/min. To monitor and quantify the levels of targeted LMs, multiple reaction monitoring was used with MS/MS, matching signature ion fragments for each molecule (6 diagnostic ions and calibration curves).
ELISA and real-time quantitative PCR
TNF and IL-12 concentrations were assessed by ELISA of culture supernatants harvested 24 h after DC stimulation using a human TNF antibody pair (TNF capture and detection; Thermo Fisher Scientific), and an IL-12 antibody pair [IL-12 capture (eBioscience, San Diego, CA USA) and IL-12 detection (R&D Systems, Minneapolis, MN, USA)], respectively. PGE2 concentrations were determined using the PGE2 EIA kit (Cayman Chemical, Ann Arbor, MI, USA). ELISA assays were done according to the manufacturer’s protocols using a spectrophotometer (Bio-Rad, Hercules, CA, USA) for readout at an absorbance of 450 nm (cytokines) and 415 nm (PGE2), and analysis was performed using Microplate Manager software (Bio-Rad).
Real-time quantitative PCR was performed using glyceraldehyde-3-phosphate as a reference gene as previously described (18). Oligonucleotides (Table 1) were designed using Primer Express 2.0 (Applied Biosystems, Bedford, MA, USA) software and synthesized by Thermo Fisher Scientific.
TABLE 1.
List of primers used for RT-PCR
| Primer, 5′–3′ | ||
|---|---|---|
| Gene | Forward | Reverse |
| P2RX7 | ACCAACGTGTCCTTGTACCCTG | CAAAACGGATCCCGAAGACTTT |
| RAB7B | TGCTCATGAACGTTTTAGGAGC | CCTCCAAGCCCATGAACAA |
| SOCS1 | TGAACTCGCACCTCCTACCTCT | CAACCCCTGGTTTGTGCAA |
| CCL2 | AATCACCAGCAGCAAGTGTCC | TCCTTGGCCACAATGGTCTT |
| CCL3 | CTACTTTGAGACGAGCAGCCAGT | TGGTTAGGAAGATGACACCGG |
| CCL8 | TGCTCATGGCAGCCACTTT | GAAACTGAATCTGGCTGAGCAAG |
| CCL19 | TCTCATCAAGGATGGCTGCA | CTCAGTGTGGTGAACACTACAGCA |
| CXCL9 | TGCAAGGAACCCCAGTAGTGA | TAGTCCCTTGGTTGGTGCTGA |
| CXCL16 | CCTATGTGCTGTGCAAGA | CACAGGTATATAATGAACCGGCAG |
Culture of T. suis in medium supplemented with labeled arachidonic acid
T. suis worms were isolated as previously described and then cultured for 24 h and washed again. Eight to 10 worms were placed in duplicate wells containing 1 ml culture medium, 50 µg/ml 13C5-labeled arachidonic acid (AA) (17336; Cayman Chemical) in DMSO (final concentration 2%) or DMSO alone was added, and the worms were cultured for 3 or 24 h. After culturing, the medium containing the secreted E/S products were collected, passed through a 0.2-µm syringe filter, and stored at −80°C for subsequent analysis by MS.
Motility and metabolic activity of T. suis cultured in medium containing different COX inhibitors
Worms were isolated as previously described and then cultured for 24 h either in culture medium containing 0–1000 µg/ml acetylsalicylic acid (ASA), 0–1000 µg/ml indomethacin, 0–200 µg/ml FR 122047, 0–200 µg/ml NS398 (all from Sigma-Aldrich) or in control medium. After 24 h, motility was assessed using a 0–3 scale as previously described (19). To confirm the inhibitory effect of ASA, metabolic activity of worms exposed to 1 mg/ml ASA was assessed using the AlamarBlue assay (Sigma-Aldrich). After 24 h culture, 2 worms from either control or ASA-treated cultures were transferred to fresh medium containing resazurin sodium salt (final concentration 12.5 µg/ml) (Sigma-Aldrich) and incubated for a further 4 h, and 200 µl medium was transferred to an opaque 96-well plate and the fluorescence emission read at 590 nm at 37°C using a spectrofluorometer (SpectraMax; Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
Results are expressed as means ± sem. Statistical analyses were performed using SPSS 15.0 software (IBM, Armonk, NY, USA). For paired comparisons of 2 groups, a paired sample Student’s t test was performed. For other parametric data, 1-way ANOVA was performed, followed by Dunnett’s multiple comparisons test.
RESULTS
Purification of TsSP components that suppress LPS-induced TNF secretion of human DCs
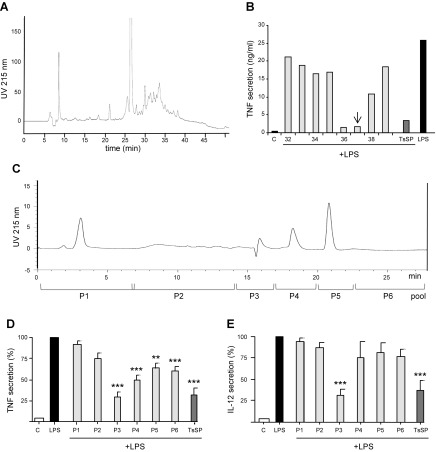
In previous studies, we found that heating TsSPs to 80°C or chymotrypsin treatment did not abolish the potential of TsSPs to suppress the production of TNF from LPS-stimulated human DCs (8). These data suggest that the suppressing compound is not a protein. We evaluated the possibility that TsSPs contain suppressive compounds with hydrophobic or amphiphilic properties by prefractionating TsSPs by Sep-Pak chromatography using a stepwise propanol gradient. The resulting fractions were tested for their ability to suppress LPS-induced TNF production in a DC assay. Profound suppressive activity was only observed in the fractions that eluted with 20–40% propanol. These fractions were pooled and further purified by C18 RP-HPLC (Fig. 1A), and all fractions obtained were tested in the DC assay (Fig. 1B). The hydrophobic fractions F36 and F37 showed most of the suppressive activity and were further purified by anion-exchange chromatography (Fig. 1C). The eluting fractions were pooled in 6 fractions (P1–P6). Only P3 strongly suppressed both LPS-induced TNF (Fig. 1D) and IL-12 (Fig. 1E) secretion from activated DCs. The neighboring fractions (P4–P6) showed suppression of TNF production, whereas other fractions did not show suppressive activity. In summary, these data suggest that P3, compared with the neighboring fractions, contains a high concentration of the active compound with amphiphilic properties.
Figure 1.
Isolation of TsSPs, which suppress LPS-induced TNF and IL-12 secretion from human DCs. A) TsSPs were fractionated by reversed-phase HPLC, and 1-ml fractions were collected. B) All fractions (Fs) were evaluated for their effect on LPS-induced TNF secretion by DCs. C–E) F37 was further purified by anion exchange chromatography (C), and all fractions were evaluated (1% v/v) for their effect on LPS-induced TNF (D) and IL-12 (E) secretion by DCs. The isolation procedure was performed twice with similar results. The data shown in D and E are derived from 3 independent experiments performed with DCs derived from different donors. Results are presented as the means ± sem. **P < 0.01; ***P < 0.001.
TsSPs contain PGE2
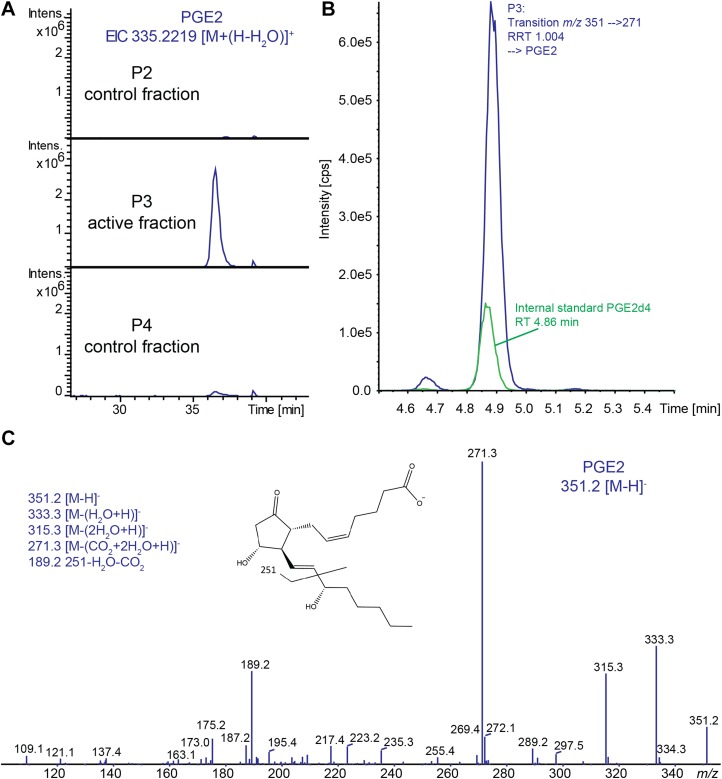
The active fraction P3 and the 2 neighboring fractions (P2 and -4) were analyzed by RPLC MS/MS in ESI+ mode (RPLC-ESI+ MS/MS) to identify the compounds that were active in the DC assay. The base peak chromatograms of all 3 samples are shown in Supplemental Fig. 1. Manual inspection of the sample runs revealed a highly abundant compound eluting at around 36.5 min in P3, which was hardly present in the other 2 fractions (Fig. 2A; Supplemental Fig. 1). The compound was characterized by m/z 335.2219 [M+H]+, and MS/MS analysis confirmed the composition C20H30O4. Together with its characteristic elution profile, this was indicative of a lipid-like molecule. Targeted analysis using a QTrap-based platform (16) of P3 resulted in a high-intensity peak in the trace of transition m/z 351→271 at a relative retention time of 1.004, compared with the internal standard PGE2d4, eluting at 4.86 min (Fig. 2B). Together with the higher-resolution MS data from the RPLC-ESI+ MS/MS analysis, this enabled the assignment of the signal as PGE2 (Fig. 2C). PGE2 has a molecular mass of 352.2244 Da, which corresponds to the observed signal in ESI+ mode of 335.2219 [M + (H-H2O)]+ with a loss of water.
Figure 2.
Identification of PGE2 in sample P3 by LC-MS/MS. A) Extracted ion chromatograms of m/z 335.2219 [M + (H-H2O)]+, which was the only discriminative peak between the active fraction P3 and the neighboring fractions P2 and -4. B) SRM transition m/z 351→271 [M-H]− of the active fraction showed a high-intensity peak at a relative retention time (RRT) corresponding to PGE2 with a RSD <0.5%. C) The presence of PGE2 in the active fraction was further confirmed by MS/MS of m/z 351.2 [M-H]−.
The major effects of TsSPs on DCs are mediated by PGE2
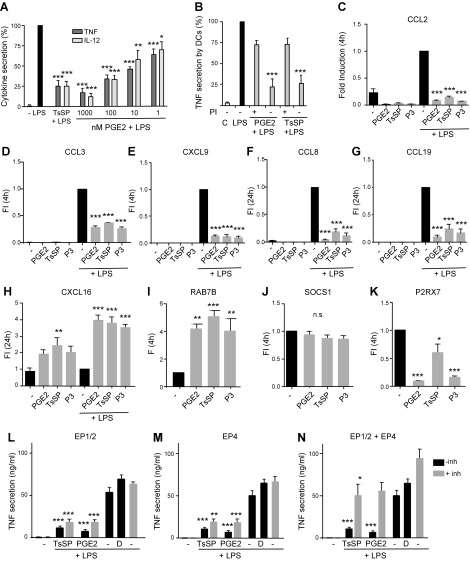
To compare the effect of pure commercially derived PGE2 with that of TsSPs on DC modulation, we performed a DC assay with different PGE2 concentrations and TsSPs (40 µg/ml, calculated to contain approximately 50 nM PGE2) and defined TNF and IL-12 levels secreted by the DCs; the suppressive effect of TsSPs most closely corresponds to the effect of 100 nM PGE2 (Fig. 3A). In a previous study, we showed that mild PI treatment of TsSPs inactivates their anti-inflammatory effect (8). To verify the effect of PI treatment, we treated PGE2 with PI, which resulted in a similar loss of potential to suppress TNF secretion in a DC assay as observed for TsSPs (Fig. 3B). A quantitative targeted MS analysis monitoring the transition m/z 351→271 showed that PGE2 was almost completely destroyed by PI treatment.
Figure 3.
TsSPs and PGE2 similarly modulate DC gene expression. A) DCs were stimulated with LPS (10 ng/ml) in the absence or presence of TsSPs (40 μg/ml) or different concentrations of PGE2. After 24 h, the levels of TNF and IL-12p70 were determined in culture supernatants by ELISA. The value for LPS activation was set at 100% to correct for donor variation in cytokine secretion levels (TNF: 27–63 ng/ml; IL-12: 20–147 ng/ml). B) DCs were stimulated with LPS (10 ng/ml) in the absence or presence of TsSPs (40 μg/ml) or PGE2 (100 nM) pretreated or not with 10 mM PI, and after 24 h TNF levels in the supernatants were determined by ELISA. C–K) mRNA levels of CCL2 (C), CCL3 (D), CXCL9 (E), CCL8 (F), CCL19 (G), CXCL16 (H), RAB7B (I), SOCS1 (J), and P2RX7 (K) were determined by real-time PCR, using glyceraldehyde-3-phosphate as a reference gene, in DCs stimulated with TsSPs (40 µg/ml), PGE2 (100 nM), or P3 (1% v/v) in the presence (C–H) or absence (I–K) of LPS (10 ng/ml). L–N) DCs were incubated with TsSPs (40 µg/ml) or PGE2 (100 nM) in the presence of LPS (10 ng/ml), ± PGE2 antagonists for EP1/2 (L), EP4 (M), or EP1/2 + EP4 (N) [inhibitors (inh) at 10 µM] for 24 h. Because the inhibitors were dissolved in DMSO, a DMSO (D) control was included. Data in C–K are expressed as fold induction (FI) relative to the LPS value (set at 1 in C–H) or untreated DCs (set at 1 in I–K). Data shown are from 3 (B) or 4 independent experiments (all others) using DCs from different donors (means ± sem). *P < 0.05, **P < 0.01, ***P < 0.001.
Next, we evaluated whether fraction P3 and/or PGE2 can induce a similar anti-inflammatory DC phenotype as TsSPs. Both P3 and PGE2 modified LPS-induced expression of CCL2, CCL3, CXCL9, CCL8, CCL19, and CXCL16 in a similar manner as TsSPs in the concentrations used (Fig. 3C–H). In addition, the expression of RAB7B was up-regulated (Fig. 3I), and P2RX7 expression was reduced (Fig. 3K) by all 3 agonists compared with control DCs, which was demonstrated previously for TsSPs (9, 18) but to our knowledge has not been reported for PGE2. Both PGE2 and P3 showed a markedly stronger reduction of P2RX7 than TsSPs, suggesting the presence of compounds within TsSPs that counteract the PGE2 effect. By contrast, the expression of SOCS1, which is induced in DCs upon contact with S. mansoni-soluble egg antigen (20), was not affected by TsSPs or PGE2 (Fig. 3J), indicating that PGE2 does not induce SOCS1.
Human monocyte-derived DCs express the PGE2 receptor subtypes (EP2–4), of which EP2 and -4 were found active with respect to cytokine production (21). Using selective EP receptor antagonists, we showed that blocking EP2 or -4 separately had only modest effects on the suppressive properties of TsSPs in a DC assay (Fig. 3L, M), whereas blocking EP3 had no effect; however, LPS-induced TNF and IL-12 secretion was significantly reduced using the combination of EP2 and -4 antagonists (Fig. 3N), indicating that the TsSPs act via both EP2 and -4 to a similar degree as PGE2.
Collectively, these data show that the TsSP-induced effects on DC modulation are similar to those induced by PGE2, which confirm that the effect of TsSPs on DCs are largely due to the presence of PGE2.
High PGE2 levels are secreted by T. suis
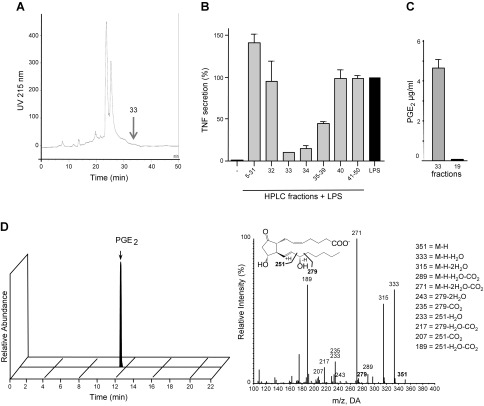
To determine the ability of T. suis to secrete PGE2 in its environment, medium from cultured T. suis was purified by C18 RP-HPLC similarly as performed for TsSP purification (Fig. 4A). Fraction 33 was identified as the major active fraction in a DC assay (Fig. 4B). Fraction 33 contained a high level of PGE2, in contrast to one of the negative fractions (F19), and control culture medium in which PGE2 was undetectable (Fig. 4C). To further confirm the presence of PGE2 and to identify the full spectrum of LMs in crude T. suis E/S products, T. suis culture medium was subjected directly to LC-MS/MS-based LM metabololipidomics. With this approach, PGs, lipoxins, leukotrienes, maresins, and resolvins, all LMs occurring in mammals, can be quantitatively determined in the low pg range (22). Again, we identified an extremely high amount of PGE2 from T. suis in comparison to other LMs (Fig. 4D and Table 2). The PGs PGD2, PGF2-α, and thromboxane B2 were detected, as well as some traces of the resolvins E2 and -3, whereas other LMs were undetectable within T. suis E/S products (Table 2). In addition, various specific bioactive LM precursors and pathway markers were identified in T. suis E/S products, including the PGE2 precursor fatty acid/arachidonic acid (AA) (Table 2).
Figure 4.
PGE2 in T. suis culture medium. T. suis worms were cultured for 24 h. A) The culture medium was purified by Sep-pak chromatography, and the 20–40% propanol elution fractions (Fs) were further purified by reversed-phase HPLC. B) F33–F39 showed significant suppression in a DC assay; the mean values of 2 DC assays (different donors) are shown. C) F33 and F19 were evaluated for their concentration of PGE2 by ELISA. D) Identification of PGE2 in T. suis E/S using lipid mediator metabololipidomics. A representative multiple reaction monitoring chromatogram of PGE2 is shown (left) along with the accompanying PGE2 MS/MS spectra (right).
TABLE 2.
Lipid mediators in T. suis E/S products.
| Lipid mediator | Q1 | Q3 | E/S (pg/ml) | Lipid mediator | Q1 | Q3 | E/S (pg/ml) |
|---|---|---|---|---|---|---|---|
| AA bioactive metabolome | E/S | DHA bioactive metabolome | |||||
| LXA4 | 351 | 115 | ND | RvD1 | 375 | 215 | — |
| LXB4 | 351 | 115 | — | RvD2 | 375 | 215 | — |
| 5,15(S)-diHETE | 335 | 235 | — | RvD3 | 375 | 181 | — |
| AT-LXA4 | 351 | 115 | — | RvD4 | 375 | 255 | — |
| AT-LXB4 | 351 | 115 | — | RvD5 | 359 | 199 | — |
| LTB4 | 335 | 195 | — | RvD6 | 359 | 101 | — |
| 20-OH-LTB4 | 351 | 195 | — | AT-RvD1 | 375 | 121 | — |
| 20-COOH-LTB4 | 365 | 195 | — | AT-RvD3 | 375 | 147 | — |
| 5,12(S)-diHETE | 335 | 195 | — | PD1 | 359 | 153 | — |
| PGD2 | 351 | 189 | 11.6 ± 3.2 | 10,17(S)-diHDHA | 359 | 153 | — |
| PGE2 | 351 | 189 | 35,945 ± 516.2 | 22-OH-PD1 | 359 | 153 | — |
| PGF2-α | 351 | 193 | 63.5 ± 22.5 | Mar1 | 359 | 221 | — |
| TXB2 | 369 | 169 | 1.9 ± 1.0 | 7,14(S)-diHDHA | 359 | 221 | — |
| 4,14(S)-diHDHA | 359 | 101 | — | ||||
| Pathway markers/FA precursors | E/S | ||||||
| 17-HDHA | 343 | 245 | — | EPA bioactive metabolome | E/S | ||
| 14-HDHA | 343 | 205 | 0.1 ± 0.0 | RvE1 | 349 | 195 | — |
| 7-HDHA | 343 | 141 | 0.2 ± 0.1 | RvE2 | 331 | 253 | 0.6 ± 0.2 |
| 4-HDHA | 343 | 101 | 0.6 ± 0.1 | RvE3 | 333 | 201 | 0.6 ± 0.3 |
| DHA | 327 | 283 | 253.4 ± 207.4 | ||||
| 18-HEPE | 317 | 259 | 0.2 ± 0.2 | ||||
| 15-HEPE | 317 | 219 | 0.2 ± 0.2 | ||||
| 12-HEPE | 317 | 179 | — | ||||
| 5-HEPE | 317 | 115 | 0.2 ± 0.1 | ||||
| EPA | 301 | 257 | 53.0 ± 22.4 | ||||
| 15-HETE | 319 | 219 | 5.1 ± 3.3 | ||||
| 12-HETE | 319 | 179 | 0.7 ± 0.3 | ||||
| 5-HETE | 319 | 115 | 1.1 ± 0.3 | ||||
| AA | 303 | 259 | 397.6 ± 201.8 |
T. suis worms were cultured for 24 h. The medium containing E/S products was harvested, and LM levels were defined by metabololipidomics. Data are categorized based on their synthesis routes in mammals. Q1, M-H (parent ion); Q3, diagnostic ion in MS-MS (daughter ion). Data are expressed as means ± sem (n = 3). ND, not detected with a limit of detection approximately 0.1 pg.
PGE2 levels in different worm species and different worm stages
To gain insight into the amounts of PGE2 present in different helminth products and different worm stages, we measured the PGE2 concentration in crude TsSPs and T. suis E/S products, as well as in S. mansoni soluble egg antigen, and in culture medium of A. suum fourth stage larvae by ELISA. The data show that TsSPs and T. suis E/S products contain much higher levels of PGE2 than the samples of the other helminths tested (Table 3). Interestingly, different larval stages of T. suis secrete PGE2 in their E/S products, indicating that PGE2 is secreted early in its development.
TABLE 3.
PGE2 concentrations in helminth products
| Helminth product | Amount of PGE2 protein (ng/mg) |
|---|---|
| Helminth extract | |
| T. suis soluble compounds | 305 |
| S. mansoni soluble egg antigen | 4 |
| Helminth culture medium | |
| T. suis E/S products, adult worms, d 50 | 90 |
| T. suis E/S products, larval stage 3, d 18 | 39 |
| T. suis E/S products, larval stage 4, d 28 | 241 |
| A. suum E/S products, larval stage 4, d 28 | ∼1 |
The amount of PGE2 is measured by ELISA and expressed relative to the amount of protein in the products (n = 2).
Synthesis pathway of PGE2 in T. suis
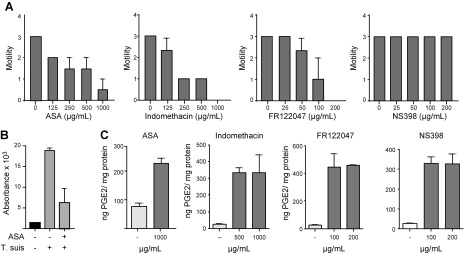
To gain insight into PGE2 synthesis by T. suis, we cultured the worms for 24 h to deplete endogenous lipids and then added 13C-labeled AA. Culture medium containing E/S products were collected 3 and 24 h after AA was added, respectively, and analyzed by targeted LC-MS/MS. Whereas a large amount of unlabeled PGE2 was detected, no trace of 13C-labeled PGE2 was observed. To evaluate the role of COX enzymes in T. suis metabolism, the worms were cultured with both nonspecific COX inhibitors (ASA and indomethacin) and isoform-specific inhibitors (FR122047 for COX1 and NS398 for COX2) for 24 h, which resulted in a dose-dependent decrease in motility compared with untreated worms for all inhibitors except NS398. At the highest concentrations tested, the worms were motionless despite extended observation, in contrast to the fully motile worms cultured without inhibitor (Fig. 5A). This was further confirmed by an Alamar blue assay, which showed a decrease in metabolic activity in the worms cultured with 1 mg/ml ASA, suggesting that COX enzymes play a key role in T. suis metabolism (Fig. 5B). Analysis of the PGE2 concentration in the culture medium of all inhibitor-treated worms showed an increased level of PGE2 compared with control medium, indicating that these COX inhibitors do not inhibit, but rather increase, PGE2 synthesis (Fig. 5C). Thus, whereas inhibitors of COX enzymes, and in particular COX1, inhibit the helminth’s motility, T. suis has the potential to synthesize PGE2, likely via a COX-independent pathway.
Figure 5.
Treatment of T. suis with COX inhibitors has anthelmintic effects but does not abrogate PGE2 secretion. A) Motility of T. suis worms after 24 h exposure to various concentrations of ASA, indomethacin, NS398, and FR122047. Data are derived from a single experiment performed in triplicate using worms (5–7 worms/ml for each inhibitor concentration) incubated in culture medium. B) After 24 h, the metabolic activity of the worms cultured with 1 mg/ml ASA was determined by Alamar blue assay. C) The PGE2 concentration in the medium was determined after 24 h by ELISA and expressed as ng PGE2/mg protein compared with the control (no inhibitor). Motility is expressed on a 0–3 scale, where 3 is fully motile and 0 is no movement despite extended observation. Control in each graph represents the motility of control worms incubated in 2% DMSO, which were fully motile at all times. Means are from 3 replicates at each concentration.
DISCUSSION
In clinical trials, the current level of administration of T. suis does not generally show sufficient therapeutic potential for the treatment of inflammation despite its clear anti-inflammatory effects (2, 4, 5). An improved treatment efficacy may be acquired with purified helminth-derived products or with synthetic mimics of such compounds. A major drawback for such an approach is that the anti-inflammatory molecules secreted by T. suis are not known. In previous studies, we showed that TsSPs polarize human DCs toward a Th2-type response and showed inhibition of proinflammatory Th1 and -17 responses (6, 8, 18). Here we report that the major compound within TsSPs mediating the reported modulation of DC function is PGE2, which belongs to a family of autocrine and paracrine acting LMs known to regulate many physiologic processes and immunologic responses in mammals.
Several reports describe that helminths, including the trematode S. mansoni (23), the cestode Taenia taeniaeformis (24), and the nematode Brugia malayi (25, 26), produce PGs, but the role of PGs in worms is less well explored than in mammals (26). In mammals, PGs are synthesized enzymatically from essential fatty acids via the COX pathway with AA as the precursor (27). Most parasitic helminths are dependent on the supply of lipid sources from their hosts for the production of eicosanoids (26), and some helminths are reported to use exogenous AA to synthesize PGE2 (24, 25). By contrast, we could not demonstrate the conversion of exogenously added 13C-labeled AA into PGE2 in T. suis, perhaps indicating that AA is not taken up and/or that the worms use endogenous AA or another lipid source for PGE2 synthesis. The mechanism of PGE2 synthesis in T. suis needs to be further investigated. Culture of T. suis in the presence of ASA, a COX inhibitor, resulted in an enhanced secretion of PGE2 despite a strongly reduced motility and metabolic activity. Similarly, indomethacin and the COX1 inhibitor FR122047 reduced the motility of the worms but did not affect PGE2 synthesis. These data suggest that PGE2 synthesis by T. suis is COX independent. In C. elegans, F-series PGs are synthesized via a COX-independent pathway, indicating that some nematodes can produce PGs via an alternative pathway (28). No COX homologs could be found in C. elegans (28), but in Oesophagostomum dentatum, the presence of COX proteins has been suggested (29). The anthelmintic effects induced by the COX inhibitors suggest that COX enzymes play a key role in the metabolism of T. suis or that these molecules inhibit another, unknown, pathway in T. suis. Clearly, PGE2 is not responsible for the observed changes in motility and metabolic activity of T. suis, but other PGs may be involved, a possibility that is partly supported by studies in the nematode O. dentatum (30).
PGE2 regulates immune responses depending on its concentration in the microenvironment, receptor usage, and other mediators acting simultaneously (21, 31). We propose that the secretion of PGE2 by T. suis contributes to its protective potential in inflammatory diseases. PGE2 modulates DC polarization, thereby directing the immunologic balance toward a Th2-type response while suppressing Th1 immunity (32), and can promote resolution of inflammation and subsequent tissue repair (33). PGE2 produced early in the inflammatory response activates enzymes that drive the production of proresolving lipid mediators in the resolution phase, a process defined as lipid-mediator class switching (34). Moreover, it has been shown that regeneration of epithelial crypts after dextran sodium sulfate-induced intestinal injury is regulated by PGE2 (35, 36). In accordance with these findings, overproduction of PGE2 is observed in Th2-associated diseases, including atopic dermatitis and asthma (37). In addition, mice with reduced PGE2 production develop systemic inflammation and translocation of gut bacteria (38). Interestingly, mechanistically PGE2 acted here via EP4 signaling, resulting in disruption of the ILC-IL-22 axis (38). Collectively, these data indicate a protective role for PGE2 to control systemic inflammation.
On the other hand, it has been reported that low endogenous PGE2 concentrations induce Th1 cell differentiation and promote Th17 cell expansion (39, 40), thereby promoting inflammation. Elevated levels of PGE2 receptors have been observed in lesions of experimental autoimmune encephalitis (EAE), an animal model for multiple sclerosis, which correlated with clinical symptoms (41). In addition, ablation of microsomal PGE synthase 1 in mice resulted in lower IL-17 and IFN-γ production associated with amelioration of pathology in EAE compared with control mice (41). Another study showed a dual role for PGE2 in murine EAE by facilitating Th1 and -17 cell generation and simultaneously protecting the blood-brain barrier, thus reducing invasion of these cells into the brain (42). By contrast, we showed previously that TsSPs, now known to contain PGE2, strongly ameliorate pathology in EAE (6). It is likely that the TsSP-associated PGE2 that we applied exogenously (4 immunizations at ∼30 ng PGE2 per injection) leads to a PGE2 concentration that greatly exceeds the endogenous levels and results in a dominant anti-inflammatory response. In vivo, T. suis worms are in proximity to the epithelial barrier of the intestines, which might result in passage of sufficient PGE2 to target intestinal DCs in the lamina propria (43). At the same time, it is possible that other compounds within the TsSPs act cooperatively with PGE2 to protect against inflammation in EAE.
The observation that T. suis secretes large amounts of PGE2 strongly suggests that the biologic function of PGE2 for T. suis in its pig host includes the modulation of the nematode’s microenvironment and the host’s immune response. Given that PGE2 favors host Th2 responses (32, 37), which are known to be host protective and to contribute to worm expulsion (44, 45), it is interesting to consider whether the strong immunomodulatory function of PGE2 represents a parasite response to the host or is an outcome of the host’s response to the parasite. This complex host-parasite interplay may be the net result of tens of thousands of years of coevolution, whereby the 2 organisms have adapted to reach an equilibrium to avoid excessive inflammation, which can be damaging to both host and parasite.
In addition to PGE2, other worm products act as immune modulators, indicating that helminths have evolved different mechanisms to modulate host immunity. Examples of such helminth products are defense molecules in Fasciola hepatica (46), the phosphorylcholine moiety of ES-62 in Acanthocheilonema viteae (47, 48), and Omega-1 in S. mansoni (49); also, helminth glycans play a role in immunoregulatory actions (49–51). We previously showed that mild PI treatment of TsSPs abolished its capacity to modulate DC function (8). Mild PI treatment is often used to abolish glycan structures, whereas protein structures are not significantly affected. These data previously led us to suggest that TsSP glycans were involved in the observed effects (8). We now show that the lipid PGE2 is PI sensitive, which is unusual. This observation raises caution in deducing a role for glycans based only on PI sensitivity of the putative compounds. Potentially, the PGE2 olefin could be oxidized, perhaps by trace metals in some fashion, similar to the Lemieux-Johnson oxidation (52), and then subsequently become PI sensitive. However, the exact chemical nature of the PI-induced degradation of PGE2 is unclear, and the reaction is the subject of ongoing studies.
In summary, we identified PGE2 as the major T. suis component in modulation of the DC phenotype and function. Our data have a major impact on our understanding the immunoregulatory capacities of PGE2. This is especially important because PGE2 biosynthesis is often targeted therapeutically by inhibiting PGE2 production. Our data suggest that we reconsider the role of PGE2 and evaluate its anti-inflammatory potential in greater detail. Furthermore, we propose that the inconsistency of therapeutic effects seen in the clinical trials may be due in part to variability in the amount of PGE2 that is released in the host. Because the viability and infectivity of the eggs supplied to the patients are high and consistent within and between batches, the variation is most likely host dependent. The establishment rate and life span of T. suis larvae in the human host is unknown, but available data suggest that the majority of larvae stay immature and live for at least several weeks and can occasionally develop into adult worms (53, 54). We showed that larvae already release PGE2 at high levels at d 18, at which time most of the larvae are expected to be alive in the human host. However, the condition of the gastrointestinal tract may influence the ability of the eggs to hatch and of the larvae to develop and settle in the mucosa and may thereby influence the concentration of worm-derived PGE2. Other medication taken and the health status of the host may have an effect as well (e.g., on the availability of essential lipid precursors for the worms to produce PGE2). Whereas PGE2 seems to be a major T. suis component to modulate DC function, other T. suis molecules and immune cells may contribute to induce an immune status that protects against inflammation, which is currently under investigation. An attractive possibility would be the design of clinical trials with a rational mix of protective helminth molecules or a combination of helminth molecules with immunobiotics derived from other sources to provide optimal protection against chronic inflammatory diseases.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Dutch MS [multiple sclerosis] Research Foundation (MS11-771 to I.V.D. and C.D.) and (MS14-878 to G.K.), and from the U.S. National Institute of Health (NIH) National Institute of Allergy and Infectious Diseases (Grant AI101982 to R.D.C.). A.R.W. was supported by the Lundbeck Foundation (Grant 14-3670A). The authors thank Dr. Paul Norris and Charles N. Serhan (NIH National Institute of General Medical Sciences Grant P01GM095467 (Center for Experimental Therapeutics and Reperfusion Injury, Harvard Institutes of Medicine, Brigham and Women's Hospital and Harvard Medical School) for help in metabololipidomic analysis and editorial assistance. The authors declare no conflicts of interest.
Glossary
- AA
arachidonic acid
- ASA
acetylsalicylic acid
- COX
cyclooxygenase
- DC
dendritic cell
- EAE
experimental autoimmune encephalitis
- E/S
excretory/secretory
- ESI
electrospray ionization
- LC
liquid chromatography
- LM
lipid mediator
- MS
mass spectrometry
- PG
prostaglandin
- PI
periodate
- Th
T helper
- TsSP
Trichuris suis soluble product
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
L. C. Laan performed most of the experimental work, purified the TsSP, and performed all experiments with dendritic cells; A. R. Williams, H. Kringel, P. Nejsum, and S. M. Thamsborg infected pigs, isolated larvae and worms, and prepared T. suis E/S; A. R. Williams designed and performed the AA labeling of T. suis and the COX inhibition studies and prepared A. suum E/S; K. Stavenhagen, M. Giera, and M. Wuhrer characterized the purified TsSP product and analyzed the periodate-treated fractions, as well as the products of the 13C AA labeling by MS; G. Kooij and I. van Die performed the lipidomics analysis of the T. suis E/S; H. Kalay helped with the chromatographic purification of TsSP and E/S products; C, D. Dijkstra, R. D. Cummings, and I. van Die designed the study; I. van Die supervised and coordinated the study and wrote most of the manuscript; and all authors contributed to the writing of the manuscript and provided critical comments.
REFERENCES
- 1.Grencis R. K. (2015) Immunity to helminths: resistance, regulation, and susceptibility to gastrointestinal nematodes. Annu. Rev. Immunol. 33, 201–225 [DOI] [PubMed] [Google Scholar]
- 2.Weinstock J. V., Elliott D. E. (2014) Helminth infections decrease host susceptibility to immune-mediated diseases. J. Immunol. 193, 3239–3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maizels R. M., Yazdanbakhsh M. (2008) T-cell regulation in helminth parasite infections: implications for inflammatory diseases. Chem. Immunol. Allergy 94, 112–123 [DOI] [PubMed] [Google Scholar]
- 4.Fleming J. O., Weinstock J. V. (2015) Clinical trials of helminth therapy in autoimmune diseases: rationale and findings. Parasite Immunol. 37, 277–292 [DOI] [PubMed] [Google Scholar]
- 5.Garg S. K., Croft A. M., Bager P. (2014) Helminth therapy (worms) for induction of remission in inflammatory bowel disease. Cochrane Database Syst. Rev. 1, CD009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuijk L. M., Klaver E. J., Kooij G., van der Pol S. M., Heijnen P., Bruijns S. C., Kringel H., Pinelli E., Kraal G., de Vries H. E., Dijkstra C. D., Bouma G., van Die I. (2012) Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol. Immunol. 51, 210–218 [DOI] [PubMed] [Google Scholar]
- 7.Ebner F., Hepworth M. R., Rausch S., Janek K., Niewienda A., Kühl A., Henklein P., Lucius R., Hamelmann E., Hartmann S. (2014) Therapeutic potential of larval excretory/secretory proteins of the pig whipworm Trichuris suis in allergic disease. Allergy 69, 1489–1497 [DOI] [PubMed] [Google Scholar]
- 8.Klaver E. J., Kuijk L. M., Laan L. C., Kringel H., van Vliet S. J., Bouma G., Cummings R. D., Kraal G., van Die I. (2013) Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int. J. Parasitol. 43, 191–200 [DOI] [PubMed] [Google Scholar]
- 9.Ottow M. K., Klaver E. J., van der Pouw Kraan T. C., Heijnen P. D., Laan L. C., Kringel H., Vogel D. Y., Dijkstra C. D., Kooij G., van Die I. (2014) The helminth Trichuris suis suppresses TLR4-induced inflammatory responses in human macrophages. Genes Immun. 15, 477–486 [DOI] [PubMed] [Google Scholar]
- 10.Kooij G., Braster R., Koning J. J., Laan L. C., van Vliet S. J., Los T., Eveleens A. M., van der Pol S. M., Förster-Waldl E., Boztug K., Belot A., Szilagyi K., van den Berg T. K., van Buul J. D., van Egmond M., de Vries H. E., Cummings R. D., Dijkstra C. D., van Die I. (2015) Trichuris suis induces human non-classical patrolling monocytes via the mannose receptor and PKC: implications for multiple sclerosis. Acta Neuropathol. Commun. 3, 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoeksema M. A., Laan L. C., Postma J. J., Cummings R. D., de Winther M. P., Dijkstra C. D., van Die I., Kooij G. (2016) Treatment with Trichuris suis soluble products during monocyte-to-macrophage differentiation reduces inflammatory responses through epigenetic remodeling. FASEB J. 30, 2826–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kringel H., Roepstorff A., Murrell K. D. (2002) A method for the recovery of immature Trichuris suis from pig intestine. Acta Vet. Scand. 43, 185–189 [PubMed] [Google Scholar]
- 13.Williams A. R., Fryganas C., Ramsay A., Mueller-Harvey I., Thamsborg S. M. (2014) Direct anthelmintic effects of condensed tannins from diverse plant sources against Ascaris suum. PLoS One 9, e97053 [Erratum] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyame A. K., Lewis F. A., Doughty B. L., Correa-Oliveira R., Cummings R. D. (2003) Immunity to schistosomiasis: glycans are potential antigenic targets for immune intervention. Exp. Parasitol. 104, 1–13 [DOI] [PubMed] [Google Scholar]
- 15.Stavenhagen K., Plomp R., Wuhrer M. (2015) Site-specific protein N- and O-glycosylation analysis by a C18-porous graphitized carbon-liquid chromatography-electrospray ionization mass spectrometry approach using pronase treated glycopeptides. Anal. Chem. 87, 11691–11699 [DOI] [PubMed] [Google Scholar]
- 16.Schlegel M., Köhler D., Körner A., Granja T., Straub A., Giera M., Mirakaj V. (2016) The neuroimmune guidance cue netrin-1 controls resolution programs and promotes liver regeneration. Hepatology 63, 1689–1705 [DOI] [PubMed] [Google Scholar]
- 17.Colas R. A., Shinohara M., Dalli J., Chiang N., Serhan C. N. (2014) Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol. 307, C39–C54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klaver E. J., van der Pouw Kraan T. C., Laan L. C., Kringel H., Cummings R. D., Bouma G., Kraal G., van Die I. (2015) Trichuris suis soluble products induce Rab7b expression and limit TLR4 responses in human dendritic cells. Genes Immun. 16, 378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen T. V., Nejsum P., Friis C., Olsen A., Thamsborg S. M. (2014) Trichuris suis and Oesophagostomum dentatum show different sensitivity and accumulation of fenbendazole, albendazole and levamisole in vitro. PLoS Negl. Trop. Dis. 8, e2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klaver E. J., Kuijk L. M., Lindhorst T. K., Cummings R. D., van Die I. (2015) Schistosoma mansoni soluble egg antigens induce expression of the negative regulators SOCS1 and SHP1 in human dendritic cells via interaction with the mannose receptor. PLoS One 10, e0124089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poloso N. J., Urquhart P., Nicolaou A., Wang J., Woodward D. F. (2013) PGE2 differentially regulates monocyte-derived dendritic cell cytokine responses depending on receptor usage (EP2/EP4). Mol. Immunol. 54, 284–295 [DOI] [PubMed] [Google Scholar]
- 22.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fusco A. C., Salafsky B., Kevin M. B. (1985) Schistosoma mansoni: eicosanoid production by cercariae. Exp. Parasitol. 59, 44–50 [DOI] [PubMed] [Google Scholar]
- 24.Leid R. W., McConnell L. A. (1983) PGE2 generation and release by the larval stage of the cestode, Taenia taeniaeformis. Prostaglandins Leukot. Med. 11, 317–323 [DOI] [PubMed] [Google Scholar]
- 25.Liu L. X., Serhan C. N., Weller P. F. (1990) Intravascular filarial parasites elaborate cyclooxygenase-derived eicosanoids. J. Exp. Med. 172, 993–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belley A., Chadee K. (1995) Eicosanoid production by parasites: from pathogenesis to immunomodulation? Parasitol. Today (Regul. Ed.) 11, 327–334 [DOI] [PubMed] [Google Scholar]
- 27.Funk C. D. (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294, 1871–1875 [DOI] [PubMed] [Google Scholar]
- 28.Hoang H. D., Prasain J. K., Dorand D., Miller M. A. (2013) A heterogeneous mixture of F-series prostaglandins promotes sperm guidance in the Caenorhabditis elegans reproductive tract. PLoS Genet. 9, e1003271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joachim A., Ruttkowski B., Daugschies A. (2001) Oesophagostomum dentatum: expression patterns of enzymes involved in eicosanoid production. Parasitol. Int. 50, 211–215 [DOI] [PubMed] [Google Scholar]
- 30.Daugschies A., Ruttkowski B. (1998) Modulation of migration of Oesophagostomum dentatum larvae by inhibitors and products of eicosanoid metabolism. Int. J. Parasitol. 28, 355–362 [DOI] [PubMed] [Google Scholar]
- 31.De Keijzer S., Meddens M. B., Torensma R., Cambi A. (2013) The multiple faces of prostaglandin E2 G-protein coupled receptor signaling during the dendritic cell life cycle. Int. J. Mol. Sci. 14, 6542–6555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalinski P. (2012) Regulation of immune responses by prostaglandin E2. J. Immunol. 188, 21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakanishi M., Rosenberg D. W. (2013) Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 35, 123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy B. D., Clish C. B., Schmidt B., Gronert K., Serhan C. N. (2001) Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2, 612–619 [DOI] [PubMed] [Google Scholar]
- 35.Cohn S. M., Schloemann S., Tessner T., Seibert K., Stenson W. F. (1997) Crypt stem cell survival in the mouse intestinal epithelium is regulated by prostaglandins synthesized through cyclooxygenase-1. J. Clin. Invest. 99, 1367–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Desai A., Yang S. Y., Bae K. B., Antczak M. I., Fink S. P., Tiwari S., Willis J. E., Williams N. S., Dawson D. M., Wald D., Chen W. D., Wang Z., Kasturi L., Larusch G. A., He L., Cominelli F., Di Martino L., Djuric Z., Milne G. L., Chance M., Sanabria J., Dealwis C., Mikkola D., Naidoo J., Wei S., Tai H. H., Gerson S. L., Ready J. M., Posner B., Willson J. K., Markowitz S. D. (2015) Tissue regeneration: inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science 348, aaa2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapsenberg M. L., Hilkens C. M., Wierenga E. A., Kalinski P. (1999) The paradigm of type 1 and type 2 antigen-presenting cells: implications for atopic allergy. Clin. Exp. Allergy 29(Suppl 2), 33–36 [PubMed] [Google Scholar]
- 38.Duffin R., O’Connor R. A., Crittenden S., Forster T., Yu C., Zheng X., Smyth D., Robb C. T., Rossi F., Skouras C., Tang S., Richards J., Pellicoro A., Weller R. B., Breyer R. M., Mole D. J., Iredale J. P., Anderton S. M., Narumiya S., Maizels R. M., Ghazal P., Howie S. E., Rossi A. G., Yao C. (2016) Prostaglandin E₂ constrains systemic inflammation through an innate lymphoid cell-IL-22 axis. Science 351, 1333–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boniface K., Bak-Jensen K. S., Li Y., Blumenschein W. M., McGeachy M. J., McClanahan T. K., McKenzie B. S., Kastelein R. A., Cua D. J., de Waal Malefyt R. (2009) Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J. Exp. Med. 206, 535–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao C., Sakata D., Esaki Y., Li Y., Matsuoka T., Kuroiwa K., Sugimoto Y., Narumiya S. (2009) Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat. Med. 15, 633–640 [DOI] [PubMed] [Google Scholar]
- 41.Kihara Y., Matsushita T., Kita Y., Uematsu S., Akira S., Kira J., Ishii S., Shimizu T. (2009) Targeted lipidomics reveals mPGES-1-PGE2 as a therapeutic target for multiple sclerosis. Proc. Natl. Acad. Sci. USA 106, 21807–21812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esaki Y., Li Y., Sakata D., Yao C., Segi-Nishida E., Matsuoka T., Fukuda K., Narumiya S. (2010) Dual roles of PGE2-EP4 signaling in mouse experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 107, 12233–12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiemstra I. H., Klaver E. J., Vrijland K., Kringel H., Andreasen A., Bouma G., Kraal G., van Die I., den Haan J. M. (2014) Excreted/secreted Trichuris suis products reduce barrier function and suppress inflammatory cytokine production of intestinal epithelial cells. Mol. Immunol. 60, 1–7 [DOI] [PubMed] [Google Scholar]
- 44.Iizuka Y., Kuwahara A., Karaki S. (2014) Role of PGE2 in the colonic motility: PGE2 generates and enhances spontaneous contractions of longitudinal smooth muscle in the rat colon. J. Physiol. Sci. 64, 85–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan W. I., Collins S. M. (2004) Immune-mediated alteration in gut physiology and its role in host defence in nematode infection. Parasite Immunol. 26, 319–326 [DOI] [PubMed] [Google Scholar]
- 46.Alvarado R., O’Brien B., Tanaka A., Dalton J. P., Donnelly S. (2015) A parasitic helminth-derived peptide that targets the macrophage lysosome is a novel therapeutic option for autoimmune disease. Immunobiology 220, 262–269 [DOI] [PubMed] [Google Scholar]
- 47.Harnett M. M., Kean D. E., Boitelle A., McGuiness S., Thalhamer T., Steiger C. N., Egan C., Al-Riyami L., Alcocer M. J., Houston K. M., Gracie J. A., McInnes I. B., Harnett W. (2008) The phosphorycholine moiety of the filarial nematode immunomodulator ES-62 is responsible for its anti-inflammatory action in arthritis. Ann. Rheum. Dis. 67, 518–523 [DOI] [PubMed] [Google Scholar]
- 48.Pineda M. A., Eason R. J., Harnett M. M., Harnett W. (2015) From the worm to the pill, the parasitic worm product ES-62 raises new horizons in the treatment of rheumatoid arthritis. Lupus 24, 400–411 [DOI] [PubMed] [Google Scholar]
- 49.Everts B., Smits H. H., Hokke C. H., Yazdanbakhsh M. (2010) Helminths and dendritic cells: sensing and regulating via pattern recognition receptors, Th2 and Treg responses. Eur. J. Immunol. 40, 1525–1537 [DOI] [PubMed] [Google Scholar]
- 50.Harn D. A., McDonald J., Atochina O., Da’dara A. A. (2009) Modulation of host immune responses by helminth glycans. Immunol. Rev. 230, 247–257 [DOI] [PubMed] [Google Scholar]
- 51.Van Die I., Cummings R. D. (2010) Glycan gimmickry by parasitic helminths: a strategy for modulating the host immune response? Glycobiology 20, 2–12 [DOI] [PubMed] [Google Scholar]
- 52.Pappo R., Allen D. S., Jr., Lemieux R. U., Johnson W. S. (1956) Osmium tetroxide-catalyzed periodate oxidation of olefinic bonds. J. Org. Chem. 21, 478–479 [Google Scholar]
- 53.Bager P., Kapel C., Roepstorff A., Thamsborg S., Arnved J., Rønborg S., Kristensen B., Poulsen L. K., Wohlfahrt J., Melbye M. (2011) Symptoms after ingestion of pig whipworm Trichuris suis eggs in a randomized placebo-controlled double-blind clinical trial. PLoS One 6, e22346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kradin R. L., Badizadegan K., Auluck P., Korzenik J., Lauwers G. Y. (2006) Iatrogenic Trichuris suis infection in a patient with Crohn disease. Arch. Pathol. Lab. Med. 130, 718–720 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.