Abstract
Secretory diarrheas caused by bacterial enterotoxins, including cholera and traveler’s diarrhea, remain a major global health problem. Inappropriate activation of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel occurs in these diarrheas. We previously reported that the benzopyrimido-pyrrolo-oxazinedione (R)-BPO-27 inhibits CFTR chloride conductance with low-nanomolar potency. Here, we demonstrate using experimental mouse models and human enterocyte cultures the potential utility of (R)-BPO-27 for treatment of secretory diarrheas caused by cholera and Escherichia coli enterotoxins. (R)-BPO-27 fully blocked CFTR chloride conductance in epithelial cell cultures and intestine after cAMP agonists, cholera toxin, or heat-stable enterotoxin of E. coli (STa toxin), with IC50 down to ∼5 nM. (R)-BPO-27 prevented cholera toxin and STa toxin-induced fluid accumulation in small intestinal loops, with IC50 down to 0.1 mg/kg. (R)-BPO-27 did not impair intestinal fluid absorption or inhibit other major intestinal transporters. Pharmacokinetics in mice showed >90% oral bioavailability with sustained therapeutic serum levels for >4 h without the significant toxicity seen with 7-d administration at 5 mg/kg/d. As evidence to support efficacy in human diarrheas, (R)-BPO-27 blocked fluid secretion in primary cultures of enteroids from human small intestine and anion current in enteroid monolayers. These studies support the potential utility of (R)-BPO-27 for therapy of CFTR-mediated secretory diarrheas.—Cil, O., Phuan, P.-W., Gillespie, A. M., Lee, S., Tradtrantip, L., Yin, J., Tse, M., Zachos, N. C., Lin, R., Donowitz, M., Verkman, A. S. Benzopyrimido-pyrrolo-oxazine-dione CFTR inhibitor (R)-BPO-27 for antisecretory therapy of diarrheas caused by bacterial enterotoxins.
Keywords: intestinal secretion, secretory diarrhea, cholera, traveler’s diarrhea
Cystic fibrosis transmembrane conductance regulator (CFTR) is a cAMP-gated chloride channel in which loss-of-function mutations cause cystic fibrosis (CF), an inherited disorder characterized by chronic pulmonary disease, pancreatic insufficiency, and male infertility. CFTR is expressed in epithelial cells in the airways, intestine, testis, sweat gland, pancreas, and other tissues (1). Pharmacological activation of CF-causing mutant CFTRs has emerged as a major therapeutic strategy in CF (2), and activation of wild-type CFTR in subjects without CF has potential utility for the treatment of constipation (3), dry eye (4), and potentially inflammatory lung diseases (5). Pharmacological inhibition of CFTR has predicted utility in the treatment of enterotoxin-mediated secretory diarrheas, including cholera and traveler’s diarrhea, and of autosomal dominant polycystic kidney disease (ADPKD) because CFTR activation causes intestinal fluid secretion in certain diarrheas (6–8) and fluid accumulation in renal cysts in ADPKD (9–12).
Fluid secretion in the intestine involves chloride entry into enterocytes from the blood side through the basolateral membrane NKCC1 cotransporter and exit into the intestinal lumen across apical membrane CFTR and other chloride transporters (13, 14). The electrochemical driving force for chloride secretion requires basolateral membrane potassium channels as well. In secretory diarrheas, chloride secretion drives secondary sodium and water secretion, which produces net fluid transport into the intestinal lumen. A considerable body of evidence implicates CFTR as the major prosecretory chloride channel in diarrheas caused by bacterial enterotoxins and certain gastrointestinal tumors.
High-throughput screens from our laboratory identified 3 classes of small-molecule CFTR inhibitors (reviewed in ref. 15), including the thiazolidinone CFTRinh-172 (16), which has been used widely as a research tool; the glycine hydrazide GlyH-101 (17); and pyrimido-pyrrolo-quinoxalinedione (PPQ)-102 (18). CFTRinh-172 stabilizes the CFTR channel closed-state by binding at or near residue Arg-347 (19, 20) and inhibits intestinal fluid secretion in mouse models of enterotoxin-induced diarrheas (21). The glycine hydrazides block the extracellular CFTR pore (17, 22), which has motivated attempts to develop nonabsorbable, luminally active antidiarrheals. Phase 1 clinical trials have been performed on a small-molecule GlyH-101 analog, iOWH032 (23), although the program was subsequently terminated; iOWH032 was probably not a good candidate because of its low CFTR inhibition potency and the rapid intestinal washout predicted for a weakly bound CFTR inhibitor (24). Various glycine hydrazide-macromolecular conjugates, including malonic acid hydrazide-lectin conjugates, were developed with IC50 for CFTR inhibition <100 nM. These conjugates resist intestinal washout and show efficacy in mouse models of cholera (25, 26). However, macromolecular conjugates are not well suited for applications in developing countries where low drug cost and stability in hot environments are important.
PPQ-102 was identified as a small-molecule CFTR inhibitor with IC50 ∼90 nM and showed efficacy in a mouse model of ADPKD (24). Medicinal chemistry produced the more potent and water-soluble benzopyrimido-pyrrolo-oxazinedione (BPO)-27 with IC50 ∼8 nM in a cell-based assay (27). Separation of BPO-27 enantiomers by chiral supercritical fluid chromatography gave active enantiomer (R)-BPO-27 with IC50 of ∼4 nM in a cell-based assay and inactive (S)-BPO-27 (28). Patch-clamp and molecular modeling suggested that (R)-BPO-27 inhibition of CFTR involves competition with ATP binding at the cytoplasmic surface of CFTR (29). Single-channel electrophysiology in inside-out patches, where inhibitor was applied directly to the cytoplasmic membrane surface, showed that (R)-BPO-27 stabilizes the CFTR channel closed state with IC50 ∼600 pM. Motivated by its high inhibition potency and drug-like properties, we investigated the utility of (R)-BPO-27 for antisecretory therapy of CFTR-dependent diarrheas, which remains a significant unmet need, particularly in developing countries.
MATERIALS AND METHODS
Compounds
Racemic BPO-27 was synthesized in 6 steps and purified as previously described (27). Enantiomerically pure (>99.5%) (R)-BPO-27 and (S)-BPO-27 were separated by chiral supercritical fluid chromatography as described (28). 8-(4-Chlorophenylthio)-cAMP (CPT-cAMP) was purchased from MP Biochemicals (Burlingame, CA, USA). Other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless specified otherwise.
Cell culture
Fischer rat thyroid (FRT) cells stably coexpressing human wild-type CFTR or TMEM16A and the halide-sensitive yellow fluorescent protein-H148Q were cultured as described (16, 30). Cells were cultured on plastic in Coon’s-modified Ham’s F12 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Primary cultures of human bronchial epithelial (HBE) cells were maintained at an air-liquid interface as described (31). Cells were grown on Snapwell porous filters (Corning Life Sciences, Lowell, MA, USA) at 37°C in 5% CO2/95% air.
Short-circuit current measurements
FRT-expressing human wild-type CFTR or TMEM16A or HBE cells were cultured on inserts (Snapwell; Corning Life Sciences). For FRT cell measurements, the basolateral solution contained (in mM) 120 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, 25 NaHCO3, and 5 HEPES (pH 7.4). In the apical bathing solution, 60 mM NaCl was replaced by Na gluconate, and CaCl2 was increased to 2 mM. The basolateral membrane was permeabilized with 250 µg/ml amphotericin B. For primary HBE cell cultures, the apical and basolateral chambers contained identical solutions (in mM): 120 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, 25 NaHCO3, and 5 HEPES (pH 7.4). For intestinal short-circuit current measurements, full-thickness midjejunum of CD1 mice was mounted as described (21). All solutions were bubbled with 95% O2/5% CO2 and maintained at 37°C. Hemichambers were connected to a DVC-1000 voltage clamp (World Precision Instruments Inc., Sarasota, FL, USA) via Ag/AgCl electrodes and 3 M KCl agar bridges.
Intestinal closed-loop model
Mice were given access to 5% dextrose in water but not solid food for 24 h before experiments. In different experiments, female CD1 mice (age 8–10 wk) were treated with various amounts of (R)-BPO-27 (0.05, 0.15, 0.5, 1.5, and 5 mg/kg), 5 mg/kg (S)-BPO-27, or vehicle (5% DMSO, 10% Kolliphor HS in saline) intraperitoneally 30 min before abdominal surgery. In a separate experiment, 5 mg/kg (R)-BPO-27 was given orally 1 h before surgery. Mice were anesthetized with isoflurane, and body temperature was maintained during surgery at 36–38°C using a heating pad. A small abdominal incision was made to expose the small intestine, and closed midjejunal loops (2–3 cm in length) were isolated by sutures. Loops were injected with 100 µl PBS containing 1 μg cholera toxin (Sigma-Aldrich) or 0.1 μg heat-stable enterotoxin of Escherichia coli (STa toxin) (Bachem Americas Inc., Torrance, CA, USA) or PBS alone. The abdominal incision was closed with sutures, and mice were allowed to recover from anesthesia. Intestinal loops were removed at 3 h, and loop length and weight were measured to quantify fluid secretion. Intestinal absorption was measured in mice given 5 mg/kg (R)-BPO-27 or vehicle intraperitoneally, in which closed loops were injected with 200 μl PBS and removed at 0 or 30 min. Absorption was calculated as (loop weight at 0 min − loop weight at 30 min)/loop weight at 0 min. Mouse studies were approved by the UCSF Institutional Animal Care and Use Committee.
Human enteroid assays
Deidentified tissues from human subjects were obtained under approval of the Johns Hopkins University School of Medicine Institutional Review Board (protocol NA_00038329). Duodenal and jejunal biopsy specimens were obtained from adults during routine endoscopy at Johns Hopkins Hospital. Crypt isolation, enteroid preparation, propagation, and culture were performed as described (32). For swelling measurements, enteroids were seeded in 35-mm dishes with bottom coverglass containing 1.5 ml media. On the day of the experiment, the media was replaced with 3 ml Advanced DMEM/F12, and enteroids were incubated with 1 mM calcein green-acetoxymethyl ester for 1 h at 37°C to label cytoplasm. Relative enteroid volume after addition of specified concentrations of forskolin was measured using a laser scanning confocal microscope (Fluoview FV10i-LIV; Olympus, Tokyo, Japan) at 37°C and 5% CO2. In some studies, (R)-BPO-27 was added 10 or 60 min before forskolin. Images were acquired every 10 min and analyzed with MetaMorph version 7.7 software (Olympus) to quantify the enteroid area. To generate planar enteroid monolayers, 50–100 enteroids were harvested from Matrigel, triturated into fragments, and seeded onto collagen IV-coated, 24-well Transwell filters (Corning Inc., Corning, NY, USA). Enteroid monolayers were maintained for 2–3 wk to 100% confluence as indicated by transepithelial resistance.
Pharmacokinetics
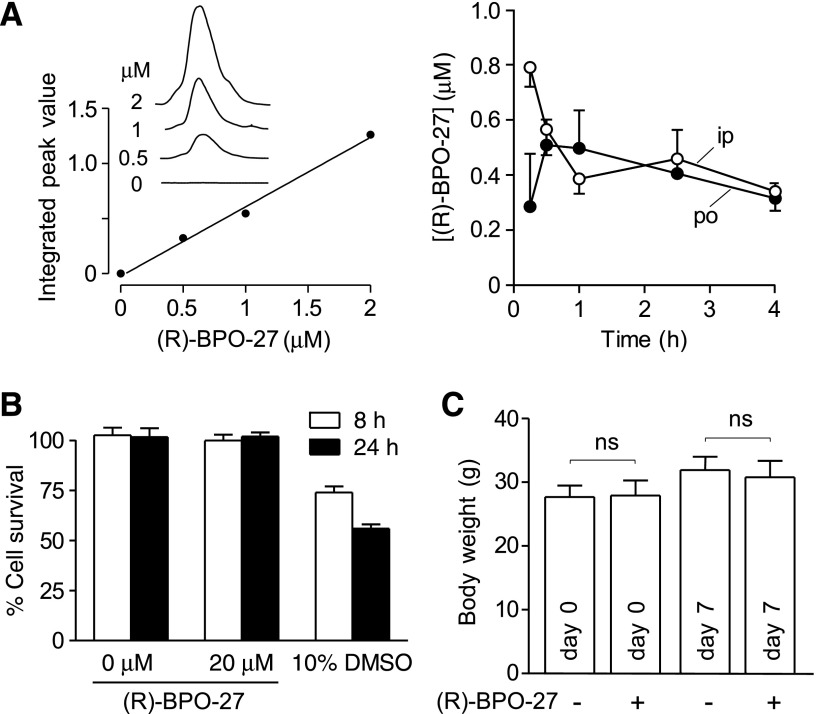
Female CD1 mice were administered 5 mg/kg (R)-BPO-27 either intraperitoneally or orally. Blood was collected at 15, 30, 60, 150, and 240 min by orbital puncture and centrifuged at 5000 rpm for 15 min to separate serum. Serum samples (60 µl) were mixed with 300 µl acetonitrile and centrifuged at 13,000 rpm for 20 min, and 90 µl of the supernatant was used for LC-MS. The solvent system consisted of a linear gradient of 5–95% acetonitrile over 16 min (0.2 ml/min flow). Mass spectra data were acquired on a mass spectrometer (Waters 2695 and Micromass ZQ; Waters, Milford, MA, USA) using electrospray (+) ionization (mass range, 100–1500 Da; cone voltage, 40 V). Calibration standards were prepared in serum from untreated mice to which known amounts of (R)-BPO-27 were added. Oral bioavailability was calculated from area under curve (AUC) ratios [AUC (oral)/AUC (intraperitoneal)].
Chronic administration and toxicity studies
Mice were administered 5 mg/kg (R)-BPO-27 or vehicle orally once a day for 7 d. In vivo toxicity was assessed by measuring serum chemistries (Idexx Laboratories Inc., Sacramento, CA, USA) 3 h after the final (R)-BPO-27 dose. In vitro cytotoxicity was measured in FRT cells incubated with 20 µM (R)-BPO-27 for 8 and 24 h using Alamar Blue assay according to the manufacturer’s instructions (Thermo Fisher Scientific, Carlsbad, CA, USA).
Statistical analysis
Experiments with 2 groups were analyzed using Student’s t test; for 3 or more groups, analysis was done with one-way ANOVA and post hoc Newman-Keuls multiple comparisons test. A value of P < 0.05 was taken as statistically significant.
RESULTS
Selective CFTR inhibition by (R)-BPO-27
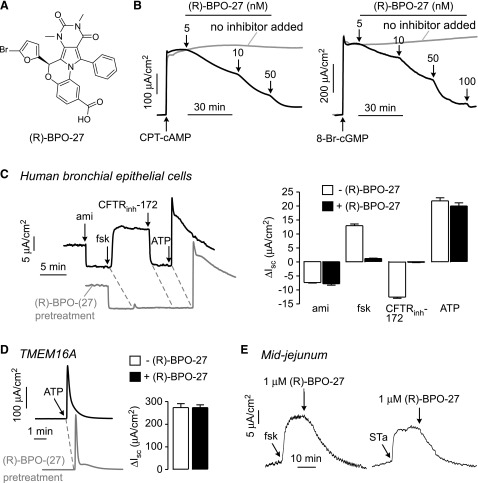
Short-circuit current measurements were done to study (R)-BPO-27 (Fig. 1A) potency and specificity. Figure 1B shows inhibition of Cl− current by (R)-BPO-27 in CFTR-expressing FRT cells after CFTR stimulation by the cell-permeable cAMP agonist CPT-cAMP (left) and the cell-permeable cGMP agonist 8-Br-cGMP (right). Measurements were done in the presence of a transepithelial chloride gradient and after basolateral membrane permeabilization with amphotericin B; therefore, short-circuit current is a linear, quantitative measure of CFTR Cl− conductance. Increasing concentrations of (R)-BPO-27 inhibited Cl− current, with apparent IC50 values of ∼5 and ∼10 nM for CPT-cAMP and 8-Br-cGMP, respectively. We previously reported an IC50 of ∼4 nM for inhibition of forskolin-stimulated CFTR Cl− current in FRT cells (27).
Figure 1.
CFTR inhibition by (R)-BPO-27. A) Chemical structure of (R)-BPO-27. B) Short-circuit current in FRT cells expressing human wild-type CFTR showing responses to 100 μM CPT-cAMP (left) and 200 μM 8-Br-cGMP (right), with indicated concentrations of (R)-BPO-27 (representative of 3 experiments). C) Left: short-circuit in HBE cells in response to agonists and inhibitors that target key ion transport processes: 20 μM amiloride (ami), 20 μM forskolin (fsk), 10 μM CFTRinh-172, and 100 µM ATP. Experiments were done in the absence and presence of (R)-BPO-27 (10 μM, 10 min pretreatment). Right: data summary (means ± se; n = 3). D) Left: short-circuit current in TMEM16A-expressing FRT cells in response to 100 µM ATP. Experiments were done in the absence and presence of (R)-BPO-27 (10 μM, 5 min pretreatment). Right: data summary (means ± se; n = 3). E) Short-circuit current in mouse midjejunal strips showing responses to 20 µM forskolin (left), 10 μg/ml STa toxin (right), and 1 μM (R)-BPO-27 (representative of 3 experiments).
To investigate (R)-BPO-27 specificity, the major ion transport pathways in HBE cells were studied by short-circuit current in which cells were sequentially treated with amiloride to inhibit the epithelial sodium channel, forskolin to activate CFTR, CFTRinh-172 to inhibit CFTR, and ATP to activate Ca2+-activated Cl− channels (CaCCs). In the absence of (R)-BPO-27, ion channel modulators produced the anticipated changes in short-circuit current (Fig. 1C). Pretreatment of cells for 10 min with a high concentration of (R)-BPO-27 (10 µM) did not alter current responses to amiloride or ATP but abolished the CFTR current. Because short-circuit current in HBE cells in this protocol depends on the activities of the epithelial sodium channel, CaCC, K+ channels, and NKCC1, it is concluded that (R)-BPO-27 does not inhibit any of these processes. Also, (R)-BPO-27 at high concentration did not inhibit the CaCC TMEM16A after ATP stimulation (Fig. 1D).
Figure 1E shows (R)-BPO-27 inhibition of forskolin- and STa toxin-stimulated short-circuit current in mouse midjejunum. Forskolin and STa toxin produced sustained Cl− currents that were fully inhibited by 1 µM (R)-BPO-27.
Efficacy in mouse models of cholera and traveler’s diarrhea
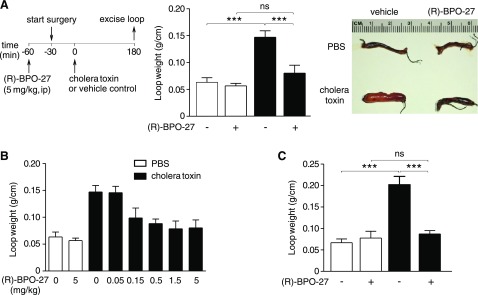
A closed intestinal loop model in mice was used to assess (R)-BPO-27 efficacy in reducing fluid secretion caused by bacterial enterotoxins. (R)-BPO-27 at 5 mg/kg administered intraperitoneally prevented fluid accumulation in closed midjejunal loops produced by cholera toxin, giving an intestinal loop weight/length ratio similar to that in PBS-injected loops (Fig. 2A). A dose-response study showed an IC50 of ∼0.1 mg/kg (Fig. 2B). Intestinal fluid secretion was also prevented by orally administered (R)-BPO-27 at 5 mg/kg given 60 min before creation of loops and administration of cholera toxin (Fig. 2C).
Figure 2.
(R)-BPO-27 prevents fluid accumulation in cholera toxin-treated intestinal closed loops. A) Experimental protocol (left), loop weight/length ratio (middle), and representative photos (right) of loops treated with PBS or cholera toxin without or with (R)-BPO-27 (means ± se; n = 5–8 loops per group). B) (R)-BPO-27 dose-response study for experiments as in A (means ± se; n = 4–8 loops per group). C) Efficacy of orally administered (R)-BPO-27 (5 mg/kg) administered 60 min before surgery (means ± se; n = 3–7 loops per group). One-way ANOVA with post hoc Newman-Keuls multiple comparisons test. ***P < 0.001. ns, not significant.
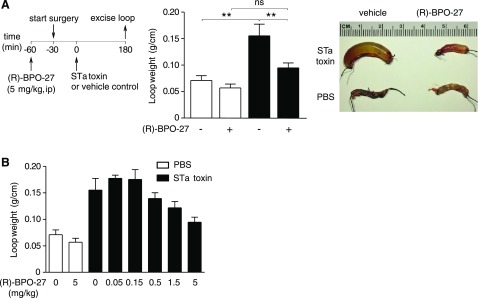
(R)-BPO-27 at 5 mg/kg (i.p.) was also effective in preventing fluid accumulation in intestinal loops injected with STa toxin (Fig. 3A). The IC50 of (R)-BPO-27 in the STa toxin model was ∼1 mg/kg (Fig. 3B).
Figure 3.
(R)-BPO-27 prevents fluid accumulation in STa toxin-treated intestinal closed loops. A) Experimental protocol (left), loop weight/length ratio (middle), and representative photos (right) of loops treated with PBS or STa toxin without or with (R)-BPO-27 (means ± se; n = 4 or 5 loops per group). B) (R)-BPO-27 dose-response study for experiments as in A (means ± se; n = 4 or 5 loops per group). One-way ANOVA with post hoc Newman-Keuls multiple comparisons test. **P < 0.01. ns, not significant.
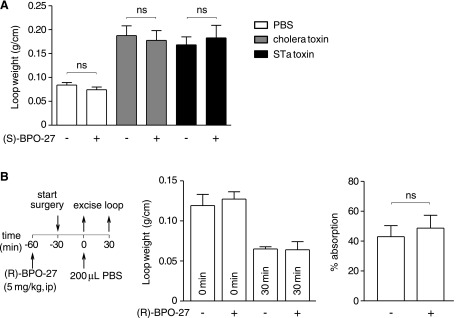
As a control, the inactive enantiomer (S)-BPO-27 did not affect fluid accumulation in intestinal loops injected with cholera toxin or STa toxin (Fig. 4A). Also, (R)-BPO-27 did not inhibit intestinal fluid absorption at 30 min after injection of PBS into loops, as measured from loop weight/length ratio (Fig. 4B).
Figure 4.
(S)-BPO-27 does not inhibit intestinal secretion, and (R)-BPO-27 does not inhibit intestinal absorption. A) Cholera toxin and STa toxin-induced intestinal secretion study done as in Figs. 2A and 3A but with (S)-BPO-27 (inactive enantiomer, 5 mg/kg, i.p.) (means ± se; n = 4–7 loops per group). One-way ANOVA with post hoc Newman-Keuls multiple comparisons test. B) Intestinal absorption study protocol (left), loop weight/length ratios at 0 and 30 min (middle), and percent intestinal absorption (right) in PBS-injected loops (means ± se; n = 4 loops per group); Student’s t test. ns, not significant.
Mouse pharmacokinetics and toxicity
Previous in vitro microsomal stability measurements showed very slow (R)-BPO-27 metabolism (28). Here, we found that administration of 5 mg/kg (R)-BPO-27, either orally or intraperitoneally, produced sustained serum (R)-BPO-27 levels for at least 4 h at concentrations much higher than its IC50 for CFTR inhibition (Fig. 5A). AUC analysis gave an oral bioavailability of ∼94% for (R)-BPO-27.
Figure 5.
(R)-BPO-27 pharmacokinetics and toxicity. A) Standard serum concentration curve determined by LC-MS (left). Time course of serum (R)-BPO-27 concentration after bolus intraperitoneal or oral administration of 5 mg/kg (R)-BPO-27 at zero time (right) (means ± se; 3 mice per group). B) Cytotoxicity measured by Alamar Blue assay in FRT cells incubated with (R)-BPO-27 for 8 or 24 h (10% DMSO as positive control) (means ± se; n = 4 cultures). C) Body weight in mice receiving 5 mg/kg (R)-BPO-27 orally for 7 d (means ± se; n = 5 mice per group); Student’s t test. ns, not significant.
Preliminary toxicological studies of (R)-BPO-27 were done in cell cultures and mice. (R)-BPO-27 at a concentration of 20 µM near its solubility limit did not show cytotoxicity as measured by the Alamar Blue assay (Fig. 5B). In mice treated for 7 d with 5 mg/kg (R)-BPO-27, no significant changes were seen in serum chemistry values (Table 1) or body weight (Fig. 5C).
TABLE 1.
Serum chemistries of mice treated for 7 d with 5 mg/kg (R)-BPO-27 (or vehicle) orally once per day
| Serum parameters | Vehicle | (R)-BPO-27 | P |
|---|---|---|---|
| Total protein (g/dl) | 4.9 ± 0.1 | 4.8 ± 0.2 | >0.05 |
| Albumin (g/dl) | 2.5 ± 0.1 | 2.5 ± 0.1 | >0.05 |
| Globulin (g/dl) | 2.4 ± 0.1 | 2.3 ± 0.1 | >0.05 |
| ALT (U/L) | 41 ± 11 | 39 ± 6 | >0.05 |
| AST (U/L) | 133 ± 23 | 139 ± 28 | >0.05 |
| ALP (U/L) | 54 ± 5 | 58 ± 4 | >0.05 |
| Total bilirubin (mg/dl) | 0.2 ± 0 | 0.16 ± 0.02 | >0.05 |
| Direct bilirubin (mg/dl) | 0.04 ± 0.02 | 0.04 ± 0.02 | >0.05 |
| Glucose (mg/dl) | 197 ± 9.3 | 207 ± 3.9 | >0.05 |
| Cholesterol (mg/dl) | 140 ± 4 | 144 ± 10 | >0.05 |
| Sodium (mM) | 147 ± 0.5 | 147 ± 0.4 | >0.05 |
| Potassium (mM) | 4.9 ± 0.1 | 5.0 ± 0.1 | >0.05 |
| Chloride (mM) | 112 ± 0.8 | 111 ± 0.6 | >0.05 |
| Calcium (mg/dl) | 8.3 ± 0.1 | 8.5 ± 0.1 | >0.05 |
| Phosphorus (mg/dl) | 5.3 ± 0.5 | 5.3 ± 0.2 | >0.05 |
| BUN (mg/dl) | 18.6 ± 3.1 | 14.0 ± 1.7 | >0.05 |
| Creatinine (mg/dl) | 0.08 ± 0.05 | 0.08 ± 0.05 | >0.05 |
| Bicarbonate (mM) | 13.2 ± 0.3 | 15.4 ± 0.9 | >0.05 |
Data represent means ± se (n = 5 mice per group). Significance determined by Student’s t test. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen.
(R)-BPO-27 inhibits fluid secretion and chloride current in human enteroid cultures
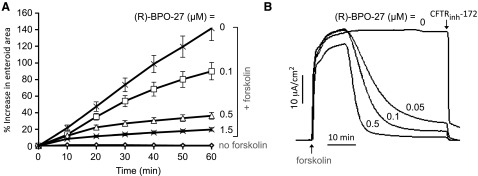
To support the potential efficacy of (R)-BPO-27 in human diarrheas, (R)-BPO-27 was tested in ex vivo primary cultures of enteroids generated from normal human jejunum. Enteroids consist of a tight, closed layer of enterocytes with the CFTR-containing apical membrane facing inward so that a secretory response is seen as enteroid swelling. Figure 6A shows the kinetics of enteroid swelling after cAMP stimulation by forskolin, in which enteroids were incubated with different concentrations of (R)-BPO-27 for 10 min before assay. The swelling response was reduced by (R)-BPO-27 in a concentration-dependent manner, with ∼75% reduction in swelling by 0.5 µM (R)-BPO-27. Similar inhibition was seen when enteroids were incubated with (R)-BPO-27 for 60 min before assay (data not shown).
Figure 6.
(R)-BPO-27 inhibits CFTR-dependent swelling of enteroids generated from human jejunum and CFTR Cl− current in planar enteroid cultures. A) Inhibition of forskolin-induced swelling of enteroids by (R)-BPO-27. Enteroids were incubated with (R)-BPO-27 for 10 min before addition of 5 µM forskolin. Enteroid swelling was quantified as areas of fluorescently stained enteroids as determined by confocal microscopy (means ± se; n = 8). B) Short-circuit current in planar cultures generated from the human enteroids. Cultures were treated with 10 µM forskolin, and then indicated concentrations of (R)-BPO-27 were added to both apical and basolateral solutions, followed by 25 µM CFTRinh-172 added to the apical solution. Representative of 2 sets of studies.
Short-circuit current measurements were done in planar monolayer cultures generated from the jejunal enteroids. Figure 6B shows robust CFTR Cl− current produced by forskolin addition, which was inhibited by ∼90% by 50 nM (R)-BPO-27 and almost completely by 100 nM (R)-BPO-27. Similar results were obtained using planar cultures generated from human duodenal enteroids with 50% inhibition at ∼5 nM (R)-BPO-27 (data not shown). The slow time course of inhibition, as also seen in Fig. 1 for CFTR-transfected FRT cells, is due to slow (R)-BPO-27 transport into cells.
DISCUSSION
Diarrheal diseases remain a major global health burden. Despite oral rehydration therapy, diarrheas are a major cause of mortality and morbidity, with ∼800,000 deaths annually (33). Diarrheas account for 9% of all deaths among children under age 5 worldwide. Mortality and morbidity due to fluid losses in secretory diarrheas is greatest in young children and in elderly persons, and repeated episodes of diarrhea in developing countries are associated with long-term impairment in physical and mental development (34, 35). There remains an unmet need for therapeutics to reduce fluid losses in severe secretory diarrheas, particularly in young and elderly patients, and when oral rehydration solution is not available or is not used properly (36). CFTR inhibition adds to drug therapies of diarrheas that target intestinal motility (µ-opioid agonists) and alternative secretory mechanisms (enkephalinase inhibitors, crofelemer) and to various potential therapeutics in the development pipeline (reviewed in ref. 14).
(R)-BPO-27 inhibited CFTR chloride conductance in cell cultures, mouse jejunum, and human enteroid cultures in response to cAMP agonists forskolin or cholera toxin and the cGMP agonists 8-Br-cGMP or STa toxin. Systemically administered (R)-BPO-27 reduced fluid accumulation in cholera- and STa toxin-treated intestinal closed loops, which is a consequence of its antisecretory action because (R)-BPO-27 did not affect fluid absorption from intestinal loops. Pharmacological studies showed high oral bioavailability, prolonged therapeutic serum levels after single oral or intraperitoneal administration, and no detectable toxicity after 1 wk of high-dose administration. The favorable efficacy and pharmacology data support preclinical development of (R)-BPO-27 as a lead candidate for antisecretory treatment of CFTR-dependent diarrheas.
Apparent IC50 values for (R)-BPO-27 inhibition of CFTR Cl− current show variability depending on the system studied. At the subcellular level in inside-out patches where (R)-BPO-27 is applied directly onto the cytoplasmic CFTR surface, the IC50 for inhibition of CFTR Cl− current was ∼0.6 nM (29), less than that of ∼5 nM in FRT cells. Several factors can influence apparent IC50 values, including membrane potential [because (R)-BPO-27 is negatively charged at physiologic pH], the presence of cellular drug export mechanisms, and cell-specific effects such as the presence of CFTR-interacting proteins. Importantly, the IC50 for inhibition of CFTR Cl− current in planar human enterocyte cultures was ∼5 nM. Another interesting observation was the greater IC50 for inhibition of cGMP vs. cAMP induced Cl− current by (R)-BPO-27, as seen using a cGMP agonist in FRT cells and STa toxin in intestinal closed loops. We speculate that a different pattern of CFTR phosphorylation in response to cAMP vs. cGMP stimulation may be responsible for the apparent difference in (R)-BPO-27 inhibition potency because cGMP-induced CFTR phosphorylation in intestinal cells involves protein kinase G-II rather than protein kinase A (37).
(R)-BPO-27 is a second-generation analog of the original class of pyrimido-pyrrolo-quinoxalinedione (PPQ) CFTR inhibitors identified in a small-molecule screen. The benzopyrimido-pyrrolo-oxazine-dione (BPO) analogs differ from the PPQ inhibitors by replacement of the secondary amine with an ether bridge in the core ring structure. There are a few prior reports on biologic activities of BPO and PPQ chemical scaffolds. Some PPQ compounds were reported to have anti-influenza activity by targeting nucleoproteins of influenza A virus H1N1 (38). Two PPQ compounds were reported to inhibit Staphylococcus aureus wall teichoic acid biosynthesis (39). Recently, a BPO compound was found to be a weak inhibitor of transcription factor MYC (40). The physico-chemical properties of the (R)-BPO-27 scaffold include the presence of multiple hydrogen bond acceptors, molecular mass of 547 Da, ClogP value of 5.9, and topologic polar surface area of 99.3 Å2. These values are within the guidelines for good oral bioavailability. In addition, the BPO/PPQ scaffold does not belong to promiscuous binders known as pan-assay interference compound molecules.
Compared with the alternatives, (R)-BPO-27 appears to be the best of the available CFTR inhibitors for the development of antidiarrheal antisecretory therapy. Glycine hydrazides that target the lumen-facing CFTR surface may not effectively block CFTR in vivo because of convective washout and relatively low CFTR inhibition potency (23, 24). Thiazolidinones are also potential development candidates, although their IC50 values for CFTR inhibition are much greater than that of (R)-BPO-27 (21). There are also natural-product preparations (41, 42) and purified compounds (43, 44) with reported CFTR inhibition activity, although their potencies are low (IC50 >> 20 µM) or their active components have not been identified.
CFTR inhibition therapy is predicted to have several indications for diarrhea therapy because CFTR plays a central role in intestinal fluid secretion. Bacteria, including Vibrio cholerae and enterotoxigenic E. coli, secrete enterotoxins, such as cholera toxin and STa toxin, that increase intracellular cyclic nucleotide concentrations, activate CFTR, and cause intestinal fluid secretion. CFTR activation is also responsible for some tumor-related diarrheas, such as adenomas that secrete vasoactive intestinal peptide (45) or prostaglandin E2 (46), and for some congenital diarrheas, such as familial diarrhea syndrome caused by guanylin receptor (guanyl cyclase C) mutation (47). In addition, because of cross-talk between cyclic nucleotide and calcium signaling in intestinal epithelia (31, 48), CFTR may also be involved to some extent in various other viral, drug-induced, congenital, inflammatory, and multifactorial diarrheas, such as HIV-related diarrhea. CFTR inhibition therapy of secretory diarrheas also has potential indications in veterinary medicine, such as in epidemic porcine diarrheas (49).
Several caveats are noted in extrapolating the experimental animal data here to efficacy and safety in treating diarrheas in humans. Although sustained CFTR loss-of-function causes CF, with pulmonary, gastrointestinal, and reproductive disease, it is unlikely that short-term, reversible, and incomplete CFTR inhibition by (R)-BPO-27 would produce CF disease. Intestinal fluid accumulation is increased in infectious diarrheas largely as a consequence of increased intestinal fluid secretion, although inhibition of intestinal fluid absorption also occurs by mechanisms that involve inhibition of sodium-proton exchanger NHE3 and possibly other proabsorptive processes (14). The precise relative contribution of increased fluid secretion vs. reduced fluid absorption in human diarrheas is not known, although it is unlikely that reduced fluid absorption in enterotoxin-mediated secretory diarrheas would reduce the efficacy of a pure antisecretory therapy because maximal fluid secretion rates far exceed absorption rates. Finally, as mentioned above, although CFTR inhibition therapy should greatly reduce fluid losses in infectious secretory diarrheas caused by bacterial enterotoxins and in some tumor and congenital diarrheas, CFTR inhibition may have limited efficacy in viral, inflammatory, and drug-induced secretory diarrheas where other prosecretory mechanisms dominate (50–52).
Notwithstanding these caveats, the data here support the potential efficacy of CFTR inhibition by (R)-BPO-27 for reducing intestinal fluid losses in cholera and traveler’s diarrhea and some tumor-related and congenital diarrheas, which could reduce morbidity and mortality. CFTR anti-secretory therapy is combinable with oral rehydration solution or future drugs targeting intestinal fluid absorption or with drugs targeting the causative organism or its secreted toxins. CFTR antisecretory therapy could be beneficial where oral rehydration solution is not available or used properly, particularly in children and in elderly patients, and may be useful in the setting of natural or human-made global disasters.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institute of Health (Grants R24 DK099803, P30 DK72517, R01 DK101373, R01 DK35124, R37 EB00415, R01 EY13574, R01 DK26523, R01 DK61765, P30 DK089502, TR000552, TR000504, and TR000504); a Research Development Program grant from the Cystic Fibrosis Foundation; a Catalyst grant from funds awarded to the University of California San Francisco Clinical and Translational Science Institute (UL1 TR000004); and a Gates Foundation Grand Challenges grant.
Glossary
- ADPKD
autosomal dominant polycystic kidney disease
- AUC
area under curve
- BPO
benzopyrimido-pyrrolo-oxazine-dione
- CaCC
calcium-activated chloride channel
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- CPT-cAMP
8-(4-chlorophenylthio)-cAMP
- FRT
Fischer rat thyroid
- HBE
human bronchial epithelial
- PPQ
pyrimido-pyrrolo-quinoxalinedione
- STa toxin
heat-stable enterotoxin of E. coli
AUTHOR CONTRIBUTIONS
A. S. Verkman conceived the idea for this study; P.-W. Phuan, S. Lee, and L. Tradtrantip performed cell cultures and functional measurements; O. Cil and A. M. Gillespie determined in vivo efficacy; O. Cil and S. Lee performed pharmacology experiments; J. Yin, M. Tse, N. C. Zachos, and R. Lin performed enteroid culture and functional measurements; and O. Cil, P.-W. Phuan, M. Donowitz, and A. S. Verkman wrote and edited the manuscript.
REFERENCES
- 1.Verkman A. S., Galietta L. J. (2009) Chloride channels as drug targets. Nat. Rev. Drug Discov. 8, 153–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon G. M., Marshall S. G., Ramsey B. W., Rowe S. M. (2015) Breakthrough therapies: cystic fibrosis (CF) potentiators and correctors. Pediatr. Pulmonol. 50(Suppl 40), S3–S13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cil O., Phuan P. W., Lee S., Tan J., Haggie P. M., Levin M. H., Sun L., Thiagarajah J. R., Ma T., Verkman A. S. (2016) CFTR activator increases intestinal fluid secretion and normalizes stool output in a mouse model of constipation. Cell Mol. Gastroenterol. Hepatol. 2, 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flores A. M., Casey S. D., Felix C. M., Phuan P. W., Verkman A. S., Levin M. H. (2016) Small-molecule CFTR activators increase tear secretion and prevent experimental dry eye disease. FASEB J. 30, 1789–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon G. M., Raju S. V., Dransfield M. T., Rowe S. M. (2016) Therapeutic approaches to acquired cystic fibrosis transmembrane conductance regulator dysfunction in chronic bronchitis. Ann. Am. Thorac. Soc. 13(Suppl 2), S169–S176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao A. C., de Sauvage F. J., Dong Y. J., Wagner J. A., Goeddel D. V., Gardner P. (1994) Activation of intestinal CFTR Cl- channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J. 13, 1065–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon C., Zhang W., Sundaram N., Yarlagadda S., Reddy V. S., Arora K., Helmrath M. A., Naren A. P. (2015) Drug-induced secretory diarrhea: a role for CFTR. Pharmacol. Res. 102, 107–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Field M. (1979) Mechanisms of action of cholera and Escherichia coli enterotoxins. Am. J. Clin. Nutr. 32, 189–196 [DOI] [PubMed] [Google Scholar]
- 9.Davidow C. J., Maser R. L., Rome L. A., Calvet J. P., Grantham J. J. (1996) The cystic fibrosis transmembrane conductance regulator mediates transepithelial fluid secretion by human autosomal dominant polycystic kidney disease epithelium in vitro. Kidney Int. 50, 208–218 [DOI] [PubMed] [Google Scholar]
- 10.Yang B., Sonawane N. D., Zhao D., Somlo S., Verkman A. S. (2008) Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol. 19, 1300–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H., Findlay I. A., Sheppard D. N. (2004) The relationship between cell proliferation, Cl- secretion, and renal cyst growth: a study using CFTR inhibitors. Kidney Int. 66, 1926–1938 [DOI] [PubMed] [Google Scholar]
- 12.Li H., Sheppard D. N. (2009) Therapeutic potential of cystic fibrosis transmembrane conductance regulator (CFTR) inhibitors in polycystic kidney disease. BioDrugs 23, 203–216 [DOI] [PubMed] [Google Scholar]
- 13.Venkatasubramanian J., Ao M., Rao M. C. (2010) Ion transport in the small intestine. Curr. Opin. Gastroenterol. 26, 123–128 [DOI] [PubMed] [Google Scholar]
- 14.Thiagarajah J. R., Donowitz M., Verkman A. S. (2015) Secretory diarrhoea: mechanisms and emerging therapies. Nat. Rev. Gastroenterol. Hepatol. 12, 446–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verkman A. S., Synder D., Tradtrantip L., Thiagarajah J. R., Anderson M. O. (2013) CFTR inhibitors. Curr. Pharm. Des. 19, 3529–3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma T., Thiagarajah J. R., Yang H., Sonawane N. D., Folli C., Galietta L. J., Verkman A. S. (2002) Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J. Clin. Invest. 110, 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muanprasat C., Sonawane N. D., Salinas D., Taddei A., Galietta L. J., Verkman A. S. (2004) Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J. Gen. Physiol. 124, 125–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tradtrantip L., Sonawane N. D., Namkung W., Verkman A. S. (2009) Nanomolar potency pyrimido-pyrrolo-quinoxalinedione CFTR inhibitor reduces cyst size in a polycystic kidney disease model. J. Med. Chem. 52, 6447–6455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caci E., Caputo A., Hinzpeter A., Arous N., Fanen P., Sonawane N., Verkman A. S., Ravazzolo R., Zegarra-Moran O., Galietta L. J. (2008) Evidence for direct CFTR inhibition by CFTR(inh)-172 based on Arg347 mutagenesis. Biochem. J. 413, 135–142 [DOI] [PubMed] [Google Scholar]
- 20.Kopeikin Z., Sohma Y., Li M., Hwang T. C. (2010) On the mechanism of CFTR inhibition by a thiazolidinone derivative. J. Gen. Physiol. 136, 659–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiagarajah J. R., Broadbent T., Hsieh E., Verkman A. S. (2004) Prevention of toxin-induced intestinal ion and fluid secretion by a small-molecule CFTR inhibitor. Gastroenterology 126, 511–519 [DOI] [PubMed] [Google Scholar]
- 22.Norimatsu Y., Ivetac A., Alexander C., O’Donnell N., Frye L., Sansom M. S., Dawson D. C. (2012) Locating a plausible binding site for an open-channel blocker, GlyH-101, in the pore of the cystic fibrosis transmembrane conductance regulator. Mol. Pharmacol. 82, 1042–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Hostos E. L., Choy R. K., Nguyen T. (2011) Developing novel antisecretory drugs to treat infectious diarrhea. Future Med. Chem. 3, 1317–1325 [DOI] [PubMed] [Google Scholar]
- 24.Jin B. J., Thiagarajah J. R., Verkman A. S. (2013) Convective washout reduces the antidiarrheal efficacy of enterocyte surface-targeted antisecretory drugs. J. Gen. Physiol. 141, 261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonawane N. D., Zhao D., Zegarra-Moran O., Galietta L. J., Verkman A. S. (2007) Lectin conjugates as potent, nonabsorbable CFTR inhibitors for reducing intestinal fluid secretion in cholera. Gastroenterology 132, 1234–1244 [DOI] [PubMed] [Google Scholar]
- 26.Sonawane N. D., Zhao D., Zegarra-Moran O., Galietta L. J., Verkman A. S. (2008) Nanomolar CFTR inhibition by pore-occluding divalent polyethylene glycol-malonic acid hydrazides. Chem. Biol. 15, 718–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder D. S., Tradtrantip L., Yao C., Kurth M. J., Verkman A. S. (2011) Potent, metabolically stable benzopyrimido-pyrrolo-oxazine-dione (BPO) CFTR inhibitors for polycystic kidney disease. J. Med. Chem. 54, 5468–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder D. S., Tradtrantip L., Battula S., Yao C., Phuan P. W., Fettinger J. C., Kurth M. J., Verkman A. S. (2013) Absolute configuration and biological properties of enantiometers of CFTR inhibitor BPO-27. ACS Med. Chem. Lett. 4, 456–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y., Anderson M. O., Park J., Lee M. G., Namkung W., Verkman A. S. (2015) Benzopyrimido-pyrrolo-oxazine-dione (R)-BPO-27 inhibits CFTR chloride channel gating by competition with ATP. Mol. Pharmacol. 88, 689–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Namkung W., Phuan P. W., Verkman A. S. (2011) TMEM16A inhibitors reveal TMEM16A as a minor component of CaCC conductance in airway and intestinal epithelial cells. J. Biol. Chem. 286, 2365–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Namkung W., Finkbeiner W. E., Verkman A. S. (2010) CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol. Biol. Cell 21, 2639–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foulke-Abel J., In J., Yin J., Zachos N. C., Kovbasnjuk O., Estes M. K., de Jonge H., Donowitz M. (2016) Human enteroids as a model of upper small intestinal ion transport physiology and pathophysiology. Gastroenterology 150, 638–649.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris J. B., LaRocque R. C., Qadri F., Ryan E. T., Calderwood S. B. (2012) Cholera. Lancet 379, 2466–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore S. R., Lima N. L., Soares A. M., Oriá R. B., Pinkerton R. C., Barrett L. J., Guerrant R. L., Lima A. A. (2010) Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology 139, 1156–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guarino A., Dupont C., Gorelov A. V., Gottrand F., Lee J. K., Lin Z., Lo Vecchio A., Nguyen T. D., Salazar-Lindo E. (2012) The management of acute diarrhea in children in developed and developing areas: from evidence base to clinical practice. Expert Opin. Pharmacother. 13, 17–26 [DOI] [PubMed] [Google Scholar]
- 36.Munos M. K., Walker C. L., Black R. E. (2010) The effect of oral rehydration solution and recommended home fluids on diarrhoea mortality. Int. J. Epidemiol. 39(Suppl 1), i75–i87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvaraj N. G., Prasad R., Goldstein J. L., Rao M. C. (2000) Evidence for the presence of cGMP-dependent protein kinase-II in human distal colon and in T84, the colonic cell line. Biochim. Biophys. Acta 1498, 32–43 [DOI] [PubMed] [Google Scholar]
- 38.Lin M. I., Su B. H., Lee C. H., Wang S. T., Wu W. C., Dangate P., Wang S. Y., Huang W. I., Cheng T. J., Lin O. A., Cheng Y. S., Tseng Y. J., Sun C. M. (2015) Synthesis and inhibitory effects of novel pyrimido-pyrrolo-quinoxalinedione analogues targeting nucleoproteins of influenza A virus H1N1. Eur. J. Med. Chem. 102, 477–486 [DOI] [PubMed] [Google Scholar]
- 39.Lee K., Campbell J., Swoboda J. G., Cuny G. D., Walker S. (2010) Development of improved inhibitors of wall teichoic acid biosynthesis with potent activity against Staphylococcus aureus. Bioorg. Med. Chem. Lett. 20, 1767–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Felsenstein K. M., Saunders L. B., Simmons J. K., Leon E., Calabrese D. R., Zhang S., Michalowski A., Gareiss P., Mock B. A., Schneekloth J. S. Jr (2016) Small molecule microarrays enable the identification of a selective, quadruplex-binding inhibitor of MYC expression. ACS Chem. Biol. 11, 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tradtrantip L., Ko E. A., Verkman A. S. (2014) Antidiarrheal efficacy and cellular mechanisms of a Thai herbal remedy. PLoS Negl. Trop. Dis. 8, e2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tradtrantip L., Namkung W., Verkman A. S. (2010) Crofelemer, an antisecretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Mol. Pharmacol. 77, 69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luan J., Zhang Y., Yang S., Wang X., Yu B., Yang H. (2015) Oridonin: a small molecule inhibitor of cystic fibrosis transmembrane conductance regulator (CFTR) isolated from traditional Chinese medicine. Fitoterapia 100, 88–94 [DOI] [PubMed] [Google Scholar]
- 44.Patanayindee J., Muanprasat C., Soodvilai S., Chatsudthipong V. (2012) Antidiarrheal efficacy of a quinazolin CFTR inhibitor on human intestinal epithelial cell and in mouse model of cholera. Indian J. Pharmacol. 44, 619–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krejs G. J. (1987) VIPoma syndrome. Am. J. Med. 82(5B), 37–48 [DOI] [PubMed] [Google Scholar]
- 46.Steven K., Lange P., Bukhave K., Rask-Madsen J. (1981) Prostaglandin E2-mediated secretory diarrhea in villous adenoma of rectum: effect of treatment with indomethacin. Gastroenterology 80, 1562–1566 [PubMed] [Google Scholar]
- 47.Fiskerstrand T., Arshad N., Haukanes B. I., Tronstad R. R., Pham K. D., Johansson S., Håvik B., Tønder S. L., Levy S. E., Brackman D., Boman H., Biswas K. H., Apold J., Hovdenak N., Visweswariah S. S., Knappskog P. M. (2012) Familial diarrhea syndrome caused by an activating GUCY2C mutation. N. Engl. J. Med. 366, 1586–1595 [DOI] [PubMed] [Google Scholar]
- 48.Hoque K. M., Woodward O. M., van Rossum D. B., Zachos N. C., Chen L., Leung G. P., Guggino W. B., Guggino S. E., Tse C. M. (2010) Epac1 mediates protein kinase A-independent mechanism of forskolin-activated intestinal chloride secretion. J. Gen. Physiol. 135, 43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin C. M., Saif L. J., Marthaler D., Wang Q. (2016) Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res. 1702, 30225–30228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ko E. A., Jin B. J., Namkung W., Ma T., Thiagarajah J. R., Verkman A. S. (2014) Chloride channel inhibition by a red wine extract and a synthetic small molecule prevents rotaviral secretory diarrhoea in neonatal mice. Gut 63, 1120–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris A. P., Scott J. K., Ball J. M., Zeng C. Q., O’Neal W. K., Estes M. K. (1999) NSP4 elicits age-dependent diarrhea and Ca(2+)mediated I(-) influx into intestinal crypts of CF mice. Am. J. Physiol. 277, G431–G444 [DOI] [PubMed] [Google Scholar]
- 52.Rufo P. A., Lin P. W., Andrade A., Jiang L., Rameh L., Flexner C., Alper S. L., Lencer W. I. (2004) Diarrhea-associated HIV-1 APIs potentiate muscarinic activation of Cl- secretion by T84 cells via prolongation of cytosolic Ca2+ signaling. Am. J. Physiol. Cell Physiol. 286, C998–C1008 [DOI] [PubMed] [Google Scholar]