Abstract
Endothelial thrombomodulin (TM) regulates coagulation and inflammation via several mechanisms, including production of activated protein C (APC). Recombinant APC and soluble fragments of TM (sTM) have been tested in settings associated with insufficiency of the endogenous TM/APC pathway, such as sepsis. We previously designed a fusion protein of TM [single-chain variable fragment antibody (scFv)/TM] targeted to red blood cells (RBCs) to improve pharmacokinetics and antithrombotic effects without increasing bleeding. Here, scFv/TM was studied in mouse models of systemic inflammation and ischemia-reperfusion injury. Injected concomitantly with or before endotoxin, scFv/TM provided more potent protection against liver injury and release of pathological mediators than sTM, showing similar efficacy at up to 50-fold lower doses. scFv/TM provided protection when injected after endotoxin, whereas sTM did not, and augmented APC production by thrombin ∼50-fold more than sTM. However, scFv/TM injected after endotoxin did not reduce thrombin/antithrombin complexes; nor did antibodies that block APC anticoagulant activity suppress the prophylactic anti-inflammatory effect of scFv/TM. Therefore, similar to endogenous TM, RBC-anchored scFv/TM activates several protective pathways. Finally, scFv/TM was more effective at reducing cerebral infarct volume and alleviated neurological deficits than sTM after cerebral ischemia/reperfusion injury. These results indicate that RBC-targeted scFv/TM exerts multifaceted cytoprotective effects and may find utility in systemic and focal inflammatory and ischemic disorders.—Carnemolla, R., Villa, C. H., Greineder, C. F., Zaitseva, S., Patel, K. R., Kowalska, M. A., Atochin, D. N., Cines, D. B., Siegel, D. L., Esmon, C. T., Muzykantov, V. R. Targeting thrombomodulin to circulating red blood cells augments its protective effects in models of endotoxemia and ischemia-reperfusion injury
Keywords: sepsis, inflammation, coagulation, pharmacokinetics, drug delivery
Thrombomodulin (TM) is a transmembrane glycoprotein expressed by endothelial cells that plays vital roles in coagulation and inflammation (1–4). TM binds thrombin and redirects its activities, enhancing its activation of protein C greater than 1000-fold, while inhibiting its cleavage of fibrinogen and other substrates. Activated protein C (APC), in turn, binds protein S and inactivates coagulation factors Va and VIIIa, thereby down-regulating 2 important positive feedback loops responsible for rapid amplification of thrombin generation. APC has antithrombotic, anti-inflammatory, vascular-barrier stabilizing, and antiapoptotic activities, including signaling to endothelial and hematopoietic cells via G-protein-coupled protease-activated receptors (5, 6). TM also has protein C-independent anti-inflammatory effects, including negative regulation of complement (7), direct inhibition of inflammatory mediators, and damage-associated molecular pattern molecules that bind to its N-terminal lectin domain, and may also be capable of sequestering soluble CD14 (8–13).
Suppression of endogenous endothelial TM has been implicated in diverse pathological processes including sepsis (14), atherosclerosis (15), and malignancies (16). Increased levels of soluble fragments of TM (sTM) (which can be shed from the endothelial surface and is considered a marker of endothelial injury) are associated with worse outcomes in acute coronary syndrome (17), acute respiratory distress syndrome (18), cerebral malaria (19), and transplantation (20), among many other clinical conditions.
Therefore, considerable effort has been expended to develop biotherapeutics capable of replenishing TM/APC activity (21–23). A recombinant form of APC showed promise in early trials of sepsis (24), but follow-up studies failed to demonstrate significant benefit, and an increased risk of bleeding was observed (25, 26). Initial results from a trial of recombinant sTM (also known as ART-123) as an alternative approach to disorders characterized by systemic inflammation and activation of coagulation (e.g., sepsis-induced disseminated intravascular coagulation) showed promising results, particularly with respect to safety, which led to marketing approval in Japan (27–29). sTM has been proposed for use in post-thrombotic syndrome (30) and stroke (31), among many other conditions, indicating that drugs that act at the nexus of inflammation and coagulation have widespread potential applications (3, 32). Nonetheless, for both APC and TM, suboptimal pharmacokinetics (i.e., rapid elimination from the circulation), modest efficacy, and safety concerns (particularly bleeding in the case of APC) have spurred investigation of strategies to prolong their circulation time, enhance their bioavailability for control of intravascular targets, and limit off-target activity. For example, an engineered form of recombinant sTM (sothromodulin alfa, solulin), which exhibits a longer enhanced plasma half-life and resists oxidation, among other features, has entered clinical trials (33). A recent meta-analysis of trials involving recombinant sTM revealed an encouraging trend toward efficacy (34, 35), which justifies further investigation into its mechanisms of action and approaches to improve its pharmacological properties.
One strategy to address these challenges involves targeting TM to pathologic sites, such as the endothelial surface or to blood cells that circulate within the vascular lumen (36–39). Regarding the latter, we have achieved safe and effective targeting of TM to the surface of red blood cells (RBCs) by fusing sTM with Ter119 scFv, a single-chain variable fragment antibody (scFv) specific for an epitope associated with murine glycophorin A (36). After intravenous injection, the RBC-targeted Ter119 scFv/TM fusion protein bound to erythrocytes, circulated in a RBC-anchored form far longer than free sTM, and was more effective at sustaining thromboprophylaxis than sTM in rodent models of thrombosis, without increasing the risk of bleeding associated with APC.
We aimed to investigate how these encouraging pharmacokinetic and antithrombotic features of RBC-targeted scFv/TM might be extended to control of inflammation and cytoprotective activity. RBC-targeted scFv/TM, similar to its endothelial-targeted counterpart and other TM biotherapeutics, may exert APC-mediated and/or APC-bypassing anti-inflammatory activity. However, RBC carriage might impair the interaction of scFv/TM with vascular receptors [e.g., protease-activated receptors and endothelial protein C receptor (40)] and with its molecular targets in blood based on limited spatial freedom in the RBC membrane or interference by the RBC glycocalyx, as has been demonstrated for other RBC-bound biotherapeutics (41). To address these questions, we investigated the efficacy, safety, and mechanism of action of scFv/TM in systemic inflammation caused by endotoxemia and in a model of cerebral ischemia/reperfusion injury.
MATERIALS AND METHODS
Animals
Animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals [National Institutes of Health, Bethesda, MD, USA (NIH)] under protocols approved by University of Pennsylvania Institutional Animal Care and Use Committee. Male C57BL/6 mice, 6–8 wk old, weighing 20–30 g (The Jackson Laboratory, Bar Harbor, ME, USA), were used for all experiments.
Antibodies and other reagents
Unless otherwise indicated, cell culture reagents were purchased from Invitrogen (Carlsbad, CA, USA). Schneider’s and Insect Express serum-free medium were purchased from Lonza (Basel, Switzerland). [125I]Na was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA, USA). Mouse anti-FLAG M2 horseradish peroxidase mAb, heparin from porcine intestinal mucosa, LPS:B4 from Escherichia coli, and anti-mouse horseradish peroxidase mAb were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bovine thrombin and enhanced chemiluminescence was purchased from GE Amersham Biosciences (Pittsburgh, PA, USA). Eight-well EIA/RIA Corning strips for human activated protein C (APC) ELISAs were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Protein C, purified from human plasma, was kindly supplied by Dr. Sriram Krishnaswamy (Children’s Hospital of Philadelphia, Philadelphia, PA, USA). Recombinant human APC, mouse monoclonal antibody against human APC (1555), and mouse APC (1591) have been previously described (42, 43). Mouse fibrinogen ELISA was purchased from AssayPro (St. Charles, MO, USA). Alanine transaminase (ALT) ELISA was purchased from Bioassay Systems (Hayward, CA, USA). TNF-α and MIP-2 Quantikine ELISAs were purchased from R&D Systems (Minneapolis, MN, USA). Antibodies to measure thrombin/antithrombin (TAT) by sandwich ELISA were purchased from Haematologic Technologies (Essex Junction, VT, USA), and the detection antibody was biotinylated with EZ-Link Sulfo-NHS biotin (Thermo Fisher Scientific).
Production of red blood cell-targeted thrombomodulin and nontargeted TM
Cloning and production of the Ter119 (anti-mouse glycophorin A) scFv/TM fusion protein and sTM has been previously described (36). Briefly, stable Drosophila S2 cell lines expressing scFv/TM and sTM were cultured, and supernatants were harvested after induction with CuSO4. Proteins were purified from medium using an M2 (anti-FLAG) affinity column and tested for purity via SDS-PAGE and HPLC (Waters, Huntingdon Valley, PA, USA) using a gel-filtration column (Biosep; Phenomenex, Torrance, CA, USA). TM and scFv/TM had similar specific activity based on their ability to generate APC in the presence of thrombin (see Supplemental Data).
Middle cerebral artery filament occlusion model
We used the middle cerebral artery (MCA) filament occlusion model of ischemia-reperfusion injury to compare the efficacy of therapeutics (44). All procedures were performed in strict accordance with the recommendations by the NIH and approved by the Massachusetts General Hospital Subcommittee on Research and Animal Care. Briefly, the external carotid artery was isolated and incised in anesthetized mice. A silicon-covered nylon filament (Doccol, Sharon, MA, USA) was advanced via the proximal external carotid artery into the internal carotid artery, occluding the MCA for 30 min, with subsequent reperfusion for 48 h documented by laser doppler monitoring of cortical blood flow. At the end of the reperfusion period, mice were examined for neurologic deficits using a 4-point scale before being humanely killed. Neurologic outcomes were graded as: 0, normal; 1, forepaw monoparesis; 2, circling to right; 3, falling to right; and 4, no spontaneous walking and depressed consciousness (45). The brain was cut into 2 mm thick coronal blocks and stained in the dark with 2,3,5-triphenyltetrazolium chloride for 1 h at 37°C, and the infarct volume was calculated (46). Where indicated, 1 mg/kg (18 nmol/kg) sTM or an equimolar dose of scFv/TM was injected into the right jugular vein 20 min before MCA occlusion.
Time course of functional activity in vivo
In vivo measurement of functional activity of TM therapeutics (i.e., APC generation) was performed as previously described (47). Briefly, mice were anesthetized with ketamine/xylazine, and various doses of sTM (1.8–90 nmol/kg; 0.1–5 mg/kg equivalent) vs. equimolar scFv/TM vs. PBS were injected into the jugular vein. At prespecified time points (0–6 h), thrombin (0.8 U/25 g) and human PC (64.5 nM) were administered intravenously. Blood was collected 10 min later from the inferior vena cava into 3.8% sodium citrate and benzamidine (v/v, 2:1) and centrifuged at 10,000 g for 10 min at 4°C to isolate plasma. Plasma levels of human APC were determined by ELISA.
LPS-induced systemic inflammation
Mice were anesthetized with ketamine/xylazine before intraperitoneal injection of LPS:B4 endotoxin (20 mg/kg, i.p.). Six h later, blood was collected from the inferior vena cava into 3.8% sodium citrate (5:1 v/v, blood/sodium citrate) and centrifuged at 10,000 g for 10 min at 4°C to isolate plasma. TNF-α, MIP-2, and the transaminase ALT were measured by ELISA. Various doses of sTM of scFv/TM (1.8–90 nmol/kg; 0.1–5 mg/kg equivalent) were injected via the jugular vein 3 h before challenge with LPS (prophylaxis), immediately before LPS (concurrent treatment), or 3 h after LPS. In other experiments, mice received the APC inhibitory antibody Ab1591 (10 mg/kg) at time of LPS injection.
RESULTS
RBC-targeted scFv/TM provides superior systemic inflammatory protection in a murine model of endotoxemia
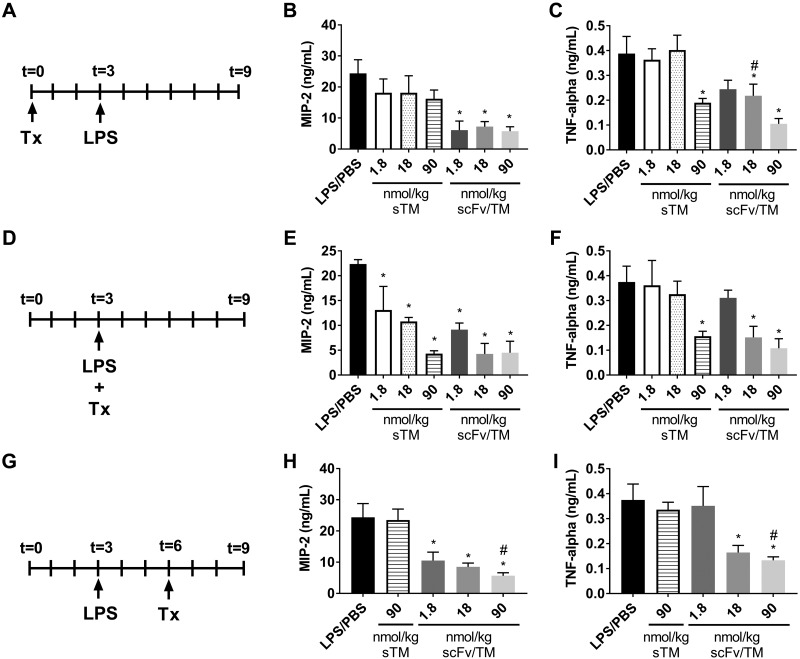
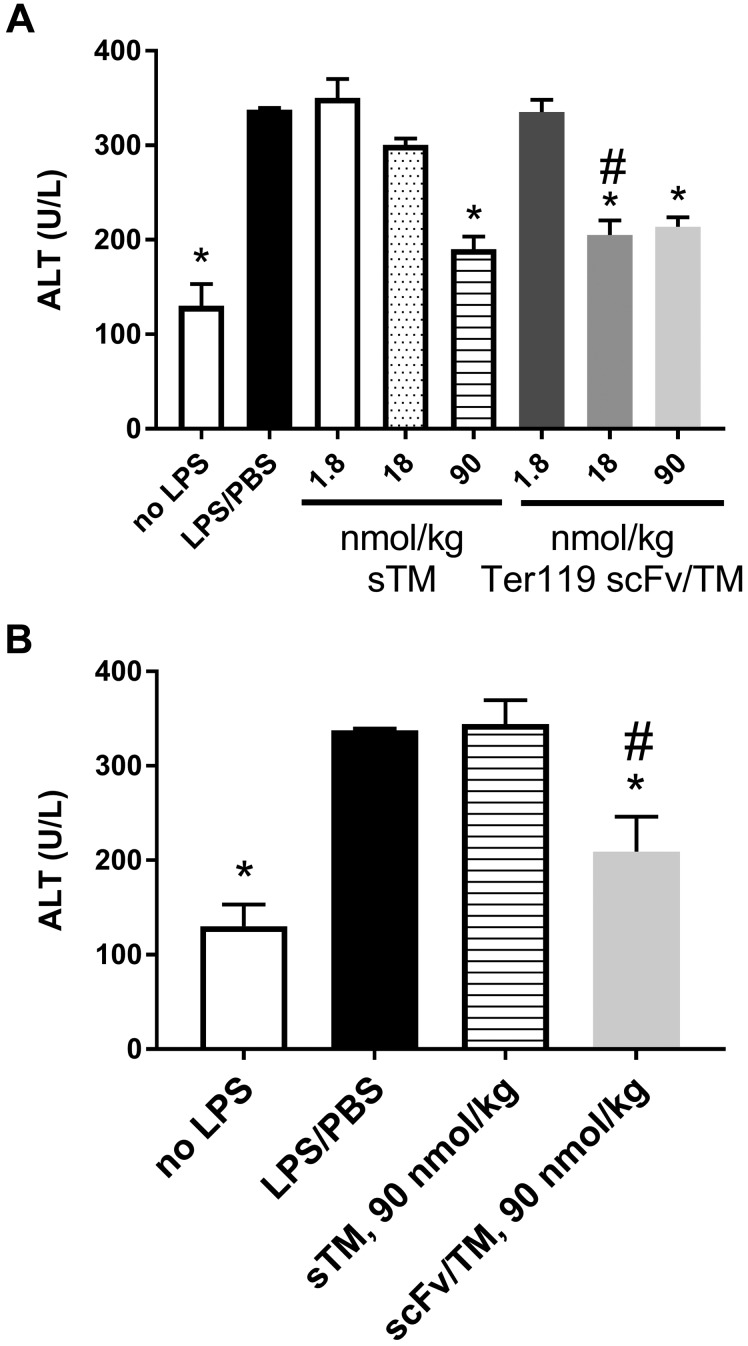
We first investigated the therapeutic effects of Ter119 scFv/TM in a mouse model of endotoxin-induced systemic inflammation and end-organ injury. Endotoxemia was induced using high-dose (20 mg/kg) intraperitoneal LPS challenge, which produces systemic elevation of proinflammatory chemokines and cytokines and markers of hepatic injury (see Supplemental Data). When given prophylactically (i.e., 3 h before LPS administration), scFv/TM significantly reduced plasma TNF-α and MIP-2 (Fig. 1A, B) even at the relatively low dose of 1.8 nmol/kg (0.1 mg/kg equivalent for sTM), whereas sTM was only effective in lowering TNF-α production at the high dose of 90 nmol/kg (5 mg/kg equivalent). A similar pattern was seen with hepatic injury (Fig. 2A). Ter119 scFv/TM nearly normalized plasma ALT to levels seen with PBS controls at a 18-nmol/kg dose, whereas sTM was effective only at the highest dose examined (90 nmol/kg).
Figure 1.
RBC-targeted scFv/TM is more potent than sTM in suppression of inflammatory markers in response to systemic endotoxemia. In a murine model of endotoxemia (LPS, 20 mg/kg, i.p.; A, D, G) mice were treated with intravenous injection of sTM, scFv/TM, or PBS at various doses as indicated. Treatments were administered prophylactically 3 h before challenge (A–C), concurrent with LPS challenge (D–F), or 3 h after LPS challenge (G–I). At 6 h after LPS challenge, plasma levels of the inflammatory chemokine MIP-2 (B, E, H) or the cytokine TNF-α (C, F, I) were measured by ELISA. Levels of TNF-α and MIP-2 in naive mice were below the limit of detection (see Supplemental Data). When given in a prophylactic mode, scFv/TM significantly prevented the rise in MIP-2 at all doses and prevented TNF-α at the 2 highest doses, whereas sTM suppressed TNF-α only and only at the highest dose. When given concurrent with LPS challenge, all doses for both treatments significantly suppressed the rise in MIP-2. scFv/TM was more potent than sTM in suppressing the rise in TNF-α, demonstrating significant suppression at 18 nmol/kg, a dose at which TM was ineffective. In the therapeutic mode, all doses of scFv/TM suppressed the rise in MIP-2, and the 2 highest doses suppressed TNF-α. However, even the highest dose of sTM did not prevent the rise in TNF-α (n = 7 ± sem). *P < 0.05 vs. LPS/PBS control; #P < 0.05 vs. sTM at the equivalent dose (1-way ANOVA with Holm-Sidak correction for multiple comparisons).
Figure 2.
RBC-targeted scFv/TM protects against liver injury induced by systemic endotoxemia more effectively than sTM in both prophylactic and therapeutic administration. Plasma levels of ALT were measured by ELISA 6 h after LPS challenge (20 mg/kg, i.p.). A) When scFv/TM and TM were administered prophylactically, scFv/TM provided significant protection against liver injury at doses of 18 and 90 nmol/kg, whereas sTM only showed an effect at a dose of 90 nmol/kg. B) In the therapeutic mode, only scFv/TM showed liver protection at 90 nmol/kg. *P < 0.05 vs. LPS/PBS control; #P < 0.05 vs. sTM at equivalent dose (1-way ANOVA with Holm-Sidak correction for multiple comparisons).
Reasoning that these differences in therapeutic efficacy were most likely due to the more prolonged circulation of RBC-targeted scFv/TM, we compared the effect of the fusion vs. untargeted sTM when given concurrently with LPS challenge (Fig. 1D–F) and when injected 3 h after LPS administration (Fig. 1G–I). The latter, in particular, best simulates the clinical setting in which treatment would be contemplated in a patient with sepsis or another syndrome and presents with acute, severe, systemic inflammation. Concurrent administration yielded similar results to prophylactic administration for scFv/TM, with improvement in efficacy over sTM (as compared with the lack of efficacy of sTM given prophylactically). Both drugs markedly blunted the rise in plasma levels of MIP-2 and TNF-α when given concurrently with LPS at the highest dose (90 nmol/kg), but scFv/TM appeared to be more potent than sTM, showing greater protection at the intermediate doses (18 nmol/kg). Somewhat surprisingly, Ter119 scFv/TM was superior to sTM when the drugs were administered after LPS challenge. sTM did not prevent the rise in inflammatory cytokine levels in this therapeutic setting after LPS challenge, even at a high dose (90 nmol/kg). In contrast, scFv/TM significantly inhibited the rise in inflammatory chemokines and cytokines even at 50-fold lower doses (1.8 nmol/kg). This benefit was also manifested in end-organ (liver) protection, as evidenced by preventing the rise in ALT after LPS challenge (Fig. 2B).
Ter119 scFv/TM has prolonged functional activity in vivo as compared with sTM
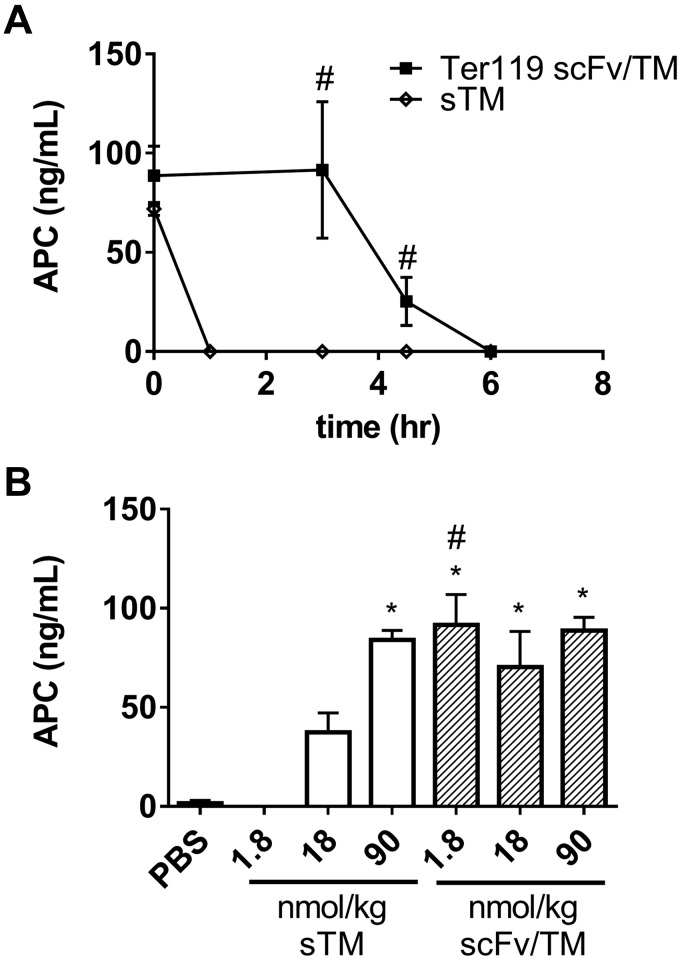
Because scFv/TM was more efficacious than sTM in our models of endotoxemia, we sought to confirm that the improved pharmacokinetics and tissue distribution of RBC-targeted TM fusion proteins observed in prior studies (36) conferred superior functional benefit with respect to sustained APC generative capacity. In prior studies, 3 h after intravenous injection, 50% of the injected dose of Ter119 scFv/TM remained bound to circulating erythrocytes, whereas <10% of sTM remained in the blood. Based on these observations, we hypothesized that the prolonged circulation of the fusion protein would result in a sustained increase in thrombin-induced APC generation. To test this, we took advantage of a technique recently reported by our laboratory, which allows for measurement of APC generation by TM-based therapeutics in vivo (47). As shown in Fig. 3A, Ter119 scFv/TM at a dose of 1.8 nmol/kg (equivalent to 0.1 mg/kg sTM) and sTM have roughly equal capacity to generate APC immediately after administration (5 min). However, the functional activity of sTM drops off quickly and is undetectable by 3 h after administration, whereas RBC-anchored scFv/TM retains detectable APC activity for up to 6 h after intravenous injection of an equimolar dose. Dose titration experiments demonstrated that the measured APC-generating potential of the scFv/TM at 3 h was saturated even at the lowest dose examined and that approximately 50-fold higher doses of sTM were needed to generate comparable levels of APC (Fig. 3B). This apparent lack of a dose response is believed to reflect saturation of the measurement of APC generation in a manner specific to this experimental setup, in which the assay reflects APC generation after administration of exogenous thrombin and human protein C and not levels of endogenous murine APC directly.
Figure 3.
RBC-targeted scFv/TM generates more APC than equimolar doses of sTM. Before thrombin challenge and injection of human PC, mice were treated with equimolar doses of sTM or scFv/TM. Immediately after injection (t = 0) and 3, 4.5, and 6 h later, thrombin (0.8 U/25 g) and human PC (64.5 nM) were injected, and blood was collected 10 min later. APC levels were measured by ELISA. A) At a dose of 1.8 nmol/kg (0.1 mg/kg sTM, 0.15 mg/kg Ter119 scFv/TM) there is sustained generation of APC in mice that received Ter119 scFv/TM. In contrast, mice given sTM show APC generation immediately after injection, but no APC generation is detected at 3 h. B) APC generation was measured at 3 h after infusion of either sTM or scFv/TM. APC generation saturates at 1.8 nmol/kg for Ter119 scFv/TM. A 50-fold higher dose of sTM is required to generate equivalent levels of APC at 3 h. *P < 0.05 vs. PBS control. #P < 0.05 vs. sTM at equivalent dose/time (1-way ANOVA with Holm-Sidak correction for multiple comparisons).
Suppression of LPS-induced inflammation by RBC-targeted scFv/TM does not require the anticoagulant activity of APC
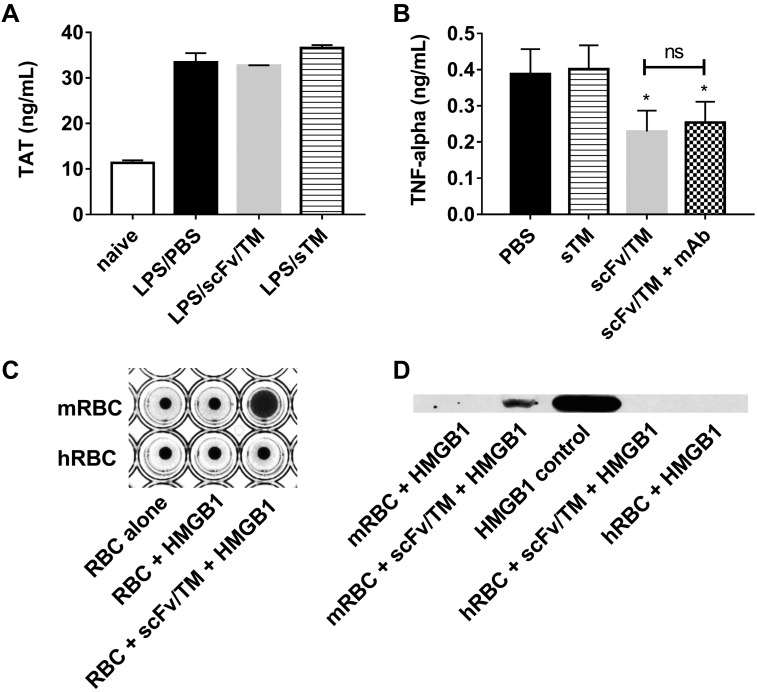
We next explored the relationship between the enhanced generation of APC by scFv/TM compared with sTM and the enhanced protection it provides against the host response to endotoxemia (particularly when administered after LPS challenge). To discern the relative contribution of the anticoagulant vs. anti-inflammatory activities of this therapeutic, we first measured levels of TAT complexes in endotoxemic mice treated with sTM or scFv/TM after the LPS challenge. TAT complexes are elevated in models of endotoxemia and serve as a plasma marker of systemic activation of coagulation (48). There was no significant difference in plasma levels of TAT in mice challenged with LPS 3 h after treatment with either sTM or scFv/TM (90 nmol/kg) compared with mice that received neither (Fig. 4A). All groups showed significantly elevated TAT compared with naive mice. This outcome contrasts with the marked differences in levels of systemic inflammatory markers in these mice using the same experimental protocol and interventions. This finding suggests that the therapeutic effect of scFv/TM does not depend on dampening or reversing antecedent activation of coagulation by LPS. We then specifically inhibited the anticoagulant activity of APC generated by scFv/TM and sTM with mAb 1591. mAb 1591 inhibits the effects of mouse APC on plasma clotting time and enhances plasma TAT levels after high doses of systemic LPS but retains the cytoprotective functions of APC (49). Coadministration of mAb 1591 did not attenuate the protective effect of Ter119 scFv/TM with respect to cytokine suppression (Fig. 4B). This finding strongly suggests that scFv/TM provides anti-inflammatory protection woithout the anticoagulant activity of APC.
Figure 4.
RBC-targeted scFv/TM suppresses LPS induced inflammation independent of anticoagulant activity. A) Levels of TAT were measured by ELISA in plasma from mice treated with PBS, sTM (90 nmol/kg), or scFv/TM (90 nmol/kg) 3 h after LPS challenge. Plasma was assayed at 6 h after LPS challenge. The results demonstrate no significant decrease in TAT for either therapeutic. B) The extent of inflammatory suppression by scFv/TM or sTM (18 nmol/kg) administered 3 h before LPS challenge was assessed by measuring plasma TNF-α by ELISA in the presence and absence of mAb 1591, which blocks the anticoagulant, but not cytoprotective, activity of APC. The results demonstrate that scFv/TM maintains its protective effect in the presence of mAb 1591. C) The ability of RBC-bound scFv/TM to sequester the inflammatory mediator HMGB-1 was assayed by hemaggultination techniques wherein mouse RBCs (that bind Ter119 scFv/TM) and human RBCs (as a nonbinding control) were coincubated with HMGB-1 followed by antibody against HMGB-1 to induce agglutination if HMGB-1 is bound to the RBC surface. The formation of an RBC carpet across the bottom of the well indicates positive agglutination, and a central button of RBCs indicates a negative result. D) Murine RBCs coated with scFv/TM captured HMGB1 in vitro as measured by Western blotting technique. Recombinant HMGB-1 is shown as a positive control; naive murine RBCs, naive human RBCs, and human RBCs treated with scFv/TM (which does not bind Ter119) are shown as negative controls. *P < 0.05 vs. LPS/PBS control (1-tailed t test).
The TM/APC axis protects against inflammation by many mechanisms, including sequestration of mediators by sTM such as CD14, TNF-α, LPS, and HMGB-1. Recent evidence implicates HMGB-1 in the pathophysiology of sepsis (50, 51), and TM is capable of binding HMGB-1, neutralizing its inflammatory signaling (13), and accelerating its breakdown by simultaneously bound thrombin (52). We hypothesized that RBC-bound scFv/TM would sequester inflammatory mediators such as HMGB-1 onto the capacious RBC surface. To test this, RBCs loaded with scFv/TM were coincubated with recombinant HMGB-1 as a representative inflammatory mediator, and the sequestration of HMGB-1 onto red cells was probed by RBC agglutination and immunoprecipitation techniques (Fig. 4C). These experiments demonstrated the RBC/scFv/TM complexes did indeed capture HMGB-1, which offers a potential mechanistic explanation for the therapeutic activity of scFv/TM that is independent of its anticoagulant activity.
RBC-targeted scFv/TM protects mice from brain infarction after MCA occlusion
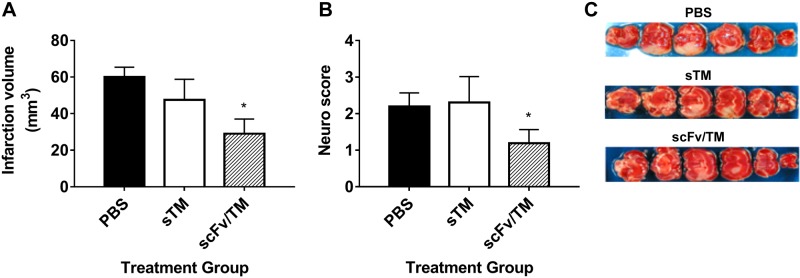
TM and APC exert anti-inflammatory and protective functions in animal models of acute stroke (53). Therefore, we investigated the efficacy of scFv/TM in the setting of CNS ischemia-reperfusion injury. The transient middle cerebral artery occlusion model was chosen because it reproduces several key features of acute stroke in humans, including a sizable ischemic penumbra amenable to studies of neuroprotection after reperfusion (54). sTM or scFv/TM (1.8 nmol/kg) was administered 20 min before occlusion of the MCA by a filament, which lasted 30 min, and the extent of infarction was measured using 2 methods 48 h after the filament was removed and reperfusion was begun: infarct volume based on tissue staining with 2,3,5-triphenyltetrazolium chloride and grading of residual neurologic function. Both outcomes were improved by prophylactic administration of scFv/TM but not sTM (Fig. 5). Representative images of the resulting areas of infarction can be seen in Fig. 5C, wherein areas of pale discoloration are significantly reduced with prior administration of scFv/TM but not sTM or PBS control.
Figure 5.
RBC-targeted scFv-TM protects against cerebral ischemia-reperfusion injury and resulting neurologic deficits. Twenty minutes before carotid artery ligation, mice were treated with PBS (black bars), 18 nmol/kg sTM (1 mg/kg equivalent, white bars), or 18 nmol/kg scFv/TM (hashed bars) given via injection into the right jugular vein. The external carotid artery was then isolated and incised, a silicon-covered nylon filament was advanced via the proximal external carotid artery into the internal carotid artery, and the MCA was occluded for 30 min with subsequent filament withdrawal and reperfusion for 48 h using laser doppler to monitor cortical blood flow. The brain was cut into 2-mm-thick coronal blocks, and the sections were stained with 2,3,5-triphenyltetrazolium chloride to calculate the infarct volume. Residual neurologic function was assessed using a 4-point scale (see Materials and Methods). A, B) The results demonstrate that scFv/TM, but not sTM, significantly reduced infarct volume (A) and neurologic deficit (B) 48 h after MCA occlusion. C) Representative images demonstrating reduced area of infarct (pale coloration) (n = 3–5; means ± sem). *P < 0.05 vs. PBS control (1-tailed Student’s t test).
DISCUSSION
TM is a multifaceted protein that plays a central role in maintaining the anticoagulant activity of normal endothelium and is increasingly recognized for its role in regulating inflammation. These properties have led to the use of sTM as a clinical therapeutic in sepsis and disseminated intravascular coagulation (27, 29, 34), and recent studies have shown its therapeutic potential in diverse disorders including stroke (31, 53, 55), radiation toxicity (56), hemolytic uremic syndrome (57), and transplantation and graft versus host disease (58, 59). Our group has developed technologies to use carrier RBCs to deliver TM and improve its bioavailability, residence time in the circulation, and efficacy. In the present study, we focused on assessing the efficacy of this novel fusion in endotoxemia and ischemia-reperfusion injury, and we investigated its therapeutic mechanism of action.
RBCs are attractive carriers because they have a prolonged circulatory lifespan, are largely restricted to the intravascular space, and may confer protection of therapeutic agents from degradation and by modulating adaptive immune responses (60). We have shown that antibodies and fusion proteins can be used to couple therapeutics to the surface of RBCs ex vivo and in vivo (36, 61–64). Although it would be expected that RBC carriage would confer pharmacokinetic benefits via prolonging the intravascular circulation of drugs, coupling to the RBC may also confer profound pharmacodynamic changes that fundamentally alter the mechanisms of the appended therapeutic, particularly so for hemostatic or thromboprophylactic agents (65). Therefore, for a multifunctional protein such as TM, it is important to assess the favorable and potentially unfavorable consequences of RBC coupling.
In previous studies, we demonstrated the efficacy of scFv/TM for thromboprophylaxis (36). Here we asked whether this novel therapeutic shows similar efficacy in models of inflammatory injury, specifically in endotoxemia, and in ischemia/reperfusion injury. We first demonstrated that in a model of endotoxemia, scFv/TM more potently attenuated the rise in the plasma levels of key inflammatory cytokine/chemokines and attenuated end-organ (liver) toxicity compared with sTM. This effect was evident when scFv/TM was administered before endotoxin challenge, concomitant with endotoxin, as well as in a therapeutic mode (i.e., 3 h after LPS). Remarkably, in the latter setting, sTM did not provide significant protection in this model. This outcome suggests that the benefit of RBC carriage extends beyond the prolongation in circulating half-life of TM and raised the question of whether enhanced APC cofactor activity is central to its therapeutic mechanism of action.
We then demonstrated that the prolonged circulation of scFv/TM observed in prior studies results in proportional prolongation of intravascular APC-generating capacity. Importantly, these data show potential for APC generation in the presence of thrombin (which was administered exogenously in this assay) and not APC levels directly. This should, in theory, mitigate safety concerns for bleeding potential in the setting of high systemic APC levels.
Systemic activation of coagulation has been demonstrated in murine models of endotoxemia, and we similarly observed elevated levels of TAT complexes after LPS exposure. However, when the scFv/TM fusion was administered as therapy (i.e., after LPS), there was no change in circulating TAT complexes, although scFv/TM suppressed the increase in TNF-α and MIP-2. Therefore, it appeared that the activity of the scFv/TM is, in part, independent of its anticoagulant activity. The anti-inflammatory activity of scFv/TM in the prophylactic setting was also not affected by concurrent administration of mAb 1591, an antibody that selectively inhibits the anticoagulant activity of APC while sparing the cytoprotective and anti-inflammatory functions of APC. This dissociation in outcomes suggests that even if scFv/TM is not able to reverse antecedent activation of coagulation in sepsis, it might prove beneficial by affecting other inflammatory pathways directly. Having previously demonstrated effective thromboprohylaxis with RBC-bound scFv/TM (36), we do not feel that the anticoagulant activity is inhibited by RBC binding, but rather, when scFv/TM is administered in a treatment setting (i.e., after endotoxin challenge), the anti-inflammatory activity is predominant for its therapeutic efficacy.
TM is comprised of several functional domains in addition to its EGF-repeat thrombin binding domains. TM contains an N-terminal lectin-like domain that binds and, in some cases, neutralizes inflammatory mediators such as HMGB1, TNF-α, and LPS (8–13). As one of several possible representative inflammatory mediators, we demonstrated that RBC-bound scFv/TM sequestered HMGB-1 onto the erythrocyte surface in vitro, likely through direct binding (13), offering a potential mechanistic rationale for its anti-inflammatory activity independent of APC generation. However, additional studies will be necessary to demonstrate if this binding (or binding of other inflammatory mediators) occurs in vivo and if it is sufficient to account for physiologic and potentially therapeutic anti-inflammatory activity. Furthermore, given the ability of TM to accelerate the inactivation of C3a and C5a, it will also be critical to probe the complement pathway in future studies and determine its contribution to the protection of scFv/TM against endotoxemia.
Finally, scFv/TM provided superior histologic and functional outcomes in a model of ischemia-reperfusion CNS injury caused by transient occlusion of the MCA. sTM was ineffective at the dose examined, although favorable outcomes have been reported in similar models. We did not compare our sTM, which consists of the native murine extracellular sequence, with other engineered soluble forms (i.e., solulin); nor did we proceed to higher doses. Nonetheless, these data affirm the efficacy of RBC delivery of TM as a therapeutic approach to reperfusion injury. Again, additional studies will be needed to delineate between the antithrombotic and anti-inflammatory actions of RBC-TM in this setting.
We used a single-chain fragment of Ter119, an antibody specific for a murine glycophorin A-associated epitope. Therefore, translation of the present approach will necessitate identification of a suitable fusion partner capable of targeting human red blood cells (in addition to fusion with human sTM). Because murine and human RBC biology differ in many important ways (size, rigidity, effects of storage, etc.), it is possible that the anticoagulant and anti-inflammatory profiles of TM bound to human RBCs will also differ. It will be critical to devise strategies to address these concerns in preclinical models. Furthermore, the potential for adverse effects on RBCs themselves, such as alteration in deformability and oxidative stress (66–68), after coupling have not been fully characterized and may depend on copy numbers and RBC epitope. Although we were able to demonstrate efficacy in the present models, mechanistic insights remain limited and will require further investigation. Resolution of these questions will help optimize strategies for translating RBC-targeted scFv/TM constructs into the clinical domain.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Mortimer Poncz, The Children’s Hospital of Philadelphia, for his scientific counsel and support of the study. This work was supported by U.S. National Institutes of Health, National Heart, Lung, and Blood Institute Grants R01 HL121134, R01 HL125462, R01 HL116916-01, R01 HL091950, K08-HL 130430, and 7UM1 HL120877-TACTIC. The authors declare no conflicts of interest.
Glossary
- ALT
alanine transaminase
- APC
activated protein C
- MCA
middle cerebral artery
- RBC
red blood cell
- sTM
soluble fragments of thrombomodulin
- TAT
thrombin/antithrombin
- TM
thrombomodulin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
R. Carnemolla, C. H. Villa, C. F. Greineder, S. Zaitsev, M. A. Kowalska, D. N. Atochin, D. B. Cines, D. L. Siegel, C. T. Esmon, and V. R. Muzykantov conceived and designed the studies; R. Carnemolla, C. H. Villa, C. F. Greineder, K. R. Patel, and S. Zaitsev synthesized and purified recombinant proteins; C. T. Esmon produced anti-APC antibodies; R. Carnemolla and C. F. Greineder performed mouse LPS experiments; D. N. Atochin performed mouse ischemia/reperfusion experiments; R. Carnemolla, C. H. Vislla, C. F. Greineder, K. R. Patel, S. Zaitsev, and M. A. Kowalska performed in vitro studies and ELISA measurements; R. Carnemolla, C. H. Villa, and C. F. Greineder wrote the manuscript; and M. A. Kowalska, D. N. Atochin, D. B. Cines, D. L. Siegel, C. T. Esmon, and V. R. Muzykantov edited the manuscript.
REFERENCES
- 1.Esmon C. T., Owen W. G. (2004) The discovery of thrombomodulin. J. Thromb. Haemost. 2, 209–213 [DOI] [PubMed] [Google Scholar]
- 2.Conway E. M. (2012) Thrombomodulin and its role in inflammation. Semin. Immunopathol. 34, 107–125 [DOI] [PubMed] [Google Scholar]
- 3.Morser J. (2012) Thrombomodulin links coagulation to inflammation and immunity. Curr. Drug Targets 13, 421–431 [DOI] [PubMed] [Google Scholar]
- 4.Ito T., Maruyama I. (2011) Thrombomodulin: protectorate god of the vasculature in thrombosis and inflammation. J. Thromb. Haemost. 9(Suppl 1), 168–173 [DOI] [PubMed] [Google Scholar]
- 5.Bouwens E. A., Stavenuiter F., Mosnier L. O. (2013) Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway. J. Thromb. Haemost. 11(Suppl 1), 242–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosnier L. O., Zlokovic B. V., Griffin J. H. (2007) The cytoprotective protein C pathway. Blood 109, 3161–3172 [DOI] [PubMed] [Google Scholar]
- 7.Delvaeye M., Noris M., De Vriese A., Esmon C. T., Esmon N. L., Ferrell G., Del-Favero J., Plaisance S., Claes B., Lambrechts D., Zoja C., Remuzzi G., Conway E. M. (2009) Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 361, 345–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang W.-E., Chang B.-Y., Ma C.-Y., Shih Y.-T., Cheng S.-E. (2015) Recombinant thrombomodulin inhibits lipopolysaccharide-induced inflammatory response by blocking the functions of CD14. J. Immunol. 194, 1905–1915 [DOI] [PubMed] [Google Scholar]
- 9.Lentz S. R., Sadler J. E., Tsiang M. (1992) Functional domains of membrane-bound human thrombomodulin: EGF-like domains four to six and the serine/threonine-rich domain are required for cofactor activity. J. Biol. Chem. 267, 6164–6170 [PubMed] [Google Scholar]
- 10.Li Y.-H., Liu S.-L., Wei H.-J., Shi G.-Y., Chang P.-C., Wu H.-L., Kuo C.-H. (2011) Thrombomodulin domains attenuate atherosclerosis by inhibiting thrombin-induced endothelial cell activation. Cardiovasc. Res. 92, 317–327 [DOI] [PubMed] [Google Scholar]
- 11.Lattenist L., Teske G., Claessen N., Florquin S., Conway E. M., Roelofs J. J. (2015) The lectin like domain of thrombomodulin is involved in the defence against pyelonephritis. Thromb. Res. 136, 1325–1331 [DOI] [PubMed] [Google Scholar]
- 12.Li Y. H., Kuo C. H., Shi G. Y., Wu H. L. (2012) The role of thrombomodulin lectin-like domain in inflammation. J. Biomed. Sci. 19, 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abeyama K., Stern D. M., Ito Y., Kawahara K., Yoshimoto Y., Tanaka M., Uchimura T., Ida N., Yamazaki Y., Yamada S., Yamamoto Y., Yamamoto H., Iino S., Taniguchi N., Maruyama I. (2005) The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J. Clin. Invest. 115, 1267–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faust S. N., Heyderman R. S., Levin M. (2001) Coagulation in severe sepsis: a central role for thrombomodulin and activated protein C. Crit. Care Med. 29, S62–S67 [DOI] [PubMed] [Google Scholar]
- 15.Laszik Z. G., Zhou X. J., Ferrell G. L., Silva F. G., Esmon C. T. (2001) Down-regulation of endothelial expression of endothelial cell protein C receptor and thrombomodulin in coronary atherosclerosis. Am. J. Pathol. 159, 797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanly A. M., Winter D. C. (2007) The role of thrombomodulin in malignancy. Semin. Thromb. Hemost. 33, 673–679 [DOI] [PubMed] [Google Scholar]
- 17.Chan S. H., Chen J. H., Li Y. H., Lin L. J., Tsai L. M. (2006) Increasing post-event plasma thrombomodulin level associates with worse outcome in survival of acute coronary syndrome. Int. J. Cardiol. 111, 280–285 [DOI] [PubMed] [Google Scholar]
- 18.Sapru A., Calfee C. S., Liu K. D., Kangelaris K., Hansen H., Pawlikowska L., Ware L. B., Alkhouli M. F., Abbott J., Matthay M. A., Network N. A.; NHLBI ARDS Network (2015) Plasma soluble thrombomodulin levels are associated with mortality in the acute respiratory distress syndrome. Intensive Care Med. 41, 470–478 [Erratum] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moxon C. A., Chisala N. V., Mzikamanda R., MacCormick I., Harding S., Downey C., Molyneux M., Seydel K. B., Taylor T. E., Heyderman R. S., Toh C. H. (2015) Laboratory evidence of disseminated intravascular coagulation is associated with a fatal outcome in children with cerebral malaria despite an absence of clinically evident thrombosis or bleeding. J. Thromb. Haemost. 13, 1653–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietrich S., Falk C. S., Benner A., Karamustafa S., Hahn E., Andrulis M., Hegenbart U., Ho A. D., Dreger P., Luft T. (2013) Endothelial vulnerability and endothelial damage are associated with risk of graft-versus-host disease and response to steroid treatment. Biol. Blood Marrow Transplant. 19, 22–27 [DOI] [PubMed] [Google Scholar]
- 21.Opal S. M., LaRosa S. P. (2013) Recombinant human activated protein C as a therapy for severe sepsis: lessons learned? Am. J. Respir. Crit. Care Med. 187, 1041–1043 [DOI] [PubMed] [Google Scholar]
- 22.Martí-Carvajal A. J., Solà I., Gluud C., Lathyris D., Cardona A. F. (2012) Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst. Rev. 12, CD004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angus D. C. (2012) Drotrecogin alfa (activated)...a sad final fizzle to a roller-coaster party. Crit. Care 16, 107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernard G. R., Vincent J. L., Laterre P. F., LaRosa S. P., Dhainaut J. F., Lopez-Rodriguez A., Steingrub J. S., Garber G. E., Helterbrand J. D., Ely E. W., Fisher C. J. Jr.; Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group (2001) Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344, 699–709 [DOI] [PubMed] [Google Scholar]
- 25.Ranieri V. M., Thompson B. T., Barie P. S., Dhainaut J. F., Douglas I. S., Finfer S., Gårdlund B., Marshall J. C., Rhodes A., Artigas A., Payen D., Tenhunen J., Al-Khalidi H. R., Thompson V., Janes J., Macias W. L., Vangerow B., Williams M. D.; PROWESS-SHOCK Study Group (2012) Drotrecogin alfa (activated) in adults with septic shock. N. Engl. J. Med. 366, 2055–2064 [DOI] [PubMed] [Google Scholar]
- 26.Annane D., Timsit J. F., Megarbane B., Martin C., Misset B., Mourvillier B., Siami S., Chagnon J. L., Constantin J. M., Petitpas F., Souweine B., Amathieu R., Forceville X., Charpentier C., Tesnière A., Chastre J., Bohe J., Colin G., Cariou A., Renault A., Brun-Buisson C., Bellissant E., Investigators A. T.; APROCCHSS Trial Investigators (2013) Recombinant human activated protein C for adults with septic shock: a randomized controlled trial. Am. J. Respir. Crit. Care Med. 187, 1091–1097 [DOI] [PubMed] [Google Scholar]
- 27.Aikawa N., Shimazaki S., Yamamoto Y., Saito H., Maruyama I., Ohno R., Hirayama A., Aoki Y., Aoki N. (2011) Thrombomodulin alfa in the treatment of infectious patients complicated by disseminated intravascular coagulation: subanalysis from the phase 3 trial. Shock 35, 349–354 [DOI] [PubMed] [Google Scholar]
- 28.Fink M. P. (2013) Recombinant soluble thrombomodulin as an adjunctive treatment for sepsis and disseminated intravascular coagulation: relatively safe and possibly effective. Crit. Care Med. 41, 2221–2223 [DOI] [PubMed] [Google Scholar]
- 29.Vincent J. L., Ramesh M. K., Ernest D., LaRosa S. P., Pachl J., Aikawa N., Hoste E., Levy H., Hirman J., Levi M., Daga M., Kutsogiannis D. J., Crowther M., Bernard G. R., Devriendt J., Puigserver J. V., Blanzaco D. U., Esmon C. T., Parrillo J. E., Guzzi L., Henderson S. J., Pothirat C., Mehta P., Fareed J., Talwar D., Tsuruta K., Gorelick K. J., Osawa Y., Kaul I. (2013) A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit. Care Med. 41, 2069–2079 [DOI] [PubMed] [Google Scholar]
- 30.Shbaklo H., Holcroft C. A., Kahn S. R. (2009) Levels of inflammatory markers and the development of the post-thrombotic syndrome. Thromb. Haemost. 101, 505–512 [PubMed] [Google Scholar]
- 31.Andreou A. P., Crawley J. T. (2011) Thrombomodulin analogues for the treatment of ischemic stroke. J. Thromb. Haemost. 9, 1171–1173 [DOI] [PubMed] [Google Scholar]
- 32.Esmon C. T. (2001) Protein C anticoagulant pathway and its role in controlling microvascular thrombosis and inflammation. Crit. Care Med. 29, S48–S51 [DOI] [PubMed] [Google Scholar]
- 33.Van Iersel T., Stroissnig H., Giesen P., Wemer J., Wilhelm-Ogunbiyi K. (2011) Phase I study of Solulin, a novel recombinant soluble human thrombomodulin analogue. Thromb. Haemost. 105, 302–312 [DOI] [PubMed] [Google Scholar]
- 34.Yamakawa K., Aihara M., Ogura H., Yuhara H., Hamasaki T., Shimazu T. (2015) Recombinant human soluble thrombomodulin in severe sepsis: a systematic review and meta-analysis. J. Thromb. Haemost. 13, 508–519 [DOI] [PubMed] [Google Scholar]
- 35.Levi M. (2015) Recombinant soluble thrombomodulin: coagulation takes another chance to reduce sepsis mortality. J. Thromb. Haemost. 13, 505–507 [DOI] [PubMed] [Google Scholar]
- 36.Zaitsev S., Kowalska M. A., Neyman M., Carnemolla R., Tliba S., Ding B. S., Stonestrom A., Spitzer D., Atkinson J. P., Poncz M., Cines D. B., Esmon C. T., Muzykantov V. R. (2012) Targeting recombinant thrombomodulin fusion protein to red blood cells provides multifaceted thromboprophylaxis. Blood 119, 4779–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding B. S., Hong N., Christofidou-Solomidou M., Gottstein C., Albelda S. M., Cines D. B., Fisher A. B., Muzykantov V. R. (2009) Anchoring fusion thrombomodulin to the endothelial lumen protects against injury-induced lung thrombosis and inflammation. Am. J. Respir. Crit. Care Med. 180, 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greineder C. F., Chacko A. M., Zaytsev S., Zern B. J., Carnemolla R., Hood E. D., Han J., Ding B. S., Esmon C. T., Muzykantov V. R. (2013) Vascular immunotargeting to endothelial determinant ICAM-1 enables optimal partnering of recombinant scFv-thrombomodulin fusion with endogenous cofactor. PLoS One 8, e80110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carnemolla R., Greineder C. F., Chacko A. M., Patel K. R., Ding B. S., Zaitsev S., Esmon C. T., Muzykantov V. R. (2013) Platelet endothelial cell adhesion molecule targeted oxidant-resistant mutant thrombomodulin fusion protein with enhanced potency in vitro and in vivo. J. Pharmacol. Exp. Ther. 347, 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohan Rao L. V., Esmon C. T., Pendurthi U. R. (2014) Endothelial cell protein C receptor: a multiliganded and multifunctional receptor. Blood 124, 1553–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganguly K., Murciano J. C., Westrick R., Leferovich J., Cines D. B., Muzykantov V. R. (2007) The glycocalyx protects erythrocyte-bound tissue-type plasminogen activator from enzymatic inhibition. J. Pharmacol. Exp. Ther. 321, 158–164 [DOI] [PubMed] [Google Scholar]
- 42.Schouten M., van ’t Veer C., Poulussen N., Meijers J. C., Levi M., Esmon C. T., van der Poll T. (2015) The cytoprotective effects of endogenous activated protein C reduce activation of coagulation during murine pneumococcal pneumonia and sepsis. Thromb. Res. 135, 537–543 [DOI] [PubMed] [Google Scholar]
- 43.Liaw P. C., Ferrell G., Esmon C. T. (2003) A monoclonal antibody against activated protein C allows rapid detection of activated protein C in plasma and reveals a calcium ion dependent epitope involved in factor Va inactivation. J. Thromb. Haemost. 1, 662–670 [DOI] [PubMed] [Google Scholar]
- 44.Shuvaev V. V., Han J., Tliba S., Arguiri E., Christofidou-Solomidou M., Ramirez S. H., Dykstra H., Persidsky Y., Atochin D. N., Huang P. L., Muzykantov V. R. (2013) Anti-inflammatory effect of targeted delivery of SOD to endothelium: mechanism, synergism with NO donors and protective effects in vitro and in vivo. PLoS One 8, e77002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atochin D. N., Schepetkin I. A., Khlebnikov A. I., Seledtsov V. I., Swanson H., Quinn M. T., Huang P. L. (2016) A novel dual NO-donating oxime and c-Jun N-terminal kinase inhibitor protects against cerebral ischemia-reperfusion injury in mice. Neurosci. Lett. 618, 45–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun P., Li Q., Zhang Q., Xu L., Han J. Y. (2013) Upregulated expression of S100A8 in mice brain after focal cerebral ischemia reperfusion. World J. Emerg. Med. 4, 210–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carnemolla R., Patel K. R., Zaitsev S., Cines D. B., Esmon C. T., Muzykantov V. R. (2012) Quantitative analysis of thrombomodulin-mediated conversion of protein C to APC: translation from in vitro to in vivo. J. Immunol. Methods 384, 21–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pawlinski R., Wang J. G., Owens A. P. III, Williams J., Antoniak S., Tencati M., Luther T., Rowley J. W., Low E. N., Weyrich A. S., Mackman N. (2010) Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood 116, 806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu J., Ji Y., Zhang X., Drake M., Esmon C. T. (2009) Endogenous activated protein C signaling is critical to protection of mice from lipopolysaccaride-induced septic shock. J. Thromb. Haemost. 7, 851–856 [DOI] [PubMed] [Google Scholar]
- 50.Bongoni A. K., Klymiuk N., Wolf E., Ayares D., Rieben R., Cowan P. J. (2016) Transgenic expression of human thrombomodulin inhibits HMGB1-induced porcine aortic endothelial cell activation. Transplantation 100, 1871–1879 [DOI] [PubMed] [Google Scholar]
- 51.Tanaka J., Seki Y., Ishikura H., Tsubota M., Sekiguchi F., Yamaguchi K., Murai A., Umemura T., Kawabata A. (2013) Recombinant human soluble thrombomodulin prevents peripheral HMGB1-dependent hyperalgesia in rats. Br. J. Pharmacol. 170, 1233–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito T., Kawahara K., Okamoto K., Yamada S., Yasuda M., Imaizumi H., Nawa Y., Meng X., Shrestha B., Hashiguchi T., Maruyama I. (2008) Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler. Thromb. Vasc. Biol. 28, 1825–1830 [DOI] [PubMed] [Google Scholar]
- 53.Su E. J., Geyer M., Wahl M., Mann K., Ginsburg D., Brohmann H., Petersen K. U., Lawrence D. A. (2011) The thrombomodulin analog Solulin promotes reperfusion and reduces infarct volume in a thrombotic stroke model. J. Thromb. Haemost. 9, 1174–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morris G. P., Wright A. L., Tan R. P., Gladbach A., Ittner L. M., Vissel B. (2016) A comparative study of variables influencing ischemic injury in the Longa and Koizumi methods of intraluminal filament middle cerebral artery occlusion in mice. PLoS One 11, e0148503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryang Y. M., Dang J., Kipp M., Petersen K. U., Fahlenkamp A. V., Gempt J., Wesp D., Rossaint R., Beyer C., Coburn M. (2011) Solulin reduces infarct volume and regulates gene-expression in transient middle cerebral artery occlusion in rats. BMC Neurosci. 12, 113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geiger H., Pawar S. A., Kerschen E. J., Nattamai K. J., Hernandez I., Liang H. P., Fernández J. A., Cancelas J. A., Ryan M. A., Kustikova O., Schambach A., Fu Q., Wang J., Fink L. M., Petersen K. U., Zhou D., Griffin J. H., Baum C., Weiler H., Hauer-Jensen M. (2012) Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat. Med. 18, 1123–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suyama K., Kawasaki Y., Miyazaki K., Kanno S., Ono A., Ohara S., Sato M., Hosoya M. (2015) The efficacy of recombinant human soluble thrombomodulin for the treatment of shiga toxin-associated hemolytic uremic syndrome model mice. Nephrol. Dial. Transplant. 30, 969–977 [DOI] [PubMed] [Google Scholar]
- 58.Tanemura A., Kuriyama N., Azumi Y., Ohsawa I., Kishiwada M., Mizuno S., Usui M., Sakurai H., Tabata M., Isaji S. (2014) Thrombomodulin administration attenuates ischemia-reperfusion injury of the remnant liver after 70% hepatectomy in rats: simulated model of small-for-size graft in living donor liver transplantation. Transplant. Proc. 46, 1107–1111 [DOI] [PubMed] [Google Scholar]
- 59.Ikezoe T., Yang J., Nishioka C., Yokoyama A. (2015) Thrombomodulin alleviates murine GVHD in association with an increase in the proportion of regulatory T cells in the spleen. Bone Marrow Transplant. 50, 113–120 [DOI] [PubMed] [Google Scholar]
- 60.Villa C. H., Anselmo A. C., Mitragotri S., Muzykantov V. (2016) Red blood cells: supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv. Drug Deliv. Rev. S0169-409X(16)30058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murciano J. C., Medinilla S., Eslin D., Atochina E., Cines D. B., Muzykantov V. R. (2003) Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat. Biotechnol. 21, 891–896 [DOI] [PubMed] [Google Scholar]
- 62.Medinilla S., Murciano J. C., Krasik T., Bdeir K., Ganguly K., Muzykantov V. R., Cines D. B. (2005) Blood clearance and activity of erythrocyte-coupled fibrinolytics. J. Pharmacol. Exp. Ther. 312, 1106–1113 [DOI] [PubMed] [Google Scholar]
- 63.Murciano J. C., Higazi A. A.-R., Cines D. B., Muzykantov V. R. (2009) Soluble urokinase receptor conjugated to carrier red blood cells binds latent pro-urokinase and alters its functional profile. J. Control. Release 139, 190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaitsev S., Spitzer D., Murciano J.-C., Ding B.-S., Tliba S., Kowalska M. A., Marcos-Contreras O. A., Kuo A., Stepanova V., Atkinson J. P., Poncz M., Cines D. B., Muzykantov V. R., Marcos-Contreras O. A., Kuo A., Atkinson J. P., Stepanova V., Kowalska M. A., Tliba S., Ding B.-S., Zaitsev S., Spitzer D., Poncz M. (2010) Sustained thromboprophylaxis mediated by an RBC-targeted pro-urokinase zymogen activated at the site of clot formation. Blood 115, 5241–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villa C. H., Muzykantov V. R., Cines D. B. (2016) The emerging role for red blood cells in haemostasis: opportunity for intervention. ISBT Sci. Ser. 11, 158 [Google Scholar]
- 66.Paulitschke M., Nash G. B., Anstee D. J., Tanner M. J., Gratzer W. B. (1995) Perturbation of red blood cell membrane rigidity by extracellular ligands. Blood 86, 342–348 [PubMed] [Google Scholar]
- 67.Chasis J. A., Mohandas N., Shohet S. B. (1985) Erythrocyte membrane rigidity induced by glycophorin A-ligand interaction: evidence for a ligand-induced association between glycophorin A and skeletal proteins. J. Clin. Invest. 75, 1919–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khoory J., Estanislau J., Elkhal A., Lazaar A., Melhorn M. I., Brodsky A., Illigens B., Hamachi I., Kurishita Y., Ivanov A. R., Shevkoplyas S., Shapiro N. I., Ghiran I. C. (2016) Ligation of glycophorin A generates reactive oxygen species leading to decreased red blood cell function. PLoS One 11, e0141206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.