Abstract
We report here that autocrine production of human growth hormone (hGH) results in a phenotypic conversion of mammary carcinoma cells such that they exhibit the morphological and molecular characteristics of a mesenchymal cell, including expression of fibronectin and vimentin. Autocrine production of hGH resulted in reduced plakoglobin expression and relocalization of E-cadherin to the cytoplasm, leading to dissolution of cell-cell contacts and decreased cell height. These phenotypic changes were accompanied by an increase in cell motility, elevated activity of specific matrix metalloproteinases, and an acquired ability to invade a reconstituted basement membrane. Forced expression of plakoglobin significantly decreased mammary carcinoma cell migration and invasion stimulated by autocrine hGH. In vivo, autocrine hGH stimulated local invasion of mammary carcinoma cells concomitant with a prominent stromal reaction in comparison with well delineated and capsulated growth of mammary carcinoma cells lacking autocrine production of hGH. Thus, autocrine production of hGH by mammary carcinoma cells is sufficient for generation of an invasive phenotype. Therapeutic targeting of autocrine hGH may provide a mechanistic approach to prevent metastatic extension of human mammary carcinoma.
Mammary epithelial carcinoma is one of the leading causes of cancer-related mortality of female residents in Western countries, predominantly due to a high metastatic frequency (1). Identification of specific molecular determinants for metastatic mammary carcinoma and subsequent development of concordant biologic therapies are therefore of primary importance. During metastatic conversion, mammary carcinoma cells acquire the ability to invade surrounding tissue with subsequent dissemination to secondary organs. The acquisition of a migratory and invasive phenotype by cells of epithelial origin is associated with gain of mesenchymal characteristics concomitant with loss of the epithelial phenotype, a phenomenon referred to as epithelial-mesenchymal transition (2). This transition from epithelial to mesenchymal cell phenotype involves specific morphological and molecular alterations, including the loss of E-cadherin-mediated cell adhesion (2-4).
The human growth hormone (hGH) gene is expressed in the normal and neoplastic human mammary epithelial cell (5, 6). Elevated hGH gene expression is observed to be associated with metastatic mammary carcinoma cells (5), suggestive of a functional contribution of autocrine hGH to the metastatic process. We have concordantly previously demonstrated that autocrine hGH dramatically enhances spreading of mammary carcinoma cells on a collagen substrate (7). These observations suggest that autocrine production of hGH may contribute to conversion of human mammary epithelial cells to a mesenchymal phenotype. We demonstrate herein that in mammary carcinoma cells with epithelial morphology, autocrine production of hGH promotes mesenchymal cellular morphology, increased cell migration, and increased matrix metalloprotease (MMP) activity with subsequent acquisition of invasive behavior both in vitro and in vivo. This phenotypic conversion occurs as a result of autocrine hGH-mediated repression of plakoglobin expression. Thus, functional antagonism of hGH will provide an additional therapeutic approach to the control of metastatic mammary carcinoma.
Materials and Methods
Cell Lines and Cell Transfection. Mammary carcinoma cell line MCF-7 was obtained from the American Type Culture Collection and stably transfected with either the hGH gene (MCF-hGH cells) or with a translation-deficient hGH gene (MCF-MUT), as described (8). Neither MCF-MUT nor MCF-hGH cells produced detectable levels of IGF-1 (8). Therefore, all of the effects of autocrine production of hGH on mammary carcinoma cell behavior described herein represent the result of direct action of hGH under conditions studied. MCF-7 cells express the hGH receptor (8), and the effects of hGH are mediated by the hGH and not the prolactin receptor (9). Cells were cultured in RPMI medium 1640 supplemented with 10% FBS/100 units/ml penicillin/100 μg/ml streptomycin at 37°C in a humidified 5% CO2 incubator. MCF-MUT and MCF-hGH cells were transiently transfected with expression vector pRK5RS containing the human plakoglobin gene (kindly provided by Axel Ullrich, Max Planck Institute for Biochemistry, Martinsried, Germany) (10) by use of Effectene transfection reagent (Qiagen, Valencia, CA).
Analysis of Cell Morphology and Confocal Laser Scanning Microscopy. MCF-MUT and MCF-hGH cells were cultivated on tissue culture plastic dishes and on dishes precoated with reconstituted basement membrane (Matrigel, Becton Dickinson Labware) and photographed by using a Zeiss Axiovert 25 microscope equipped with a Nikon (Tokyo) Coolpix 995 digital camera. For localization of E-cadherin, cells were cultured on glass coverslips, on coverslips precoated with Matrigel, or on filters with 0.4-μm pore size (Becton Dickinson). After methanol fixation for 5 min at -20°C, cells were incubated with mouse monoclonal antibody against E-cadherin (Transduction Laboratories, Lexington, KY), followed by incubation with goat anti-mouse Alexa-488 antibody (Molecular Probes). Mouse anti-vimentin monoclonal antibody (Transduction Laboratories) was used for localization of vimentin. Cells were examined under a Carl Zeiss Axioplan microscope equipped with epifluorescence optics and a Bio-Rad MRC1040 confocal laser system.
In Vitro Cell Migration and Invasion Assays. Assays were performed in BD BioCoat Matrigel invasion chambers according to the manufacturer's instructions, with uncoated porous filters (8 μm pore size) for estimation of cell migration and filters precoated with Matrigel to examine cell invasion. Cells were serum-deprived for 24 h before experimentation. MCF-MUT and MCF-hGH cell suspension (5 × 105 cells per ml) was placed into the upper chamber in 0.5 ml of RPMI serum-free medium or with one of the following reagents: hGH (Novo-Nordisk, Copenhagen), generic Src family kinase inhibitor PP1, the specific Src kinase inhibitor PP2, and the structurally related noninhibitory PP3 (50 μM); broad-spectrum inhibitor of MMPs GM 6001 (10 μM), its inactive structural homologue (10 μM), or MMP-2/9 inhibitor (100 μM). RPMI medium 1640 supplemented with 10% FBS was placed in the lower chamber as a chemoattractant. After incubation for 30 h, cells that had migrated to the lower surface of the filters were fixed in methanol for 5 min at room temperature, visualized with Diff Quik reagent (Lab Aids, Narabeen, Australia) and counted. Values for cell migration or invasion were expressed as the average number of cells per microscopic field over four fields per one filter for triplicate experiments, as described previously (11). Experiments were repeated three to five times. All data are expressed as mean ± SE, and the comparison was made using Student's t test. For the wound migration assay, confluent monolayers of MCF-MUT and MCF-hGH cells were scraped with pipette tips, washed with PBS, and incubated in culture medium supplemented with 5% FBS for 75 h.
Preparation of Total RNA and RT-PCR. Total RNA was isolated from MCF-MUT and MCF-hGH cells using the RNeasy kit (Qiagen) and treated with DNase I using RNase-free DNase kit (Qiagen). RT-PCR of total RNA (1.6 μg) was performed using OneStep RT-PCR kit (Qiagen). Sequences of the oligonucleotide primers used for RT-PCR were as follows: 5′-AGGCAGGCTCAGCAAATG-3′ and 5′-TTAGGACGCTCATAAGTGTCACCC-3′ for fibronectin (260 bp), 5′-GGGGAATTCACTTCAGGCAGCCTCG-3′ and 5′-GGGAAGCTTTTCTATGTTTTCTGTCTA-3′ for occludin (510 bp), 5′-TTTGTACAGATGGGGTCTTGC-3′ and 5′-CAAGCCCACTTTTCATAGTTCC-3′ for E-cadherin (466 bp), 5′-GACAGACTTTACCCGAGGTAAAGGACCAC-3′ and 5′-ATAGCTTTGAACTCGCTGAGGGCCTGCAC-3′ for α-catenin (531 bp), 5′-AAGGTCTGAGGAGCAGCTTC-3′ and 5′-TGGACCATAACTGCAGCCTT-3′ for β-catenin (668 bp), 5′-CGACGGGCTGCAAAAGAT-3′ and 5′-ATGTGGTCTGCAGTGGGGTA-3′ for plakoglobin (1428 bp), and 5′-ATGATATCGCCGCGCTCG-3′ and 5′-CGCTCGGTGAGGATCTTCA-3′ for β-actin (581 bp). Amplified PCR products were visualized on a 1.2% agarose gel.
Western Blot Analysis. MCF-MUT and MCF-hGH cells were lysed with hot sample buffer containing 1% SDS and then centrifuged for 15 min at room temperature. Protein extracts were separated by 10% SDS/PAGE and transferred to nitrocellulose membranes (Hybond C-extra, Amersham Pharmacia). After blocking, membranes were incubated with primary monoclonal antibodies (all from Transduction Laboratories), followed by anti-mouse antibody conjugated with horseradish peroxidase (Upstate Biotechnology, Lake Placid, NY). Blots were stripped and reprobed by using anti-actin antibody (Santa Cruz Biotechnology). Protein bands were detected by the Phototype horse-radish peroxidase Western blot detection system (Cell Signaling Technology, Beverly, MA).
Substrate Gel Electrophoresis (Zymography). Secreted MMP-2 and MMP-9 were detected by the method of gelatin zymography (12) with several modifications. Conditioned media were obtained by incubation of MCF-MUT and MCF-hGH cells with serum-free medium for 24 h, and volume was normalized to protein concentration in total cell extracts. Samples were loaded on 7.5% SDS/PAGE gels containing 0.2% gelatin. Electrophoresis was performed under nonreducing conditions at 100 V and 4°C. Gels were washed in 2.5% Triton X-100, incubated in substrate buffer (50 mM Tris·HCl, pH 8.0/50 mM NaCl/10 mM CaCl2/0.05% Brij 35) for 40 h at 37°C, and stained with Coomassie stain solution (Bio-Rad).
Xenograft Analyses. Severe combined immunodeficient 4-week-old female mice were purchased from the animal holding facility of the National University of Singapore and acclimated for 10 days. MCF-MUT or MCF-hGH cells (5 × 106) were suspended in 100 μl of Matrigel (1:1) and injected into the first mammary fat pad of 10 mice without estrogen supplementation. Mice were killed 9 weeks after injection, and the whole primary tumor with adjacent skin, muscle, and connective tissues was collected, fixed in 4% paraformaldehyde in PBS, and paraffin-embedded. Deparaffinized and rehydrated 7-μm sections were visualized with hematoxylin/eosin for morphology. Human xenografts were identified by immunohistochemical localization with a cytokeratin antibody (CAM 5.2, BD Biosciences), specific for human, but not mouse, cytokeratin (11).
Results
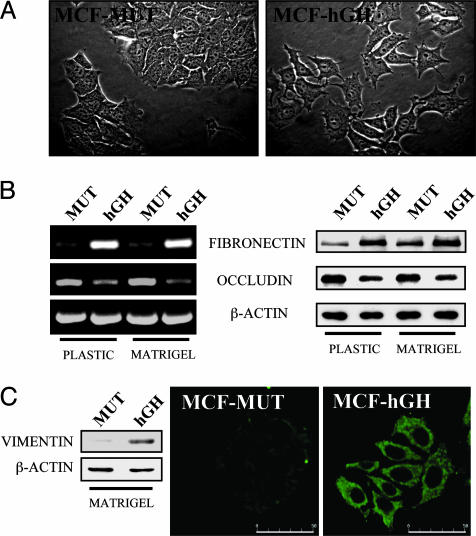
Autocrine Production of hGH in Mammary Carcinoma Cells Promotes a Mesenchymal Phenotype. During the progression of carcinoma toward a less differentiated and more malignant state, cells lose their epithelial characteristics and acquire a mesenchymal morphology, together with concomitant changes in gene expression (3, 4). Such a phenotypic conversion is referred to as epithelial-mesenchymal transition. We have previously demonstrated that autocrine production of hGH by human mammary cells dramatically altered their cellular morphology and ability to spread on a collagen substrate (7). Human mammary carcinoma (MCF-7) cells exhibit a well differentiated epithelioid morphology (4, 13). To determine the effect of autocrine hGH on mammary epithelial phenotype, we therefore used MCF-7 cells stably transfected with either the hGH gene (MCF-hGH cells) or with a translation-deficient hGH gene (MCF-MUT) (8). Cells were cultured on plastic dishes or on dishes precoated with the reconstituted basement membrane Matrigel. On both substrates, MCF-MUT cells exhibited epithelial characteristics (Fig. 1A) similar to the parental MCF-7 cell line (data not shown). In contrast, MCF-hGH cells demonstrated loss of cell-cell contact and formation of multiple cellular protrusions. Epithelial-mesenchymal transition is associated with down-regulation of epithelial marker genes such as E-cadherin, α- and γ-catenins, and occludin and an induction of mesenchymal markers such as fibronectin and vimentin (4, 14). MCF-hGH cells exhibited decreased levels of the mRNA and protein of the tight junction component occludin and increased expression of the extracellular matrix protein fibronectin in comparison to MCF-MUT cells (Fig. 1B). In MCF-hGH cells, we also observed an increased level of the intermediate filament vimentin (Fig. 1C), a structural protein in cells of mesenchymal origin, which is also expressed in poorly differentiated and highly invasive human breast carcinoma cells (4). Thus, autocrine production of hGH in human mammary carcinoma cells results in a phenotypic conversion to that reminiscent of a mesenchymal cell.
Fig. 1.
Autocrine hGH stimulation of human mammary carcinoma cells produces a mesenchymal phenotype. (A) The morphology of MCF-MUT and MCF-hGH cells cultured on Matrigel was examined by phase-contrast microscopy under ×400 magnification. (B) Fibronectin and occludin expression in MCF-MUT (MUT) and MCF-hGH cells (hGH) was examined by RT-PCR (Left) and Western blot (Right). (C) Western blot analysis and confocal laser scanning microscopic detection of vimentin expression in MCF-MUT and MCF-hGH cells cultured on Matrigel. (Bar = 50 μm.)
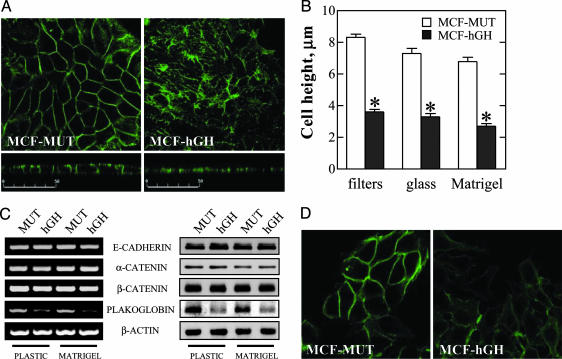
E-cadherin possesses a pivotal function in the establishment and stabilization of adherens junctions (AJs) that are essential for the maintenance of cell-cell adhesion in epithelial cells (2). In MCF-MUT cells cultured on permeable filters to ensure an epithelial phenotype, E-cadherin was localized as expected predominantly at the sites of cell-cell contact on the lateral membrane (Fig. 2A, horizontal and vertical optical sections). Production of autocrine hGH in MCF-hGH cells resulted in relocalization of E-cadherin to the cytoplasm and disruption of cell-cell junctions (Fig. 2 A), in agreement with the morphological changes observed in cells by phase-contrast microscopy (Fig. 1 A). Similar differences between MCF-MUT and MCF-hGH cells were also observed for cells cultured on glass or Matrigel-coated coverslips (data not shown). In addition to the loss of cell-cell contacts, MCF-hGH cells exhibited a flattened morphology as compared with MCF-MUT cells (Fig. 2 A, vertical sections) with resultant decrease in cell height (Fig. 2B).
Fig. 2.
Autocrine production of hGH in mammary carcinoma cells disrupts AJs. (A) MCF-MUT and MCF-hGH cells were grown on permeable filters, and E-cadherin was localized by immunofluorescence. Confocal laser scanning microscopic images of horizontal (Upper) and vertical (Lower) optical sections through cell layers are shown. (Bars = 50 μm.) (B) The height of MCF-MUT and MCF-hGH cells grown on permeable filters, glass coverslips, or coverslips precoated with Matrigel. Results represent average height of cell ± SEM (*, P < 0.01 for difference between MCF-MUT and MCF-hGH cell height). (C) RT-PCR (Left) and Western blot (Right) analysis of the expression of components of the AJs in MCF-MUT (MUT) and MCF-hGH (hGH) cells. (D) Confocal laser scanning microscopic analysis of plakoglobin expression and localization in MCF-MUT and MCF-hGH cells cultured on Matrigel-coated coverslips.
One of the causes of AJ dysfunction often detected in tumor cells of epithelial origin is decreased expression of E-cadherin, α-, and γ-catenin (plakoglobin) (15, 16). Upon examination of the expression of components of the AJs, we observed that neither mRNA nor protein levels of E-cadherin, α-catenin, and β-catenin were affected by autocrine production of hGH (Fig. 2C). However, both the mRNA and protein levels of plakoglobin were significantly decreased in MCF-hGH cells (Fig. 2 C and D). Thus, autocrine production of hGH in human mammary carcinoma cells resulted in decreased plakoglobin expression associated with relocalization of E-cadherin to the cytoplasm, disruption of cell-cell contacts, and conversion to a mesenchymal phenotype.
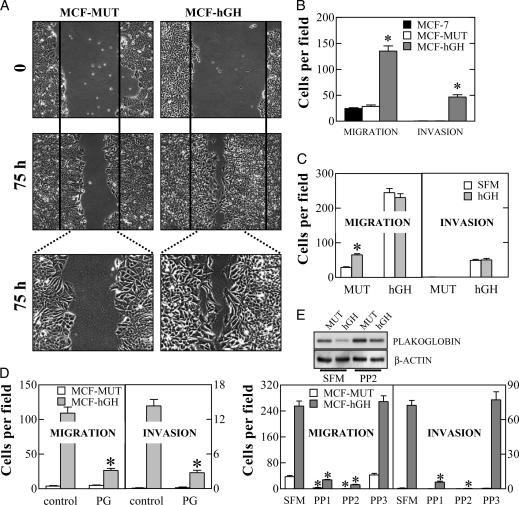
Autocrine Production of hGH in Mammary Carcinoma Cells Results in Increased Cell Migration and Acquisition of an Invasive Phenotype. The observed mesenchymal conversion of human mammary carcinoma cells expressing hGH was suggestive of an increased potential for migration and invasion. When cell motility was examined by the wound-healing assay, we observed that autocrine hGH did indeed stimulate cell migration with a more rapid closing of the wound than observed in MCF-MUT cells (Fig. 3A). Increased migration of MCF-hGH cells in comparison with MCF-MUT and the MCF-7 parental cell line was also confirmed by the Transwell assay (Fig. 3B). Noninvasive MCF-7 and MCF-MUT cells used in this study were unable to pass through Matrigel, whereas MCF-hGH cells exhibited significant ability to do so (Fig. 3B). Transient transfection of the hGH gene into MCF-7 cells was similarly able to promote cell migration and invasion, thereby negating the possibility of clonal selection artifact (data not shown). Interestingly, exogenously applied hGH (10-100 nM) also stimulated mammary carcinoma cell migration but was not able to produce an invasive phenotype (Fig. 3C).
Fig. 3.
Autocrine production of hGH in human mammary carcinoma cells results in increased cell motility and acquisition of an invasive phenotype. (A) Wound-healing assay. The wounded areas were examined under ×100 (Upper) or ×200 (Lower) magnification. (B) The motility and invasion of parental MCF-7, MCF-MUT, and MCF-hGH cells were determined by Transwell chamber assay (*, P < 0.01). (C) Effect of exogenous hGH (10 nM) on MCF-MUT and MCF-hGH cell migration and invasion (*, P < 0.01 for difference between nonstimulated cells and cells in the presence of 10 nM hGH). (D) MCF-MUT and MCF-hGH cells were transiently transfected with either an expression vector for plakoglobin (PG) or the control empty vector (control). Cell migration and invasion were examined by Transwell chamber assay (*, P < 0.01 for difference between migration and invasion of control and plakoglobin-transfected cells). (E) Effect of src inhibitors PP1 and PP2 and the inactive structural homologue PP3 on MCF-MUT and MCF-hGH cell migration and invasion (*, P < 0.01 for the difference between migration and invasion of cells in the presence of inhibitors in comparison with SFM). (Inset) Effect of PP2 on the level of expression of plakoglobin determined by Western blot analysis.
Increased invasiveness of certain mammary carcinoma cell lines has been correlated with decreased plakoglobin expression (13). We therefore examined whether decreased expression of plakoglobin in MCF-hGH cells was responsible for their migratory and invasive behavior. Forced expression of plakoglobin did not affect the migratory ability of MCF-MUT cells nor did it alter their noninvasive phenotype (Fig. 3D). In contrast, forced expression of plakoglobin in MCF-hGH cells resulted in a dramatic abrogation of the ability of MCF-hGH cells to migrate and invade through Matrigel (Fig. 3D). Thus, decreased plakoglobin expression as a consequence of autocrine production of hGH is required for conversion of a noninvasive mammary carcinoma cell to an invasive phenotype.
We subsequently examined the mechanism of autocrine hGH-stimulated down-regulation of plakoglobin expression. Human GH has previously been demonstrated to initiate cellular signal transduction by activation of either Janus kinase 2 (JAK2) or Src kinase (17). We observed that the inhibitor of the Src family of tyrosine kinases PP2 restored plakoglobin expression in MCF-hGH cells and inhibited the migration and Matrigel invasion of MCF-hGH cells (Fig. 3E). A pharmacologic inhibitor of JAK2, AG490, failed to alter the expression level of plakoglobin in MCF-hGH cells despite the ability of AG490 to inhibit autocrine hGH-stimulated STAT5-mediated transcription (data not shown). It is worth noting that Src kinase inhibitors PP1 and PP2 also suppressed MCF-MUT cell migration, in accordance with previous data demonstrating the importance of Src family kinases in cell motility (18). Thus, autocrine hGH repression of plakoglobin expression in mammary carcinoma cells is c-Src mediated.
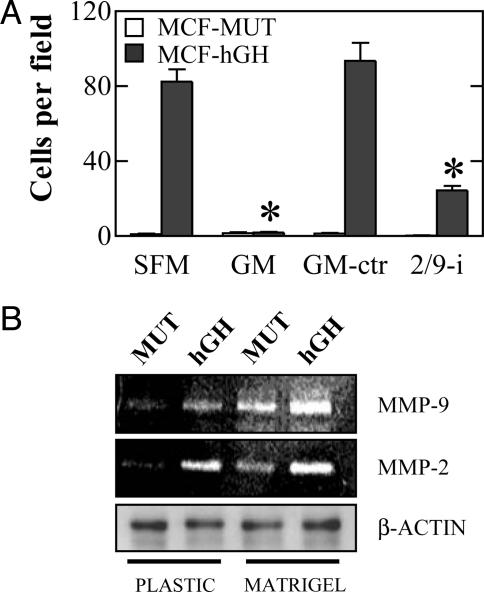
Autocrine hGH-Stimulated Mammary Carcinoma Cell Invasion Depends on MMP-2 and MMP-9 Activity. MMPs possess a pivotal role in the ability of cells to degrade extracellular matrix proteins to allow subsequent invasion to surrounding tissues (19). Autocrine hGH-stimulated mammary carcinoma cell invasion was completely prevented by exposure of cells to the MMP inhibitor GM 6001 (Ilomastat, Calbiochem), but not with its inactive structural homologue (Fig. 4A). Neither compound affected the migratory ability of MCF-MUT or MCF-hGH cells (data not shown). The activity of two gelatinases, MMP-2 and -9, has been positively correlated with the invasive capacity of mammary carcinoma cell lines (11, 20). A specific MMP-2/9 inhibitor significantly decreased invasion of MCF-hGH cells (Fig. 4A), indicative that MMP-2 and MMP-9 were primarily responsible for the autocrine hGH-stimulated conversion to an invasive phenotype. These data were concordant with increased activity of both gelatinases determined by zymography of the conditioned media of MCF-hGH cells compared with MCF-MUT cells (Fig. 4B). Thus, enhanced MMP-2 and MMP-9 activity is required for the establishment of an invasive phenotype by autocrine hGH in mammary carcinoma cells.
Fig. 4.
Effect of autocrine hGH on cell invasion depends on MMP-2 and MMP-9 activity. (A) Invasion of MCF-MUT and MCF-hGH cells was estimated in the presence of the broad-spectrum MMP inhibitor GM 6001 (GM), its structural homologue lacking inhibitor activity (GM-ctr), or the specific MMP-2/9 inhibitor (2/9-i) (*, P < 0.01 for the difference between MCF-hGH cell invasion in the presence of inhibitors in comparison with SFM). (B) Gelatin zymography analysis of MMP-2 and MMP-9 activity in the conditioned media of MCF-MUT (MUT) and MCF-hGH (hGH) cells. Volumes of conditioned media samples were normalized to the concentration of protein in total cell extracts (β-actin control).
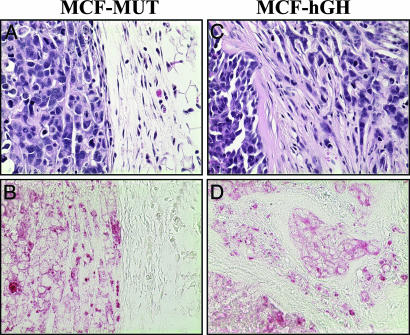
Autocrine Production of hGH in Mammary Carcinoma Cells Stimulates Local Invasion in Vivo. To determine whether hGH production resulted in increased local invasion of mammary carcinoma cells in vivo, we orthotopically implanted MCF-MUT and MCF-hGH cells into the first mammary fat pads of female severe combined immunodeficient mice. It has previously been demonstrated that wild-type MCF-7 cells were able to form tumors in nude mice (11). Similarly, both MCF-MUT- and MCF-hGH-injected severe combined immunodeficient mice developed visible solid tumors. Eight of 10 MCF-MUT-injected animals and 10 of 10 MCF-hGH-injected animals formed tumors. Mammary tumors formed by MCF-MUT cells (eight of eight) were macroscopically well defined and loosely attached to surrounding tissue. Histologically, they exhibited solid cellular architecture surrounded by a vascular and fibrous capsule, which formed a clear boundary with normal adjacent tissue (Fig. 5 A and B). The tumors formed by MCF-hGH cells were firmly attached to surrounding tissues, including the underlying axillary muscle. Histologically, the tumors did not display well defined margins from surrounding tissues, cells were observed to be diffusely infiltrated into surrounding tissues, and islands of tumor cells removed from the main tumor mass, reminiscent of invasive ductal carcinoma, were common (Fig. 5 C and D). In addition, the tumors formed by MCF-hGH cells (10 of 10) were accompanied by a pronounced fibroblastic stromal reaction to the tumor cells with consequent disruption of normal tissue architecture. Cells of human origin were identified by immunohistochemical labeling with a human specific cytokeratin antibody (Fig. 5 B and D). The above observations confirmed our in vitro data, and therefore autocrine production of hGH in human mammary carcinoma cells results in the acquisition of an invasive phenotype.
Fig. 5.
Autocrine production of hGH in mammary carcinoma cells stimulates local invasion in vivo. Tumors with adjacent tissues were stained by hematoxylin/eosin (A and C) or human specific anti-cytokeratin monoclonal antibody (B and D) and examined under ×400 magnification.
Discussion
We have demonstrated here that autocrine production of hGH by human mammary carcinoma cells results in the acquisition of a mesenchymal phenotype with subsequent enhanced migratory ability and invasion through extracellular matrices. In vivo, autocrine hGH consequently produced mammary carcinoma cells with local invasive capacity. These results are concordant with our recent demonstration that the level of hGH gene expression is increased in metastatic mammary carcinoma cells with extension to axillary lymph nodes as compared with mammary epithelial cells in the normal mammary gland and other less aggressive proliferative disorders (5). Thus, autocrine hGH represents a potential therapeutic target to abrogate local invasion of, and eventual progression to, metastatic mammary carcinoma.
Metastasis is a complex process that involves several coordinated events including shedding of cells from the primary tumor due to the loss of cell-cell contacts, increased cell motility, and production of proteases resulting in the ability to invade the surrounding tissue and enter the vascular or lymphatic system (19, 21). We observed that autocrine production of hGH by human mammary carcinoma cells resulted in the disruption of cell-cell contacts. In epithelial cells, AJs play a significant role in the maintenance of cell-cell adhesion. Loss of AJ function results in cell-cell dissociation (2) and is strongly implicated in tumor progression and metastasis (22, 23). We observed that autocrine hGH specifically decreased the expression of plakoglobin in human mammary carcinoma cells. This was accompanied by the relocalization of E-cadherin to the cytoplasm, dissolution of cell-cell contacts, and altered cellular morphology and motility. Plakoglobin is a multifunctional protein involved in transcriptional regulation and cell-cell adhesion. Structural homology between plakoglobin and β-catenin enables competitive binding to transcriptional coactivators, and it has been suggested that plakoglobin may regulate the β-catenin/Tcf transcriptional pathway (24, 25). Plakoglobin participates in the maintenance of mature AJs (26) and also regulates the organization of desmosomes (27), another type of adhesive cell-cell junction, formation of which is inhibitory to cell invasion (28). We observed that forced expression of plakoglobin in MCF-hGH cells prevented the autocrine hGH-stimulated increase in cell motility and invasion, indicating that repression of plakoglobin expression may represent one mechanism that autocrine hGH utilizes to execute conversion to a mesenchymal phenotype. These results are concordant with previous findings that suggested an inhibitory role of plakoglobin in cell invasion and tumor progression associated with an adverse clinical outcome (13, 16, 29). The precise mechanism mediating the tumor-suppressive effects of plakoglobin has not been determined. One possibility is that plakoglobin acts through stabilization of AJs and desmosomes. However, overexpression of plakoglobin suppressed tumorigenicity of both simian virus-40-transformed 3T3 cells expressing other components of AJ and human renal carcinoma cells in the absence of cadherins and α- and β-catenin, suggesting the existence of other mechanisms (30).
Conclusion
We have demonstrated that autocrine hGH production by human mammary carcinoma cells results in conversion to a mesenchymal and invasive phenotype. These observations are therapeutically relevant, given that mortality consequent to mammary carcinoma is predominantly associated with metastatic extension. A specific hGH receptor antagonist has already been approved for other purposes (31), and the clinical utility of antagonism of autocrine hGH as an adjuvant therapeutic tool for control of mammary carcinoma warrants investigation.
Acknowledgments
We thank Zhu Zhe and Gan Bin Qi for excellent technical help. We also thank Dr. A. Ullrich (Max Planck Institute for Biochemistry, Martinsreid, Germany) and Dr. A. Ben-Ze'ev (Weizman Institute of Science, Rehovot, Israel) for plakoglobin constructs and Dr. S. Oliferenko for helpful discussion. This work was supported by the National Science and Technology Board of Singapore, the National Research Centre in Growth and Development (New Zealand), and the School of Medicine Foundation (Auckland University, Auckland.
Abbreviations: hGH, human growth hormone; MMP, matrix metalloprotease; AJ, adherens junctions.
See Commentary on page 14992.
References
- 1.Baselga, J. & Norton, L. (2002) Cancer Cell 1, 319-322. [DOI] [PubMed] [Google Scholar]
- 2.Savagner, P. (2001) BioEssays 23, 912-923. [DOI] [PubMed] [Google Scholar]
- 3.Thiery, J. P. (2002) Nat. Rev. Cancer 2, 442-454. [DOI] [PubMed] [Google Scholar]
- 4.Sommers, C. L., Byers, S. W., Thompson, E. W., Torri, J. A. & Gelmann, E. P. (1994) Breast Cancer Res. Treat. 31, 325-335. [DOI] [PubMed] [Google Scholar]
- 5.Raccurt, M., Lobie, P. E., Moudilou, E., Garcia-Caballero, T., Frappart, L., Morel, G. & Mertani, H. C. (2002) J. Endocrinol. 175, 307-318. [DOI] [PubMed] [Google Scholar]
- 6.Mol, J. A., Henzen-Logmans, S. C., Hageman, P., Misdorp, W., Blankenstein, M. A. & Rijnberk, A. (1995) J. Clin. Endocrinol. Metab. 80, 3094-3096. [DOI] [PubMed] [Google Scholar]
- 7.Kaulsay, K. K., Mertani, H. C., Lee, K. O. & Lobie, P. E. (2000) Endocrinology 141, 1571-1584. [DOI] [PubMed] [Google Scholar]
- 8.Kaulsay, K. K., Mertani, H. C., Tornell, J., Morel, G., Lee, K. O. & Lobie, P. E. (1999) Exp. Cell Res. 250, 35-50. [DOI] [PubMed] [Google Scholar]
- 9.Kaulsay, K. K., Zhu, T., Bennett, W., Lee, K. O. & Lobie, P. E. (2001) Endocrinology 142, 767-777. [DOI] [PubMed] [Google Scholar]
- 10.Muller, T., Choidas, A., Reichmann, E. & Ullrich, A. (1999) J. Biol. Chem. 274, 10173-10183. [DOI] [PubMed] [Google Scholar]
- 11.Hazan, R. B., Phillips, G. R., Qiao, R. F., Norton, L. & Aaronson, S. A. (2000) J. Cell Biol. 148, 779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menshikov, M., Elizarova, E., Plakida, K., Timofeeva, A., Khaspekov, G., Beabealashvilli, R., Bobik, A. & Tkachuk, V. (2002) Biochem. J. 367, 833-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommers, C. L., Gelmann, E. P., Kemler, R., Cowin, P. & Byers, S. W. (1994) Cancer Res. 54, 3544-3552. [PubMed] [Google Scholar]
- 14.Reichert, M., Muller, T. & Hunziker, W. (2000) J. Biol. Chem. 275, 9492-9500. [DOI] [PubMed] [Google Scholar]
- 15.Ghadimi, B. M., Behrens, J., Hoffmann, I., Haensch, W., Birchmeier, W. & Schlag, P. M. (1999) Eur. J. Cancer 35, 60-65. [DOI] [PubMed] [Google Scholar]
- 16.Pantel, K., Passlick, B., Vogt, J., Stosiek, P., Angstwurm, M., Seen-Hibler, R., Haussinger, K., Thetter, O., Izbicki, J. R. & Riethmuller, G. (1998) J. Clin. Oncol. 16, 1407-1413. [DOI] [PubMed] [Google Scholar]
- 17.Zhu, T., Ling, L. & Lobie, P. E. (2002) J. Biol. Chem. 277, 45592-45603. [DOI] [PubMed] [Google Scholar]
- 18.Klinghoffer, R. A., Sachsenmaier, C., Cooper, J. A. & Soriano, P. (1999) EMBO J. 18, 2459-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCawley, L. J. & Matrisian, L. M. (2000) Mol. Med. Today 6, 149-156. [DOI] [PubMed] [Google Scholar]
- 20.Tester, A. M., Ruangpanit, N., Anderson, R. L. & Thompson, E. W. (2000) Clin. Exp. Metastasis 18, 553-560. [DOI] [PubMed] [Google Scholar]
- 21.Chambers, A. F., Groom, A. C. & MacDonald, I. C. (2002) Nat. Rev. Cancer 2, 563-572. [DOI] [PubMed] [Google Scholar]
- 22.Behrens, J., Mareel, M. M., van Roy, F. M. & Birchmeier, W. (1989) J. Cell Biol. 108, 2435-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirohashi, S. (1998) Am. J. Pathol. 153, 333-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winn, R. A., Bremnes, R. M., Bemis, L., Franklin, W. A., Miller, Y. E., Cool, C. & Heasley, L. E. (2002) Oncogene 21, 7497-7506. [DOI] [PubMed] [Google Scholar]
- 25.Miravet, S., Piedra, J., Miro, F., Itarte, E., Garcia, d. H. & Dunach, M. (2002) J. Biol. Chem. 277, 1884-1891. [DOI] [PubMed] [Google Scholar]
- 26.Lampugnani, M. G., Corada, M., Caveda, L., Breviario, F., Ayalon, O., Geiger, B. & Dejana, E. (1995) J. Cell Biol. 129, 203-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis, J. E., Wahl, J. K., III, Sass, K. M., Jensen, P. J., Johnson, K. R. & Wheelock, M. J. (1997) J. Cell Biol. 136, 919-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tselepis, C., Chidgey, M., North, A. & Garrod, D. (1998) Proc. Natl. Acad. Sci. USA 95, 8064-8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amitay, R., Nass, D., Meitar, D., Goldberg, I., Davidson, B., Trakhtenbrot, L., Brok-Simoni, F., Ben Ze'ev, A., Rechavi, G. & Kaufmann, Y. (2001) Am. J. Pathol. 159, 43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simcha, I., Geiger, B., Yehuda-Levenberg, S., Salomon, D. & Ben Ze'ev, A. (1996) J. Cell Biol. 133, 199-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drake, W. M., Parkinson, C., Besser, G. M. & Trainer, P. J. (2001) Trends Endocrinol. Metab. 12, 408-413. [DOI] [PubMed] [Google Scholar]