Significance
Warfare is one of the most pervasive problems among human societies, and understanding mechanisms involved in in-group cooperation and favoritism is of paramount importance. Wild chimpanzees share key features of humans’ intergroup conflict, in terms of in-group coordination, coalitionary support, and out-group hostility. The hormone oxytocin may regulate humans’ intergroup conflict, although tests in natural settings are lacking. We found strong evidence that, like in humans, oxytocin is involved in chimpanzee intergroup conflict. Both intergroup conflict anticipation and participation involved high urinary oxytocin levels, irrespective of intragroup affiliations or potential threat by rivals. These results are indicative of similar physiological processes involved in intergroup violence and intragroup support in both species, likely supporting behavior that is adaptive during intergroup conflicts.
Keywords: Pan troglodytes, cooperation, group cohesion, neuropeptide, parochial altruism
Abstract
Intergroup conflict is evident throughout the history of our species, ubiquitous across human societies, and considered crucial for the evolution of humans’ large-scale cooperative nature. Like humans, chimpanzee societies exhibit intragroup coordination and coalitionary support during violent intergroup conflicts. In both species, cooperation among group members is essential for individuals to gain access to benefits from engaging in intergroup conflict. Studies suggest that a contributive mechanism regulating in-group cooperation during intergroup conflicts in humans involves the neuropeptide hormone oxytocin, known to influence trust, coordination, and social cognition, although evidence from natural settings is lacking. Here, applying a noninvasive method, we investigate oxytocinergic system involvement during natural intergroup conflicts in wild chimpanzees. We found that chimpanzees of both sexes had significantly higher urinary oxytocin levels immediately before and during intergroup conflict compared with controls. Also, elevated hormone levels were linked with greater cohesion during intergroup conflicts, rather than with the level of potential threat posed by rival groups, intragroup affiliative social interactions, or coordinated behavior alone. Thus, the oxytocinergic system, potentially engendering cohesion and cooperation when facing an out-group threat, may not be uniquely human but rather a mechanism with evolutionary roots shared by our last common ancestor with chimpanzees, likely expediting fitness gains during intergroup conflict.
Recent evolutionary models suggest that parochial altruism, the link between in-group favoritism and the benefit of others at a cost to oneself, is key to understanding the evolution of humans’ cooperative traits and propensity for intergroup violence (1, 2). Intergroup conflict is ubiquitous across human societies (2), repeatedly leading to devastating results of prejudice, war, and genocide (2, 3). Individuals contribute to these patterns both by supporting in-group members and acting with hostility toward the out-group. When such a combination contributes to success in intergroup conflicts, parochial altruism could have evolved (1), and biological mechanisms that sustain and promote it are likely adaptive (4).
One such proposed biological mechanism involves the neuropeptide hormone oxytocin, previously linked with various aspects of human sociality, particularly the development of mother–offspring bonds, but also tolerance, coordination, and cooperation between nonkin adults (5–7). Owing to its anxiolytic and prosocial effects, oxytocin is proposed to facilitate cooperation during risk, a mechanism potentially co-opted from maternal defense circuitry (4). Intranasal administration of oxytocin enhances in-group cooperation and trust (8, 9) and out-group defensive, but not offensive, competition in men (8). This suggests that oxytocin triggers a “tend and defend” form of parochial altruism, accentuating cooperative behavior toward the in-group as well as defensive behavior toward out-groups (4). However, these results were obtained in laboratory settings using intergroup social dilemma games and focusing on human male participants. Few, if any, studies have involved intergroup contexts and oxytocin in captive or wild nonhuman animals. Therefore, additional evidence is essential for corroborating oxytocinergic system involvement in an ecologically relevant setting.
Wild chimpanzees in almost all long-term field sites engage in competitive intergroup conflicts (10, 11), which are characterized by two sets of behavior (Movie S1), intergroup encounters and border patrols. Intergroup encounters (direct out-group contact) are characterized by coordinated attacks, with synchronous vocalizations and charges toward and combat against chimpanzees from rival groups (12, 13). In border patrols (no direct out-group contact), chimpanzees’ typical foraging and traveling movements change to become more cohesive and quiet while they vigilantly scout the peripheral areas of their territory, often continuing for several kilometers (12, 13). Feeding and vocalizations are minimal. Travel is slow, often in single file, interrupted by frequent pauses in which chimpanzees may sniff forest items, such as out-group chimpanzee feces or food remnants, and are unusually alert to sounds beyond the immediate group (12, 13). Individuals appear to search for signs indicative of recent rival-group chimpanzee presence, such as vocal presence, or recent physical presence, potentially assessing the strength of their opponents.
Chimpanzee group defense is energetically costly in terms of reduced foraging and increased traveling (14), and risky, as it may lead to injury or death (10). Successful attacks on rival groups, however, potentially increase the territory size of the in-group and the reproductive output of its members (14, 15), thereby increasing fitness. Access to benefits of intergroup conflict can be maintained through cooperation, providing benefits to both actor and recipient regardless of potential short-term costs to the actor (16). Chimpanzee in-group behavior during intergroup conflict is considered to be cooperative (11, 17), as it encompasses prolonged coordination and cohesion, as well as coalitionary support, which can involve individuals safe from the out-group running forward to defend an in-group member under attack from the out-group (11). In humans, cohesion and support also intensify under life-threatening situations, such as war (18). These parallels highlight key features shared by chimpanzee and human group defense and intergroup conflict (14). Also similar to humans, the oxytocinergic system in chimpanzees is involved in prosocial and cooperative behavior between both kin and nonkin group members, such as grooming (19) and food sharing (20), suggesting parallels in oxytocinergic system involvement between humans and chimpanzees.
Here we investigate whether chimpanzees’ in-group behavior during border patrols and intergroup encounters (intergroup conflict) enhances group cohesion and involves the oxytocinergic system. Due to the competitive, aggressive nature of chimpanzee intergroup interactions and in accordance with the human literature, we use the term intergroup conflict (2) to describe border patrols and intergroup encounters. We assessed (i) whether chimpanzees’ group cohesion is greater during intergroup conflicts than control periods (“defection” model). Chimpanzees live in a fission–fusion social system in which individuals from the same group split into small and dynamic subgroups of varying size, composition, and duration (12). Accordingly, we measured fissions of adult individuals as an estimate of defection. Fission may be affected by ecological, social, and spatial factors (21). Nonetheless, if intergroup conflicts require cohesion, we expected fewer fissions during intergroup conflicts than during control periods, regardless of subgroup size and composition, which may affect fission patterns, or proximity to territory border areas, where intergroup encounters often occur. We also investigated whether in-group activity during border patrols and intergroup encounters engaged the oxytocinergic system. The oxytocinergic system influences attributes likely to assist cooperation and hence successful intergroup conflict, such as in-group trust and coordination (5–7). Accordingly, we hypothesized that high oxytocin levels immediately before and during intergroup conflicts would be adaptive when influencing group cooperation. We expected both (ii) high oxytocin levels during intergroup conflicts (“event” model) and (iii) high anticipatory oxytocin levels before border patrol initiation (“anticipation” model). We expected high oxytocin levels to persist, even when controlling for the occurrence of in-group affiliative behavior, proximity to border areas, or behavior involving in-group coordination in the absence of out-group threat, specifically hunting events where chimpanzees coordinate to capture monkeys (22).
We investigated our hypotheses using a within-subjects design, sampling naturally occurring events during intra- and intergroup interactions in wild chimpanzees (Pan troglodytes verus) in the Taï National Park, Ivory Coast. We conducted focal animal sampling (23) for 20 adult male and female chimpanzees of two neighboring groups, and measured the oxytocin concentration of urine samples from all individuals using an established method to sample specific events (19, 20).
Results and Discussion
As with humans, chimpanzee group defense requires coordination and coalitionary support to be effective (11, 17), and when such cooperative behavior is maintained, access to benefits is more likely. For instance, in chimpanzees, intergroup lethal violence occurs predominantly at times of power imbalance in favor of attackers (10), and thus group cohesion may reduce the likelihood of suffering costs. To investigate the influence of intergroup conflict on group cohesion (defection model), we counted fissions per individual as an estimate of defection (Table 1). We determined for each adult individual the number of times it left the subgroup from the onset to the end of the intergroup conflict (n = 23), or during a matched control period (n = 23). Controls were defined as periods with similar duration, subgroup size, and composition on days that did not include intergroup conflicts or hunting behavior. We found similar numbers of leaves per individual during intergroup conflict periods that included direct interactions with rival groups (intergroup encounters n = 11) and those that did not (border patrols n = 12) (Table 1). Accordingly, we fitted a Poisson generalized linear mixed model (GLMM) (24) to analyze how the response varied between both types of intergroup conflict and controls. We controlled for group identity, proximity to border areas where encounters with rivals are more likely, and subgroup duration.
Table 1.
Behavioral fission data: Comparison of fission numbers between intergroup conflict and control periods
| Fission events* | Individual fissions† | Subgroup size‡ | Proportion of adult leaves§ | |||||||
| Events | n | Mean | SD | n | Mean | SD | Mean | SD | Mean | SD |
| Control, n = 23 | 65 | 2.826 | 2.145 | 312 | 1.079 | 0.974 | 10.043 | 3.268 | 0.697 | 0.329 |
| Intergroup conflict, n = 23 | 25 | 1.089 | 1.276 | 91 | 0.365 | 0.694 | 10.304 | 3.096 | 0.257 | 0.279 |
| Border patrol, n = 12 | 16 | 1.454 | 0.934 | 44 | 0.382 | 0.539 | 9.333 | 2.269 | 0.341 | 0.234 |
| Intergroup encounter, n = 11 | 9 | 0.750 | 1.484 | 47 | 0.350 | 0.806 | 11.363 | 3.613 | 0.173 | 0.298 |
Adult individuals leaving the subgroup within at most 1 min were counted as having left during the same fission event.
Number of times each adult individual left the subgroup.
Subgroup size at the start of each event.
The proportion of adult individuals that left the subgroup at least once to the total adult individuals present in the subgroup.
We found that during intergroup conflicts, individuals were significantly less likely to leave the subgroup than during control periods (GLMM, likelihood ratio test: χ2 = 13.484, df = 1, P < 0.001; estimate ± SE −1.732 ± 0.446; Table S1). This effect was not driven by proximity to border areas, where encounters with rivals are more likely. We also found an effect of group identity (Table S1), with South group individuals being significantly less likely to leave the subgroup than East group individuals; however, this parameter was associated with some instability. Contextual variation in defection numbers showed that, in comparison with control contexts, intergroup conflict promoted group cohesion. Similarly, human soldiers increase their cohesion and affiliation when going into battle (18). Although cohesion is fundamental in promoting group cooperation, it remains unclear whether intergroup hostilities have contributed to the proliferation of chimpanzee cooperative capacities, as is theorized for humans.
Table S1.
Defection model: Effect of intergroup conflict on subgroup cohesion
| Term | Estimate | SE | CIlower | CIupper | χ2* | P |
| Intercept | −0.475 | 0.241 | −0.962 | −0.026 | – | – |
| Intergroup conflict† | −1.732 | 0.446 | −2.586 | −0.809 | 13.484 | <0.001 |
| Group‡,§ | −1.358 | 0.671 | −3.113 | −0.311 | 4.700 | 0.030 |
| Proximity¶ | 0.184 | 0.201 | −0.190 | 0.587 | 0.829 | 0.363 |
Statistically significant results (P ≤ 0.05) appear in bold.
Degrees of freedom are 1.
,‡χ2 and P values refer to comparison with the reference categories †control and ‡East group.
Group identity was associated with large uncertainty due to the small sample size of South group.
z-transformed, mean ± SD of the original variables: 72.57 ± 24.19 (range 5 to 99).
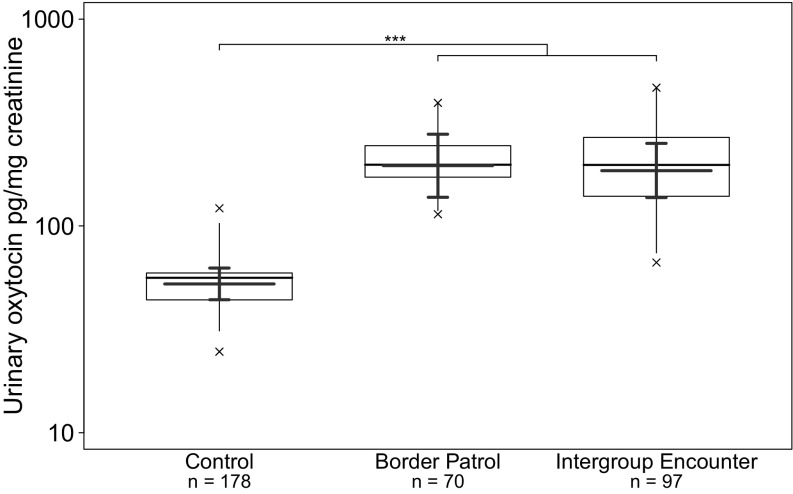
At an endocrinological level, we investigated whether chimpanzee in-group activity during border patrols and intergroup encounters engaged the oxytocinergic system. In case a direct contact with rival groups occurred within the time window for oxytocin secretion, urine samples were assigned as intergroup encounters, and those that did not include any contact as border patrols. Both types of intergroup conflict had a significant positive effect on log-transformed urinary oxytocin levels (pg/mg creatinine), in contrast to control situations with no positive social interactions [“type” model; linear mixed model (LMM), likelihood ratio test: χ2 = 44.600, df = 2, P < 0.001; Fig. S1 and Table S2]. However, log-transformed urinary oxytocin levels did not differ between the two types of intergroup conflict (Table S3; see SI Methods for tests separating these two contexts). Thus, although border patrols represent only a covert out-group threat, they are subject to similar urinary oxytocin and cohesion levels as time periods including intergroup encounters. Hence, we combine them as one context in the following event model (see SI Methods, Fig. S2, and Table S4 for tests only including intergroup encounters).
Fig. S1.
Effects of the type of intergroup conflict on urinary oxytocin levels in wild chimpanzees in East and South groups (n = 345 samples, 16 subjects, 194 events). Shown are medians (thin horizontal lines), quartiles (boxes), percentiles (2.5 and 97.5%; vertical lines), minimum and maximum (laying crosses), as well as the fitted model and its 95% confidence intervals (thick lines). ***P < 0.001.
Table S2.
Intergroup conflict type model: Effect of the type of intergroup conflict, namely border patrol or intergroup encounter, on urinary oxytocin levels, log-transformed
| Term | Estimate | SE | χ2* | P |
| Intercept | 4.399 | 0.214 | – | – |
| Test predictor | ||||
| Border patrol† | 1.321 | 0.221 | 22.227 | <0.001 |
| Intergroup encounter† | 1.261 | 0.189 | 37.522 | <0.001 |
| Control predictors | ||||
| Group‡ | −0.358 | 0.134 | 7.014 | 0.008 |
| Sex§ | −0.359 | 0.209 | 2.788 | 0.095 |
| Proximity¶ | −0.058 | 0.068 | 0.714 | 0.398 |
| Subgroup size# | 0.077 | 0.054 | 2.010 | 0.156 |
| Rank|| | 0.086 | 0.099 | 0.614 | 0.433 |
Statistically significant results (P ≤ 0.05) appear in bold.
Degrees of freedom are 1.
,‡,§χ2 and P values refer to comparison with the reference categories †control, ‡East group, and §female.
,#,||z-transformed, mean ± SD of the original variables: ¶65.86 ± 29.35 (range 5 to 99), #11.45 ± 5.96, and ||0.61 ± 0.24 (range 0 to 1, with 1 being the highest social rank).
Table S3.
Post hoc intergroup conflict type model: Post hoc analysis of the effect of the type of intergroup conflict, namely border patrol or intergroup encounter, on urinary oxytocin levels, log-transformed
| Term | Estimate | SE | χ2* | P |
| Intercept | 5.660 | 0.248 | – | – |
| Test predictor | ||||
| Control† | −1.261 | 0.189 | 39.372 | <0.001 |
| Intergroup Encounter† | 0.060 | 0.192 | 0.097 | 0.756 |
| Control predictors | ||||
| Group‡ | −0.358 | 0.134 | 7.014 | 0.008 |
| Sex§ | −0.359 | 0.209 | 2.788 | 0.095 |
| Proximity¶ | −0.058 | 0.068 | 0.714 | 0.398 |
| Subgroup size# | 0.077 | 0.054 | 2.010 | 0.156 |
| Rank|| | 0.086 | 0.099 | 0.614 | 0.433 |
Statistically significant results (P ≤ 0.05) appear in bold.
Degrees of freedom are 1.
,‡,§χ2 and P values refer to comparison with the reference categories †border patrol, ‡East group, and §female.
,#,||z-transformed.
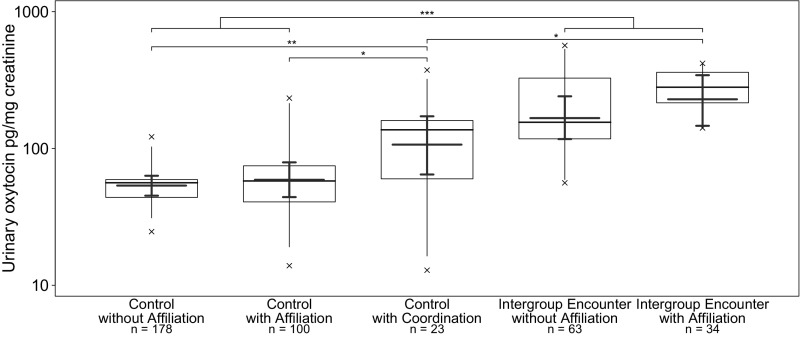
Fig. S2.
Effects of intergroup encounters with and without in-group affiliation on urinary oxytocin levels in wild chimpanzees in East and South groups (n = 398 samples, 20 subjects, 282 events). ***P < 0.001, **P < 0.01, *P < 0.05.
Table S4.
Reduced event model: Effect of intergroup encounters and in-group affiliation on urinary oxytocin levels, log-transformed
| Term | Estimate | SE | CIlower | CIupper | χ2* | P |
| Intercept | 4.484 | 0.224 | 4.037 | 4.986 | – | – |
| Test predictor levels | ||||||
| Control with affiliation† | 0.097 | 0.163 | −0.241 | 0.414 | 0.340 | 0.560 |
| Control with coordination† | 0.689 | 0.249 | 0.198 | 1.200 | 4.902 | 0.027 |
| Intergroup encounter without affiliation† | 1.136 | 0.199 | 0.723 | 1.559 | 28.920 | <0.001 |
| Intergroup encounter with affiliation† | 1.452 | 0.226 | 0.949 | 1.923 | 23.401 | <0.001 |
| Control predictors | ||||||
| Group‡ | −0.349 | 0.131 | −0.624 | −0.075 | 6.521 | 0.011 |
| Sex§ | −0.438 | 0.214 | −0.905 | −0.015 | 3.943 | 0.047 |
| Proximity¶ | −0.054 | 0.058 | −0.173 | 0.064 | 0.858 | 0.354 |
| Subgroup size# | 0.116 | 0.058 | 0.001 | 0.224 | 3.715 | 0.054 |
| Rank|| | 0.174 | 0.109 | −0.054 | 0.391 | 2.351 | 0.125 |
Statistically significant results (P ≤ 0.05) appear in bold.
Degrees of freedom are 1.
,‡,§χ2 and P values refer to comparison with the reference categories †control without affiliation, ‡East group, and §female.
,#,||z-transformed, mean ± SD of the original variables: ¶56.46 ± 28.43 (range 5 to 99), #11.21 ± 5.95, and ||0.618 ± 0.24 (range 0 to 1, with 1 being the highest social rank).
Here we tested the impact of different events and confounding factors on urinary oxytocin levels (event model). Because affiliation frequently occurs during in-group out-group contexts and might impact urinary oxytocin levels (19), we divided intergroup conflict samples into two categories: group members participating in intergroup conflict (i) without in-group affiliation (11 subjects, n = 103 samples of 37 events), or (ii) with in-group affiliation (grooming or play with multiple partners; 15 subjects, n = 64 samples of 24 events). We contrasted intergroup conflict with three control events excluding intergroup conflict or food sharing, as the latter shows association with high oxytocin levels in chimpanzees (20): (i) 90-min time periods in which no positive social interactions, except vocalizations, occurred (“control without affiliation”; 20 subjects, n = 178 samples of 150 events); (ii) multipartner grooming of at least 10-min duration (“control with affiliation”; 19 subjects, n = 100 samples of 87 events); and (iii) participation in group hunting of monkeys, a coordinated behavior (22) that does not involve in-group out-group contexts (“control with coordination”; 9 subjects, n = 23 samples of 17 events). The two latter contexts allowed us to control for the in-group affiliative and coordinated behavior often observed during intergroup conflicts, respectively. All samples relating to a target context were collected within the time window of oxytocin excretion into urine at least 15 min after the start and up to 60 min after the end of interactions (19, 20). We fitted an LMM (event model) (24) to test for the influence of intergroup conflict, coordination, and affiliation on log-transformed urinary oxytocin levels (pg/mg creatinine). To control for other factors that might influence hormone levels, we included individuals’ sex and rank, subgroup size, group identity, and proximity to border areas to evaluate potential risk. Our dataset for the event model included 468 samples from 20 different individuals from 296 different events.
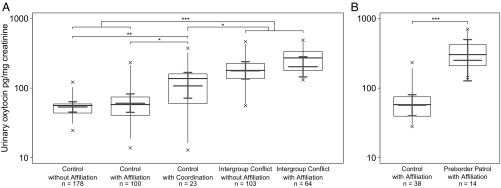
Overall, the full-null model comparison was significant (LMM, likelihood ratio test: χ2 = 51.253, df = 4, P < 0.001; Fig. 1A and Table 2). More specifically, intergroup conflicts with and without affiliation were associated with higher urinary oxytocin levels than the three controls (Table 2 and Tables S5, S6, and S7). We also found a positive effect of group coordinated hunting behavior on urinary oxytocin levels compared with both the control with and without affiliation, although less pronounced than the effect of intergroup conflict (Table S7). These effects were neither driven by individual rank or sex, subgroup size, nor proximity to border areas. However, we found a group effect, with East group having higher urinary oxytocin levels than South group (Table 2), despite having similar group sizes and little ecological variance or genetic differentiation (25). Moreover, in post hoc analyses comparing intergroup conflict with and without affiliation, we found no significant effect of affiliation on urinary oxytocin levels within this context (LMM: χ2 = 0.136, df = 1, P = 0.334; Table S5).
Fig. 1.
(A) Effects of intergroup conflict with and without in-group affiliation on urinary oxytocin levels in wild chimpanzees in East and South groups (n = 468 samples, 20 subjects, 296 events). (B) Effects of imminent intergroup conflict in East group chimpanzees on urinary oxytocin levels (n = 52 samples, 9 subjects, 43 events). Shown are medians (thin horizontal lines), quartiles (boxes), percentiles (2.5 and 97.5%; vertical lines), minimum and maximum (laying crosses), as well as the fitted model and its 95% confidence intervals (thick lines). ***P < 0.001, **P < 0.01, *P < 0.05.
Table 2.
Event model: Effect of intergroup conflict and in-group affiliation on urinary oxytocin levels, log-transformed
| Term | Estimate | SE | CIlower | CIupper | χ2* | P |
| Intercept | 4.353 | 0.212 | 3.943 | 4.783 | – | – |
| Test predictor levels | ||||||
| Control with affiliation† | 0.122 | 0.160 | −0.199 | 0.411 | 0.549 | 0.459 |
| Control with coordination† | 0.698 | 0.222 | 0.254 | 1.137 | 6.556 | 0.010 |
| Intergroup conflict without affiliation† | 1.197 | 0.171 | 0.840 | 1.576 | 36.044 | <0.001 |
| Intergroup conflict with affiliation† | 1.333 | 0.193 | 0.928 | 1.718 | 27.298 | <0.001 |
| Control predictors | ||||||
| Group‡ | −0.319 | 0.127 | −0.579 | −0.048 | 5.916 | 0.015 |
| Sex§ | −0.307 | 0.200 | −0.717 | 0.054 | 2.292 | 0.130 |
| Proximity¶ | −0.035 | 0.057 | −0.153 | 0.070 | 0.378 | 0.538 |
| Subgroup size# | 0.082 | 0.049 | −0.010 | 0.186 | 2.714 | 0.099 |
| Rank|| | 0.077 | 0.100 | −0.105 | 0.269 | 0.574 | 0.449 |
Statistically significant results (P ≤ 0.05) appear in bold. CI, confidence interval.
Degrees of freedom are 1.
,‡,§χ2 and P values refer to comparison with the reference categories: †control without affiliation, ‡East group, and §female.
,#,||z-transformed, mean ± SD of the original variables: ¶61.59 ± 29.21 (range 5 to 99), #11.77 ± 5.87, and ||0.62 ± 0.24 (range 0 to 1, with 1 being the highest social rank).
Table S5.
Post hoc event model 1: Post hoc analysis of the effect of intergroup conflict and in-group affiliation on urinary oxytocin levels, log-transformed, with intergroup conflict without affiliation as the reference category
| Term | Estimate | SE | χ2* | P |
| Intercept | 5.549 | 0.236 | – | – |
| Test predictor levels | ||||
| Control without affiliation† | −1.197 | 0.171 | 43.294 | <0.001 |
| Control with affiliation† | −1.075 | 0.208 | 22.343 | <0.001 |
| Control with coordination† | −0.499 | 0.245 | 4.085 | 0.043 |
| Intergroup conflict with affiliation† | 0.136 | 0.14 | 0.933 | 0.334 |
| Control predictors | ||||
| Group‡ | −0.319 | 0.127 | 5.916 | 0.015 |
| Sex§ | −0.307 | 0.200 | 2.292 | 0.130 |
| Proximity¶ | −0.035 | 0.057 | 0.378 | 0.538 |
| Subgroup size# | 0.082 | 0.049 | 2.714 | 0.099 |
| Rank|| | 0.077 | 0.100 | 0.574 | 0.449 |
Statistically significant results (P ≤ 0.05) appear in bold.
Degrees of freedom are 1.
,‡,§χ2 and P values refer to comparison with the reference categories †intergroup conflict without affiliation, ‡East group, and §female.
,#,||z-transformed.
Table S6.
Post hoc event model 2: Post hoc analysis of the effect of intergroup conflict and in-group affiliation on urinary oxytocin levels, log-transformed, with intergroup conflict with affiliation as the reference category
| Term | Estimate | SE | χ2* | P |
| Intercept | 5.677 | 0.242 | – | – |
| Test predictor levels | ||||
| Control without affiliation† | −1.328 | 0.187 | 44.160 | <0.001 |
| Control with affiliation† | −1.205 | 0.219 | 25.042 | <0.001 |
| Control with coordination† | −0.633 | 0.256 | 5.973 | 0.015 |
| Intergroup conflict without affiliation† | −0.128 | 0.130 | 0.968 | 0.325 |
| Control predictors | ||||
| Group‡ | −0.321 | 0.126 | 6.110 | 0.013 |
| Sex§ | −0.302 | 0.198 | 2.274 | 0.132 |
| Proximity¶ | −0.037 | 0.058 | 0.411 | 0.521 |
| Subgroup size# | 0.082 | 0.049 | 2.770 | 0.096 |
| Rank|| | 0.072 | 0.098 | 0.521 | 0.471 |
Statistically significant results (P ≤ 0.05) appear in bold.
Degrees of freedom are 1.
,‡,§χ2 and P values refer to comparison with the reference categories †intergroup conflict with affiliation, ‡East group, and §female.
,#,||z-transformed.
Table S7.
Post hoc event model 3: Post hoc analysis of the effect of intergroup conflict and in-group affiliation on urinary oxytocin levels, log-transformed, with control with coordination as the reference category
| Term | Estimate | SE | χ2* | P |
| Intercept | 5.051 | 0.283 | – | – |
| Test predictor levels | ||||
| Control without affiliation† | −0.698 | 0.222 | 9.517 | 0.002 |
| Control with affiliation† | −0.576 | 0.255 | 5.000 | 0.025 |
| Intergroup conflict without affiliation† | 0.499 | 0.245 | 4.085 | 0.043 |
| Intergroup conflict with affiliation† | 0.635 | 0.261 | 5.799 | 0.016 |
| Control predictors | ||||
| Group‡ | −0.319 | 0.127 | 5.916 | 0.015 |
| Sex§ | −0.307 | 0.200 | 2.292 | 0.130 |
| Proximity¶ | −0.035 | 0.057 | 0.378 | 0.538 |
| Subgroup size# | 0.082 | 0.049 | 2.714 | 0.099 |
| Rank|| | 0.077 | 0.100 | 0.574 | 0.449 |
Statistically significant results (P ≤ 0.05) appear in bold.
Degrees of freedom are 1.
,‡,§χ2 and P values refer to comparison with the reference categories †control with coordination, ‡East group, and §female.
,#,||z-transformed.
When facing rival groups, chimpanzee in-group behavior was positively linked with urinary oxytocin levels. This was true even when accounting for affiliative interactions and potential threat from rival groups, suggesting that, similar to humans, the oxytocinergic system is an influential mechanism involved in chimpanzee in-group out-group contexts. The stimulus that triggers oxytocin release in intergroup contexts, however, remains unknown for either humans or chimpanzees. Affiliative contact has been proposed as an oxytocin trigger (26), but our results concur with other studies, of both humans and chimpanzees, suggesting that physical contact is not necessarily required (20, 27) nor sufficient (19) for oxytocin secretion. Here, neither the presence of affiliation during intergroup conflict nor multipartner affiliation without intergroup conflict (Table 2) led to urinary oxytocin levels that differed from nonaffiliative intergroup conflict or control samples, respectively. This is in agreement with recent evidence that the mere act of grooming is not linked with an oxytocin response but rather the social context in which the grooming occurs (19). Also, proximity to border areas, where encounters with rival groups are more likely, did not affect urinary oxytocin levels. The latter suggests that a potential threat by out-groups alone is not sufficient for triggering oxytocin excretion. The finding that both coordinated activities (i.e., hunting and intergroup conflict) showed higher urinary oxytocin levels than control with and without affiliation suggests that coordinated behavior is linked to the oxytocinergic system. Given that intergroup conflicts had significantly higher oxytocin levels than hunting indicates that this effect is likely reinforced in the context of out-group threat. It is therefore possible that in-group coordinated activity and the perception of an in-group out-group context act in synergy during intergroup conflicts. However, because we are lacking a behavioral measure of the degree of coordination, we cannot rule out that the oxytocinergic system reactivity observed in intergroup conflicts is a mere function of a greater level of coordination when facing hostile rival groups. Nonetheless, whether in-group out-group perception drives group coordination, oxytocin secretion, or both, our results suggest that chimpanzee in-group cohesive behavior in the face of out-group threat is likely supported by the same physiological mechanism suggested for human parochial altruism, the oxytocinergic system.
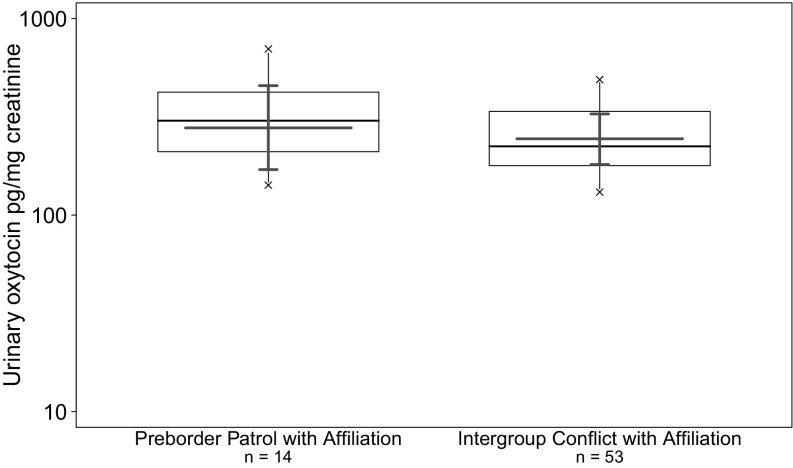
Moreover, it is unclear whether oxytocin release results from or precipitates participation in intergroup conflicts. Owing to the beneficial value of cooperative group action during intergroup conflict, we expect that an anticipatory oxytocin increase would be adaptive when influencing group cohesion. Because in the Taï Forest, chimpanzee border patrols are often preceded by grooming with multiple group members, the majority of preborder patrol samples collected involved grooming interactions. Accordingly, to investigate anticipatory oxytocin increase (anticipation model), we contrasted urine samples collected after multipartner grooming sessions: (i) shortly before the initiation of border patrols (“preborder patrol with affiliation”; 6 subjects, n = 14 samples of 10 intergroup conflict events) or, as a control, (ii) on days without intergroup conflict (control with affiliation; 9 individuals, n = 38 samples of 34 events). This model included samples from a single chimpanzee group, East group, because no preborder patrol with affiliation samples were attained for South group. We fitted an LMM controlling for duration of grooming, subgroup size, individuals’ sex and rank, and proximity to border areas. We found a significant positive impact of imminent border patrols on urinary oxytocin levels (LMM, likelihood ratio test: χ2 = 11.132, df = 1, P < 0.001; Fig. 1B and Table S8), an effect that was not driven by the duration of multipartner grooming or other control predictors.
Table S8.
Anticipation model: Effect of intergroup conflict anticipation on urinary oxytocin levels, log-transformed
| Term | Estimate | SE | CIlower | CIupper | χ2* | P |
| Intercept | 4.369 | 0.393 | 3.586 | 5.157 | – | – |
| Test predictor | ||||||
| Preborder patrol with affiliation† | 1.483 | 0.410 | 0.681 | 2.282 | 11.132 | <0.001 |
| Control predictors | ||||||
| Sex‡ | −0.418 | 0.484 | −1.418 | 0.554 | 0.697 | 0.404 |
| Proximity§ | 0.261 | 0.166 | −0.081 | 0.610 | 2.331 | 0.127 |
| Subgroup size¶ | −0.012 | 0.143 | −0.311 | 0.270 | 0.007 | 0.935 |
| Rank# | −0.150 | 0.199 | −0.564 | 0.272 | 0.528 | 0.467 |
| Affiliation duration|| | 0.167 | 0.172 | −0.195 | 0.526 | 0.862 | 0.353 |
Statistically significant results (P ≤ 0.05) appear in bold.
Degrees of freedom are 1.
,‡χ2 and P values refer to comparison with the reference categories †control with affiliation and ‡female.
,¶,#,||z-transformed, mean ± SD of the original variables: §60.9 ± 25.97 (range 5 to 99), ¶15.04 ± 5.21, #0.628 ± 0.27 (range 0 to 1, with 1 being the highest social rank), and ||1357.64 ± 791.9 (s).
Our results demonstrate an anticipatory increase in urinary oxytocin in a similar manner to the anticipatory testosterone increase found in intergroup conflicts (28) and intragroup competition (29) in chimpanzees. The observed high urinary oxytocin levels before border patrol initiation suggest that individuals may anticipate imminent intergroup conflict. Moreover, when comparing preborder patrol with affiliation with intergroup conflict with affiliation, we found no significant effect on urinary oxytocin levels (LMM: χ2 = 0.181, df = 1, P = 0.669; Fig. S3 and Table S9). This suggests that the observed anticipatory increase remains high throughout the intergroup conflict. The anxiolytic effect of oxytocin is proposed to facilitate social cohesion during highly risky situations that might otherwise precipitate defection away from threat (4). Accordingly, when group defense provides individuals with fitness advantages, mechanisms involving anticipatory high oxytocin potentially maintain cooperation and safeguard against defection.
Fig. S3.
Effects of intergroup conflict anticipation and participation on log-transformed urinary oxytocin levels in wild chimpanzees of East group (n = 67 samples, 7 subjects, 29 events). Shown are medians (thin horizontal lines), quartiles (boxes), percentiles (2.5 and 97.5%; vertical lines), minimum and maximum (laying crosses), as well as the fitted model and its 95% confidence intervals (thick lines).
Table S9.
Persistence model: Effect of intergroup conflict anticipation and participation on urinary oxytocin levels, log-transformed
| Term | Estimate | SE | χ2* | P |
| Intercept | 5.902 | 0.427 | – | – |
| Test predictor | ||||
| Intergroup conflict with affiliation† | −0.128 | 0.299 | 0.182 | 0.670 |
| Control predictors | ||||
| Sex‡ | −0.318 | 0.424 | 0.559 | 0.455 |
| Proximity§ | 0.123 | 0.127 | 0.921 | 0.337 |
| Subgroup size¶ | 0.094 | 0.116 | 0.615 | 0.433 |
| Rank# | −0.169 | 0.138 | 1.357 | 0.244 |
Degrees of freedom are 1.
,‡χ2 and P values refer to comparison with the reference categories †preborder patrol with affiliation and ‡female.
,¶,#z-transformed, mean ± SD of the original variables: §87.55 ± 11.87 (range 5 to 99), ¶15.71 ± 4.34, and #0.64 ± 0.22 (range 0 to 1, with 1 being the highest social rank).
Possibly resulting from a male propensity to participate in intergroup hostility (30), experimental approaches investigating cooperation during intergroup conflict have been mainly limited to male behavior (31). However, in a recent study, intranasal administration of oxytocin enhanced in-group cooperation during an in-group out-group setting in both men and women (32). This suggests oxytocin as an influential physiological mechanism in both sexes in in-group out-group contexts. Accordingly, we investigated sex differences during natural intergroup conflicts in chimpanzees. In the Taï Forest, both sexes participate in intergroup conflict (12), and females participated in 91% of intergroup conflicts in this study (proportion of all adult females and parous females 0.44 ± 0.21 and 0.35 ± 0.2, respectively). An interaction between event and sex was not significant, showing that female and male chimpanzees have similar oxytocin reactivity across events. Male chimpanzees are more likely to be the victims of lethal intergroup aggression (10). Nonetheless, the risk of intergroup infanticide and female hostage taking by rival groups are substantial threats for females (10, 11) and may increase the likelihood of female avoidance of intergroup conflicts (13). However, in the Taï Forest, rates of intergroup lethal violence are low in comparison with other chimpanzee field sites (10). Whether this is a cause or an effect of the likelihood of both sexes to cooperate in threatening situations remains unknown. Nonetheless, our findings emphasize that selective pressures may have led to similar oxytocinergic system involvement in intergroup conflict in both sexes. Future investigations of the hormonal mechanisms involving in-group out-group contexts in women in natural settings will aid understanding of the reported sex differences (30) in human intergroup violence.
This study used a within-subjects design, requiring repeated sampling of the same wild chimpanzees when engaged in different natural events. We achieved this by using peripheral oxytocin measures that could be noninvasively collected. The biological validity of peripheral oxytocin measurements with respect to central oxytocin patterns nonetheless is debated (33–35). However, an increasing body of evidence shows that oxytocin pathways can involve coordinated central and peripheral oxytocin release, indicating that high peripheral measures reflect the release of central oxytocin. Furthermore, an increasing number of studies demonstrate the same effects of behavior, social context, and social relationships on both central and peripheral oxytocin measures (35–37).
Using an ecologically relevant paradigm, we found that the oxytocinergic system, a highly conserved physiological mechanism (5), is involved in cohesion during intergroup conflict in chimpanzees, as suggested for humans (8). Given that in-group favoritism in humans and chimpanzees may be underpinned by the same physiological mechanism, the most parsimonious explanation for such similarities is that this mechanism was present in our common ancestor, regulating in-group bias. Accordingly, it may be that some aspects thought to play a role in human parochial altruism rest on more ancient evolutionary origins than has been presumed.
The fundamental need for within-group support in times of rising between-group conflict is not uniquely human but apparently also present in one of our closest living relatives, the chimpanzee (11, 17). The link between human intergroup violence, group division, categorization, and attribution clearly is a current and pressing topic (3, 38). Understanding the evolutionary mechanisms underlying in-group out-group interactions, the pressures that switch intergroup collaboration to conflict and vice versa, and the interplay between behavior and hormones in these contexts may eventually assist the building of cooperation rather than destruction in fragile human between-group relations.
SI Methods
Urine Sample Collection and Analysis.
During focal follows, we systematically collected every urine sample possible of the focal subjects from leaf litter using a plastic pipette. We then transferred the urine (200 to 1,000 µL) into a cryovial containing 100 µL 0.5 N H3PO4 (19) to prevent hormone degradation, using a 1-mL Eppendorf pipette. We kept the samples cool by storing them in a thermos can with frozen cold packs until arrival at the camp, where we stored all samples in liquid nitrogen (within 12 h of collection). Samples were then shipped frozen on dry ice to the Laboratory of Endocrinology at the Max Planck Institute for Evolutionary Anthropology, where we stored them at −80 °C until analysis. Before the analysis, we assigned urine samples according to the behavior that occurred within the 15 to 60 min time window of oxytocin excretion (19, 43). Samples were excluded if other social behaviors occurred within the excretion window to ensure greater interpretation accuracy of the results in relation to the specific control and social events.
Accordingly, n = 19 of the samples collected in the context of intergroup conflict that overlapped with other behaviors that might influence urinary oxytocin levels (n = 15 overlapped with hunting behavior and n = 4 overlapped with food sharing) were thus not included in the analysis. Similarly, in the context of control with affiliation, n = 14 samples were not included in the analysis due to overlap with play behavior.
Sample extraction and analysis followed the protocol used by Crockford et al. (19), incorporating minor changes. Accordingly, we thawed samples while keeping them cool using an IsoPack (0 °C; Eppendorf), vortexed them for 10 s, and centrifuged for 1 min at 214.55 × g. We performed a solid-phase extraction with Chromabond HR-X SPE cartridges (1 mL, 30 mg). First, we conditioned the cartridges with 1 mL 100% methanol followed by 1 mL distilled HPLC water. Then, thawed urine samples were diluted 1:2 using 0.1% trifluoroacetic acid (TFA) and loaded onto the cartridge. We continued to wash the cartridge with 1 mL 10% (vol/vol) acetonitrile (ACN) containing 1% TFA in water, and eluted using 1 mL 80% (vol/vol) ACN. Extracted samples were then evaporated with an air stream at 50 °C, reconstituted with 300 µL 100% ethanol, and vortexed for 10 s. Samples were left at 4 °C for 60 min and evaporated again using the same procedure. Once dried, the samples were reconstituted in 250 µL of the assay buffer supplied in the commercially available enzyme immunoassay kit (Assay Designs; 901-153A-0001). We again vortexed the samples for 10 s and then centrifuged for 1 min at 9391 × g. Samples were added as 100-µL duplicates to the assay, following the instructions of the assay provider.
The assay standard curve ranged from 15.62 to 1,000 pg/mL, and assay sensitivity was 15 pg/mL. Oxytocin validations of parallelism and accuracy were conducted and appeared satisfactory (19). Interassay coefficients of variation of low- (50 pg/mL) and high- (250 pg/mL) value quality controls were 19.1 and 7.6% (n = 44), respectively, whereas intraassay coefficients of variation of low- (50 pg/mL) and high- (250 pg/mL) value quality controls were 12.9 and 8.9%, respectively.
For cases that produced results outside of the linear range of the oxytocin standard curve, we repeated the extraction and analysis, applying less volume. Overall, we excluded 23 cases for which remeasurement produced results outside of the linear range or for which no material was left over for remeasurement.
Statistical Analysis.
We fitted a Poisson generalized linear mixed model (GLMM) with log link function (24) to investigate the effect of the type of event (intergroup conflict or control) on the number of times each adult individual left the subgroup. In the model, we included group identity and proximity to border areas as control predictors. Border proximity was expressed as the mean value of proximity to the border throughout the course of each period. We included the duration from an individual’s first arrival in the subgroup to the end of the event (log-transformed) as an offset term. Identities of subjects that were present in the subgroup and event identities were included as random effects. To keep type I error rate at the nominal 5%, we included random slopes (46, 47) for both proximity and period type within-subject. The R script formula for the defection model: glmer(number of times each adult individual left ∼ event type + z-transformed border proximity + group + offset(log(duration)) + (1 | event identity) + (1 + dummy-coded event type 2 + dummy-coded event type 3 + z-transformed border proximity || subject identity), family = Poisson).
We then investigated whether chimpanzee in-group behavior during and before hostile intergroup conflicts engaged the oxytocinergic system. All urine samples associated with intergroup conflicts were collected from individuals that participated in the intergroup conflict. We fitted LMMs (24) with Gaussian error structure and identity link function, and log-transformed the response variable, urinary oxytocin levels (pg/mg creatinine). Our test predictor for the models was the type of events sampled (event model, n = 468: control with and without affiliation, control with coordination, and intergroup conflict with and without affiliation; anticipation model, n = 52: control with affiliation and preborder patrol with affiliation). To control for factors that might influence hormone levels, we included in each model subgroup size as well as individuals’ sex and rank. We also included proximity to border areas by assigning each urine sample a value according to the minimum polygon in which it was excreted. This provided the relative distance from the border areas of the territory where intergroup encounters are more likely, to evaluate potential risk and its possible effect on oxytocin excretion. The anticipation model included samples from a single chimpanzee group (East) because no preborder patrol with affiliation samples were attained for South group, hence the reduction in sample size from n = 100 samples to n = 38 for control with affiliation. Therefore, we included group identity (i.e., East or South) as a control predictor only in the event model. We also included duration of affiliative contact as a control predictor in the anticipation model (affiliation duration) to control for its effects on urinary oxytocin levels (26). We initially included an interaction between our test predictor (event type) and sex in the event model; however, because it did not reveal a significant effect (P = 0.580), we excluded this variable and reran the model. Event and subject identity were included as random effects to control for having several samples from the same events and subjects. Furthermore, we included random slopes (46, 47) for the test predictors (i.e., event or anticipation) as well as for subgroup size, rank, and proximity within-subject. For the anticipation model, we also included the random slope of affiliation duration within-subject. We did not include the correlation among the random slopes and random intercepts in any of the fitted models (46). Furthermore, we investigated the effect of affiliation with or without intergroup conflict, and coordination, by conducting a post hoc analysis for the event model. This was done by dummy coding the test predictor and subsequently changing the reference categories (Tables S5, S6, and S7).
Before fitting the models, we checked all predictors and the response for their distribution and, as a consequence, log-transformed urinary oxytocin levels to achieve a more symmetrical distribution. We then proceeded by z transforming the covariates of subgroup size, rank, proximity, and affiliation duration to a mean of zero and an SD of one (49). Visual inspection of qqplots and residuals plotted against fitted values did not reveal obvious deviations from the assumptions of normally distributed and homogeneous residuals.
The R script formula for the event model: lmer(log-transformed urinary oxytocin ∼ event + z-transformed border proximity + z-transformed subgroup size + group identity + subject sex + z-transformed subject rank + (1 + dummy-coded event 2 + dummy-coded event 3 + dummy-coded event 4 + dummy-coded event 5 + z-transformed border proximity + z-transformed subgroup size + z-transformed subject rank||subject identity) + (1|event identity).
The R script formula for the anticipation model: lmer(log-transformed urinary oxytocin ∼ event + z-transformed border proximity + z-transformed subgroup size + subject sex + z-transformed subject rank + z-transformed affiliation duration + (1 + dummy-coded event 2 + z-transformed border proximity + z-transformed subgroup size + z-transformed subject rank + z-transformed affiliation duration||subject identity) + (1|event identity).
Moreover, to determine how similar contexts influenced urinary oxytocin levels, we fitted three additional models: (i) the type of intergroup conflict (i.e., control versus border patrol or intergroup encounter; intergroup conflict type model; Fig. S1 and Table S2), (ii) a reduced event model excluding intergroup conflict samples of border patrols (Fig. S2 and Table S4), and (iii) pre versus during intergroup conflict affiliation (“persistence” model; Fig. S3 and Table S9). We fitted LMMs (24) with Gaussian error structure and identity link function, with the response being log-transformed urinary oxytocin levels (pg/mg creatinine). We included subgroup size, proximity to border areas, and individuals’ sex and rank as control predictors in both models. We included group identity (i.e., East or South) as a control predictor only in the intergroup conflict type model and in the reduced event model, including samples from individuals of both groups. Event and subject identity were included as random effects to control for having several samples from the same events and subjects. Furthermore, to keep type I error rate at the nominal 5%, we included random slopes (46, 47) for the two test predictors, as well as for subgroup size, rank, and proximity within-subject. We did not include the correlation among the random slopes and the respective random intercept in any of the fitted models (46).
We fitted all models in R [version 3.3.0 (42)] using the functions lmer and glmer of the R package lme4 (45) and derived variance inflation factor (VIF) values using the function vif of the R package car (50), applied to a standard linear model lacking the random effects. We determined model stability for all models by excluding subjects and event identities one at a time. We then compared the estimates derived for these data with those derived for the full dataset. This indicated no influential subjects or event identities to exist. We derived confidence intervals by means of parametric bootstraps (function bootMer of the package lme4). VIFs did not reveal collinearity problems, as indicated by the largest value being <4 (51) (event model 2.59; anticipation model 2.43; defection model 1.98; intergroup conflict type model 2.74; persistence model 2.03; reduced event model 2.72).
Methods
Fieldwork was conducted with the Taï Chimpanzee Project located at the Taï National Park, Ivory Coast (5°52′N, 7°20′E), between October 2013 and April 2014 as well as between September 2014 and May 2015, observing the well-habituated East and South neighboring chimpanzee (P. t. verus) groups. We conducted all-day focal animal sampling (23) on 20 individuals (5 males and 5 parous females in each group) for a total of 2,278 observation hours in East group and 2,164 in South group, along with noninvasively collected urine samples (analysis included n = 482 samples, 23.4 ± 14.55 samples per individual). During focal follows, we documented changes in the behavior, social interactions, and vocalizations emitted by and directed toward the focal individual, using CyberTracker software (version 3.389; www.cybertracker.org/). We continuously updated the subgroup composition and size. Every occurrence of a border patrol or an intergroup encounter was recorded ad libitum. During the study period, 67 instances of intergroup conflict were observed in East group, a rate of 1 every 5 d, out of which 28 involved both a border patrol (with no direct out-group contact) and an intergroup encounter (with direct out-group contact; 42%; 1 every 11 d); 25 instances of intergroup conflict were observed in South group, a rate of 1 every 12 d, out of which 17 involved both a border patrol and an intergroup encounter and a single instance involving only an intergroup encounter (72%; 1 every 16 d). None of the intergroup encounters observed resulted in lethal aggression.
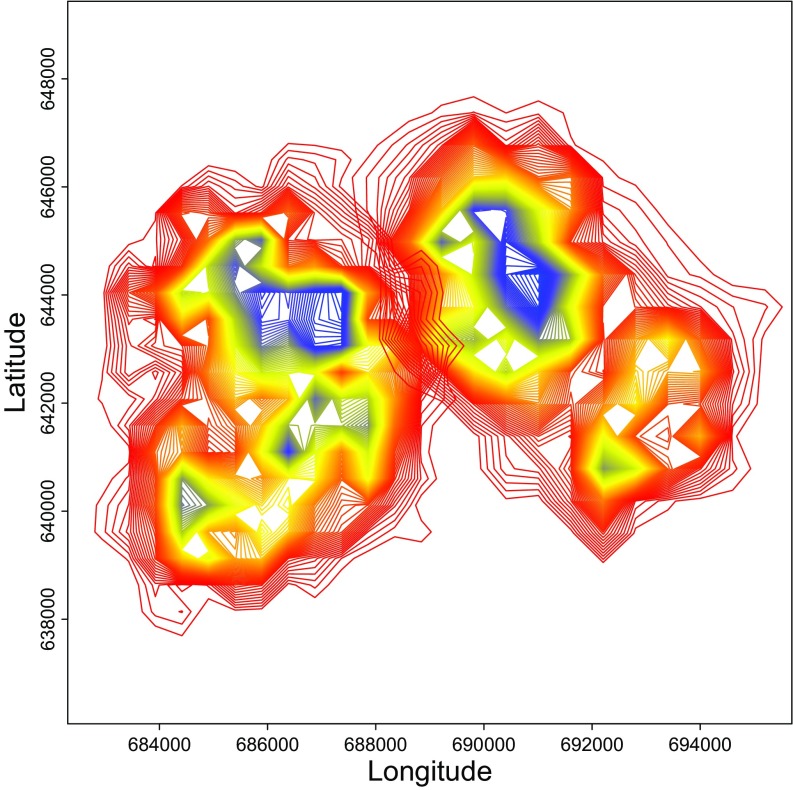
To determine dynamic changes in dominance relationships over time within each group, we used the Elo-rating (39), based on unidirectional submissive pant grunt vocalizations (40). We continuously recorded the location of the focal subject using a Garmin Rino 610 global positioning system set on the automatic tracklog recording function. This was done to control for changes in the chimpanzee’s endocrinological response in relation to proximity to peripheral territorial areas and, thus, the potential to encounter rival chimpanzee groups. We then assessed the proximity of the focal individual to the border areas of the territory. We used a kernel density estimate (41) in R (version 3.2.3) (42) to construct polygons representing the percentage of home-range use kernels ranging from 5 to 99, with 5 representing the very core of the home range and 99 being the border area (Fig. S4).
Fig. S4.
Kernel density estimate constructing polygons representing the percentage of home-range use in South (west of longitude 688500) and East (east of longitude 688500) groups. The polygons range from 5 to 99, with 5 (blue) representing the very core of the home range and 99 (red) being the border areas.
As an estimate of defection, we measured for each adult individual the number of times it left the subgroup of the focal individual during instances of prolonged intergroup conflicts (East group n = 21; South group n = 2; duration, mean ± SD 102.4 ± 41.48 min; Table 1). All intergroup conflict periods used in this model included border patrol behavior. Whereas some periods included direct contact with rival groups and were labeled as intergroup encounters (n = 11), others did not include out-group contact and were labeled as border patrols (n = 12). We defined separate fission events as any adult who left the subgroup per min, such that leaves that occurred >1 min apart were counted as separate fission events (Table 1). Accordingly, n = 25 fission events occurred during intergroup conflicts at a rate of 1 every 96 min (fissions per period, mean ± SD 1.089 ± 1.276, with 3.64 ± 2.36 individuals leaving during each fission event). We compared this with matched control periods of the same duration and within 1 to 3 d before or after the intergroup conflict (East group n = 21; South group n = 2), on days that did not include intergroup conflicts or hunting behavior, and with similar subgroup size and composition. A total of n = 65 fission events occurred during matched control periods at a rate of 1 every 37 min (fissions per period, mean ± SD 2.826 ± 2.145, with 4.8 ± 3.23 individuals leaving during each fission event).
Urine Sample Collection and Analysis.
We took the clearance of oxytocin into urine in chimpanzees to be 15 to 60 min after secretion (19, 20), adapted from a human clearance study (43). Urinary oxytocin measures show biobehaviorally relevant levels following target behaviors or social interactions that occur within this time window (19, 20). Sample collection, extraction, and analysis followed the event sampling protocol used by Crockford et al. (19), incorporating minor changes (SI Methods). Analysis was done using a commercially available enzyme immunoassay kit (Assay Designs; 901-153A-0001; SI Methods). We measured creatinine levels in all urine samples and expressed urinary oxytocin values as pg/mg creatinine, to control for variation in urine volume and concentration (44). Because very low creatinine values may lead to overestimation of urinary oxytocin levels, we excluded all urine samples with creatinine levels ≤0.04 mg/mL (n = 5, <1.2% of the samples included).
All methods were noninvasive and approved by the Ministries of Research and Environment of Ivory Coast and Office Ivorien des Parcs et Reserves. Our study complies with the ethics of both the Max Planck Society and the Max Planck Institute for Evolutionary Anthropology primatology department ethics policy (www.eva.mpg.de/primat/ethical-guidelines.html).
Statistical Analysis.
We conducted a series of linear mixed models (24) with Gaussian error structure and identity link function, and a Poisson generalized linear mixed model (24) with log link function in R [version 3.3.0 (42)], using the functions lmer and glmer of the R package lme4 (45). In each model, we included factors that might influence hormone levels (as described above; Datasets S1–S3). Furthermore, to keep type I error rate at the nominal 5%, we included random slopes (46, 47) (SI Methods). We compared the fit of the full models with those of a respective null model lacking only the test predictors of event or period type but otherwise identical to the respective full model in all other terms (48), using a likelihood ratio test (SI Methods).
Supplementary Material
Acknowledgments
We thank the Centre Suisse de Recherches Scientifiques and staff members of the Taï Chimpanzee Project for support, Christophe Boesch, Linda Vigilant, and Kevin Langergraber as well as Alexander Mielke, Michal Samuni-Blank, and two anonymous reviewers for their helpful comments. This study was funded by the Minerva Foundation (L.S.), Leakey Foundation (A.P.), and Max Planck Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616812114/-/DCSupplemental.
References
- 1.Choi J-K, Bowles S. The coevolution of parochial altruism and war. Science. 2007;318(5850):636–640. doi: 10.1126/science.1144237. [DOI] [PubMed] [Google Scholar]
- 2.Bowles S. Did warfare among ancestral hunter-gatherers affect the evolution of human social behaviors? Science. 2009;324(5932):1293–1298. doi: 10.1126/science.1168112. [DOI] [PubMed] [Google Scholar]
- 3.Cohen TR, Insko CA. War and peace: Possible approaches to reducing intergroup conflict. Perspect Psychol Sci. 2008;3(2):87–93. doi: 10.1111/j.1745-6916.2008.00066.x. [DOI] [PubMed] [Google Scholar]
- 4.De Dreu CKW. Oxytocin modulates cooperation within and competition between groups: An integrative review and research agenda. Horm Behav. 2012;61(3):419–428. doi: 10.1016/j.yhbeh.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322(5903):900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 6.Insel TR. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65(6):768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arueti M, et al. When two become one: The role of oxytocin in interpersonal coordination and cooperation. J Cogn Neurosci. 2013;25(9):1418–1427. doi: 10.1162/jocn_a_00400. [DOI] [PubMed] [Google Scholar]
- 8.De Dreu CKW, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328(5984):1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- 9.Israel S, Weisel O, Ebstein RP, Bornstein G. Oxytocin, but not vasopressin, increases both parochial and universal altruism. Psychoneuroendocrinology. 2012;37(8):1341–1344. doi: 10.1016/j.psyneuen.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Wilson ML, et al. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature. 2014;513(7518):414–417. doi: 10.1038/nature13727. [DOI] [PubMed] [Google Scholar]
- 11.Boesch C, et al. Intergroup conflicts among chimpanzees in Taï National Park: Lethal violence and the female perspective. Am J Primatol. 2008;70(6):519–532. doi: 10.1002/ajp.20524. [DOI] [PubMed] [Google Scholar]
- 12.Boesch C, Boesch-Achermann H. The Chimpanzees of the Taï Forest: Behavioural Ecology and Evolution. Oxford Univ Press; New York: 2000. [Google Scholar]
- 13.Watts DP, Mitani JC. Boundary patrols and intergroup encounters in wild chimpanzees. Behaviour. 2001;138(3):299–327. [Google Scholar]
- 14.Wilson ML. Chimpanzees, warfare, and the invention of peace. In: Fry DP, editor. War, Peace, and Human Nature: The Convergence of Evolutionary and Cultural Views. Oxford Univ Press; New York: 2013. pp. 361–388. [Google Scholar]
- 15.Mitani JC, Watts DP, Amsler SJ. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr Biol. 2010;20(12):R507–R508. doi: 10.1016/j.cub.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Axelrod R, Hamilton WD. The evolution of cooperation. Science. 1981;211(4489):1390–1396. doi: 10.1126/science.7466396. [DOI] [PubMed] [Google Scholar]
- 17.Mitani JC. Cooperation and competition in chimpanzees: Current understanding and future challenges. Evol Anthropol Issues News Rev. 2009;18(5):215–227. [Google Scholar]
- 18.Mawson AR. Understanding mass panic and other collective responses to threat and disaster. Psychiatry. 2005;68(2):95–113. doi: 10.1521/psyc.2005.68.2.95. [DOI] [PubMed] [Google Scholar]
- 19.Crockford C, et al. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc Biol Sci. 2013;280(1755):20122765. doi: 10.1098/rspb.2012.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wittig RM, et al. Food sharing is linked to urinary oxytocin levels and bonding in related and unrelated wild chimpanzees. Proc Biol Sci. 2014;281(1778):20133096. doi: 10.1098/rspb.2013.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aureli F, et al. Fission‐fusion dynamics: New research frameworks. Curr Anthropol. 2008;49(4):627–654. [Google Scholar]
- 22.Boesch C. Cooperative hunting in wild chimpanzees. Anim Behav. 1994;48(3):653–667. [Google Scholar]
- 23.Altmann J. Observational study of behavior: Sampling methods. Behaviour. 1974;49(3):227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- 24.Baayen RH. Analyzing Linguistic Data: A Practical Introduction to Statistics Using R. 1st Ed Cambridge Univ Press; Cambridge, UK: 2008. [Google Scholar]
- 25.Luncz LV, Mundry R, Boesch C. Evidence for cultural differences between neighboring chimpanzee communities. Curr Biol. 2012;22(10):922–926. doi: 10.1016/j.cub.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 26.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50(4):506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 27.Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proc Biol Sci. 2010;277(1694):2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobolewski ME, Brown JL, Mitani JC. Territoriality, tolerance and testosterone in wild chimpanzees. Anim Behav. 2012;84(6):1469–1474. [Google Scholar]
- 29.Wobber V, et al. Differential changes in steroid hormones before competition in bonobos and chimpanzees. Proc Natl Acad Sci USA. 2010;107(28):12457–12462. doi: 10.1073/pnas.1007411107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durrant R. Collective violence: An evolutionary perspective. Aggress Violent Behav. 2011;16(5):428–436. [Google Scholar]
- 31.Rusch H. The evolutionary interplay of intergroup conflict and altruism in humans: A review of parochial altruism theory and prospects for its extension. Proc Biol Sci. 2014;281(1794):20141539. doi: 10.1098/rspb.2014.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ten Velden FS, Daughters K, De Dreu CKW. Oxytocin promotes intuitive rather than deliberated cooperation with the in-group. Horm Behav. June 8, 2016 doi: 10.1016/j.yhbeh.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Leng G, Ludwig M. Intranasal oxytocin: Myths and delusions. Biol Psychiatry. 2016;79(3):243–250. doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Macdonald K, Feifel D. Helping oxytocin deliver: Considerations in the development of oxytocin-based therapeutics for brain disorders. Front Neurosci. 2013;7:35. doi: 10.3389/fnins.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: Can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev. 2013;37(8):1485–1492. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Crockford C, Deschner T, Ziegler TE, Wittig RM. Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: A review. Front Behav Neurosci. 2014;8:68. doi: 10.3389/fnbeh.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30(4):534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tropp LR, editor. The Oxford Handbook of Intergroup Conflict. 1st Ed Oxford Univ Press; Oxford: 2012. [Google Scholar]
- 39.Neumann C, et al. Assessing dominance hierarchies: Validation and advantages of progressive evaluation with Elo-rating. Anim Behav. 2011;82(4):911–921. [Google Scholar]
- 40.Wittig RM, Boesch C. Food competition and linear dominance hierarchy among female chimpanzees of the Taï National Park. Int J Primatol. 2003;24(4):847–867. [Google Scholar]
- 41.Worton BJ. Kernel methods for estimating the utilization distribution in home-range studies. Ecology. 1989;70(1):164–168. [Google Scholar]
- 42.R Core Team 2016 R: A Language and Environment for Statistical Computing (R Found Stat Comput, Vienna). https://www.R-project.org/
- 43.Amico JA, Ulbrecht JS, Robinson AG. Clearance studies of oxytocin in humans using radioimmunoassay measurements of the hormone in plasma and urine. J Clin Endocrinol Metab. 1987;64(2):340–345. doi: 10.1210/jcem-64-2-340. [DOI] [PubMed] [Google Scholar]
- 44.Bahr NI, Palme R, Möhle U, Hodges JK, Heistermann M. Comparative aspects of the metabolism and excretion of cortisol in three individual nonhuman primates. Gen Comp Endocrinol. 2000;117(3):427–438. doi: 10.1006/gcen.1999.7431. [DOI] [PubMed] [Google Scholar]
- 45.Bates B, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67(1):1–48. [Google Scholar]
- 46.Barr DJ, Levy R, Scheepers C, Tily HJ. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J Mem Lang. 2013;68(3):255–278. doi: 10.1016/j.jml.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schielzeth H, Forstmeier W. Conclusions beyond support: Overconfident estimates in mixed models. Behav Ecol. 2009;20(2):416–420. doi: 10.1093/beheco/arn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forstmeier W, Schielzeth H. Cryptic multiple hypotheses testing in linear models: Overestimated effect sizes and the winner’s curse. Behav Ecol Sociobiol. 2011;65(1):47–55. doi: 10.1007/s00265-010-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol. 2010;1(2):103–113. [Google Scholar]
- 50.Fox J, Weisberg S. An R Companion to Applied Regression. SAGE; Thousand Oaks, CA: 2010. [Google Scholar]
- 51.Quinn GP, Keough MJ. Experimental Design and Data Analysis for Biologists. Cambridge Univ Press; Cambridge, UK: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.