Significance
The unfolded protein response regulator activating transcription factor 6 (ATF6) was recently identified as a novel genetic cause of the cone photoreceptor disease achromatopsia. ATF6 upregulates genes that help cells cope with endoplasmic reticulum stress. We identified the pathomechanisms of all ATF6 achromatopsia mutations. Class 1 ATF6 mutants show impaired endoplasmic reticulum (ER)-to-Golgi trafficking and diminished production of the transcriptional activator fragment. Class 2 mutants encode the intact ATF6 transcriptional activator domain with full activity. Class 3 mutants have defective basic leucine zipper (bZIP) domains with abrogated function. Patient fibroblasts show increased apoptosis after ER stress. Our findings reveal that human ATF6 mutations interrupt distinct steps of ATF6 activation. ER stress-associated damage may underlie the pathology of achromatopsia arising from ATF6.
Keywords: cone photoreceptor, achromatopsia, endoplasmic reticulum stress, ATF6, unfolded protein response
Abstract
Achromatopsia is an autosomal recessive disorder characterized by cone photoreceptor dysfunction. We recently identified activating transcription factor 6 (ATF6) as a genetic cause of achromatopsia. ATF6 is a key regulator of the unfolded protein response. In response to endoplasmic reticulum (ER) stress, ATF6 migrates from the ER to Golgi to undergo regulated intramembrane proteolysis to release a cytosolic domain containing a basic leucine zipper (bZIP) transcriptional activator. The cleaved ATF6 fragment migrates to the nucleus to transcriptionally up-regulate protein-folding enzymes and chaperones. ATF6 mutations in patients with achromatopsia include missense, nonsense, splice site, and single-nucleotide deletion or duplication changes found across the entire gene. Here, we comprehensively tested the function of achromatopsia-associated ATF6 mutations and found that they group into three distinct molecular pathomechanisms: class 1 ATF6 mutants show impaired ER-to-Golgi trafficking and diminished regulated intramembrane proteolysis and transcriptional activity; class 2 ATF6 mutants bear the entire ATF6 cytosolic domain with fully intact transcriptional activity and constitutive induction of downstream target genes, even in the absence of ER stress; and class 3 ATF6 mutants have complete loss of transcriptional activity because of absent or defective bZIP domains. Primary fibroblasts from patients with class 1 or class 3 ATF6 mutations show increased cell death in response to ER stress. Our findings reveal that human ATF6 mutations interrupt distinct sequential steps of the ATF6 activation mechanism. We suggest that increased susceptibility to ER stress-induced damage during retinal development underlies the pathology of achromatopsia in patients with ATF6 mutations.
Achromatopsia is a heritable blinding disease caused by cone photoreceptor dysfunction that spares the rod system. Using next-generation whole-exome sequencing, we recently discovered autosomal recessive mutations in the activating transcription factor 6 (ATF6) gene in patients with achromatopsia (1). ATF6 mutations span the entire coding region and include missense, nonsense, splice site, and single-nucleotide deletion and duplication changes (1–3). We previously showed that a missense mutation that introduced an arginine-to-cysteine substitution at amino acid residue 324 of the ATF6 protein compromised ATF6 activity in patient fibroblasts obtained from an achromatopsia family (1). However, the functional consequences of the other ATF6 mutations found in patients with achromatopsia remain unknown.
In humans, ATF6 is a 670-amino acid glycosylated transmembrane protein found in the endoplasmic reticulum (ER) (4). In response to protein misfolding in the ER or other forms of ER stress, ATF6 migrates from the ER to the Golgi apparatus, where the site 1 protease (S1P) and site 2 protease (S2P) cleave ATF6 in the transmembrane domain to liberate the cytosolic domain of ATF6 (4–6). The cytosolic domain contains a transcription factor of the basic leucine zipper (bZIP) family (4). Upon release from the Golgi membrane, the free ATF6 cytosolic transcriptional activator fragment migrates to the nucleus to bind DNA and transcriptionally up-regulate target genes that include ER protein folding chaperones and enzymes (4, 7, 8). Via this signal transduction mechanism, ATF6 activation helps restore ER protein folding homeostasis and alleviates ER stress (9).
Here, we investigated how ATF6 mutations found in patients with achromatopsia affect ATF6’s molecular mechanism of signaling and activation, using patient fibroblasts and recombinant mutant ATF6 proteins. We identified a class of ATF6 mutations in the luminal domain that reduce ATF6 signaling by impairing ER-to-Golgi trafficking of full-length ATF6 during ER stress. We identified a second class of ATF6 mutations near the transmembrane domain that have the potential to produce intact ATF6 cytosolic fragments with fully functional transcriptional activator properties. Last, we identified a third class of ATF6 mutations in the cytosolic domain that cause the loss of ATF6 function by deletion or mutation of the bZIP and/or transcriptional activator domain. Patient fibroblasts with loss-of-function ATF6 mutations exhibited significantly increased cell death in response to ER stress.
Results
Class 1 ATF6[Y567N] Mutation Impairs ER-to-Golgi Trafficking During ER Stress.
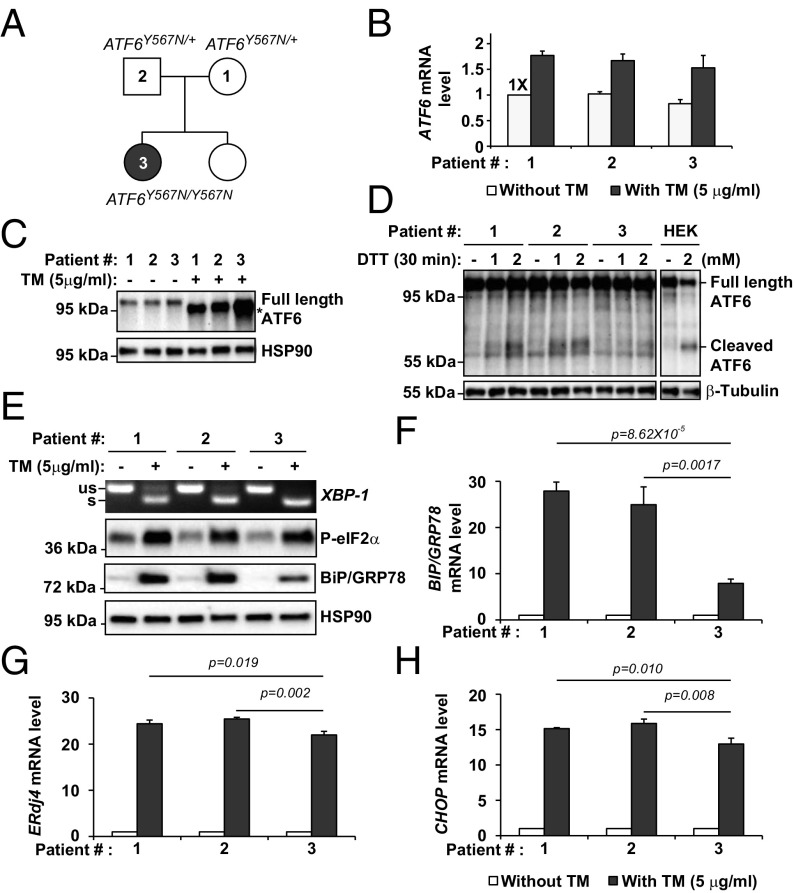
Four achromatopsia-associated ATF6 mutations introduce missense or frame shift changes in exons encoding the ER luminal domain of ATF6 (Fig. S1) (1, 3). We previously identified a family with a tyrosine-to-asparagine substitution at amino acid position 567 in the luminal domain of ATF6 (1). We obtained fibroblasts from two unaffected heterozygous (ATF6Y567N/+) parents, probands 1 and 2, and a homozygous (ATF6Y567N/Y567N) affected child, patient 3 (Fig. 1A). We found comparable levels of ATF6 mRNA and full-length ATF6 protein in heterozygous and homozygous fibroblasts under standard cell culturing conditions (Fig. 1 B and C). During ER stress conditions experimentally induced by tunicamycin application, ATF6 mRNA levels and the full-length ATF6 protein levels were up-regulated in both heterozygous and homozygous fibroblasts compared with the untreated samples (Fig. 1 B and C). However, significantly reduced levels of the cleaved ATF6 cytosolic fragment were seen in homozygous ATF6Y567N/Y567N fibroblasts compared with heterozygous controls in response to experimental ER stress induced by DTT (Fig. 1D). These findings showed that ATF6 mRNA and full-length protein were normally generated in the ATF6Y567N mutant, but during ER stress, lower levels of the cleaved functional transcriptional activator domain of ATF6 were present in ATF6Y567N/Y567N fibroblasts despite normal to increased levels of full-length ATF6 protein compared with heterozygous controls. Consistent with reduction of the ATF6 transcriptionally active fragment, we found reduced levels of BIP/GRP78 mRNA and protein, an ER chaperone transcriptionally up-regulated by ATF6 (4, 7, 10), in ATF6Y567N/Y567N fibroblasts compared with heterozygous controls in response to experimental ER stress (Fig. 1 E and F). These findings revealed that the luminal ATF6[Y567N] mutation resulted in the impairment of ATF6 signaling in ATF6Y567N/Y567N fibroblasts during ER stress.
Fig. S1.
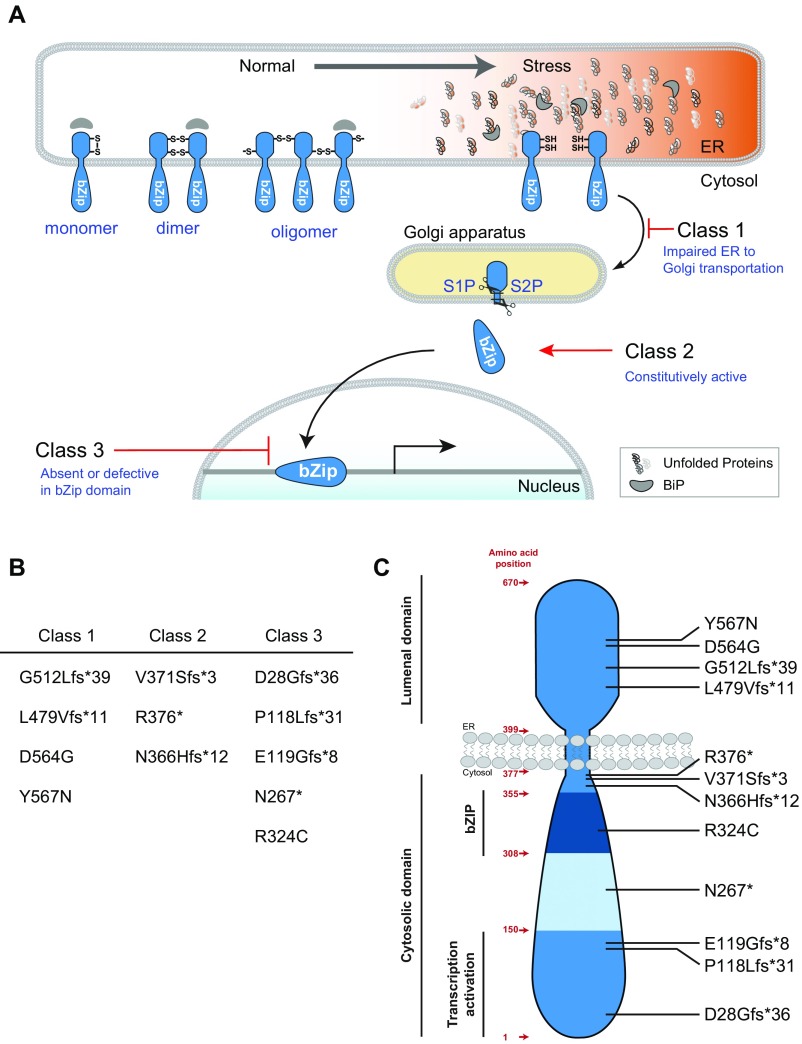
Functional classification of ATF6 mutants. (A) Reduced ATF6 monomers traffic from ER to Golgi in response to ER stress. S1P and S2P proteases cleave ATF6 in the Golgi apparatus to liberate the cytosolic bZIP transcriptional activator ATF6 fragment. Cleaved ATF6 migrates to nucleus to transcribe target genes. Class 1 ATF6 mutants D564G and Y567N show impaired ER-to-Golgi trafficking. G512Lfs*39 and L479Vfs*11 mutations are also proposed as class 1 mutations, because these mutations also occur in the luminal domain of ATF6. Class 2 ATF6 mutants V371Sfs*3 and R376* have fully intact ATF6 cytosolic domain and show constitutive transcriptional activator function. N366Hfs*12 is also proposed as a class 2 mutation because it contains the entire active ATF6 cytosolic domain. Class 3 ATF6 mutants do not have a functional bZIP domain and fail to bind and up-regulate ATF6 target genes. (B) List of class 1, 2, and 3 ATF6 mutants associated with achromatopsia. (C) Topography of ATF6 and the location of the disease-causing mutations identified in patients with achromatopsia.
Fig. 1.
Impaired cleavage of ATF6 in response to ER stress in class 1 mutant ATF6[Y567N] patient fibroblasts. (A) Pedigree of the family carrying ATF6Y567N alleles. The heterozygous parents (ATF6Y567N/+) were indicated as patient 1 and 2 for the mother and the father, respectively. The homozygous (ATF6Y567N/Y567N) female child was indicated as patient 3. (B) ATF6Y567N/+ or ATF6Y567N/Y567N patient fibroblasts were challenged with tunicamycin (TM) at the indicated concentration for 24 h. mRNA was collected from the fibroblasts. ATF6 mRNA levels were measured by real-time quantitative PCR and normalized to the level in the untreated ATF6Y567N/+ parental control (patient #1) fibroblast cells. (C) Patient fibroblasts expressing ATF6[Y567N] were challenged with TM at the indicated concentration for 24 h. Endogenous ATF6 protein levels were detected by immunoblotting. *Position of the deglycosylated full-length ATF6 protein produced after TM treatment. (D) Patient fibroblasts expressing ATF6[Y567N] were challenged with DTT for 30 min, and ATF6 protein levels were detected by immunoblotting using anti-ATF6 antibody. To help identify the electrophoretic migration patterns of full-length and cleaved ATF6 fragments, HEK293 cells were also challenged with DTT, and lysates were immunoblotted with anti-ATF6 antibody. (E) Patient fibroblasts were challenged with TM at the indicated concentration for 24 h. XBP1 mRNA splicing was assessed by RT-PCR. The level of phosphorylated-eIF2α and BiP/GRP78 were detected by immunoblotting. (F–H) The mRNA levels of ATF6 downstream target gene BIP/GRP78 (F), IRE1 downstream target gene ERdj4 (G), and PERK downstream target gene CHOP (H) were assessed by real-time quantitative PCR and normalized to mRNA levels in untreated samples.
ATF6 signaling is a key component of the unfolded protein response (UPR) and operates in parallel with UPR signal transduction pathways controlled by the inositol-requiring enzyme 1 (IRE1) and PKR-like endoplasmic reticulum kinase (PERK) proteins to ensure normal ER function in mammalian cells (11, 12). We next examined whether the other branches of the UPR were also dysregulated in ATF6Y567N/Y567N fibroblasts. We assayed two specific molecular events of the IRE1 UPR signaling pathway, XBP1 mRNA splicing, an early proximal event specifically initiated by IRE1 activation (13, 14), and ERdj4 transcription, a downstream target gene induced by IRE1 signaling (Fig. 1 E and G) (7, 8, 15, 16). For the UPR signaling pathway regulated by PERK, we examined levels of phosphorylated eIF2α protein, an early proximal event in the PERK signal transduction pathway (17, 18), and CHOP mRNA transcript, a downstream target gene potently up-regulated by PERK signaling (Fig. 1 E and H) (19). For these IRE1 and PERK pathway markers, we observed a small (<10%) but statistically significant decrease in ERdj4 and CHOP transcript levels between ATF6Y567N/Y567N fibroblasts and heterozygous controls (Fig. 1 E–H). These studies revealed that ATF6Y567N/Y567N fibroblasts with compromised ATF6 signaling also showed mild impairment of the transcriptional output of other UPR pathways during ER stress.
Next, we investigated the mechanism underlying the reduction in levels of the cleaved ATF6 fragment observed during ER stress in ATF6Y567N/Y567N fibroblasts. To ensure that the ATF6 protein level differences were not a result of fibroblast cell line differences, we expressed FLAG-tagged full-length wild-type ATF6 or mutant ATF6[Y567N] in HEK293 cells. Similar to our findings in the primary patient fibroblasts, we observed significantly reduced levels of cleaved ATF6 in response to ER stress induced by DTT in cells expressing ATF6[Y567N] compared with wild-type ATF6 (Fig. 2A). In response to ER stress, full-length ATF6 traffics from ER to Golgi, where the Golgi-resident S1P and S2P proteases cleave the full-length protein to liberate the cytosolic transcriptional activator ATF6 fragment. We examined whether defects in ER-to-Golgi trafficking were responsible for the reduced production of cleaved ATF6 seen with the ATF6[Y567N] mutant. First, we compared the sensitivity with endoglycosidase H (Endo H) of wild-type ATF6 and the ATF6[Y567N] mutant. Prior studies had demonstrated that ATF6 was glycosylated with high mannose N-glycan in the ER and that this glycosylated ATF6 isoform was sensitive to Endo H (4, 5). When ATF6 traveled to Golgi during ER stress, the high mannose N-glycan of ATF6 was trimmed by glycosidases in the Golgi to produce an Endo H-resistant full-length ATF6 that could be transiently visualized by SDS/PAGE in cells before undergoing S1P protease cleavage (5).
Fig. 2.
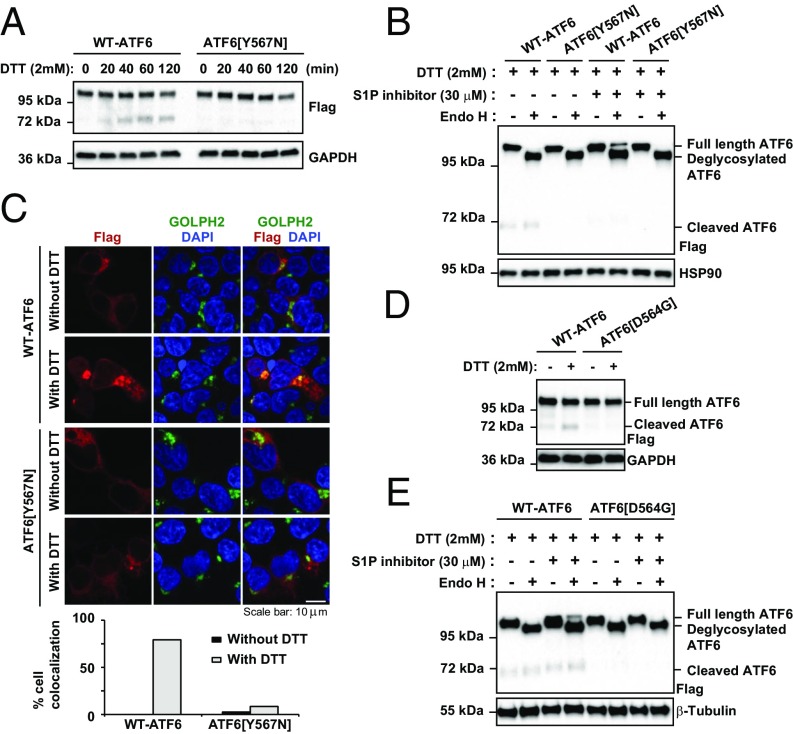
Impaired ER-to-Golgi trafficking of class 1 mutants ATF6[Y567N] and ATF6[D564G] during ER stress. (A) Recombinant FLAG-tagged ATF6[Y567N] was expressed in HEK293 cells for 20 h and then challenged with DTT as indicated. ATF6 protein levels were detected by immunoblotting with anti-FLAG. The band above 95 kDa and the band at 72 kDa represent the full-length FLAG-tagged ATF6 and cleaved FLAG-tagged ATF6, respectively. (B) Recombinant FLAG-tagged wild-type ATF6 and ATF6[Y567N] were expressed in HEK293 cells for 20 h and then challenged with DTT with or without S1P inhibitor, as indicated, for 90 min. Cell lysates were treated with Endo H, as indicated. ATF6 protein levels were detected by immunoblotting with anti-FLAG. The bands above 95 kDa and the band at 72 kDa represent the full-length FLAG-tagged ATF6 (either Endo H sensitive or insensitive) and cleaved FLAG-tagged ATF6, respectively. (C) ATF6[Y567N] was expressed in HEK293 cells for 20 h and then challenged with 2 mM DTT for 40 min. The subcellular localization of ATF6 was visualized by immunofluorescence labeling and confocal microscopy by anti-FLAG antibody (shown in red). The Golgi apparatus was visualized by GOLPH2 immunostaining (shown in green). The nucleus was visualized by DAPI staining (shown in blue). The percentage of cells showing FLAG and GOLPH2 colocalization was quantified and shown in the bottom graph. (Scale bar, 10 μm.) (D) Recombinant FLAG-tagged ATF6[D564G] was expressed in HEK293 cells for 20 h and then challenged with DTT for 1 h. ATF6 protein levels were detected by immunoblotting with anti-FLAG. (E) Recombinant FLAG-tagged wild-type ATF6 and ATF6[D564G] were expressed in HEK293 cells for 20 h and then challenged with DTT with or without S1P inhibitor, as indicated, for 90 min. Cell lysates were treated with Endo H, as indicated.
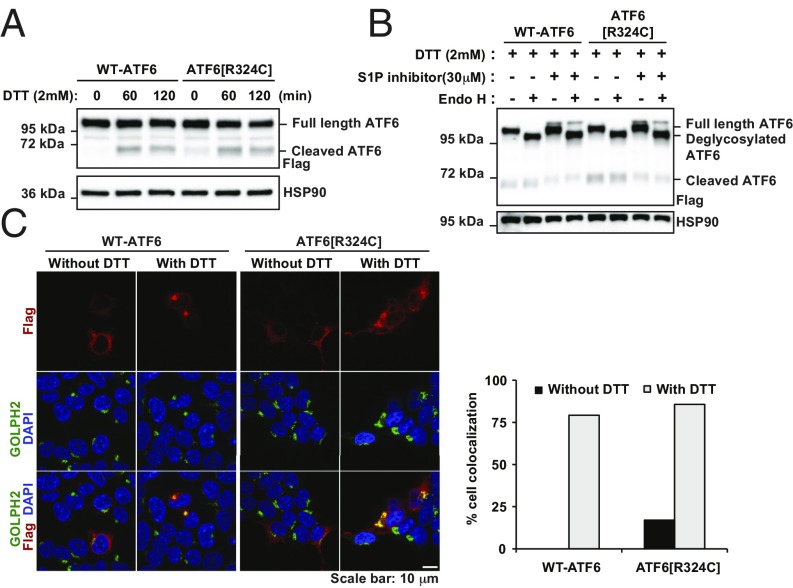
We found that both wild-type ATF6 and the mutant ATF6[Y567N] underwent glycosylation in the ER and showed identical Endo H sensitivity profiles (Fig. 2B). However, when we added S1P inhibitor and analyzed protein lysates collected from cells treated with DTT, we saw an Endo H-resistant ATF6 isoform only with wild-type ATF6 (Fig. 2B). This finding provided biochemical evidence that the mutant ATF6[Y567N] trafficked poorly from ER to Golgi compared with the wild-type protein during ER stress. Consistent with these biochemical results, when we examined the subcellular localization of ATF6 by fluorescence confocal microscopy, we found colocalization of wild-type ATF6 and the Golgi marker Golgi membrane protein 1 (GOLPH2), in response to ER stress induced by DTT (Fig. 2C, upper two rows). However, the mutant ATF6[Y567N] and GOLPH2 remained in separate subcellular compartments with no colocalization of fluorescent signals under the same experimental conditions (Fig. 2C, bottom two rows). We confirmed that both wild-type and mutant ATF6[Y567N] were localized in the ER under resting (nonstressed) conditions, as evident by colocalization with the ER resident protein, PDI (Fig. S2). Another luminal domain mutant, ATF6[D564G], also showed impaired cleavage and trafficking in analogous assays (Fig. 2 D and E). In sum, our studies revealed that the mechanism underlying attenuation of transcriptional activity in class 1 ATF6 luminal domain mutants arose from an unexpected defect in the process of ER-to-Golgi trafficking in response to ER stress, leading to reduced production of the cleaved transcriptional activator domain of ATF6.
Fig. S2.
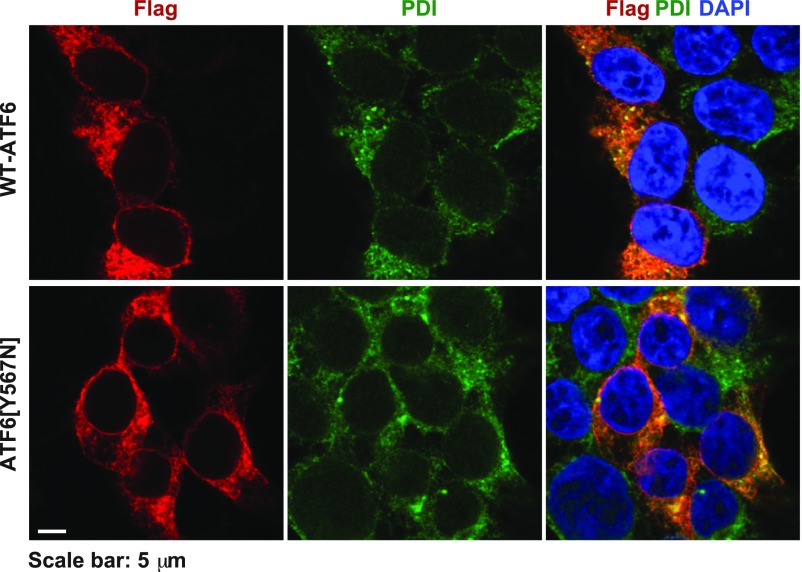
FLAG-tagged wild-type ATF6 and ATF6[Y567N] localize to the ER at resting conditions. FLAG-tagged wild-type ATF6 or ATF6[Y567N] were expressed in HEK293 cells for 20 h. The subcellular localization of ATF6 was visualized by immunofluorescence labeling and confocal microscopy by anti-FLAG antibody (shown in red). The endoplasmic reticulum was visualized by PDI immunostaining (shown in green). The nucleus was visualized by DAPI staining (shown in blue). (Scale bar, 5 μm.)
Constitutive Transcriptional Activity in Class 2 ATF6 Mutants.
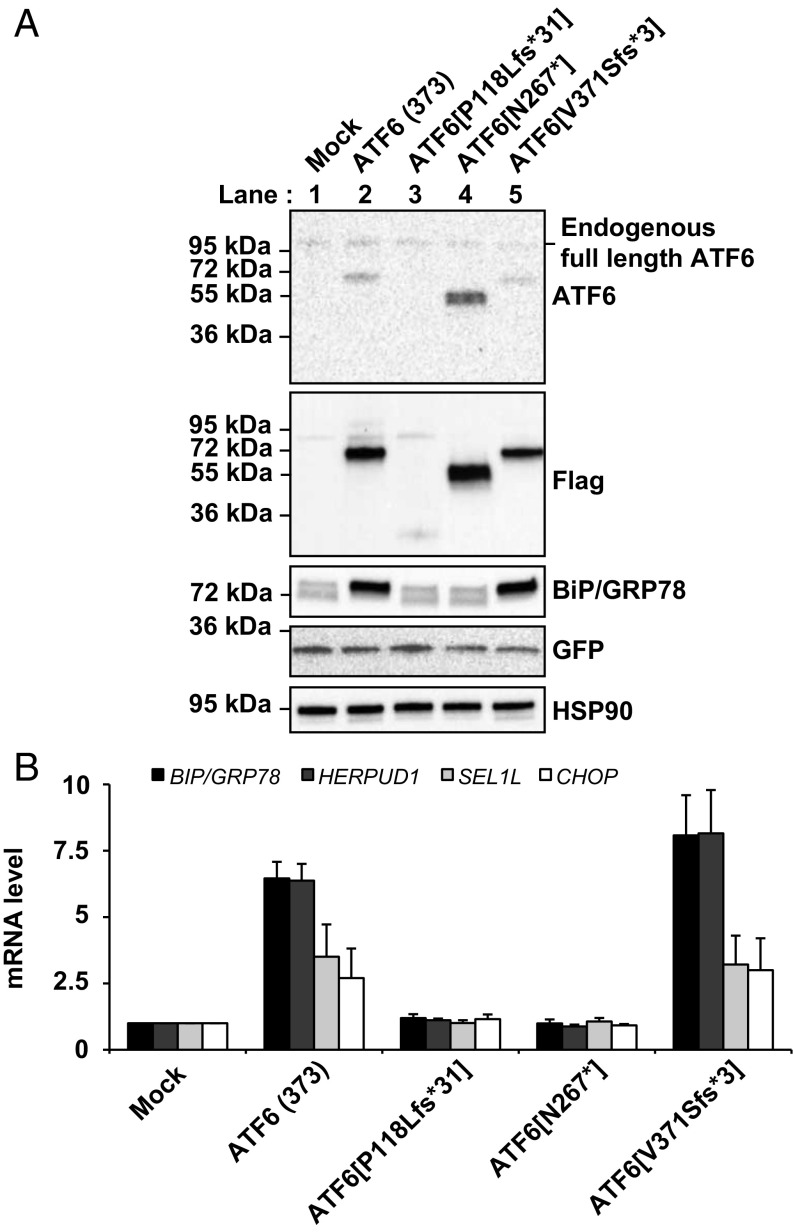
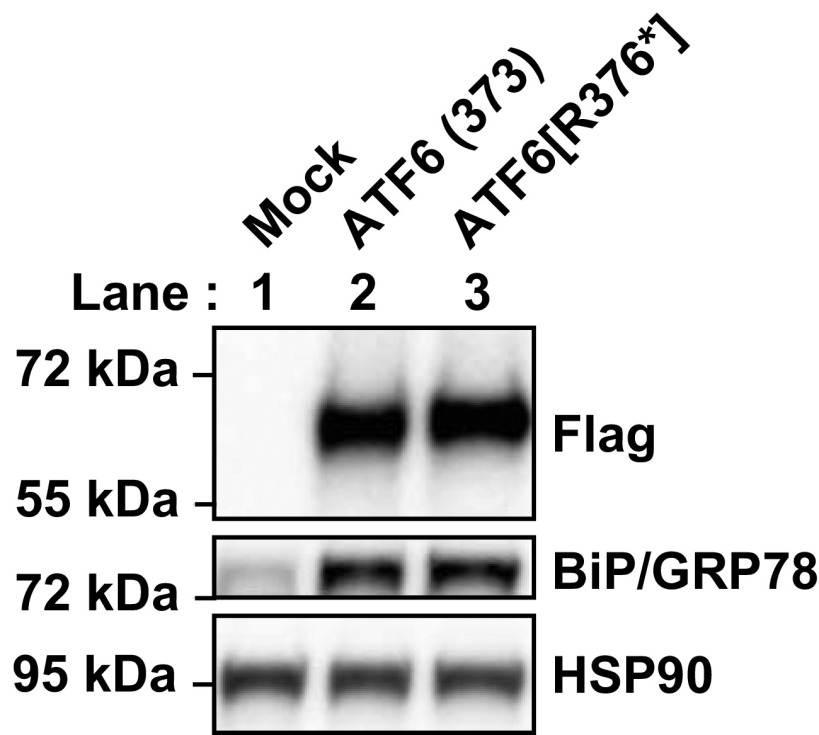
The amino terminal domain of ATF6 is a 370–380-amino acid bZIP transcription factor, and expression of recombinant ATF6 protein bearing only the first 373 amino acids from ATF6’s cytosolic domain reconstituted the transcriptional activity of ATF6 in mammalian cells (4, 20). Intriguingly, three of the ATF6 mutations identified in patients with achromatopsia were predicted to create ATF6 fragments of similar length to the ATF6(373) transcriptionally active fragment (Fig. S1 and refs. 1 and 3). To determine whether this group of ATF6 mutations showed functional activity, we expressed a recombinant FLAG-tagged ATF6 bearing a frame-shift mutation that caused a valine-to-serine substitution at amino acid 371 of ATF6, followed by a stop codon three amino acids distally, ATF6[V371Sfs*3]. We found that the ATF6[V371Sfs*3] protein showed nearly identical size mobility to ATF6(373) in transfected HEK293 cells (Fig. 3A, compare lane 5 and lane 2). We also found that ATF6[V371Sfs*3] transcriptionally induced ATF6 downstream target genes, BIP/GRP78, HERPUD1, and SEL1L, as potently as ATF6(373) (21) (Fig. 3B). The expression of CHOP was also up-regulated by both ATF6(373) and ATF6[V371Sfs*3] (Fig. 3B). In analogous studies, we also found that the ATF6[R376*] mutant showed nearly identical size mobility to ATF6(373), and induction of ATF6 downstream target gene, BIP/GRP78 (Fig. S3). These findings demonstrated that the recombinant mutant ATF6[V371Sfs*3] and ATF6[R376*] proteins are fully functional transcriptional activators in vitro. In vivo, the amount of functional truncated ATF6[V371Sfs*3] and ATF6[R376*] proteins may be less than wild-type ATF6 protein levels because the nonsense and premature stop codons found in these mutants may subject their mRNA transcripts to nonsense-mediated mRNA decay (NMD) (1).
Fig. 3.
Transcriptional activator properties of class 2 and class 3 mutant ATF6 proteins. (A) FLAG epitope-tagged ATF6(373), ATF6[V371Sfs*3] (a class 2 mutant), and ATF6[P118Lfs*31] and ATF6[N267*] (class 3 mutants) were cotransfected with GFP into HEK293 cells. ATF6 protein levels were detected by immunoblotting, using anti-ATF6 and anti-FLAG antibodies. BiP/GRP78 and GFP protein levels were detected by immunoblotting. GFP levels were assessed as a transfection control. (B) ATF6(373), ATF6[P118Lfs*31], ATF6[N267*], and ATF6[V371Sfs*3] were expressed in the HEK293 cells and mRNA was collected. Levels of genes transcriptionally regulated by ATF6, including BIP/GRP78, HERPUD1, and SEL1L, were measured by real-time quantitative PCR and normalized to levels in mock transfected cells. CHOP, a gene that is regulated by PERK activation, was also assessed.
Fig. S3.
Transcriptional activator properties of class 2 mutant ATF6[R376*]. FLAG epitope-tagged ATF6(373) and ATF6[R376*], a class 2 mutant, were transfected into HEK293 cells. ATF6 protein levels were detected by immunoblotting using anti-FLAG antibody. BiP/GRP78 protein level was detected by immunoblotting.
Class 3 ATF6 Mutants Are Transcriptionally Inactive.
Five ATF6 mutations found in patients with achromatopsia introduce nonsense or premature stop codons in exons encoding the cytosolic domain of ATF6 and are predicted to produce ATF6 cytosolic protein fragments with nonfunctional bZIP or lacking the entire bZIP and transcriptional activator domains (Fig. S1 B and C and refs. 1 and 2). To test whether this group of ATF6 mutations compromised ATF6 function, we expressed recombinant FLAG-tagged ATF6 bearing several of these mutations, including a proline-to-leucine mutation at position 118, followed by a premature stop codon 31 amino acids distally, ATF6[P118Lfs*31], which lacks the bZIP domain and part of the transcription activator domain, and a nonsense mutation of an asparagine residue at position 267, ATF6[N267*], which lacks only the bZIP domain. We detected protein expression of these truncated ATF6 mutants after transfection into HEK293 cells (Fig. 3A), but found no transcriptional induction of ATF6 target proteins with either mutant (Fig. 3 A and B). Coupled with our prior studies of the ATF6[R324C] mutant (1), these results identify a class of disease-associated ATF6 mutations that cause loss of function through disruption of transcriptional activity by truncating the bZIP and/or transcriptional activator domains or by directly mutating critical residues in the bZIP domain in the ATF6 cytosolic domain.
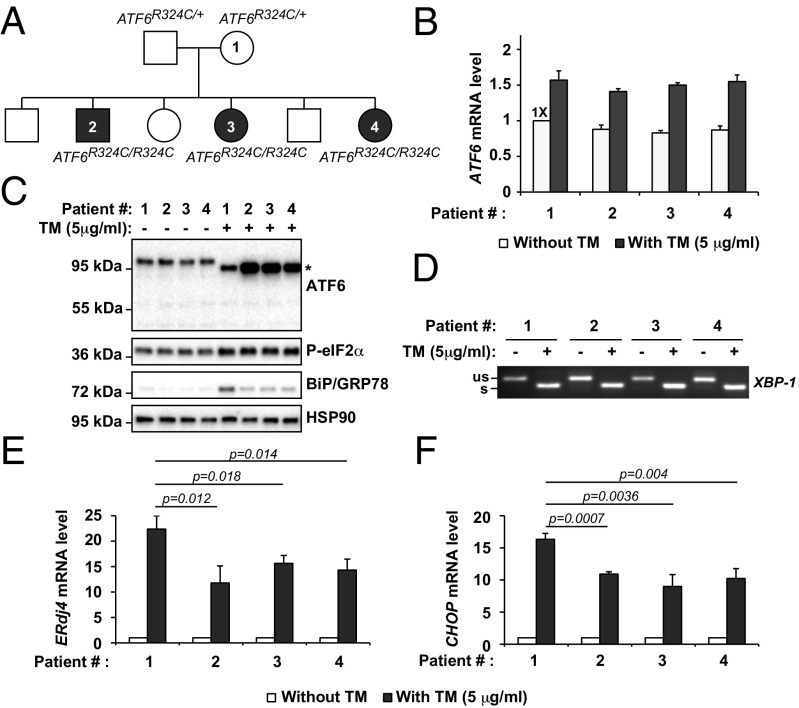
To determine how the loss of ATF6 activity affected other signaling arms of the UPR in patient cells with this class of mutations, we examined fibroblasts previously collected from a family expressing the ATF6[R324C] mutation (1). These included an unaffected heterozygous parent (ATF6R324C/+), proband 1, and three affected homozygous achromatic children (ATF6R324C/R324C), patients 2, 3, and 4 (Fig. 4A). We found comparable levels of ATF6 mRNA and full-length ATF6 protein in heterozygous and homozygous fibroblasts (Fig. 4 B and C). During experimentally induced ER stress conditions, we found increased levels of ATF6 mRNA and protein in heterozygous and homozygous fibroblasts (Fig. 4 B and C). However, we found reduced levels of BiP/GRP78 protein in ATF6R324C/R324C fibroblasts compared with heterozygous controls in response to experimental ER stress consistent with our prior studies that showed that the ATF6[R324C] mutation compromised ATF6 signaling activity (Fig. 4C). This signaling defect arose from impairment of the bZIP transcriptional activator domain itself, and not at earlier steps in the trafficking or production of the cytosolic ATF6 fragment, because full-length ATF6[R324C] protein underwent ER-to-Golgi trafficking (Fig. 5 B and C) and produced the cleaved ATF6 transcriptional activator fragment with similar kinetics to the wild-type protein in response to ER stress (Fig. 5A).
Fig. 4.
ATF6, IRE1, and PERK activity in class 3 mutant, ATF6R324C, patient fibroblasts. (A) Pedigree of the family carrying ATF6R324C alleles. The heterozygous mother (ATF6R324C/+) was indicated as patient 1. The homozygous (ATF6R324C/R324C) children were indicated as patients 2, 3, and 4. (B) ATF6R324C/+ or ATF6R324C/R324C patient fibroblasts were challenged with TM at the indicated concentration for 24 h. mRNA was collected from the fibroblasts. ATF6 mRNA levels were measured by real-time quantitative PCR and normalized to the level in the untreated ATF6R324C/+ parental control (patient #1) fibroblast cells. (C) ATF6R324C/+ or ATF6R324C/R324C patient fibroblasts were challenged with TM for 24 h. ATF6, phosphorylated-eIF2α, and BiP/GRP78 were detected in protein lysates by immunoblotting. *Position of the deglycosylated full-length ATF6 protein produced after TM treatment. (D) XBP1 mRNA splicing was visualized by semiquantitative RT-PCR from patient fibroblasts treated with TM for 24 h, as indicated. (E and F) Patient fibroblasts were challenged with TM as indicated for 24 h. The mRNA levels of IRE1 pathway downstream target gene, ERdj4 (E), and PERK pathway downstream target gene, CHOP (F), were measured by real-time quantitative PCR and normalized to levels in untreated cells.
Fig. 5.
Normal ER-to-Golgi trafficking of class 3 mutant, ATF6R324C during ER stress. (A) Recombinant FLAG-tagged ATF6[R324C] was expressed in HEK293 cells for 20 h and then challenged with DTT, as indicated. ATF6 protein levels were detected by immunoblotting with anti-FLAG. (B) Recombinant FLAG-tagged wild-type ATF6 and ATF6[R324C] were expressed in HEK293 cells for 20 h and then challenged with DTT with or without S1P inhibitor, as indicated, for 90 min. Cell lysates were treated with Endo H, as indicated. ATF6 protein levels were detected by immunoblotting with anti-FLAG. (C) ATF6[Y567N] was expressed in HEK293 cells for 20 h and then challenged with 2 mM DTT for 40 min. The subcellular localization of ATF6 was visualized by immunofluorescence labeling and confocal microscopy by anti-FLAG antibody (shown in red). The Golgi apparatus was visualized by GOLPH2 immunostaining (shown in green). The nucleus was visualized by DAPI staining (shown in blue). The percentage of cells showing FLAG and GOLPH2 colocalization was quantified and shown in the graph. (Scale bar, 10 μm.)
We next examined whether the IRE1 and PERK signaling branches of the UPR were dysregulated in ATF6R324C/R324C fibroblasts. For IRE1 pathway markers, we saw no difference in XBP1 mRNA levels or XBP1 mRNA splicing between ATF6R324C/R324C fibroblasts and heterozygous control (Fig. 4D and Fig. S4). However, we saw significantly reduced transcriptional induction of ERdj4 in ATF6R324C/R324C fibroblasts compared with heterozygous control (Fig. 4 D and E). For the PERK pathway markers, we saw no difference in eIF2α phosphorylation between ATF6R324C/R324C fibroblasts and heterozygous control (Fig. 4C). However, we saw significantly reduced transcriptional induction of CHOP in ATF6R324C/R324C fibroblasts compared with heterozygous control (Fig. 4 C and F). These studies revealed that ATF6R324C/R324C fibroblasts show compromised ATF6 transcriptional activity as well as significant impairment of the transcriptional output from the IRE1 and PERK signaling branches of the UPR during ER stress.
Fig. S4.
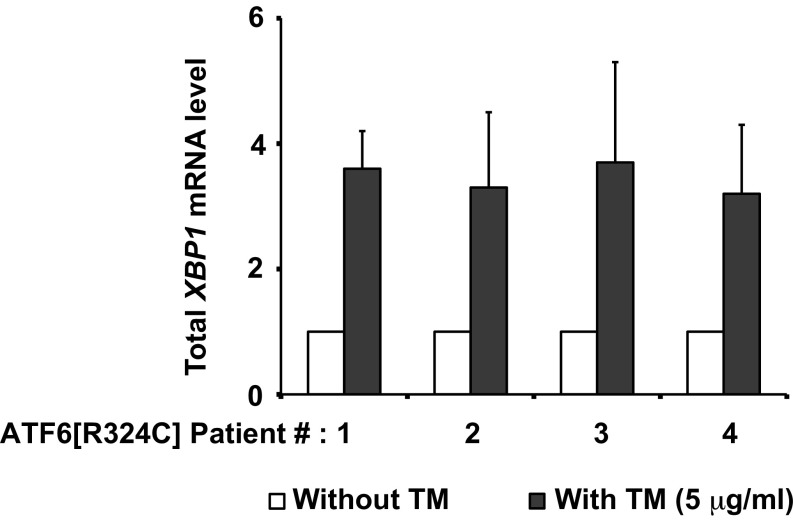
Total XBP1 mRNA levels in class 3 mutant, ATF6R324C, patient fibroblasts. Total XBP1 mRNA level was quantified by qPCR from patient fibroblasts after treatment with TM for 24 h and normalized to levels in untreated cells.
Class 1 and Class 3 ATF6 Mutant Fibroblasts Are More Susceptible to ER Stress-Mediated Cell Death.
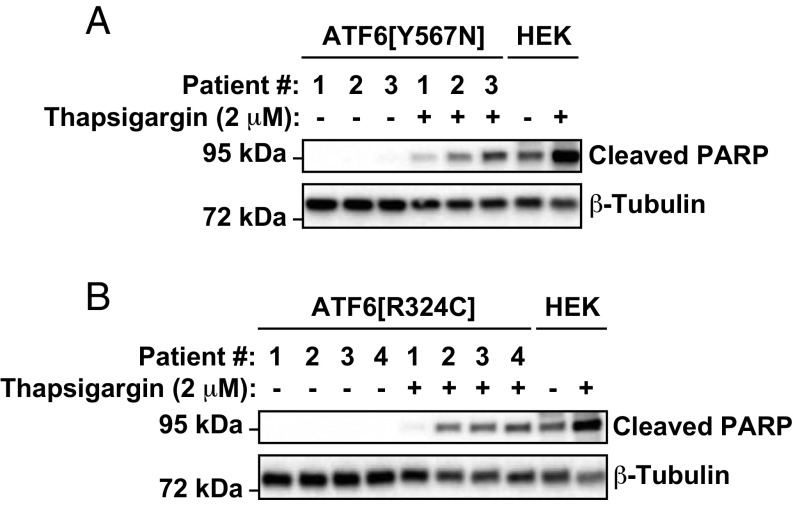
Our studies revealed that class 1 and class 3 ATF6 mutations both impaired ATF6 function. ATF6 ameliorates ER stress through its transcriptional induction of ER protein folding chaperones and enzymes (7). If ER stress is not alleviated, cells ultimately undergo cell death (22, 23). We compared kinetics of cell death in patient fibroblasts expressing the class 1 and class 3 ATF6 mutants to see how the loss of ATF6 function affected survival during extended ER stress. In response to thapsigargin exposure, we observed significantly increased levels of the apoptosis marker, cleaved poly(ADP-ribose) polymerase (PARP), in ATF6Y567N/Y567N fibroblasts compared with heterozygous controls (Fig. 6A, cf. patient 3’s fibroblasts with those of patients 1 and 2). Similarly, we observed increased levels of PARP cleavage in ATF6R324C/R324C fibroblasts compared with heterozygous parental control (Fig. 6B, cf. patients 2, 3, and 4 with patient 1’s cells). These studies revealed that a physiologic consequence of the loss of ATF6 function caused by class 1 and class 3 ATF6 mutations was heightened cell death in response to ER stress.
Fig. 6.
Class 1 or class 3 mutant ATF6 fibroblasts show increased susceptibility to ER stress-induced cell death. Class 1 ATF6Y567N/+ or ATF6Y567N/Y567N human fibroblasts (A) and class 3 ATF6R324C/+ or ATF6R324C/R324C human fibroblasts (B) were treated with thapsigargin at the indicated concentration for 3 or 6 d, respectively. Cleaved PARP protein was detected by immunoblotting. As a control for the position of cleaved PARP protein, HEK293 cells were treated with thapsigargin, and lysates were probed for PARP.
Discussion
Our studies provide a framework for functional and pathomechanistic classification of ATF6 mutations identified in achromatopsia (Fig. S1). Class 1 mutations affect the luminal domain of ATF6 and lead to loss of function. The pathomechanism underlying loss of function in class 1 ATF6 mutations is inefficient trafficking from ER to Golgi during ER stress, leading to poor production of the ATF6 transcriptional activator fragment (Fig. S1A). Class 2 mutations cluster near the transmembrane domain of ATF6. These mutations produce the entire cytosolic ATF6 fragment unbound to membrane and show fully intact ATF6 transcriptional activity (Fig. S1A). The class 2 ATF6 transcripts all bear premature stop codons and are likely targets of nonsense-mediated mRNA decay machinery (1). Nonsense-mediated decay reduces mRNA transcript levels by ∼50–85%, depending on tissue type and environmental factors (24). Therefore, whether class 2 mutations produce significant amounts of cytosolic ATF6 transcriptional activator fragment and show constitutive signaling in vivo requires further analysis in patients with these mutations. Class 3 mutations affect the cytosolic domain of ATF6 and lead to loss of function. The pathomechanism underlying loss of function in this class of mutations is deletion or mutation of the bZIP and/or transcriptional activator domain.
Do the mechanistic differences we identified between ATF6 mutations lead to phenotypic differences? In particular, we found that a class 3 mutant had loss of ATF6 signaling plus significant impairment of the transcriptional output of IRE1 and PERK signaling (Fig. 4), whereas the class 1 mutant only showed minor impairment of the transcriptional output of IRE1 and PERK signaling (Fig. 1). One possibility is that up-regulation of ER stress genes, such as ERdj4 and CHOP, requires the production of functional ATF6 cytosolic bZIP transcriptional activator domain. In this view, class 1 mutant ATF6 can still produce a functional cytosolic domain under ER stress, albeit with reduced efficiency. In contrast, class 3 mutant ATF6 produces a nonfunctional cytosolic domain. This could explain why the up-regulation of ERdj4 and CHOP under ER stress is more severely impaired in class 3 mutant ATF6s. Our identification of distinct pathomechanisms of ATF6 disease mutations enables prospective longitudinal study of retinal structure and phenotype to identify possible differences in achromatopsia disease phenotype and severity between carriers with different classes of ATF6 mutations. Evaluation with adaptive optics and other retinal imaging modalities may reveal cone phenotype differences corresponding to different classes of ATF6 mutations (25, 26).
What are the therapeutic implications of differences between ATF6 mutations’ mechanisms of pathology? Class 1 ATF6 mutants inefficiently traffic from ER to Golgi but have normal bZIP transcriptional activator domains. For this class of mutations, our findings indicate that therapeutic strategies should focus on improving protein trafficking out of the ER so the full-length ATF6 protein can be cleaved by the S1P and S2P Golgi-resident proteases. Once cleaved and separated from the defective luminal domain, the cytosolic ATF6 domain can engage in its normal transcriptional activator role. In contrast, the class 3 ATF6 mutants require a different therapeutic approach than class 1 trafficking mutants because class 3 mutants completely lack or bear defective bZIP/transcriptional activator domains. Gene therapy to introduce functional ATF6 bZIP transcriptional activator or gene editing to repair the primary ATF6 nucleotide alterations may be potential therapeutic strategies for patients with these types of ATF6 mutations.
Our current study highlights the surprisingly diverse molecular defects in the mechanism of ATF6 activation caused by achromatopsia-associated mutations. Patient fibroblasts with several of these mutations showed increased sensitivity to ER stress-induced cell death and damage. Diverse environmental insults have been found to trigger ER stress, including hypoxia, infection, inflammation, protein misfolding, and light damage (27–33). Exposure to these insults during retinal development may contribute to the cone dysfunction and vision loss that arises in children with mutations that compromise ATF6 function.
Materials and Methods
Cell Culture and Transfection.
The maintenance and drug treatments of human primary fibroblast cells or HEK293 cells were described in detail in SI Materials and Methods. The generation of ATF6 mutant plasmids and the expression of these ATF6 mutants were described in detail in SI Materials and Methods.
Molecular Biology.
Cells were lysed, and total RNA was collected using the RNeasy mini kit according to manufacturer’s instructions (Qiagen). mRNA was reverse-transcribed, using the iScript cDNA Synthesis Kit (Bio-Rad). The methods for analyzing the level of XBP1 mRNA splicing and the qPCR analysis of gene expression are described in detail in SI Materials and Methods.
Immunoblotting Analysis.
Human fibroblasts or HEK293 cells expressing wild-type or mutant ATF6 were lysed in SDS lysis buffer [2% (g/vol) SDS, 62.5 mM Tris⋅HCl at pH 6.8, containing protease inhibitors (Sigma-Aldrich) and phosphatase inhibitor (Thermo Scientific)]. Protein concentrations of the total cell lysates were determined by bicinchoninic acid (BCA) protein assay (Pierce). Equal amounts of protein were loaded onto 10% or 4–15% Mini-PROTEAN TGX precast gels (Bio-Rad) and analyzed by Western blot, as described in SI Materials and Methods.
Endo H (New England Biolabs) digestion was performed on precleared total cell lysate for 1 h at 37 °C in the buffer supplied by the manufacturer. To preclear the cellular debris from the total lysate, cell lysate were spun at 21,900 × g at 4 °C for 1 h.
Immunofluorescence and Confocal Microscopy.
Cells were grown on poly-d-lysine coated glass coverslips and transfected with wild-type ATF6 or ATF6[Y567N]. For immunofluorescence analysis, cells were fixed for 20 min at room temperature in 4% paraformaldehyde in PBS at pH 7.4, washed briefly with PBS, and permeabilized with 0.1% Triton X-100 in PBS. Cells were then washed two times with 1% BSA in PBS and blocked with 5% (vol/vol) goat serum in 1% BSA/PBS for 20 min. The coverslips were then incubated at room temperature for 1 h with primary antibodies followed by secondary antibody incubation as described in SI Materials and Methods. The coverslips were mounted in ProLong Gold antifade reagent with DAPI (Invitrogen), and images were collected with an Olympus FluoView-1000 confocal microscope.
SI Materials and Methods
Cell Culture.
Human primary fibroblast cells or HEK293 cells were maintained at 37 °C, 5% CO2 in Dulbecco’s modified Eagle medium (Mediatech), supplemented with 10% FCS (Mediatech), and 1% penicillin/streptomycin (Invitrogen). To induce ER stress, TM or thapsigargin (Calbiochem EMD Bioscience Inc.) were dissolved in dimethylformamide, and DTT (BioPioneer Inc.) was dissolved in water and added to the cell culture media at the indicated concentration. S1P inhibitor PF 429242 (Tocris Bioscience) was dissolved in DMSO and added to the cell culture media at the indicated concentration.
Plasmid Construction and Transfection.
To generate ATF6[P118Lfs*31], ATF6[N267*], and ATF6[V371Sfs*3] expression plasmids, nucleotide mutation, 353delC, 797dupC, or 1110dupA was introduced into the coding region of FLAG-tag-ATF6(373).pcDNA3.1 plasmid by site-direct mutagenesis, using QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies Inc.). To generate ATF6[R324C], ATF6[R376*], ATF6[D564G], and ATF6[Y567N] expression plasmids, mutations were introduced into the coding region of full-length ATF6.pcDNA3.1 plasmid by site-direct mutagenesis, using QuikChange II Site-Directed Mutagenesis Kit. To express wild-type or mutant ATF6 in the cell culture system, plasmids containing the cDNA of those genes were transiently transfected to HEK293, using Lipofectamine 2000 (Invitrogen).
Molecular Biology.
Cells were lysed and total RNA collected using the RNeasy mini kit, according to manufacturer’s instructions (Qiagen). mRNA was reverse-transcribed using the iScript cDNA Synthesis Kit (Bio-Rad). To analyze the level of XBP1 mRNA splicing, cDNA was used as template for PCR amplification across the fragment of the XBP1 cDNA bearing the intron target of IRE1’s RNase activity. Primers used included: human XBP1, 5′-TTACGAGAGAAAACTCATGGC-3′ and 5′- GGGTCCAAGTTGTCCAGAATGC-3′. PCR conditions were: 95 °C for 5 min, 95 °C for 1 min, 58 °C for 30 s, 72 °C for 30 s, and 72 °C for 5 min with 35 cycles of amplification. PCR products were resolved on a 2.5% agarose/1× TAE (Tris base, acetic acid, and EDTA) gel.
For quantitative PCR analysis, cDNA was used as template in SYBR green qPCR supermix (Bio-Rad). Primers used include: human RPL19, 5′-ATGTATCACAGCCTGTACCTG-3′ and 5′-TTCTTGGTCTCTTCCTCCTTG-3′; human BIP/GRP78, 5′-GCCTGTATTTCTAGACCTGCC-3′ and 5′-TTCATCTTGCCAGCCAGTTG-3′; human HERPUD1, 5′-AACGGCATGTTTTGCATCTG-3′ and 5′-GGGGAAGAAAGGTTCCGAAG-3′; human SEL1L, 5′-ATCTCCAAAAGGCAGCAAGC-3′ and 5′-TGGGAGAGCCTTCCTCAGTC-3′; human CHOP, 5′-ACCAAGGGAGAACCAGGAAACG-3′ and 5′-TCACCATTCGGTCAATCAGAGC-3′; human ERdj4, 5′-GGAAGGAGGAGCGCTAGGTC-3′ and 5′-ATCCTGCACCCTCCGACTAC-3′; human ATF6, 5′- GCTTTACATTCCTCCACCTCCTTG -3′ and 5′- ATTTGAGCCCTGTTCCAGAGCAC-3′; human total XBP1, 5′-GCAAGCGACAGCGCCT-3′ and 5′-TTTTCAGTTTCCTCCTCAGCG-3′. Rpl19 mRNA levels served as internal normalization standards. qPCR condition was 95 °C for 5 min, 95 °C for 10 s, 60 °C for 10 s, 72 °C for 10 s, with 40 cycles of amplification.
Immunoblotting Analysis.
Human fibroblasts or HEK293 cells expressing wild-type or mutant ATF6 were lysed in SDS lysis buffer (2% SDS, 62.5 mM Tris⋅HCl at pH 6.8, containing protease inhibitors (Sigma-Aldrich), and phosphatase inhibitor (Thermo Scientific). Protein concentrations of the total cell lysates were determined by BCA protein assay (Pierce). Equal amounts of protein were loaded onto 10% or 4–15% Mini-PROTEAN TGX precast gels (Bio-Rad) and analyzed by Western blot. The following antibodies and dilutions were used: anti-FLAG at 1:5,000 (Sigma-Aldrich); anti-GFP at 1:1,000 (Santa Cruz Biotechnologies); anti-human ATF6α antibody at 1:1,000 (Abcam); anti-phosph-eIF2α and anti-cleaved PARP at 1:1,000 (Cell Signaling); anti-BiP/GRP78 at 1:1,000, anti-GAPDH at 1:5,000, anti-HSP90 at 1:5,000, and anti-β-tubulin at 1:5,000 (GeneTex Inc). After overnight incubation with primary antibody, membranes were washed in TBS with 0.1% Tween-20, followed by incubation of a horseradish peroxidase-coupled secondary antibody (Cell Signaling). Immunoreactivity was detected using the SuperSignal West chemiluminescent substrate (Pierce). HSP90, GAPDH, or β-tubulin levels were assessed as a loading control as indicated.
Endo H (New England Biolabs) digestion was performed on precleared total cell lysate for 1 h at 37 °C in the buffer supplied by the manufacturer. To preclear the cellular debris from the total lysate, cell lysate were spun at 21,900 × g at 4 °C for 1 h.
Immunofluorescence and Confocal Microscopy.
Cells were grown on poly-d-lysine-coated glass coverslips and transfected with wild-type ATF6 or ATF6[Y567N]. For immunofluorescence analysis, cells were fixed for 20 min at room temperature in 4% paraformaldehyde in PBS at pH 7.4, washed briefly with PBS, and permeabilized with 0.1% Triton X-100 in PBS. Cells were then washed two times with 1% BSA in PBS and blocked with 5% goat serum in 1% BSA/PBS for 20 min. The coverslips were then incubated at room temperature for 1 h with the mouse monoclonal anti-FLAG antibody (Sigma-Aldrich), used at a dilution of 1:500; the rabbit polyclonal anti-GOLPH2 antibody (GeneTex Inc.), used at a dilution of 1:500; and the rabbit polyclonal anti-PDI antibody (Enzo Life Sciences, Inc.), used at a dilution of 1:500. After washing in 0.1% BSA in PBS three times, cells were incubated with secondary antibodies. Secondary antibodies included Alexa546 goat anti-mouse (green) antibody (Molecular Probes) and Alexa488 goat anti-rabbit (red) antibody (Molecular Probes), and these were used at a dilution of 1:250. After washing in PBS three times, the coverslips were mounted in ProLong Gold antifade reagent with DAPI (Invitrogen), and images were collected with an Olympus FluoView-1000 confocal microscope and processed using Olympus FluoView Ver.2.0a Viewer software at University of California, San Diego, School of Medicine Microscopy Core.
Statistical Analysis.
All results are expressed as mean ± SD of at least three independent experiments. Two-tailed t tests were performed to determine P values for paired samples.
Acknowledgments
We thank M. Wilkinson for helpful discussions on NMD and members of the J.H.L. laboratory for helpful review of the manuscript. S.H.T. is supported by the Barbara & Donald Jonas Laboratory of Regenerative Medicine; the Bernard & Shirlee Brown Glaucoma Laboratory; NIH Grants 5P30EY019007,R01EY018213, R01EY024698, R01EY026682, R21AG050437, and 5P30CA013696; the Research to Prevent Blindness Physician-Scientist Award; unrestricted funds from Research to Prevent Blindness; the Tistou and Charlotte Kerstan Foundation; the Crowley Family Fund; the Schneeweiss Stem Cell Fund; New York State (C029572); the Foundation Fighting Blindness New York Regional Research Center Grant (C-NY05-0705-0312); and the Gebroe Family Foundation. R.J.K. is supported by NIH Grants DK042394, DK103185, and DK088227. J.H.L. is supported by NIH Grants EY020846, NS088485, and U54OD020351, and VA Grant BX002284. The University of California, San Diego, School of Medicine Microscopy Core is supported by NIH Grants P30NS047101 and P30EY022589.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606387114/-/DCSupplemental.
References
- 1.Kohl S, et al. Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat Genet. 2015;47(7):757–765. doi: 10.1038/ng.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansar M, et al. University of Washington Center for Mendelian Genomics Mutation of ATF6 causes autosomal recessive achromatopsia. Hum Genet. 2015;134(9):941–950. doi: 10.1007/s00439-015-1571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu M, et al. ATF6 Is Mutated in Early Onset Photoreceptor Degeneration With Macular Involvement. Invest Ophthalmol Vis Sci. 2015;56(6):3889–3895. doi: 10.1167/iovs.15-16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10(11):3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye J, et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6(6):1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 6.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3(1):99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, et al. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13(3):351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto K, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13(3):365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Nadanaka S, Yoshida H, Kano F, Murata M, Mori K. Activation of mammalian unfolded protein response is compatible with the quality control system operating in the endoplasmic reticulum. Mol Biol Cell. 2004;15(6):2537–2548. doi: 10.1091/mbc.E03-09-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, et al. Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J Biol Chem. 2000;275(35):27013–27020. doi: 10.1074/jbc.M003322200. [DOI] [PubMed] [Google Scholar]
- 11.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529(7586):326–335. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
- 13.Calfon M, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415(6867):92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 15.Shoulders MD, et al. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Reports. 2013;3(4):1279–1292. doi: 10.1016/j.celrep.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassler JR, et al. The IRE1α/XBP1s Pathway Is Essential for the Glucose Response and Protection of β Cells. PLoS Biol. 2015;13(10):e1002277. doi: 10.1371/journal.pbio.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5(5):897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 18.Scheuner D, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 19.Harding HP, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 20.Chiang WC, Hiramatsu N, Messah C, Kroeger H, Lin JH. Selective activation of ATF6 and PERK endoplasmic reticulum stress signaling pathways prevent mutant rhodopsin accumulation. Invest Ophthalmol Vis Sci. 2012;53(11):7159–7166. doi: 10.1167/iovs.12-10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bommiasamy H, et al. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J Cell Sci. 2009;122(Pt 10):1626–1636. doi: 10.1242/jcs.045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin JH, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318(5852):944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutkowski DT, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4(11):e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zetoune AB, et al. Comparison of nonsense-mediated mRNA decay efficiency in various murine tissues. BMC Genet. 2008;9:83. doi: 10.1186/1471-2156-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aboshiha J, et al. A prospective longitudinal study of retinal structure and function in achromatopsia. Invest Ophthalmol Vis Sci. 2014;55(9):5733–5743. doi: 10.1167/iovs.14-14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genead MA, et al. Photoreceptor structure and function in patients with congenital achromatopsia. Invest Ophthalmol Vis Sci. 2011;52(10):7298–7308. doi: 10.1167/iovs.11-7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroeger H, et al. Induction of endoplasmic reticulum stress genes, BiP and chop, in genetic and environmental models of retinal degeneration. Invest Ophthalmol Vis Sci. 2012;53(12):7590–7599. doi: 10.1167/iovs.12-10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang LP, Wu LM, Guo XJ, Li Y, Tso MO. Endoplasmic reticulum stress is activated in light-induced retinal degeneration. J Neurosci Res. 2008;86(4):910–919. doi: 10.1002/jnr.21535. [DOI] [PubMed] [Google Scholar]
- 29.Nakanishi T, et al. Role of endoplasmic reticulum stress in light-induced photoreceptor degeneration in mice. J Neurochem. 2013;125(1):111–124. doi: 10.1111/jnc.12116. [DOI] [PubMed] [Google Scholar]
- 30.Zhang SX, Ma JH, Bhatta M, Fliesler SJ, Wang JJ. The unfolded protein response in retinal vascular diseases: implications and therapeutic potential beyond protein folding. Prog Retin Eye Res. 2015;45:111–131. doi: 10.1016/j.preteyeres.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang SX, Sanders E, Fliesler SJ, Wang JJ. Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration. Exp Eye Res. 2014;125(0):30–40. doi: 10.1016/j.exer.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alavi MV, et al. In Vivo Visualization of Endoplasmic Reticulum Stress in the Retina Using the ERAI Reporter Mouse. Invest Ophthalmol Vis Sci. 2015;56(11):6961–6970. doi: 10.1167/iovs.15-16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiang WC, et al. Robust Endoplasmic Reticulum-Associated Degradation of Rhodopsin Precedes Retinal Degeneration. Mol Neurobiol. 2015;52(1):679–695. doi: 10.1007/s12035-014-8881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]