Significance
Shoot-branching patterns affect key aspects of plant life and are important targets for crop breeding. However, we are still ignorant of the genetic mechanisms controlling locally an important decision during branch development: whether the axillary bud grows out to give a lateral shoot or remains dormant. Here we show that the TEOSINTE BRANCHED1, CYCLOIDEA, PCF (TCP) transcriptional regulator BRANCHED1 (BRC1), which acts inside axillary buds, binds and activates three genes encoding Homeodomain leucine zipper (HD-ZIP) transcription factors. These factors, together with BRC1, trigger a cascade leading to local abscisic acid (ABA) accumulation and response, essential for bud dormancy under light-limiting conditions. This finding demonstrates a direct relationship between BRC1 and ABA signaling and places ABA downstream of BRC1 in the control of axillary bud dormancy.
Keywords: abscisic acid, TCP proteins, HD-ZIP proteins, bud dormancy, Arabidopsis
Abstract
Shoot-branching patterns determine key aspects of plant life and are important targets for crop breeding. However, we are still largely ignorant of the genetic networks controlling locally the most important decision during branch development: whether the axillary bud, or branch primordium, grows out to give a lateral shoot or remains dormant. Here we show that, inside the buds, the TEOSINTE BRANCHED1, CYCLOIDEA, PCF (TCP) transcription factor BRANCHED1 (BRC1) binds to and positively regulates the transcription of three related Homeodomain leucine zipper protein (HD-ZIP)-encoding genes: HOMEOBOX PROTEIN 21 (HB21), HOMEOBOX PROTEIN 40 (HB40), and HOMEOBOX PROTEIN 53 (HB53). These three genes, together with BRC1, enhance 9-CIS-EPOXICAROTENOID DIOXIGENASE 3 (NCED3) expression, lead to abscisic acid accumulation, and trigger hormone response, thus causing suppression of bud development. This TCP/HD-ZIP genetic module seems to be conserved in dicot and monocotyledonous species to prevent branching under light-limiting conditions.
In flowering plants, lateral shoots develop from axillary buds formed at the base of leaves. These buds, comprising a meristem, a few leaf primordia, and sometimes flower meristems, can become quiescent at this stage or can continue their development to form branches. Bud growth arrest, or dormancy, is promoted by various environmental and developmental factors including a canopy shade rich in far-red (FR) light or an actively growing shoot apex. When these stimuli are suppressed, growth can resume, and the bud develops into a shoot.
In Arabidopsis thaliana, the class II TEOSINTE BRANCHED1, CYCLOIDEA, PCF (TCP) gene BRANCHED1 (BRC1) functions inside axillary buds (1) to prevent constitutive branch outgrowth, and it mediates bud dormancy induced by FR-rich light or apical dominance. In brc1 mutants most buds complete their development without restraint and have a reduced response to changes in the red (R):FR light ratio or decapitation. Moreover, BRC1 mRNA levels are increased within hours of treatment with white light (W) highly supplemented with FR (W+FR) and are decreased shortly after decapitation (1–4). BRC1’s inhibitory effect on growth and development is spatially restricted to axillary buds, and it can be turned off by appropriate signals. However, when ectopically expressed in seedlings, BRC1 also can cause a rapid growth cessation in shoot and root apical meristem and leaf primordia (3). Likewise, generalized overexpression of the Solanum tuberosum ortholog, StBRC1a, produces dwarf plants with very small leaves and short internodes in potato (5). Despite these remarkable effects on growth and development and their well-known, critical role in the suppression of shoot branching, the downstream pathways by which BRC1-like genes promote bud dormancy are still largely unknown.
Transcriptomic data of wild-type and brc1 Arabidopsis axillary buds treated with W+FR light revealed two BRC1-dependent gene-regulatory networks (GRNs) down-regulated in response to BRC1. One is enriched in DNA synthesis-, cell cycle-, and cytokinesis-related genes, the other in protein synthesis-related genes (3). Gene promoters of both GRNs have a significant overrepresentation of TCP-binding sites, and therefore it was proposed that BRC1 represses these GRNs directly, or indirectly by competition with other TCP factors (3). The same study revealed a strong induction of abscisic acid (ABA)-specific marker genes in response to BRC1 (3), indicating that BRC1 enhances or maintains ABA signaling in buds. Indeed, ABA accumulation is required for bud growth suppression in wild-type plants: plants bearing mutations in genes involved in ABA synthesis [9-CIS-EPOXYCAROTENOID DIOXYGENASE3 (NCED3) and ABA DEFICIENT2 (ABA2)] display enhanced bud outgrowth (4, 6). Furthermore, a meta-analysis of three transcriptomic studies of active vs. dormant buds revealed that a GRN of ABA-related genes is induced in dormant buds regardless the stimuli involved (7).
Here we have investigated further the relationship between BRC1 activity and ABA signaling. We have found that BRC1 directly activates a group of phylogenetically related genes, HB21 (HOMEOBOX PROTEIN 21, At2g18550), HB40 (HOMEOBOX PROTEIN 40, At4g36740), and HB53 (HOMEOBOX PROTEIN 53, At5g66700), encoding class I Homeodomain leucine zipper (HD-ZIP) transcription factors (TFs). These genes are necessary and sufficient for enhanced expression of NCED3, a key ABA biosynthesis gene, and for normal ABA accumulation inside axillary buds in conditions of low R:FR or short photoperiods. This pathway has a strong influence on the expression and maintenance of an ABA-related GRN induced in dormant buds, and is essential for negative regulation of bud development and branch outgrowth under limiting light conditions.
Results
HB21, HB40, and HB53 are BRC1-Dependent Genes.
In dormant buds, a GRN of ABA-related genes is induced (7). Because BRC1 promotes the expression of ABA-marker genes in buds (3), we investigated the relationship between BRC1 and ABA signaling. To do so, we searched for BRC1-dependent genes (defined as genes induced in wild-type but not in brc1 buds treated with W+FR) (3) among the genes of the ABA-related GRN (SI Appendix, Fig. S1A and Dataset S1). We found 26 BRC1-dependent genes in the ABA-related GRN (SI Appendix, Fig. S1B). This list included 12 genes encoding TFs of the HD-ZIP, MYB, basic leucine zipper (bZIP), AP2, and NAM, ATAF1,2, CUC2 (NAC) families. Because TFs can have a strong influence on transcriptional networks, we hypothesized that some of these genes could play an important role in the local response to ABA in axillary buds. We focused on three closely related HD-ZIP protein-encoding genes: HB21, HB40, and HB53 (SI Appendix, Fig. S2).
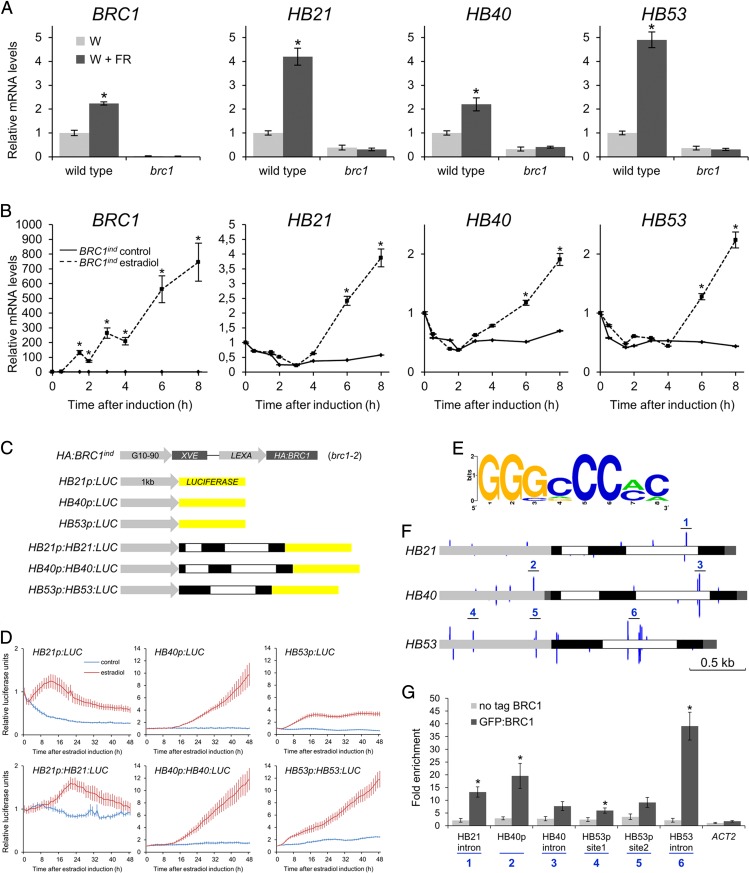
HB21, HB40, and HB53 mRNA levels correlated with BRC1 levels and bud growth arrest. Like BRC1, they were up-regulated in wild-type buds treated with W+FR light (R:FR = 0.2) for 8 h relative to plants treated with W light (R:FR = 11.7). This response was abolished in brc1 mutants (Fig. 1A). Also, they were down-regulated in active buds 24 h after decapitation (SI Appendix, Fig. S3A). When decapitated plants were apically treated with auxin, mRNA levels reverted to those of intact plants (HB40 and HB53) or to higher levels (HB21) SI Appendix, Fig. S3A). Sucrose treatments on buds caused down-regulation of BRC1 (SI Appendix, Fig. S3B) as described in pea and rose (8, 9). Likewise, HB21 and HB53 (but not HB40) mRNA levels decreased after a sugar treatment (SI Appendix, Fig. S3B). Then we investigated whether the expression of these genes correlated with BRC1 activity in tissues other than buds. Estradiol-inducible BRC1 (BRC1ind) seedlings displayed a strong induction of BRC1 mRNA (Fig. 1B) and accumulation of the BRC1 protein (SI Appendix, Fig. S4) 2–4 h after estradiol application. Likewise, HB21, HB40, and HB53 mRNA levels rose 4–6 h after estradiol application in these seedlings (Fig. 1B and Fig. S5).
Fig. 1.
BRC1 binds HB21, HB40, and HB53 and controls their transcription. (A and B) HB21, HB40, and HB53 mRNA levels correlate with BRC1 levels. mRNA levels of BRC1, HB21, HB40, and HB53 were analyzed by quantitative PCR in wild-type and brc1 buds treated with W or W+FR light for 8 h (A) and in 7-d-old BRC1ind seedlings after treatment with 10 µM estradiol (B). (C) Schematic representation of reporter constructs transformed into HA:BRC1ind; brc1-2 lines. (D) LUC activity after BRC1 induction with 10 µM estradiol. Levels are relative to t = 0 after induction. Error bars show SEM of eight plants per line for each treatment. (E) Logo representing the frequency matrix of the consensus motif obtained from the alignment of the 10 best-scored binding sites in PBM assays. (F) BRC1-binding motifs in a 1-kb region upstream of the ATG start codon (gray) and genomic regions (exons black, introns white) of HB21, HB40, and HB53. Peak height is proportional to the similarity between sequence and consensus. Numbers indicate the peaks with the highest Rsat score (12). (G) Relative enrichment of GFP:BRC1 binding to sites 1–6. ACT2 was used as a negative control. Error bars show the SEM of three biological replicates (A and B), eight biological replicates (D), and three biological replicates with two technical repetitions (G). Asterisks indicate significant differences (P < 0.05; student’s t-test) between control and treated plants (A) and between untagged BRC1ind and GFP:BRC1ind lines (G).
To test their response to BRC1 further, we introduced a LUCIFERASE (LUC) reporter fused to either the promoter (HBp:LUC) or the genomic sequence (promoter and coding region with introns, HBp:HB:LUC) of each gene into HA:BRC1ind ; brc1-2 lines (Fig. 1C). We monitored LUC activity in 7-d-old seedlings after estradiol induction of HA:BRC1. LUC activity increased following treatment in all lines (Fig. 1D). These results indicate that BRC1 is sufficient to cause up-regulation of HB21, HB40, and HB53 not only in axillary buds where BRC1 is expressed (1) but also in tissues where BRC1 usually is not expressed, such as seedlings.
HB21, HB40, and HB53 Are Direct BRC1 Targets.
To elucidate whether HB21, HB40, and HB53 were BRC1 direct targets, we looked for BRC1-binding sites in their genomic regions. We first studied BRC1 DNA-binding specificity using protein-binding microarray (PBM11) assays (10, 11) by incubating BRC1 fused to the MALTOSE-BINDING PROTEIN (BRC1:MBP) with PBM11 microarrays. The consensus binding motif obtained was GGgcCCmc (Fig. 1E). We used the position weight matrix obtained to search for BRC1-binding sites in the HB21/40/53 genomic regions including 1 kb upstream of the ATG start codon of each gene. We found the BRC1-binding motif in all three promoters and introns as well as in some exons (Fig. 1F). These sequences and their locations were partially conserved in closely related Brassicaceae species (SI Appendix, Figs. S6 and S7). To verify that BRC1 bound directly to these regions, we performed ChIP assays using GFP:BRC1ind seedlings (SI Appendix, Fig. S8). We tested BRC1 binding to the six potential BRC1 regions with the highest scores for the BRC1 position weight matrix (Fig. 1F, sites 1–6). We compared the immunoprecipitated DNA of estradiol-treated GFP:BRC1ind vs. untagged BRC1ind lines and found a significant enrichment for sites 1, 2, 4, and 6 (Fig. 1G), indicating that BRC1 bound directly to these genomic regions in vivo.
HB21, HB40, and HB53 Are Expressed in Axillary Buds.
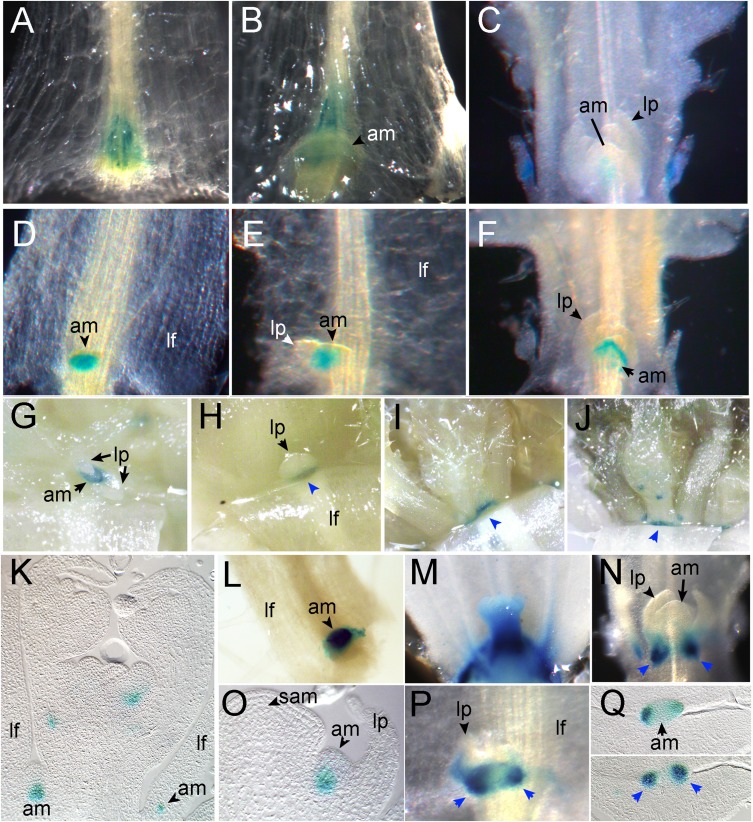
If these three genes are bona fide BRC1 direct targets, they should be expressed in regions at least partially overlapping with BRC1 expression domains (1). To investigate whether these regions do overlap, we studied HB21, HB40, and HB53 expression patterns in developing axillary buds in more than 10 representative Arabidopsis transgenic lines carrying HBp:β-GLUCURONIDASE (HBp:GUS) transcriptional fusions (with 1-, 1-, and 2-kb regions upstream of the ATG start codon of HB21, HB40, and HB53, respectively). All three gene promoters drove GUS expression in young axillary buds in overlapping but not identical patterns (Fig. 2). Expression of the three genes often was associated with provascular and vascular tissues. Expression was detectable from very early stages in the leaf vascular tissue at the position where axillary meristems initiate (Fig. 2 A and B), in young axillary meristems (Fig. 2 D, K, L, and O), and at the base of young axillary buds (Fig. 2, H–J, N, P, and Q). GUS usually was excluded from bud leaf primordia (Fig. 2 C and E–H). In older buds the signal became restricted to the base of buds (Fig. 2 H–J, N, and Q). These expression patterns overlapped with those described for BRC1 (1) and are in agreement with a potential transcriptional regulation of these genes by BRC1. The HB40p:GUS and HB53p:GUS lines also showed GUS activity in stomata of floral tissues (SI Appendix, Fig. S9 A, B, D, and E), the HB40p:GUS line showed GUS activity in pollen grains (SI Appendix, Fig. S9C), and the HB53p:GUS line showed GUS activity in developing lateral roots (SI Appendix, Fig. S9 F–H).
Fig. 2.
HB21, HB40, and HB53 are expressed in axillary buds. GUS activity in axillary buds of transgenic HB21p:GUS (A–C), HB40p:GUS (D–J), and HB53p:GUS (K–Q) lines. HB21p:GUS leaf vascular tissue stained at the position where the axillary meristem will initiate (A), underneath young axillary meristem (B), and at the inner layers of young axillary buds but absent from leaf primordia (C). (D–G) HB40p:GUS activity is present in the axillary meristem but is excluded from leaf primordia. (H–J) In HB40p:GUS older axillary buds, GUS signal is restricted to the base of the bud (arrowheads). (K–M) HB53p:GUS activity in young axillary meristems. (N–Q) HB53p:GUS activity at the base of leaf primordia in buds (arrowheads). K, O, and Q are sections of stained material embedded in plastic. am, axillary meristem; lf, leaf; lp, leaf primordia; sam, shoot apical meristem.
HB21, HB40, and HB53 Redundantly Repress Shoot Branching.
To study the role of these HD-ZIP proteins during axillary bud development, we obtained homozygous transfer-DNA (T-DNA) insertion lines for the three genes (hb21-1, hb40-1, and hb53-1 and hb53-2) (SI Appendix, Fig. S10A). These mutants were predicted to generate truncated proteins lacking a putative AHA activation domain (13) identified in the C-terminal end of each protein. In the case of hb40-1, the mutant protein also lacked the HD-ZIP domain. Moreover, insertions disrupted the transcription of each gene but not that of the other two or of BRC1 (SI Appendix, Fig. S10B), suggesting that transcriptional cross-regulation did not take place between these HD-zip genes and that they did not affect BRC1 expression.
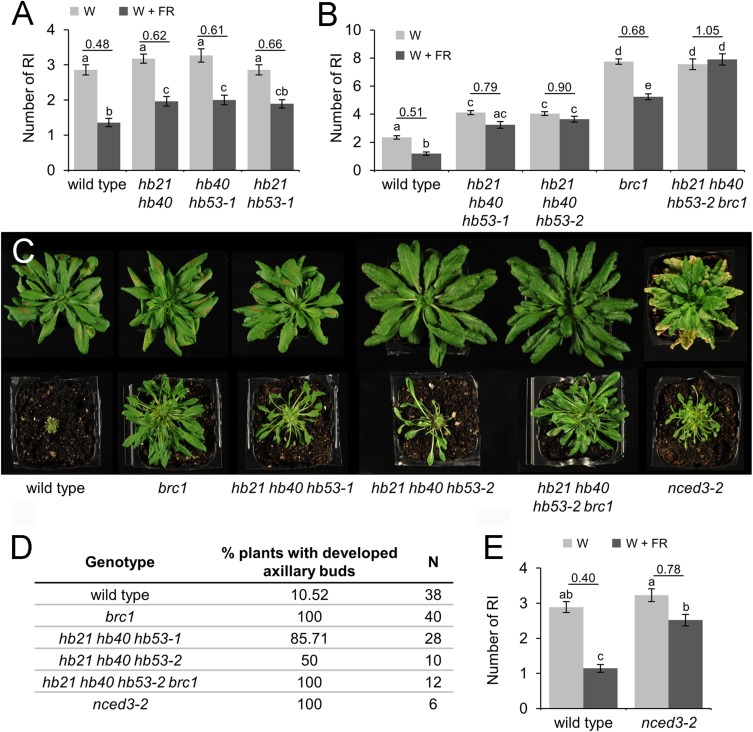
We studied the branching phenotype of single, double, and triple mutants bearing these insertions in W and W+FR light. We grew wild-type and mutant plants in W light and long days until flowering. Then we transferred half of the plants to W+FR light and maintained the other half in W light. Two weeks later, we counted the number of primary rosette branches (RI) of each plant set. As described (3), wild-type plants grown in W light had around three RI, whereas wild-type plants grown in W+FR light had one or two RI (a 40–50% reduction in the number of RI relative to the number in plants grown in W light) (Fig. 3A). The number of RI in single mutants and in hb21 hb40, hb21 hb53-1, and hb40 hb53-1 double mutants grown in W light was similar to the number of RI in wild-type plants (Fig. 3A and SI Appendix, Fig. S11A). However, hb21 hb40 and hb40 hb53-1 double mutants had a reduced response to W+FR light (62 and 61%, respectively) (Fig. 3A), and hb21 hb40 hb53-1 and hb21 hb40 hb53-2 triple mutants had an even more reduced response (79 and 90%, respectively) (Fig. 3B). Moreover, unlike wild-type plants, the hb21 hb40 hb53-2 triple mutant had a similar number of secondary cauline (CII) branches in both light regimes (SI Appendix, Fig. S11B). In summary, the branch-suppression response to W+FR light was significantly reduced in double and triple mutants of HB21, HB40, and HB53. The reduced response of the triple mutants was even more reduced than that of brc1 mutants (Fig. 3B) (3).
Fig. 3.
HB21, HB40, and HB53 act redundantly to repress shoot branching. Branching phenotypes of plants grown in W or W+FR light for 2 wk after flowering (n = 25–52). (A) Wild-type plants and double hb mutants. (B) Wild-type plants, brc1 mutants, hb triple mutants, and quadruple hb21 hb40 hb53-2 brc1 mutants. (C, Upper) Rosettes of plants grown until flowering in short-day conditions viewed from above. (Lower) The same plants after the removal of all of the rosette leaves to display axillary bud leaves. (D) Percentage of plants grown in short-day conditions that display axillary buds with developed leaves. (E) Branching phenotype of wild-type plants and nced3-2 mutants 2 wk after bolting. n = 21–28. Error bars show SEM. Letters denote significant differences among means (P < 0.05; one-way ANOVA).
We then investigated whether these genes affected early (vegetative) bud development, as described for BRC1 (1). In long days (16 h light/8 h dark) we could not find significant phenotypic differences between the mutant and wild-type plants (SI Appendix, Fig. S11C). Then we grew the triple mutants under short-day conditions (8 h light/16 h dark), in which axillary buds undergo vegetative development for several weeks before flowering of the main shoot, and found that leaves of mutant buds were remarkably more developed than those of wild-type buds (Fig. 3 C and D). This phenotype resembled but was milder than that of the brc1 mutants (Fig. 3 C and D) (1). Furthermore, the branching phenotype of the quadruple mutant hb21 hb40 hb53-2 brc1 was not more extreme than that of the parents, either in W+FR light or in short-day photoperiods (Fig. 3 B–D), indicating that HB21/40/53 and BRC1 act in the same pathway.
Taken together, these results suggest that HB21, HB40, and HB53 act redundantly to slow axillary bud development and branch outgrowth in the same genetic pathway as BRC1 and that their functions are essential in low R:FR light conditions and in short-day photoperiods.
BRC1, HB21, HB40, and HB53 Regulate NCED3 Expression and ABA Levels in Buds.
Another BRC1-dependent gene coregulated with HB21, HB40, and HB53 is NCED3 (At3g14440) (SI Appendix, Fig. S1B and Dataset S1) (3, 7). NCED3 encodes an enzyme that catalyzes the cleavage of 9-cis-epoxycarotenoids to xanthoxin, a key regulatory step of ABA biosynthesis (14). NCED3 also plays a role in the control of branch outgrowth: nced3-2 mutants have increased branching in low and high R:FR light (4, 6). In our W conditions, the number of branches in nced3-2 mutants was similar to that of wild-type plants, but their branch suppression response to W+FR light was reduced (78%) (Fig. 3E), and they displayed accelerated bud development in short days (Fig. 3 C and D). These phenotypes resemble those of hb21 hb40 hb53 triple mutants.
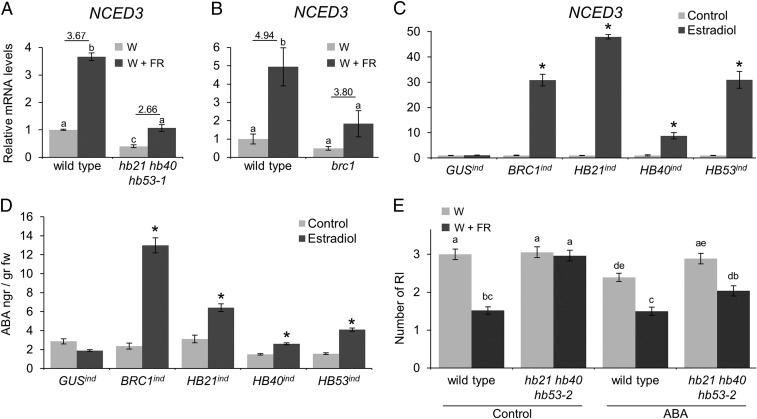
Therefore we investigated whether HB21, HB40, and HB53 could influence NCED3 expression. First we studied whether NCED3 up-regulation in W+FR light was reduced in hb21 hb40 hb53 mutants relative to wild-type plants. To do so, we treated wild-type plants and triple mutants with either W or W+FR light and compared NCED3 mRNA levels in buds. Indeed, NCED3 induction was reduced in the triple mutants (Fig. 4A), and this reduction resembled the brc1 mutant response (Fig. 4B) (3). Furthermore, in W light, NCED3 mRNA levels were significantly lower in the triple hb21 hb40 hb53 mutants than in the wild-type plants (Fig. 4A). These results indicated that HB21, HB40, and HB53 (and BRC1) are necessary for the expression of wild-type levels of NCED3 in buds in W+FR, and to some extent, in W light.
Fig. 4.
BRC1, HB21, HB40, and HB53 promote ABA accumulation via NCED3. (A and B) NCED3 mRNA levels analyzed by quantitative PCR measured in axillary buds of wild-type plants and hb21 hb40 hb53-1 mutants (A) and brc1 mutants (B) treated with W or W+FR light for 8 h. (C) NCED3 mRNA levels in 7-d-old GUSind, HA:BRC1ind, HA:HB21ind, HA:HB40ind, and HA:HB53ind seedlings after an 8-h treatment with 10 µM estradiol. (D) ABA levels measured in estradiol-treated seedlings of the genotypes in C. (E) Branching phenotype of wild-type and hb21 hb40 hb53-2 plants treated with W or W+FR light and 50 µM ABA or mock (control) for 2 wk after bolting (n = 28). Error bars show the SEM of three biological replicates. Asterisks show significant differences (P < 0.05; student’s t-test) between control and treated plants. Letters denote significant differences (P < 0.05; one-way ANOVA) among means.
Next, we investigated whether BRC1, HB21, HB40, and HB53 were not only necessary but also sufficient for NCED3 up-regulation. To do so, we used seedlings, a stage in which BRC1, HB21, HB40, and HB53 are hardly or not expressed, using estradiol-inducible lines (HA:BRC1ind, HA:HB21 ind, HA:HB40 ind, and HA:HB53 ind) (SI Appendix, Figs. S4A and S12). We treated 7-d-old seedlings with estradiol for 8 h and quantified NCED3 transcripts (Fig. 4C). Induction of BRC1, HB21, HB40, or HB53 caused a 31-, 48-, nine-, and 31-fold increase, respectively, in NCED3 mRNA levels. Changes in NCED3 mRNA levels have been shown to correlate positively with changes in ABA levels (14). We confirmed this correlation by measuring ABA in these samples. Indeed, induced plants displayed a significant increase in ABA levels after the estradiol treatment (Fig. 4D).
Transcriptional induction of NCED3 by BRC1, HB21, HB40, and HB53 may be direct. DNA affinity purification sequencing (Dap-Seq) data of HB21, HB40, and HB53 (15) indicates that these proteins directly bind a genomic region 800–900 bp upstream of the NCED3 transcription start site (SI Appendix, Fig. S13 A–C). In addition ChIP assays using GFP:BRC1ind seedlings indicate that BRC1 also binds directly to the NCED3 promoter (SI Appendix, Fig. S13 D and E).
BRC1, HB21, HB40, and HB53 mRNA levels were not affected in axillary buds of nced3-2 mutants (SI Appendix, Fig. S14A), in agreement with NCED3 acting downstream of this pathway (see Fig. 6). However, HB21 and, to a lesser extent, HB40 and HB53 were responsive to ABA application in buds (SI Appendix, Fig. S14B), indicating that although NCED3 (and ABA) are not essential for the induction of these genes, ABA nevertheless could help maintain their expression. In contrast, BRC1 mRNA levels did not change in response to ABA (SI Appendix, Fig. S14B) and were not significantly affected in the hb21 hb40 hb53 triple mutants (SI Appendix, Fig. S14C), confirming that BRC1 is the most upstream gene of this pathway (see Fig. 6).
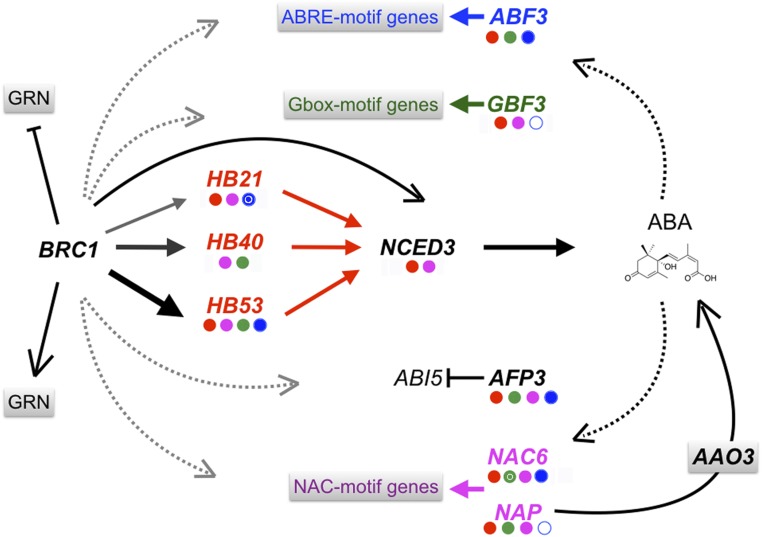
Fig. 6.
BRC1 regulation of ABA signaling in dormant buds. A working model is shown of how BRC1 regulates ABA signaling and response in buds. BRC1 and some core transcriptional regulators of the GRN (SI Appendix, Fig. S1B) are represented. Solid arrows indicate (i) direct protein–DNA interactions based on ChIP (this work) or DAP-seq data (15); (ii) protein–protein interactions (24); and (iii) known metabolic pathways (14, 28). Potential cross-regulation between HB21/40/53, ABF3, NAP, GBF3, and NAC6 is indicated by the colored dots below genes based on direct binding (15, 29) of the TF with the same color coding. Red dots, HB21/40/53 binding; green dots, GBF3 binding; purple dots, NAP/NAC6 binding; open blue circles, binding only without ABA; white inner circles, binding only with ABA. Dotted arrows indicate indirect or untested regulation, e.g., BRC1 also may promote ABA signaling via direct control of ABF3, NAP, NAC6, GBF3, and AFP3 (also see SI Appendix, Fig. S18). In addition, BRC1 controls GRNs other than the one analyzed in this work (SI Appendix, Fig. S17).
All these results suggest that BRC1, HB21, HB40, and HB53 are necessary and sufficient to cause NCED3 induction and ABA accumulation and that BRC1-dependent transcriptional activation of HB21, HB40, and HB53 could boost local ABA signaling and response in axillary buds.
ABA Rescues the Excess-Branching Phenotype of hb21 hb40 hb53 Triple Mutants.
If the excessive branching phenotype of hb21 hb40 hb53 triple mutants in W+FR light is caused mostly by a failure to accumulate ABA in buds, ABA application to buds should rescue the phenotype of the triple mutants. We tested this notion by applying 50 μM ABA directly to buds of wild-type and hb21 hb40 hb53-2 triple-mutant plants every day for 15 d after bolting and quantified their branching phenotypes in W and W+FR light (Fig. 4E). In the triple mutants ABA restored the wild-type response to W+FR light. In wild-type plants, ABA application led to a further reduction in branch number in W light but had no effect in W+FR light. These results are consistent with the possibility that the increased branching phenotype of hb21 hb40 hb53 triple mutants in low R:FR light is caused mainly by a failure to accumulate ABA in buds.
HB21, HB40, and HB53 Promote Expression of the ABA-Related GRN.
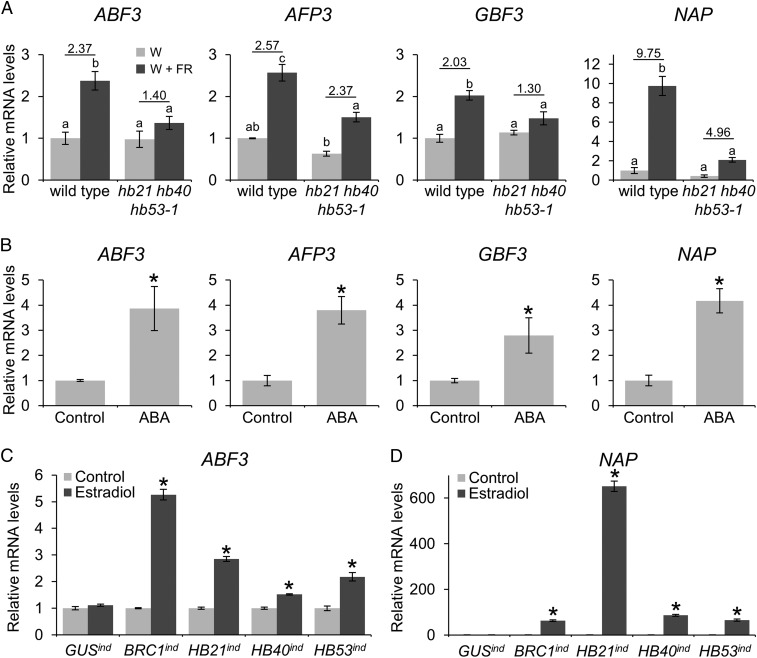
BRC1, HB21, HB40, and HB53 could promote ABA accumulation, which could in turn enhance the expression of the ABA-related GRN induced in dormant buds. To test this possibility, we studied the expression of genes of this GRN under conditions of loss/gain of BRC1 or HB21/40/53 function. We selected four genes encoding proteins associated with ABA signaling and response: ABA RESPONSIVE ELEMENTS-BINDING FACTOR 3 (ABF3; At4g34000), encoding a bZIP master regulator of ABA signaling (16), ABI FIVE BINDING PROTEIN 3 (AFP3; At3g29575), G-BOX BINDING FACTOR 3 (GBF3; At2g46270), and NAC-LIKE, ACTIVATED BY AP3/PI (NAP; At1g69490) (SI Appendix, Fig. S1B). These factors could modulate and amplify the transcriptional responses of the GRN. We compared induction levels of these genes in wild-type plants and hb21 hb40 hb53-1 mutants after a W+FR light treatment. The four genes showed a reduced induction in the triple mutants (Fig. 5A). These results resembled those obtained in brc1 mutants (3) (SI Appendix, Fig. S15A) and suggested that BRC1 and its direct targets, HB21, HB40, and HB53, are necessary for the normal expression of these factors in W+FR light-treated dormant buds.
Fig. 5.
BRC1, HB21, HB40, and HB53 regulate the expression of ABA-related genes encoding TFs. (A and B) mRNA levels of ABF3, AFP3, GBF3, and NAP analyzed by quantitative PCR in axillary buds in wild-type and hb21 hb40 hb53-1 buds treated with W or W+FR light for 8 h (A) or in wild-type buds after an application of 50 µM ABA for 8 h (B). (C and D) Expression of ABF3 (C) and NAP (D) in 7-d-old GUSind, HA:BRC1ind, HA:HB21ind, HA:HB40ind, and HA:HB53ind seedlings treated for 8 h with 10 µM estradiol. Error bars show the SEM of three biological replicates. Asterisks indicate significant differences (P < 0.05; student’s t-test) between control and treated plants. Letters denote significant differences (P < 0.05; one-way ANOVA) among means.
We then studied their response to W+FR light in nced3-2 mutants and found reduced up-regulation compared with the response in wild-type plants (SI Appendix, Fig. S15B). This reduced response suggested that NCED3, and presumably ABA, are required for the full induction of these genes. Therefore, we studied their mRNA levels after direct application of ABA to axillary buds. All four genes were responsive to ABA (Fig. 5B), confirming that NCED3-induced ABA accumulation could contribute to their transcriptional induction.
Using estradiol-inducible lines, we also examined whether BRC1, HB21, HB40, or HB53 alone was sufficient to boost their expression in seedlings. We treated 7-d-old seedlings with estradiol for 8 h and measured ABF3, AFP3, GBF3, and NAP mRNA levels. All four genes were significantly up-regulated after BRC1 or HB53 induction, and ABF3 and NAP also were up-regulated by HB21 and HB40 (Fig. 5 C and D and SI Appendix, Fig. S15 C and D).
HB21, HB40, and HB53 Do Not Mediate All BRC1-Induced Responses.
HB21, HB40, and HB53 do not seem to mediate all the BRC1-induced responses. The expression of a group of BRC1-dependent genes related to cell division and consistently down-regulated after BRC1 induction (3) was not affected in buds of the hb21 hb40 hb53-1 triple mutants (SI Appendix, Fig. S16A) or in estradiol-induced HB21ind, HB40ind, or HB53ind lines (SI Appendix, Fig. S16B). These results indicate that HB21, HB40, and HB53 mediate only a subset of the gene responses promoted by BRC1 (SI Appendix, Fig. S17).
Discussion
Little is known about the genetic mechanisms acting inside axillary buds during the growth-to-dormancy transition. In Arabidopsis, this process is regulated by the class II TCP transcription factor BRC1, which regulates the expression of several GRNs (SI Appendix, Fig. S17) and which, among other effects, causes a local enhancement of the ABA response (3). Although ABA has been classically associated with dormancy in seeds and buds in many different species, including Arabidopsis (4, 6), this hormone is not yet fully integrated into the current molecular and genetic models of the hormonal control of shoot branching. Moreover, how BRC1 controls the response to ABA was completely unknown.
Here we provide evidence that Arabidopsis BRC1 directly triggers an HD-ZIP–mediated cascade that results in a local boost of NCED3 expression and ABA biosynthesis inside axillary buds in conditions of low R:FR or short photoperiods. ABA, along with BRC1 and HD-ZIP activity, may promote the induction of a GRN required for bud dormancy (Fig. 6). NCED3 encodes an enzyme catalyzing a key regulatory, rate-limiting step of ABA biosynthesis: NCED3 loss of function compromises ABA accumulation, and its overexpression is sufficient to increase ABA levels (14, 17). We have shown that HB21, HB40, and HB53 are essential for wild-type NCED3 transcription levels in buds. Moreover, ectopic expression of BRC1, HB21, HB40, or HB53 in seedlings is sufficient to cause a significant accumulation of NCED3 transcripts and ABA within 8 h, providing compelling evidence of the causal relationship between the function of these genes and NCED3 activity. Furthermore, these four genes may control NCED3 transcription directly, because our ChIP data and available Dap-Seq data (15) indicate that all four proteins bind the NCED3 promoter.
ABA measurements have confirmed an inverse correlation between bud growth potential and bud ABA levels in Arabidopsis (4, 6). The increased branching phenotypes of nced3 mutants further support a critical role for ABA in this process (refs. 4 and 6 and this work). Moreover, the observation that the hb21 hb40 hb53 mutant phenotype in low R:FR is rescuable by ABA application supports the idea that this phenotype is caused by a failure to accumulate this hormone in buds, indicating that an important role of HB21/40/53 is to cause a local rise in ABA. It is noteworthy that HB40 and HB53 also are expressed in stomata guard cells, where cell-autonomous ABA synthesis occurs to control stomata closure (18). Local ABA synthesis contrasts with that of auxin, known to control shoot branching systemically, and with strigolactones that can be transported from the roots to suppress branching (19). ABA transport nevertheless may contribute to strengthen ABA accumulation in buds because the nitrate transporter NRT1.2 (At1g69850), which also mediates ABA cellular uptake (20), is induced in dormant buds and repressed in active buds (3, 21).
A rise in ABA may activate a positive feed-back loop, because several ABA synthesis genes including NCED3 and HB21/40/53 (but not BRC1) are induced by ABA (Fig. 6) (ref. 22 and this work). Furthermore, most of the genes in the ABA-related GRN are induced by ABA, according to public microarray data (23), so it is likely that ABA accumulation causes a general up-regulation of the GRN. Indeed the response to NCED3 and ABA of four members of the GRN encoding TFs (ABF3, GBF3, NAP) or TF-interacting proteins (AFP3) supports this model (refs. 16 and 24 and this work). ABF3 is a bZIP master regulator of ABA signaling that controls ABRE-dependent gene expression (16), GBF3 is a bZIP factor that binds G-box motifs (25), and AFP3 interacts with the bZIP protein ABI5 to fine-tune the ABA response (24). NAP, an NAC TF associated with stress and senescence (26, 27), controls the expression of ABSCISIC ALDEHYDE OXIDASE3 (AAO3), an enzyme that catalyzes the final steps of ABA synthesis (28), and thereby may contribute further to ABA accumulation (Fig. 6). Up-regulation of these genes probably has a great impact in the GRN: ABF3, GBF3, and NAP bind 76, 83, and 29% of the GRN promoters, respectively (SI Appendix, Fig. S18 A and B) (15, 29). Cross-talk also may involve HB21, HB40, and HB53, which bind 46, 63, and 42% of the GRN gene promoters, respectively (15), including those of GBF3, NAP, AFP3, and NAC6 (SI Appendix, Fig. S18 A and B). Further ChIP-seq studies and high-resolution transcriptomic analyses of mutants and ABA-treated and inducible lines will allow a better understanding of the sequential gene activation and the relationships among the genes of the GRN.
This response seems essential in low R:FR light and short photoperiods, conditions associated with reduced photosynthesis, energy deprivation, and stress in which ABA is required (30). The molecular mechanisms by which this hormone controls bud growth are yet to be determined. However, the antagonistic roles of ABA and cytokinin, which locally promotes bud activity, in several developmental processes such as seed germination and seedling development are well known (31, 32). Moreover, it is worth noting that loss-of-function mutants of the ortholog gene of HB21/40/53 in maize, GRASSY TILLERS1 (GT1), have increased branching and that GT1 has been proposed to act genetically downstream of TEOSINTE BRANCHED1 (TB1) in the control of shoot branching. Moreover GT1 orthologs in both sorghum and teosinte are induced in plants treated with FR-rich light, and this gene has been proposed to mediate the reduced branching associated with the shade-avoidance response in the grasses (33). It remains to be tested whether this pathway also controls ABA synthesis and response in monocots. Nevertheless these results indicate that the genetic module TB1/GT1, BRC1/HB21/40/53, which is enhanced under shade conditions, is probably conserved throughout flowering plants. Conservation of the BRC1-binding sites in the genomic regions of the corresponding HD-ZIP genes in several Brassicaceae supports the conservation of this regulatory module and suggests the existence of a general strategy to promote branch suppression as a plant adaptation to light-limiting conditions.
Materials and Methods
Details about plant lines, growth conditions, cloning, LUC assays, histochemical analyses, treatments, quantitative PCR, ABA measurements, and other techniques are in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Javier Paz Ares and Desmond Bradley for critically reading the manuscript, Miguel Moreno for advice about HB53p:GUS expression in roots, Esther Carrera and Isabel Lopez-Diaz for ABA quantification at the Plant Hormone Quantification Service, and K. Shinozaki (Riken) for nced3-2 seeds. P.C. is supported by Ministry of Economy, Industry, and Competitivity Grants BIO2011-25687 and BIO2014-57011-R. R.G.H.I. is supported by Netherlands Organization for Scientific Research Joint Research Projects Grant 833.13.008, and R.G.H.I. and A.P. are supported by ERA-Coordinating Action in Plant Sciences Grant 849.13.005. E.G.-G. was a Predoctoral Fellow of Fundación Ramón Areces and a Consejo Superior de Investigaciones Científicas JAE-Predoctoral Fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613199114/-/DCSupplemental.
References
- 1.Aguilar-Martínez JA, Poza-Carrión C, Cubas P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell. 2007;19(2):458–472. doi: 10.1105/tpc.106.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finlayson SA. Arabidopsis Teosinte Branched1-like 1 regulates axillary bud outgrowth and is homologous to monocot Teosinte Branched1. Plant Cell Physiol. 2007;48(5):667–677. doi: 10.1093/pcp/pcm044. [DOI] [PubMed] [Google Scholar]
- 3.González-Grandío E, Poza-Carrión C, Sorzano COS, Cubas P. BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell. 2013;25(3):834–850. doi: 10.1105/tpc.112.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy SK, Holalu SV, Casal JJ, Finlayson SA. Abscisic acid regulates axillary bud outgrowth responses to the ratio of red to far-red light. Plant Physiol. 2013;163(2):1047–1058. doi: 10.1104/pp.113.221895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolas M, Rodríguez-Buey ML, Franco-Zorrilla JM, Cubas P. A recently evolved alternative splice site in the BRANCHED1a gene controls potato plant architecture. Curr Biol. 2015;25(14):1799–1809. doi: 10.1016/j.cub.2015.05.053. [DOI] [PubMed] [Google Scholar]
- 6.Yao C, Finlayson SA. Abscisic acid is a general negative regulator of Arabidopsis axillary bud growth. Plant Physiol. 2015;169(1):611–26. doi: 10.1104/pp.15.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González-Grandío E, Cubas P. Identification of gene functions associated to active and dormant buds in Arabidopsis. Plant Signal Behav. 2014;9(2):e27994. doi: 10.4161/psb.27994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason MG, Ross JJ, Babst BA, Wienclaw BN, Beveridge CA. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc Natl Acad Sci USA. 2014;111(16):6092–6097. doi: 10.1073/pnas.1322045111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbier F, et al. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J Exp Bot. 2015;66(9):2569–2582. doi: 10.1093/jxb/erv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godoy M, et al. Improved protein-binding microarrays for the identification of DNA-binding specificities of transcription factors. Plant J. 2011;66(4):700–711. doi: 10.1111/j.1365-313X.2011.04519.x. [DOI] [PubMed] [Google Scholar]
- 11.Franco-Zorrilla JM, et al. DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc Natl Acad Sci USA. 2014;111(6):2367–2372. doi: 10.1073/pnas.1316278111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medina-Rivera A, et al. RSAT 2015: Regulatory sequence analysis tools. Nucleic Acids Res. 2015;43(W1):W50–W56. doi: 10.1093/nar/gkv362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capella M, Ré DA, Arce AL, Chan RL. Plant homeodomain-leucine zipper I transcription factors exhibit different functional AHA motifs that selectively interact with TBP or/and TFIIB. Plant Cell Rep. 2014;33(6):955–967. doi: 10.1007/s00299-014-1576-9. [DOI] [PubMed] [Google Scholar]
- 14.Iuchi S, et al. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001;27(4):325–333. doi: 10.1046/j.1365-313x.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- 15.O’Malley RC, et al. Cistrome and epicistrome features shape the regulatory DNA landscape. Cell. 2016;165(5):1280–1292. doi: 10.1016/j.cell.2016.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida T, et al. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010;61(4):672–685. doi: 10.1111/j.1365-313X.2009.04092.x. [DOI] [PubMed] [Google Scholar]
- 17.Thompson AJ, et al. Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes over-production of abscisic acid. Plant J. 2000;23(3):363–374. doi: 10.1046/j.1365-313x.2000.00789.x. [DOI] [PubMed] [Google Scholar]
- 18.Bauer H, et al. The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol. 2013;23(1):53–57. doi: 10.1016/j.cub.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Domagalska MA, Leyser O. Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol. 2011;12(4):211–221. doi: 10.1038/nrm3088. [DOI] [PubMed] [Google Scholar]
- 20.Kanno Y, et al. Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc Natl Acad Sci USA. 2012;109(24):9653–9658. doi: 10.1073/pnas.1203567109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tatematsu K, Ward S, Leyser O, Kamiya Y, Nambara E. Identification of cis-elements that regulate gene expression during initiation of axillary bud outgrowth in Arabidopsis. Plant Physiol. 2005;138(2):757–766. doi: 10.1104/pp.104.057984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrero JM, et al. Both abscisic acid (ABA)-dependent and ABA-independent pathways govern the induction of NCED3, AAO3 and ABA1 in response to salt stress. Plant Cell Environ. 2006;29(10):2000–2008. doi: 10.1111/j.1365-3040.2006.01576.x. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 2004;136(1):2621–2632. doi: 10.1104/pp.104.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia ME, Lynch T, Peeters J, Snowden C, Finkelstein R. A small plant-specific protein family of ABI five binding proteins (AFPs) regulates stress response in germinating Arabidopsis seeds and seedlings. Plant Mol Biol. 2008;67(6):643–658. doi: 10.1007/s11103-008-9344-2. [DOI] [PubMed] [Google Scholar]
- 25.Uno Y, et al. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA. 2000;97(21):11632–11637. doi: 10.1073/pnas.190309197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HJ, et al. Gene regulatory cascade of senescence-associated NAC transcription factors activated by ETHYLENE-INSENSITIVE2-mediated leaf senescence signalling in Arabidopsis. J Exp Bot. 2014;65(14):4023–4036. doi: 10.1093/jxb/eru112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindemose S, et al. A DNA-binding-site landscape and regulatory network analysis for NAC transcription factors in Arabidopsis thaliana. Nucleic Acids Res. 2014;42(12):7681–7693. doi: 10.1093/nar/gku502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Worley E, Udvardi M. A NAP-AAO3 regulatory module promotes chlorophyll degradation via ABA biosynthesis in Arabidopsis leaves. Plant Cell. 2014;26(12):4862–4874. doi: 10.1105/tpc.114.133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song L, Huang SC, Wise A, Castanon R, Nery JR, Chen H, Watanabe M, Thomas J, Bar-Joseph Z, Ecker JR. A transcription factor hierarchy defines an environmental stress response network. Science. 2016;354(6312):aag1550-aag1550. doi: 10.1126/science.aag1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues A, et al. ABI1 and PP2CA phosphatases are negative regulators of Snf1-related protein kinase1 signaling in Arabidopsis. Plant Cell. 2013;25(10):3871–3884. doi: 10.1105/tpc.113.114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, et al. Cytokinin antagonizes ABA suppression to seed germination of Arabidopsis by downregulating ABI5 expression. Plant J. 2011;68(2):249–261. doi: 10.1111/j.1365-313X.2011.04683.x. [DOI] [PubMed] [Google Scholar]
- 32.Guan C, et al. Cytokinin antagonizes abscisic acid-mediated inhibition of cotyledon greening by promoting the degradation of abscisic acid insensitive5 protein in Arabidopsis. Plant Physiol. 2014;164(3):1515–1526. doi: 10.1104/pp.113.234740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whipple CJ, et al. grassy tillers1 promotes apical dominance in maize and responds to shade signals in the grasses. Proc Natl Acad Sci USA. 2011;108(33):E506–E512. doi: 10.1073/pnas.1102819108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.