Significance
In the setting of glioblastoma multiforme (GBM), invasion of cells into normal brain and the unlikeliness of complete surgical removal contributes to GBM lethality and recurrence. “Gold standard” GBM treatment includes adjuvant radiotherapy. Unfortunately, cells surviving radiation demonstrate increased invasion and therapeutic resistance. Melanoma differentiation-associated gene 9 (MDA-9/Syntenin) expression is elevated in patient-derived tumors and GBM cell lines, which correlates with decreased survival and poor response to radiation. Genetic suppression of MDA-9/Syntenin sensitizes GBM to radiation by inhibiting radiation-induced invasion gains and signaling changes. Additionally, intraperitoneal administration of a small-molecule MDA-9/Syntenin inhibitor, PDZ1i, developed using innovative fragment-based drug design and NMR approaches, improved survival of brain tumor-bearing mice. Survival was enhanced further when used with radiation, supporting MDA-9/Syntenin as a therapeutic target for this deadly disease.
Keywords: MDA-9/Syntenin, glioblastoma multiforme, radiation, EGFR, PDZ1 inhibitor
Abstract
Glioblastoma multiforme (GBM) is an intractable tumor despite therapeutic advances, principally because of its invasive properties. Radiation is a staple in therapeutic regimens, although cells surviving radiation can become more aggressive and invasive. Subtraction hybridization identified melanoma differentiation-associated gene 9 [MDA-9/Syntenin; syndecan-binding protein (SDCBP)] as a differentially regulated gene associated with aggressive cancer phenotypes in melanoma. MDA-9/Syntenin, a highly conserved double-PDZ domain-containing scaffolding protein, is robustly expressed in human-derived GBM cell lines and patient samples, with expression increasing with tumor grade and correlating with shorter survival times and poorer response to radiotherapy. Knockdown of MDA-9/Syntenin sensitizes GBM cells to radiation, reducing postradiation invasion gains. Radiation induces Src and EGFRvIII signaling, which is abrogated through MDA-9/Syntenin down-regulation. A specific inhibitor of MDA-9/Syntenin activity, PDZ1i (113B7), identified through NMR-guided fragment-based drug design, inhibited MDA-9/Syntenin binding to EGFRvIII, which increased following radiation. Both genetic (shmda-9) and pharmacological (PDZ1i) targeting of MDA-9/Syntenin reduced invasion gains in GBM cells following radiation. Although not affecting normal astrocyte survival when combined with radiation, PDZ1i radiosensitized GBM cells. PDZ1i inhibited crucial GBM signaling involving FAK and mutant EGFR, EGFRvIII, and abrogated gains in secreted proteases, MMP-2 and MMP-9, following radiation. In an in vivo glioma model, PDZ1i resulted in smaller, less invasive tumors and enhanced survival. When combined with radiation, survival gains exceeded radiotherapy alone. MDA-9/Syntenin (SDCBP) provides a direct target for therapy of aggressive cancers such as GBM, and defined small-molecule inhibitors such as PDZ1i hold promise to advance targeted brain cancer therapy.
Despite advances in surgical, pharmacological, and radiation therapeutic approaches, glioblastoma multiforme (GBM) remains a particularly aggressive and ultimately an invariably fatal tumor, with a median survival of less than 15 mo and 5-y survival at 5% (1, 2). The current standard of care includes maximal surgical resection followed by radiation and temozolomide chemotherapy. However, inevitable recurrence occurs near resection margins and within the high-dose radiation field, implying that intrinsic invasiveness and radioresistance contribute significantly to relapse (3, 4). Sublethal radiation has repeatedly been shown to induce invasion and migration in surviving tumor cells, enhancing the very property that makes curative treatment so difficult (5, 6). A contributing factor to tumor relapse and recurrence is the ability of tumor cells to escape from the primary tumor mass (7), which underscores the importance of developing antiinvasive therapies that complement, and ideally enhance, conventional therapeutic approaches (8).
Melanoma differentiation-associated gene 9 (mda-9), also known as syntenin (syndecan-binding protein; SDCBP), is involved in invasion and metastatic signaling in multiple cancer settings (9–11). MDA-9/Syntenin serves critical roles in signal transduction, as well as in cell–cell and cell–matrix adhesion (12, 13). In addition to its roles in melanoma metastasis and tumor progression, MDA-9/Syntenin is highly expressed and involved in breast, gastric, and urothelial cell cancers (10, 13). MDA-9/Syntenin is an important regulator of GBM invasion, angiogenesis, and tumor progression (14), and inhibiting MDA-9/Syntenin expression decreases GBM invasion (14, 15) and enhances survival. Moreover, MDA-9/Syntenin is also overexpressed in glioma stem cells (GSCs), and is a regulator of GSC survival and stemness (16). Gene expression analysis of The Cancer Genome Atlas (TCGA) database revealed that patients whose tumors express high levels of MDA-9/Syntenin have a poorer prognosis and reduced survival compared with low-expressing MDA-9/Syntenin tumors (11). mda-9/syntenin expression correlates positively with astrocytoma grade, as analyzed through tissue samples and gene expression databases (11), and is most highly expressed in GBM. In both melanoma and glioma, MDA-9/Syntenin is involved in NF-κB activation through a proto-oncogene tyrosine-protein kinase Src (c-Src)/p38 MAPK signaling pathway (13, 15). Inhibiting MDA-9/Syntenin expression reduces NF-κB target gene expression such as matrix metalloproteinase-2 (MMP-2), a critical secreted metalloproteinase involved in GBM invasion.
A vital characteristic of MDA-9/Syntenin is the inclusion of two tandem PDZ domains, so named for their discovery in PSD-95/SAP90, DLGA, and ZO-1 (15). PDZ domains are common to a number of scaffolding proteins, and are critical for facilitating protein–protein interactions throughout various regions of the cell. MDA-9/Syntenin uses these motifs to successfully facilitate the interaction of c-Src–focal adhesion kinase (FAK) kinase complexes, noted for involvement in proinvasive signaling in cancer (13, 15). In these contexts, inhibiting the interaction between MDA-9/Syntenin and its targets in GBM might provide a strategy to inhibit invasive functions of this gene. Fragment-based lead design, or fragment-based drug design (FBDD), is an emerging and useful strategy for developing biologically active compounds (17). Using an NMR-guided FBDD approach (18) combined with in silico modeling and synthetic medicinal chemistry, we developed a PDZ1 MDA-9/Syntenin–binding small molecule, represented by compound 113B7 (herein referred to as PDZ1i), that binds with micromolar affinity to the PDZ1 domain of MDA-9/Syntenin (Fig. S1). PDZ1i does not bind appreciably to the PDZ2 domain of MDA-9/Syntenin (Fig. S1), and is long-lived in vivo after both i.v. and i.p. administration in mice (Table S1). These data indicated that PDZ1i was a suitable potential pharmacological tool to investigate the role of the PDZ1 domain of MDA-9/Syntenin in cellular mechanistic and in vivo efficacy studies.
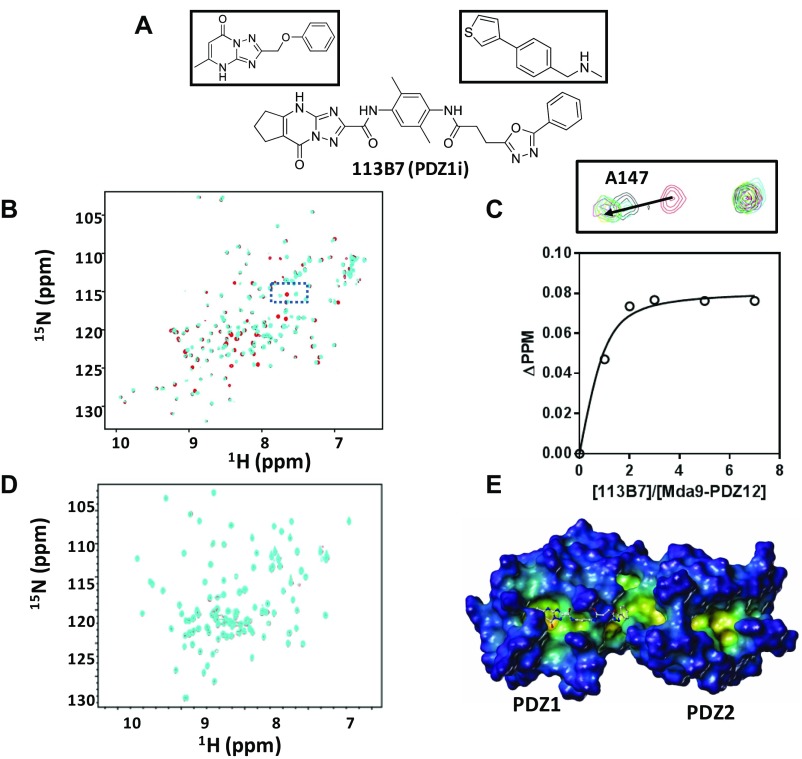
Fig. S1.
(A) Chemical structures of the two initial fragment hits (Top) and the structure of the resulting bidentate molecule 113B7 (PDZ1i). (B) Superposition of 2D [15N,1H] HSQC spectra of 50 µM MDA-9 PDZ1/2 tandem domain in the absence (red) and presence of 350 µM 113B7 (cyan). (C) Binding curve and representative spectra for the titration of 113B7 against the MDA-9 PDZ1/2 tandem domain. (C, Top) Zoom of the boxed region in B after titrating 113B7 into the MDA-9 PDZ tandem domain. The cross-peak corresponding to the backbone amide of residue A147 experiences a gradual shift upon the addition of 113B7 (red, 0 µM; black, 50 µM; green, 100 µM; orange, 150 µM; magenta, 250 µM; cyan, 350 µM). (C, Bottom) Titration curve of 113B7 for residue A147. The dissociation constant for the binding of 113B7 to MDA-9 was calculated to be 21.37 ± 8.62 µM. (D) Superposition of 2D [15N,1H] HSQC spectra of 50 µM PDZ domain from X11/mint scaffold protein (20 mM; 33% identity with PDZ1) is shown in the absence (cyan) and presence of 100 mM 113B7 (red). No appreciable binding is detected. (E) Docked structure of 113B7 in complex with the MDA-9 PDZ tandem domain (Protein Data Bank ID code 1W9E). The pose was obtained with GOLD (Cambridge Crystallographic Data Centre). Two PDZ domains of MDA-9 are labeled. The surface of the MDA-9 PDZ tandem domain is displayed and color-coded according to cavity depth: blue, shallow; yellow, deep (MOLCAD) (SYBYL-X, Certara USA, Inc.). The structure of 113B7 is displayed as balls and sticks.
Table S1.
Pharmacokinetic data for PDZ1i
| PK parameters | Mean ± SD | CV, % |
| I.v. study | ||
| Body weight | 25.1 ± 0.173 g | 0.690 |
| I.v. time, h | ||
| 0 | 35,033 ± 9,424 ng/mL | 26.9 |
| 0.250 | 29,533 ± 7,387 ng/mL | 25.0 |
| 1.00 | 17,733 ± 3,630 ng/mL | 20.5 |
| 2.00 | 11,600 ± 985 ng/mL | 8.49 |
| 6.00 | 6,113 ± 531 ng/mL | 8.68 |
| 12.0 | 3,360 ± 927 ng/mL | 27.6 |
| 24.0 | 541 ± 79.6 ng/mL | 14.7 |
| C0 | 35,033 ± 9,424 ng/mL | 26.9 |
| T1/2* | 5.06 ± 0.285 h | 5.64 |
| AUC0-last | 120,000 ± 17,692 ng·h−1⋅mL−1 | 14.7 |
| AUC0-inf | 124,000 ± 17,349 ng·h−1⋅mL−1 | 14.0 |
| I.p. study | ||
| Body weight | 24.5 ± 0.252 g | 1.03 |
| I.p. time, h | ||
| 0 | ND | ND |
| 0.250 | 98,233 ± 10,289 ng/mL | 10.5 |
| 1.00 | 69,233 ± 9,757 ng/mL | 14.1 |
| 2.00 | 58,967 ± 7,257 ng/mL | 12.3 |
| 6.00 | 54,600 ± 6,351 ng/mL | 11.6 |
| 12.0 | 42,833 ± 7,129 ng/mL | 16.6 |
| 24.0 | 15,133 ± 2,754 ng/mL | 18.2 |
| Cmax | 98,233 ± 10,289 ng/mL | 10.5 |
| Tmax | 0.250 ± 0.000 h | 0.00 |
| T1/2* | 9.42 ± 0.585 h | 6.22 |
| AUC0-last | 974,667 ± 127,602 ng·h−1⋅mL−1 | 13.1 |
| AUC0-inf | 1,183,333 ± 180,093 ng·h−1⋅mL−1 | 15.2 |
| Bioavailability, i.p./i.v. | 81.2 ± 10.6% |
Plasma concentration (ng/mL) in mice (n = 3) after i.v. (3 mg/kg) and i.p. (30 mg/kg) administration, measured at time points reported under I.v. time and I.p. time, respectively. BALB/c mice (n = 3 per group) were injected with 3 mg/kg and 30 mg/kg PDZ1i i.v. or i.p., respectively, and the average concentration of intact compound in plasma was determined via HPLC at the indicated time points. AUC0-inf, area under the curve of plasma concentration versus time calculated between time 0 and an extrapolated infinite time point; AUC0-last, area under the curve of plasma concentration versus time calculated between time 0 and last measured time point; C0 = concentration at time 0; Cmax = maximal concentration observed; CV, coefficient of variation; ND, not determined; SD, standard deviation.
Three time points were used for T1/2.
Here we demonstrate the efficacy of supplementing radiotherapy by targeting, either genetically [small hairpin RNA for mda-9/syntenin (shmda-9)] or pharmacologically (PDZ1i), MDA-9/Syntenin in GBM. By counteracting gains in Src, FAK, EPH receptor A2 (EphA2), and epidermal growth factor receptor (EGFR) signaling, MDA-9/Syntenin inhibition can reduce radiation-induced invasion as well as radiosensitize GBM cells, ideal properties to complement radiation treatment.
Results
MDA-9/Syntenin Inhibition Leads to Radiosensitization and Inhibition of Radiation-Induced Invasion.
MDA-9/Syntenin was shown previously to be a valuable target in addressing one of the deadliest aspects of GBM, its propensity to invade (14). Because radiation therapy has been demonstrated to induce invasion in GBM cells, targeting MDA-9/Syntenin could be a useful approach to complement conventional treatment. Gliomas with higher expression of MDA-9/Syntenin are more likely to be high-grade, and patients whose tumors had high levels of MDA-9/Syntenin had a worse prognosis (14). With this in mind, we explored possible connections between MDA-9/Syntenin expression and radiotherapy in glioma using the Repository for Molecular Brain Neoplasia Data (REMBRANDT) database, a publicly available dataset with information on tumor gene expression, treatment history, and survival (https://gdoc.georgetown.edu/gdoc/). In glioma patients with no history of radiation who then underwent subsequent radiotherapy, we probed whether survival time correlated with MDA-9/Syntenin expression. Patients whose tumors had higher expression of MDA-9/Syntenin had significantly shorter survival (Fig. 1A; P < 0.01). Median survival was reduced nearly threefold in patients with gliomas expressing high levels of MDA-9/Syntenin (15.2 mo) compared with others (43.6 mo).
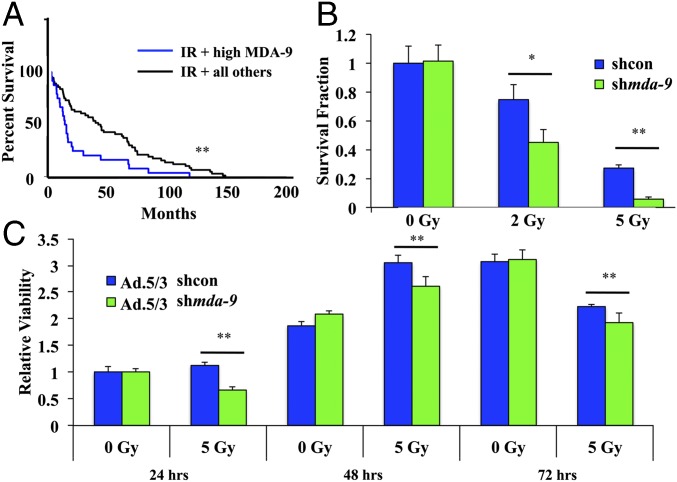
Fig. 1.
MDA-9/Syntenin is involved in radiosensitivity. (A) The REMBRANDT database was mined for glioma patients with no prior radiation treatment who then underwent radiation therapy. These were stratified by SDCBP (mda-9/syntenin) expression (high was set at >1.5-fold overexpression). High tumor MDA-9/Syntenin led to a worse prognosis in those undergoing radiotherapy. (B) U1242-shcon and U1242-shmda-9 cells were analyzed for colony formation 14 d postradiation treatment. (C) U1242 cells treated with either Ad.5/3-shcon or Ad.5/3-shmda-9 were analyzed for viability at the indicated time points via MTT assay. Error bars indicate ±SD. *P < 0.05, **P < 0.01.
We then asked whether inhibiting the expression of MDA-9/Syntenin would result in changes in radiosensitivity in vitro. Using GBM cells expressing a control shRNA, U1242-shcon, or shRNA targeting mda-9/syntenin, U1242-shmda-9, we found that low levels of MDA-9/Syntenin radiosensitized GBM in a colony-formation assay (Fig. 1B). Furthermore, proliferation was inhibited in cells transiently reduced in MDA-9/Syntenin expression via adenoviral vector Ad.5/3-shmda-9 (Fig. 1C), indicating that MDA-9/Syntenin may play a central role in cell survival following radiation exposure.
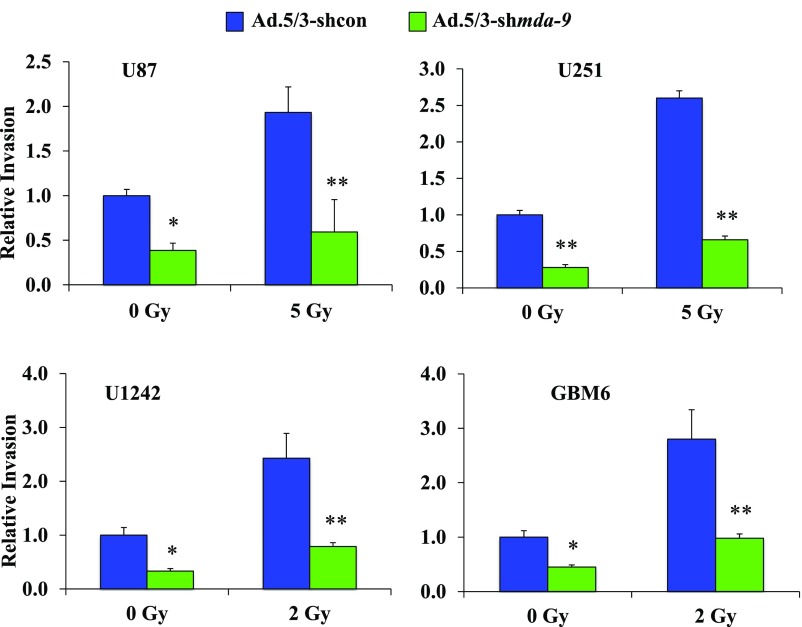
Radiation consistently increases motility and invasion in GBM cells (5, 6, 19). Four cell lines were exposed to radiation after treatment with control adenovirus, Ad.5/3-shcon, or Ad.5/3-shmda-9. Each of the cell lines received a range of radiation doses, and the dose that demonstrated maximal radiation gains was used for MDA-9/Syntenin knockdown studies. As anticipated, knockdown of MDA-9/Syntenin levels reduced invasion with no radiation exposure. After irradiation, each GBM cell line exhibited at least a twofold invasion gain, whereas MDA-9/Syntenin knockdown significantly curtailed these gains (Fig. S2). GBM invasion following radiation was reduced with MDA-9/Syntenin knockdown to about 33% of levels observed in controls, representing a substantial reduction to an undesirable side effect of conventional radiotherapy.
Fig. S2.
MDA-9/Syntenin knockdown inhibits radiation-induced invasion. U87, U251, U1242, and GBM6 cells were treated with either Ad.5/3-shcon or Ad.5/3-shmda-9 and irradiated 48 h later. Cells were then seeded in a Transwell Matrigel invasion assay and stained after 18 h. Relative invasion was quantified from five random fields. Error bars indicate ±SD. *P < 0.05, **P < 0.01.
Knockdown of mda-9/syntenin Inhibits Src and EphA2 Activation Postradiation.
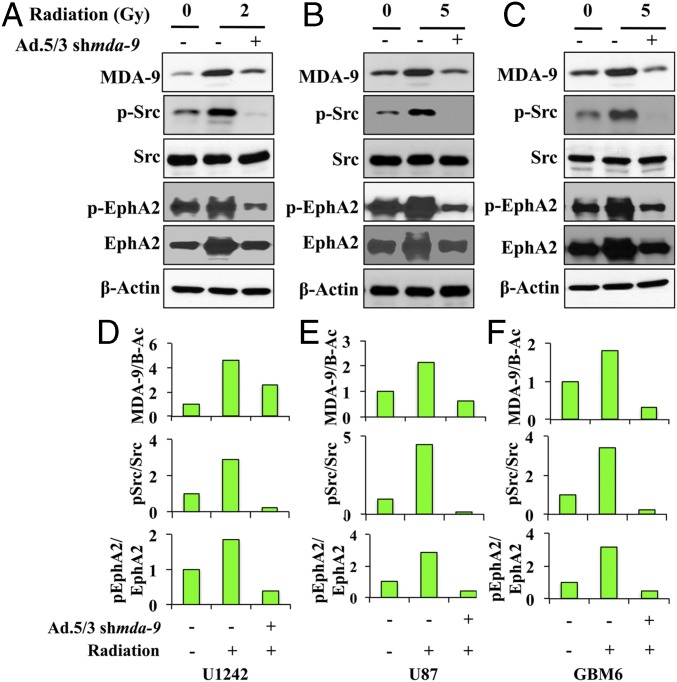
MDA-9/Syntenin amplifies Src and NF-κB signaling in melanoma and glioma (13, 14, 20). Src is a recognized enhancer of invasive signals in cancer settings, and has demonstrated involvement in postradiation signaling (19). Whereas a modest increase in MDA-9/Syntenin levels 24 h postradiation was observed, we detected a significant increase in activated levels of Src (Fig. 2). MDA-9/Syntenin knockdown reduced those activation levels in each of the GBM cell lines tested. EphA2 is a member of the Eph receptor family, the largest subfamily of receptor tyrosine kinases, and is involved in numerous cellular processes, including angiogenesis and motility (21). EphA2 overexpression correlates with poor prognosis and increased metastasis, and is implicated in the pathogenesis of numerous tumors, including those of the brain (22). EphA2 has been shown to interact with Src in invasion signaling (23), is up-regulated following radiation, and enhances the malignant phenotype in melanoma (24). Both total and activated EphA2 levels increased after radiation exposure, yet MDA-9/Syntenin inhibition effectively reduced these gains (Fig. 2). Because MDA-9/Syntenin inhibition negated the gains in Src and EphA2 signaling postradiation, it can be considered a valuable target in controlling radiation-induced pathogenesis.
Fig. 2.
Inhibition of MDA-9/Syntenin impairs Src/EphA2 signaling. (A–C) Immunoblot analysis of GBM cells treated with either Ad.5/3-shcon or Ad.5/3-shmda-9 and irradiated 48 h later. Cell lysates were collected 24 h postradiation. (D–F) Changes in protein levels were quantified. β-Actin is the protein loading control.
A Small Molecule Targeting MDA-9/Syntenin Inhibits Invasion and Radiosensitizes GBM.
FBDD is an important technique used with success in efforts to develop small-molecule inhibitors of challenging drug targets, including those involved in protein–protein interactions (18). NMR-based screening of an in-house assembled fragment library of about 5,000 compounds using [15N,1H] heteronuclear single-quantum coherence spectroscopy (HSQC) spectra with a 15N-labeled PDZ1/2 tandem domain from MDA-9/Syntenin identified two hit compounds (Fig. S1). Chemical shift mapping studies revealed that these two molecules interacted mainly with the PDZ1 domain and in a region at the interface between the domains, whereas no viable fragment hits were found binding to the PDZ2 domain (Fig. S1). A combination of molecular docking studies and structure–activity relationship studies led to the synthesis of the bidentate molecule 113B7 (PDZ1i) (Fig. S1). The proposed docked structure of PDZ1i in the PDZ1 domain and interdomain of MDA-9/Syntenin is shown in Fig. S1, and the chemical structure of PDZ1i is also shown in Fig. S1. In agreement with the data with the individual fragments that compose 113B7, the molecule does not appreciably bind to the PDZ2 domain of MDA-9/Syntenin, indicating selectivity (Fig. S1). Larger-scale amounts of the compound (>500 mg) were synthesized and used for cellular and in vivo characterizations, including pharmacokinetics (PK) and efficacy studies in mice (Table S1).
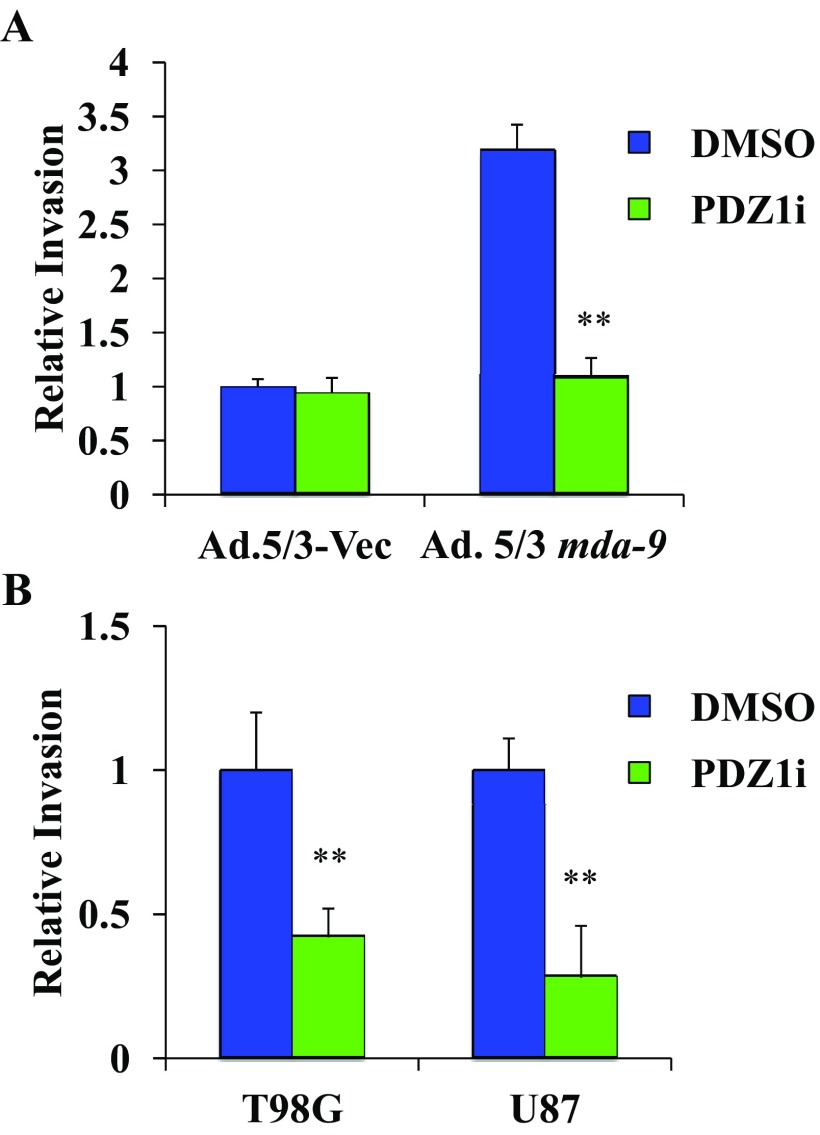
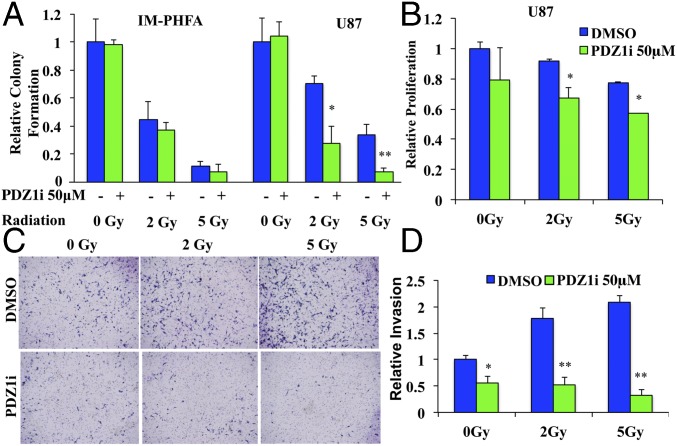
Initial studies revealed that PDZ1i effectively inhibited invasion in T98G and U87 cells (Fig. S3). Moreover, it inhibited MDA-9/Syntenin–induced invasion following MDA-9/Syntenin overexpression (Fig. S3). We then tested whether PDZ1i treatment would radiosensitize glioma cells by exposing immortalized primary human fetal astrocytes (Im-PHFAs) and the GBM cell line U87 to 0-, 2-, and 5-Gy radiation after a 2-h pretreatment with PDZ1i. Normal astrocytes showed significant radiosensitivity following radiation exposure, yet PDZ1i treatment did not further radiosensitize these cells (Fig. 3A). However, U87 cells showed markedly more radiosensitivity when combined with PDZ1i treatment. Proliferation following radiation was also significantly decreased in U87 cells that combined radiation and PDZ1i compared with radiation with control DMSO treatment (Fig. 3B). Finally, U87 cells were exposed to 2 and 5 Gy of radiation, with or without PDZ1i pretreatment, which increased the ability of these cells to invade, whereas PDZ1i pretreatment abolished these invasion gains (Fig. 3 C and D).
Fig. S3.
(A) Im-PHFAs were infected with an MDA-9/Syntenin expression plasmid carrying adenovirus. Forty-eight hours postinfection, both the control and MDA-9/Syntenin–overexpressing cells were treated with DMSO or PDZ1i followed by seeding in a Transwell Matrigel invasion assay and stained after 24 h. (B) T98G and U87 cells were treated with DMSO or PDZ1i, seeded in a Transwell Matrigel invasion assay, and stained after 24 h. Invasion was quantified from four random fields. Data represent fold changes and average ± SD. **P < 0.01.
Fig. 3.
PDZ1i produces similar effects to mda-9/syntenin knockdown postradiation. (A) Im-PHFAs and U87 cells were treated with 50 μM PDZ1i 2 h before radiation and analyzed for colony formation after 14 d. (B) U87 cells were treated with 50 μM PDZ1i 2 h before radiation and analyzed via MTT assay after 24 h. (C) U87 cells were pretreated with 50 μM PDZ1i 2 h before radiation and subsequently seeded in a Transwell Matrigel invasion assay and stained after 18 h. (D) Invasion was quantified from five random fields. Error bars indicate ±SD. *P < 0.05, **P < 0.01.
PDZ1i Inhibits EGFRvIII-Driven Signaling in GBM and Reduces MMP Secretion.
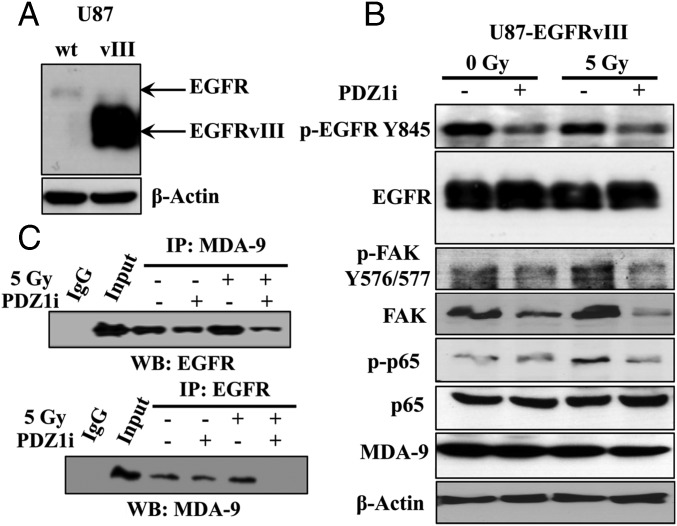
EGFR amplification and mutation are both a common and important alteration in GBM (25, 26). A particular mutation of this receptor, EGFR variant III (EGFRvIII), is often coexpressed along with EGFR amplification in GBM. EGFRvIII and FAK interact as part of a complex to mediate EGFRvIII-mediated MAPK activation (27). Given the demonstrated role of MDA-9/Syntenin in enhancing downstream signaling of the FAK–Src complex in melanoma (13), we determined whether PDZ1i could inhibit EGFRvIII signaling in GBM. We used U87 cells stably expressing EGFRvIII, U87-EGFRvIII (Fig. 4A), and treated them with PDZ1i before radiation. In both radiated and nonradiated cells, phospho-EGFR was significantly reduced in the presence of PDZ1i. FAK activation was enhanced after radiation, and this activation was nullified by PDZ1i treatment (Fig. 4B). Downstream, we observed that NF-κB activation, enhanced following radiation, was reduced in the presence of PDZ1i. Additionally, treatment with the PDZ1i disrupted MDA-9 interactions with EGFR in both radiated and nonradiated cells (Fig. 4C). From these findings, we propose that PDZ1i disrupts EGFRvIII–FAK signaling, which ultimately reduces the observed postradiation gains in NF-κB activation.
Fig. 4.
PDZ1i treatment impairs EGFRvIII and FAK signaling as well as EGFRvIII–MDA-9 interaction. (A) Immunoblot analysis showing expression of mutated EGFR in U87 or U87-EGFRvIII cells. (B) Cells were treated with either DMSO or PDZ1i 2 h before radiation. Cells were subsequently seeded on fibronectin-coated plates and cell lysates were collected after 1 h and analyzed for protein expression. (C) Lysates from cells treated with and without radiation or PDZ1i as indicated were analyzed via immunoprecipitation (IP). Anti–MDA-9 (Top) and anti-EGFR (Bottom) were used to pull down associated complexes, and Western blotting (WB) used the indicated antibodies. (Left) IgG and control input samples.
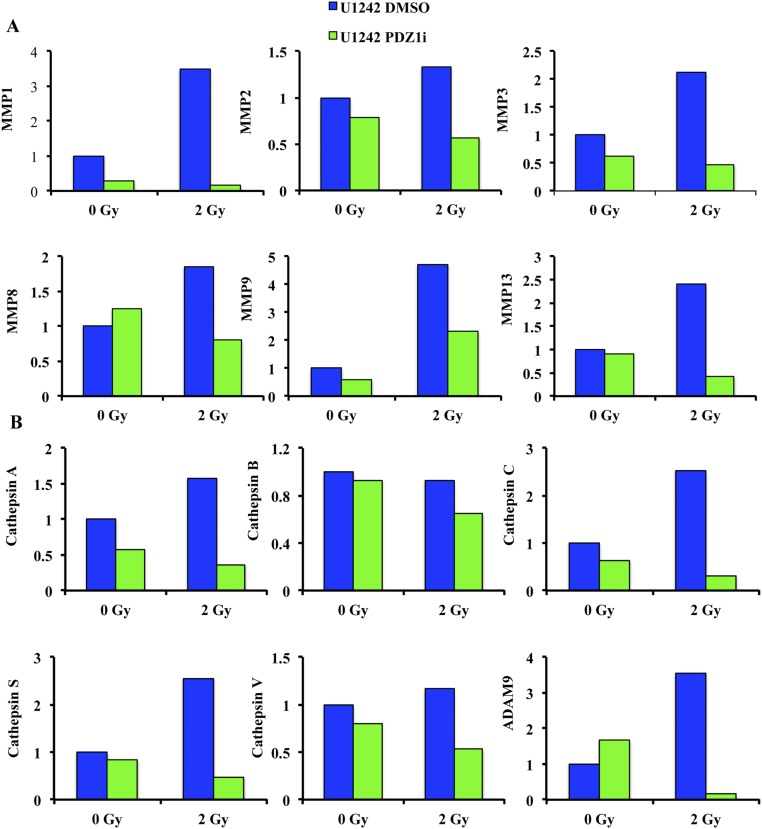
Secreted factors significantly impact on cancer cell invasion, and MDA-9/Syntenin affects the secretion of important factors such as MMP-2 and VEGF in glioma (14). Radiation enhances the release of important enzymes, including those involved in invasion, such as the matrix metalloproteinase family (19). We determined whether PDZ1i could affect the secretion of invasion-related proteins following radiation therapy. U1242 cells were treated for 2 h before radiation therapy, and media were collected after 48 h. Expression of several MMP family members significantly increased following radiation therapy, including MMP-2 and MMP-9. PDZ1i treatment reduced the levels of these enzymes following radiation therapy (Fig. S4), an important aspect of reducing radiation-induced invasion gains. Additionally, radiation therapy increased expression of several cathepsin family members as well as ADAM9, whereas PDZ1i eliminated these gains (Fig. S4). Cathepsin family proteases modulate tumor invasion and metastasis in a variety of malignancies, including melanoma and breast cancer metastatic to the brain. Notably, the expression of this family is important in microenvironment-mediated chemo- and radioresistance and in facilitating supportive tumor stroma (28).
Fig. S4.
PDZ1i inhibits key invasion proteins. (A) Metalloproteinase, (B) Cathepsin family, and ADAM9 expression. U1242 cells were treated with either DMSO or 50 μM PDZ1i for 2 h before radiation in serum-free media. After 48 h, media were collected and analyzed via a Proteome Profiler Human Protease Array Kit (R&D Systems). Relative protein amounts were quantified via ImageJ.
Stability in the circulation and bioavailability in vivo are critical properties of a potential therapeutic agent. PK studies were performed administering 3.0 mg/kg (i.v.) and 30.0 mg/kg (i.p.) in mice (n = 3), and the compound concentration in serum was measured at various times (15 min to 24 h after drug administration). Noteworthy were the lack of adverse signs of toxicity during the experiment, the very slow clearance with T1/2 >9 h in each route, and the 80% bioavailability for the compound administered i.p. vs. i.v. (Table S1). Based on these data, we anticipated that one to three weekly doses of the drug i.p. at 30 mg/kg would result in an effective dose for achieving a constant inhibition of the target, despite the relatively low affinity (micromolar) of the compound for the target. We did not anticipate issues inhibiting brain exposure, because mice with GBM have a leaky blood–brain barrier (29). Before in vivo efficacy studies, we evaluated the ability of the compound to cross the blood–brain barrier using a target-based surrogate cellular assay. This method is preferred to direct in vivo measurements of compound concentration in the brain because of low sensitivity of the detection methods and because of the presence of drug in various vascular spaces, namely the residual blood of the brain (30). Accordingly, we used human brain microvascular endothelial cells (HBMECs) (30) seeded with PDZ1i in the upper chamber of Transwell inserts placed on top of wells containing GBM6 cells. After 24 h, the invasive ability of the treated GBM6 cells was compared with pretreated controls. PDZ1i effectively crossed the HBMEC barrier to inhibit invasion in GBM6 cells comparably to the pretreated control with no barrier (Fig. S5). Taken together, these results suggest that PDZ1i is a potent inhibitor of invasion with radiosensitizing effects and favorable properties for CNS treatment.
Fig. S5.
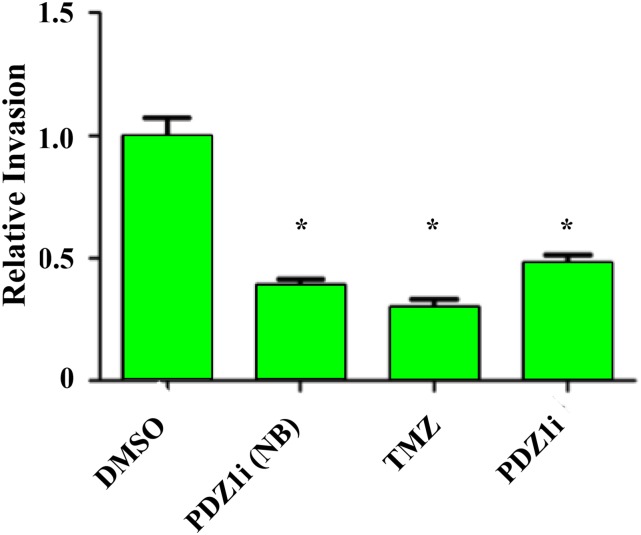
PDZ1i crosses the blood–brain barrier. Primary human malignant glioma cells (GBM6) were seeded in the bottom well of a Transwell chamber. In the top chamber, a monolayer of HBMECs separated GBM6 cells from media containing DMSO, 50 μM PDZ1i, or temozolomide (500 μM). After a 24-h incubation in these conditions, invasion of GBM6 cells was analyzed by seeding in a Transwell Matrigel invasion assay and stained after 24 h. NB, PDZ1i treatment and invasion assay without HBMEC barrier. Error bars indicate ±SD. *P < 0.05.
PDZ1i Is Effective in Reducing Tumor Invasion in an Animal Model of GBM.
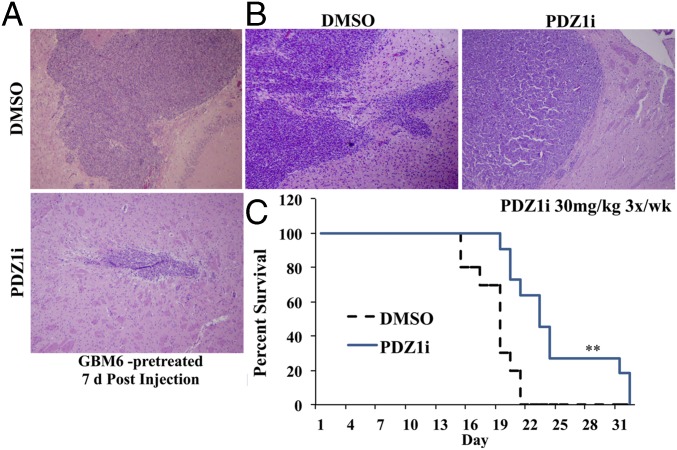
Orthotopic xenograft models with GBM6 cells were pretreated with either DMSO or PDZ1i and injected intracranially to form tumors. Seven days postinjection, tumor and brain tissue were isolated for sectioning. Tumor cells treated with PDZ1i developed noticeably smaller and more demarcated neoplasms compared with controls (Fig. 5A). Separately, untreated cells were injected and tumors were established, and then mice were treated i.p. with either DMSO or PDZ1i (30 mg/kg) three times per wk for 2 wk. Survival was significantly increased in treated mice compared with controls (Fig. 5C), and tumors were well-circumscribed and less infiltrative on analysis than those of control-treated mice (Fig. 5B). Similar to previous studies knocking down MDA-9/Syntenin expression in an in vivo model (14), PDZ1i was effective in reducing tumor invasion and extending survival in an animal model of GBM.
Fig. 5.
Effect of PDZ1i on survival in an in vivo model of glioma. (A) GBM6 cells were pretreated for 2 h before intracranial injection. After 7 d, brain tissue was isolated and sectioned at the injection site. (B) Seven days after tumor implantation, mice received either vehicle or PDZ1i (30 mg/kg) three times per wk for 2 wk. Brain tissue was isolated and analyzed via H&E stain. (C) Kaplan–Meier curves of these groups based on animal survival. **P < 0.01.
PDZ1i Combined with Radiation Extends Survival and Reduces GBM Invasion in Vivo.
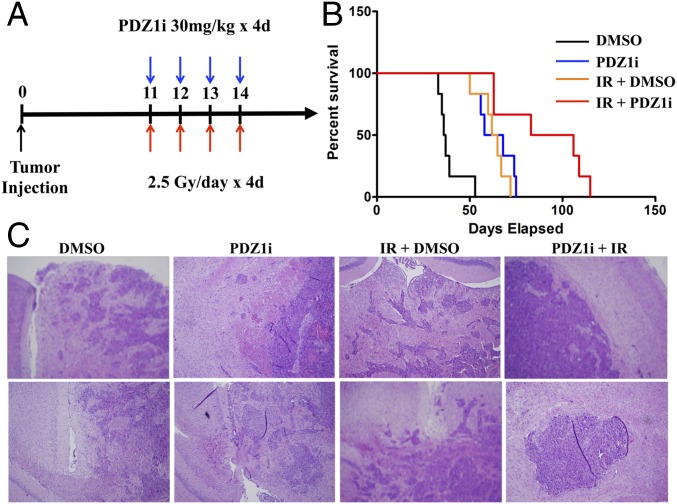
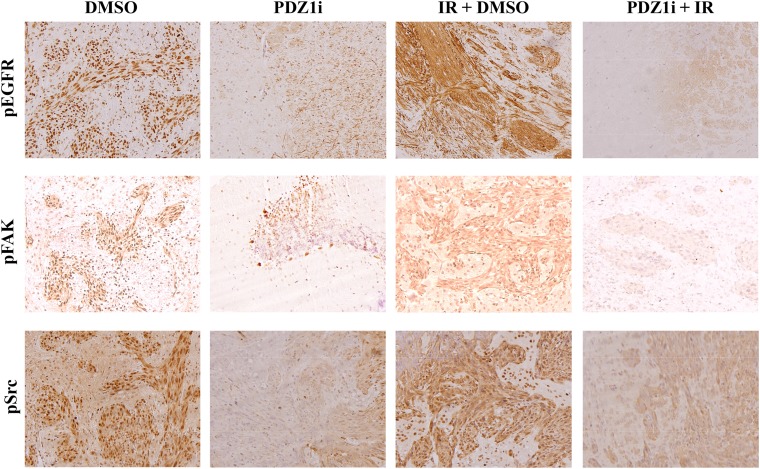
We investigated the combination of PDZ1i with radiotherapy in vivo. U1242-luc cells were injected intracranially under anesthesia and formed tumors, confirmed by imaging at 7 d postinjection. At this point, mice were randomized to four groups: DMSO treatment, PDZ1i treatment (30 mg/kg), DMSO + ionizing radiation (IR), and PDZ1i + IR. Treatment began at 11 d postinjection, and mice receiving radiation were treated with 2.5 Gy for 4 consecutive days. PDZ1i was administered 2 h before radiation treatment on each of the 4 treatment days (Fig. 6A). Control-treated mice had an average survival of 41.3 d, whereas PDZ1i treatment extended that to 54.7 d. Radiation alone extended survival to 62.8 d, whereas PDZ1i treatment + IR therapy led to a survival of 78.8 d (Fig. 6B). To investigate whether tumor morphology and invasion pattern changed in treatment groups, brain tissue sections were collected and H&E stains were analyzed. Indeed, U1242-luc cells developed diffusely infiltrating tumors in control groups. Tumor margins were slightly more demarcated in groups treated with PDZ1i (Fig. 6C). Radiation treatment led to generally smaller, yet still diffuse, tumor patterns, which frequently crossed the midline of the brain with invasive outgrowths. The combination of PDZ1i with radiation led to tumors with markedly more circumscribed margins, less invasive outgrowths, and less spread to the leptomeninges (Fig. 6C). Analysis of tumor sections from these treatment groups indicated that activated forms of EGFR, FAK, and Src correlated with PDZ1i treatment status (Fig. S6). Those mice treated with only radiation showed marked increases in activation of these proteins, whereas groups that underwent treatment with PDZ1i showed decreased signal intensity compared with both control- and radiation-treated groups. Collectively, these data support the hypothesis that targeting MDA-9/Syntenin is a promising approach to enhancing conventional radiation treatment.
Fig. 6.
PDZ1i treatment combined with radiation in an in vivo model of GBM. (A) U1242-luc cells were injected intracranially into nude mice. After 7 d, mice were randomized to four groups, and mice receiving therapy were treated on days 11 to 14 as pictured. (B) Kaplan–Meier survival curves for each treatment group. Median survival is as listed. (C) Brain tissue was isolated and sectioned, and H&E staining is shown.
Fig. S6.
PDZ1i reduces EGFR, FAK, and Src activation. Tumor sections from DMSO-, PDZ1i-, IR-, and PDZ1i + IR-treated mice were collected and stained for pEGFR (Top), pFAK (Middle), and pSrc (Bottom).
Discussion
We demonstrate that MDA-9/Syntenin is an important mediator of the postradiation signaling process, eliminating invasion enhancement induced by radiation. Database analysis of REMBRANDT clinical samples supports the idea that high MDA-9/Syntenin expression can lead to a more radioresistant phenotype compared with tumors with lower MDA-9/Syntenin expression. Interesting links in glioma to MDA-9/Syntenin–related signaling have emerged recently. Database expression profiles comparing long-term survivors (LTSs) of GBM (>48 mo) with short-term survivors (<12 mo) revealed a 40% decrease in IGFBP-2, a noted downstream product of MDA-9/Syntenin signaling (9), in LTS tumors (31). Additionally, identification of a radiosensitive gene signature via expression data from the NCI-60 cancer cell panel revealed that a recognized MDA-9/Syntenin binding partner, integrin-linked kinase (32), was significantly down-regulated in radiosensitive cells (33). An ideal complement to radiotherapy would sensitize tumor cells to the cytotoxic effects of radiation while abrogating the unwanted proinvasive effects. We now establish MDA-9/Syntenin as a promising therapeutic target by showing that knockdown potentiates radiosensitivity and abolishes radiation-induced invasion.
MDA-9/Syntenin activates multiple signaling pathways, including enhancing Src signaling. Src is involved in numerous cancer-related processes, including survival, motility, and invasion (34). Especially relevant for GBM, Src interacts with EGFR, including wild-type and mutant versions, activating EGFR through phosphorylation (34, 35). Notably, radiation induces Src-dependent activation of EGFR in GBM, leading to MMP-2 expression and invasion (19), supporting our hypothesis that targeting MDA-9/Syntenin could inhibit radiation-induced invasion signaling. Although extensive preclinical data support the targeting of Src directly for cancer therapy, the results from clinical trials have overall been disappointing (34). Low or no response rates as a single agent or in combination were reported in breast cancer and GBM (34), and GBM patients progressing while on bevacizumab also did not respond on Src inhibitors (36). A proposed method of escape and resistance to Src inhibitors involves up-regulation of IGFBP-2/FAK signaling (37). High levels of IGFBP-2 correlated with resistance, and exogenous IGFBP-2 rendered cells less sensitive to dasatinib. Additionally, FAK activation has been linked to radioresistance (37–39). Targeting MDA-9/Syntenin reduces FAK phosphorylation as well as IGFBP-2 production in addition to Src activation. Therefore, our approach of inhibiting the action of MDA-9/Syntenin may preempt resistance stemming from Src inhibition.
Recent efforts reveal novel mechanisms by which cells evade the cytotoxic effects of radiation therapy. Exosome signaling is a potential pathway by which cancer cells survive under the stress of radiation damage (40, 41). Among exosome cargoes are full-length protein receptors, ligands, RNA, and DNA, including oncogenes (40). Radiation therapy induces greater exosome release from both GBM cells and normal astrocytes. These exosomes contain much higher levels of IGFBP-2 and enhance migration in recipient cells. Furthermore, radiation increases cellular uptake of exosomes (41), and uptake following radiation promotes FAK and Src activation in target cells (40). This is relevant to our work, because MDA-9/Syntenin gain enhances exosome yield whereas MDA-9/Syntenin knockdown reduces exosome release (42). MDA-9/Syntenin targeting is an effective inhibitor of Src activation in glioma and reduces the release of MMP family proteins. Thus, PDZ1i (113B7) could prove useful in reducing both the efficiency of exosome release as well as their effect on the ECM in recipient cells.
The combination of PDZ1i and radiotherapy in a model of GBM showed improvement over each method alone. In vivo, important interactions occur within the tumor microenvironment, including extracellular communication between tumor cells, but also between tumor cells and normal cells, such as endothelial cells. Recent work suggests a mechanism of radioresistance through endothelial cell FAK–NF-κB signaling (43). In normal endothelial cells within the tumor vasculature, DNA-damaging therapy induced FAK activation and NF-κB translocation and activation, leading to secretion of protective cytokines and the development of a sheltering perivascular niche following radiation (43). Therefore, our PDZ1i has the potential to have positive therapeutic effects on both tumor and normal cells within the tumor microenvironment.
Overall, we now show that MDA-9/Syntenin targeting is a viable approach for combating radiation-induced invasion and can radiosensitize GBM. A targeted inhibitor leads to a reduction in phenotypes enhanced by MDA-9/Syntenin, and can be combined effectively with conventional radiotherapy in vivo. This study highlights an MDA-9/Syntenin PDZ1 inhibitor with profound antiinvasive effects, good stability without overt toxicity in vivo, a potential ability to pass the blood–brain barrier, and the capacity to both radiosensitize and block invasion gains of radiation in aggressive GBM cells in vivo. Because MDA-9/Syntenin is involved in many important cancer-related cellular processes and signaling events, further investigation into the spectrum of activities of PDZ1i will be enlightening, as will the potential further development of this class of inhibitors into potential drugs for potentiating the therapeutic utility of radiation for the therapy of GBM, a currently invariably fatal cancer.
Materials and Methods
Reagents, Plasmids, Adenoviruses, and Stable Cell Lines.
The chemical structures of two initial fragment hits, structure of the resulting bidentate molecule 113B7 (PDZ1i), binding curve and representative spectra for the titration of 113B7 against the MDA-9 PDZ1/2 tandem domain, and docked structure of 113B7 in complex with the MDA-9/Syntenin PDZ tandem domain are shown in Fig. S1. Description of the synthesis of 113B7 (PDZ1i) is provided in SI Materials and Methods. Small hairpin RNA for mda-9/syntenin (shmda-9) and plasmids pAd.5/3-shmda-9 and pAd.5/3-shcon were constructed as described (14). Details are in SI Materials and Methods.
Cell Lines, Cell Culture, and Treatments.
Human malignant glioma cell lines and culture conditions are described in SI Materials and Methods. The U1242 human glioma cell line exhibits an invasive and very aggressive phenotype when grown in nude mice (44). Cells were treated with PDZ1i 2 h before irradiation. Irradiations were done using an MDS Nordion Gammacell 40 research irradiator with a 137Cs source delivering a dose rate of 0.896 Gy/min.
Preparation of Whole-Cell Lysates and Western Blotting Analysis.
Preparation of whole-cell lysates and Western blotting analysis were performed as described (45). For densitometry evaluation, X-ray films were scanned and analyzed with ImageJ software (NIH).
Extraction of Total RNA and Real-Time PCR.
Total RNA was extracted from cells using the QIAGEN miRNeasy Mini Kit. Details are in SI Materials and Methods.
Immunohistochemistry.
Hematoxylin & eosin staining and immunohistochemistry were conducted using a standard protocol (20). Details are in SI Materials and Methods.
Invasion and Migration Assays.
Invasion and migration assays were performed following standard procedures. Details are in SI Materials and Methods.
Survival and Viability Analysis.
Clonogenic radiosurvival experiments were carried out as described (44). Cell viability was assessed through tetrazolium dye, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium) reduction analysis as described (46). Details are in SI Materials and Methods.
Viral Infections.
Viral infection conditions and protocols were performed as described (20). Details are in SI Materials and Methods.
Intracranial Implant of Cells in Nude Mice.
The Institutional Animal Care and Use Committee at Virginia Commonwealth University School of Medicine approved the animal studies. Intracranial xenograft implantation was performed following standard procedures. Details are in SI Materials and Methods.
Database Mining.
The REMBRANDT database (now housed as part of the Georgetown Database of Cancer https://gdoc.georgetown.edu/gdoc/) was accessed and mined for all astrocytoma and GBM patients. Details are in SI Materials and Methods.
Statistical Analysis.
The data are reported as the mean ± SD of the values from three independent determinations, and significance analysis was performed using the Student’s t test in comparison with corresponding controls. Probability values <0.05 were considered statistically significant. Survival curves were analyzed using Cox proportional hazards survival regression using GraphPad Prism.
SI Materials and Methods
Reagents, Plasmids, Adenoviruses, and Stable Cell Lines.
PDZ1i (113B7) (N-(2,5-dimethyl-4-(3-(5-phenyl-1,3,4-oxadiazol-2-yl)propanamido)phenyl)-8-oxo-5,6,7,8-tetrahydro-4H-cyclopenta[d][1,2,4]triazolo[1,5-a]pyrimidine-2-carboxamide) was synthesized from 3-(5-phenyl-1,3,4-oxadiazol-2-yl)propanoic acid (418 mg, 1.92 mmol), 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate (875 mg, 2.30 mmol), and N,N-diisopropylethylamine (0.83 mL, 4.8 mmol) in DMF (10 mL) (room temperature for 24 h). PDZ1i was purified via a C18 column (CombiFlash Rf; Teledyne Isco) using acetonitrile/water as an eluent. This compound was used for all subsequent cellular and in vivo studies, including PK and efficacy studies (Table S1).
Small hairpin RNA for mda-9/syntenin (shmda-9) and plasmids pAd.5/3-shmda-9 and pAd.5/3-shcon was constructed with pSilencer hygro expression vectors according to the manufacturer’s protocol (Ambion) as previously described (14), and the resultant plasmids were cleaved with PacI to release the recombinant adenovirus genomes and then transfected to HEK-293 cells to rescue the corresponding Ad.5/3-based vectors. The rescued viruses were upscaled using HEK-293 cells and purified by cesium chloride double ultracentrifugation (Beckman SW28 rotor) using a standard protocol (OD260 Inc., Boise, ID), and the titers of infectious viral particles were determined by plaque assay using HEK-293 cells as described (47).
Cell Lines, Cell Culture, and Treatments.
Human malignant glioma cells U87, U251, and T98G as well as grade III astrocytoma lines Sw1088 and Sw1783 were purchased from the American Type Culture Collection. Human primary GBM6 cells were described previously and provided by C. David James, University of California, San Francisco (48). These and U1242 cells (44) were cultured in DMEM + F12 supplemented with 10% (vol/vol) FCS in a 37-°C incubator supplemented with 5% (vol/vol) CO2. Primary human fetal astrocytes were obtained from preterm abortions as previously described with institutional review board approval, and h-TERT–immortalized primary human fetal astrocytes (Im-PHFAs) were produced and cultured as described (14). All cells were routinely screened for mycoplasma contamination. Cells were treated with PDZ1i at the indicated concentrations 2 h before irradiation. Irradiations were done using an MDS Nordion Gammacell 40 research irradiator with a 137Cs source delivering a dose rate of 0.896 Gy/min.
Antibodies and Reagents.
Antibodies against FAK, phospho-FAK (Tyr576/577), EphA2, phospho-EphA2 (S897), EGFR, phospho-EGFR (Y845), p65, phospho-p65(S536), Src, and phospho-Src (Tyr416) were obtained from Cell Signaling. β-Actin and α-tubulin were obtained from Abcam, and MDA-9/Syntenin was from Abnova. Fibronectin was purchased from Sigma-Aldrich.
Preparation of Whole-Cell Lysates and Western Blotting Analysis.
Preparation of whole-cell lysates and Western blotting analysis were performed as described (45). For densitometry evaluation, X-ray films were scanned and analyzed with ImageJ software (NIH).
Extraction of Total RNA and Real-Time PCR.
Total RNA was extracted from cells using the QIAGEN miRNeasy Mini Kit. cDNA preparation was done using a cDNA synthesis kit (Applied Biosystems). Real-time PCR was performed using a ViiA 7 fast real-time PCR system and TaqMan gene expression assays according to the manufacturer’s protocol (Applied Biosystems).
Immunohistochemistry.
A portion of the frozen tumor specimen was fixed in phosphate-buffered formalin and preserved as paraffin sections following a standard procedure for the maintenance of histological structure. Paraffin-embedded sections were dewaxed and rehydrated through incubations in xylene and a gradient series of alcohol. Antigen retrieval was processed in 10 mM citric acid (pH 6.0) with microwave treatment for 20 min. Endogenous hydrogen peroxidase was quenched with 3% (vol/vol) H2O2 for 20 min. After blocking nonspecific binding sites with 5% (vol/vol) normal sera, the sections were incubated overnight with antibody. The sections were incubated with appropriate biotinylated secondary antibody and subsequently with avidin–biotin complex peroxidase (Vector Elite; Vector Laboratories). Colorimetric reactions were developed by incubation in DAB substrate [0.02% DAB (3,3′-diaminobenzidine), 0.005% hydrogen peroxide] and counterstained with 10% (vol/vol) Harris hematoxylin. Hematoxylin & eosin staining was conducted following a standard protocol (20). The images were analyzed under an Olympus BX41 microscope system equipped with DP25 digital camera and software.
Invasion and Migration Assays.
Invasion was measured using 24-well BioCoat cell-culture inserts (BD Biosciences) with an 8-μm-porosity polyethylene terephthalate membrane coated with Matrigel Basement Membrane Matrix (100 μg/cm2) (BD Biosciences). Briefly, the Matrigel was allowed to rehydrate for 2 h at room temperature by adding warm, serum-free DMEM. The wells of the lower chamber were filled with medium containing 10% (vol/vol) FBS. Cells (5 × 104) were seeded in the upper compartment (6.5-mm membrane size) in serum-free medium. The invasion assay was done at 37 °C in a 5% (vol/vol) CO2 humidified incubator for 18 or 24 h. At the end of the invasion assay, filters were removed, fixed, and stained with a Diff-Quick Staining Kit (IMEB). Cells remaining on the upper surface of the filters were removed by wiping with a cotton swab, and invasion was determined by counting the cells that migrated to the lower side of the filter using at least five fields per insert at 100× magnification. Wound-healing scratch motility assays were done as described previously (20). Data from triplicate experiments are expressed as mean ± 95% confidence interval.
Survival and Viability Analysis.
Clonogenic radiosurvival experiments were carried out as described previously (44). Briefly, cells were diluted, seeded on 6-cm dishes, and, after the cells attached, treated with DMSO or PDZ1i (12.5, 25, or 50 μM) in the medium for 2 h before irradiation. Cells were incubated overnight, and the medium was changed to non–drug-containing medium 16 h postirradiation. Cells were incubated further for 14 d and stained with 2% (vol/vol) Giemsa solution, and colonies consisting of 50 or more cells were counted. Cell viability was assessed through MTT analysis as described previously (46).
Conditioned Medium Isolation and Analysis.
Conditioned medium was harvested from an overnight culture of plated cells in serum-free DMEM + F12, filtered with 0.2-μm filters, and further concentrated 10-fold on an Amicon Ultra centrifugal filter unit, 3 kDa (Millipore). Protein levels were analyzed via a Proteome Profiler Human Protease Array Kit (R&D Systems). Relative quantification was performed by densitometry evaluation. X-ray films were scanned and analyzed with ImageJ software (NIH).
Virus Infections.
Viral infection conditions and protocols were performed as delineated previously (20).
Intracranial Implant of Cells in Nude Mice.
Athymic female NCr-nu/nu mice (National Cancer Institute at Frederick) weighing ∼25 g were used for this study. Mice were maintained under pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the US Department of Agriculture, US Department of Health and Human Services, and NIH. At least 5 mice per group were used, with duplicate replications for a minimum total of 10 mice per group. Mice were anesthetized through i.p. administration of ketamine (40 mg/kg) and xylazine (3 mg/kg) and immobilized in a stereotactic frame (Stoelting). A 24-gauge needle attached to a Hamilton syringe was inserted into the right basal ganglia to a depth of 3.5 mm and then withdrawn 0.5 mm to make space for tumor cell accumulation. The entry point at the skull was 2-mm lateral and 1-mm dorsal to the bregma. Intracerebral injections of 1.5 × 104 cells in 2 μL per mouse were completed over 10 min using an automated injector (Stoelting). The skull opening was enclosed with sterile bone wax and the skin incision was closed using sterile surgical staples. Postsacrifice, the tumors were resected, weighed, and preserved for immunohistochemistry staining.
Database Mining.
The Repository for Molecular Brain Neoplasia Data (REMBRANDT) database (now housed as part of the Georgetown Database of Cancer, https://gdoc.georgetown.edu/gdoc/) was accessed and mined for all astrocytoma and GBM patients. These were filtered for those with no previous history of radiation therapy but who had a record of undergoing subsequent radiation. This population was stratified for SDCBP (mda-9/syntenin) expression. The survival data for the resulting groups were downloaded and analyzed via GraphPad Prism.
Acknowledgments
The present study was supported in part by NIH/NCI Grant R01 CA168517 (to M.P. and P.B.F.), NCI Cancer Center Support Grant to the VCU Massey Cancer Center (MCC) P30 CA016059 (to P.B.F. and D.S.), and the National Foundation for Cancer Research (W.K.C. and P.B.F.). M.P. holds the Daniel Hays Chair in Cancer Research. P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research at the MCC.
Footnotes
Conflict of interest statement: The authors have applied for patent protection for the small-molecule PDZ inhibitor reported in this paper.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616100114/-/DCSupplemental.
References
- 1.Johnson DR, O’Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107(2):359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(Suppl 4):iv1–iv63. doi: 10.1093/neuonc/nou223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SW, et al. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: A quantitative dosimetric study. Int J Radiat Oncol Biol Phys. 1999;43(1):79–88. doi: 10.1016/s0360-3016(98)00266-1. [DOI] [PubMed] [Google Scholar]
- 4.Chan JL, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20(6):1635–1642. doi: 10.1200/JCO.2002.20.6.1635. [DOI] [PubMed] [Google Scholar]
- 5.Wild-Bode C, Weller M, Rimner A, Dichgans J, Wick W. Sublethal irradiation promotes migration and invasiveness of glioma cells: Implications for radiotherapy of human glioblastoma. Cancer Res. 2001;61(6):2744–2750. [PubMed] [Google Scholar]
- 6.Kargiotis O, Geka A, Rao JS, Kyritsis AP. Effects of irradiation on tumor cell survival, invasion and angiogenesis. J Neurooncol. 2010;100(3):323–338. doi: 10.1007/s11060-010-0199-4. [DOI] [PubMed] [Google Scholar]
- 7.Fiveash JB, Spencer SA. Role of radiation therapy and radiosurgery in glioblastoma multiforme. Cancer J. 2003;9(3):222–229. doi: 10.1097/00130404-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Vehlow A, Cordes N. Invasion as target for therapy of glioblastoma multiforme. Biochim Biophys Acta. 2013;1836(2):236–244. doi: 10.1016/j.bbcan.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Das SK, et al. MDA-9/syntenin and IGFBP-2 promote angiogenesis in human melanoma. Cancer Res. 2013;73(2):844–854. doi: 10.1158/0008-5472.CAN-12-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasgupta S, et al. Novel role of MDA-9/syntenin in regulating urothelial cell proliferation by modulating EGFR signaling. Clin Cancer Res. 2013;19(17):4621–4633. doi: 10.1158/1078-0432.CCR-13-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacolod MD, et al. Examination of epigenetic and other molecular factors associated with mda-9/Syntenin dysregulation in cancer through integrated analyses of public genomic datasets. Adv Cancer Res. 2015;127:49–121. doi: 10.1016/bs.acr.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das SK, et al. MDA-9/syntenin: A positive gatekeeper of melanoma metastasis. Front Biosci (Landmark Ed) 2012;17:1–15. doi: 10.2741/3911. [DOI] [PubMed] [Google Scholar]
- 13.Kegelman TP, et al. Targeting tumor invasion: The roles of MDA-9/Syntenin. Expert Opin Ther Targets. 2015;19(1):97–112. doi: 10.1517/14728222.2014.959495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kegelman TP, et al. MDA-9/syntenin is a key regulator of glioma pathogenesis. Neuro Oncol. 2014;16(1):50–61. doi: 10.1093/neuonc/not157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar D, Boukerche H, Su Z-Z, Fisher PB. mda-9/Syntenin: More than just a simple adapter protein when it comes to cancer metastasis. Cancer Res. 2008;68(9):3087–3093. doi: 10.1158/0008-5472.CAN-07-6210. [DOI] [PubMed] [Google Scholar]
- 16.Talukdar S, et al. Novel function of MDA-9/Syntenin (SDCBP) as a regulator of survival and stemness in glioma stem cells. Oncotarget. July 26, 2016 doi: 10.18632/oncotarget.10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rees DC, Congreve M, Murray CW, Carr R. Fragment-based lead discovery. Nat Rev Drug Discov. 2004;3(8):660–672. doi: 10.1038/nrd1467. [DOI] [PubMed] [Google Scholar]
- 18.Barile E, Pellecchia M. NMR-based approaches for the identification and optimization of inhibitors of protein-protein interactions. Chem Rev. 2014;114(9):4749–4763. doi: 10.1021/cr500043b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park CM, et al. Ionizing radiation enhances matrix metalloproteinase-2 secretion and invasion of glioma cells through Src/epidermal growth factor receptor-mediated p38/Akt and phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res. 2006;66(17):8511–8519. doi: 10.1158/0008-5472.CAN-05-4340. [DOI] [PubMed] [Google Scholar]
- 20.Boukerche H, et al. mda-9/Syntenin: A positive regulator of melanoma metastasis. Cancer Res. 2005;65(23):10901–10911. doi: 10.1158/0008-5472.CAN-05-1614. [DOI] [PubMed] [Google Scholar]
- 21.Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: Embryogenesis to tumorigenesis. Oncogene. 2000;19(49):5614–5619. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- 22.Larsen AB, Stockhausen M-T, Poulsen HS. Cell adhesion and EGFR activation regulate EphA2 expression in cancer. Cell Signal. 2010;22(4):636–644. doi: 10.1016/j.cellsig.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama N, et al. EphA2 cleavage by MT1-MMP triggers single cancer cell invasion via homotypic cell repulsion. J Cell Biol. 2013;201(3):467–484. doi: 10.1083/jcb.201205176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Udayakumar D, et al. EphA2 is a critical oncogene in melanoma. Oncogene. 2011;30(50):4921–4929. doi: 10.1038/onc.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huse JT, Holland EC. Targeting brain cancer: Advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10(5):319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 26.Kegelman TP, et al. In vivo modeling of malignant glioma: The road to effective therapy. Adv Cancer Res. 2014;121:261–330. doi: 10.1016/B978-0-12-800249-0.00007-X. [DOI] [PubMed] [Google Scholar]
- 27.Zheng Q, et al. JAK2/STAT3 targeted therapy suppresses tumor invasion via disruption of the EGFRvIII/JAK2/STAT3 axis and associated focal adhesion in EGFRvIII-expressing glioblastoma. Neuro Oncol. 2014;16(9):1229–1243. doi: 10.1093/neuonc/nou046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson OC, Joyce JA. Cysteine cathepsin proteases: Regulators of cancer progression and therapeutic response. Nat Rev Cancer. 2015;15(12):712–729. doi: 10.1038/nrc4027. [DOI] [PubMed] [Google Scholar]
- 29.Watkins S, et al. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun. 2014;5:4196. doi: 10.1038/ncomms5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson HK, Shusta EV. Human-based in vitro brain endothelial cell models. In: Di L, Kerns EH, editors. Blood-Brain Barrier in Drug Discovery: Optimizing Brain Exposure of CNS Drugs and Minimizing Brain Side Effects for Peripheral Drugs. John Wiley & Sons; Hoboken, NJ: 2015. pp. 238–273. [Google Scholar]
- 31.Gerber NK, et al. Transcriptional diversity of long-term glioblastoma survivors. Neuro Oncol. 2014;16(9):1186–1195. doi: 10.1093/neuonc/nou043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwangbo C, Park J, Lee J-H. mda-9/Syntenin protein positively regulates the activation of Akt protein by facilitating integrin-linked kinase adaptor function during adhesion to type I collagen. J Biol Chem. 2011;286(38):33601–33612. doi: 10.1074/jbc.M110.206789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HS, et al. Identification of a radiosensitivity signature using integrative metaanalysis of published microarray data for NCI-60 cancer cells. BMC Genomics. 2012;13:348. doi: 10.1186/1471-2164-13-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Creedon H, Brunton VG. Src kinase inhibitors: Promising cancer therapeutics? Crit Rev Oncog. 2012;17(2):145–159. doi: 10.1615/critrevoncog.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- 35.Chung BM, et al. The role of cooperativity with Src in oncogenic transformation mediated by non-small cell lung cancer-associated EGF receptor mutants. Oncogene. 2009;28(16):1821–1832. doi: 10.1038/onc.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu-Emerson C, et al. Retrospective study of dasatinib for recurrent glioblastoma after bevacizumab failure. J Neurooncol. 2011;104(1):287–291. doi: 10.1007/s11060-010-0489-x. [DOI] [PubMed] [Google Scholar]
- 37.Lu H, et al. IGFBP2/FAK pathway is causally associated with dasatinib resistance in non-small cell lung cancer cells. Mol Cancer Ther. 2013;12(12):2864–2873. doi: 10.1158/1535-7163.MCT-13-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nalla AK, et al. Suppression of uPAR retards radiation-induced invasion and migration mediated by integrin β1/FAK signaling in medulloblastoma. PLoS One. 2010;5(9):e13006. doi: 10.1371/journal.pone.0013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kasahara T, Koguchi E, Funakoshi M, Aizu-Yokota E, Sonoda Y. Antiapoptotic action of focal adhesion kinase (FAK) against ionizing radiation. Antioxid Redox Signal. 2002;4(3):491–499. doi: 10.1089/15230860260196290. [DOI] [PubMed] [Google Scholar]
- 40.Arscott WT, et al. Ionizing radiation and glioblastoma exosomes: Implications in tumor biology and cell migration. Transl Oncol. 2013;6(6):638–648. doi: 10.1593/tlo.13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hazawa M, et al. Radiation increases the cellular uptake of exosomes through CD29/CD81 complex formation. Biochem Biophys Res Commun. 2014;446(4):1165–1171. doi: 10.1016/j.bbrc.2014.03.067. [DOI] [PubMed] [Google Scholar]
- 42.Baietti MF, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 43.Tavora B, et al. Endothelial-cell FAK targeting sensitizes tumours to DNA-damaging therapy. Nature. 2014;514(7520):112–116. doi: 10.1038/nature13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biddlestone-Thorpe L, et al. ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation. Clin Cancer Res. 2013;19(12):3189–3200. doi: 10.1158/1078-0432.CCR-12-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emdad L, et al. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc Natl Acad Sci USA. 2009;106(50):21300–21305. doi: 10.1073/pnas.0910936106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhutia SK, et al. Astrocyte elevated gene-1 induces protective autophagy. Proc Natl Acad Sci USA. 2010;107(51):22243–22248. doi: 10.1073/pnas.1009479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70(11):7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yacoub A, et al. MDA-7/IL-24 plus radiation enhance survival in animals with intracranial primary human GBM tumors. Cancer Biol Ther. 2008;7(6):917–933. doi: 10.4161/cbt.7.6.5928. [DOI] [PubMed] [Google Scholar]