Abstract
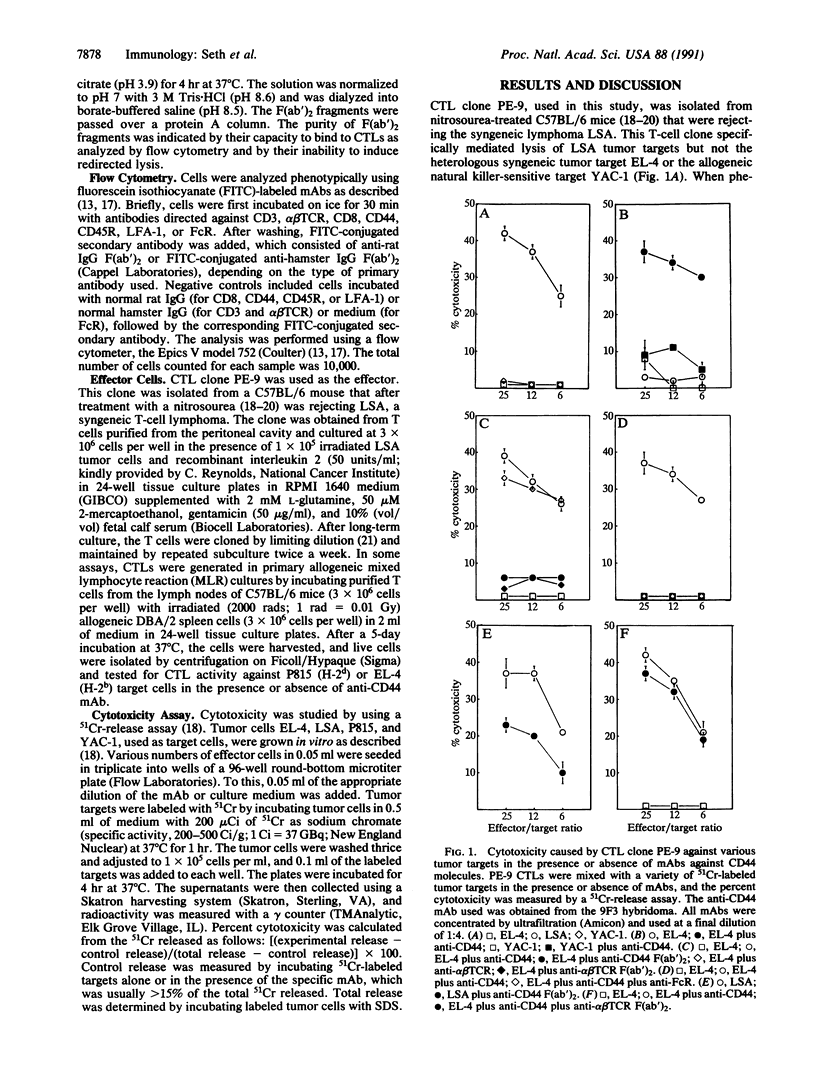
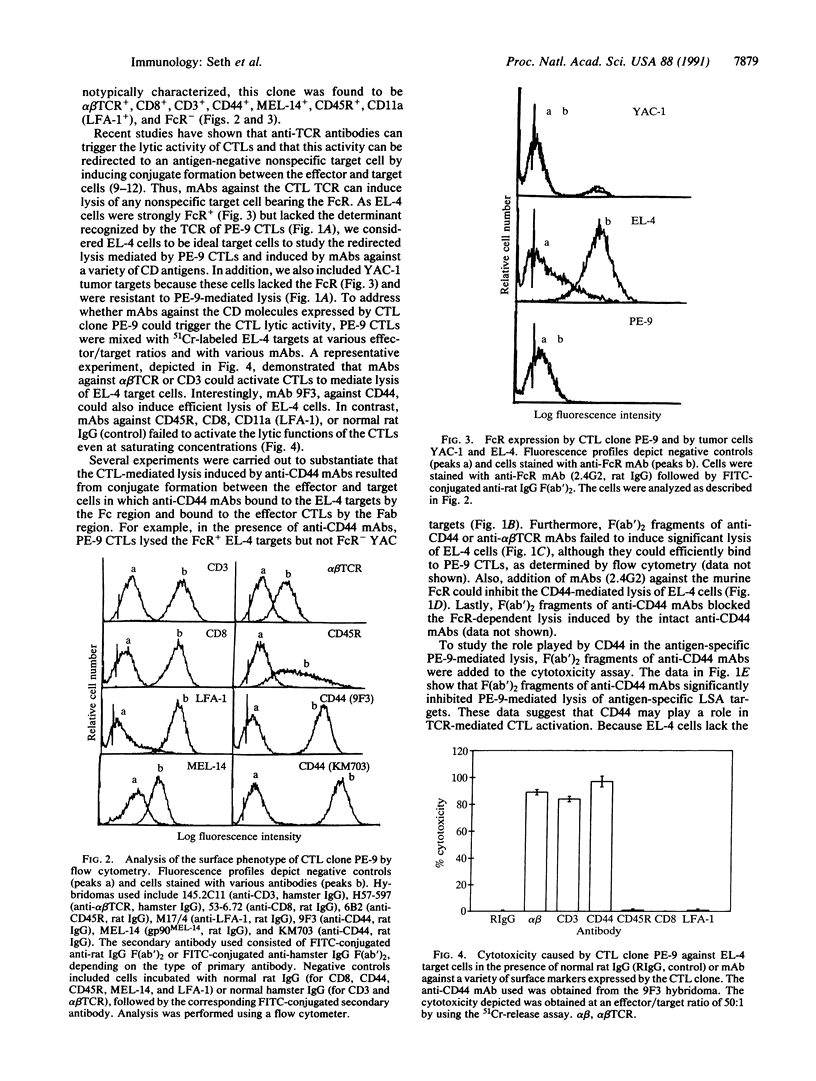
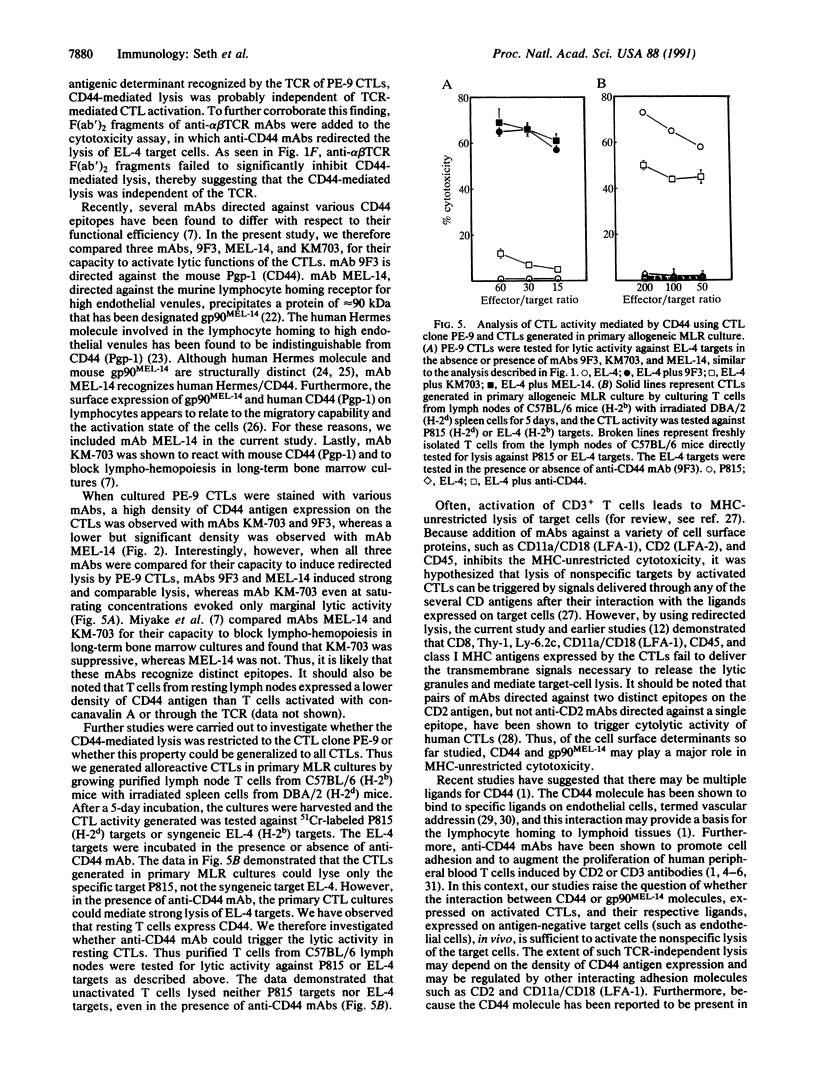
CD44 is a transmembrane glycoprotein found on a variety of cells including those of myeloid and lymphoid origin. CD44 is highly conserved among various species and is involved in the homing of lymphocytes and monocytes to lymph nodes, Peyer's patches, and sites of inflammation. In the present study, we demonstrate that monoclonal antibody (mAb) 9F3, directed against murine phagocytic glycoprotein 1 (CD44) expressed on cytotoxic T lymphocytes (CTLs), can trigger the lytic activity of CTLs and redirect CTL-mediated lysis to antigen-negative Fc receptor-positive target cells. Similar redirected lysis was also inducible using mAb MEL-14, directed against the lymphocyte homing receptor for endothelium (gp90MEL-14). The redirected lysis induced by mAbs 9F3 and MEL-14 was similar to that induced by mAbs against the alpha beta T-cell receptor or CD3. In contrast, mAbs directed against CD8, CD45R, and CD11a (LFA-1, lymphocyte function-associated antigen 1) failed to evoke lytic activity. The current study demonstrates that CD44 and gp90MEL-14 molecules, in addition to participating in T-cell homing and adhesion, may play a major role in delivering the transmembrane signal to the CTL that triggers the lytic activity, even when the T-cell receptor is not occupied. Such a mechanism may account for the nonspecific tissue damage seen at sites of CTL-mediated inflammation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belitsos P. C., Hildreth J. E., August J. T. Homotypic cell aggregation induced by anti-CD44(Pgp-1) monoclonal antibodies and related to CD44(Pgp-1) expression. J Immunol. 1990 Mar 1;144(5):1661–1670. [PubMed] [Google Scholar]
- Damle N. K., Doyle L. V., Bender J. R., Bradley E. C. Interleukin 2-activated human lymphocytes exhibit enhanced adhesion to normal vascular endothelial cells and cause their lysis. J Immunol. 1987 Mar 15;138(6):1779–1785. [PubMed] [Google Scholar]
- Denning S. M., Le P. T., Singer K. H., Haynes B. F. Antibodies against the CD44 p80, lymphocyte homing receptor molecule augment human peripheral blood T cell activation. J Immunol. 1990 Jan 1;144(1):7–15. [PubMed] [Google Scholar]
- Doherty P. C., Allan J. E., Lynch F., Ceredig R. Dissection of an inflammatory process induced by CD8+ T cells. Immunol Today. 1990 Feb;11(2):55–59. doi: 10.1016/0167-5699(90)90019-6. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A. M., Horst E., Pals S. T., Rouse B. N., Steere A. C., Picker L. J., Meijer C. J., Butcher E. C. High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am J Pathol. 1988 Jan;130(1):147–155. [PMC free article] [PubMed] [Google Scholar]
- Dumont F. J., Habbersett R. C., Nichols E. A. A new lymphocyte surface antigen defined by a monoclonal antibody (9F3) to the T cell population expanding in MRL/Mp-lpr/lpr mice. J Immunol. 1984 Aug;133(2):809–815. [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Goldstein L. A., Zhou D. F., Picker L. J., Minty C. N., Bargatze R. F., Ding J. F., Butcher E. C. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989 Mar 24;56(6):1063–1072. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Telen M. J., Hale L. P., Denning S. M. CD44--a molecule involved in leukocyte adherence and T-cell activation. Immunol Today. 1989 Dec;10(12):423–428. doi: 10.1016/0167-5699(89)90040-6. [DOI] [PubMed] [Google Scholar]
- Hirsch R., Gress R. E., Pluznik D. H., Eckhaus M., Bluestone J. A. Effects of in vivo administration of anti-CD3 monoclonal antibody on T cell function in mice. II. In vivo activation of T cells. J Immunol. 1989 Feb 1;142(3):737–743. [PubMed] [Google Scholar]
- Huet S., Groux H., Caillou B., Valentin H., Prieur A. M., Bernard A. CD44 contributes to T cell activation. J Immunol. 1989 Aug 1;143(3):798–801. [PubMed] [Google Scholar]
- Kakkanaiah V. N., Nagarkatti M., Nagarkatti P. S. Evidence for the existence of distinct heterogeneity among the peripheral CD4-CD8- T cells from MRL-lpr/lpr mice based on the expression of the J11d marker, activation requirements, and functional properties. Cell Immunol. 1990 May;127(2):442–457. doi: 10.1016/0008-8749(90)90145-h. [DOI] [PubMed] [Google Scholar]
- Koopman G., van Kooyk Y., de Graaff M., Meyer C. J., Figdor C. G., Pals S. T. Triggering of the CD44 antigen on T lymphocytes promotes T cell adhesion through the LFA-1 pathway. J Immunol. 1990 Dec 1;145(11):3589–3593. [PubMed] [Google Scholar]
- Kranz D. M., Tonegawa S., Eisen H. N. Attachment of an anti-receptor antibody to non-target cells renders them susceptible to lysis by a clone of cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7922–7926. doi: 10.1073/pnas.81.24.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Lancki D. W., Ma D. I., Havran W. L., Fitch F. W. Cell surface structures involved in T cell activation. Immunol Rev. 1984 Oct;81:65–94. doi: 10.1111/j.1600-065x.1984.tb01105.x. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch F., Ceredig R. Ly-24 (Pgp-1) expression by thymocytes and peripheral T cells. Immunol Today. 1988 Jan;9(1):7–10. doi: 10.1016/0167-5699(88)91347-3. [DOI] [PubMed] [Google Scholar]
- Miyake K., Medina K. L., Hayashi S., Ono S., Hamaoka T., Kincade P. W. Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow cultures. J Exp Med. 1990 Feb 1;171(2):477–488. doi: 10.1084/jem.171.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti M., Clary S. R., Nagarkatti P. S. Characterization of tumor-infiltrating CD4+ T cells as Th1 cells based on lymphokine secretion and functional properties. J Immunol. 1990 Jun 15;144(12):4898–4905. [PubMed] [Google Scholar]
- Nagarkatti M., Kaplan A. M. The role of suppressor T cells in BCNU-mediated rejection of a syngeneic tumor. J Immunol. 1985 Aug;135(2):1510–1517. [PubMed] [Google Scholar]
- Nagarkatti M., Toney D. M., Nagarkatti P. S. Immunomodulation by various nitrosoureas and its effect on the survival of the murine host bearing a syngeneic tumor. Cancer Res. 1989 Dec 1;49(23):6587–6592. [PubMed] [Google Scholar]
- Nagarkatti P. S., Snow E. C., Kaplan A. M. Characterization and function of autoreactive T-lymphocyte clones isolated from normal, unprimed mice. Cell Immunol. 1985 Aug;94(1):32–48. doi: 10.1016/0008-8749(85)90083-8. [DOI] [PubMed] [Google Scholar]
- Perez P., Hoffman R. W., Shaw S., Bluestone J. A., Segal D. M. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature. 1985 Jul 25;316(6026):354–356. doi: 10.1038/316354a0. [DOI] [PubMed] [Google Scholar]
- Picker L. J., De los Toyos J., Telen M. J., Haynes B. F., Butcher E. C. Monoclonal antibodies against the CD44 [In(Lu)-related p80], and Pgp-1 antigens in man recognize the Hermes class of lymphocyte homing receptors. J Immunol. 1989 Mar 15;142(6):2046–2051. [PubMed] [Google Scholar]
- Seth A., Pyle R. H., Nagarkatti M., Nagarkatti P. S. Expression of the J11d marker on peripheral T lymphocytes of MRL-lpr/lpr mice. J Immunol. 1988 Aug 15;141(4):1120–1125. [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Siraganian R., Wahl L., Shaw S. Dual role of the CD44 molecule in T cell adhesion and activation. J Immunol. 1989 Oct 15;143(8):2457–2463. [PubMed] [Google Scholar]
- Siegelman M. H., van de Rijn M., Weissman I. L. Mouse lymph node homing receptor cDNA clone encodes a glycoprotein revealing tandem interaction domains. Science. 1989 Mar 3;243(4895):1165–1172. doi: 10.1126/science.2646713. [DOI] [PubMed] [Google Scholar]
- Siliciano R. F., Pratt J. C., Schmidt R. E., Ritz J., Reinherz E. L. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature. 1985 Oct 3;317(6036):428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Streeter P. R., Berg E. L., Rouse B. T., Bargatze R. F., Butcher E. C. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988 Jan 7;331(6151):41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- Thiele D. L., Lipsky P. E. The role of cell surface recognition structures in the initiation of MHC-unrestricted 'promiscuous' killing by T cells. Immunol Today. 1989 Nov;10(11):375–381. doi: 10.1016/0167-5699(89)90271-5. [DOI] [PubMed] [Google Scholar]



