Significance
Zf-GRF domains are found in more than 100 eukaryotic architectures, including key proteins modulating DNA damage response and transcription. We establish the apurinic/apyrimidinic endonuclease 2 (APE2) Zf-GRF domain as a prototypical member of the Zf-GRF class of nucleic acid-binding modules, and through structural analysis reveal that the APE2 protein is composed of a compacted three-stranded β-sheet and a CHCC Zn2+-binding site, harboring structure-specific ssDNA-binding activity. Notably, the ssDNA-binding region of APE2 Zf-GRF is required for the 3′-5′ end resection of oxidative DNA damage and activation of the ATR-Chk1 DNA damage response pathway following oxidative stress. This distinct regulatory mechanism of APE2 exonuclease activity by ssDNA binding via Zf-GRF may extend to other Zf-GRF–containing proteins.
Keywords: oxidative stress, APE2, Zf-GRF, crystallography, Xenopus laevis
Abstract
The Xenopus laevis APE2 (apurinic/apyrimidinic endonuclease 2) nuclease participates in 3′-5′ nucleolytic resection of oxidative DNA damage and activation of the ATR-Chk1 DNA damage response (DDR) pathway via ill-defined mechanisms. Here we report that APE2 resection activity is regulated by DNA interactions in its Zf-GRF domain, a region sharing high homology with DDR proteins Topoisomerase 3α (TOP3α) and NEIL3 (Nei-like DNA glycosylase 3), as well as transcription and RNA regulatory proteins, such as TTF2 (transcription termination factor 2), TFIIS, and RPB9. Biochemical and NMR results establish the nucleic acid-binding activity of the Zf-GRF domain. Moreover, an APE2 Zf-GRF X-ray structure and small-angle X-ray scattering analyses show that the Zf-GRF fold is typified by a crescent-shaped ssDNA binding claw that is flexibly appended to an APE2 endonuclease/exonuclease/phosphatase (EEP) catalytic core. Structure-guided Zf-GRF mutations impact APE2 DNA binding and 3′-5′ exonuclease processing, and also prevent efficient APE2-dependent RPA recruitment to damaged chromatin and activation of the ATR-Chk1 DDR pathway in response to oxidative stress in Xenopus egg extracts. Collectively, our data unveil the APE2 Zf-GRF domain as a nucleic acid interaction module in the regulation of a key single-strand break resection function of APE2, and also reveal topologic similarity of the Zf-GRF to the zinc ribbon domains of TFIIS and RPB9.
Oxidative stress is the imbalance between the production of reactive oxygen species (ROS) and antioxidant defenses (1, 2). This presents a major challenge to genomic stability (3–6) and is implicated in the pathogenesis of multiple human diseases, including cancer and neurodegenerative disorders (7, 8). Abundant DNA damage from oxidative stress constitutes ∼10% of all DNA lesions and includes oxidative DNA damage, such as base damage, sugar moiety damage, apurinic/apyrimidinic (AP) sites, DNA single-strand breaks (SSBs), and double-strand breaks (DSBs) (2, 6). Repair of oxidative lesions involves primarily the base excision repair (BER) pathway (9, 10). Although the details of DNA damage recognition and corrective processes are well documented, the molecular mechanisms for activation of oxidative DNA-damage linked cell-cycle checkpoints remain comparatively less well defined.
The Ataxia-Telangiectasia Mutated (ATM)-Checkpoint Kinase 2 (ATM-Chk2) and the ATM- and Rad3-Related (ATR)-Checkpoint Kinase 1 (ATR-Chk1) pathways are the two major DNA damage response (DDR) kinase cascades activated in response to DNA damage and DNA replication stress (3, 5, 11). Whereas ATM is activated by autophosphorylation and dimer dissociation in response to DSBs (12, 13), ATR is activated by primed single-stranded DNA (ssDNA) from the functional uncoupling of minichromosome maintenance (MCM) helicase and DNA polymerase activities at stalled replication forks or DNA end resection of DNA strand breaks in the 5′-3′ direction (3, 14). ATR activation requires several mediator proteins, such as ATRIP (ATR-interaction protein), TopBP1, and the 9–1-1 (Rad9-Rad1-Hus1) complex (15–18). Activated ATR phosphorylates a variety of substrates, including Chk1 (19). Chk1 phosphorylation serves as an indicator of ATR activation, and activated Chk1 kinase phosphorylates its own substrates, such as Cdc25, to arrest cell cycle progression (20, 21). We recently established that the ATR-Chk1 checkpoint is triggered by oxidative DNA damage in Xenopus egg extracts (2, 22). Here, checkpoint activation critically requires the APE2 (apurinic/apyrimidinic endonuclease 2) 3′-5′ resection nuclease. Our understanding of APE2 molecular functions in this process remains poorly delineated, however.
APE2 (also termed APEX2, or Apn2 in yeast) is a minor AP endonuclease (23–25) that, compared with APE1, harbors weak AP endonuclease activity but robust proliferating cell nuclear antigen (PCNA)-stimulated 3′-5′ exonuclease and 3′-phosphodiesterase activities (24, 26, 27). APE2-null mice exhibit growth retardation and dyslymphopoiesis accompanied by G2/M arrest, suggesting its importance for proper cell cycle progression (28). In mammalian cells, APE2 is also necessary for normal B-cell development and recovery from chemotherapy drug-induced DNA damage (29). It was recently proposed that differential expression of APE2 in germinal centers promotes error-prone repair and mutations during somatic hypermutation (30). Importantly, APE2 is a key player in the PCNA-dependent repair of hydrogen peroxide-induced oxidative DNA damage (31–33).
Although APE2 enzymatic activities and associated functions in DNA repair and oxidative stress have been characterized in different experimental systems, including Arabidopsis thaliana, Trypanosoma cruzi, Saccharomyces cerevisiae, Schizosaccharomyces pombe, and Homo sapiens (33–39), a detailed mechanistic understanding of conserved APE2 functions is lacking. In particular, compared with other EEP (endonuclease/exonuclease/phosphatase) nuclease family members, APE2 is distinguished by the presence of a highly conserved zinc finger motif containing GRxF residues (hereinafter designated Zf-GRF) of unknown function. Herein we provide evidence that the APE2 C-terminal Zf-GRF preferentially associates with ssDNA and plays a central, unexpected role in regulating the 3′-5′ exonuclease activity. Using combined structural, biochemical, and functional approaches, we further establish that the Zf-GRF domain is crucial for APE2’s role in activating the ATR-Chk1 DDR pathway following oxidative stress.
Results
Mapping APE2 Functional Domains.
The molecular underpinnings for APE2 resection activities are ill-defined. To delineate APE2 functional domains, we coupled predictions of protein order/disorder to limited trypsin proteolysis and small-angle X-ray scattering (SAXS) studies of APE2. We purified full-length recombinant Xenopus laevis APE2 (APE2FL, residues 1–517) and subjected it to limited chymotrypsin proteolysis that identified stable regions of APE2 encompassing the EEP catalytic domain core (residues 1–336) and a folded domain bounding a predicted “Zf-GRF” region (amino acids 445–517). Proteolysis results closely correlate with predicted regions of protein disorder derived from D2P2 (40) (Fig. 1 A and B). Additional observations from SAXS (SI Appendix, Results and Discussion and Figs. S1–S4) are also consistent with the EEP nuclease core of APE2 being flexibly tethered to a structured C-terminal Zf-GRF domain via a flexible region that includes a PCNA-interacting protein (PIP) box motif.
Fig. 1.
Functional domains of APE2. (A) Mapping functional regions of Xl APE2. The APE2 domain structure is displayed with a catalytic EEP core (blue), a PCNA-interacting motif (purple), and a Zf-GRF domain (red). The D2P2 plot marks the consensus protein order prediction, with folded protein in white and predicted unstructured, low-complexity peptide regions in gray. Chymotrypsin-stable domains are in beige, corresponding to domain boundaries identified by mass spectrometry. (B) Limited chymotrypsin proteolysis of APE2FL identified structurally ordered regions in APE2. Stable regions encompass the EEP core nuclease fold and the predicted Zf-GRF domain.
Molecular Architecture of the Zf-GRF.
The C-terminal Zf-GRF is a defining feature of APE2 that distinguishes it from other EEP-containing enzymes (SI Appendix, Fig. S5A). Zf-GRF domains are 45- to 50-residue domains widely distributed throughout Eukarya that are found in >100 unique domain architectures (pfam.xfam.org/family/zf-GRF), including several human proteins involved in DDR, transcriptional regulation, and RNA processing (SI Appendix, Fig. S5B). The function of Zf-GRF domains in APE2 and other proteins remains undefined, however. To characterize the Zf-GRF molecular structure, we first crystallized and determined the X-ray structure of a fragment of APE2 encompassing the chymotrypsin stable Zf-GRF core (Fig. 1B), plus an N-terminal extension to this domain that is conserved among APE2 homologs (XlApe2 amino acids 436–517) (Fig. 2A). Consistent with a predicted Zn2+-binding fold, we detected a strong anomalous signal at the Zn2+ absorption edge in our crystals. Accordingly, we solved the structure using Zn2+ single-wavelength anomalous dispersion (SAD) phasing (to 2.60 Å; SI Appendix, Table S1).
Fig. 2.
Structure of the APE2 Zn-GRF domain. (A) Crystal structure of the C-terminal Zf-GRF domain. SAD phasing using the Zn edge wavelength was used to solve the structure. This domain is composed of an N-terminal helix (α1) connected by a polyproline helix hinge to three antiparallel β-sheets (β1–β3) containing the Zn coordination site and the GRF motif. The model phased anomalous difference Fourier map is indicated in red (5.0 σ), and the final 2Fo-Fc map is in blue (2.0 σ). The polyproline helix of the Zf-GRF structure overlaid with the collagen crystal structure showing high structural similarity. (B) Electrostatic surface representation of the front and side views of the Zf-GRF domain. The electrostatic potential was calculated in APBS and rendered in PYMOL to display ±3 kT/e. An electropositive patch that interacts with a bound sulfate molecule is shown as yellow and red sticks. Key conserved lysine and arginine residues are labeled.
Overall, the Zf-GRF comprises a three-stranded anti-parallel β-sheet (β1–β3) that folds into a crescent-shaped claw-like structure. A single bound Zn2+ ion plays a central structural role in this domain, and is coordinated with tetrahedral geometry by a “CHCC” sequence motif. The identity of these Zn2+ ligands is conserved for the majority of Zf-GRF–containing proteins; however, a subset of Zf-GRF proteins [e.g., Top3α (Topoisomerase 3α)] substitute the His of this motif with a Cys residue (CCCC-coordination). The first two Zn2+ ligands (C463 and H466) are found in a loop preceding β1, whereas the second half of the motif (C489 and C503) maps to the β2-β3 connecting loop. N-terminal to the Zf-GRF, APE2 homologs also contain a proline-rich region. Intriguingly, five consecutive prolines in APE2 adopt a collagen-like helical conformation and bridge the Zf-GRF to an extended α-helix, α1 (Fig. 2A, Inset). These three elements the N-terminal α1 helix, polyproline helix, and Zf-GRF—combine to form an elongated crutch-shaped molecule in the crystal (Fig. 2A). Although the polyproline region adopts an extended conformation in the crystallization lattice, we suspect that this structure may be stabilized through crystal lattice packing.
Zf-GRF Is a Structure-Specific DNA-Binding Domain.
The APE2 Zf-GRF bears a distinctive electropositive groove on its concave surface (Fig. 2B). We observed a bound sulfate (SO42-) molecule salt-bridged to the conserved basic residues K476 and R473. With K477, R484, R491, and R502, these positively charged residues combine and form a contiguous extended basic surface that appears appropriate for binding nucleic acid (Fig. 2B). To test this hypothesis using electrophoretic mobility shift assays (EMSA), we evaluated the ability of maltose binding protein (MBP)–Zf-GRF fusion proteins to interact with variable DNA secondary structures, including ssDNA, dsDNA, DNA bubbles, 5′ and 3′ flaps, and forked DNA duplexes (SI Appendix, Fig. S6A). Strikingly, the Zf-GRF displayed high-affinity DNA binding to substrates containing single-stranded regions. Substrates with considerable ssDNA character bound best, in the order ssDNA > fork > 3′ and 5′ flaps > dsDNA. Similarly, quantification of DNA-binding affinities using fluorescence polarization equilibrium binding analysis of FAM-labeled DNA substrates showed that Zf-GRF binds ssDNA (180 ± 40 nM), or a 3′-recessed substrate (560 ± 74 nM, a presumed biologically relevant substrate for APE2), in the nanomolar range, but does not interact appreciably with dsDNA (SI Appendix, Fig. S6B).
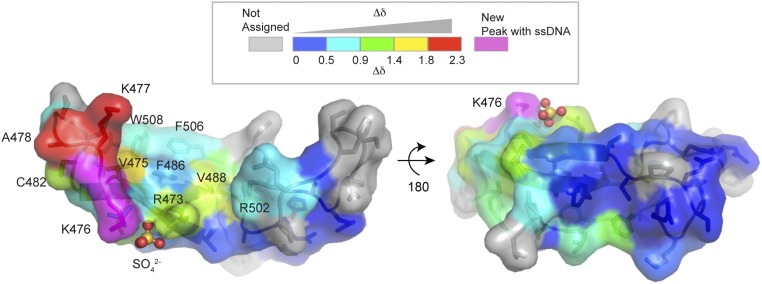
To more directly evaluate DNA binding in solution, we examined interactions of the Zf-GRF with ssDNA by chemical shift perturbation of 15N-labeled Zf-GRF protein (amino acids 460–511). Overall, we observed a pronounced chemical shift response of Zf-GRF to incubation with a 10-nt ssDNA target (Fig. 3 and SI Appendix, Fig. S7A). This analysis indicates that the concave surface of the Zf-GRF likely embraces the nucleic acid (Fig. 3, Left). In contrast, the backside (convex surface) of Zf-GRF is relatively unresponsive to ligand (Fig. 3, Right). Three ssDNA-binding clusters, denoted DNA-binding regions 1–3 (DBR 1–3), are identified with significant chemical shift perturbation on incubation with ssDNA (SI Appendix, Fig. S7 A and B). Intriguingly, these regions include the conserved Zf-GRF basic residue clusters R473/K476/K477, which bind sulfate in our crystals, and R502 in the β2-β3 connecting loop, which we predict will interact with the DNA phosphate backbone. In addition, a clustering of conserved aromatic residues from β1 (V475), β2 (F486 and V488), and β3 (F506 and W508) display moderate ssDNA-induced chemical shift perturbation of the backbone amide. Together, these residues form a contiguous hydrophobic surface that may serve to base stack with the exposed ssDNA nucleobases (Fig. 3).
Fig. 3.
Chemical shift mapping of the Zf-GRF–ssDNA interaction. Mapping of chemical shifts onto the Zf-GRF crystal structure. Regions of high perturbation are marked in red, and regions of no change are in blue. Magenta corresponds to new resonances that appeared on the addition of DNA. The region perturbed by ssDNA binding maps to a contiguous surface on the concave edge of the Zf-GRF.
Zf-GRF DNA Binding Is Critical for APE2 DNA Resection Functions.
To evaluate the functional significance of Zf-GRF DNA binding, we carried out mutational analyses of the Zf-GRF DNA-binding surface by specifically targeting the DNA interaction surface encompassing residues R473, K477, and R502. EMSA DNA-binding assays on ssDNA showed marked impairment of binding for all mutants tested (R473A, R473E, R502A, R502E, K477A, and K477E), consistent with a crucial role for this basic surface in mediating ssDNA interactions (SI Appendix, Fig. S8).
We next evaluated whether Zf-GRF DBR1–3 regions support APE2 nuclease function. To test this, we analyzed the PCNA-stimulated 3′-5′ nuclease activity of WT-APE2FL on a 3′ recessed DNA substrate. Similar to human APE2 (31) and S. cerevisiae Apn2 (41), in the absence of PCNA, Xenopus laevis APE2 (XlAPE2) carries out nuclease cleavage limited to one or two nucleotides from the 3′ end of a 5′ FAM-labeled substrate (Fig. 4A). The APE2 PIP box is known to promote PCNA binding (31, 36, 41). Accordingly, we observed robust stimulation of XlAPE2 nuclease activity by PCNA (Fig. 4A, lane 3). We further found that, similar to human APE2 (27), XlAPE2 has a preference for the 3′ recessed ends over blunt-ended substrates, in both the presence and the absence of PCNA (SI Appendix, Fig. S9). Therefore, the Zf-GRF’s preference for binding ssDNA and ssDNA-dsDNA junctions correlates with full-length vertebrate APE2 substrate specificity.
Fig. 4.
Zf-GRF DNA binding is critical for APE2 function. (A) 3′-5′ exonuclease assays using a 3′ recessed ssDNA/dsDNA structure with FAM-labeling on the 5′ terminus of the short strand. Zf-GRF mutations of the positive residues in the crescent platform reveal effects on full-length exonuclease activity. (B) Whereas sperm chromatin and hydrogen peroxide were added to mock-depleted egg extracts, WT APE2 or ΔZF APE2 was added to APE2-depleted egg extracts supplemented with sperm chromatin and hydrogen peroxide. After a 45-min incubation, total egg extracts were examined as indicated via immunoblotting analysis. “Endo APE2” represents endogenous APE2. A star indicates a nonspecific protein overlapping with WT Myc-APE2. (C) WT APE2 or ΔZF APE2 was added to APE2-depleted egg extracts supplemented with sperm chromatin and hydrogen peroxide. After a 45-min incubation, chromatin fractions (“chromatin”) were isolated and examined as indicated via immunoblotting analysis. Total egg extracts were analyzed via immunoblotting analysis. (D) WT APE2 or R502E APE2 was added to APE2-depleted egg extracts supplemented with sperm chromatin and hydrogen peroxide. After a 45-min incubation, chromatin fractions and total egg extracts were analyzed via immunoblotting analysis as in C. (E) WT Zf-GRF or R502E Zf-GRF was added to egg extracts, to a final concentration of 35 nM. Egg extracts were then supplemented with sperm chromatin and hydrogen peroxide. After a 45-min incubation, total egg extracts were examined via immunoblotting analysis.
Disruption of the Zf-GRF by mutations at R473, K477, or R502 impairs nucleolytic activity in the absence of PCNA, but under the conditions examined, partial PCNA-stimulated activity can be recovered for the R473E, K477A, K477E, and R502A mutants (Fig. 4A, lanes 4–12). In contrast, the R502E mutation shows nearly complete loss of nuclease activity even in the presence of PCNA (Fig. 4A, lanes 13 and 14). The R502E mutant does not significantly impair APE2 endonucleolytic incision of an apurinic site mimic (tetrahydrofuran) containing oligonucleotide (SI Appendix, Fig. S10). The purified R502E mutant protein is monomeric in solution as assessed by gel filtration, suggesting that this mutation does not result in gross unfolding and aggregation of APE2; however, an increase in the apparent molecular weight of this mutant as an MBP fusion (12% size increase), or for the purified Zf-GRF (10% increase) (SI Appendix, Fig. S11) suggests that in addition to mutating the DNA-binding surface, this variant may influence conformation of the Zf-GRF. Thus, overall our mutational work suggests that mutants that inhibit DNA binding in the Zf-GRF also impair PCNA-stimulated nucleolytic activity of APE2, underscoring a critical role for Zf-GRF DNA interactions in modulating APE2 DNA resection activity in vitro.
APE2 Zf-GRF DNA Binding Is Important for the ATR-Chk1 DDR Pathway Activation in Oxidative Stress.
To determine the biological significance of APE2’s Zf-GRF in DNA binding and nuclease resection, we tested whether it is important for the oxidative stress-induced ATR-Chk1 checkpoint activation in Xenopus egg extracts. Consistent with our previous results (22), WT APE2 rescued H2O2-induced Chk1 phosphorylation in APE2-depleted Xenopus egg extracts (Fig. 4B). Furthermore, the initiation of DNA replication was also needed for activation of the ATR-Chk1 DDR pathway in oxidative stress (SI Appendix, Results and Discussion and Fig. S12A). Complementation of APE2-depleted egg extracts with a ΔZF APE2 protein that deletes the Zf-GRF failed to rescue the H2O2-induced Chk1 phosphorylation in APE2-depleted egg extracts, however (Fig. 4B). Further evaluation of chromatin-bound fractions showed that RPA32, ATR, ATRIP, and Rad9 were recruited to H2O2-damaged chromatin when WT APE2, but not ΔZF APE2, was added back to the APE2-depleted egg extracts. This occurred even though both WT APE2 and ΔZF APE2 were associated with H2O2-damaged chromatin (Fig. 4C). Histone H3 was used as a loading control for chromatin-bound fractions. These observations suggest that Zf-GRF–stimulated 3′-5′ exonuclease activity of APE2 is important for the generation of RPA-ssDNA; assembly of the checkpoint protein complex including ATR, ATRIP, and 9–1-1 complex; and subsequent Chk1 phosphorylation by activated ATR (22). Similarly, WT APE2, but not the Zf-GRF R502E nuclease-deficient mutant, was able to rescue H2O2-induced Chk1 phosphorylation in APE2-depleted egg extracts (Fig. 4D). Although R502E APE2 was efficiently recruited to H2O2-damaged chromatin, WT APE2, but not R502E APE2, rescued the recruitment of RPA32, ATR, ATRIP, and Rad9 to H2O2-damaged chromatin in APE2-depleted egg extracts (Fig. 4D). Similar to the complete deletion of the Zf-GRF, these observations are consistent with a model in which the Zf-GRF DNA binding and regulation of nuclease resection play key roles in ATR-Chk1 checkpoint activation in oxidative stress.
We hypothesized that given the high-affinity DNA interactions mediated by Zf-GRF, exogenous addition of purified Zf-GRF to egg extracts at a concentration similar to that of endogenous APE2 may modulate APE2 functions in a dominant-negative manner. The addition of purified Zf-GRF compromised H2O2-induced Chk1 phosphorylation (Fig. 4E), suggesting that the WT Zf-GRF motif may compete with endogenous APE2 for ssDNA in egg extracts. Notably, addition of the DNA-binding–deficient R502E mutant Zf-GRF to egg extracts had no effect on H2O2-induced Chk1 phosphorylation (Fig. 4E). Furthermore, WT Zf-GRF, but not R502E Zf-GRF, was recruited to hydrogen peroxide-damaged chromatin (SI Appendix, Fig. S12B). These observations suggest that WT Zf-GRF may compete with endogenous APE2 binding to presumptive ssDNA regions on chromatin under oxidative stress conditions. Taken together, the foregoing results indicate that the WT Zf-GRF fragment functions in a dominant-negative manner to modulate the ATR-Chk1 pathway activation following oxidative stress.
Discussion
Our APE2 structure-function analyses reveal that APE2 contains an ordered Zf-GRF in its extreme C terminus, and that this region is critical for APE2 function in cellular responses to oxidative stress. Along with Zf-GRF, it has been established that the APE2-PCNA interface is mediated via a PIP–PCNA interaction (31, 36, 41). Structural order/disorder predictions, limited proteolysis, and SAXS results indicate that the intrinsically unstructured regions between the PCNA-binding PIP box and Zf-GRF flexibly connect the EEP–PIP–Zf-GRF architecture. The flexible connection of the PIP box to the catalytic domain and Zf-GRF appears suited to enable the Zf-GRF to tether ssDNA and direct the nuclease resection reaction (SI Appendix, Fig. S13).
Although the precise mode of molecular assembly of the APE2-PCNA-DNA complexes requires further investigation, we propose a model for the role and mechanism of the APE2 Zf-GRF in response to oxidative stress that involves the following: (i) APE2 is recruited to DNA damage with PCNA via its PIP box interaction and/or the Zf-GRF DNA sensing of ssDNA, or DNA gaps (e.g., oxidative single-strand breaks); (ii) APE2’s Zf-GRF stimulates its 3′-5′ exonucleolytic resection activity through interaction with ssDNA or ssDNA/dsDNA junctions; and (iii) an extended stretch of exposed ssDNA is produced, facilitating RPA binding, checkpoint protein complex assembly, and ATR-Chk1 activation (SI Appendix, Fig. S13).
Disruption of either the APE2 Zf-GRF DNA-binding surface or its PCNA-binding PIP box (22) compromises downstream chromatin recruitment of RPA, checkpoint kinases, and checkpoint activation (SI Appendix, Fig. S13); however, deletion or mutation of the APE2 Zf-GRF has no detectable impact on APE2 association with chromatin following oxidative stress. We conclude that the catalytic domain and PIP box are necessary and sufficient for the recruitment of APE2 to DNA damage. Previous work has also demonstrated that the C terminus of budding yeast Apn2 bounding both the PIP box and Zf-GRF is dispensable for a weak Apn2 AP-endonuclease activity, but is critically required for the repair of MMS-induced AP sites in vivo (42). Thus, we envisage that DNA binding and regulation of the nuclease reaction by the Zf-GRF, in combination with the PCNA–DNA interface is required to support robust APE2/Apn2 3′ end processing of DNA damage following oxidative stress and other forms of DNA damage.
Zn-finger domains participate in diverse functions, including peptide/protein binding, gene regulation, and lipid, RNA, and DNA binding (43–46). Zf-GRF–containing proteins are grouped as a distinct PFAM protein family (pfam.xfam.org/family/zf-GRF); however, DALI protein structural similarity searches show that the Zf-GRF is related to the eukaryotic RNA polymerase subunit RPB9, a member of the TFIIS C-terminal Zn-ribbon superfamily (47–49) (pfam.xfam.org/family/PF01096) (SI Appendix, Fig. S14). Although DALI failed to detect a structural relatedness of TFIIS to APE2 Zf-GRF, manual overlays confirmed the structural relatedness of the overall topology of these domains (SI Appendix, Fig. S14 B and C). Interestingly, in the Zf-GRF family, the “GRF” consensus, along with a neighboring highly conserved “GPN” motif, structurally distinguishes APE2 from the TFIIS family, and these sequence elements scaffold the DNA interaction surface loops and floor of the Zf-GRF DNA-binding groove and characteristic claw-like structure (SI Appendix, Fig. S14A). Thus, although clearly related to the TFIIS Zn-ribbon, the structural divergence of the interstrand connector loops is tailored for nucleic acid binding functionality, possibly giving rise to distinct nucleic acid binding modes built on this common Zn-ribbon scaffold. An overall topological similarity to TFIIS is also noted through the extended morphology of the N-terminal sequences flanking the APE2 Zf-GRF that adopt a polyproline extended-arm conformation in Zf-GRF, or extended structures in the TFIIS-RNA polymerase II complex (SI Appendix, Fig. S14C), or the extended domain linker in the case of the RPB9 Zn-ribbon (SI Appendix, Fig. S14B). Notably, the “GRxF” motif, GPN, and β1-β2 connector loop basic DNA-binding motifs are well conserved in other DNA Zf-GRF damage response proteins, including the NEIL3 (Nei-like DNA glycosylase 3) DNA glycosylase and TOP3α (SI Appendix, Fig. S7B), indicating a possible conserved DNA-binding function in these human Zf-GRF–containing proteins.
This work establishes the Zf-GRF as a structure-specific DNA-binding element in regulating APE2 resection functions. This function highlights the diverse roles for Zn-finger DNA-binding domains in modulating the DDR. For example, recognition of DNA ends and nicks for reversal of adenylation DNA damage is dependent on structure-specific DNA binding the C2H2/C2HE Aprataxin Zn-finger domain (50–52). For APE2, the Zf-GRF ssDNA-binding element is flexibly linked to the APE2 catalytic domain, yet is critical for robust catalytic activity. Conservation of the size and predicted disorder of the interdomain linkages in APE2 homologs from yeast to man suggests that this flexible association is needed for activity and/or dynamic regulation of APE2 activity. In line with this idea, an additional striking feature of the crystal lattice observed in our APE2 Zf-GRF structures is that the helical region (α1) of one protomer occupies the DNA-binding surface of the neighboring protomer (SI Appendix, Fig. S15). This helical contact surface occludes the hydrophobic cleft that we identify as important for binding ssDNA. We speculate that protein mimicry of Zf-GRF–ssDNA interactions might regulate APE2 Zf-GRF–DNA interactions and catalytic function, as has been observed in UNG–UGI interactions (53), among others (54). Taken together, our findings demonstrate that APE2 Zf-GRF is a distinct ssDNA-binding domain that facilitates the 3′-5′ resection of DNA damage for ATR-Chk1 DDR pathway activation following oxidative stress.
Materials and Methods
APE2 Protein Expression.
The APE2FL and APE2cat were PCR-amplified and subcloned into the pET MBP His6 LIC cloning vector (2Cc-T), containing a C-terminal MBP fusion tag (Addgene). The Zf-GRF domain was amplified and inserted into the pMCSG9 N-terminal MBP expression vector (MCSG). BL21-AI cells (Life Technologies) were transformed with all three protein constructs for protein expression overnight at 17 °C in Terrific Broth. Induction of expression was carried out by the primary addition of 0.1% (wt/vol; final concentration) l-arabinose (GoldBio), followed by isopropyl β-d-1-thiogalactopyranoside.
APE2 protein purification, Zf-GRF crystallization data collection, structure solution and refinement, preparation of oligonucleotides substrates, electrophoretic mobility shift assays, fluorescence DNA-binding assays, exonuclease cleavage assays, SAXS, and NMR methods are described in SI Appendix, Materials and Methods.
X. laevis Egg Extracts and related Experiments.
The care and use of X. laevis were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Charlotte. X. laevis egg extracts were prepared as described previously (55, 56). The X. laevis egg extracts and related experiments with recombinant proteins and antibodies are described in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Matthew Michael, Karlene Cimprich, and Howard Lindsay for reagents; Jason Williams of the National Institute of Environmental Health Sciences (NIEHS) protein microcharacterization core for mass spectrometry analysis, and Lars Pedersen of the NIEHS X-ray crystallography core for help with X-ray data collection. We also thank Drs. Robin Stanley and Jun Zhang for comments on the manuscript. This study was supported in part by funds provided by University of North Carolina at Charlotte and the National Institute of General Medical Sciences of the National Institutes of Health (NIH; Grants R15 GM101571 and R15 GM114713) to S.Y. The SAXS experiments were conducted at the Advanced Light Source, a national user facility operated by Lawrence Berkeley National Laboratory on behalf of the Department of Energy (DOE), Office of Basic Energy Sciences, through the Integrated Diffraction Analysis Technologies program, supported by the DOE’s Office of Biological and Environmental Research. Additional support came from the NIH Project MINOS (Grant R01 GM105404). X-ray crystallographic data were collected at Southeast Regional Collaborative Access Team beamline 22-ID at the Advanced Photon Source, Argonne National Laboratory. Use of the Advanced Photon Source was supported by the DOE, Office of Science, Office of Basic Energy Sciences, under Contract No. W-31-109-Eng-38. This research was also supported by the NIH Intramural Program and the NIEHS (Grants 1Z01ES102765, to R.S.W., and 1Z01ES050111, to R.E.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Atomic coordinates and structure factors have been deposited to the RCSB Protein Data Bank (accession no. 5U6Z).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610011114/-/DCSupplemental.
References
- 1.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8(9-10):1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 2.Yan S, Sorrell M, Berman Z. Functional interplay between ATM/ATR-mediated DNA damage response and DNA repair pathways in oxidative stress. Cell Mol Life Sci. 2014;71(20):3951–3967. doi: 10.1007/s00018-014-1666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciccia A, Elledge SJ. The DNA damage response: Making it safe to play with knives. Mol Cell. 2010;40(2):179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison JC, Haber JE. Surviving the breakup: The DNA damage checkpoint. Annu Rev Genet. 2006;40:209–235. doi: 10.1146/annurev.genet.40.051206.105231. [DOI] [PubMed] [Google Scholar]
- 6.Andres SN, Schellenberg MJ, Wallace BD, Tumbale P, Williams RS. Recognition and repair of chemically heterogeneous structures at DNA ends. Environ Mol Mutagen. 2015;56(1):1–21. doi: 10.1002/em.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson C, Yan S, Vestal CG. Oxidative stress, bone marrow failure, and genome instability in hematopoietic stem cells. Int J Mol Sci. 2015;16(2):2366–2385. doi: 10.3390/ijms16022366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thanan R, et al. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci. 2014;16(1):193–217. doi: 10.3390/ijms16010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baute J, Depicker A. Base excision repair and its role in maintaining genome stability. Crit Rev Biochem Mol Biol. 2008;43(4):239–276. doi: 10.1080/10409230802309905. [DOI] [PubMed] [Google Scholar]
- 10.Krokan HE, Bjørås M. Base excision repair. Cold Spring Harb Perspect Biol. 2013;5(4):a012583. doi: 10.1101/cshperspect.a012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartek J, Lukas C, Lukas J. Checking on DNA damage in S phase. Nat Rev Mol Cell Biol. 2004;5(10):792–804. doi: 10.1038/nrm1493. [DOI] [PubMed] [Google Scholar]
- 12.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421(6922):499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308(5721):551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 14.Cimprich KA, Cortez D. ATR: An essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9(8):616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300(5625):1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 16.Kumagai A, Lee J, Yoo HY, Dunphy WG. TopBP1 activates the ATR-ATRIP complex. Cell. 2006;124(5):943–955. doi: 10.1016/j.cell.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 17.Yan S, Michael WM. TopBP1 and DNA polymerase-alpha directly recruit the 9-1-1 complex to stalled DNA replication forks. J Cell Biol. 2009;184(6):793–804. doi: 10.1083/jcb.200810185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delacroix S, Wagner JM, Kobayashi M, Yamamoto K, Karnitz LM. The Rad9-Hus1-Rad1 (9-1-1) clamp activates checkpoint signaling via TopBP1. Genes Dev. 2007;21(12):1472–1477. doi: 10.1101/gad.1547007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuoka S, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316(5828):1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Sanchez Y. Chk1 in the DNA damage response: Conserved roles from yeasts to mammals. DNA Repair (Amst) 2004;3(8-9):1025–1032. doi: 10.1016/j.dnarep.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Zeman MK, Cimprich KA. Causes and consequences of replication stress. Nat Cell Biol. 2014;16(1):2–9. doi: 10.1038/ncb2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willis J, Patel Y, Lentz BL, Yan S. APE2 is required for ATR-Chk1 checkpoint activation in response to oxidative stress. Proc Natl Acad Sci USA. 2013;110(26):10592–10597. doi: 10.1073/pnas.1301445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boiteux S, Guillet M. Abasic sites in DNA: Repair and biological consequences in Saccharomyces cerevisiae. DNA Repair (Amst) 2004;3(1):1–12. doi: 10.1016/j.dnarep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The many functions of APE1/Ref-1: Not only a DNA repair enzyme. Antioxid Redox Signal. 2009;11(3):601–620. doi: 10.1089/ars.2008.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hegde ML, et al. Oxidative genome damage and its repair: Implications in aging and neurodegenerative diseases. Mech Ageing Dev. 2012;133(4):157–168. doi: 10.1016/j.mad.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson DM, 3rd, Barsky D. The major human abasic endonuclease: Formation, consequences and repair of abasic lesions in DNA. Mutat Res. 2001;485(4):283–307. doi: 10.1016/s0921-8777(01)00063-5. [DOI] [PubMed] [Google Scholar]
- 27.Burkovics P, Szukacsov V, Unk I, Haracska L. Human Ape2 protein has a 3′-5′ exonuclease activity that acts preferentially on mismatched base pairs. Nucleic Acids Res. 2006;34(9):2508–2515. doi: 10.1093/nar/gkl259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ide Y, et al. Growth retardation and dyslymphopoiesis accompanied by G2/M arrest in APEX2-null mice. Blood. 2004;104(13):4097–4103. doi: 10.1182/blood-2004-04-1476. [DOI] [PubMed] [Google Scholar]
- 29.Guikema JE, et al. Apurinic/apyrimidinic endonuclease 2 is necessary for normal B cell development and recovery of lymphoid progenitors after chemotherapeutic challenge. J Immunol. 2011;186(4):1943–1950. doi: 10.4049/jimmunol.1002422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stavnezer J, et al. Differential expression of APE1 and APE2 in germinal centers promotes error-prone repair and A:T mutations during somatic hypermutation. Proc Natl Acad Sci USA. 2014;111(25):9217–9222. doi: 10.1073/pnas.1405590111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burkovics P, Hajdú I, Szukacsov V, Unk I, Haracska L. Role of PCNA-dependent stimulation of 3′-phosphodiesterase and 3′-5′ exonuclease activities of human Ape2 in repair of oxidative DNA damage. Nucleic Acids Res. 2009;37(13):4247–4255. doi: 10.1093/nar/gkp357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandaru V, Sunkara S, Wallace SS, Bond JP. A novel human DNA glycosylase that removes oxidative DNA damage and is homologous to Escherichia coli endonuclease VIII. DNA Repair (Amst) 2002;1(7):517–529. doi: 10.1016/s1568-7864(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 33.Hadi MZ, Wilson DM., 3rd Second human protein with homology to the Escherichia coli abasic endonuclease exonuclease III. Environ Mol Mutagen. 2000;36(4):312–324. [PubMed] [Google Scholar]
- 34.Sepúlveda S, et al. Expression, functionality, and localization of apurinic/apyrimidinic endonucleases in replicative and non-replicative forms of Trypanosoma cruzi. J Cell Biochem. 2014;115(2):397–409. doi: 10.1002/jcb.24675. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, et al. AP endonucleases process 5-methylcytosine excision intermediates during active DNA demethylation in Arabidopsis. Nucleic Acids Res. 2014;42(18):11408–18. doi: 10.1093/nar/gku834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsuchimoto D, et al. Human APE2 protein is mostly localized in the nuclei and to some extent in the mitochondria, while nuclear APE2 is partly associated with proliferating cell nuclear antigen. Nucleic Acids Res. 2001;29(11):2349–2360. doi: 10.1093/nar/29.11.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson RE, et al. Identification of APN2, the Saccharomyces cerevisiae homolog of the major human AP endonuclease HAP1, and its role in the repair of abasic sites. Genes Dev. 1998;12(19):3137–3143. doi: 10.1101/gad.12.19.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin J, Hwang BJ, Chang PW, Toth EA, Lu AL. Interaction of apurinic/apyrimidinic endonuclease 2 (Apn2) with Myh1 DNA glycosylase in fission yeast. DNA Repair (Amst) 2014;15:1–10. doi: 10.1016/j.dnarep.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadi MZ, Ginalski K, Nguyen LH, Wilson DM., 3rd Determinants in nuclease specificity of Ape1 and Ape2, human homologues of Escherichia coli exonuclease III. J Mol Biol. 2002;316(3):853–866. doi: 10.1006/jmbi.2001.5382. [DOI] [PubMed] [Google Scholar]
- 40.Oates ME, et al. D2P2: Database of disordered protein predictions. Nucleic Acids Res. 2013;41(Database issue):D508–D516. doi: 10.1093/nar/gks1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Unk I, et al. Stimulation of 3′→5′ exonuclease and 3′-phosphodiesterase activities of yeast apn2 by proliferating cell nuclear antigen. Mol Cell Biol. 2002;22(18):6480–6486. doi: 10.1128/MCB.22.18.6480-6486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unk I, Haracska L, Johnson RE, Prakash S, Prakash L. Apurinic endonuclease activity of yeast Apn2 protein. J Biol Chem. 2000;275(29):22427–22434. doi: 10.1074/jbc.M002845200. [DOI] [PubMed] [Google Scholar]
- 43.Klug A. Zinc finger peptides for the regulation of gene expression. J Mol Biol. 1999;293(2):215–218. doi: 10.1006/jmbi.1999.3007. [DOI] [PubMed] [Google Scholar]
- 44.Brown RS. Zinc finger proteins: Getting a grip on RNA. Curr Opin Struct Biol. 2005;15(1):94–98. doi: 10.1016/j.sbi.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 45.Gamsjaeger R, Liew CK, Loughlin FE, Crossley M, Mackay JP. Sticky fingers: Zinc-fingers as protein-recognition motifs. Trends Biochem Sci. 2007;32(2):63–70. doi: 10.1016/j.tibs.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Matthews JM, Sunde M. Zinc fingers—folds for many occasions. IUBMB Life. 2002;54(6):351–355. doi: 10.1080/15216540216035. [DOI] [PubMed] [Google Scholar]
- 47.Cheung AC, Cramer P. Structural basis of RNA polymerase II backtracking, arrest and reactivation. Nature. 2011;471(7337):249–253. doi: 10.1038/nature09785. [DOI] [PubMed] [Google Scholar]
- 48.Qian X, Jeon C, Yoon H, Agarwal K, Weiss MA. Structure of a new nucleic-acid-binding motif in eukaryotic transcriptional elongation factor TFIIS. Nature. 1993;365(6443):277–279. doi: 10.1038/365277a0. [DOI] [PubMed] [Google Scholar]
- 49.Grishin NV. C-terminal domains of Escherichia coli topoisomerase I belong to the zinc-ribbon superfamily. J Mol Biol. 2000;299(5):1165–1177. doi: 10.1006/jmbi.2000.3841. [DOI] [PubMed] [Google Scholar]
- 50.Rass U, Ahel I, West SC. Actions of aprataxin in multiple DNA repair pathways. J Biol Chem. 2007;282(13):9469–9474. doi: 10.1074/jbc.M611489200. [DOI] [PubMed] [Google Scholar]
- 51.Tumbale P, et al. Structure of an aprataxin-DNA complex with insights into AOA1 neurodegenerative disease. Nat Struct Mol Biol. 2011;18(11):1189–1195. doi: 10.1038/nsmb.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tumbale P, Williams JS, Schellenberg MJ, Kunkel TA, Williams RS. Aprataxin resolves adenylated RNA-DNA junctions to maintain genome integrity. Nature. 2014;506(7486):111–115. doi: 10.1038/nature12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Putnam CD, et al. Protein mimicry of DNA from crystal structures of the uracil-DNA glycosylase inhibitor protein and its complex with Escherichia coli uracil-DNA glycosylase. J Mol Biol. 1999;287(2):331–346. doi: 10.1006/jmbi.1999.2605. [DOI] [PubMed] [Google Scholar]
- 54.Putnam CD, Tainer JA. Protein mimicry of DNA and pathway regulation. DNA Repair (Amst) 2005;4(12):1410–1420. doi: 10.1016/j.dnarep.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Willis J, DeStephanis D, Patel Y, Gowda V, Yan S. Study of the DNA damage checkpoint using Xenopus egg extracts. J Vis Exp. 2012;(69):e4449. doi: 10.3791/4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan S, Lindsay HD, Michael WM. Direct requirement for Xmus101 in ATR-mediated phosphorylation of Claspin-bound Chk1 during checkpoint signaling. J Cell Biol. 2006;173(2):181–186. doi: 10.1083/jcb.200601076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.