Abstract
Objectives
We examined florbetapir positron emission tomography (PET) amyloid scans across stages of preclinical Alzheimer’s disease (AD) in cortical, allocortical, and subcortical regions. Stages were characterized using empirically defined methods.
Methods
A total of 312 cognitively normal Alzheimer’s Disease Neuroimaging Initiative participants completed a neuropsychological assessment and florbetapir PET scan. Participants were classified into stages of preclinical AD using (1) a novel approach based on the number of abnormal biomarkers/cognitive markers each individual possessed, and (2) National Institute on Aging and the Alzheimer’s Association (NIA-AA) criteria. Preclinical AD groups were compared to one another and to a mild cognitive impairment (MCI) sample on florbetapir standardized uptake value ratios (SUVRs) in cortical and allocortical/subcortical regions of interest (ROIs).
Results
Amyloid deposition increased across stages of preclinical AD in all cortical ROIs, with SUVRs in the later stages reaching levels seen in MCI. Several subcortical areas showed a pattern of results similar to the cortical regions; however, SUVRs in the hippocampus, pallidum, and thalamus largely did not differ across stages of preclinical AD.
Conclusions
Substantial amyloid accumulation in cortical areas has already occurred before one meets criteria for a clinical diagnosis. Potential explanations for the unexpected pattern of results in some allocortical/subcortical ROIs include lack of correspondence between (1) cerebrospinal fluid and florbetapir PET measures of amyloid, or between (2) subcortical florbetapir PET SUVRs and underlying neuropathology. Findings support the utility of our novel method for staging preclinical AD. By combining imaging biomarkers with detailed cognitive assessment to better characterize preclinical AD, we can advance our understanding of who is at risk for future progression.
Keywords: Dementia, Beta-amyloid peptides, Florbetapir, Positron emission tomography, Neuropsychology, Biomarkers, Alzheimer disease
INTRODUCTION
The ability to accurately identify individuals at risk for progression to Alzheimer’s disease (AD) is dependent on detecting and characterizing its earliest manifestations. Efforts to characterize early stages of AD have focused on identifying biomarkers that become abnormal well before an individual demonstrates clinical symptoms. Beta-amyloid (Aβ) peptides are the primary component of amyloid plaques, a hallmark feature of AD. These peptides are thought to accumulate very early in the pathogenesis of AD (Jansen et al., 2015) and to drive other downsteam effects, including a progressive loss of neurons and cognitive symptoms (Jack et al., 2010; Jack, Knopman, et al., 2013). More recently, it has been proposed that pathways promoting Aβ and neurodegeneration may arise independently and then converge, leading to further acceleration of neurodegeneration and cognitive impairment (Sperling, Mormino, & Johnson, 2014).
Florbetapir is an amyloid imaging tracer that has been shown through in vivo and ex vivo studies to measure cortical fibrillar Aβ (Clark et al., 2012). Within the Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset, we recently examined cortical amyloid burden as measured by florbetapir positron emission tomography (PET) amyloid scans in empirically derived subtypes of mild cognitive impairment (MCI) (Bangen et al., 2016). We found that 65% of MCI participants with impaired neuropsychological performance and 34% of normal controls demonstrated an abnormal scan that was positive for amyloid (Bangen et al., 2016). These findings are consistent with the literature showing a high prevalence of amyloid deposition in cognitively normal samples (Balasubramanian, Kawas, Peltz, Brookmeyer, & Corrada, 2012; Bennett, Schneider, Bienias, Evans, & Wilson, 2005; Davis, Schmitt, Wekstein, & Markesbery, 1999; Price et al., 2009; Rodrigue et al., 2012; Rowe et al., 2007), suggesting some level of amyloid burden may be nonspecific and not necessarily a sign of early AD. On the other hand, it is possible that at least some of these individuals with high amyloid levels are already in a “preclinical” stage of AD.
“Preclinical” AD is a phase in which individuals are classified as “cognitively normal” yet they demonstrate abnormalities in biomarkers or subtle cognitive markers associated with AD. The National Institute on Aging and the Alzheimer’s Association (NIA-AA) have proposed a method of staging preclinical AD based on the presence or absence of these particular markers. Stage 1 involves amyloidosis; Stage 2 involves amyloidosis plus neurodegeneration; Stage 3 involves amyloidosis, neurodegeneration, and evidence of “subtle cognitive decline” (from one’s own baseline) defined as “very subtle cognitive impairment” on sensitive cognitive measures (Sperling et al., 2011). Studies that have attempted to apply these NIA-AA criteria have also included two additional classifications: “suspected non-AD pathophysiology” (SNAP; individuals with normal amyloid levels but evidence of neurodegeneration) and “Unclassified” (individuals with subtle cognitive decline but no neurodegeneration) (Jack et al., 2012).
A limitation to this staging system, which is based on the amyloid cascade hypothesis (Jack et al., 2010; Jack, Knopman, et al. 2013), is that many individuals do not follow this proposed sequence of events, as there is growing evidence that neurodegeneration (Edmonds, Delano-Wood, Galasko et al., 2015; Jack, Wiste, et al., 2013) and/or cognitive changes (Edmonds, Delano-Wood, Galasko, et al., 2015; Jedynak et al., 2012; Landau et al., 2010) may precede amyloidosis as the first sign of prodromal AD.
We proposed an alternative classification method for staging preclinical AD (Edmonds, Delano-Wood, Galasko, et al., 2015). Our staging method is based simply on a tally of the number of abnormal biomarkers (i.e., amyloidosis, neurodegeneration) or cognitive markers (i.e., subtle cognitive/functional decline) associated with preclinical AD that each individual possesses without regard for their temporal order of occurrence. This method does not adhere to the amyloid cascade hypothesis (Jack et al., 2010; Jack, Knopman, et al., 2013), which requires a specific temporal order of biomarker/cognitive marker abnormalities (although there is recent acknowledgement that amyloid and neurodegeneration do not have to occur in a fixed sequence; Jack, Knopman, et al., 2013; Sperling, Mormino, & Johnson, 2014). Rather, our classification method is based on the work of Braak, Zetterberg, Del Tredici, and Blennow (2013) who have proposed an alternative model to the amyloid cascade hypothesis. This model posits that AD pathologic markers (Aβ deposition, tau pathology, neurodegeneration) co-occur nearly simultaneously, and the perceived differences in timing are thought to be due to varying sensitivity of the biomarkers or of our ability to detect change, rather than a true difference in the sequence of these neurobiological changes. Cognition has traditionally been viewed as the last marker to be affected in preclinical AD due to the routine use of insensitive measures (i.e., rating scales or screening measures). However, sensitive episodic memory measures (i.e., verbal list learning and memory) may be the earliest markers to become abnormal in the progression to AD (Jedynak et al., 2012). We found that our approach to classification was as predictive of progression to MCI or AD as the method proposed by the NIA-AA criteria (Edmonds, Delano-Wood, Galasko, et al., 2015).
The current study aimed to build upon our previous findings in MCI (Bangen et al., 2016) by examining Aβ burden using florbetapir PET amyloid scans in individuals who are earlier in the disease process—those with preclinical AD. We hypothesized that we would observe an increase in cortical Aβ burden across stages of preclinical AD, with relatively low levels in early stages of preclinical AD and levels approaching MCI participants by the later stages. An exploratory aim was to examine amyloid burden in several allocortical and subcortical regions, including the accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus. We expected that subcortical amyloid would largely follow the same pattern as cortical amyloid, with levels increasing over stages of preclinical AD.
METHODS
Data used in the preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public–private partnership. This study was approved by an ethical standards committee on human experimentation at each institution. Written informed consent was obtained from all participants or authorized representatives participating in the study. For additional information, see www.adni-info.org.
Participants
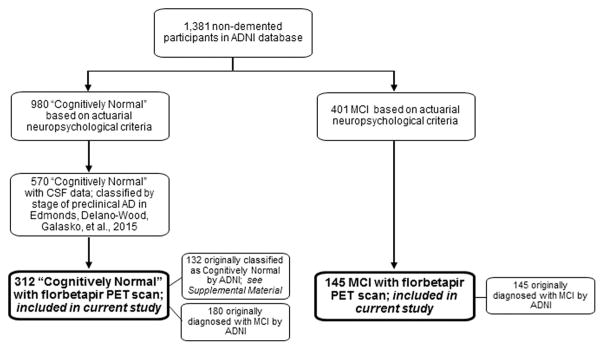
This cross-sectional study included 312 “cognitively normal” participants and 145 MCI participants enrolled in ADNI (mean age = 72.3 years; SD = 7.2). The sample of 312 cognitively normal individuals is a subset of 570 ADNI participants who we had previous classified by stage of pre-clinical AD (Edmonds, Delano-Wood, Galasko, et al., 2015). For the current study, we included those participants who had a florbetapir PET scan with processed data available for download as of December 1, 2015. See Figure 1 for a flowchart showing which participants from the overall ADNI database were included in the current study.
Fig. 1.
Flowchart showing which participants from the overall ADNI database were included in the current study.
The classification of “cognitively normal” versus MCI for this study was determined by an actuarial neuropsychological diagnostic method (Bondi et al., 2014; Jak et al., 2009) applied to participants’ baseline neuropsychological test data. We have previously demonstrated that conventional diagnostic methods for MCI (which are based on subjective complaints, rating scales, cognitive screening measures, and a single memory test; Petersen, 2004; Petersen et al., 2010) are highly susceptible to false-positive diagnostic errors, with over one-third of MCI samples being better classified as “cognitively normal” due to normal cognitive functioning, normal AD biomarkers, and low progression rates to AD (Bangen et al., 2016; Bondi et al., 2014; Clark et al., 2013; Edmonds, Delano-Wood, Cark, et al., 2015; Edmonds et al., in press). MCI diagnosed via our actuarial neuropsychological method, which assigns diagnoses of MCI based on multiple objective neuropsychological tests assessing a range of cognitive domains, has been shown to produce greater diagnostic stability (Jak et al., 2009) and stronger relationships between cognition, biomarkers, and rates of progression to AD (Bondi et al., 2014; Clark et al., 2013; Edmonds et al., 2016).
MATERIALS AND PROCEDURE
Preclinical AD Staging Based on Number of Abnormal Biomarkers
Participants underwent neuropsychological testing and lumbar puncture for cerebrospinal fluid (CSF) collection at the same visit during their baseline assessment. We classified all cognitively normal participants as “normal” or “abnormal” for (1) cerebral amyloidosis, (2) neurodegeneration, and (3) subtle cognitive/functional decline. Abnormal cerebral amyloid accumulation was defined as CSF Aβ1–42 level of < 192 pg/mL, and the presence of neurodegeneration was defined as CSF tau level of >93 pg/mL or CSF p-tau181p level of >23 pg/mL (Shaw et al., 2009).
We operationalized subtle cognitive decline based on two measures of language (Animal Fluency; Boston Naming Test), two measures of attention/executive function (Trail Making Test, Parts A & B), and two scores from a memory measure (Rey Auditory Verbal Learning Test [AVLT] 30-min delayed free recall and recognition). Each score was converted to an age-corrected standard score (Ivnik et al., 1992; Shirk et al., 2011; Weintraub et al., 2009). Subtle cognitive or functional decline was defined as having (1) scores > 1 SD below the age-corrected normative mean (i.e., “impaired”) on two of the six neuropsychological measures in different cognitive domains (patients with two impaired scores within the same cognitive domain were considered to have MCI and excluded from the study; see Edmonds, Delano-Wood, Galasko, et al., 2015), or (2) a Functional Activities Questionnaire (FAQ) score of 2, indicative of some decline in daily activities (patients with an FAQ score of ≥3 were considered to have MCI and excluded from the study). The number of abnormal biomarkers or cognitive markers that each individual possessed was tallied to determine their stage of preclinical AD. For comparison purposes, we also classified participants based on the NIA-AA criteria (Sperling et al., 2011).
Florbetapir PET Data Acquisition and Processing
All participants underwent florbetapir PET imaging within 2 weeks of their baseline neuropsychological assessment. A detailed description of ADNIs florbetapir PET imaging data acquisition and processing methods can be found online (http://adni.loni.usc.edu/methods/pet-analysis/pre-processing/). Briefly, florbetapir images consisting of four or six frames were acquired post-injection of florbetapir F18. Each scan was reviewed for quality control before being co-registered, averaged, reoriented into a standard 160 × 160 × 96 voxel image grid with 1.5-mm cubic voxels, and smoothed to a uniform isotropic resolution of 8 mm full width at half maximum. Structural MR images were skull-stripped, segmented, parcellated using Freesurfer (version 5.3.0; surfer.nmr.mgh.harvard.edu) and then co-registered to each participant’s first florbetapir image. Freesurfer was used to delineate cortical and subcortical regions.
The ADNI database provides the mean florbetapir uptake within several cortical and subcortical regions. The four cortical regions of interest (ROI) were: (1) frontal, (2) anterior/posterior cingulate, (3) lateral parietal, and (4) lateral temporal cortex. ADNI extracts florbetapir means from gray matter in each subregion within these four large ROIs (Jagust et al., 2009; Mormino et al., 2009). For the current study, standardized uptake value ratios (SUVRs) were calculated using the procedure recommended by ADNI: dividing the florbetapir mean for each of the cortical ROIs by the mean florbetapir uptake value for the reference region (i.e., whole cerebellum). ADNI provides a global cortical summary SUVR, which is calculated by creating a conventional (non-weighted) average across the four main cortical ROIs and dividing by the mean florbetapir uptake value of the whole cerebellum. Increased retention of florbetapir is thought to reflect increased cortical amyloid load.
The ADNI database also includes florbetapir uptake values for allocortical and subcortical ROIs. The following seven regions were examined: (1) acumbens, (2) amygdala, (3) caudate, (4) hippocampus, (5) pallidum, (6) putamen, and (7) thalamus. We created SUVRs by averaging the left and right values for each ROI and dividing by the mean florbetapir uptake value for the reference region (i.e., whole cerebellum).
Statistical Analyses
Differences between stages of preclinical AD (based on number of abnormal biomarkers) in demographics, apolipoprotein E (APOE) genotype, and baseline neuropsychological performance were examined using analysis of variance (ANOVA) and chi-square analyses. To examine our hypotheses related to cortical and allocortical/subcortical Aβ burden across preclinical AD stages, group differences in regional florbetapir SUVRs were examined using multivariate analysis of covariance (MANCOVA). Bonferroni-corrected post hoc comparisons were conducted for significant omnibus tests (four preclinical AD groups based on number of abnormal biomarkers; six comparisons; p = .05/6 = .008). We also used MANCOVA to compare preclinical AD groups to the 145 MCI participants.
All cognitively normal participants were also classified based on the NIA-AA criteria (Sperling et al., 2011). Group differences in regional florbetapir SUVRs were examined using MANCOVA with Bonferroni-corrected post hoc comparisons (six NIA-AA stages; 15 comparisons; p = .05/15 = .003). Lastly, to ensure that our results were not simply due to our method of classifying “cognitively normal,” we examined florbetapir SUVRs using only those individuals who were originally classified as cognitively normal by ADNI based on conventional diagnostic criteria (Petersen et al., 2010); see Supplemental Materials for further description of this subset (n = 132).
RESULTS
Clinical Characteristics of Preclinical AD Stages
For the 312 cognitively normal participants, 46.2% of the sample (n = 144) was positive for amyloidosis, 68.6% (n = 214) for neurodegeneration, and 17.3% (n = 54) for subtle cognitive/functional decline. There was no difference in age, education, gender, or APOE status between participants who demonstrated subtle cognitive decline (n = 30) versus subtle functional decline (n = 24; p’s >.05).
The number of abnormal biomarkers or cognitive markers that each individual possessed was tallied to determine their stage of preclinical AD. Using this classification strategy, 55 participants (17.6%) had no abnormal biomarkers or cognitive markers (“0 Biomarkers”); 127 (40.7%) had one abnormal marker (“1 Biomarker”); 105 (33.7%) had two abnormal markers (“2 Biomarkers”); and 25 (8.0%) had abnormalities on all three markers (“3 Biomarkers”; see Table 1). Of those with one abnormal biomarker, 23 had amyloidosis, 91 had neurodegeneration, and 13 had subtle cognitive/functional decline. Of those with two abnormal biomarkers, 89 had amyloidosis and neurodegeneration, 7 had amyloidosis and subtle/functional cognitive decline, and 9 had neurodegeneration and subtle cognitive/functional decline.
Table 1.
Demographic and neuropsychological characteristics for participants in each stage of preclinical AD (based on number of abnormal biomarkers)
| 0 Biomarkers (n = 55) | 1 Biomarker (n = 127) | 2 Biomarkers (n = 105) | 3 Biomarkers (n = 25) | F or χ2 | Sig. | Effect size | ||
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age (years) | 70.7 (6.7) | 71.3 (7.0) | 73.6 (7.3) | 72.1 (6.0) | F = 2.86 | p = .04 |
|
|
| Education (years) | 16.6 (2.4) | 16.9 (2.5) | 16.4 (2.5) | 16.0 (2.8) | F = 1.35 | p = .26 |
|
|
| Gender (% female) | 50.9% | 52.0% | 46.7% | 64.0% | χ2 = 2.53 | p = .47 | φc = .09 | |
| APOE (% ε4 positive) | 14.5% | 22.8% | 49.5% | 68.0% | χ2 = 40.50 | p <.001 | φc = .36 | |
| Neuropsychological (raw) | ||||||||
| Animal Fluency | 21.3 (4.2) | 21.4 (4.9) | 20.5 (4.8) | 16.5 (3.6) | F = 9.16 | p <.001 |
|
|
| BNT | 28.2 (1.5) | 28.5 (1.6) | 27.9 (2.2) | 27.0 (2.2) | F = 6.05 | p = .001 |
|
|
| TMT, Part A (s) | 30.5 (11.1) | 30.7 (8.1) | 33.3 (10.3) | 44.7 (14.7) | F = 15.10 | p <.001 |
|
|
| TMT, Part B (s) | 73.2 (23.7) | 74.4 (27.8) | 87.9 (47.6) | 104.9 (52.1) | F = 8.37 | p <.001 |
|
|
| AVLT Recall | 8.4 (4.1) | 8.1 (4.0) | 6.0 (3.4) | 3.7 (3.4) | F = 14.80 | p <.001 |
|
|
| AVLT Recognition | 12.7 (2.0) | 13.0 (1.9) | 12.7 (2.2) | 11.7 (2.0) | F = 3.36 | p = .06 |
|
|
Note. Data are summarized as mean (SD) unless otherwise noted.
APOE = apolipoprotein E; BNT = Boston Naming Test; TMT = Trail Making Test; AVLT = Rey Auditory Verbal Learning Test.
Demographic and neuropsychological characteristics for each stage of preclinical AD based on number of abnormal biomarkers are presented in Table 1. Age, education, and gender did not differ significantly between groups. The prevalence of APOE-ε4 carriers increased across preclinical AD stages, with the 0 Biomarkers and 1 Biomarker groups differing significantly from the 2 Biomarkers and 3 Biomarkers groups (p <.001; φc = .36). All analyses comparing preclinical AD stages controlled for APOE status.
Comparison of the 312 cognitively normal and the 145 MCI participants revealed no significant age difference (p >.05). However, the MCI group had less education (mean = 15.7 years; SD = 2.8; p = .001), more males (60.0%; p = .03), and a greater prevalence of APOE-ε4 carriers (62.7%; p <.001). Analyses comparing the preclinical AD stages to MCI controlled for education, gender, and APOE status.
Cortical Amyloid in Preclinical AD Stages
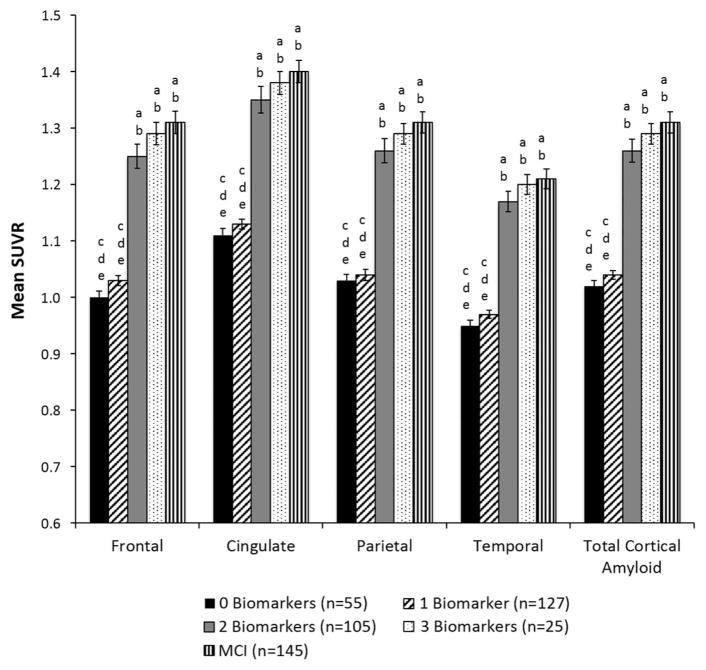
The mean florbetapir SUVRs for each cortical ROI, as well as the mean global cortical florbetapir SUVRs, are shown as a function of preclinical AD stage (based on number of abnormal biomarkers) in Table 2 and Figure 2. There were significant group differences for global cortical SUVR and for SUVRs in all cortical ROIs (ps <.001). Post hoc comparisons showed the same pattern of results across all cortical ROIs: the 0 Biomarkers and 1 Biomarker groups did not differ from each other (p >.05), but both had significantly lower SUVRs than the 2 Biomarkers and 3 Biomarkers groups (p <.001). The 2 Biomarkers and 3 Biomarkers groups did not differ from each other (p >.05). Comparison of the preclinical AD stages (based on number of abnormal biomarkers) to MCI revealed that the 2 Biomarkers and 3 Biomarkers groups had mean florbetapir SUVRs that were not significantly different from MCI for all cortical ROIs (p >.05); see Figure 2.
Table 2.
Mean florbetapir SUVR for cortical and subcortical regions for participants in each stage of preclinical AD (based on number of abnormal biomarkers)
| 0 Biomarkers (n = 55) | 1 Biomarker (n = 127) | 2 Biomarkers (n = 105) | 3 Biomarkers (n = 25) | F or χ2 | Sig. | Effect size | ||
|---|---|---|---|---|---|---|---|---|
| Cortical regions | ||||||||
| Frontal | 1.00 (.08) | 1.03 (.10) | 1.25 (.22) | 1.29 (.19) | F = 45.75 | p <.001 |
|
|
| Cingulate | 1.11 (.09) | 1.13 (.10) | 1.35 (.24) | 1.38 (.20) | F = 37.32 | p <.001 |
|
|
| Parietal | 1.03 (.08) | 1.04 (.11) | 1.26 (.22) | 1.29 (.18) | F = 42.89 | p <.001 |
|
|
| Temporal | 0.95 (.07) | 0.97 (.08) | 1.17 (.19) | 1.20 (.17) | F = 50.81 | p <.001 |
|
|
| Total Cortical Amyloid | 1.02 (.07) | 1.04 (.09) | 1.26 (.21) | 1.29 (.18) | F = 46.74 | p <.001 |
|
|
| Allocortical/subcortical regions | ||||||||
| Accumbens | 0.93 (.09) | 0.97 (.08) | 1.18 (.23) | 1.22 (.21) | F = 39.31 | p <.001 |
|
|
| Amygdala | 0.98 (.08) | 0.99 (.07) | 1.05 (.12) | 1.06 (.11) | F = 9.24 | p <.001 |
|
|
| Caudate | 1.05 (.10) | 1.09 (.10) | 1.15 (.14) | 1.14 (.13) | F = 6.52 | p <.001 |
|
|
| Hippocampus | 1.09 (.10) | 1.10 (.08) | 1.11 (.12) | 1.09 (.09) | F = 0.89 | p = .45 |
|
|
| Pallidum | 1.37 (.13) | 1.40 (.11) | 1.44 (.13) | 1.39 (.13) | F = 3.78 | p = .01 |
|
|
| Putamen | 1.22 (.09) | 1.24 (.09) | 1.36 (.17) | 1.36 (.16) | F = 19.46 | p <.001 |
|
|
| Thalamus | 1.20 (.09) | 1.21 (.09) | 1.23 (.13) | 1.19 (.11) | F = 1.25 | p = .29 |
|
|
Note. Data are summarized as mean (SD). All SUVRs use whole cerebellum as reference region.
Fig. 2.
Mean regional and global florbetapir standard uptake ratio (SUVR) for cortical regions in preclinical AD stages (based on number of abnormal biomarkers) and MCI. Error bars denote standard error of the mean. Letters denote significant group differences: a = different than 0 Biomarkers; b = different than 1 Biomarker; c = different than 2 Biomarkers; d = different than 3 Biomarkers; e = different than MCI.
Allocortical and Subcortical Amyloid in Preclinical AD Stages
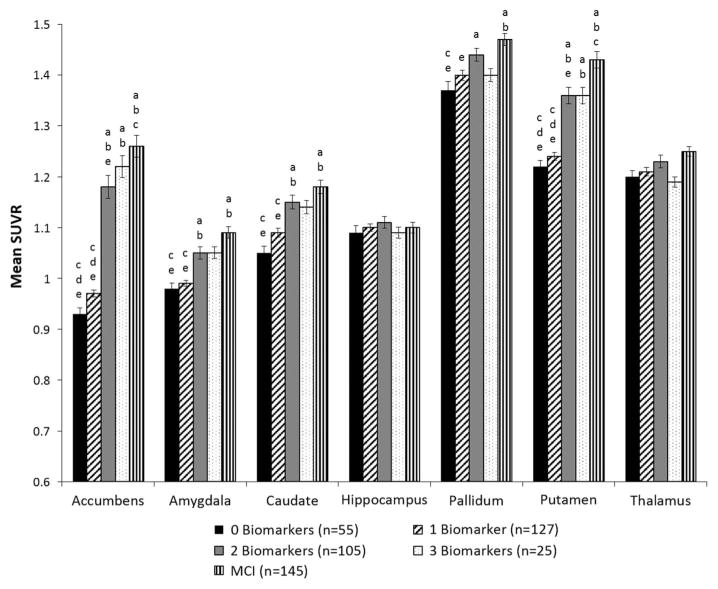
Mean florbetapir SUVRs for the seven allocortical and subcortical ROIs are shown as a function of preclinical AD stage (based on number of abnormal biomarkers) in Table 2 and Figure 3. There were significant group differences in the accumbens, amygdala, caudate, and putamen (p < .001). Post hoc tests showed a pattern of results that was similar to the cortical regions for each of these four allocortical/subcortical areas: the 0 Biomarkers and 1 Biomarker groups did not differ (p > .05), and the 2 Biomarkers and 3 Biomarkers groups did not differ (p > .05). However, the 0 Biomarkers and 1 Biomarker groups had significantly lower SUVRs than the 2 Biomarkers group for all four regions (ps < .001) and lower SUVRs than the 3 Biomarkers group for the accumbens and putamen (ps < .001).
Fig. 3.
Mean standard uptake ratio (SUVR) for allocortical/subcortical regions in preclinical AD stages (based on number of abnormal biomarkers) and MCI. Error bars denote standard error of the mean. Letters denote significant group differences: a = different than 0 Biomarkers; b = different than 1 Biomarker; c = different than 2 Biomarkers; d = different than 3 Biomarkers; e = different than MCI.
A different pattern emerged for the other three allocortical and subcortical regions. There were no differences among the groups in SUVRs in the hippocampus (omnibus p = .45) or the thalamus (omnibus p = .29). While the omnibus test for the pallidum was significant at p = .01, Bonferroni-corrected post hoc tests showed the only significant group difference was between the 0 Biomarkers and 2 Biomarkers group.
Comparison of the preclinical AD stages to MCI (see Figure 3) revealed that the 2 Biomarkers and 3 Biomarkers groups had mean florbetapir SUVRs that did not differ from the MCI group for the amygdala, caudate, and pallidum (ps ≥ .05). The 3 Biomarkers group also did not differ from MCI for the accumens and putamen (ps ≥ .04). SUVRs in the hippocampus and thalamus did not differ between MCI and any of the preclinical AD stages (based on number of biomarkers) (omnibus ps >.05).
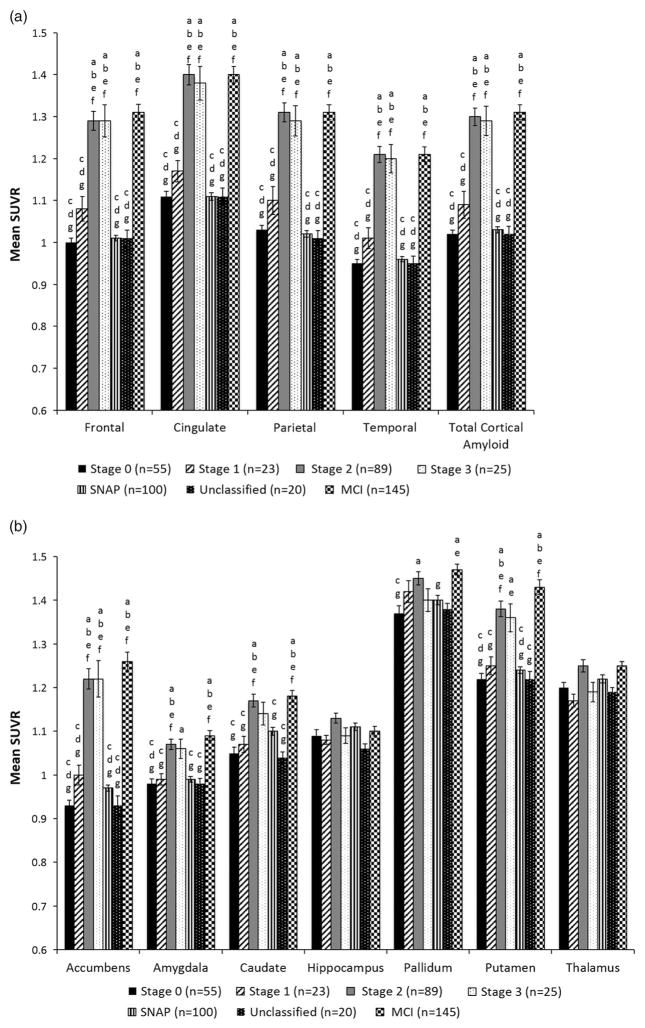
Comparison to Preclinical AD Stages Based on NIA-AA Criteria
The NIA-AA criteria for preclinical AD (Sperling et al., 2011) was applied to stage all 312 cognitively normal participants. Comparison of the number of participants classified at each stage of preclinical based on the two staging systems is shown in Table 3. Mean florbetapir SUVRs for cortical and allocortical/subcortical regions are shown as a function of NIA-AA preclinical AD stage in Figure 4. MANCOVA with APOE status included as a covariate (as this variable differed significantly between groups based on NIA-AA criteria) showed that the SNAP and Unclassified groups did not differ from one another in global cortical SUVR or SUVRs in any cortical ROIs (ps > .05), nor did they statistically differ from Stage 0 (ps > .05) or Stage 1 (ps >.01). Similarly, for the subcortical ROIs, the SNAP and Unclassified groups did not differ significantly from each other (ps > .02), or from Stage 0 (ps > .03) or Stage 1 (ps >.04). The SNAP and Unclassified groups had significantly lower SUVRs than Stages 2 and 3 for all cortical ROIs (ps <.001); lower SUVRs than Stage 2 for the accumbens, amygdala, caudate, and putamen (ps < .002); and lower SUVRs than Stage 3 for the accumbens (ps <.001).
Table 3.
Number of participants classified at each stage of preclinical AD based on the two staging systems
| Staging system based on number of abnormal biomarkersb
|
|||||
|---|---|---|---|---|---|
| 0 Biomarkers | 1 Biomarker | 2 Biomarkers | 3 Biomarkers | ||
| NIA-AA Staging Systema | Stage 0 | 55 | – | – | – |
| Stage 1 | – | 23 | – | – | |
| Stage 2 | – | – | 89 | – | |
| Stage 3 | – | – | – | 25 | |
| SNAPc | – | 91 | 9 | – | |
| Unclassifiedd | – | 13 | 7 | – | |
Note. NIA-AA = National Institute on Aging–Alzheimer’s Association; SNAP = suspected non-AD pathophysiology.
Staging system based on the biomarkers/cognitive markers an individual possesses and requires a specific temporal order (i.e., amyloidosis first, then neurodegeneration, then subtle cognitive decline).
Staging system based on the number of biomarkers/cognitive markers an individual possesses without regard for their temporal order of occurrence.
SNAP participants had neurodegeneration with normal amyloid levels.
Unclassified participants had subtle cognitive/functional decline with no neurodegeneration.
Fig. 4.
Mean standard uptake ratio (SUVR) for (a) cortical and (b) subcortical regions when participants were classified based on NIA-AA criteria for preclinical AD. Error bars denote standard error of the mean. Letters denote significant group differences: a = different than Stage 0; b = different than Stage 1; c = different than Stage 2; d = different than Stage 3; e = different than SNAP; f = different than Unclassified; g = different than MCI.
Comparison of the NIA-AA stages to MCI participants revealed that Stages 2 and 3 had mean florbetapir SUVRs that were not significantly different from MCI for all cortical ROIs (ps > .05); see Figure 4. Stages 2 and 3 also did not differ from the MCI group for allcortical/subcortical ROIs (p ≥ .04). Stage 1 and the Unclassified group did not differ from MCI for the pallidum (p ≥ .03). There were no group differences for the hippocampus (omnibus p > .05) or thalamus (p >.01).
Comparison to Conventional Diagnostic Methods
We examined florbetapir SUVRs in only those individuals classified as “cognitively normal” by ADNI’s diagnostic criteria (n = 132) (Petersen et al., 2010; see Supplemental Material). The pattern of results for amyloid deposition in the cortical and allocortical/subcortical ROIs across stages of preclinical AD in this subsample was remarkably similar to the results found in the full sample of 312 participants who were classified as “cognitively normal” based on actuarial neuropsychological criteria. This was the case both when stages of preclinical AD were based on the number of abnormal biomarkers (see Supplementary Figure 1) or the NIA-AA criteria (see Supplementary Figure 2).
DISCUSSION
We examined florbetapir PET amyloid scans in preclinical AD to characterize the prevalence and pattern of cerebral amyloid burden. Preclinical AD stages were based on empirically defined methods (Edmonds, Delano-Wood, Galasko, et al., 2015). In cortical ROIs, amyloid deposition increased across stages of preclinical AD, consistent with our hypothesis. This is not surprising, given that the preclinical AD stages themselves were based on three markers, one of which was participants’ CSF Aβ1–42 level. The correspondence between florbetapir PET imaging and CSF Aβ has been shown to be quite high in the ADNI dataset, with 86% agreement between the two measurements (Landau, Lu, et al., 2013). Thus, it follows that cortical SUVRs would be higher in the 2 and 3 Biomarkers groups, where nearly everyone (97% of the 2 and 3 Biomarkers groups combined) had abnormal CSF Aβ1–42, relative to the 0 and 1 Biomarker groups where the rate of CSF Aβ1–42 abnormality was much lower (14% of the 0 and 1 Biomarker groups combined).
Levels of florbetapir PET Aβ in the later stages of pre-clinical AD were not significantly different from MCI participants, although it should be noted that this does not necessarily imply equivalence and there was a trend for the 2 and 3 Biomarkers groups to have somewhat lower amyloid levels than the MCI group (see Figures 2 and 3). These findings are consistent with the notion that cerebral amyloid pathology may often, although not invariantly, occur early in the pathogenesis of AD, perhaps as many as 20–30 years before expression of clinical AD (Jansen et al., 2015), and that substantial accumulation has already occurred before one meets criteria for a clinical diagnosis for even mild forms of cognitive impairment.
The most intriguing finding from this study was the pattern of results seen in the allocortical and subcortical gray-matter regions. Several of these regions followed the same general pattern as the cortical areas, including the accumbens, amygdala, caudate, and putamen. However, the SUVRs observed in the hippocampus, pallidum, and thalamus largely did not differ across stages of preclinical AD. This finding indicates that neither our staging system nor the NIA-AA staging system adequately captures the progression of amyloid in these allocortical and subcortical regions. One possible explanation for the discrepancy is that CSF Aβ1–42 may not correspond well to florbetapir PET SUVRs in these particular subcortical structures. Previous research has shown that CSF and PET markers of amyloid are indeed associated with one another, but in a nonlinear way. Specifically, the relationship between CSF Aβ1–42 and florbetapir PET in the ADNI sample was found to be strong only when values were in the midrange on both measures; they did not closely correlate in the low and high range of values (Toledo et al., 2015). This suggests that CSF and florbetapir PET bio-markers are measuring different aspects of AD amyloid pathology (Toledo et al., 2015), which may account for our unexpected findings in some of the allocortical/subcortical regions.
An SUVR of 1.11 has been suggested as a cutoff value for “amyloid positivity” in cortical regions (Joshi et al., 2012; Landau, Breault, et al., 2013). However, it is unclear what cutoff value would be most appropriate for determining “positivity” in subcortical regions. One consideration in establishing such a cutoff is that the cortical uptake measure samples from a larger number of voxels relative to the smaller subcortical ROIs, which may make subcortical SUVRs less reliable and more sensitive to variation. Thus, a single cutoff value for regions of different sizes may not be ideal, perhaps necessitating different normative values for each region. On the other hand, perhaps abnormality should be determined by a range of SUVRs values rather than a particular cutoff, given the drawbacks of dichotomizing a continuous predictor in biomarker research (Royston, Altman, & Sauerbrei, 2006).
Despite not having a clear cutoff for “positivity,” the pallidum, putamen, and thalamus all appear to have high SUVRs across preclinical AD stages. The accumbens and caudate are also quite high in the later stages. Older neuropathologic studies in AD patients have shown that the striatum is particularly vulnerable to amyloid deposition and diffuse plaques (Braak & Braak, 1990; Suenaga, Hirano, Llena, Yen, & Dickson, 1990), especially the caudate, rostral putamen, and accumbens (Brilliant, Elble, Ghobrial, & Struble, 1997). The hippocampus, on the other hand, is an area that shows a low level of amyloid deposition until late in the disease (Arriagada, Growdon, Hedley-Whyte, & Hyman, 1992; Giannakopoulos, Hof, Michel, Guimon, & Bouras, 1997; Price, Davis, Morris, & White, 1991), consistent with our finding that SUVR levels in the hippocampus did not increase across stages of preclinical AD.
The pattern of observed SUVRs raises questions regarding the timing and/or sequence of amyloid accumulation between cortical and some allocortical and subcortical regions. At face value, our results appear to suggest that subcortical amyloid deposition occurs early in the disease process, and that the buildup of amyloid may be more complete in subcortical areas relative to cortical areas, even by the earliest phases of preclinical AD. However, such a sequence of amyloid accumulation would contradict the cascade of events that has been described in the literature which is a downward progression of Aβ from neocortex to subcortical regions (e.g., thalamus and striatum) (Braak & Del Tredici, 2015; Thal, Rüb, Orantes, & Braak, 2002).
Although studies have shown early subcortical Aβ deposition in the basal ganglia and thalamus in autosomal dominant forms of AD (Klunk et al., 2007; Bateman et al., 2012; Cho et al., 2013), this has not been described in late-onset sporadic AD. Therefore, rather than being indicative of an alternate sequence of amyloid accumulation in late-onset sporadic AD, the current findings may point to a lack of correspondence between subcortical florbetapir PET SUVRs and the underlying neuropathology. Hatsuta et al. (2015) found that 11C-Pittsburgh compound B (PiB) uptake in cortical regions was highly correlated with amyloid deposition and neuritic plaques at autopsy in patients with dementia; however, PiB uptake in subcortical grey matter (i.e., basal ganglia, thalamus, amygdala) did not show these associations. In a previous study, subcortical PiB uptake in the putamen and thalamus were found to be high regardless of whether a patient had amyloid aggregates at biopsy (Leinonen et al., 2008). The current findings dovetail nicely with this work and suggest that the discrepancy between subcortical SUVRs and histopathological measures of amyloid deposition/neuritic plaques could extend to a preclinical AD group, although clearly more work is needed to explore this hypothesis.
An alternative interpretation of our findings is that amyloid deposition in certain subcortical regions is non-specific and unrelated to risk for future development of AD. Previous studies have reported amyloid positivity in up to one-third of cognitively normal older adults (Chételat et al., 2013; Sperling et al., 2014); however, the clinical implications of these elevations in asymptomatic individuals remain uncertain (Leuzy, Zimmer, Heurling, Rosa-Neto, & Gauthier, 2014; Sperling et al., 2014). It is also possible that subcortical disease may be contributing to our findings of high SUVRs in some subcortical regions. Although participants with significant vascular burden (Hachinski Ischemic Score of > 4) were excluded from the ADNI sample, previous research has shown the presence of vascular pathology in ADNI’s cognitively normal participants (Nettiksimmons et al., 2013) and MCI participants (Toledo et al., 2013). Future studies examining vascular risk factors and vascular biomarkers in stages of preclinical AD are needed to address this possibility.
In sum, our analysis of the florbetapir PET imaging data in the ADNI cohort demonstrates unique patterns of amyloid burden in cortical and subcortical regions before a clinical diagnosis. Longitudinal research will be important to further understand how biomarkers of subcortical amyloid are related to the mechanistic pathways underlying AD, and whether the presence of amyloid in striatal or other subcortical regions may add predictive power in determining who is most likely to progress to AD. Beach and colleagues (2016) have recently suggested that amyloid imaging of the cerebral cortex and striatum together may increase accuracy in making a clinicopathological diagnosis of AD and in the pathology-based clinical staging of AD. Perhaps this type of clinical staging could be applied even earlier in the disease process if longitudinal findings ultimately show that “cognitively normal” individuals with both cortical and striatal amyloid burden have an increased risk of progressing to MCI or AD.
A limitation of our study was ADNI’s use of Freesurfer to delineate the ROIs. Although scans underwent a quality control process by ADNI, previous research has shown that Freesurfer’s segmentation accuracy is decreased in subcortical structures (e.g., thalamus; Eggert, Sommer, Jansen, Kircher, & Konrad, 2012). An additional limitation is that neuropsychological measures were corrected for age only, as normative data correcting for age, education, and sex were not available for all measures. A strength of our study was our ability to compare the two classification systems for preclinical AD. We demonstrated that the NIA-AA method essentially produced the same pattern of results as our novel staging method. The “SNAP” and “Unclassified” groups were largely comparable to Stages 0 and 1; therefore, separating these two groups based on their sequence of biomarker abnormalities neither improved nor informed the characterization of preclinical AD at baseline. Our previous work has also shown that these additional categories did not improve the prediction of who progressed to MCI/AD, since most participants who progressed did not follow the temporal order proposed by NIA-AA criteria (Edmonds, Delano-Wood, Galasko, et al., 2015). Similarly, other studies have shown that both “amyloid-first” and “neurodegeneration-first” (i.e., SNAP) biomarker profile pathways to preclinical AD exist (Jack, Wiste, et al., 2013), and that individuals with subtle cognitive decline but no neurodegeneration (i.e., Unclassified) progress to MCI/AD at a relatively high rate (Toledo et al., 2014). By combining sophisticated imaging biomarkers with detailed cognitive assessment to better characterize stages of preclinical AD, we can advance our understanding of which individuals are at risk for future progression, with the hope that eventually disease-modifying interventions can be provided early in the course of the disease.
Acknowledgments
Dr. Salmon serves as a consultant for Bristol-Myers Squibb. The other authors report no disclosures. This work was supported by the NIH grants R01 AG049810, K24 AG026431 and P50 AG05131. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S1355617716000928
References
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Balasubramanian AB, Kawas CH, Peltz CB, Brookmeyer R, Corrada MM. Alzheimer disease pathology and longitudinal cognitive performance in the oldest-old with no dementia. Neurology. 2012;79(9):915–921. doi: 10.1212/WNL.0b013e318266fc77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Clark AL, Werhane M, Edmonds E, Nation DA, Evangelista N, … Delano-Wood L. Cortical amyloid burden in empirically-derived MCI subtypes. Journal of Alzheimer’s Disease. 2016;52:849–861. doi: 10.3233/JAD-150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, … Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. New England Journal of Medicine. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Thal DR, Zanette M, Smith A, Buckley C. Detection of striatal amyloid plaques with [18F] flutemetamol: Validation with postmortem histopathology. Journal of Alzheimer’s Disease. 2016;52:863–873. doi: 10.3233/JAD-150732. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64(5):834–841. doi: 10.1212/01.wnl.0000152982.47274.9e. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, … Salmon DP. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and prediction of progression. Journal of Alzheimer’s Disease. 2014;42(1):275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Alzheimer’s disease: Striatal amyloid deposits and neurofibrillary changes. Journal of Neuropathology and Experimental Neurology. 1990;49(3):215–224. [PubMed] [Google Scholar]
- Braak H, Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain. 2015;138(10):2814–2833. doi: 10.1093/brain/awv236. [DOI] [PubMed] [Google Scholar]
- Braak H, Zetterberg H, Del Tredici K, Blennow K. Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid-β changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathologica. 2013;126(5):631–641. doi: 10.1007/s00401-013-1139-0. [DOI] [PubMed] [Google Scholar]
- Brilliant MJ, Elble RJ, Ghobrial M, Struble RG. The distribution of amyloid beta protein deposition in the corpus striatum of patients with Alzheimer’s disease. Neuropathology and Applied Neurobiology. 1997;23(4):322–325. [PubMed] [Google Scholar]
- Chételat G, La Joie R, Villain N, Perrotin A, da La Sayette V, Eustache F, Vandenberghe R. Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. Neuroimage: Clinical. 2013;2:356–365. doi: 10.1016/j.nicl.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Seo SW, Kim JH, Suh MK, Lee JH, Choe YS, … Na DL. Amyloid deposition in early onset versus late onset Alzheimer’s disease. Journal of Alzheimer’s Disease. 2013;35(4):813–821. doi: 10.3233/JAD-121927. [DOI] [PubMed] [Google Scholar]
- Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, … Skovronsky DM. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: A prospective cohort study. Lancet Neurology. 2012;11(8):669–678. doi: 10.1016/s1474-4422(12)70142-4. [DOI] [PubMed] [Google Scholar]
- Clark LR, Delano-Wood L, Libon DJ, McDonald CR, Nation DA, Bangen KJ, … Bondi MW. Are empirically derived subtypes of mild cognitive impairment consistent with conventional subtypes? Journal of the International Neuropsychological Society. 2013;19(6):635–645. doi: 10.1017/S1355617713000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. Journal of Neuropathology and Experimental Neurology. 1999;58(4):376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, … Bondi MW. Susceptibility of the conventional criteria for mild cognitive impairment to false positive diagnostic errors. Alzheimer’s & Dementia. 2015;11(4):415–424. doi: 10.1016/j.jalz.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW. Subtle cognitive decline and biomarker staging in preclinical Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2015;47(1):231–242. doi: 10.3233/JAD-150128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Jak AJ, Galasko DR, Salmon DP, Bondi MW. “Missed” mild cognitive impairment: High false-negative error rate based on conventional diagnostic criteria. Journal of Alzheimer’s Disease. 2016;52:685–691. doi: 10.3233/JAD-150986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Eppig J, Bondi MW, Leyden KM, Goodwin B, Delano-Wood L, McDonald CR. Heterogeneous cortical atrophy patterns in MCI not captured by conventional diagnostic criteria. Neurology. doi: 10.1212/WNL.0000000000003326. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert LD, Sommer J, Jansen A, Kircher T, Konrad C. Accuracy and reliability of automated gray matter segmentation pathways on real and simulated structural magnetic resonance images of the human brain. PLoS One. 2012;7:e45081. doi: 10.1371/journal.pone.0045081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos P, Hof PR, Michel JP, Guimon J, Bouras C. Cerebral cortex pathology in aging and Alzheimer’s disease: A quantitative survey of large hospital-based geriatric and psychiatric cohorts. Brain Research Reviews. 1997;25(2):217–245. doi: 10.1016/s0165-0173(97)00023-4. [DOI] [PubMed] [Google Scholar]
- Hatsuta H, Takao M, Ishii K, Ishiwata K, Saito Y, Kanemaru K, … Murayama S. Amyloid β accumulation assessed with 11C-Pittsburgh compound B PET and postmortem neuropathology. Current Alzheimer Research. 2015;12(3):278–286. doi: 10.2174/1567205012666150302155930. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo’s older Americans normative studies: Updated AVLT norms for ages 56 to 97. Clinical Neuropsychologist. 1992;6:83–104. [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, … Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, … Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurology. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, … Petersen RC. An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Annals of Neurology. 2012;71(6):765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Wiste HJ, Weigand SD, Knopman DS, Lowe V, Vemuri P, … Petersen RC. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology. 2013;81(20):1732–1740. doi: 10.1212/01.wnl.0000435556.21319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, … Mathis CA. Relationships between biomarkers in aging and dementia. Neurology. 2009;73(15):1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC. Quantification of five neuropsychological approaches to defining mild cognitive impairment. American Journal of Geriatric Psychiatry. 2009;17(5):368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, … Zetterberg H. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. Journal of the American Medical Association. 2015;313(19):1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak BM, Lang A, Liu B, Katz E, Zhang Y, Wyman BT, … Prince JL. A computational neurodegenerative disease progression score: Method and results with the Alzheimer’s disease neuroimaging initiative cohort. Neuroimage. 2012;63(3):1478–1486. doi: 10.1016/j.neuroimage.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, … Skovronsky DM. Performance characteristics of amyloid PET with florbetapir F 18 in patients with Alzheimer’s disease and cognitively normal subjects. Journal of Nuclear Medicine. 2012;53(3):378–384. doi: 10.2967/jnumed.111.090340. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Price JC, Mathis CA, Tsopelas ND, Lopresti BJ, Ziolko SK, … DeKosky ST. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. Journal of Neuroscience. 2007;27(23):6174–6184. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Jack CR, Jr, Wiste HJ. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78(20):1576–1582. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Breault C, Joshi AD, Pontecorvo M, Mathis CA, Jagust WJ, Mintun MA. Amyloid-beta imaging with Pittsburgh compound B and florbetapir: Comparing radiotracers and quantification methods. Journal of Nuclear Medicine. 2013;54(1):70–77. doi: 10.2967/jnumed.112.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, … Jagust WJ. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75(3):230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Lu M, Joshi AD, Pontecorvo M, Mintun MA, Trojanowski JQ, … Shaw LM. Comparing PET imaging and CSF measurements in Aβ. Annals of Neurology. 2013;74(6):826–836. doi: 10.1002/ana.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen V, Alafuzoff I, Aalto S, Suotunen T, Savolainen S, Nagren K, … Rinner JO. Assessment of beta-amyloid in a frontal cortical brain biopsy specimen and by positron emission tomography with carbon 11-labeled Pittsburgh Compound B. Archives of Neurology. 2008;65(10):1304–1309. doi: 10.1001/archneur.65.10.noc80013. [DOI] [PubMed] [Google Scholar]
- Leuzy A, Zimmer ER, Heurling K, Rosa-Neto P, Gauthier S. Use of amyloid PET across the spectrum of Alzheimer’s disease: Clinical utility and associated ethical issues. Amyloid. 2014;21(3):143–148. doi: 10.3109/13506129.2014.926267. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, … Jagust MJ. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettiksimmons J, Beckett L, Schwarz C, Carmichael O, Fletcher E, Decarli C. Subgroup of ADNI normal controls characterized by atrophy and cognitive decline associated with vascular damage. Psychology and Aging. 2013;28:191–201. doi: 10.1037/a0031063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, … Weiner MW. Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiology of Aging. 1991;12(4):295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, … Morris JC. Neuropathology of nondemented aging: Presumptive evidence for preclinical Alzheimer disease. Neurobiology of Aging. 2009;30(7):1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD, Sr, Rieck JR, Hebrank AC, Diaz-Arrastia R, … Park DC. β-Amyloid burden in healthy aging: Regional distribution and cognitive consequences. Neurology. 2012;78(6):387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, … Villemagne VL. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68(20):1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: A bad idea. Statistics in Medicine. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, … Trojanowski JQ. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Annals of Neurology. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirk SD, Mitchell MB, Shaughnessy LW, Sherman JC, Locascio JJ, Weintraub S, Atri A. A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimer’s Research & Therapy. 2011;3(6):32. doi: 10.1186/alzrt94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, … Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer’s disease: Implications for prevention trials. Neuron. 2014;84(3):608–622. doi: 10.1016/j.neuron.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga T, Hirano A, Llena JF, Yen SH, Dickson DW. Modified Bielschowsky stain and immunohistochemical studies on striatal plaques in Alzheimer’s disease. Acta Neuropathologica. 1990;80(3):280–286. doi: 10.1007/BF00294646. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–2000. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Toledo JB, Bjerke M, Da X, Landau SM, Foster NL, Jagust W, … Trojanowski JQ. Nonlinear association between cerebrospinal fluid and florbetapir F-18 β-amyloid measures across the spectrum of Alzheimer disease. JAMA Neurology. 2015;72(5):571–581. doi: 10.1001/jamaneurol.2014.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, … Trojanoswki JQ. Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathologica Communications. 2013;1:65. doi: 10.1186/2051-5960-1-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Weiner MW, Wolk DA, Da X, Chen K, Arnold SE, … Trojanowski JQ. Neuronal injury biomarkers and prognosis in ADNI subjects with normal cognition. Acta Neuropathologica Communications. 2014;2:26. doi: 10.1186/2051-5960-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, … Morris JC. The Alzheimer’s disease centers’ uniform data set (UDS): The neuropsychologic test battery. Alzheimer Disease and Associated Disorders. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]