Abstract
Considering the scarce information on occurrences of Toxoplasma gondii and Neospora caninum in domestic animals from Turkey, the aim of this study was to investigate the seroprevalence of these parasite infections in cattle, horses, sheep, goats and dogs in Turkey. The specific antibodies against T. gondii and N. caninum were detected by iELISAs based on the recombinant TgSAG2 or NcSAG1 in a total of 2,039 serum samples from eleven provinces. The seroprevalence of T. gondii infections was 46.3%, 4.0%, 20.0%, 12.9% and 19.8%, that of N. caninum infections was 0.3%, 7.4%, 2.1%, 3.2% and 16.6% in the horses, cattle, sheep, goats and dogs, respectively. These results indicated that T. gondii and N. caninum infections are prevalent in Turkish domestic animals.
Keywords: ELISA, Neospora caninum, Toxoplasma gondii, Turkey
Toxoplasma gondii (T. gondii) and Neospora caninum (N. caninum) are two closely related apicomplexan protozoa that infect many warm blood animals. T. gondii shares structural, genetic and immunological similarities to N. caninum, but they are antigenically different [10, 23]. T. gondii infections cause abortion or neonatal mortalities in human and warm-blooded animals [7, 28]. N. caninum is the causative agent of neosporosis, an infection that always causes reproductive failure in cattle, sheep, goats and horses, and neurological alterations in dogs [1, 10, 26]. Numerous epidemiologic studies of toxoplasmosis and neosporosis have been reported in many areas worldwide [2, 6, 20, 27]. However, the epidemiological information about the seroprevalence of T. gondii and N. caninum is limited in Turkey [19]. Therefore, the objective of this study was to determine the seroprevalence of T. gondii and N. caninum in a wide range of domestic animals in Turkey. Recombinant antigens are usually available in pure form, which provide better options in serological diagnosis [5, 16]. Surface antigen 2 of T. gondii (TgSAG2) and surface antigen 1 of N. caninum (NcSAG1) have been identified as important candidate of serological diagnosis for toxoplasmosis and neosporosis, respectively [5, 13, 15,16,17, 21]. In this study, we determined the seroprevalence of T. gondii and N. caninum in a wide range of domestic animals in Turkey using ELISA based on the recombinant TgSAG2 and NcSAG1, respectively.
The field samples analyzed in this study were collected from 11 provinces that located in the 6 regions of Turkey (Fig. 1). The serum samples were obtained from a total of 2,039 animals (616 horses, 377 cattle, 610 sheep, 249 goats and 187 dogs) within the study area. The horses were adult draft type belonging to the farmers in Adana, Bursa, Gaziantep, Istanbul, Izmir and Konya provinces. The cattle that raised primarily for milk production (dairy breed/cross breed) were selected from Adana, Afyon, Diyarbakir, Karaman, Kirklareli, Konya and Zonguldak provinces. Sheep that raised principally for the meat, wool and breeding were selected from Karaman, Konya and Zonguldak provinces. Most of the selected sheep were females and over one year old. The goats selected from the herds in Karaman and Konya provinces were reared for family milk and meat consumption. The dogs in the present study were house and stray dogs across the urban-rural areas of Konya province. All animal experiments were approved by the Scientific and Technological Research Council of Turkey. Care of animals and animal experimentation were performed in accordance with Animal Welfare Approved Standards for Turkeys (http://animalwelfareapproved.org/).
Fig. 1.
Regions for collecting samples in Turkey. The serum samples from six historical regions were studied: Karaman and Konya (Central Anatolia region); Zonguldak (Black sea region); Bursa, Istanbul and Kirklareli (Marmara region); Afyon and Izmir (Aegean region); Adana and Gaziantep (Mediterranean region); Diyarbakir (Southeastern Anatolia region). The provinces, from which the serum samples were collected, are presented with grey.
The recombinant TgSAG2-GST and NcSAG1-GST proteins used in this study were generated according to the method described previously [5, 16]. In brief, the PCR products of truncated TgSAG2 and NcSAG1 were inserted into the pGEX-4T vector (Amersham Pharmacia Biotech, San Francisco, CA, U.S.A.) and expressed in an E. coli BL-21 strain. A fresh 10 ml overnight culture of transformed E. coli was grown in 1 L of LB base broth containing 50 µg/ml of ampicillin at 37°C with shaking at 250 rpm until the optical density (OD) at 600 nm reached to 0.5. The expression of these proteins was induced by 5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) followed by incubation at 27°C overnight. The E. coli culture was centrifuged at 8,000 g for 15 min, and the cell pellet was then suspended in TNE buffer (50 mM Tris-HCl, pH 8.0, 100 mM NaCl, 2 mM EDTA and 1% Triton X-100) containing 50 mg/ml lysozyme, 1% (w/v) N-Lauroylsarcosine sodium and protease inhibitors. These recombinant proteins were purified from the soluble fractions using Glutathione-Sepharose 4B beads, according to the manufacturer’s instructions (Amersham Pharmacia Biotech). Protein expressions were verified by SDS-PAGE stained with Coomassie blue. The concentration of the expressed protein was measured using the BCA assay.
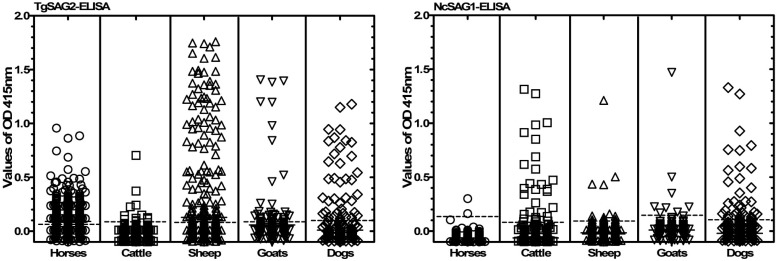
Indirect ELISAs were carried out to detect specific antibodies to T. gondii (rTgSAG2-ELISA) and N. caninum (rNcSAG1-ELISA) according to the previous reports, with modifications [5, 16]. Briefly, rTgSAG2-GST, rNcSAG1-GST and rGST were diluted to a final concentration of 4 µg/ml, 4 µg/ml and 4 µg/ml, in coating buffer (0.05 M carbonate-bicarbonate buffer, pH 9.6), respectively. The wells of the ELISA plate were coated with 100 µl of these antigens and incubated overnight at 4°C. Typically, after removing the coating solution, the plates were then blocked with PBS containing 3% (w/v) skim milk for 1 hr at 37°C. After washing, the plates were incubated with serum samples (diluted 1:100). The bound antibody was detected by treating with horseradish peroxidase (HRP)-conjugated (Bethyl, Montgomery, AL, U.S.A.) to anti-horse IgG, anti-bovine IgG, anti-sheep/goat IgG or anti-dog IgG (1:4,000) and ABTS [2,2’-azinobis (3-ethylbenzthiazolinesulfonic acid)] (Sigma, St. Louis, MO, U.S.A.). The color was allowed to develop at room temperature. And, 50 µl stop solution was added (2 M sulfuric acid) to each well to stop the action of horseradish peroxidase in the substrate. Optical density (OD) was measured by an MTP-500 microplate reader (Corona Electric, Hitachinaka, Japan) at 415 nm. The ELISA results were determined for each sample by subtracting OD415 value of GST protein from OD415 value of recombinant TgSAG2 or NcSAG1 proteins. The cut-off values were determined using the T. gondii or N. caninum-negative sera from different animals and calculated as the mean of OD415 value of negative sera plus three standard deviations. The cut off values were determined as 0.098, 0.103, 0.107, 0.102 and 0.118 for TgSAG2-ELISA; and 0.118, 0.093, 0.102, 0.141 and 0.124 for NcSAG1-ELISA in horses (n=30), cattle (n=30), sheep (n=50), goats (n=50) and dogs (n=20), respectively (Fig. 2). A sample was considered positive when the absorbance value of sample was higher than the cut-off value. The prevalence of anti-T. gondii and anti-N. caninum antibodies was estimated from the ratio of positive results to the total number of different animal examined with the exact binomial confidence interval of 95% (95% CI). Odds ratio (OR) with 95% confidence intervals based on likelihood ratio statistics is reported. Difference in the prevalence of these pathogens among different provinces was calculated using the binary logistic regression in SPSS (Release 18.0 standard version, SPSS Inc., Chicago, IL, U.S.A.).
Fig. 2.
Enzyme-linked immunosorbent assay (ELISA) results of the recombinant TgSAG2 and NcSAG1.
The prevalence of T. gondii and N. caninum in horses, cattle, sheep, goats and dogs from 11 provinces of Turkey is summarized in Table 1. The overall seroprevalence of T. gondii (24.1%, 95% CI: 22.3–26.0%) is significantly higher than N. caninumn (4.0%, 95% CI: 3.3–5.0%) in the surveyed regions (P<0.0001; OR: 7.49; 95% CI: 5.88–9.56). Furthermore, the anti-Toxoplasma antibodies were detected in all of the 11 provinces (100%). The overall seroprevalence of T. gondii in horses (46.3%, 95% CI: 42.4–50.2%) was higher than that of sheep (20.0%, 95% CI: 17.0–23.4%), dogs (19.8%, 95% CI: 14.7–26.1%), goats (12.9%, 95% CI: 9.3–17.6%) and cattle (4.0%, 95% CI: 2.4–6.5%). This investigation revealed a high T. gondii seroprevalence of horses in Turkey, indicating a potential threat to equine health in this country. Under natural conditions, seroprevalence of Toxoplasma in horses may vary from 0 to 90% [25]. In this study, the seroprevalence of T. gondii was detected in all the surveyed regions ranged from 3.7 to 56.5%, which was consistent with a previous study. Cattle have a high natural resistance to T. gondii, and infection in them does not usually cause clinical symptoms [8]. Therefore, the seroprevalence of T. gondii in cattle was low. Seroprevalence of T. gondii infection in cattle in Brazil and Iran was 1.03 and 4.0%, respectively, in a previous study [20, 22]. The present study also showed a low seroprevalence (4.0%) of T. gondii in cattle. The role of canine toxoplasmosis in the epidemiology of this parasitic disease has not been clarified, however, these animals can act as sentinels of environmental contamination with oocysts. Seroprevalence of T. gondii infections in free-living animals, such as stray dogs, has been investigated worldwide [4, 18, 29]. However, the information about the distribution and density of T. gondii in dogs from Turkey is limited. Therefore, we estimated the distribution and transmission dynamics of T. gondii in the environment by examining the seroprevalence of stray dogs as indicators in Konya province of Turkey. The seroprevalence of T. gondii antibodies in dogs was 19.8% in this study, which was lower than the previous reports in other countries [4, 18, 29].
Table 1. Seroprevalence of Toxoplasma gondii and Neospora caninum among horses, cattle, sheep, goats and dogs in Turkey.
| Animal species | Regions | No. of animals tested | T. gondii-seropositive | N. caninum-seropositive | Both seropositive | |||
|---|---|---|---|---|---|---|---|---|
| Frequency | Prevalence (95% CI) | Frequency | Prevalence (95% CI) | Frequency | Prevalence (95% CI) | |||
| Horses | Adana | 54 | 17 | 31.5 (20.7−44.7) | 0 | 0 (0−6.6) | 0 | 0 (0−6.6) |
| Bursa | 370 | 209 | 56.5 (51.4−61.5) | 1 | 0.3 (0.1−1.5) | 1 | 0.3 (0.1−1.5) | |
| Gaziantep | 19 | 5 | 26.3 (11.8−49.0) | 0 | 0 (0−16.8) | 0 | 0 (0−16.8) | |
| Istanbul | 39 | 19 | 48.7 (33.9−63.8) | 0 | 0 (0−9.0) | 0 | 0 (0−9.0) | |
| Izmir | 54 | 2 | 3.7 (1.0−12.5) | 0 | 0 (0−6.6) | 0 | 0 (0−6.6) | |
| Konya | 80 | 33 | 41.3 (31.1−52.2) | 1 | 1.3 (0.2−6.8) | 0 | 0 (0−4.6) | |
| Total | 616 | 285 | 46.3 (42.4−50.2) | 2 | 0.3 (0.1−1.2) | 1 | 0.2 (0−0.9) | |
| Cattle | Adana | 39 | 0 | 0 (0−9.0) | 3 | 7.7 (2.7−20.3) | 0 | 0 (0−9.0) |
| Afyon | 80 | 1 | 1.3 (0.2−6.8) | 3 | 3.8 (1.3−10.5) | 0 | 0 (0−4.6) | |
| Diyarbakir | 17 | 0 | 0 (0−18.4) | 1 | 5.9 (1.1−27.0) | 0 | 0 (0−18.4) | |
| Karaman | 10 | 0 | 0 (0−27.8) | 0 | 0 (0−27.8) | 0 | 0 (0−27.8) | |
| Kirklareli | 78 | 2 | 2.6 (0.7−8.9) | 6 | 7.7 (3.6−15.8) | 0 | 0 (0−4.7) | |
| Konya | 130 | 12 | 9.2 (5.4−15.4) | 6 | 4.6 (2.1−9.7) | 1 | 0.8 (0.1−4.2) | |
| Zonguldak | 23 | 0 | 0 (0−14.3) | 9 | 39.1 (22.2−59.2) | 0 | 0 (0−14.3) | |
| Total | 377 | 15 | 4.0 (2.4−6.5) | 28 | 7.4 (5.2−10.5) | 1 | 0.3 (0.1−1.5) | |
| Sheep | Karaman | 120 | 11 | 9.2 (5.2−15.7) | 1 | 0.8 (0.2−4.6) | 0 | 0 (0−3.1) |
| Konya | 450 | 108 | 24.0 (20.3−28.2) | 12 | 2.7 (1.5−4.6) | 3 | 0.7 (0.2−2.0) | |
| Zonguldak | 40 | 3 | 7.5 (2.6−19.9) | 0 | 0 (0−8.8) | 0 | 0 (0−8.8) | |
| Total | 610 | 122 | 20.0 (17.0−23.4) | 13 | 2.1 (1.3−3.6) | 3 | 0.5 (0.2−1.4) | |
| Goats | Karaman | 60 | 5 | 8.3 (3.6−18.1) | 0 | 0 (0−6.0) | 0 | 0 (0−6.0) |
| Konya | 189 | 27 | 14.3 (10.0−20.0) | 8 | 4.2 (2.2−8.1) | 2 | 1.1 (0.3−3.8) | |
| Total | 249 | 32 | 12.9 (9.3−17.6) | 8 | 3.2 (1.6−6.2) | 2 | 0.8 (0.2−2.9) | |
| Dogs | Konya | 187 | 37 | 19.8 (14.7−26.1) | 31 | 16.6 (11.9−22.6) | 8 | 4.3 (2.2−8.2) |
| Total | 2,039 | 491 | 24.1 (22.3−26.0) | 82 | 4.0 (3.3−5.0) | 15 | 0.7 (0.5−1.2) | |
The specific antibodies of N. caninum were detected in the sera of 82 out of 2,039 (4.0%) animals in this study. Neosporosis is a serious disease of cattle and dogs worldwide, and it also affects sheep, goats, water buffaloes and horses [3]. The overall seroprevalence of N. caninum in dogs (16.6%; 95% CI: 11.9–22.6%) was higher than that of cattle (7.4%; 95% CI: 5.2–10.5%), goats (3.2%; 95% CI: 1.6–6.2%), sheep (2.1%; 95% CI: 1.3–3.6%) and horses (0.3%; 95% CI: 0.1–1.2%), which demonstrated that the infection of N. caninum is not common in horses, sheep and goats in Turkey. Many studies about the prevalence of N. caninum in sheep and goats have been reported. The Neospora was reported at a prevalence of 3.3% and 6% in goats from Brazil and Czech Republic, respectively. Furthermore, 8.1% of sheep from Uberlandia, and 16.8% of sheep and 6.9% of goats from Greece showed seropositive for anti-N. caninum antibodies in a previous study [2, 12, 24]. The seroprevalence of N. caninum in sheep and goats obtained by us is similar with other reports from different countries. Antibodies to Neospora species in equine populations were reported in many parts of the world, such as Brazil [7, 9], U.S.A. [11] and Iran [14]. However, little is known on the prevalence of antibodies to Neospora in subclinically infected horses in Turkey. Several studies have detected the antibodies against N. caninum even more than 30% of horses [11]. The current study found that the proportion of horses with antibodies against N. caninum was quite low (0.3%), which was lower than previously reports for other regions. The finding of this study indicates that the horses in Turkey are at risk of neosporosis.
The highest prevalence of toxoplasma in cattle was found in Konya province (9.2%; 95% CI: 5.4–15.4%), and followed by Kirklareli (2%; 95% CI: 0.7–15.4%) and Afyon (1.3%; 95% CI: 0.2–6.8%). The specific antibody against T. gondiis could not be detected in other four provinces. For neosporosis, Zonguldak showed the highest prevalence (39.1%; 95% CI: 22.2–59.2%) in cattle. The seroprevalence of N. caninum in cattle was 7.7% (95% CI: 2.7–20.3%), 3.8% (95% CI: 1.3–10.5%), 5.9% (95% CI: 1.1–27.0%), 7.7% (95% CI: 3.6–15.8%) and 4.6 (95% CI: 2.1–9.7%) in Adana, Afyon, Diyarbakir, Kirklareli and Konya provinces, respectively. Mixed infection was found in one cow. The sheep from Konya showed the highest prevalence (24.0%; 95% CI: 20.3–28.2%) of toxoplasma, and followed by Karaman (9.2%; 95% CI: 5.2–15.7%) and Zonguldak (7.5%; 95% CI: 2.6–19.9%) provinces. The seropositivity of neospora in sheep from Karaman and Konya was 0.8% (95% CI: 0.2–4.6%) and 2.7% (95% CI: 1.5–4.6%). Mixed infection was found in 3 sheep. In goat samples, the antibodies aganist T. gondii were found to be 8.3% (95% CI: 3.6–18.1%) in Karaman and 14.3% (95% CI: 10.0–20.0%) in Konya. The specific antibodies against N. caninum were detected in the goats only from Konya province showing low level of infection rate with 4.2% (95% CI: 2.2–8.1%). Mixed infection was found in 2 goats. The seroprevalence of T. gondii (19.8%; 95% CI: 14.7–26.1) and N. caninum (16.6%; 95% CI: 11.9–22.6) was similar in dogs. In this study, the dog samples were collected from 50 stray and 137 registered dogs in Konya province. The seroprevalence of these two parasites in stray dogs was higher than that of registered dogs (data not shown). Mixed infection was detected in 8 dogs. These results indicated that the prevalence of these parasites was different among the different animal species and regions. Furthermore, the co-infection may affect transmission and recovery from infection, and therefore, the co-infection should be considered in a disease control and treatment decisions.
In summary, the results of this study confirm that T. gondii and N. caninum affect some species of domestic animals in Turkey. In the case of toxoplasmosis, the relative high prevalence was evidenced, emphasizing the necessity of more detailed studies for the control of this infection in these regions. In respect to neosporosis, the results obtained in this study showed a higher prevalence of dogs and cattle than those of horses, sheep and goats. For preventing N. caninum infection, the livestock need to be quarantined with dogs, which play an important role in the transmission and dissemination of the disease.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. This work was also supported by the Scientific and Technological Research Council of Turkey (TUBITAK 1001-113O336).
REFERENCES
- 1.Barber J. S., Trees A. J.1996. Clinical aspects of 27 cases of neosporosis in dogs. Vet. Rec. 139: 439–443. doi: 10.1136/vr.139.18.439 [DOI] [PubMed] [Google Scholar]
- 2.Bártová E., Sedlak K.2012. Toxoplasma gondii and Neospora caninum antibodies in goats in the Czech Republic. Vet. Med. 57: 111–114. [Google Scholar]
- 3.Bártová E., Sedlák K., Literák I.2009. Toxoplasma gondii and Neospora caninum antibodies in sheep in the Czech Republic. Vet. Parasitol. 161: 131–132. doi: 10.1016/j.vetpar.2008.12.022 [DOI] [PubMed] [Google Scholar]
- 4.Cedillo-Peláez C., Díaz-Figueroa I. D., Jiménez-Seres M. I., Sánchez-Hernández G., Correa D.2012. Frequency of antibodies to Toxoplasma gondii in stray dogs of Oaxaca, México. J. Parasitol. 98: 871–872. doi: 10.1645/GE-3095.1 [DOI] [PubMed] [Google Scholar]
- 5.Chahan B., Gaturaga I., Huang X., Liao M., Fukumoto S., Hirata H., Nishikawa Y., Suzuki H., Sugimoto C., Nagasawa H., Fujisaki K., Igarashi I., Mikami T., Xuan X.2003. Serodiagnosis of Neospora caninum infection in cattle by enzyme-linked immunosorbent assay with recombinant truncated NcSAG1. Vet. Parasitol. 118: 177–185. doi: 10.1016/j.vetpar.2003.10.010 [DOI] [PubMed] [Google Scholar]
- 6.Diakoua A., Papadopoulos E., Panousis N., Karatzias C., Giadinis N.2013. Toxoplasma gondii and Neospora caninum seroprevalence in dairy sheep and goats mixed stock farming. Vet. Parasitol. 198: 387–390. doi: 10.1016/j.vetpar.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 7.Dubey J. P., Beattie C. P.1988. General biology. p.220. In: Toxoplasmosis of animals and man. CRC Press, Boca Raton. [Google Scholar]
- 8.Dubey J. P., Thulliez P.1993. Persistence of tissue cysts in edible tissues of cattle fed Toxoplasma gondii oocysts. Am. J. Vet. Res. 54: 270–273. [PubMed] [Google Scholar]
- 9.Dubey J. P., Kerber C. E., Granstrom D. E.1999. Serologic prevalence of Sarcocystis neurona, Toxoplasma gondii, and Neospora caninum in horses in Brazil. J. Am. Vet. Med. Assoc. 215: 970–972. [PubMed] [Google Scholar]
- 10.Dubey J. P.2003. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 41: 1–16. doi: 10.3347/kjp.2003.41.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubey J. P., Mitchell S. M., Morrow J. K., Rhyan J. C., Stewart L. M., Granstrom D. E., Romand S., Thulliez P., Saville W. J., Lindsay D. S.2003. Prevalence of antibodies to Neospora caninum, Sarcocystis neurona, and Toxoplasma gondii in wild horses from central Wyoming. J. Parasitol. 89: 716–720. doi: 10.1645/GE-66R [DOI] [PubMed] [Google Scholar]
- 12.Faria E. B., Gennari S. M., Pena H. F., Athayde A. C., Silva M. L., Azevedo S. S.2007. Prevalence of anti-Toxoplasma gondii and anti-Neospora caninum antibodies in goats slaughtered in the public slaughterhouse of Patos city, Paraíba State, Northeast region of Brazil. Vet. Parasitol. 149: 126–129. doi: 10.1016/j.vetpar.2007.07.009 [DOI] [PubMed] [Google Scholar]
- 13.Hemphill A., Fuchs N., Sonda S., Gottstein B., Hentrich B.1997. Identification and partial characterization of a 36 kDa surface protein on Neospora caninum tachyzoites. Parasitology 115: 371–380. doi: 10.1017/S0031182097001455 [DOI] [PubMed] [Google Scholar]
- 14.Hosseini M., Moraveji M., Tahamtan Y., Rahimian A., Mohammadi G., Namavari M.2011. Seroprevalence of Neospora spp. in Horses in North East of Iran. Iran. J. Parasitol. 6: 64–68. [PMC free article] [PubMed] [Google Scholar]
- 15.Howe D. K., Crawford A. C., Lindsay D., Sibley L. D.1998. The p29 and p35 immunodominant antigens of Neospora caninum tachyzoites are homologous to the family of surface antigens of Toxoplasma gondii. Infect. Immun. 66: 5322–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X., Xuan X., Kimbita E. N., Battur B., Miyazawa T., Fukumoto S., Mishima M., Makala L. H., Suzuki H., Sugimoto C., Nagasawa H., Fujisaki K., Mikami T., Igarashi I.2002. Development and evaluation of an enzyme-linked immunosorbent assay with recombinant SAG2 for diagnosis of Toxoplasma gondii infection in cats. J. Parasitol. 88: 804–807. doi: 10.1645/0022-3395(2002)088[0804:DAEOAE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim H. M., Huang P., Salem T. A., Talaat R. M., Nasr M. I., Xuan X., Nishikawa Y.2009. Short report: prevalence of Neospora caninum and Toxoplasma gondii antibodies in northern Egypt. Am. J. Trop. Med. Hyg. 80: 263–267. [PubMed] [Google Scholar]
- 18.Kamani J., Mani A. U., Kumshe H. A., Dogo G. I., Yidawi J. P., Pauline D. K., Nnabuife H. E., Joan P., Egwu G. O.2009. Serosurvey for Toxoplasma gondii in dogs in Maiduguri, Borno State, Nigeria. J. Infect. Dev. Ctries. 4: 15–18. [DOI] [PubMed] [Google Scholar]
- 19.Oncel T., Vural G.2006. Occurrence of Toxoplasma gondii antibodies in sheep in Istanbul, Turkey. Vet. Arh. 76: 547–553. [Google Scholar]
- 20.Pita Gondim L. F., Barbosa H. V., Jr, Ribeiro Filho C. H., Saeki H.1999. Serological survey of antibodies to Toxoplasma gondii in goats, sheep, cattle and water buffaloes in Bahia State, Brazil. Vet. Parasitol. 82: 273–276. doi: 10.1016/S0304-4017(99)00033-3 [DOI] [PubMed] [Google Scholar]
- 21.Prince J. B., Auer K. L., Huskinson J., Parmley S. F., Araujo F. G., Remington J. S.1990. Cloning, expression, and cDNA sequence of surface antigen P22 from Toxoplasma gondii. Mol. Biochem. Parasitol. 43: 97–106. doi: 10.1016/0166-6851(90)90134-8 [DOI] [PubMed] [Google Scholar]
- 22.Raeghi S., Akaberi A., Sedeghi S.2011. Seroprevalence of Toxoplasma gondii in Sheep, Cattle and Horses in Urmia North-West of Iran. Iran. J. Parasitol. 6: 90–94. [PMC free article] [PubMed] [Google Scholar]
- 23.Reid A. J., Vermont S. J., Cotton J. A., Harris D., Hill-Cawthorne G. A., Könen-Waisman S., Latham S. M., Mourier T., Norton R., Quail M. A., Sanders M., Shanmugam D., Sohal A., Wasmuth J. D., Brunk B., Grigg M. E., Howard J. C., Parkinson J., Roos D. S., Trees A. J., Berriman M., Pain A., Wastling J. M.2012. Comparative genomics of the apicomplexan parasites Toxoplasma gondii and Neospora caninum: Coccidia differing in host range and transmission strategy. PLoS Pathog. 8: e1002567. doi: 10.1371/journal.ppat.1002567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salaberry S. R., Okuda L. H., Nassar A. F., de Castro J. R., Lima-Ribeiro A. M.2010. Prevalence of Neospora caninum antibodies in sheep flocks of Uberlândia county, MG. Rev. Bras. Parasitol. Vet. 19: 148–151. doi: 10.1590/S1984-29612010000300004 [DOI] [PubMed] [Google Scholar]
- 25.Tassi P.2007. Toxoplasma gondii infection in horses. A review. Parassitologia 49: 7–15. [PubMed] [Google Scholar]
- 26.Trees A. J., Davison H. C., Innes E. A., Wastling J. M.1999. Towards evaluating the economic impact of bovine neosporosis. Int. J. Parasitol. 29: 1195–1200. doi: 10.1016/S0020-7519(99)00093-4 [DOI] [PubMed] [Google Scholar]
- 27.Wanha K., Edelhofer R., Gabler-Eduardo C., Prosl H.2005. Prevalence of antibodies against Neospora caninum and Toxoplasma gondii in dogs and foxes in Austria. Vet. Parasitol. 128: 189–193. doi: 10.1016/j.vetpar.2004.11.027 [DOI] [PubMed] [Google Scholar]
- 28.Webster Joanne P.2010. Review of “Toxoplasmosis of Animals and Humans (Second Edition)” by J. P. Dubey. Parasites & Vectors3: 112. [Google Scholar]
- 29.Yan C., Fu L. L., Yue C. L., Tang R. X., Liu Y. S., Lv L., Shi N., Zeng P., Zhang P., Wang D. H., Zhou D. H., Zhu X. Q., Zheng K. Y.2012. Stray dogs as indicators of Toxoplasma gondii distributed in the environment: the first report across an urban-rural gradient in China. Parasit. Vectors 5: 5. doi: 10.1186/1756-3305-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]