Abstract
Neurological signs and serum acetylcholine receptor antibody (AChR-Ab) levels before and after thymectomy were monitored in a 6-year-old male cat with acquired Myasthenia Gravis (MG) as a paraneoplastic syndrome of thymoma. Soon after surgery, the neurological symptoms relapsed, and the cholinesterase inhibitor was administered to control them. The AChR-Ab levels increased postoperatively until 90 days after surgery. This is the first report on long term measurements of serum AChR-Ab levels in a cat with MG. Although thymectomy is valuable for the removal of thymoma, it may not resolve MG symptoms, neurological signs and serum AChR-Ab levels, without medication early after surgery. Also, this case report indicates that the AChR-Ab level might be a guide to detect a deterioration of MG symptoms.
Keywords: acetylcholine receptor antibody, cat, myasthenia gravis, thymectomy, thymoma
Acquired myasthenia gravis (MG) is a disorder characterized by inefficient neuromuscular transmission caused by autoantibodies, usually IgG, against acetylcholine receptors (AChR) [4]. These autoantibodies interfere the interaction between acetylcholine and its receptor: direct interfering, accelerating the normal turnover rate of receptors or activating complement-mediated lysis of the postsynaptic membrane [4].
Two retrospective studies have previously reported reviews of MG cases in cats using relatively large sample sizes (105 and 235 cases) [3, 16]. Although Abyssinian and Somali cats have a predilection to develop primary MG [16], any cat breed may develop MG secondary to thymoma, hypothyroidism, hypoadrenocorticism and other neoplasms [21].
Thymoma is the most frequent cause of secondary MG in cats, accounting for more than 25% of all reported feline MG cases [21]. To diagnose MG, serum antibodies are measured that react with α-bungarotoxin extracted acetylcholine receptors [15]. When MG is diagnosed, the patients are examined the existence of thymoma. If there is a thymoma, the initial treatment is surgery in both veterinary and human medicine: thymectomy [1, 6, 11]. To the best of our knowledge, no previous reports have assessed the clinical signs and the changes in serum AChR antibody (AChR-Ab) levels repeatedly before and after resection of thymoma in cats. In this report, serial changes in serum AChR-Ab levels and clinical status after thymectomy in a feline MG case were comparatively monitored.
A 6-year-old castrated male domestic shorthair cat, weighing 6.9 kg, was referred to our animal hospital due to anorexia, sudden hypersalivation and low head carriage. The vital signs were normal. Neurological examination confirmed low head carriage and torticollis, but showed that the other reflexes were normal. The complete blood cell counts and serum chemistry were generally within reference ranges, with the exception of a slight increase in segmented neutrophils and a mild decrease in blood urea nitrogen. Thoracic radiography revealed a prominent mass lesion in the anterior thorax (Fig. 1). Thymoma or mediastinal lymphoma was presumed, and we performed cytological examination of a fine-needle aspiration sample. From the epithelial cell-like aspect of the tumor cells, a tentative diagnosis of thymoma-associated MG was made. Serum AChR-Ab level (IDEXX laboratories, Tokyo, Japan) was shown to be high (2.75 nM; reference range, 0.0–0.3 nM) at this time. As computed tomography scanning revealed no prominent swelling of lymph nodes in the mediastinum or metastasis, a median sternotomy was conducted to resect the mass. Propofol (6 mg/kg, Fuji Pharma, Toyama, Japan) was injected for induction, and the cat was maintained with isoflurane and oxygen. Lactate Ringer’s solution (LRS; 10 ml/kg/hr) was infused during surgery, and 20 mg/kg ampicillin sodium (Amipenix; Kyoritsu Seiyaku,Tokyo, Japan) was administered intravenously for every 8 hr. For analgesia, fentanyl citrate (Fentanyl, Daiichi-Sankyo,Tokyo, Japan) was infused intravenously for 5–30 µg/kg/hr during surgery. The mass, which was covered in a thin capsule, appeared immediately beneath the sternum. The whole mass was grossly resected, and subjected to histopathological evaluation. The recovery was uneventful, and fentanyl citrate was infused intravenously for 5–10 µg/kg/hr postoperatively until the next day. The mass consisted of scattered parenchyma and fluid-filled lumina of varying size (Fig. 2). Small and mature lymphocytes were predominant in the parenchymal area (Fig. 3), whereas medium-sized lymphoblasts were very rare; abnormal mitosis was not observed. In addition, the parenchymal area contained neoplastic epithelial cells of varying size with round or oval-shaped nuclei and clear and large cytoplasm. The surgical margin was clear, and tumor infiltration to the surrounding tissue was not detected. Based on these findings, the resected mass was determined to be a thymoma.
Fig. 1.
Thoracic radiographs of the cat. (A) Lateral view. Black arrows indicate the shape of a thoracic mass lesion. (B) Ventral- Dorsal view. M: mass, H: heart, L: liver.
Fig. 2.

Low-power image of the resected mass (hematoxylin-eosin stain, original magnification ×100). Multiple lumina were observed in the marginal area of the mass, and islands of parenchyma were profuse in the center area.
Fig. 3.
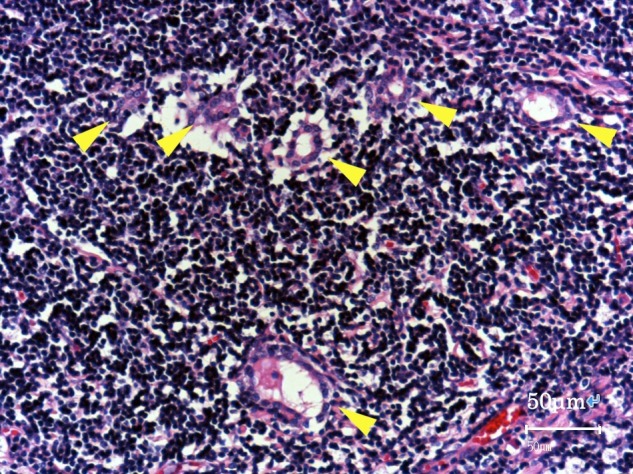
High-power image of the parenchymal area in the resected mass (hematoxylin-eosin stain, original magnification ×400). Small lymphocytes were predominant. Arrowheads indicate neoplastic epithelial cells forming small lumina.
Thereafter, the clinical status and the serum AChR-Ab concentrations were serially monitored (Fig. 4). After surgery, all neurological signs briefly disappeared, and the cat was discharged from the hospital with no major complications and neurological signs at 16 days post-surgery. Despite the improvements to clinical status, the serum AChR-Ab concentration was measured to be 4.93 nM at 7 days post-surgery, which was higher than the preoperative concentration. From 28 days after surgery, the cat’s hind limbs and eyelids had become intolerant to prolonged exercise and movements. At the same time, the AChR-Ab level had increased more to 6.12 nM. As the neurological signs had deteriorated, we began the oral administration of an anticholinesterase drug (Neostigmine 0.4 mg/kg q12h [Vagostigmin, Shionogi & Co., Ltd., Osaka, Japan]) at 56 days post-surgery. The neurological signs diminished dramatically from the next day after an anticholinesterase drug administration started. By 61 days post-surgery, almost all of the neurological signs had disappeared. However, the serum AChR-Ab level continued to increase until 90 days post-surgery. It took 465 days after surgery until the serum AChR-Ab level returned to the preoperative level. The anticholinesterase drug was still being administered approximately 16 months after surgery without any neurological signs or adverse effects.
Fig. 4.
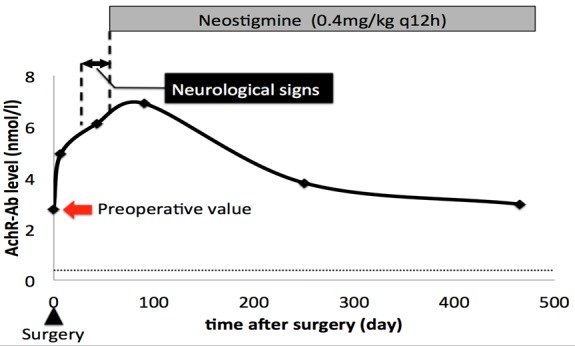
Time-course levels of AChR-Ab after surgery. The horizontal dashed line indicates the reference level. Neurological signs are indicated by a two-headed black arrow above the figure, and neostigmine (0.4 mg/kg q12hr) administration is indicated by a gray box above the figure.
In general, surgical resections of thymoma are conducted together with the administration of both anticholinesterase drugs and immunosuppressive drugs for thymoma-associated MG in small animal medicine [4] and human medicine [6]. In this case, the administration of neostigmine was unaccompanied by an immunosuppressive drug, though other reports on feline MG have employed the concurrent use of prednisolone [17, 18]. Our findings implied that immunosuppressive drugs might not be necessary to control neurological signs of MG, if the anticholinesterase drug is sufficiently effective.
This is the first report in the veterinary field to document the serial changes in neurological signs and serum AChR-Ab concentrations in a cat with MG resulting from thymoma. Thymectomy was performed to remove a thymoma and ameliorate the clinical signs, and the elevated AChR-Ab levels derived from MG. This case did not show any recurrence symptoms for at least one year after surgery, and one purpose of surgery, removal of tumor, was accomplished. Thymectomy is the initial treatment for the patients with thymoma in veterinary and human medicine [1, 6, 11]. As for the neurological signs, this cat showed a temporal deterioration after surgery, but was successfully treated by administration of an anticholinesterase drug. Although the underlying reason for the temporal deterioration of neurological signs after surgery remains undetermined, our case indicated that thymectomy might not resolve the clinical signs derived from MG without a valid medication at least during early postoperative period. There are several articles that showed the need of medication after thymectomy to control the clinical symptoms in animals with MG [3, 13, 17, 19]. In human and veterinary medicine, some patients with thymoma develop postthymectomy MG and require the medication [2, 20, 23]. This case and these articles may imply the thymectomy has a risk to deteriorate the clinical signs of MG. Serum AChR-Ab levels also increased and lasted for more than three months after surgery. At 465 days after surgery, the AChR-Ab level returned to preoperative level. In the study of Shelton and his colleague, serum AChR-Ab levels in some acquired MG cats increased or increased and then decreased after thymectomy [3]. The correlation of the clinical signs and serum AChR-Ab levels is still controversial in human and veterinary medicine [5, 7,8,9,10, 12, 14, 22, 23]. This case showed some relations of them early after surgery. Because the spontaneous remission of acquired MG is scarce in cats [3], the decrease of AChR-Ab levels may be achieved from thymectomy, though we can not compare the case with and without surgery.
In this paper, the clinical signs and serum AChR-Ab concentrations were monitored before and after surgery and following anticholinesterase drug administration in a cat with MG associated with thymoma. The temporal deterioration of neurological signs could be controlled through the administration of anticholinesterase drug without any immunosuppressive agents. Serum AChR-Ab concentration was elevated over a long term after thymectomy and returned to pretreatment level at 465 days after surgery. In summary, this case suggests that 1) though thymectomy for a cat with thymoma and MG is an appropriate treatment, the clinical signs may deteriorate temporarily and 2) the increase of serum AChR-Ab concentration may correlate to the deterioration of clinical symptoms in some cases.
REFERENCES
- 1.Binks S., Vincent A., Palace J.2016. Myasthenia gravis: a clinical-immunological update. J. Neurol. 263: 826–834. doi: 10.1007/s00415-015-7963-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gores B. R., Berg J., Carpenter J. L., Aronsohn M. G.1994. Surgical treatment of thymoma in cats: 12 cases (1987-1992). J. Am. Vet. Med. Assoc. 204: 1782–1785. [PubMed] [Google Scholar]
- 3.Hague D. W., Humphries H. D., Mitchell M. A., Shelton G. D.2015. Risk factors and outcomes in cats with acquired myasthenia gravis (2001–2012). J. Vet. Intern. Med. 29: 1307–1312. doi: 10.1111/jvim.13596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inzana K. D.2000. Myasthenia Gravis. pp. 675–676. In: Textbook of Veterinary Internal Medicine, 5th ed. (Ettinger, S. J. and Feldman, E. C. eds.), Saunders, Philadelphia. [Google Scholar]
- 5.Khorzad R., Whelan M., Sisson A., Shelton G. D.2011. Myasthenia gravis in dogs with an emphasis on treatment and critical care management. J. Vet. Emerg. Crit. Care (San Antonio) 21: 193–208. doi: 10.1111/j.1476-4431.2011.00636.x [DOI] [PubMed] [Google Scholar]
- 6.Kumar V., Kaminski H. J.2011. Treatment of myasthenia gravis. Curr. Neurol. Neurosci. Rep. 11: 89–96. doi: 10.1007/s11910-010-0151-1 [DOI] [PubMed] [Google Scholar]
- 7.Meriggioli M. N., Sanders D. B.2012. Muscle autoantibodies in myasthenia gravis: beyond diagnosis? Expert Rev. Clin. Immunol. 8: 427–438. doi: 10.1586/eci.12.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakajima J., Murakawa T., Fukami T., Sano A., Takamoto S., Ohtsu H.2008. Postthymectomy myasthenia gravis: relationship with thymoma and antiacetylcholine receptor antibody. Ann. Thorac. Surg. 86: 941–945. doi: 10.1016/j.athoracsur.2008.04.070 [DOI] [PubMed] [Google Scholar]
- 9.Oosterhuis H. J., Limburg P. C., Hummel-Tappel E., The T. H., The T. H.1983. Anti-acetylcholine receptor antibodies in myasthenia gravis. Part 2. Clinical and serological follow-up of individual patients. J. Neurol. Sci. 58: 371–385. doi: 10.1016/0022-510X(83)90096-5 [DOI] [PubMed] [Google Scholar]
- 10.Oosterhuis H. J., Limburg P. C., Hummel-Tappel E., Van den Burg W., The T. H.1985. Anti-acetylcholine receptor antibodies in myasthenia gravis. Part 3. The effect of thymectomy. J. Neurol. Sci. 69: 335–343. doi: 10.1016/0022-510X(85)90144-3 [DOI] [PubMed] [Google Scholar]
- 11.Radlinsky M. A. G.2012. Thoracic Cavity. pp. 1787-1812. In: Veterinary Surgery: Small Animal (Tobias, K. M. and Johnston, S. A. eds.), Saunders, Philadelphia. [Google Scholar]
- 12.Sanders D. B., Burns T. M., Cutter G. R., Massey J. M., Juel V. C., Hobson-Webb L., Muscle Study Group 2014. Does change in acetylcholine receptor antibody level correlate with clinical change in myasthenia gravis? Muscle Nerve 49: 483–486. doi: 10.1002/mus.23944 [DOI] [PubMed] [Google Scholar]
- 13.Scott-Moncrieff J. C., Cook J. R., Jr, Lantz G. C.1990. Acquired myasthenia gravis in a cat with thymoma. J. Am. Vet. Med. Assoc. 196: 1291–1293. [PubMed] [Google Scholar]
- 14.Shelton G. D.2010. Routine and specialized laboratory testing for the diagnosis of neuromuscular diseases in dogs and cats. Vet. Clin. Pathol. 39: 278–295. doi: 10.1111/j.1939-165X.2010.00244.x [DOI] [PubMed] [Google Scholar]
- 15.Shelton G. D.1992. Canine Myasthenia Gravis. p. 1039. In: Kirk’s Current Veterinary Therapy XI (Kirk, R. W. and Bonagura, J. D. eds.), Saunders, Philadelphia. [Google Scholar]
- 16.Shelton G. D., Ho M., Kass P. H.2000. Risk factors for acquired myasthenia gravis in cats: 105 cases (1986-1998). J. Am. Vet. Med. Assoc. 216: 55–57. doi: 10.2460/javma.2000.216.55 [DOI] [PubMed] [Google Scholar]
- 17.Shilo Y., Pypendop B. H., Barter L. S., Epstein S. E.2011. Thymoma removal in a cat with acquired myasthenia gravis: a case report and literature review of anesthetic techniques. Vet. Anaesth. Analg. 38: 603–613. doi: 10.1111/j.1467-2995.2011.00648.x [DOI] [PubMed] [Google Scholar]
- 18.Singh A., Boston S. E., Poma R.2010. Thymoma-associated exfoliative dermatitis with post-thymectomy myasthenia gravis in a cat. Can. Vet. J. 51: 757–760. [PMC free article] [PubMed] [Google Scholar]
- 19.Somnier F. E.1994. Exacerbation of myasthenia gravis after removal of thymomas. Acta Neurol. Scand. 90: 56–66. doi: 10.1111/j.1600-0404.1994.tb02680.x [DOI] [PubMed] [Google Scholar]
- 20.Sun X. G., Wang Y. L., Liu Y. H., Zhang N., Yin X. L., Zhang W. J.2011. Myasthenia gravis appearing after thymectomy. J. Clin. Neurosci. 18: 57–60. doi: 10.1016/j.jocn.2010.05.018 [DOI] [PubMed] [Google Scholar]
- 21.Taylor S. M.2003. pp. 1059-1060. Disorders of peripheral nerves and the neuromuscular junction. In: Small Animal Internal Medicine 3rd ed. (Nelson, R. W. and Couto, C. G. eds.), Mosby, Maryland Heights. [Google Scholar]
- 22.Tindall R. S., Tindall M. D.1981. Humoral immunity in myasthenia gravis: biochemical characterization of acquired antireceptor antibodies and clinical correlations. Ann. Neurol. 10: 437–447. doi: 10.1002/ana.410100506 [DOI] [PubMed] [Google Scholar]
- 23.Yamada Y., Yoshida S., Iwata T., Suzuki H., Tagawa T., Mizobuchi T., Kawaguchi N., Yoshino I.2015. Risk factors for developing postthymectomy myasthenia gravis in thymoma patients. Ann. Thorac. Surg. 99: 1013–1019. doi: 10.1016/j.athoracsur.2014.10.068 [DOI] [PubMed] [Google Scholar]



