Abstract
Purpose
A phase III trial assessing response-based therapy in intermediate-risk Hodgkin lymphoma, mandated real-time central review of involved field radiotherapy and imaging records by a centralized review center to maximize protocol compliance. We report the impact of centralized radiotherapy review upon protocol compliance.
Methods
Review of simulation films, port films, and dosimetry records was required pre-treatment and after treatment completion. Records were reviewed by study-affiliated or review center-affiliated radiation oncologists. A 6–10% deviation from protocol-specified dose was scored as “minor”; >10% was “major”. A volume deviation was scored as “minor” if margins were less than specified, or “major” if fields transected disease-bearing areas. Interventional review and final compliance review scores were assigned to each radiotherapy case and compared.
Results
Of 1712 patients enrolled, 1173 underwent IFRT at 256 institutions in 7 countries. An interventional review was performed in 88% and a final review in 98%. Overall, minor and major deviations were found in 12% and 6%, respectively. Among the cases for which ≥ 1 pre-IFRT modification was requested by QARC and subsequently made by the treating institution, 100% were made compliant on final review. In contrast, among the cases for which ≥ 1 modification was requested but not made by the treating institution, 10% were deemed compliant on final review.
Conclusion
In a large trial with complex treatment pathways and heterogeneous radiotherapy fields, central review was performed in a large percentage of cases pre-IFRT and identified frequent potential deviations in a timely manner. When suggested modifications were performed by the institutions, deviations were almost eliminated.
Keywords: Radiotherapy quality assurance, pediatric Hodgkin Lymphoma, involved field radiation therapy
Introduction
The primary objectives of a recently-closed phase III trial of dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk Hodgkin lymphoma were to determine whether, first, involved-field radiotherapy could be safely eliminated in select patients based upon early response to standard chemotherapy without compromising event-free survival and, second, whether augmented chemotherapy for patients with slow early response could improve outcomes over to standard chemotherapy. Four-year event-free survival and overall survival are reported [1].
Such a large and multi-national trial with a complicated protocol schema and a diverse group of investigators from numerous institutions introduced unique challenges in terms of overcoming treatment heterogeneity. Yet, treatment uniformity is critically important for establishing trial validity and generalizability. Over the history of cooperative group trials, it is well established that a lack of high-quality protocol compliance, particularly as it pertains to quality assurance for radiation therapy, has not only been associated with a consequent lack of confidence in study results and poor generalizability, but has also led to inferior outcomes [2–8].
Real-time interventional review of the quality of involved field radiation therapy as prescribed at a centralized facility in the US was instrumental in ensuring the success of radiotherapy and imaging response assessments on this protocol with its multiple response-based randomization points, including a no-RT randomization arm. Herein, we investigate the feasibility of real-time interventional radiotherapy review and its impact on overall protocol compliance and outcome from one of the largest pediatric cooperative group trials performed to date.
Methods and Materials
Patients
The trial enrolled patients age 0 to 21 inclusive, with newly diagnosed intermediate-risk Hodgkin lymphoma defined by protocol guidelines as stage I-IIA with bulk disease, I-IIB, I-IIAE, IIIA-IVA, III-IVAE, IIIAS, or IIIAE+S. In total, 1712 eligible patients from 256 institutions across 7 countries were entered onto the protocol, which opened to accrual on September 23, 2002 and permanently closed on October 9, 2009. The study was approved by the institutional ethics committees of all the participating centers.
Protocol treatment and response assessments
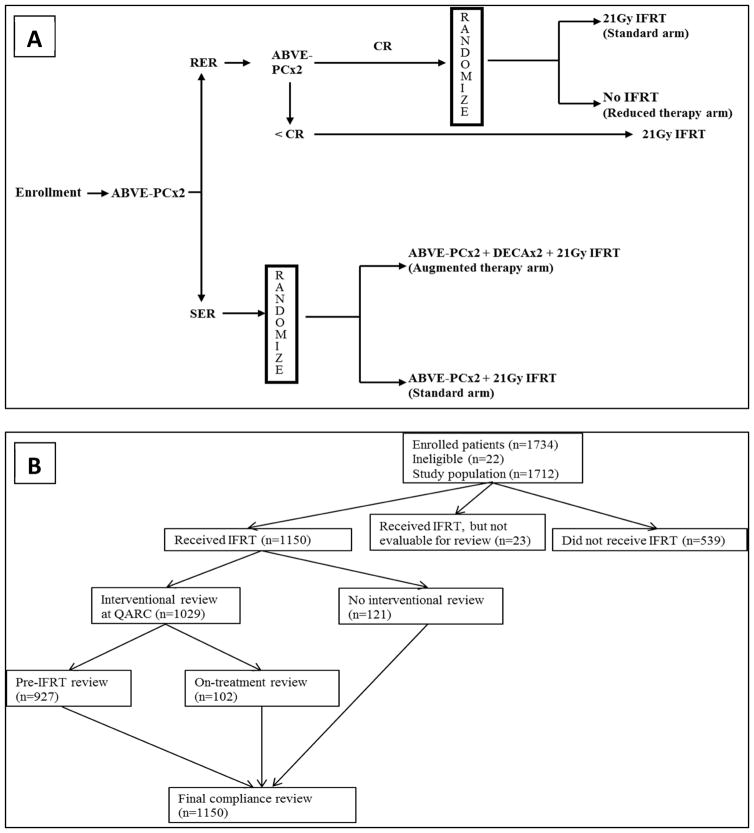
The study objectives were accomplished by a response-based two-randomization design. The basic sequence of the trial is shown in Fig. 1A. Patients were treated with multi-agent chemotherapy consisting of doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide (ABVE-PC). All patients would receive four cycles of ABVE-PC chemotherapy. Imaging response was assessed after two and after four cycles by computed tomography (CT) and functional imaging (positron emission tomography (FDG-PET) or gallium scintigraphy). Patients were categorized as rapid early responders (RER) if CT imaging demonstrated at least 60% response or as slow early responders (SER) if CT imaging demonstrated less than 60% response. RER patients were further subcategorized if they demonstrated complete response (CR), defined as having at least 80% response on CT imaging, no new lesions, no residual disease, and no abnormal uptake on PET or gallium scans. Patients who were RER after two cycles and demonstrated a sustained CR after four cycles were randomized to involved field radiation therapy (IFRT) or no further therapy. RER patients with less than CR were non-randomly assigned to IFRT. Patients who were SER after two cycles of ABVE-PC were randomized to two additional cycles of ABVE-PC or two additional cycles of ABVE-PC preceded by two cycles of augmented chemotherapy with dexamethasone, etoposide, cisplatin, and cytarabine (DECA). All SER patients would receive consolidative IFRT.
Figure 1.
Panel A AHOD0031 treatment and randomization schema. ABVE-PC – Doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide; RER – Rapid Early Response; SER – Slow Early Response; CR – Complete Response; IFRT – Involved Field Radiation Therapy; DECA – Dexamethasone, etoposide, cisplatin, cytarabine. Panel B: Sequence of QARC reviews.
Involved field radiation therapy
Involved field radiation therapy (IFRT) was stipulated to begin within four weeks of completion of chemotherapy. The prescribed dose was 21 Gy, given in fourteen 1.5 Gy daily fractions with balanced anterior and posterior fields. The gross tumor volume included any sites of disease involved at presentation and was defined as any lymph node measuring > 1.5cm in single-axis on CT imaging. The clinical target volume (CTV) was defined as the involved anatomical nodal region. For instance, any cervical node involvement would mandate treatment to the entire ipsilateral but not bilateral cervical chain. Radiotherapy for mediastinal involvement would include the post-chemotherapy mediastinal width plus the bilateral hila. The axillae were excluded unless initially involved. When para-aortic nodes were involved, the spleen was also included in the field. However, splenic radiation was done without inclusion of para-aortic nodes if they were not involved. The planning target volume was a 1 centimeter margin around the CTV. If solitary bone was involved, it was treated to 21 Gy with a 2 cm margin on the initial extent of disease. If bone marrow or multiple bone involvement was present, these sites were not irradiated as part of treatment for stage IV disease. Whole organ radiotherapy was used for parenchymal metastases to lung and liver as well as for extensive pericardial involvement. Whole organ doses in those cases were limited to 10.5 Gy for lung and heart, and 15 Gy for the liver using partial transmission blocking. Dose at midplane was stipulated to be kept within a range of −5% to +7% of the prescribed dose. Reference points varied depending on the volume treated. When necessary, appropriate compensating filters or boosting/blocking was performed to achieve dose uniformity.
Protocol compliance and quality assurance
Quality control of response assessments and IFRT parameters was performed centrally in real-time at centralized facility in the US by study radiologists and radiation oncologists. For assessment of imaging response, centers were required to submit copies of the CT neck, chest, abdomen, and pelvis, gallium scans, FDG-PET imaging, bone scans, and upright chest x-rays. Imaging was required from pretreatment (baseline) staging and after cycle 2 of chemotherapy from all patients. For RER patients, an additional set of imaging was required after cycle 4 of chemotherapy before randomization to +/− IFRT.
For assessment of radiotherapy data, centers were required to submit copies of simulator films and/or digitally reconstructed radiographs for each field, a dosimetry summary form for each target volume including required reference points and critical organ doses, worksheets used for monitor unit calculations, and verification images. A rapid turn-around review of the treatment plan and suggestions for improvement were provided in real-time. Within one week of completion of radiotherapy, centers were required to submit any revised documentation including additional simulation and verification films for any field or dose modifications made after the initial review of radiotherapy objects. Radiotherapy records were mandated to be sent and reviewed both before beginning and after completing IFRT.
Radiotherapy data were evaluated for adherence to the protocol treatment guidelines in terms of appropriateness of dose and treatment volume. A dose to the prescription point differing from protocol guidelines by 6–10%, a margin less than specified, or an excessively large field were considered minor deviations. A dose to the prescription point differing by more than 10% from protocol guidelines, or fields transecting gross tumor or potentially tumor bearing areas were considered major deviations.
Analysis
At the conclusion of radiation treatment reviews, the review center assigned two scores to each case to describe overall compliance. The Interventional Review assessed whether any RT modifications which had been requested by the review center before a subject began IFRT was ever made by the treating institution, thereby impacting treatment. The Performance Evaluation, assigned at the end of treatment, assessed the overall radiotherapy protocol compliance for each case upon final review (e.g. treatment appropriate, minor deviation, major deviation, or not evaluable). Deviations upon final review were counted and reported as frequencies. The Interventional Review data were analyzed in terms of the impact on the number and type of protocol deviations seen on final review and reported in terms of frequencies. Overall radiotherapy compliance was compared across the years of the protocol from 2003 to 2009 and by number of patients enrolled per institution. Chi-square tests were used where appropriate to test statistical significance of categorical variables.
Results
Sequence of reviews
Of 1734 patients enrolled onto the study, 1712 were deemed trial eligible. Of these, 1173 underwent IFRT at 256 participating institutions in the United States, Canada, Israel, Switzerland, Netherlands, Australia, and New Zealand. Radiotherapy objects were successfully reviewed pre-treatment in 927 (79%) patients and on-treatment in 102 (9%) patients. A final compliance review assessing overall radiotherapy performance was conducted in 1150 (98%) patients. The sequence of radiotherapy reviews performed at the review center is described in Fig 1B. On-treatment reviews occurred after radiotherapy had already begun but before the patient reached the mid-way point of treatment.
Interventional Reviews
An interventional review was defined as a real-time assessment of protocol compliance to RT wherein RT data were reviewed and any modifications requested by QARC were made before a patient began RT. Table 1 summarizes the results of the interventional review as it relates to the overall compliance of radiotherapy as judged by the Overall Performance Evaluation determined upon final review. In 354 cases, ≥ 1 pre-treatment modifications were requested by the review center and were made by the treating institution. Upon final review of these cases, none were found to have deviations and RT was delivered appropriately in all cases. In 84 cases, ≥ 1 treatment modifications requested by the review center were not made by the treating institution. Upon the final compliance review of these cases, 45 (53%) were scored as having minor deviations, 31 (37%) were scored as having major deviations, and only 8 (10%) were scored as being appropriate. In 121 cases, an interventional review was required but was not performed due to delinquent data submission or failure of data submission by the treating institution. Of these, 39% resulted in a major (n=27) or minor (n=20) deviation on final review.
Table 1.
Impact of Interventional Review on Overall Radiotherapy Compliance
| Interventional Review | Final Performance Evaluation | |||
|---|---|---|---|---|
| Compliant | Major Deviation | Minor Deviation | Total | |
| Interventional review not done | 74 | 27 | 20 | 121 |
| Modification(s) not required | 494 | 0 | 0 | 494 |
| Modification(s) required and made | 354 | 0 | 0 | 354 |
| Modification(s) required and NOT made | 8 | 31 | 45 | 84 |
| Non-characterizable deviations* | 8 | 15 | 74 | 97 |
Deviations were not detected during pre- or on-treatment review, or interventional review and final review scores were not in agreement.
Final compliance reviews
In total, 73 (6%) cases were scored as having major deviations upon final review and 139 (12%) were scored as having minor deviations. Table 2 breaks down the deviations scored upon final compliance review based on when and whether an interventional review took place. Not surprisingly, deviations were significantly higher in cases when an interventional review was not performed (p <0.05).
Table 2.
Deviations on Final Review
| Timing of Interventional Review | Deviation Type | P-value | |
|---|---|---|---|
| Major Deviation | Minor Deviation | ||
| If pre-IFRT evaluation performed (n=927) | 40 (4%) | 101 (11%) | 0.03 |
| If evaluation performed during IFRT (n=102) | 2 (2%) | 2 (2% ) | |
| If only post-IFRT evaluation performed (n=121) | 31 (26%) | 36 (30%) | |
| Total deviations (n=1150) | 73 (6%) | 139 (12%) | |
Protocol deviations as a function of time and institutional experience
Of the 256 institutions enrolling patients on the study, 224 (88%) treated 10 or fewer patients each, and 135 (53%) treated 5 or fewer patients each. Institutional experience, determined by the number of cases enrolled per institution, did not appear to be a significant factor in overall compliance of the radiotherapy plans though a trend towards significance is suggested. Results are described in Table 3.
Table 3.
Deviations per Institutional Enrollment
| Patients enrolled per institution | Compliant | Major Deviation | Minor Deviation | Not evaluable | p-value* |
|---|---|---|---|---|---|
| ≤10 patients | 747 | 54 | 100 | 20 | 0.07 |
| >10 patients | 191 | 19 | 39 | 3 |
p-value is based on a chi-square test comparing major deviations, minor deviations, and compliant treatments between the two patient cohorts.
Additionally, an analysis was conducted to evaluate whether an institution’s familiarity with the protocol over time impacted the frequency of protocol deviations (Table 4). From 2003 to 2005, 82% (286 of 350) of IFRT cases that underwent interventional review were compliant, 4% (15 of 350) were scored with major deviations, and 14% (49 of 350) were scored with minor deviations. From 2006 until the last patient was entered onto the protocol in 2009, 88% (578 of 679) were compliant, 5% (31 of 679) were scored with major deviations, and 10% (70 of 679) were scored with minor deviations. These results demonstrate a slightly higher frequency of compliant cases in later years and correspondingly a lower frequency of deviations, though the difference is not statistically significant. Similar results were seen for those that did not undergo an interventional review. From 2003–2005, 52% were compliant (29 of 56), 25% had major deviations (14 of 56), and 23% had minor deviations (13 of 56). From 2006–2009, 69% were compliant (45 of 65), 20% had major deviations (13 of 65), and 11% had minor deviations (7 of 65). P-values did not reach significance.
Table 4.
Protocol Compliance by Year
| Year | Interventional Review Performed | Interventional Review Not Performed | ||||
|---|---|---|---|---|---|---|
| Compliant | Major Deviation | Minor Deviation | Compliant | Major Deviation | Minor Deviation | |
| 2003 | 73 | 5 | 13 | 7 | 4 | 2 |
| 2004 | 90 | 4 | 16 | 11 | 4 | 3 |
| 2005 | 123 | 6 | 20 | 11 | 6 | 8 |
| 2006 | 134 | 7 | 13 | 16 | 4 | 3 |
| 2007 | 148 | 7 | 20 | 12 | 3 | 1 |
| 2008 | 152 | 10 | 13 | 11 | 3 | 2 |
| 2009 | 144 | 7 | 24 | 6 | 3 | 1 |
| Total | 864 | 46 | 119 | 74 | 27 | 20 |
Deviations in Relapsed vs. Non-relapsed patients
We analyzed the frequency of deviations in the cohort of 153 patients who underwent IFRT and experienced relapse during follow-up, and we compared this with the cohort of 1020 non-relapsed patients who also underwent IFRT. We found no significant difference in the rates of compliance or deviations between the two groups (Table 5).
Table 5.
Deviations by Relapsed vs. Non-relapsed Patients
| Compliant | Major Deviation | Minor Deviation | Not Evaluable | Total | p-value* | |
|---|---|---|---|---|---|---|
| Relapsed cohort | 119 | 13 | 19 | 2 | 153 | 0.44 |
| Non-relapsed cohort | 819 | 60 | 120 | 21 | 1020 |
p-value is based on a chi-square test comparing major deviations, minor deviations, and compliant treatments between the two patient cohorts.
Discussion
The results of this compliance analysis from this large study illustrate the success of the radiotherapy quality assurance program of one of the largest children’s oncology group trials to date and demonstrate the feasibility of executing real-time, rapid turn-around QA of the highest standard despite the trial’s size and complexity. Eighty-eight percent of IFRT cases on study underwent an interventional review and 98% underwent a final compliance review. Systematic central review pre-IFRT identified potential deviations in a timely manner. When modifications were suggested by the review center and made by the treating institution, 100% of the IFRT plans were scored as compliant on the final review. The majority of institutions enrolled 10 or fewer patients each yet still delivered compliant RT as long as they participated in the real-time interventional review process. Deviations did not differ significantly between relapsed and non-relapsed patient cohorts.
The importance of robust quality metrics in clinical trials, particularly with regard to radiation therapy, has been investigated previously. Problems with radiotherapy compliance have resulted in poorer outcomes of the patients treated on those trials and resultant difficulty in drawing practice-changing conclusions from trial results themselves. One of the earliest cooperative group trials to highlight the importance of radiotherapy compliance was the Pediatric Oncology Group 8725 protocol, which randomized patients with advanced stage Hodgkin lymphoma after chemotherapy to +/− radiation therapy. The published results from the trial found no benefit to adding radiation; however, retrospective analysis found a 10% survival benefit in patients who underwent radiation treatment in a study-compliant fashion [7, 9, 10]. In fact, these issues set the stage and informed the priority for integration of pre-treatment rapid review QA into future pediatric cooperative trials.
Results of several adult cooperative group trials have been clouded by poor QA. Lack of radiotherapy quality assurance on the European Study Group for Pancreatic Cancer (ESPAC-1), which randomized resected pancreatic cancer patients to adjuvant chemotherapy or chemoradiation, has been touted as a major weakness in the study’s design and has consequently led other investigators to question the validity of the conclusion that there was no benefit of chemoradiation in these patients [2, 3, 8]. A previous Southwest Oncology Group study of IFRT after chemotherapy for advanced Hodgkin lymphoma reported major radiotherapy deviations were found in 56% of relapsing patients [11], and a quality control analysis of the German Hodgkin Study Group HD 10 and HD11 trials reported suboptimal RT delivered in 47% of the reviewed cases [12].
In each of these trials, radiotherapy review was either not performed, or was performed after the patient had already completed treatment. A recent metaanalysis of 8 trials by Ohri et al. found that RT protocol deviations were associated with lower overall survival and increased risks of treatment failure. The frequencies of radiotherapy deviation ranged from 8% to 71%. Interventional radiotherapy review was performed in only one of the eight trials analyzed in this metanalaysis. In the remainder, radiotherapy was evaluated in a post-hoc fashion [5]. In our analysis, major protocol deviations were significantly fewer in number if an interventional review had been performed compared with if only a final review had been performed (4% vs. 14%), again emphasizing the critical importance of the interventional review process.
Despite the process of interventional review, radiotherapy protocol compliance may still not be optimal in some cases. One such example is the TROG 02.02 trial, which tested the addition of tirapazamine to cisplatin-based chemoradiotherapy for patients with locally advanced squamous cell carcinoma of the head and neck [13]. While the rates of interventional and final reviews were similar to those on this protocol, the rate of radiotherapy noncompliance was higher at 25%. Most strikingly, overall survival and locoregional failure-free survival were significantly lower in patients with non-compliant radiotherapy plans. It is important to note that this study had on-treatment but not pre-treatment reviews. While potential deviations were caught with on-treatment reviews, there was not always time or incentive to modify the radiotherapy plans and it was not mandated by the study QA guidelines. This may explain the higher rate of non-compliance. Pre-treatment reviews, on the other hand, allows or requires modifications before treatment.
A factor associated with unsatisfactory radiotherapy in the TROG 02.02 trial was the number of patients enrolled per institution. Centers enrolling fewer than five patients had significantly higher rates of predicted major adverse impact vs. centers enrolling more than 20 patients [4]. Similarly, a radiotherapy quality report from POG 9404 of prophylactic cranial irradiation for T-cell acute lymphoblastic leukemia/non-Hodgkin lymphoma found that centers enrolling more than 5 patients were more likely to be treating with compliant radiotherapy [14]. In our study, we did not find a significant difference in compliance between institutions enrolling fewer patients versus those enrolling larger numbers, though a trend toward increased compliance in higher enrolling institutions was suggested. The consistency in compliance may be due to the interventional review process which detected potential deviations in cases from low-enrolling institutions, which were subsequently corrected before final review.
Real-time rapid turnaround radiotherapy review is arguably the quality assurance method of the highest standard. It is costly to maintain. However, when performed successfully as we have shown, it substantially reduces the frequency of potentially suboptimal RT which can have a detrimental impact on the patient treated and confound study outcomes, particularly when RT is a central question in the protocol. The main findings of the protocol concluded that IFRT could be safely eliminated in a select group of patients who experienced a rapid early response to ABVE-PC chemotherapy who were randomized to IFRT or no IFRT without compromising event-free survival. Given our analysis of favorable radiotherapy compliance on this protocol, we do not have to worry whether suboptimally delivered radiotherapy could have washed out the effect at the radiotherapy randomization point. We can rest assured as to the validity of the trial results and thus incorporate the practice-changing conclusions into our everyday clinics.
Conclusion
In a large trial with complicated protocol procedures and use of heterogeneous radiotherapy fields, a remarkable amount of cooperation between the central review facility and the large number of treating institutions was necessary for timely central review of RT data. The fact that 88% of patients underwent an interventional review within the appropriate time-frame is a testament to the fact that centralized RT review is not only feasible in a large cooperative group trial but is capable of successfully averting a large number of potential radiotherapy deviations. When suggested interventional review modifications were performed, protocol deviations were nearly eliminated.
Summary.
A recently closed Phase III study, a randomized response-based study with over 1700 intermediate Hodgkin Lymphoma patients, utilized a real-time radiotherapy review process at a centralized location to maximize protocol compliance. We investigated the impact of interventional radiotherapy reviews on overall protocol compliance and found extremely high rates of radiotherapy compliance when interventional reviews were performed. When no interventional review was performed, radiotherapy compliance was low.
Acknowledgments
Funding
Supported in part by grants Chair’s Grant - U10 CA98543-08, Statistics and Data Center Grant – U10 CA98413-08, COG NCTN Network Group Operations Center Grant - 1U10CA180886, COG NCTN Statistics & Data Center - 1U10CA180899 and U10 CA29511 from the National Cancer Institute/National Institutes of Health
Footnotes
Conflicts: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedman DL, Wolden S, Constine L, et al. AHOD0031: a phase III study of dose intensive therapy for intermediate risk Hodgkin Lymphoma: a report from the Children’s Oncology Group. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.52.5410. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodman KA. Quality assurance for radiotherapy: a priority for clinical trials. J Natl Cancer Inst. 2013;105(6):376–7. doi: 10.1093/jnci/djt031. [DOI] [PubMed] [Google Scholar]
- 3.Koshy MC, Landry JC, Cavanaugh SX, et al. A challenge to the therapeutic nihilism of ESPAC-1. Int J Radiat Oncol Biol Phys. 2005;61(4):965–6. doi: 10.1016/j.ijrobp.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Peters LJ, O’Sullivan B, Giralt J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol. 2010;28(18):2996–3001. doi: 10.1200/JCO.2009.27.4498. [DOI] [PubMed] [Google Scholar]
- 5.Ohri N, Shen X, Dicker AP, et al. Radiotherapy protocol deviations and clinical outcomes: a meta-analysis of cooperative group clinical trials. J Natl Cancer Inst. 2013;105(6):387–93. doi: 10.1093/jnci/djt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Million L, Anderson J, Breneman J, et al. Influence of noncompliance with radiation therapy protocol guidelines and operative bed recurrences for children with rhabdomyosarcoma and microscopic residual disease: a report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys. 2011;80(2):333–8. doi: 10.1016/j.ijrobp.2010.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner MA, Leventhal B, Brecher ML, et al. Randomized study of intensive MOPP-ABVD with or without low-dose total-nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin’s disease in pediatric patients: a Pediatric Oncology Group study. J Clin Oncol. 1997;15(8):2769–79. doi: 10.1200/JCO.1997.15.8.2769. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 9.FitzGerald TJ, et al. Processes for quality improvements in radiation oncology clinical trials. Int J Radiat Oncol Biol Phys. 2008;71(1 Suppl):S76–9. doi: 10.1016/j.ijrobp.2007.07.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzgerald TJ, Urie M, Ulin K, et al. Quality of radiotherapy reporting in randomized controlled trials of Hodgkin’s lymphoma and non-Hodgkin’s lymphoma: in regard to Bekelman and Yahalom. Int J Radiat Oncol Biol Phys. 2009;73:492–498. doi: 10.1016/j.ijrobp.2008.04.058. [DOI] [PubMed] [Google Scholar]; Int J Radiat Oncol Biol Phys. 2010;77(1):315–6. doi: 10.1016/j.ijrobp.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabian CJ, Mansfield CM, Dalhberg S, et al. Low-dose involved field radiation after chemotherapy in advanced Hodgkin disease. A Southwest Oncology Group randomized study. Ann Intern Med. 1994;120(11):903–12. doi: 10.7326/0003-4819-120-11-199406010-00002. [DOI] [PubMed] [Google Scholar]
- 12.Eich HT, Engenhart-Cabillic R, Hansemann K, et al. Quality control of involved field radiotherapy in patients with early-favorable (HD10) and early-unfavorable (HD11) Hodgkin’s lymphoma: an analysis of the German Hodgkin Study Group. Int J Radiat Oncol Biol Phys. 2008;71(5):1419–24. doi: 10.1016/j.ijrobp.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Rischin D, Peters LJ, O’Sullivan B, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol. 2010;28(18):2989–95. doi: 10.1200/JCO.2009.27.4449. [DOI] [PubMed] [Google Scholar]
- 14.Halperin EC, Laurie F, Fitzgerald TJ. An evaluation of the relationship between the quality of prophylactic cranial radiotherapy in childhood acute leukemia and institutional experience: a Quality Assurance Review Center-Pediatric Oncology Group study. Int J Radiat Oncol Biol Phys. 2002;53(4):1001–4. doi: 10.1016/s0360-3016(02)02833-x. [DOI] [PubMed] [Google Scholar]