Abstract
Observations that dopaminergic antagonists are beneficial in bipolar disorder and that dopaminergic agonists can produce mania suggest that bipolar disorder involves excessive dopaminergic transmission. Thus, mood stabilizers used to treat the disease might act in part by downregulating dopaminergic transmission. In agreement, we reported that dopamine D2-like receptor mediated signaling involving arachidonic acid (AA, 20:4n-6) was downregulated in rats chronically treated with lithium. To see whether chronic carbamazepine, another mood stabilizer, did this as well, we injected i.p. saline or the D2-like receptor agonist, quinpirole (1 mg/kg), into unanesthetized rats that had been pretreated for 30 days with i.p. carbamazepine (25 mg/kg/day) or vehicle, and used quantitative autoradiography to measure regional brain incorporation coefficients (k*) for AA, markers of signaling. We also measured brain prostaglandin E2 (PGE2), an AA metabolite. In vehicle-treated rats, quinpirole compared with saline significantly increased k* for AA in 35 of 82 brain regions examined, as well as brain PGE2 concentration. Affected regions belong to dopaminergic circuits and have high D2-like receptor densities. Chronic carbamazepine pretreatment prevented the quinpirole-induced increments in k* and in PGE2. These findings are consistent with the hypothesis that effective mood stabilizers generally downregulate brain AA signaling via D2-like receptors, and that this signaling is upregulated in bipolar disorder.
Keywords: Bipolar disorder, Carbamazepine, Phospholipase A2, D2-like receptors, Quinpirole, Arachidonic acid, Prostaglandin E2, Brain imaging
Introduction
Lithium, valproic acid and carbamazepine (5H-dibenz[b,f]azepine-5-carboxamide) (CBZ) are used to treat mania in bipolar disorder, but whether they have a common mechanism of action is not agreed on [3]. One possibility is that these agents correct a neurotransmission imbalance that contributes to bipolar symptoms. Clinical evidence suggests that excessive or abnormal dopaminergic signaling contributes to this neurotransmission imbalance [13, 28, 31]. Thus, drugs that inhibit dopaminergic transmission (e.g., haloperidol) have an antimanic action in bipolar disorder [20], whereas drugs that stimulate dopamine synthesis (levodopa), bind to dopamine receptors (bromocriptine), or reduce dopamine reuptake (amphetamine), often precipitate mania [1, 40].
Brain signal transduction mediated by dopaminergic D2-like (D2, D3 and D4) receptors can be coupled to the activation of Ca2+-dependent cytosolic phospholipase A2 (cPLA2), to selectively release arachidonic acid (AA, 20:4n-6), from the stereospecifically numbered (sn)-2 position of membrane phospholipid [53]. The signaling process can be imaged in unanesthetized rats by injecting intravenously [1-14C]AA and measuring tracer AA uptake into brain using quantitative autoradiography. Regional brain AA incorporation coefficients k* (brain radioactivity/integrated plasma radioactivity) are calculated and, if multiplied by unlabeled unesterified plasma AA concentrations, are converted to regional incorporation rates Jin that represent regional brain AA consumption. Both k* and Jin are independent of changes in cerebral blood flow [44, 46]. Increments in k* caused by drug reflect the quantity of unesterified AA released and then metabolized to eicosanoids (e.g. prostaglandin E2 (PGE2), thromboxane B2 (TXB2)) and other products [9, 10]. Unesterified AA as well as its eicosanoid metabolites are bioactive and can influence many physiological processes, including membrane excitability, gene transcription, apoptosis, sleep, brain blood flow and behavior [49].
Consistent with inhibition of dopaminergic receptor-mediated signaling by mood stabilizers, we reported that chronic LiCl feeding, sufficient to produce therapeutically relevant plasma and brain lithium concentrations, blocked k* signals caused by administration of the D2-like receptor agonist, quinpirole, to unanesthetized rats [5]. In control animals fed a LiCl-free diet, quinpirole-induced increases in k* are robust and widespread in brain regions within dopaminergic circuits, and can be blocked by pre-treatment with the D2-like receptor antagonists, butaclamol or raclopride [16, 29]. In addition to LiCl, chronic CBZ has been reported to attenuate dopamine function in rats, suggesting that normalization of a dysfunctional dopamine neurotransmission may underlie CBZ effects in bipolar disorder [2, 4, 35, 38].
In the present study, we determined if chronic CBZ administration, like chronic LiCl, would block the k* increments in response to quinpirole, and would influence brain PGE2 or TXB2 concentrations at rest or following drug. We measured these global brain concentrations, as well as k* for AA in 82 brain regions, in unanesthetized rats that had been treated daily for 30 days with i.p. vehicle or CBZ 25 mg/kg, then administered saline (control) or quinpirole (1.0 mg/kg i.v.). The CBZ regimen produces a plasma CBZ concentration of 54 μM, at the high end found in CBZ-treated bipolar patients (51 μM), and decreases AA turnover in brain phospholipids and the brain PGE2 concentration [2, 11, 18, 27]. The quinpirole dose increases k* for AA significantly in brain dopaminergic circuits [5, 15].
Experimental Procedures
Animals and Diets
The study was approved by the National Institutes of Health (NIH) Animal Care and Use Committee in accordance with NIH Guidelines on the Care and Use of Laboratory Animals. Two-month-old male Fischer CDF (F-344)/CrlBR rats (Charles River Laboratories, Wilmington, MA, USA) were acclimatized for 1 week in an animal facility in which temperature, humidity and light cycle were regulated, and had ad libitum access to food (NIH-31 diet, Zeigler, Gardners, PA, USA) and water. The diet contained (as percent of total fatty acids): 20.1% saturated, 22.5% monounsaturated, 47.9% linoleic, 5.1% α-linolenic, 0.02% AA, 2.0% eicosapentaenoic, and 2.3% docosahexaenoic acid.
Drugs
[1-14C]AA in ethanol (53 mCi/mmol, >98% pure, Moravek Biochemicals, Brea, CA, USA) was evaporated and resuspended in HEPES buffer, pH 7.4, containing 50 mg/ml fatty acid-free bovine serum albumin (Sigma-Aldrich, St Louis, MO, USA). CBZ-treated rats received 25 mg/kg intraperitoneally once daily for 30 days (Sigma-Aldrich). The CBZ was dissolved in a 50:50 (v/v) dimethyl sulfoxide (DMSO, ≥99.9% Sigma-Aldrich): saline (0.9% NaCl, Hospira Inc., Lake Forest, IL, USA) mixture and kept at 37°C as described previously [2, 11, 27]. A control group received the same volume of DMSO:saline (vehicle) under parallel conditions. The acute 1 mg/kg i.v. dose of (−)-quinpirole hydrochloride (Sigma-Aldrich), a selective D2-like dopamine receptor agonist [47], was chosen because it does not cause convulsions but produces widespread significant increments in k* for AA in the brain of unanesthetized rats that can be blocked by D2-like receptor antagonists [5, 15].
Surgical Procedures and Tracer Infusion
On the morning of day 30 of chronic treatment, a rat was injected with the last CBZ or vehicle dose and then anesthetized with 2–3% halothane in O2. Polyethylene catheters (PE 50) were inserted into the right femoral artery and vein as described previously [5]. The wound was closed with surgical clips and the rat was wrapped loosely, with its upper body remaining free, in a fast-setting plaster cast taped to a wooden block. Surgery lasted 20–25 min. The rat was allowed to recover from anesthesia for 4 h in an environment maintained at 25°C. Body temperature was maintained at 36.4–37.1°C using a feedback-heating device and rectal thermometer. Arterial blood pressure and heart rate were measured with a blood pressure recorder (CyQ 103/302; Cybersense, Inc., Nicholasville, KY, USA). Arterial blood pH, pO2 and pCO2 were measured with a blood gas analyzer (Model 248, Bayer Health Care, Norwood, MA, USA).
One minute after an i.v. injection of quinpirole or saline, [1-14C]AA (170 μCi/kg) in 2 ml was infused into the femoral vein for 5 min at a rate of 400 μl/min, using an infusion pump (Harvard Apparatus Model 22, Natick, MA, USA). Twenty min after beginning tracer infusion, the rat was killed with an overdose of Nembutal® (100 mg/kg, i.v.) and decapitated. The brain was removed (<30 s), frozen in 2-methylbutane maintained at −40°C with dry ice, and stored at −80°C until sectioned.
Chemical Analysis
Thirteen arterial blood samples were collected before, during and after [1-14C]AA infusion, and were centrifuged immediately (30 s at 18,000g). Total lipids were extracted from 30 μl of plasma with 3 ml chloroform:methanol (2:1, by vol) and 1.5 ml 0.1 M KCl using the Folch procedure [26]. Radioactivity was determined at an efficiency of 88% in 100 μl of the lower organic phase by liquid scintillation counting. As reported, following 5 min [1-14C]AA infusion, 98% of total plasma radioactivity was radiolabeled AA [11]. Concentrations of unesterified fatty acids were determined in 100–150 μl of the frozen arterial plasma. Total lipids were extracted by the method of Folch et al. [26], and were separated by thin layer chromatography on silica gel 60 plates (Whatman, Clifton, NJ, USA) using the solvent system, heptane:diethylether:glacial acetic acid (60:40:3, by vol). Unesterified fatty acids were scraped from the plate and methylated with 1% H2SO4 in anhydrous methanol for 3 h at 70°C. Fatty acid methyl esters were then separated and quantified by gas chromatography using an internal standard, heptadecanoic acid (17:0) [11].
Quantitative Autoradiography
Frozen brains were cut in serial 20-μm thick coronal sections in a cryostat at −20°C. Sections were placed for 5 weeks with calibrated [14C]methylmethacrylate standards on Kodak Ektascan C/RA film (Eastman Kodak Company, Rochester, NY, USA). Brain regions from autoradiographs were identified from a stereotaxic rat brain atlas [39], and were sampled in both hemispheres. The average of bilateral measurements for each region from three consecutive brain sections was used to calculate regional radioactivity (nCi/g of brain) by digital quantitative densitometry, using a Macintosh computer and the public domain NIH Image program 1.62 (developed at the US National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). Regional incorporation coefficients k* (ml plasma/s/g brain) of AA were calculated as [46],
| (1) |
equals plasma radioactivity determined by scintillation counting (nCi/ml), equals brain radioactivity (nCi/g of brain), and t equals time (min) after beginning of [1-14C]AA infusion.
Rates of incorporation of unesterified AA from plasma into brain phospholipids, Jin (fmol/s/g) were calculated as,
| (2) |
where cplasma is the plasma concentration of unlabeled unesterified AA (nmol/ml).
Brain PGE2 and TXB2 Concentrations
In separate experiments, on the morning of day 30, rats received the last injection of CBZ or vehicle, and 3 h and 30 min later were injected i.v. with quinpirole (1 mg/kg) or saline. Twenty-one minutes later, they were anesthetized with Nembutal® (50 mg/kg, i.p.) and subjected to high-energy head-focused microwave irradiation (5.5 kW, 3.8 s; Cober Electronics, Stamford, CT, USA) to stop post-mortem changes, such as formation of prostaglandins and fatty acid release from phospholipid [41]. Half-brains were weighed, homogenized with 18 volumes of hexane:isopropanol (3:2, by vol) using a glass Tenbroeck homogenizer and the homogenate was centrifuged for 5 min at 800g. Tissue residues were rinsed with 3 × 2 vol of the same solvent. The resultant lipid extract was concentrated to dryness under nitrogen and resuspended in the enzyme immunoassay buffer provided with a polyclonal PGE2 or TXB2 kit (Oxford Biochemical Research, Oxford, MI, USA).
Statistical Analyses
An unpaired two-tailed t-test was used to compare mean physiological parameters in CBZ- and vehicle-treated rats, using GraphPad Prism version 4.0b (GraphPad Software, San Diego, CA, www.graphpad.com). A standard two-way ANOVA, comparing CBZ administration (CBZ vs. vehicle) with drug (quinpirole vs. saline) was performed to compare arterial plasma radioactivity input functions, plasma unesterified fatty acid concentrations, brain eicosanoid concentrations and regional values of k* and Jin using SPSS 11.0 (SPSS Inc., Chicago, IL, USA, http://www.spss.com). Where interactions between CBZ and quinpirole were statistically insignificant, probabilities of effects of CBZ and quinpirole were reported. Where interactions were statistically significant, these probabilities were not reported because they cannot be interpreted [51]. Instead, unpaired two-tailed t-tests were used to compare quinpirole and saline responses between CBZ- and vehicle-treated rats as well as saline responses in CBZ-compared with vehicle-treated rats. Other comparisons were not considered relevant. A post-hoc test was not used to avoid a correction for multiple comparisons. However, when a Bonferroni post-hoc test with correction for three comparisons was performed, statistical significance of differences were not changed. Data are reported as means ± SD, with statistical significance taken as P ≤ 0.05.
Results
Physiology, Behavior and Arterial Plasma Radioactivity
At surgery, CBZ-treated rats had a lower mean body weight than vehicle-treated rats, 269 ± 11 g (n = 12) versus 281 ± 11 g (n = 12) (P = 0.02). Quinpirole (1 mg/kg) provoked behavioral cycles, each consisting of an “activity” period (repetitive sniffing, mouth and head-turning) followed by a “calm” period, whereas saline did not obviously affect behavior (Table 1). No significant difference in mean cycling periods was observed in CBZ-treated compared to vehicle-treated rats (Table 1). Compared with saline, quinpirole did not significantly affect arterial pH, pCO2 or pO2, or blood pressure or heart rate (Table 1).
Table 1.
Effects of carbamazepine and quinpirole on physiological parameters
| Vehicle
|
Carbamazepine
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Saline
|
Quinpirole
|
Saline
|
Quinpirole
|
|||||
| Before | After | Before | After | Before | After | Before | After | |
| Body temperature (°C) | 36.5 ± 0.2 | 36.1 ± 0.2 | 36.4 ± 0.3 | 36.6 ± 0.3 | 36.5 ± 0.2 | 36.8 ± 0.4 | 36.5 ± 0.5 | 36.7 ± 0.2 |
| Heart rate (beats/min) | 384 ± 40 | 410 ± 34 | 441 ± 21 | 442 ± 31 | 417 ± 30 | 423 ± 23 | 421 ± 18 | 427 ± 18 |
| Arterial blood pressure (mm Hg) | ||||||||
| Systolic | 149 ± 7 | 152 ± 6 | 160 ± 7 | 154 ± 13 | 142 ± 13 | 144 ± 11 | 142 ± 6 | 149 ± 12 |
| Diastolic | 94 ± 12 | 92 ± 8 | 103 ± 9 | 95 ± 12 | 99 ± 5 | 97 ± 7 | 105 ± 5 | 102 ± 7 |
| pH | 7.461 ± 0.024 | 7.435 ± 0.012 | 7.465 ± 0.021 | 7.451 ± 0.024 | 7.447 ± 0.021 | 7.368 ± 0.182 | 7.462 ± 0.012 | 7.441 ± 0.012 |
| pO2 (mm Hg) | 94.7 ± 12.5 | 97.2 ± 4.7 | 97.1 ± 10.0 | 108 ± 18.0 | 94.4 ± 6.8 | 98.5 ± 3.9 | 99.6 ± 5.0 | 103.2 ± 3.8 |
| pCO2 (mm Hg) | 37.4 ± 3.6 | 42.4 ± 2.2 | 37.2 ± 3.1 | 35.5 ± 6.3 | 39.1 ± 2.6 | 42.4 ± 3.2 | 37.2 ± 2.0 | 39.9 ± 1.8 |
| Orofacial activity duration (s) | ||||||||
| Orofacial activity | 15 ± 4 | 14 ± 3 | ||||||
| Calm period | 34 ± 5 | 36 ± 4 | ||||||
Values are means ± SD (n = 6) measured before and 11 min after saline or quinpirole (1 mg/kg, i.v.) injection. None of these physiological parameters was statistically significantly affected by chronic carbamazepine or quinpirole administration
Following intravenous [1–14C]AA infusion, neither CBZ nor quinpirole modified the integral of plasma radioactivity in the organic fraction, the input function for determining k* in Eq. 1. The mean integral, (nCi × s)/ml (n = 5–6), did not differ significantly between groups: vehicle plus saline, 213,888 ± 28,329; vehicle plus quinpirole, 253,791 ± 32,823; CBZ plus saline, 194,933 ± 15,343; CBZ plus quinpirole, 215,594 ± 14,957.
Plasma Concentrations of Unlabeled Unesterified Fatty Acids
A two-way ANOVA showed statistically insignificant interactions between CBZ and quinpirole with regard to eight of the measured plasma concentrations of unesterified fatty acids, including AA (Table 2). Chronic CBZ compared with vehicle had a negative main effect on oleic, linoleic, α-linolenic and docosahexaenoic acid concentrations (Table 2). Quinpirole did not have a main effect on any concentration.
Table 2.
Effects of quinpirole and carbamazepine on plasma concentrations of unlabeled unesterified fatty acid in rats
| Fatty acid | Vehicle
|
CBZ
|
CBZ × quinpirole interaction P-value |
CBZ effect P-value |
Quinpirole effect P-value |
||
|---|---|---|---|---|---|---|---|
| Saline (n = 5) |
Quinpirole (n = 6) |
Saline (n = 6) | Quinpirole (n = 6) |
||||
| Concentration, nmol/ml plasma | |||||||
| Palmitic (16:0) | 213.1 ± 47.2 | 247.4 ± 101.4 | 219.3 ± 81.1 | 142.2 ± 33.3 | 0.080 | 0.117 | 0.487 |
| Palmitoleic (16:1 n–7) | 20.5 ± 5.5 | 26.5 ± 11.7 | 19.2 ± 10.4 | 14.9 ± 7.6 | 0.202 | 0.115 | 0.831 |
| Stearic (18:0) | 37.8 ± 6.9 | 37.7 ± 10.5 | 40.5 ± 5.9 | 35.9 ± 2.6 | 0.456 | 0.882 | 0.430 |
| Oleic (18:1 n–9) | 104.9 ± 24.0 | 117.3 ± 52.0 | 90.7 ± 18.7 | 71.1 ± 21.0 | 0.250 | 0.038 | 0.790 |
| Linoleic (18:2 n–6) | 225.9 ± 52.3 | 277.9 ± 110.7 | 158.4 ± 36.6 | 125.2 ± 34.9 | 0.144 | 0.001 | 0.740 |
| α-Linolenic (18:3 n–3) | 14.1 ± 3.4 | 18.8 ± 7.9 | 8.0 ± 2.3 | 7.1 ± 3.3 | 0.183 | <0.001 | 0.354 |
| Arachidonic (20:4 n–6) | 17.2 ± 3.3 | 22.1 ± 9.1 | 16.2 ± 3.4 | 14.4 ± 2.1 | 0.150 | 0.067 | 0.497 |
| Docosahexaenoic (22:6 n–3) | 36.2 ± 5.5 | 33.0 ± 15.4 | 21.8 ± 8.1 | 17.9 ± 2.7 | 0.931 | 0.001 | 0.375 |
Values are mean ± SD measured from arterial plasma collected at 19 min after the beginning of [1-14C]AA infusion
Regional Brain AA Incorporation Coefficients, k*
Figure 1 presents coronal autoradiographs of brains from rats treated chronically with vehicle or CBZ, then injected acutely with either saline or quinpirole. It illustrates that quinpirole increased k* for AA in multiple brain regions in the vehicle- but not CBZ-pretreated rats. Data from such autoradiographs were collated and analyzed in Table 3.
Fig. 1.
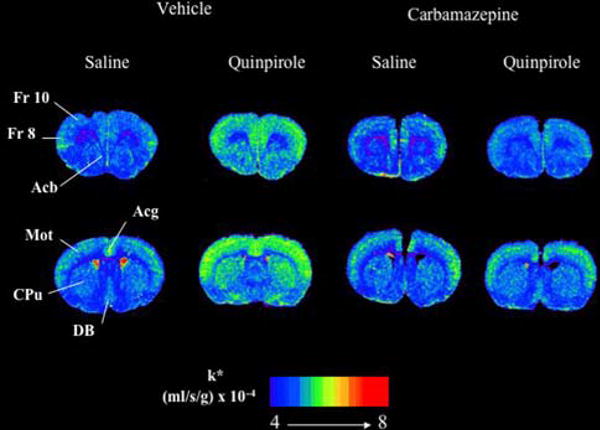
Coronal autoradiographs of brains showing effects of quinpirole and carbamazepine on regional AA incorporation coefficients k* in rats. Values of k* (ml/s/g brain × 10−4) are given on a color scale from 4 (blue) to 8 (yellow-orange). Abbreviations: Acb, nucleus accumbens; Acg, anterior cingulate cortex; CPu, caudate-putamen; DB, diagonal band; Fr 8, frontal cortex area 8; Fr 10, frontal cortex area 10; Mot, motor cortex. Note: For interpretation of the references to color in this figure legend, the reader is referred to the online version of this article
Table 3.
Effect of chronic carbamazepine on quinpirole-induced regional AA incorporation coefficients, k* in rat brain
| Brain region | Vehicle
|
Carbamazepine (CBZ)
|
CBZ × Quinpirole Interaction P-value |
CBZ Effect P-value |
CBZ Effect P-value |
||
|---|---|---|---|---|---|---|---|
| Saline (n = 5) | Quinpirole (n = 6) |
Saline (n = 6) |
Quinpirole (n = 6) |
||||
| Prefrontal cortex layer I | 5.03 ± 0.75 | 5.36 ± 0.66 | 5.12 ± 0.36 | 5.38 ± 0.25 | 0.302 | 0.523 | 0.745 |
| Prefrontal cortex layer IV | 5.42 ± 0.77 | 6.42 ± 0.62* | 5.48 ± 0.52 | 5.86 ± 0.52 | 0.005 | ||
| Primary olfactory cortex | 4.50 ± 0.31 | 5.24 ± 0.82 | 4.33 ± 0.61 | 4.40 ± 0.35 | 0.020 | ||
| Frontal cortex (10) | |||||||
| Layer I | 4.86 ± 0.28 | 5.66 ± 0.40** | 4.20 ± 0.63 | 3.62 ± 0.36 | 0.002 | ||
| Layer IV | 5.34 ± 0.50 | 6.68 ± 0.67** | 4.71 ± 0.69 | 3.99 ± 0.54 | 0.001 | ||
| Frontal cortex (8) | |||||||
| Layer I | 5.00 ± 0.28 | 6.15 ± 0.50** | 4.35 ± 0.55 | 3.99 ± 0.54 | 0.001 | ||
| Layer IV | 5.52 ± 0.66 | 7.52 ± 0.76** | 4.76 ± 0.50 | 4.36 ± 0.70 | <0.001 | ||
| Pyriform cortex | 4.50 ± 0.55 | 4.30 ± 0.76 | 4.13 ± 0.15 | 4.10 ± 0.32 | 0.697 | 0.584 | 0.688 |
| Anterior cingulate cortex | 5.83 ± 0.76 | 6.79 ± 0.41** | 5.32 ± 0.51 | 5.46 ± 0.53 | 0.003 | ||
| Motor cortex | |||||||
| Layer I | 5.09 ± 0.56 | 6.18 ± 0.67* | 5.26 ± 0.29 | 5.12 ± 0.33 | 0.007 | ||
| Layer II–III | 4.71 ± 0.21 | 6.26 ± 0.78** | 4.30 ± 0.37 | 4.93 ± 0.32 | <0.001 | ||
| Layer IV | 5.18 ± 0.45 | 7.45 ± 1.16** | 4.35 ± 0.42 | 4.71 ± 0.31 | 0.001 | ||
| Layer V | 4.30 ± 0.32 | 5.34 ± 0.78* | 3.97 ± 0.36 | 3.66 ± 0.65 | 0.011 | ||
| Layer VI | 4.02 ± 0.21 | 5.43 ± 0.61*** | 3.72 ± 0.59 | 3.28 ± 0.23 | <0.001 | ||
| Somatosensory cortex | |||||||
| Layer I | 5.10 ± 0.49 | 6.18 ± 0.96* | 4.81 ± 0.44 | 4.31 ± 0.63 | 0.048 | ||
| Layer II–III | 5.60 ± 0.77 | 7.36 ± 0.89** | 4.93 ± 0.68 | 4.19 ± 0.34* | 0.001 | ||
| Layer IV | 5.72 ± 0.85 | 9.41 ± 1.33*** | 4.83 ± 0.47 | 4.30 ± 0.60 | <0.001 | ||
| Layer V | 5.16 ± 0.38 | 7.28 ± 0.85*** | 4.63 ± 0.56 | 4.02 ± 0.52 | <0.001 | ||
| Layer VI | 4.71 ± 0.23 | 7.32 ± 0.73*** | 4.13 ± 0.42 | 3.77 ± 0.42 | <0.001 | ||
| Auditory cortex | |||||||
| Layer I | 5.54 ± 0.51 | 6.59 ± 0.69* | 4.97 ± 0.41 | 4.74 ± 0.38 | 0.037 | ||
| Layer IV | 6.21 ± 0.45 | 7.62 ± 1.12* | 5.59 ± 0.55 | 4.91 ± 0.36* | <0.001 | ||
| Layer VI | 5.43 ± 0.28 | 6.02 ± 0.90 | 4.92 ± 0.45 | 4.46 ± 0.36 | 0.037 | ||
| Visual cortex | |||||||
| Layer I | 5.25 ± 0.30 | 5.51 ± 0.67 | 4.81 ± 0.37 | 4.69 ± 0.27 | 0.302 | 0.003 | 0.703 |
| Layer IV | 5.64 ± 0.50 | 6.74 ± 0.85* | 4.99 ± 0.47 | 4.55 ± 0.35 | 0.003 | ||
| Layer VI | 5.32 ± 0.24 | 5.55 ± 0.68 | 4.74 ± 0.48 | 4.76 ± 0.55 | 0.799 | 0.021 | 0.427 |
| Preoptic area (LPO/MPO) | 4.33 ± 0.24 | 4.56 ± 0.68 | 4.65 ± 0.42 | 4.76 ± 0.55 | 0.801 | 0.512 | 0.478 |
| Suprachiasmatic nu | 4.94 ± 0.35 | 4.34 ± 0.66 | 4.30 ± 0.52 | 4.29 ± 0.56 | 0.217 | 0.148 | 0.195 |
| Globus pallidus | 3.94 ± 0.99 | 4.62 ± 0.45 | 3.88 ± 0.29 | 4.16 ± 0.57 | 0.442 | 0.307 | 0.074 |
| Bed nu stria terminalis | 4.34 ± 0.44 | 4.60 ± 0.41 | 4.11 ± 0.48 | 4.29 ± 0.45 | 0.319 | 0.054 | 0.064 |
| Olfactory tubercle | 4.52 ± 0.32 | 5.28 ± 0.52 | 4.26 ± 0.42 | 4.31 ± 0.47 | 0.072 | 0.004 | 0.044 |
| Diagonal band dorsal | 4.66 ± 0.21 | 5.39 ± 0.84 | 4.56 ± 0.48 | 4.01 ± 0.38 | 0.011 | ||
| Ventral | 4.54 ± 0.22 | 5.40 ± 0.67* | 4.14 ± 0.45 | 4.09 ± 0.52 | 0.042 | ||
| Amygdala basolateral/medial | 5.18 ± 0.33 | 4.82 ± 0.65 | 4.64 ± 0.48 | 4.55 ± 0.29 | 0.490 | 0.052 | 0.262 |
| Hippocampus | |||||||
| CA1 | 4.29 ± 0.38 | 4.20 ± 0.58 | 3.77 ± 0.31 | 3.76 ± 0.39 | 0.835 | 0.014 | 0.773 |
| CA2 | 4.22 ± 0.41 | 4.57 ± 0.65 | 4.05 ± 0.45 | 4.19 ± 0.60 | 0.666 | 0.244 | 0.290 |
| CA3 | 4.32 ± 0.35 | 4.41 ± 0.49 | 4.03 ± 0.28 | 4.15 ± 0.49 | 0.745 | 0.301 | 0.456 |
| Dentate gyrus | 4.62 ± 0.27 | 5.21 ± 0.95 | 4.45 ± 0.25 | 4.50 ± 0.38 | 0.588 | 0.245 | 0.055 |
| SLM | 5.89 ± 0.70 | 5.76 ± 1.06 | 5.24 ± 0.28 | 5.28 ± 0.61 | 0.773 | 0.077 | 0.884 |
| Accumbens nucleus | 4.92 ± 0.24 | 6.79 ± 0.89** | 4.66 ± 0.38 | 4.18 ± 0.28 | 0.001 | ||
| Caudate putamen | |||||||
| Dorsal | 4.66 ± 0.30 | 5.63 ± 0.57** | 4.24 ± 0.42 | 4.16 ± 0.30 | 0.007 | ||
| Ventral | 4.81 ± 0.26 | 5.89 ± 0.59** | 4.44 ± 0.35 | 4.31 ± 0.35 | 0.002 | ||
| Lateral | 4.72 ± 0.32 | 6.20 ± 0.70** | 4.29 ± 0.40 | 4.14 ± 0.25 | <0.001 | ||
| Medial | 4.83 ± 0.37 | 6.19 ± 0.54** | 4.27 ± 0.38 | 4.16 ± 0.26 | <0.001 | ||
| Septal nu lateral | 4.68 ± 0.48 | 4.37 ± 0.57 | 4.05 ± 0.34 | 4.10 ± 0.48 | 0.360 | 0.034 | 0.496 |
| Septal nu medial | 5.02 ± 0.35 | 6.42 ± 0.60** | 4.65 ± 0.31 | 4.40 ± 0.25 | <0.001 | ||
| Habenular nu lateral | 6.61 ± 0.40 | 7.39 ± 0.56 | 6.23 ± 0.22 | 6.34 ± 0.50 | 0.089 | 0.001 | 0.026 |
| Habenular nu medial | 6.96 ± 0.26 | 7.59 ± 0.61 | 6.48 ± 0.37 | 6.78 ± 0.93 | 0.469 | 0.010 | 0.050 |
| Lateral geniculate nu dorsal | 5.83 ± 0.26 | 7.39 ± 1.48 | 5.32 ± 0.29 | 5.58 ± 0.52 | 0.075 | 0.003 | 0.017 |
| Medial geniculate nu | 6.27 ± 0.51 | 6.28 ± 0.89 | 6.57 ± 0.31 | 6.65 ± 0.23 | 0.883 | 0.745 | 0.836 |
| Thalamus | |||||||
| Ventroposterior lateral nu | 4.77 ± 0.42 | 5.44 ± 0.23 | 4.60 ± 0.44 | 4.37 ± 0.32 | 0.175 | 0.001 | 0.009 |
| Ventroposterior medial nu | 5.56 ± 0.24 | 6.16 ± 0.41* | 5.27 ± 0.42 | 5.31 ± 0.29 | 0.041 | ||
| Paratenial nu | 4.81 ± 0.55 | 5.98 ± 1.09 | 4.23 ± 0.37 | 4.27 ± 0.29 | 0.055 | 0.001 | 0.042 |
| Anteroventral nu | 7.39 ± 1.17 | 8.44 ± 1.11 | 6.87 ± 0.85 | 6.95 ± 0.67 | 0.381 | 0.236 | 0.301 |
| Anteromedial nu | 4.83 ± 0.21 | 6.14 ± 0.89* | 4.39 ± 0.51 | 4.14 ± 0.65 | 0.008 | ||
| Reticular nu | 5.29 ± 0.69 | 5.45 ± 1.10 | 5.28 ± 0.31 | 5.64 ± 0.75 | 0.766 | 0.311 | 0.432 |
| Paraventricular nu | 4.54 ± 0.91 | 4.35 ± 0.77 | 3.94 ± 0.47 | 3.83 ± 0.56 | 0.107 | 0.546 | 0.216 |
| Parafascicular nu | 3.83 ± 0.59 | 4.10 ± 0.45 | 4.22 ± 0.86 | 4.12 ± 0.59 | 0.506 | 0.467 | 0.753 |
| Subthalamic nu | 5.70 ± 1.43 | 5.26 ± 0.58 | 5.58 ± 0.52 | 5.85 ± 0.38 | 0.232 | 0.453 | 0.914 |
| Hypothalamus | |||||||
| Supraoptic nu | 4.11 ± 0.62 | 4.09 ± 0.60 | 3.51 ± 0.33 | 3.97 ± 0.57 | 0.299 | 0.421 | 0.338 |
| Lateral | 3.67 ± 0.56 | 3.78 ± 0.61 | 3.42 ± 0.24 | 3.10 ± 0.42 | 0.299 | 0.325 | 0.609 |
| Anterior | 3.63 ± 0.74 | 3.25 ± 0.26 | 3.42 ± 0.19 | 2.95 ± 0.53 | 0.835 | 0.523 | 0.589 |
| Periventricular | 2.59 ± 0.48 | 2.99 ± 0.42 | 2.79 ± 0.14 | 2.49 ± 0.38 | 0.758 | 0.458 | 0.562 |
| Arcuate | 3.00 ± 0.51 | 3.42 ± 0.38 | 3.44 ± 0.32 | 3.83 ± 0.32 | 0.914 | 0.658 | 0.745 |
| Ventromedial | 3.52 ± 0.57 | 4.00 ± 0.51 | 3.26 ± 0.26 | 3.36 ± 0.25 | 0.251 | 0.569 | 0.645 |
| Posterior | 4.72 ± 0.91 | 4.70 ± 0.84 | 4.70 ± 0.54 | 4.71 ± 0.42 | 0.965 | 0.982 | 0.859 |
| Mammillary nu | 4.04 ± 0.44 | 4.45 ± 0.23 | 3.90 ± 0.51 | 4.15 ± 0.44 | 0.857 | 0.759 | 0.589 |
| Interpeduncular nu | 7.96 ± 0.83 | 8.07 ± 0.51 | 7.32 ± 0.34 | 7.61 ± 0.65 | 0.2456 | 0.489 | 0.357 |
| Substantia nigra | 4.28 ± 0.75 | 5.21 ± 0.42* | 4.21 ± 0.90 | 3.95 ± 0.69 | 0.008 | ||
| Pretectal area | 4.77 ± 1.60 | 6.08 ± 0.94 | 4.55 ± 0.53 | 4.58 ± 0.47 | 0.124 | 0.053 | 0.105 |
| Grey layer superior colliculus | 5.77 ± 0.26 | 5.41 ± 0.38 | 4.97 ± 0.47 | 5.20 ± 0.38 | 0.086 | 0.006 | 0.661 |
| Superior colliculus | 5.57 ± 1.17 | 5.11 ± 0.47 | 4.97 ± 1.10 | 4.99 ± 1.00 | 0.558 | 0.382 | 0.581 |
| Inferior colliculus | 7.32 ± 0.26 | 7.85 ± 0.78 | 7.24 ± 0.30 | 7.51 ± 0.51 | 0.857 | 0.569 | 0.645 |
| Flocculus | 5.63 ± 0.33 | 5.89 ± 0.51 | 5.39 ± 0.35 | 5.22 ± 0.40 | 0.179 | 0.459 | 0.768 |
| Cerebellar gray matter | 5.03 ± 0.97 | 5.61 ± 1.11 | 5.09 ± 0.35 | 5.17 ± 0.79 | 0.493 | 0.645 | 0.368 |
| Molecular layer cerebellar gray | 7.10 ± 0.51 | 7.65 ± 0.51 | 6.52 ± 0.47 | 6.49 ± 1.02 | 0.458 | 0.485 | 0.414 |
| White matter | |||||||
| Corpus callosum | 3.38 ± 0.45 | 3.39 ± 0.45 | 3.11 ± 0.46 | 2.95 ± 0.34 | 0.630 | 0.061 | 0.695 |
| Zona incerta | 3.69 ± 0.50 | 3.53 ± 0.32 | 3.47 ± 0.63 | 3.55 ± 0.27 | 0.530 | 0.612 | 0.848 |
| Internal capsule | 2.65 ± 0.25 | 2.84 ± 0.38 | 2.42 ± 0.48 | 2.69 ± 0.41 | 0.819 | 0.259 | 0.176 |
| Cerebellar white matter | 3.04 ± 0.21 | 2.73 ± 0.25 | 2.77 ± 0.56 | 3.03 ± 0.43 | 0.546 | 0.569 | 0.651 |
| Non-blood-brain barrier regions | |||||||
| Subfornical organ | 5.85 ± 0.19 | 5.77 ± 0.34 | 5.49 ± 0.31 | 5.24 ± 0.42 | 0.551 | 0.311 | 0.238 |
| Median eminence | 4.53 ± 0.99 | 5.02 ± 0.51 | 4.87 ± 0.51 | 4.80 ± 0.74 | 0.347 | 0.489 | 0.845 |
| Choroid plexus | 20.9 ± 3.25 | 19.6 ± 2.01 | 19.1 ± 1.69 | 19.0 ± 2.03 | 0.477 | 0.333 | 0.480 |
Abbreviations: nu, nucleus; k* = (ml/s/g) × 10−4. Each k* value is a mean ± SD
Main effects are not reported if statistically significant CBZ × quinpirole interaction, when unpaired t-tests were performed.
P < 0.05;
P < 0.01;
P < 0.001; Vehicle plus quinpirole versus vehicle plus saline, CBZ plus saline versus vehicle plus saline, and CBZ plus quinpirole versus CBZ plus saline
Quinpirole Administration in Vehicle-Treated Rats
Mean AA incorporation coefficients, k*, determined in each of 82 brain regions, were subjected to a two-way ANOVA (Table 3). Statistically significant interactions between quinpirole and CBZ were found in 30 regions. In 29 of these, t-tests showed that quinpirole compared with saline significantly increased k* in the vehicle-treated rats. The regions, many of which belong to dopamine circuits [21], include prefrontal layer IV (18%), frontal 10 and 8 (16–36%), anterior cingulate (16%), motor (21–44%), somatosensory (21–65%), auditory (19–23%) and visual layer IV cortical areas (20%), diagonal band ventral (19%), nucleus accumbens (38%), caudate-putamen (21–31%), medial septal nuclei (28%), 2 regions of the thalamus (11–27%), and the substantia nigra (22%).
Quinpirole also significantly increased k* for AA in 6 regions having statistically insignificant CBZ × quinpirole interactions—lateral and medial habenular nuclei (12% and 9%, respectively), dorsal lateral geniculate nucleus (27%), ventroposterior thalamus nucleus lateral (14%), paratenial thalamus nucleus (24%), and olfactory tubercle (17%). In total, then, 35 brain regions were significantly activated by quinpirole in vehicle-treated control rats. The pattern of significant activations is illustrated in a sagittal representation of the brain in Fig. 2a.
Fig. 2.
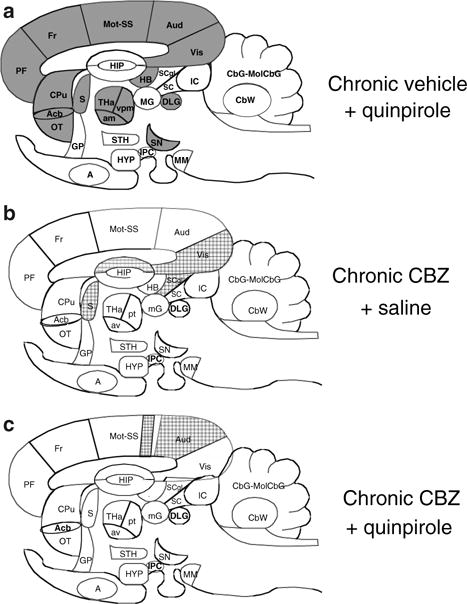
Difference patterns of k* responses to quinpirole and carbamazepine in sagittal representation of rat brain. Regions in which k* was increased significantly (P < 0.05) compared with chronic vehicle + saline are solid black, regions in which k* was decreased significantly are hatched. List of regions: A, amygdala; Acb, nucleus accumbens; Aud, auditory cortex; av, anteroventral thalamic nucleus; CbG, cerebellar gray matter; CbW, crebellar white matter; CPu, caudate putamen; DLG, dorsal lateral geniculate nucleus; Fr, frontal cortex; GP, globus pallidus; HB, habenular nuclei; HIP, hippocampus; HYP, hypothalamus; IC, inferior colliculus; IPC, interpeduncular nucleus; MM, mammillary nucleus; mG, medial geniculate nucleus; MolCBG, molecular layer of cerebellar gray matter; Mot, motor cortex; OT, olfactory tubercle; PF, prefrontal cortex; pt, paratenial thalamic nucleus; SN, substantia nigra; S, septum; SS, somatosensory cortex; SC, superior colliculus; SCgl, gray layer of superior colliculus; STH, subthalamic nucleus; THa, thalamus; Vis, visual cortex
Effects of Chronic CBZ Administration at Baseline
In the 30 regions in which CBZ × quinpirole interactions were statistically significant, t-tests showed that chronic CBZ did not significantly change mean baseline (acute saline) k* in any region. Where CBZ × quinpirole interactions were statistically insignificant, chronic CBZ reduced k* in 4 regions: visual cortex layer VI (−11%), hippocampus CA1 (−12%), lateral septal nucleus (−13%) and grey layer of the superior colliculus (−14%) (Table 3, Fig. 2b).
Effects of Quinpirole in Chronic CBZ-Treated Rats
Of the 30 regions in which CBZ × quinpirole interactions were statistically significant, quinpirole compared with saline reduced k* in somatosensory cortex layer II–III (−15%) and auditory cortex layer IV (−12%) (Fig. 2c). In the 6 regions in which CBZ × quinpirole interactions were statistically insignificant and in which quinpirole had a significant effect in vehicle-treated rats, chronic CBZ had a main effect by preventing the quinpirole-induced k* increments (Table 3).
Regional Rates of Incorporation of Unlabeled Unesterified AA into Brain
Baseline (following saline)- and quinpirole-induced regional values of Jin were calculated by Eq. 2 (data not shown). Because the mean plasma concentration of unlabeled unesterified AA did not differ significantly between chronic CBZ- and vehicle-treated rats (Table 2), baseline differences and percent changes in Jin corresponded to the differences and percent changes in respective values of k* (Table 3). In vehicle-treated rats, baseline values of Jin ranged from 4.5 fmol/s/g in the internal capsule to 36.1 fmol/s/g in the choroid plexus. In CBZ-treated rats, no baseline value of Jin differed significantly from its respective value in vehicle-treated rats; Jin ranged from 3.9 fmol/s/g in the periventricular of the hypothalamus to 30.9 fmol/s/g in the choroid plexus. As noted above, Jin increments following quinpirole in the vehicle-treated rats did not differ significantly from respective increments the CBZ-treated rats (data not shown).
Brain PGE2 and TXB2 Concentrations
A two-way ANOVA demonstrated both significant and insignificant interactions between CBZ and quinpirole with regard to brain PGE2 and TXB2 (Table 4). Consequent t-tests showed that chronic CBZ decreased the basal PGE2 concentration by 25% (P = 0.048). Acute quinpirole increased brain PGE2 by 67% (P = 0.011) in vehicle-treated rats, whereas chronic CBZ prevented this increase. CBZ and quinpirole had main effects on TXB2 (Table 4). Chronic CBZ decreased the basal TXB2 concentration by 35%. Quinpirole reduced the TXB2 concentration by 23% in vehicle-treated rats but had no effect in the CBZ-treated rats (Table 4).
Table 4.
Effect of quinpirole on brain PGE2 and TXB2 concentrations in vehicle- and CBZ-treated rats
| Vehicle
|
CBZ
|
CBZ × quinpirole interaction P-value |
CBZ effect P-value |
Quinpirole effect P-value |
|||
|---|---|---|---|---|---|---|---|
| Saline | Quinpirole | Saline | Quinpirole | ||||
| PGE2 (ng/g brain) | 13.8 ± 2.3 | 23.1 ± 4.5* | 10.4 ± 1.5* | 9.8 ± 0.6 | 0.003 | ||
| TXB2 (pg/g brain) | 60.0 ± 2.6 | 46.3 ± 9.1 | 39.0 ± 8.9 | 33.0 ± 1.1 | 0.261 | <0.001 | 0.011 |
Each value is a mean ± SD (n = 4).
P < 0.05; Vehicle plus quinpirole versus vehicle plus saline, CBZ plus saline versus vehicle plus saline, and CBZ plus quinpirole versus CBZ plus saline
Discussion
Chronic administration of CBZ, sufficient to produce a plasma CBZ concentration therapeutically relevant to bipolar disorder, blocked the increments in k* for AA and in whole brain PGE2 and TXB2 concentrations that were produced in chronic vehicle-treated rats injected with quinpirole. Chronic CBZ by itself reduced k* in four regions as well as global brain concentrations of PGE2 and TXB2.
The effects in rats of chronic CBZ on baseline brain AA cascade markers, and on quinpirole-induced changes in these markers, are comparable to those produced by chronic LiCl feeding [5]. For example, chronic LiCl like chronic CBZ blocked quinpirole-induced increments in k* for AA (we have not as yet examined lithium’s ability to block the PGE2 increment following quinpirole). Both chronic LiCl and CBZ reduced AA turnover in rat brain phospholipids, brain mRNA, protein and activity levels of cPLA2, and the DNA-binding capacity and protein level of a cPLA2 transcription factor, activator protein-2 [11, 19, 27, 42, 43, 45]. These observations, plus clinical data that dopaminergic neurotransmission is disturbed in bipolar disorder [13, 28, 31], and that dopamine receptor antagonists can be therapeutic whereas drugs that stimulate dopamine synthesis, bind to dopamine receptors or reduce dopamine reuptake often precipitate mania (see “Introduction”), suggest that mood stabilizers are therapeutic in bipolar disorder in part by suppressing excessive D2-like receptor signaling involving AA.
CBZ could have downregulated the D2-like receptor-initiated AA signal by reducing synaptic dopamine release and synthesis, D2 receptor density, D2-like coupled Gαo/i, D2-like receptor phosphorylation, or histone deacetylation by histone deacetylase [2, 14, 32, 34, 35, 38]. CBZ also could have altered G-protein receptor kinase translocation from cytosol to cell membrane, and thus densitiztion of D2-like receptors [24]. CBZ’s ability to reduce rat brain cPLA2 transcription and COX activity also could have contributed to the reduced signaling, associated with reduced basal PGE2 and TXB2 concentrations and reduced quinpirole-induced changes in these concentrations [8, 27]. PGE2 and TXB2 are converted preferentially from AA by COX-2 and COX-1, respectively. When these enzymes are pharmacologically inhibited or knocked out in rodent models, k* responses to drugs acting at cPLA2-coupled neuroreceptors are reduced or lost, as are the increments in brain PGE2 and/or TXB2 concentrations [9, 10]. Our finding that quinpirole elevated brain PGE2 in vehicle-treated rats agrees with prior in vitro and in vivo observations [23]. The mechanism for the reduction of brain TXB2 by quinpirole is not apparent but might be elucidated by studying the drug effect on COX-1 and thromboxane synthase expression in brain.
The baseline values of k* for AA in vehicle-treated rats, which ranged from 2.65 to 20.9 × 10−4 ml/s/g brain, are similar to previously reported values [5, 6, 8, 10, 15]. Quinpirole significantly increased k* in 35 regions, many of which belong to dopaminergic circuits containing D2 receptors (e.g. caudate-putamen and substantia nigra) [33], D3 receptors (e.g. nucleus accumbens and olfactory tubercle) [50] or D4 receptors (e.g. cerebral cortex) [55]. Giving selective D2, D3 and D4 agonists or antagonists might identify the contributions of the different receptor subtypes to the k* signal. Furthermore, regional baseline values of Jin in vehicle-treated rats, 4.5–36.1 fmol/s/g, agree with a published global value of 6.57 fmol/s/g [11]. Given that Jin represents the regional rate of metabolic AA loss from brain [10, 44], our data on Jin indicate comparable baseline rates of AA loss in vehicle- and CBZ-treated rats.
Chronic CBZ, unlike chronic lithium [5, 12] did not prevent the quinpirole induced hyperactivity or stereotypy (Table 1). Chronic CBZ or chronic valproate also do not affect quinpirole-induced locomotor activity [48]. As each of the three anti-bipolar agents downregulates the brain AA cascade, their different effects on quinpirole-induced behaviors suggest that these behaviors don’t involve AA signaling and, moreover, that the quinpirole-induced activity cycles are not modeling bipolar disorder [48].
In addition to attenuating D2-like receptor-mediated AA signaling, chronic lithium, CBZ and valproic acid [6–8] each attenuates AA signaling mediated by glutamatergic N-methyl-D-aspartate (NMDA) receptors in unanesthetized rats [6, 8]. As D2-like and NMDA receptors are often functionally coupled and co-localized on the same neurons in brain [52, 54], these data suggest that mood stabilizers that are effective against mania suppress AA signaling coupled to both D2-like and NMDA receptors. In this regard lamotrigine, which is preferred for treating bipolar depression and rapid recycling, is considered to act in part by reducing presynaptic glutamate release [22]. A role for both receptor subtypes is consistent with evidence of disturbed dopaminergic and NMDA transmission in bipolar disorder [1, 13, 20, 28, 31, 36, 37, 40].
It now is possible to measure k* for AA in the human brain with positron emission tomography following the intravenous injection of [1-11C]AA [25]. Thus, it would be of interest to see if our findings in rats can be extrapolated to bipolar disorder patients off and on treatment with mood stabilizers, by giving them dopamine receptor agonists to stimulate the AA signal, such as apomorphine or ropinerole [17, 30, 56].
In conclusion, chronic CBZ blocked the increments in k* for AA as well in the global brain PGE2 concentration seen in response to quinpirole in chronic-vehicle treated rats. Those and related observations regarding chronic lithium and valproic acid support the hypothesis that mood stabilizers of proven efficacy against bipolar disorder may act by downregulating brain AA signaling coupled to both D2-like and NMDA receptors.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Abbreviations
- AA
Arachidonic acid
- PLA2
Phospholipase A2
- cPLA2
Cytosolic PLA2
- sn
Stereospecifically numbered
- NMDA
N-methyl-D-aspartate
- CBZ
Carbamazepine
- PGE2
Prostaglandin E2
- TXB2
Thromboxane B2
- COX
Cyclooxygenase
Footnotes
None of the authors has a financial or other conflict of interest related to this work.
References
- 1.Anand A, Verhoeff P, Seneca N, et al. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry. 2000;157:1108–1114. doi: 10.1176/appi.ajp.157.7.1108. [DOI] [PubMed] [Google Scholar]
- 2.Baf MH, Subhash MN, Lakshmana KM, et al. Alterations in monoamine levels in discrete regions of rat brain after chronic administration of carbamazepine. Neurochem Res. 1994;19:1139–1143. doi: 10.1007/BF00965147. [DOI] [PubMed] [Google Scholar]
- 3.Barchas J, Hamblin M, Malenka R. Biochemical hypotheses of mood and anxiety disorders. In: Siegel GJ, Agranoff BW, Albers RW, Molinoff PB, editors. Basic neurochemistry. 5th. Raven Press; New York: 1994. pp. 979–1001. [Google Scholar]
- 4.Barros HM, Leite JR. Effects of acute and chronic carbamazepine administration on apomorphine-elicited stereotypy. Eur J Pharmacol. 1986;123:345–349. doi: 10.1016/0014-2999(86)90707-7. [DOI] [PubMed] [Google Scholar]
- 5.Basselin M, Chang L, Bell JM, et al. Chronic lithium chloride administration to unanesthetized rats attenuates brain dopamine D2-like receptor-initiated signaling via arachidonic acid. Neuropsychopharmacology. 2005;30:1064–1075. doi: 10.1038/sj.npp.1300671. [DOI] [PubMed] [Google Scholar]
- 6.Basselin M, Chang L, Bell JM, et al. Chronic lithium chloride administration attenuates brain NMDA receptor-initiated signaling via arachidonic acid in unanesthetized rats. Neuropsychopharmacology. 2006;31:1659–1674. doi: 10.1038/sj.npp.1300920. [DOI] [PubMed] [Google Scholar]
- 7.Basselin M, Chang L, Chen M, et al. Chronic valproic acid reduces NMDA receptor-initiated signaling via arachidonic acid in rat brain (Abstract) J Neurochem. 2007;102(Suppl 1):146. [Google Scholar]
- 8.Basselin M, Villacreses NE, Chen M, et al. Chronic carbamazepine administration reduces NMDA receptor-initiated signaling via arachidonic acid in rat brain. Biol Psychiatry. 2007;62:934–943. doi: 10.1016/j.biopsych.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basselin M, Villacreses NE, Langenbach R, et al. Resting and arecoline-stimulated brain metabolism and signaling involving arachidonic acid are altered in the cyclooxygenase-2 knockout mouse. J Neurochem. 2006;96:669–679. doi: 10.1111/j.1471-4159.2005.03612.x. [DOI] [PubMed] [Google Scholar]
- 10.Basselin M, Villacreses NE, Lee HJ, et al. Flurbiprofen, a cyclooxygenase inhibitor, reduces the brain arachidonic acid signal in response to the cholinergic muscarinic agonist, arecoline, in awake rats. Neurochem Res. 2007;32:1857–1867. doi: 10.1007/s11064-007-9372-3. [DOI] [PubMed] [Google Scholar]
- 11.Bazinet RP, Rao JS, Chang L, et al. Chronic carbamazepine decreases the incorporation rate and turnover of arachidonic acid but not docosahexaenoic acid in brain phospholipids of the unanesthetized rat: relevance to bipolar disorder. Biol Psychiatry. 2006;59:401–407. doi: 10.1016/j.biopsych.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Beaulieu JM, Sotnikova TD, Yao WD, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berk M, Dodd S, Kauer-Sant’anna M, et al. Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr Scand. 2007;(Suppl):41–49. doi: 10.1111/j.1600-0447.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 14.Beutler AS, Li S, Nicol R, et al. Carbamazepine is an inhibitor of histone deacetylases. Life Sci. 2005;76:3107–3115. doi: 10.1016/j.lfs.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharjee AK, Chang L, Lee HJ, et al. D2 but not D1 dopamine receptor stimulation augments brain signaling involving arachidonic acid in unanesthetized rats. Psychopharmacology (Berl) 2005;180:735–742. doi: 10.1007/s00213-005-2208-4. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharjee AK, Chang L, White L, et al. D-amphetamine stimulates D2 dopamine receptor-mediated brain signaling involving arachidonic acid in unanesthetized rats. J Cereb Blood Flow Metab. 2006;26:1378–1388. doi: 10.1038/sj.jcbfm.9600290. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharjee AK, Chang L, White L, et al. Imaging apomorphine stimulation of brain arachidonic acid signaling via D2-like receptors in unanesthetized rats. Psychopharmacology. doi: 10.1007/s00213-008-1073-3. [DOI] [PubMed] [Google Scholar]
- 18.Bialer M, Levy RH, Perucca E. Does carbamazepine have a narrow therapeutic plasma concentration range? Ther Drug Monit. 1998;20:56–59. doi: 10.1097/00007691-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Chang MC, Grange E, Rabin O, et al. Lithium decreases turnover of arachidonate in several brain phospholipids. Neurosci Lett. 1996;220:171–174. doi: 10.1016/s0304-3940(96)13264-x. Erratum in: Neurosci Lett 1997 31:222:141. [DOI] [PubMed] [Google Scholar]
- 20.Cipriani A, Rendell JM, Geddes JR. Haloperidol alone or in combination for acute mania. Cochrane Database Syst Rev. 2006;3:CD004362. doi: 10.1002/14651858.CD004362.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Cooper JR, Bloom FE, Roth RH. The biochemical basis of neuropharmacology. 8th. Oxford University Press; Oxford: 2003. [Google Scholar]
- 22.Cunningham MO, Jones RS. The anticonvulsant, lamotrigine decreases spontaneous glutamate release but increases spontaneous GABA release in the rat entorhinal cortex in vitro. Neuropharmacology. 2000;39:2139–2146. doi: 10.1016/s0028-3908(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 23.Di Marzo V, Piomelli D. Participation of prostaglandin E2 in dopamine D2 receptor-dependent potentiation of arachidonic acid release. J Neurochem. 1992;59:379–382. doi: 10.1111/j.1471-4159.1992.tb08915.x. [DOI] [PubMed] [Google Scholar]
- 24.Ertley RN, Bazinet RP, Lee HJ, et al. Chronic treatment with mood stabilizers increases membrane GRK3 in rat frontal cortex. Biol Psychiatry. 2007;61:246–249. doi: 10.1016/j.biopsych.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Esposito G, Giovacchini G, Der M, et al. Imaging signal transduction via arachidonic acid in the human brain during visual stimulation, by means of positron emission tomography. Neuroimage. 2006;34:1342–1351. doi: 10.1016/j.neuroimage.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 27.Ghelardoni S, Tomita YA, Bell JM, et al. Chronic carbamazepine selectively downregulates cytosolic phospholipase A2 expression and cyclooxygenase activity in rat brain. Biol Psychiatry. 2004;56:248–254. doi: 10.1016/j.biopsych.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Greenwood TA, Schork NJ, Eskin E, et al. Identification of additional variants within the human dopamine transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Mol Psychiatry. 2006;11:125–133, 115. doi: 10.1038/sj.mp.4001764. [DOI] [PubMed] [Google Scholar]
- 29.Hayakawa T, Chang MC, Rapoport SI, et al. Selective dopamine receptor stimulation differentially affects [3H]arachidonic acid incorporation, a surrogate marker for phospholipase A2-mediated neurotransmitter signal transduction, in a rodent model of Parkinson’s disease. J Pharmacol Exp Ther. 2001;296:1074–1084. [PubMed] [Google Scholar]
- 30.Hosey LA, Thompson JL, Metman LV, et al. Temporal dynamics of cortical and subcortical responses to apomorphine in Parkinson disease: an PET study. Clin Neuropharmacol. 2005;28:18–27. doi: 10.1097/01.wnf.0000154220.30263.0e. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa M, Mizukami K, Iwakiri M, et al. Immunohistochemical and immunoblot analysis of dopamine and cyclic AMP-regulated phosphoprotein, relative molecular mass 32,000 (DARPP-32) in the prefrontal cortex of subjects with schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1177–1181. doi: 10.1016/j.pnpbp.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Jensen JB, Mork A. Altered protein phosphorylation in the rat brain following chronic lithium and carbamezepine treatments. Eur Neuropsychopharmacol. 1997;7:173–179. doi: 10.1016/s0924-977x(96)00396-3. [DOI] [PubMed] [Google Scholar]
- 33.Khan ZU, Gutierrez A, Martin R, et al. Differential regional and cellular distribution of dopamine D2-like receptors: an immunocytochemical study of subtype-specific antibodies in rat and human brain. J Comp Neurol. 1998;402:353–371. doi: 10.1002/(sici)1096-9861(19981221)402:3<353::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Kim KM, Valenzano KJ, Robinson SR, et al. Differential regulation of the dopamine D2 and D3 receptors by G proteincoupled receptor kinases and beta-arrestins. J Biol Chem. 2001;276:37409–37414. doi: 10.1074/jbc.M106728200. [DOI] [PubMed] [Google Scholar]
- 35.Lesch KP, Aulakh CS, Tolliver TJ, et al. Differential effects of long-term lithium and carbamazepine administration on Gs alpha and Gi alpha protein in rat brain. Eur J Pharmacol. 1991;207:355–359. doi: 10.1016/0922-4106(91)90011-6. [DOI] [PubMed] [Google Scholar]
- 36.Martucci L, Wong AH, De Luca V, et al. N-methyl-d-aspartate receptor NR2B subunit gene GRIN2B in schizophrenia and bipolar disorder: polymorphisms and mRNA levels. Schizophr Res. 2006;84:214–221. doi: 10.1016/j.schres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 37.McCullumsmith RE, Kristiansen LV, Beneyto M, et al. Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Res. 2007;1127:108–118. doi: 10.1016/j.brainres.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montezinho LP, Castro MM, Duarte CB, et al. The interaction between dopamine D2-like and beta-adrenergic receptors in the prefrontal cortex is altered by mood-stabilizing agents. J Neurochem. 2006;96:1336–1348. doi: 10.1111/j.1471-4159.2005.03654.x. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1987. [DOI] [PubMed] [Google Scholar]
- 40.Peet M, Peters S. Drug-induced mania. Drug Saf. 1995;12:146–153. doi: 10.2165/00002018-199512020-00007. [DOI] [PubMed] [Google Scholar]
- 41.Poddubiuk ZM, Blumberg JB, Kopin IJ. Brain prostaglandin content in rats sacrificed by decapitation vs focused microwave irradiation. Experientia. 1982;38:987–988. doi: 10.1007/BF01953694. [DOI] [PubMed] [Google Scholar]
- 42.Rao JS, Bazinet RP, Rapoport SI, et al. Chronic administration of carbamazepine downregulates AP-2 DNA binding activity and AP-2α protein expression in rat frontal cortex. Biol Psychiatry. 2007;61:154–161. doi: 10.1016/j.biopsych.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 43.Rao JS, Rapoport SI, Bosetti F. Decrease in the AP-2 DNA-binding activity and in the protein expression of AP-2α and AP-2β in frontal cortex of rats treated with lithium for 6 weeks. Neuropsychopharmacology. 2005;30:2006–2013. doi: 10.1038/sj.npp.1300740. [DOI] [PubMed] [Google Scholar]
- 44.Rapoport SI. In vivo approaches to quantifying and imaging brain arachidonic and docosahexaenoic acid metabolism. J Pediatr. 2003;143:S26–S34. doi: 10.1067/s0022-3476(03)00399-8. [DOI] [PubMed] [Google Scholar]
- 45.Rintala J, Seemann R, Chandrasekaran K, et al. 85 kDa cytosolic phospholipase A2 is a target for chronic lithium in rat brain. Neuroreport. 1999;10:3887–3890. doi: 10.1097/00001756-199912160-00030. [DOI] [PubMed] [Google Scholar]
- 46.Robinson PJ, Noronha J, DeGeorge JJ, et al. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res Brain Res Rev. 1992;17:187–214. doi: 10.1016/0165-0173(92)90016-f. [DOI] [PubMed] [Google Scholar]
- 47.Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends Pharmacol Sci. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 48.Shaldubina A, Einat H, Szechtman H, et al. Preliminary evaluation of oral anticonvulsant treatment in the quinpirole model of bipolar disorder. J Neural Transm. 2002;109:433–440. doi: 10.1007/s007020200035. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu T, Wolfe LS. Arachidonic acid cascade and signal transduction. J Neurochem. 1990;55:1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. [DOI] [PubMed] [Google Scholar]
- 50.Sokoloff P, Giros B, Martres MP, et al. Molecular cloning and characterization of a novel dopamine receptor D3 as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 51.Tabachnick BG, Fidell LS. In: Computer-assisted research design and analysis. Allyn, Bacon, editors. Boston: 2001. [Google Scholar]
- 52.Tarazi FI, Baldessarini RJ. Regional localization of dopamine and ionotropic glutamate receptor subtypes in striatolimbic brain regions. J Neurosci Res. 1999;55:401–410. doi: 10.1002/(SICI)1097-4547(19990215)55:4<401::AID-JNR1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 53.Vial D, Piomelli D. Dopamine D2 receptors potentiate arachidonate release via activation of cytosolic, arachidonic-specific phospholipase A2. J Neurochem. 1995;64:2765–2772. doi: 10.1046/j.1471-4159.1995.64062765.x. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Zhong P, Gu Z, et al. Regulation of NMDA receptors by dopamine D4 signaling in prefrontal cortex. J Neurosci. 2003;23:9852–9861. doi: 10.1523/JNEUROSCI.23-30-09852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wedzony K, Chocyk A, Mackowiak M, et al. Cortical localization of dopamine D4 receptors in the rat brain—immunocytochemical study. J Physiol Pharmacol. 2000;51:205–221. [PubMed] [Google Scholar]
- 56.Whone AL, Watts RL, Stoessl AJ, et al. Slower progression of Parkinson’s disease with ropinirole versus levodopa: the REAL-PET study. Ann Neurol. 2003;54:93–101. doi: 10.1002/ana.10609. [DOI] [PubMed] [Google Scholar]


