Abstract
Arteriovenous fistula (AVF) is the preferred hemodialysis access type because it has better patency rates and fewer complications than other access types. However, primary failure remains a common problem impeding AVF maturation and adding to patients’ morbidity and mortality. Juxta-anastomotic (or inflow) stenosis is the most common reason leading to primary failure, and percutaneous transluminal angioplasty (PTA) continues to be the gold standard treatment with excellent success rates. Intimal hyperplasia (IH) has been traditionally blamed as the main pathophysiologic culprit, but new evidence raises doubts regarding the contribution of IH alone to primary failure. We report a 64-year-old man with a two-stage brachio-basilic AVF that was complicated by failure four months after creation. Angiogram showed multiple juxta-anastomotic and mid-fistula stenotic lesions. PTA was successful in assisting maturation and subsequently cannulating AVF for hemodialysis treatment. We failed to identify the underlying cause of stenosis as biopsy specimens from fistula tissue obtained at the time of transposition revealed no occlusive IH. This case emphasizes the need for additional research on factors contributing to AVF failure besides IH, and highlights the need for more therapeutic options to reduce AVF failure rate.
INDEX WORDS: Arteriovenous fistula (AVF), primary failure, vascular remodeling, intimal hyperplasia, vascular access dysfunction
Introduction
Arteriovenous fistula (AVF) is the preferred hemodialysis vascular access type. Once functional, AVFs have better patency rates and fewer complications than arteriovenous grafts and hemodialysis catheters. (1, 2) Nevertheless, newly created AVFs are susceptible to primary failure. This common complication is defined as the AVF that never matures to support cannulation with 2 hemodialysis needles, or fails within the first 3 months after its initial cannulation. (3) Primary failure rates range from 30 to 50% (4, 5), most often secondary to juxta-anastomotic stenosis which is routinely attributed to intimal hyperplasia (IH). (6, 7)
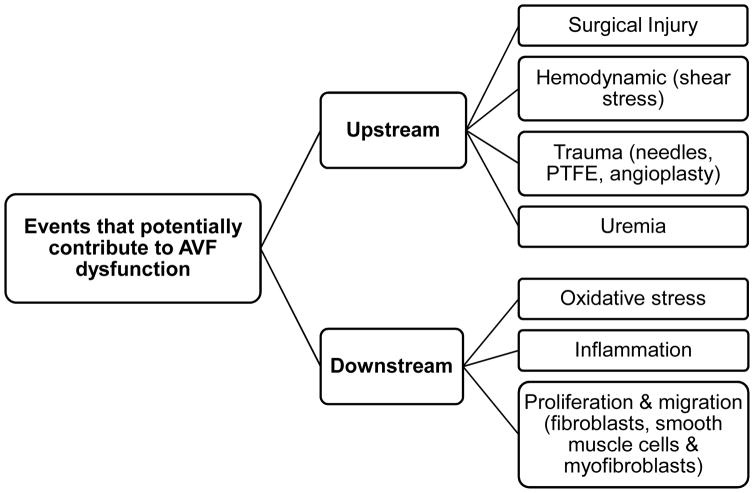
IH is caused by the hyperplastic accumulation of myofibroblast-like cells in the venous intima. (8) The etiology of IH in hemodialysis patients remains poorly understood. (9) Roy-Chaudhury et al (10) have proposed a group of upstream and downstream events as causes of IH development. The upstream factors include comorbidities, surgical trauma, endothelial dysfunction, inflammation, and abnormal flow conditions after anastomosis. (11) Downstream factors are secondary to upstream events, and mostly include biologic events reflecting the adaptive, and maladaptive, response of the vein to injury and new flow conditions (Figure 1). Of note, the contribution of each of these factors to AVF failure is not certain and further research is warranted to determine the role of upstream and downstream events on stenosis. While IH is historically associated with stenosis and early failure of vascular accesses based on histologic observations, a recent study showed that it is improbable that this lesion alone causes critical AVF stenosis (12). This may explain why drug eluting stents or local delivery of anti-proliferative agents have shown promising results in animal models (13), but have failed to improve human AVF maturation outcome (14, 15)
Figure 1.
Upstream and downstream events as causes of intimal hyperplasia development proposed by Roy-Chaudhury et al (10). The upstream factors include comorbidities, surgical trauma, endothelial dysfunction, inflammation, and abnormal flow conditions after anastomosis. Downstream factors are secondary to upstream events and mostly include biologic events reflecting the adaptive, and maladaptive, response of the vein to injury and new flow conditions. Abbreviations: AVF, arteriovenous fistula; PTFE, polytetrafluoroethylene.
An AVF with primary failure requires additional maturation procedures in order to be successfully cannulated for hemodialysis, which increases the use of hemodialysis catheters. Hence, primary failure increases hemodialysis patients’ morbidities and mortality, and adds significantly to hemodialysis access care costs (16). Remarkably, creation and maintenance of a vascular access represents 14–20% of the total health care cost for these patients (16). Therefore, increasing the functional AVF rate in the hemodialysis population is part of a national effort to improve patients’ health and quality of life, and minimize their economic burden. Percutaneous transluminal angioplasty (PTA) remains the gold standard intervention to salvage primary failed AVFs and has excellent success rates in primary failure (17). PTA reduces the need for surgical interventions or AVF abandonment and the creation of the potentially more costly arteriovenous graft, given the historically greater need for maintenance intervention (16). However recent studies have shown benefits of different vascular access types, including arteriovenous graft, in patient populations with low likelihood of AVF success that may improve the cost differential (18).
Although the mechanism by which PTA causes acute dilation of the stenotic area is not completely understood, it is likely that the biologic response to the mechanical injury secondary to radial stretching of the vein, and intimal and medial compression, spark positive remodeling of the vascular wall. We present a 64-year-old white man that had AVF failure four months after creation with extensive fistula stenosis on AVF angiogram while having no evident occlusive IH on tissue analysis.
Case Report
Clinical History and Initial Laboratory Data
A 64-year-old man was referred for evaluation of his newly created AVF due to progressive difficulty with AVF cannulation that was first observed a few weeks after his first cannulation, and further complicated with hematoma formation. He had initiated hemodialysis via a 23-cm cuff to tip right internal jugular tunneled hemodialysis catheter. Soon after, vessel mapping was performed and led to the creation of a two-stage brachio-basilic AVF with no intra- or postoperative complications. A surgical follow-up showed adequate size AVF with a blood flow of 750 ml per minute evaluated by Duplex ultrasound, and the AVF was cleared to be cannulated for hemodialysis. However, soon after initial AVF cannulation, the patient required referral to the Section of Interventional Nephrology due to the difficulty with cannulation and hematoma formation. Subsequent evaluation with Duplex ultrasound showed a decline in AVF blood flow to 425 ml per minute with findings consistent with inflow stenosis on physical examination, including a weak AVF pulse by palpation, discontinuous AVF thrill with no audible thrill during diastole, poor augmentation test, and complete collapse of the AVF on arm elevation. Specifically, the augmentation test (19) showed weak response with an increase from 2/10 to 4/10 of the pulse after manual access occlusion.
Imaging Studies
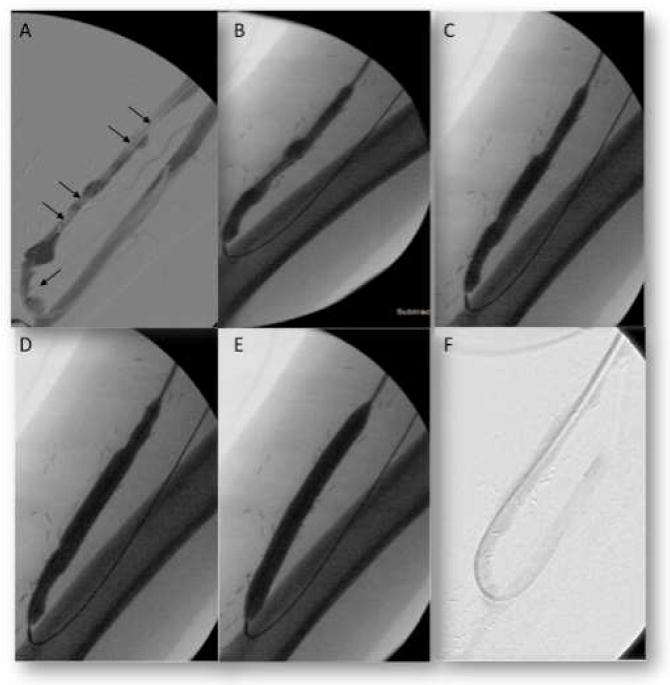
Retrograde cannulation and AVF angiogram was performed, which showed long stenotic lesions at the juxta anastomotic area and mid fistula (Figure 2). Sequential PTA with a 6mm × 4cm followed by an 8mm × 8cm access angioplasty balloons (Bard Peripheral Vascular, Inc.) was performed for all lesions. Post PTA angiogram showed complete resolution of the stenosis (Figure 2) with significant improvement on physical examination, normal pulse and continuous (systolic-diastolic) thrill.
Figure 2.
Angiogram results showing arteriovenous fistula (AVF) stenosis predominantly at the Juxta-Anastomosis Mid-fistula. (A) Angiography showing 70–90% long segment stenosis (arrows) at the Juxta-Anastomosis and Mid-fistula. (B–E) Percutaneous transluminal angioplasty with 8mm × 8cm balloon and increasing pressure from nominal pressure to rated burst pressure of 18 atm (at which full effacement is achieved). (F) AVF showing resolution of the vascular stenosis after angioplasty.
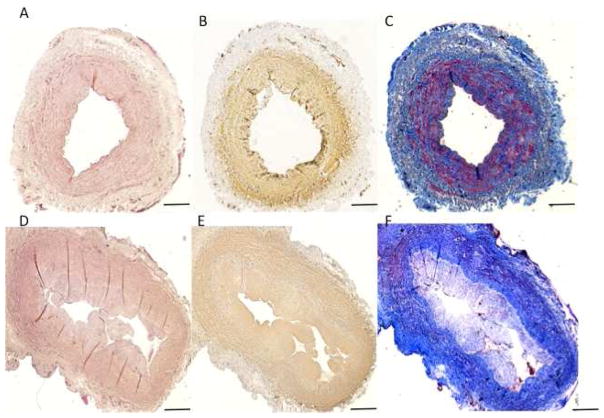
An AVF tissue sample of 2 mm was collected around the juxta anastomotic area at the time of transposition. Samples were formalin-fixed and paraffin-embedded for histopathology and immunohistochemistry with smooth muscle actin (clone 1A4, DAKO) and smooth muscle myosin heavy chain antibodies (clone hSM-V, Sigma Aldrich). Masson trichrome stained tissue sections showed eccentric development of NIH (N/M ratio of 0.297) that compromised only 30% of calculated luminal area with a marked atrophy of the vascular wall. The loss of medial smooth muscle cell and increased medial fibroses in the AVF wall was noticeable (Figure 3).
Figure 3.
Vascular wall changes and intimal hyperplasia in matched-paired vein and arteriovenous fistula (AVF). Representative of a two-stage brachial-basilic AVF matched-pair. (A–C) vein collected before anastomosis with mild NIH and (D–F) AVF at the time of superficialization with mild to moderate NIH. Sections were (A, D) Hematoxilin and Eosin stained, (B, E) Smooth Muscle cell stained and (C, F) Mason Trichrome stained. Scale bars denote 200μm.
Diagnosis
Primary failure of AVF maturation evident by a rapid decline in AVF blood flow by Duplex ultrasound within 3 months of initial cannulation.
Clinical Follow-up
The patient had successful subsequent cannulations with no reported difficulties. Hemodialysis treatment sessions were delivered with the prescribed blood flow of 400 ml per minute. It is important to mention that this treatment with PTA did not require any need for bridging hemodialysis catheter placement.
Discussion
In this report, we describe a case of AVF failure with a unique multidimensional analysis involving AVF physical examination, pre-surgical vein tissue biopsy analysis, fistula tissue biopsy analysis, and pre- and post- PTA angiographic evaluation. Such an evaluation enables a more comprehensive investigation into this common and costly complication of AVFs (20). Primary AVF failure leads to an unpredictable lag time between AVF creation and its readiness for successful cannulation entailing an intervention (sometimes more than one) to assist maturation process (4, 21, 22). While this intervention is usually an outpatient procedure, it carries its own spectrum of complications and adds inconvenience to a patient population with an already reduced quality of life. In addition, any delay in using the AVF may lead to higher usage of the less preferable hemodialysis access type, catheters. Current guidelines recommend referral for an AVF creation 6 months before the expected start of hemodialysis to allow sufficient time for AVF maturation and reduce the need for catheters. Such a strategy aims to reduce catheter use and related complications including hospitalization, and therefore minimize hemodialysis access related costs and maximize patient outcomes (23). Evidence suggests that risk factors such as advanced age, peripheral vascular disease, coronary artery disease, diabetes mellitus, and immune conditions may lead to delayed AVF maturation (24, 25).
AVF maturation depends on blood flow volume, blood vessel radius, length, and blood viscosity as postulated by Poiseuille’s and Laplace’s laws (26, 27). After AVF creation, blood flow may increase up to 20–50 fold, with as much as a 60% increase occurring within the first 24 hours after creation. Blood flow is a significant, if not key, determinant of AVF maturation (28, 29). Inflow stenosis, as we found in our patient, constitutes the most common reason behind primary failure (7). As mentioned before, vascular stenosis has been traditionally attributed to IH (30), limiting AVF blood flow from increasing sufficiently to start the maturation process.
Physical examination of the AVF by qualified clinicians is a valuable method to evaluate and diagnose potential causes of primary failure, with sensitivity and specificity of 85% and 71%, respectively, and 80% concordance with angiography findings (19). The augmentation test is a useful assessment tool, which is performed by occluding the AVF and monitoring the changes to AVF pulse and thrill before and after access occlusion. Pulse is expected to increase in intensity and thrill should disappear after AVF occlusion. Therefore, suboptimal increase in pulse intensity after AVF occlusion (like in this case) reflects inflow stenosis. Pulse intensity (before and after access occlusion) can be graded on scale of 1 – 10 (19).
Intimal hyperplasia is not only present in AVFs but also can be commonly found in veins prior to AVF creation (pre-existing) (31, 32). Our patient’s tissue sections showed non-occlusive IH at the time of transposition. Given that the AVF failed soon after creation in spite of non-occlusive IH suggests additional contributing factors leading to AVF failure. A prospective study from our center of paired tissue samples from 51 patients collected at the first stage (vein sample) and second stage (fistula sample) of transposed brachio-basilic AVF creation surgeries showed that that neither pre-existing nor postoperative IH affected AVF outcomes (12). These findings, similar to the reported case, demonstrate that AVF failure is a more complex process than what was initially thought, and the balance of outward expansion and luminal narrowing of the vessel due to IH is what ultimately determines clinically significant stenosis. These counterintuitive results may reflect the disparity between a presumptive functional interpretation of histologic observations and the actual stenotic capacity of the hypertrophic intima.
In conclusion, AVF failure remains a common complication that impedes our national goal to increase AVF rate. Even though PTA has provided an excellent therapeutic option to salvage failed AVFs, its associated complications, costs and patients’ inconvenience add emphasis that future research should aim to discover non-invasive treatment options to reduce or prevent primary AVF failure. Without a doubt, understanding the clinical factors and vascular characteristics that affect maturation and determine AVF failure is the first step of a long journey towards non-invasive therapeutic discovery.
Acknowledgments
The authors would like to thank Fadi Fayad for contributing to this case.
Support: This work was supported by the National Institutes of Health grant R01-DK-098511 to R.I.V.-P and L.H.S.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Peer Review: Evaluated by 3 external peer reviewers, the Deputy Editor, the Feature Editor, the Education Editor, and the Editor-in-Chief.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney international. 2001;60:1–13. doi: 10.1046/j.1523-1755.2001.00765.x. [DOI] [PubMed] [Google Scholar]
- 2.Woo K, Doros G, Ng T, Farber A. Comparison of the efficacy of upper arm transposed arteriovenous fistulae and upper arm prosthetic grafts. Journal of vascular surgery. 2009;50:1405–1411. e1402. doi: 10.1016/j.jvs.2009.07.090. [DOI] [PubMed] [Google Scholar]
- 3.Asif A, Roy-Chaudhury P, Beathard GA. Early arteriovenous fistula failure: a logical proposal for when and how to intervene. Clinical Journal of the American Society of Nephrology. 2006;1:332–339. doi: 10.2215/CJN.00850805. [DOI] [PubMed] [Google Scholar]
- 4.Huijbregts HJ, Bots ML, Wittens CH, Schrama YC, Moll FL, Blankestijn PJ. Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative. Clinical Journal of the American Society of Nephrology. 2008;3:714–719. doi: 10.2215/CJN.02950707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisoni R, Barker-Finkel J, Allo M. Statin therapy is not associated with improved vascular access outcomes. Clinical Journal of the American Society of Nephrology. 2010;5:1447–1450. doi: 10.2215/CJN.02740310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huijbregts HJ, Bots ML, Moll FL, Blankestijn PJ. Hospital specific aspects predominantly determine primary failure of hemodialysis arteriovenous fistulas. Journal of vascular surgery. 2007;45:962–967. doi: 10.1016/j.jvs.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Beathard GA, Arnold P, Jackson J, Litchfield T. Aggressive treatment of early fistula failure. Kidney international. 2003;64:1487–1494. doi: 10.1046/j.1523-1755.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 8.Skartsis N, Manning E, Wei Y, et al. Seminars in dialysis. Wiley Online Library; 2011. Origin of neointimal cells in arteriovenous fistulae: bone marrow, artery, or the vein itself? pp. 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duque JC, Vazquez-Padron RI. Myofibroblasts: the ideal target to prevent arteriovenous fistula failure? Kidney international. 2014;85:234–236. doi: 10.1038/ki.2013.384. [DOI] [PubMed] [Google Scholar]
- 10.Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis Vascular Access Dysfunction: A Cellular and Molecular Viewpoint. Journal of the American Society of Nephrology. 2006;17:1112–1127. doi: 10.1681/ASN.2005050615. [DOI] [PubMed] [Google Scholar]
- 11.Lee T, Roy-Chaudhury P. Advances and new frontiers in the pathophysiology of venous neointimal hyperplasia and dialysis access stenosis. Advances in chronic kidney disease. 2009;16:329–338. doi: 10.1053/j.ackd.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabbara M, Duque JC, Martinez L, et al. Pre-existing and Postoperative Intimal Hyperplasia and Arteriovenous Fistula Outcomes. American Journal of Kidney Diseases. 2016;68:455–64. doi: 10.1053/j.ajkd.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly B, Melhem M, Zhang J, et al. Perivascular paclitaxel wraps block arteriovenous graft stenosis in a pig model. Nephrology Dialysis Transplantation. 2006;21:2425–2431. doi: 10.1093/ndt/gfl250. [DOI] [PubMed] [Google Scholar]
- 14.Peden EK1, Leeser DB, Dixon BS, et al. A multi-center, dose-escalation study of human type I pancreatic elastase (PRT-201) administered after arteriovenous fistula. J Vasc Access. 2013;14:143–51. doi: 10.5301/jva.5000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Kassem M, Alghamdi I, Vazquez-Padron RI, et al. The Role of Endovascular Stents in Dialysis Access Maintenance. Advances in chronic kidney disease. 2015;22:453–458. doi: 10.1053/j.ackd.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stolic R. Most important chronic complications of arteriovenous fistulas for hemodialysis. Medical Principles and Practice. 2013;22:220–228. doi: 10.1159/000343669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nassar GM, Nguyen B, Rhee E, Achkar K. Endovascular treatment of the “failing to mature” arteriovenous fistula. Clinical Journal of the American Society of Nephrology. 2006;1:275–280. doi: 10.2215/CJN.00360705. [DOI] [PubMed] [Google Scholar]
- 18.Drew DA, Lok CE. Strategies for planning the optimal dialysis access for an individual patient. Current opinion in nephrology and hypertension. 2014;23:314–320. doi: 10.1097/01.mnh.0000444815.49755.d9. [DOI] [PubMed] [Google Scholar]
- 19.Salman L, Beathard G. Interventional nephrology: Physical examination as a tool for surveillance for the hemodialysis arteriovenous access. Clinical Journal of the American Society of Nephrology. 2013;8:1220–1227. doi: 10.2215/CJN.00740113. [DOI] [PubMed] [Google Scholar]
- 20.Perera GB, Mueller MP, Kubaska SM, Wilson SE, Lawrence PF, Fujitani RM. Superiority of autogenous arteriovenous hemodialysis access: maintenance of function with fewer secondary interventions. Annals of vascular surgery. 2004;18:66–73. doi: 10.1007/s10016-003-0094-y. [DOI] [PubMed] [Google Scholar]
- 21.Brunori G, Ravani P, Mandolfo S, Imbasciati E, Malberti F, Cancarini G. Fistula maturation: doesn’t time matter at all? Nephrology Dialysis Transplantation. 2005;20:684–687. doi: 10.1093/ndt/gfh777. [DOI] [PubMed] [Google Scholar]
- 22.Lok CE, Sontrop JM, Tomlinson G, et al. Cumulative patency of contemporary fistulas versus grafts (2000–2010) Clinical Journal of the American Society of Nephrology. 2013;8:810–818. doi: 10.2215/CJN.00730112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimball TA, Barz K, Dimond KR, Edwards JM, Nehler MR. Efficiency of the kidney disease outcomes quality initiative guidelines for preemptive vascular access in an academic setting. Journal of vascular surgery. 2011;54:760–765. doi: 10.1016/j.jvs.2011.03.006. discussion 765–766. [DOI] [PubMed] [Google Scholar]
- 24.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D. Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I) Journal of the American Society of Nephrology. 2006;17:3204–3212. doi: 10.1681/ASN.2006030190. [DOI] [PubMed] [Google Scholar]
- 25.Duque JC, Martinez L, Mesa A, et al. CD4(+) lymphocytes improve venous blood flow in experimental arteriovenous fistulae. Surgery. 2015;158:529–536. doi: 10.1016/j.surg.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGrogan DG, Maxwell AP, Khawaja AZ, Inston NG. Current tools for prediction of arteriovenous fistula outcomes. Clinical Kidney Journal. 2015;8:282–289. doi: 10.1093/ckj/sfv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgakarakos EI, Kapoulas KC, Georgiadis GS, Tsangaris AS, Nikolopoulos ES, Lazarides MK. An overview of the hemodynamic aspects of the blood flow in the venous outflow tract of the arteriovenous fistula. The journal of vascular access. 2012;13:271–278. doi: 10.5301/jva.5000037. [DOI] [PubMed] [Google Scholar]
- 28.Tessitore N, Mansueto G, Bedogna V, et al. A prospective controlled trial on effect of percutaneous transluminal angioplasty on functioning arteriovenous fistulae survival. Journal of the American Society of Nephrology. 2003;14:1623–1627. doi: 10.1097/01.asn.0000069218.31647.39. [DOI] [PubMed] [Google Scholar]
- 29.Tessitore N, Bedogna V, Gammaro L, et al. Diagnostic accuracy of ultrasound dilution access blood flow measurement in detecting stenosis and predicting thrombosis in native forearm arteriovenous fistulae for hemodialysis. American Journal of Kidney Diseases. 2003;42:331–341. doi: 10.1016/s0272-6386(03)00659-0. [DOI] [PubMed] [Google Scholar]
- 30.Salman LH. How is Arteriovenous Fistula Longevity Best Prolonged?: The role of surveillance. Seminars in dialysis. 2015;28:33–34. doi: 10.1111/sdi.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wali MA, Eid RA, Dewan M, Al-Homrany MA. Intimal changes in the cephalic vein of renal failure patients before arterio-venous fistula (AVF) construction. Journal of Smooth Muscle Research. 2003;39:95–105. doi: 10.1540/jsmr.39.95. [DOI] [PubMed] [Google Scholar]
- 32.Allon M, Robbin ML, Young CJ, et al. Preoperative venous intimal hyperplasia, postoperative arteriovenous fistula stenosis, and clinical fistula outcomes. Clinical Journal of the American Society of Nephrology. 2013;8:1750–1755. doi: 10.2215/CJN.02740313. [DOI] [PMC free article] [PubMed] [Google Scholar]