Abstract
Store-operated Ca2+ entry (SOCE) inhibitors are emerging as an attractive new generation of anti-cancer drugs. Here, we report that SKF-96365, an SOCE inhibitor, exhibits potent anti-neoplastic activity by inducing cell-cycle arrest and apoptosis in colorectal cancer cells. In the meantime, SKF-96365 also induces cytoprotective autophagy to delay apoptosis by preventing the release of cytochrome c (cyt c) from the mitochondria into the cytoplasm. Mechanistically, SKF-96365 treatment inhibited the calcium/calmodulin-dependent protein kinase IIγ (CaMKIIγ)/AKT signaling cascade in vitro and in vivo. Overexpression of CaMKIIγ or AKT abolished the effects of SKF-96365 on cancer cells, suggesting a critical role of the CaMKIIγ/AKT signaling pathway in SFK-96365-induced biological effects. Moreover, Hydroxychloroquine (HCQ), an FDA-approved drug used to inhibit autophagy, could significantly augment the anti-cancer effect of SFK-96365 in a mouse xenograft model. To our best knowledge, this is the first report to demonstrate that calcium/CaMKIIγ/AKT signaling can regulate apoptosis and autophagy simultaneously in cancer cells, and the combination of the SOCE inhibitor SKF-96365 with autophagy inhibitors represents a promising strategy for treating patients with colorectal cancer.
Keywords: SKF-96365, Autophagy, Apoptosis, Colorectal cancer, CaMKII
Introduction
Store-operated Ca2+ entry (SOCE), which is initiated by the emptying of intracellular calcium (iCa2+) stores, is one of the most common and ubiquitous Ca2+ influx routes in non-excitable cells, including tumor cells [1]. SOCE regulates a broad range of cellular functions, such as cell proliferation, cell death and enzymatic activity. Several studies have shown that remodeling or deregulation of iCa2+ homeostasis via aberrant SOCE is involved in carcinogenesis, cancer cell proliferation, metastasis and chemoresistance [2,3]. Various molecular targets have been implicated in the process of SOCE, including stromal interacting molecule (STIM)-1 and Orai1. STIM1, as a calcium-sensing protein, is predominantly located in the endoplasmic reticulum (ER) membrane, whereas Orai1 is usually located in the plasmalemma [4]. Recently, STIM1-mediated SOCE has been found to promote CRC metastasis and progression. CRC patients with high STIM1 expression had a poorer prognosis than those with low expression. Mechanistically, STIM1 promoted epithelial-to-mesenchymal transition (EMT) in CRC cells through the increased expression of cyclooxygenase-2 (COX-2) and the production of prostaglandin E2 (PGE2) [5,6].
As a calcium antagonist, SKF-96365 is widely used to explore the pathophysiological function of SOCE in non-excitable cells [7]. Previous studies have shown the cytotoxic potential of SKF-96365 in several types of human cancer. Cai R reported that SKF-96365 induced cell cycle arrest in G2/M phase and suppressed cell growth in gastric cancer via inhibition of TRPC channels [8]. Intraperitoneal injection of SKF-96365 significantly retarded esophageal squamous cell carcinoma (ESCC) growth in xenografted nude mice [9]. Selvaraj S demonstrated that SKF-96365 suppressed cell growth and induced autophagic cell death via downregulation of STIM1 in prostate cancer cells [10]. However, its anti-tumor activity and molecular mechanism in colon cancer is still unclear.
In the present study, we demonstrated that the SOCE inhibitor SKF-96365 suppressed CRC cell growth both in vitro and in vivo. Mechanistically, SKF-96365 induced G2/M cell-cycle arrest and apoptosis with concomitant autophagy in HCT116 and HT29 cells. We also showed that SKF-96365-induced apoptosis resulted in the death of cancer cells, whereas autophagy played a cytoprotective role to delay the initiation of apoptosis. Additionally, we found that the calcium/calmodulin-dependent protein kinase IIγ (CaMKIIγ)/AKT signaling pathway is a key determinant of SFK-96365-induced biological effects on colon cancer cells and that CaMKIIγ represents a novel potential diagnostic and therapeutic target for patients with CRC.
Materials and methods
Measurements of intracellular Ca2+ concentration and SOCE
The iCa2+ concentration was monitored using a Ca2+-sensitive fluorescence indicator, fluo-3/AM (Invitrogen), following a previously published procedure [11]. ER Ca2+ stores were depleted by treatment with 2 µM thapsigargin in BSS solution (140 mM NaCl, 2.8 mM KCl, 2 mM MgCl2, 10 mM HEPES, pH 7.4) containing 1 mM EGTA. Ca2+ entry was accomplished by addition of CaCl2 (2 mM). The iCa2+ concentration in colon cancer cells was monitored by a Nikon A1 confocal microscopy (Nikon Instruments Inc, Melville, USA) at an excitation of 488 nm and an emission of 530 nm. Changes of intracellular fluorescence intensity compared with the baseline were indicative of the changes in iCa2+. Images were obtained at a rate of one image per 4 s and were analyzed with Nikon confocal software.
Mitochondrial membrane potential assay
The mitochondrial membrane potential was measured using JC-1 as previously described [12]. Briefly, the treated cells were incubated with an equal volume of JC-1 staining solution (5 µg/ml) at 37 °C for 20 min, rinsed twice with PBS, and analyzed by flow cytometry.
Intracellular signaling array
Intracellular signaling molecules were detected using a PathScan intracellular signaling array kit (Cell Signaling Technology) according to the manufacturer’s instructions.
Xenograft assays
Five to six-week-old female athymic BALB/c mice were inoculated into the right oxter with HCT116 cells. When the diameter of the subcutaneous tumor reached approximately 0.5 centimeters, animals were randomly assigned to the vehicle, SKF-96365 alone, HCQ alone or SKF-96365 + HCQ. SKF-96365 was applied (20 mg/kg) and HCQ was applied (60 mg/kg) daily for 14 successive days by intraperitoneal injection. Tumor sizes were determined by measuring 2 diameters with a caliper. Tumor volume (V) was estimated using the equation V = ab2 /2, where a is the largest diameter and b is the smallest diameter. Eight mice were included in each group. Mice were sacrificed 24 h after the last treatment. The tumors were weighed and processed for western blot analysis or paraffin embedding. This animal study was approved by the Institutional Animal Ethics Committee of Zhejiang University with Approval No. zju-2013-1-01-066.
Statistical analysis
All experiments were performed at least three times, and the data were presented as the mean ± standard deviation. GraphPad Prism 5.0 was used for statistical analysis. A difference was considered significant when P < 0.05.
Other methods
Please see Appendix: Supplementary materials.
Results
SKF-96365 inhibits SOCE and suppresses CRC cell growth
We first examined the inhibitory effect of SKF-96365 on SOCE and viability in colon cancer cell lines. Thapsigargin (TG), an inhibitor of endoplasmic reticulum Ca2+-ATPase, was used to deplete intracellular calcium stores in a Ca2+-free solution. After addition of 2 µM TG in Ca2+-free solution, HCT116 and HT29 cells exhibited a rapid rise in iCa2+. Subsequent reintroduction of 2 mM CaCl2 to the extracellular solution resulted in a sustained increase in iCa2+ from baseline, which is consistent with a characteristic SOCE-mediated Ca2+ influx from the extracellular solution. As expected, pretreatment with SKF-96365 inhibited SOCE-mediated Ca2+ influx in HCT116 and HT29 cells in a dose-dependent manner (Fig. 1A and B). In the following study, SKF-96365 significantly inhibited the growth of cancer cells in a dose- and time-dependent manner (Fig. 1C), with a half maximal inhibitory concentration (IC50) of 10.88 µM for HCT116 and 14.56 µM for HT29 after 48 h of exposure. Colony-formation experiments also indicated that SKF-96365 dramatically inhibited the growth of CRC cells (Fig. S1). We also measured the cytotoxicity of SKF-96365 on normal human colon epithelial cells. The results demonstrated that SKF-96365 was significantly more toxic toward HCT116 and HT29 cancer cells than NCM460 cells, a normal colon epithelial cell line (Fig. S2).
Fig. 1.
SKF-96365 inhibits SOCE and suppresses the growth of colon cancer cells. (A) Representative time-course recording of intracellular Ca2+ fluorescence showing the inhibitory effect of SKF-96365 on the SOCE response to 2 µM TG in the absence or presence of extracellular Ca2+ in HCT116 and HT29 cells. (B) Summarized data of cells from different coverslips showing intracellular fluorescence intensity changes compared with the baseline (Fmax/Fbase-1; n = 30 cells for each group) in HCT116 and HT29 cells. (C) Inhibitory effect of SKF-96365 on HCT116 and HT29 cells. The percentage of viable cells was measured by the MTS assay. *P < 0.05, ***P < 0.001.
SKF-96365 induces cell cycle arrest at the G2/M phase in CRC cells
To determine whether the growth inhibition induced by SKF-96365 is a result of cell cycle arrest, we analyzed the effect of SKF-96365 on cell cycle progression. The percentage of the cell population at G2/M was significantly increased after SKF-96365 treatment, with a concomitant reduction of the percentage in G1 (Fig. S3A and B). A small decrease in the S phase was also observed. Next, we examined the expression of cell cycle-related proteins. The results clearly showed that SKF-96365 produced an increase in p21waf/Cip1 and a decrease in p-Cdc25c, Cdc25c and Cyclin B (Fig. S3C). However, there was little change in the expression of Cyclin A. Taken together, these data show that SKF-96365 triggers G2/M cell cycle arrest by regulating several key G2/M transition-phase proteins.
SKF-96365 induces apoptosis in CRC cells via the intrinsic mitochondrial pathway
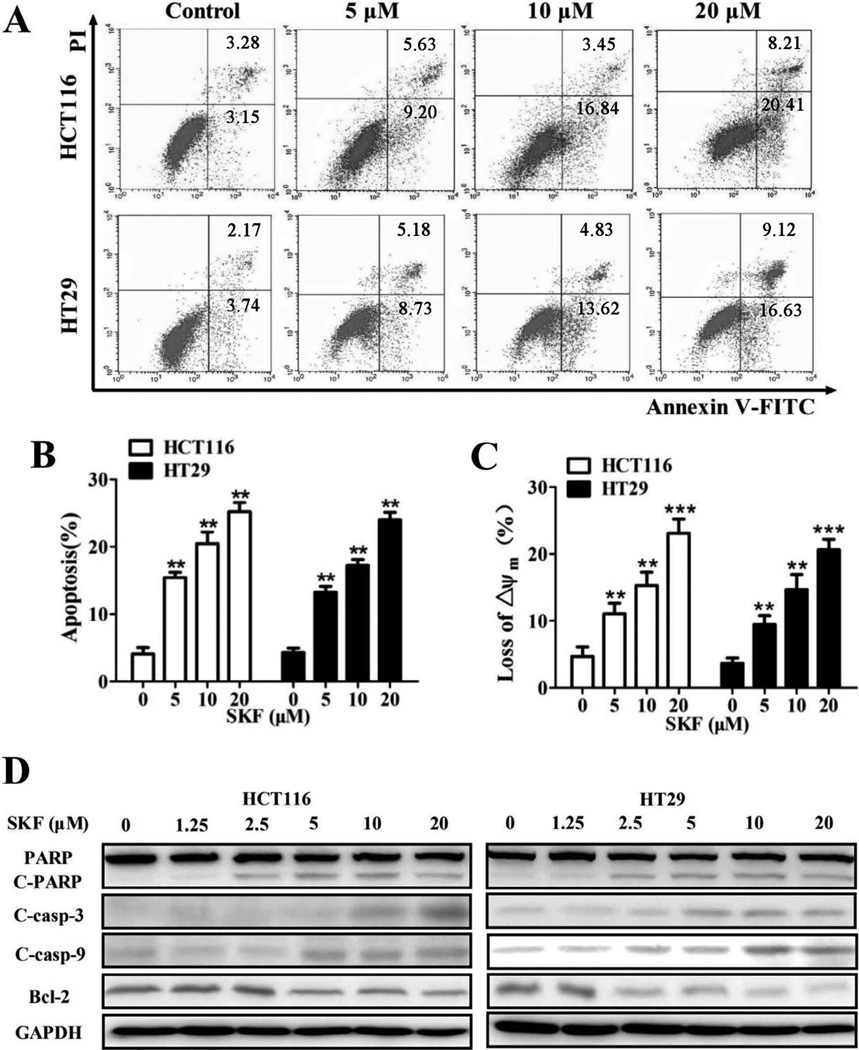
To examine whether the cell growth inhibition induced by SKF-96365 is also dependent on apoptosis, SKF-96365-treated cells were stained with propidium iodide (PI)/Annexin V-FITC and quantified by flow cytometry. SKF-96365 induced remarkable apoptosis in treated cells (Fig. 2A and B). Then we measured the mitochondrial membrane potential (Δψm) by flow cytometry and found that SKF-96365 treatment led to depolarization of Δψm in a dose-dependent manner (Fig. 2C).Western blot analyses showed that cleaved caspase-9, cleaved caspase-3 and cleaved poly (ADP-ribose) polymerase (PARP) were also increased after treatment with SKF-96365 for 24 h (Fig. 2D). In the following study, depolarization of Δψm was detected as early as 12 h after SKF-96365 treatment (Fig. 3A). At this time, the re-localization of pro-apoptotic protein Bax within mitochondria was also observed. Bax was exclusively cytoplasmic, which did not co-localize with mitochondria in untreated cells (Fig. S4). However, SKF-96365 treatment resulted in an intense green Bax staining that co-localized with the mitochondria.
Fig. 2.
SKF-96365 induces apoptosis in colorectal cancer cells. Cells were treated with SKF-96365 for 48 h before staining with Annexin V (AV) and propidium iodide (PI) (A), and the apoptotic rates were determined by flow cytometry (B). (C) Cells were treated with SKF-96365 for 48 h before staining with JC-1, and the mitochondrial membrane potential was evaluated by flow cytometry. (D) The expression of apoptosis-associated proteins in cells treated with SKF-96365 for 24 h. **P < 0.01, ***P < 0.001.
Fig. 3.
Mitochondrial membrane potential collapse and cytochrome c release occurs much earlier than the induction of apoptosis in cells treated with SKF-96365. (A) Cells were treated with 10 µM SKF-96365 for the indicated intervals, and the mitochondrial membrane potential was analyzed by flow cytometry. (B) Cells were treated with DMSO or 10 µM SKF-96365 for 12 h before labeling with fluorescence and imaged by fluorescence microscopy. Green: FITC-labeled cytochrome c; Red: Mito-Tracker-labeled mitochondria; Blue: DAPI-labeled nuclei. (C) Quantification of cells exhibiting overlap of cytochrome c and mitochondria. (D) The cleavage of PARP and caspase-3 in cells treated with SKF-96365 for the indicated intervals was determined by western blot. (E) Cells were treated with 10 µM SKF-96365 for the indicated intervals before staining with AV and PI, and the apoptotic rates were determined by flow cytometry. **P < 0.01, ***P < 0.001.
It is well known that Δψm and Bax relocation plays an important role in the release of cytochrome c (cyt c). Thus, cyt c was further investigated by immunofluorescence. As shown in Fig. 3B and C, cyt c localizes to the inner mitochondrial membrane of the untreated cells. However, it was released into the cytosol to form a diffuse staining pattern after treatment with SKF-96365 for 12 h. These results demonstrated that SKF-96365 induced mitochondrial membrane depolarization and cyt c release, indicating that SKF-96365 triggered apoptosis via the mitochondrial pathway.
As described above, Bax mitochondrial translocation and depolarization of Δψm was observed 12 h after SKF-96365 treatment; however, activation of caspase-3 and cleavage of PARP were only detected after 24 h-treatment (Fig. 3D). Quantitative analysis of apoptosis by AV/PI dual staining also showed that marked apoptosis was induced only after 24 h-treatment (Fig. 3E). Notably, the SFK-96365-inducedmitochondrial membrane potential collapse and cyt c release occurred much earlier than the induction of apoptosis.
SKF-96365 induces autophagy to counteract apoptosis by controlling cyt c release in CRC cells
Emerging evidence has demonstrated that autophagy can delay multiple stimuli-induced apoptosis by clearance of damaged mitochondria [13,14]. To examine whether SKF-96365 could induce autophagy, the treated cells were analyzed by electron microscopy. After treatment with SKF-96365 for 24 h, most of the HCT116 and HT29 cells displayed an extensive accumulation of double or multimembraned structures with a broad range of morphologies, indicating the formation of autophagosomes (Fig. 4A and B). For further confirmation, the cells were incubated with an antibody against microtubule-associated protein 1 light chain 3 (LC3). The punctate LC3 II-labeled autophagolysosome vacuoles were frequently observed in cells treated with SKF-96365 for 24 h compared with their controls (Fig. 4C and S5). In addition, the significantly increased expression of LC3-II, Beclin-1, and Atg5 and the attenuated expression of p62/SQSTM1 were observed in cells treated with SKF-96365 for 24 h (Fig. 4D). Interestingly, we noticed that SKF-96365-induced autophagy preceded apoptosis. As shown in Fig. 4E, after 12 h of treatment, the autophagy markers were apparent, but there was no evidence of apoptosis at this time (Fig. 3D and E).
Fig. 4.
SKF-96365 activates autophagy to counteract apoptosis by controlling cytochrome c release in CRC cells. (A) Numerous autophagosomes appeared in cells treated with 10 µM SKF-96365 for 24 h. Scale bar = 1 µm. (B) The number of autophagosomes was calculated by continuous counts within 10 fields under high resolution. (C) The percentage of puncta-positive cells was quantified by automated image acquisition and analysis using a threshold of >5 dots/cell. (D) Immunoblotting for p62, Beclin-1, ATG5 and LC3 using lysates from cells treated with SKF-96365 for 24 h. (E) Immunoblotting for LC3 and p62 using lysates from cells treated with 10 µM SKF-96365 for the indicated intervals. (F) Cells were treated with 10 µM SKF-96365 for 12 h before labeling with fluorescence and imaged by fluorescence microscopy. Green: FITC-labeled LC3; Red: Mito-Tracker-labeled mitochondria; Blue: DAPI-labeled nuclei. (G) Quantification of cells exhibiting overlap of LC3 and mitochondria. (H) Western blot analysis was used to detect cytochrome c levels in cytosolic fractions at the indicated intervals. **P < 0.01, ***P < 0.001.
Next, we investigated the cross talk between SKF-96365-induced autophagy and apoptosis. After 12 h of SKF-96365 treatment, most of green signals of LC3 were co-localized with red signals of Mito-Tracker, indicating fusion of the mitochondria with the autophagic vacuoles (Fig. 4F and G). The cytosolic cyt c increased after 12 h of SKF-96365 treatment (Fig. 4H), followed by a significant reduction, which was in concurrence with the initiation of robust autophagy. Since there was no evidence of apoptosis at this time, we thought that SKF-96365 treatment utilized autophagy to counteract apoptosis by controlling cyt c release from the mitochondria into the cytoplasm.
Inhibition of autophagy enhances SKF-96365-induced apoptotic cell death
To further investigate the effect of autophagy on the cytotoxicity of SKF-96365, HCT116 and HT29 cells were combined treated with two autophagy inhibitors: 3-methyladenine (3-MA) and chloroquine (CQ). As shown in Fig. 5A and B, 3-MA or CQ significantly augmented the SKF-96365-induced growth inhibition and apoptosis. The cleaved PARP and caspase-3 were significantly upregulated when cells were co-treated with 3-MA or CQ (Fig. 5C and D). Moreover, this combined treatment resulted in a significant induction of apoptosis after 12 h of SKF-96365 exposure (Fig. 5E). Next, siRNA against Beclin-1 was used to block autophagy, and similar results were obtained (Fig. 5F and G). We also evaluated the effect of autophagy on cell cycle arrest induced by SKF-96365. The inhibition of autophagy by CQ had no significant effect on SKF-96365-induced cell cycle arrest (Fig. S6), indicating that autophagy modulate biological effect of SKF-96365 is independent of cell cycle regulation.
Fig. 5.
Inhibition of autophagy promotes SKF-96365-induced apoptosis in CRC cells. Cells were treated with 10 µM SKF-96365 in the presence or absence of CQ (5 µM) or 3-MA (5 mM) for 48 h. Cell viability (A) and apoptotic rate (B) were measured by MTS assay and flow cytometry, respectively. Immunoblotting for PARP, cleaved caspase-3 and LC3 using lysates from cells treated with 10 µM SKF-96365 in the presence or absence of 3-MA (C) or CQ (D). (E) Cells were treated with 10 µM SKF-96365 in the presence or absence of autophagy inhibitor for the indicated intervals, and the apoptotic rates were determined by flow cytometry. Cells were transfected with siRNA against Beclin-1 for 24 h, then treated with SKF-96365 for 24 h. Cell viability (F) and apoptotic rate (G) were measured by MTS assay and flow cytometry, respectively. *P < 0.05, **P < 0.01, ***P < 0.001.
AKT is a critical mediator in regulating SKF-96365-mediated biological effects
To decipher the molecular mechanisms by which SKF-96365 affects the viability of CRC cells, a PathScan intracellular signaling array was used to detect the changes of 18 important, well-characterized signaling molecules. As shown in Fig. 6A, the phosphorylated levels of many components in AKT signaling pathway, including AKT (Ser473, Thr308), mTOR (Ser2448), GSK-3β (Ser9), BAD (Ser112), PRASP40 (Thr246) and S6 ribosomal protein (Ser235/236)were significantly decreased in SKF-96365-treated cells, whereas the level of PARP (Asp214) was increased. These phosphorylation antibody data suggest AKT pathway is involved in SKF-96365 induced biological effects in colon cancer cells. Western blot re-confirmed that the levels of phosphorylated AKT (Ser473), mTOR and its down-stream effector p70S6K and p-4EBP1 were significantly reduced by SKF-96365 (Fig. 6B).
Fig. 6.
AKT signaling is a critical mediator in regulating SKF-96365-mediated biological effects. (A) PathScan intracellular signaling antibody array kit was used to detect the changes in signaling molecules in cells treated with SKF-96365 for 24 h. (B) Immunoblotting for phospho-AKT, mTOR, S6K, 4EBP1, total AKT, and mTOR in cells treated with SKF-96365 for 24 h. After transient overexpression of AKT, cells were treated with SKF-96365 for 48 h, and then cell viability (C), cell cycle and apoptotic rate (D) were determined by MTS assay or flow cytometry. (E) Autophagy and apoptosis-associated proteins, including p62, LC3, cleaved PARP, cleaved caspase-3, phospho- and total AKT were analyzed by immunoblotting. (F) After transient overexpression of constitutively active AKT, cells were treated with SKF-96365 for 48 h, and then cell viability was determined by MTS. Western blot bands was quantified by ImageJ densitometric analysis and normalized by GAPDH. *P < 0.05. **P < 0.01.
To further identify the role of AKT in SKF-96365-induced biological effects, HCT116 and HT29 cells were transiently transfected with pcDNA3-AKT-T7 plasmid. As shown in Fig. 6C and D, enforced expression of AKT significantly attenuated SKF-96365-induced growth inhibition, cell cycle arrest and apoptosis. Furthermore, SKF-96365-induced autophagy was also decreased in the transfected cells (Fig. 6E and S7). Similarly, overexpression of constitutively active AKT (T308D/S473D; CA-AKT) markedly reduced the cytotoxicity of SKF-96365 on HCT116 and HT29 cells (Fig. 6F).
These results suggest that overexpression of AKT can override the SKF-96365-induced biological effects in CRC cells. In addition, AKT inhibitor LY294002 significantly augmented the cytotoxicity of SKF-96365 in HCT116 and HT29 cells (Fig. S8). Taken together, these data demonstrate that AKT is a critical mediator in regulating SKF-96365-mediated biological effects.
CaMKII couples store-operated Ca2+ influx to the downstream AKT/mTOR pathway
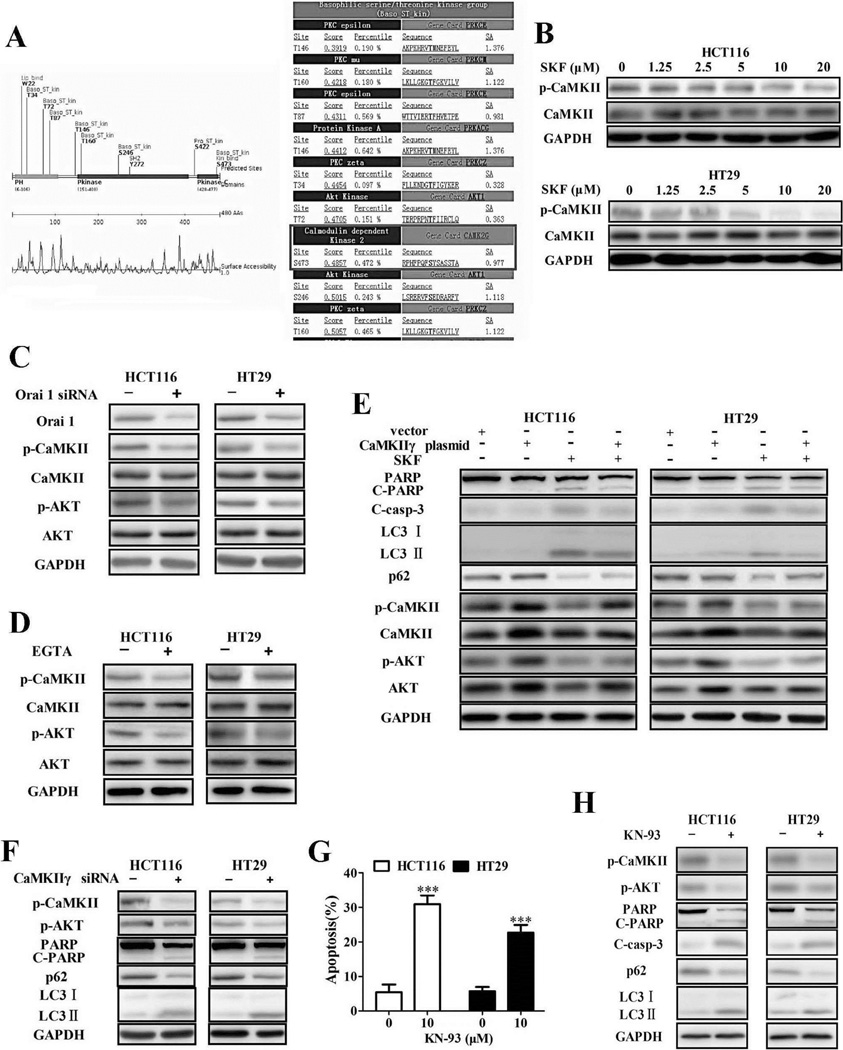
Recent studies have shown that AKT activity is regulated by a pathway that involves increased Ca2+ influx via the store-operated Ca2+ channels (SOC) [15,16]. However, the crucial genes linking Ca2+ influx to modulate AKT activity remains unknown. Here, Scansite, an online tool for predicting kinase-substrate interaction, was used to search for kinases that can phosphorylate AKT at the Ser 473 site. Of the potential regulators, we found that CaMKII could phosphorylate AKT at Ser 473 (http://scansite.mit.edu) (Fig. 7A). CaMKII is encoded by four different genes, α, β, γ, and δ. Using isoform-specific primers, we found that the γ-isoform is the major isoform in CRC cells (Fig. S9).
Fig. 7.
CaMKII couples store-operated Ca2+ influx to the downstream AKT/mTOR pathway. (A) The phosphorylation sites of AKT and their potential regulators. (B) Immunoblotting for phospho- or total CaMKII in cells treated with SKF-96365 for 24 h. Immunoblotting for phospho- or total AKT and CaMKII in cells transfected with siRNA targeting Orai1 (C) or in cells treated with 0.5 mM EGTA (D). (E) Cells with stable expression of active CaMKIIγ were incubated with 10 µM SKF-96365 for 24 h. Immunoblotting for p62, LC3, PARP, cleaved caspase-3, phospho- or total AKT and CaMKII. (F) Immunoblotting for phospho-AKT/CaMKII, PARP, cleaved caspase-3, and p62 using lysates from cells transfected with siRNA against CaMKIIγ for 24 h. (G) Cells were treated with KN-93 for 48 h before staining with Annexin V (AV) and propidium iodide (PI), and the apoptotic rates were determined by flow cytometry. (H) Autophagy and apoptosis-associated proteins, including p62, LC3, PARP, cleaved caspase-3, and phospho- AKT/CaMKII, were analyzed by immunoblotting in cells treated with 10 µM KN-93 for 24 h. ***P < 0.001.
Next, we evaluated the effect of SKF-96365 on CaMKIIγ. As shown in Fig. 7B, the phosphorylated levels of CaMKIIγ was greatly decreased by SKF-96365. Similarly, inhibition of SOCE through knockdown of Orai1 or treatment with the Ca2+ chelator EGTA also reduced the phosphorylation of CaMKIIγ and AKT, indicating that CaMKII couples SOCE-mediated Ca2+ influx to AKT activation (Fig. 7C and D). In another study, we found that CaMKIIγ overexpression led to an increase of p-AKT; a decrease of cleaved PARP, cleaved caspase-3 and LC3-II; and the accumulation of p62 in SKF-96365-treated cells (Fig. 7E). By contrast, genetic or pharmacological inhibition of CaMKIIγ by siRNA or KN93 resulted in a decrease of p-AKT and the induction of apoptosis and autophagy (Fig. 7F–H), similar to the observations derived from the SKF-96365-treated cells. Taken together, these results indicate that CaMKIIγ is the crucial target of SKF-96365 and couples store-operated Ca2+ entry to the downstream AKT/mTOR signaling cascade.
SKF-96365 inhibits CRC cell growth in vivo
To further determine the therapeutic potential of SKF-96365 in vivo, BALB/c nude mice were inoculated into the right oxter with HCT116 cells. When the xenografted tumors were measurable, mice were randomly assigned to receive hydroxychloroquine (HCQ), SKF-96365 or a combination of HCQ with SKF-96365. As shown in Fig. 8A–C, HCQ alone had no effect on the growth of tumors, and intraperitoneal injections of SKF-96365 at 20 mg/kg/day alone yielded moderate anti-tumor activity. Consistent with the in vitro results, a combination with the autophagy inhibitor HCQ significantly reduced tumor growth compared with SKF-96365 alone, indicating that SKF-96365 also activated cytoprotective autophagy in vivo. Immunohistochemistry (IHC) analysis showed that SKF-96365 decreased the expression of p-AKT and proliferating cell nuclear antigen (PCNA) but increased the expression of cleaved caspase-3 and LC3-II (Fig. 8D). Mechanistically, western blot analysis in lysate from xenografted tumors showed that SKF-96365 treatment resulted in a decrease of p-CaMKII and p-AKT as well as an increase in LC3-II, cleaved PARP, caspase-3, and caspase-9 (Fig. 8E). Moreover, no significant weight loss or liver and kidney injury were observed by combined treatments (Fig. S10). These data suggested that SKF-96365 inhibits CRC cells growth in vivo through inhibition of the CaMKIIγ/AKT-mediated pathway, which results in cell cycle arrest, apoptosis and cytoprotective autophagy. The combination with the autophagy inhibitor significantly increased the anti-cancer effect of SKF-96365.
Fig. 8.
Autophagy inhibition enhances the anti-tumor effect of SKF-96365 in HCT116 xenograft models. (A) Tumors from nude mice in each treatment group. Tumor volume (B) and weight (C) in each group. (D) Immunohistochemical staining of phospho-AKT, LC3, PCNA, and cleaved caspase-3 using paraffin-embedded sections. Bar = 20 µm. (E) Tumor lysate was subjected to immunoblotting for LC3, p62, PARP, cleaved caspase-9, cleaved caspase-3, and phospho- or total AKT/CaMKII. Each lane represents a lysate from eight mice. Western blot bands was quantified by ImageJ densitometric analysis and normalized by GAPDH. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Growing evidence supports the contribution of altered Ca2+ signaling to tumor progression and metastasis, suggesting that agents targeting calcium influx and calcium influx-driven downstream signaling may hopefully offer alternative approaches for cancer therapy [17,18]. Several calcium channels blockers have been investigated in clinical trials [19–21]; however, their efficacy has not been satisfactory for unclear reasons. Understanding the molecular mechanisms may help improve the efficacy of these agents.
In this study, we found that SKF-96365 induced mitochondria-dependent apoptosis in CRC cells. Bax translocation-mediated release of cyt c is a necessary and early event in the activation of intrinsic apoptosis [22]. Cytosolic cyt c initiates the apoptotic process by activating a downstream cascade of caspases through processing of procaspase-9 [23]. It is intriguing that the early appearance of cytosolic cyt c in SKF-96365-treated cells did not result in evident simultaneous caspases activation, indicating the presence of a cytoprotective mechanism. As a cellular catabolic mechanism in response to metabolic and therapeutic stresses, autophagy has a prodeath or a pro-survival role [24]. Previous studies have shown that the mitochondria play an important role in the cross talk between autophagy and apoptosis [25,26]. Mitochondrial membrane depolarization can promote sequestration of the mitochondria into autophagosomes, which results in inhibition of cyt c release from damaged mitochondria and thus blockade of intrinsic pro-apoptotic cascades [27,28]. In the current study, the depolarization of Δψm and autophagy emerged 12 h after SKF-96365-treatment, whereas apoptosis appeared 24 h later. We hypothesize that depolarization of Δψm and cyt c translocation induced by SKF-96365 can trigger apoptosis and cytoprotective autophagy simultaneously. During the early stages, autophagy can eliminate damaged mitochondria and then inhibit sustained release of cyt c (Fig. 4H). However, after SKF-96365 treatment is prolonged, the cytoprotective effect of autophagy seems to be saturated and then the apoptosis activation leads to cell death (Fig. 3D and E). In this respect, autophagy delays the occurrence of apoptosis. Moreover, we found that suppression of autophagy by pharmacological inhibitors (3-MA and CQ) or Beclin-1 siRNA markedly increased the cytotoxic effect of SKF-96365 in CRC cells by promoting apoptosis (Fig. 5). Interestingly, autophagy inhibition made apoptosis occur in 12 h after SKF-96365 treatment, indicating SKF-96365-induced autophagy preceded apoptosis (Fig. 5E).
Recent studies report that accelerated Ca2+ influx via the store-operated Ca2+ channels (SOC) could promote the AKT activity [29]. SOCE sustained constitutive activation of AKT and augmented survival of malignant melanoma cells [30,31]. AKT can downregulate radiation-induced autophagy and apoptosis in breast cancer cells [32]. The natural alkaloid piperlongumine was shown to activate apoptosis and autophagy in lung cancer cells through inhibition of AKT/mTOR pathway [33]. The antitumor enzyme asparaginase triggers apoptosis and cytoprotective autophagy in chronic myeloid leukemia cells by inhibition of AKT/mTOR signaling pathway [34]. In addition, the AKT/mTOR pathway can induce cell cycle progression by modulating the protein stability of cyclin B, Cdc25c, CDK-2/4, p27 and p21 [35,36]. Here, we demonstrated that an SOCE inhibitor, SKF-96365, exerts its anti-cancer effects by inducing cell-cycle arrest, apoptosis and autophagy in CRC cells through the inhibition of the AKT/mTOR signaling pathway, and overexpression of AKT significantly attenuated the biological effect of SKF-96365.
Currently, the networks linking Ca2+ influx to AKT activation are unknown. In the current study, we found that CaMKII has the potential to phosphorylate AKT at the Ser 473 site. CaMKII was shown to be a multifunctional calcium-dependent serine/threonine protein kinase that responds to the fluctuation of Ca2+ concentrations. There are four CaMKII homologs, each encoded by distinct genes (CaMKII α/β/γ/δ). Accumulating reports demonstrate that CaMKIIγ is preferentially expressed in some tumors to regulate cancer growth and survival [37–40]. Our results clearly demonstrated that CaMKIIγ is the major isoform of CaMKII in colon cancer cells (Fig. S11), and its overexpression stimulated AKT phosphorylation at Ser473, which impaired SKF-96365-induced apoptosis and autophagy (Fig. 7E). Furthermore, pharmacological or genetic inhibition of CaMKIIγ inhibited the phosphorylation of AKT and induced apoptosis and autophagy in HCT116 and HT29 cells (Fig. 7F–H), similar to the effect of SKF-96365, indicating the critical role of CaMKIIγ/AKT pathway in regulation of SOCE inhibitor-activated apoptotic or autophagic signaling. A recent study further supports our hypothesis for demonstrating that CaMKIIγ directly binds to AKT for phosphorylation at Ser 473 [41]. The current study is the first report to show that CaMKIIγ is an upstream regulator of apoptosis and autophagy, and inhibition of CaMKIIγ represents a promising strategy for treatment of CRC. Additionally, in the cohort from the GEO database (GSE17538), the upregulation of CaMKIIγ is strongly correlated with a dramatically shorter overall survival (the mean survival time was 75.7 months for patients with high CaMKIIγ levels versus 100.4 months for patients with low CaMKII levels; P = 0.0043) (Fig. S11).
Interestingly, we also observed that SKF-96365 inhibited the phosphorylated ERK1/2 (Thr202/Tyr204) remarkably in the PathScan intracellular signaling array, which was re-confirmed by western blot (data not shown). Transfection with constitutively active ERK1/2 plasmids could also reduce SKF-96365-induced growth inhibition significantly, like enforced expression of AKT in the treated cells (data not shown). The above results may explain the phenomenon that overexpression of AKT only partially attenuated the cytotoxicity of SKF-96365 in the treated cells. It has been reported that the CaMKIIγ directly or indirectly upregulated multiple signaling pathways such as ERK1/2, NF-κB, and β-catenin [39], all of which are important for cancer survival. The exact role of ERK1/2 in SKF-96365-induced cytotoxicity remains to be further investigated.
In conclusion, our results demonstrate that inhibition of SOCE by SKF-96365 suppresses cell proliferation and induces autophagy and apoptosis in CRC cells (Fig. S12). Autophagy and apoptosis contribute contrarily to SKF-96365-induced cytotoxicity. Apoptosis causes cancer cell death, whereas autophagy plays a cytoprotective role to delay apoptosis. Moreover, we demonstrated that SKF-96365 triggered cross talk between apoptosis and autophagy via inhibition of the calcium/CaMKII-mediated AKT/mTOR pathway (Fig. S12). Our data support the hypothesis that enhanced SOCE-mediated Ca2+ influx serves to activate downstream AKT/mTOR pathways, which contributes to colorectal carcinogenesis. Hence, our discovery, for the first time, not only provides further insights into the anticancer potential of SOCE inhibitors but also provides an alternative strategy for combining SOCE inhibitors with autophagy inhibitors for CRC patients.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81272593, 81572592), the Zhejiang Medicines and Health Platform Program (2014ZDA012), and the National Health and Family Planning Commission Fund (2015112271) to H. Pan, the National Natural Science Foundation of China (81372621, 81572361), and Zhejiang Province Preeminence Youth Fund (LR16H160001) to W. Han.
Abbreviations
- Δψm
membrane potential
- 3-MA
3-methyladenine
- Ca2+
calciumion
- CaMKIIγ
calmodulin-dependent kinase IIγ
- COX-2
cyclooxygenase-2
- CQ
chloroquine
- CRC
colorectal cancer
- cyt c
cytochrome c
- EMT
epithelial-to-mesenchymal transition
- ER
endoplasmic reticulum
- ESCC
esophageal squamous cell carcinoma
- HCQ
hydroxychloroquine
- iCa2+
intracellular calcium
- IHC
immunohistochemistry
- LC3
microtubule-associated protein 1 light chain 3
- PARP
poly (ADP-ribose) polymerase
- PCNA
proliferating cell nuclear antigen
- PGE2
prostaglandin E2
- PI
propidium iodide
- SOCE
store-operated Ca2+ entry
- STIM
stromal interacting molecule
- TG
thapsigargin
- TRPC
transient receptor potential channel
Footnotes
Competing financial interests
The authors declare no competing financial interests.
Author contribution statement
W.H. and H.P. conceived the concept and designed the study; Z.J., X.S., J.Y., J.X., and L.J. performed the experiments; Z.J., Y.Z. and W.H. analyzed the data and prepared the figures; W.H., Z.J., and X.S. wrote the paper; all authors reviewed the manuscript.
Conflicts of interest statement
Zhao Jing, Xinbin Sui, Junlin Yao, Jiansheng Xie, Liming Jiang, Yubin Zhou, Hongming Pan and Weidong Han declare that: we have no proprietary, financial, professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled: SKF-96365 activates cytoprotective autophagy to delay apoptosis in colorectal cancer cells through inhibition of the calcium/ CaMKIIγ/AKT-mediated pathway.
Appendix: Supplementary materials
Supplementary data to this article can be found online at doi:10.1016/j.canlet.2016.01.006.
References
- 1.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Fahrenholtz CD, Greene AM, Beltran PJ, Burnstein KL. A novel calcium-dependent mechanism of acquired resistance to IGF-1 receptor inhibition in prostate cancer cells. Oncotarget. 2014;5:9007–9021. doi: 10.18632/oncotarget.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y, Wang J, Zhou H, Yu X, Hu L, Meng F, et al. Effects of calcium signaling on coagulation factor VIIa-induced proliferation and migration of the SW620 colon cancer cell line. Mol. Med. Rep. 2014;10:3021–3026. doi: 10.3892/mmr.2014.2665. [DOI] [PubMed] [Google Scholar]
- 4.Stathopulos PB, Schindl R, Fahrner M, Zheng L, Gasmi-Seabrook GM, Muik M, et al. STIM1/Orai1 coiled-coil interplay in the regulation of store-operated calcium entry. Nat Commun. 2013;4:2963. doi: 10.1038/ncomms3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang JY, Sun J, Huang MY, Wang YS, Hou MF, Sun Y, et al. STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression. Oncogene. 2015;34:4358–4367. doi: 10.1038/onc.2014.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Liu X, Feng B, Liu N, Wu Q, Han Y, et al. STIM1, a direct target of microRNA-185, promotes tumor metastasis and is associated with poor prognosis in colorectal cancer. Oncogene. 2015;34:4808–4820. doi: 10.1038/onc.2014.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung YM, Kwan CY. Current perspectives in the pharmacological studies of store-operated Ca2+ entry blockers. Jpn J. Pharmacol. 1999;81:253–258. doi: 10.1254/jjp.81.253. [DOI] [PubMed] [Google Scholar]
- 8.Cai R, Ding X, Zhou K, Shi Y, Ge R, Ren G, et al. Blockade of TRPC6 channels induced G2/M phase arrest and suppressed growth in human gastric cancer cells. Int. J. Cancer. 2009;125:2281–2287. doi: 10.1002/ijc.24551. [DOI] [PubMed] [Google Scholar]
- 9.Zhu H, Zhang H, Jin F, Fang M, Huang M, Yang CS, et al. Elevated Orai1 expression mediates tumor-promoting intracellular Ca2+ oscillations in human esophageal squamous cell carcinoma. Oncotarget. 2014;5:3455–3471. doi: 10.18632/oncotarget.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selvaraj S, Sun Y, Sukumaran P, Singh BB. Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway. Mol. Carcinog. 2015 doi: 10.1002/mc.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Wang QL, Zhan YZ, Duan HJ, Cao YJ, He LC. Role of store-operated calcium entry in imperatorin-induced vasodilatation of rat small mesenteric artery. Eur. J. Pharmacol. 2010;647:126–131. doi: 10.1016/j.ejphar.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Guthrie HD, Welch GR. Determination of high mitochondrial membrane potential in spermatozoa loaded with the mitochondrial probe 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1) by using fluorescence-activated flow cytometry. Methods Mol. Biol. 2008;477:89–97. doi: 10.1007/978-1-60327-517-0_8. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Syed GH, Khan M, Chiu WW, Sohail MA, Gish RG, et al. Hepatitis C virus triggers mitochondrial fission and attenuates apoptosis to promote viral persistence. Proc. Natl. Acad. Sci. U.S.A. 2014;111:6413–6418. doi: 10.1073/pnas.1321114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinsay MN, Lee Y, Rikka S, Sayen MR, Molkentin JD, Gottlieb RA, et al. Bnip3 mediates permeabilization of mitochondria and release of cytochrome c via a novel mechanism. J. Mol. Cell. Cardiol. 2010;48:1146–1156. doi: 10.1016/j.yjmcc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt S, Liu G, Liu G, Yang W, Honisch S, Pantelakos S, et al. Enhanced Orai1 and STIM1 expression as well as store operated Ca2+ entry in therapy resistant ovary carcinoma cells. Oncotarget. 2014;5:4799–4810. doi: 10.18632/oncotarget.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa A, Firth AL, Smith KA, Maliakal MV, Yuan JX. PDGF enhances store-operated Ca2+ entry by upregulating STIM1/Orai1 via activation of Akt/mTOR in human pulmonary arterial smooth muscle cells. Am. J. Physiol. Cell Physiol. 2012;302:C405–C411. doi: 10.1152/ajpcell.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balasubramaniam SL, Gopalakrishnapillai A, Gangadharan V, Duncan RL, Barwe SP. Sodium-calcium exchanger 1 regulates epithelial cell migration via calcium-dependent extracellular signal-regulated kinase signaling. J. Biol. Chem. 2015;290:12463–12473. doi: 10.1074/jbc.M114.629519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu KH, Yang ST, Lin YK, Lin JW, Lee YH, Wang JY, et al. Fluoxetine, an antidepressant, suppresses glioblastoma by evoking AMPAR-mediated calcium-dependent apoptosis. Oncotarget. 2015;6:5088–5101. doi: 10.18632/oncotarget.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson EA, Marks RS, Mandrekar SJ, Hillman SL, Hauge MD, Bauman MD, et al. Phase III randomized, double-blind study of maintenance CAI or placebo in patients with advanced non-small cell lung cancer (NSCLC) after completion of initial therapy (NCCTG 97-24-51) Lung Cancer. 2008;60:200–207. doi: 10.1016/j.lungcan.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Kohn EC, Reed E, Sarosy G, Christian M, Link CJ, Cole K, et al. Clinical investigation of a cytostatic calcium influx inhibitor in patients with refractory cancers. Cancer Res. 1996;56:569–573. [PubMed] [Google Scholar]
- 21.Mross K, Bohn C, Edler L, Jonat W, Queisser W, Heidemann E, et al. Randomized phase II study of single-agent epirubicin +/− verapamil in patients with advanced metastatic breast cancer. An AIO clinical trial. Arbeitsgemeinschaft Internistische Onkologie of the German Cancer Society. Ann. Oncol. 1993;4:45–50. doi: 10.1093/oxfordjournals.annonc.a058356. [DOI] [PubMed] [Google Scholar]
- 22.Jiang X, Jiang H, Shen Z, Wang X. Activation of mitochondrial protease OMA1 by Bax and Bak promotes cytochrome c release during apoptosis. Proc. Natl. Acad. Sci. U.S.A. 2014;111:14782–14787. doi: 10.1073/pnas.1417253111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D, Armstrong JS. Bax and the mitochondrial permeability transition cooperate in the release of cytochrome c during endoplasmic reticulum-stress-induced apoptosis. Cell Death Differ. 2007;14:703–715. doi: 10.1038/sj.cdd.4402072. [DOI] [PubMed] [Google Scholar]
- 24.Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014;344:174–179. doi: 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Booth LA, Tavallai S, Hamed HA, Cruickshanks N, Dent P. The role of cell signalling in the crosstalk between autophagy and apoptosis. Cell. Signal. 2014;26:549–555. doi: 10.1016/j.cellsig.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 28.Elmore SP, Nishimura Y, Qian T, Herman B, Lemasters JJ. Discrimination of depolarized from polarized mitochondria by confocal fluorescence resonance energy transfer. Arch. Biochem. Biophys. 2004;422:145–152. doi: 10.1016/j.abb.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 29.Zou W, Meng X, Cai C, Zou M, Tang S, Chu X, et al. Store-operated Ca2+ entry (SOCE) plays a role in the polarization of neutrophil-like HL-60 cells by regulating the activation of Akt, Src, and Rho family GTPases. Cell. Physiol. Biochem. 2012;30:221–237. doi: 10.1159/000339059. [DOI] [PubMed] [Google Scholar]
- 30.Feldman B, Fedida-Metula S, Nita J, Sekler I, Fishman D. Coupling of mitochondria to store-operated Ca(2+)-signaling sustains constitutive activation of protein kinase B/Akt and augments survival of malignant melanoma cells. Cell Calcium. 2010;47:525–537. doi: 10.1016/j.ceca.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Fedida-Metula S, Feldman B, Koshelev V, Levin-Gromiko U, Voronov E, Fishman D. Lipid rafts couple store-operated Ca2+ entry to constitutive activation of PKB/Akt in a Ca2+/calmodulin-, Src- and PP2A-mediated pathway and promote melanoma tumor growth. Carcinogenesis. 2012;33:740–750. doi: 10.1093/carcin/bgs021. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Zhao D, Xie Z, Qi Y. Down-regulation of AKT combined with radiation-induced autophagy and apoptosis roles in MCF-7 cells. Biomed Mater. Eng. 2015;26(Suppl. 1):S2259–S2265. doi: 10.3233/BME-151532. [DOI] [PubMed] [Google Scholar]
- 33.Wang F, Mao Y, You Q, Hua D, Cai D. Piperlongumine induces apoptosis and autophagy in human lung cancer cells through inhibition of PI3K/Akt/mTOR pathway. Int. J. Immunopathol. Pharmacol. 2015;28:362–373. doi: 10.1177/0394632015598849. [DOI] [PubMed] [Google Scholar]
- 34.Song P, Ye L, Fan J, Li Y, Zeng X, Wang Z, et al. Asparaginase induces apoptosis and cytoprotective autophagy in chronic myeloid leukemia cells. Oncotarget. 2015;6:3861–3873. doi: 10.18632/oncotarget.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viola G, Bortolozzi R, Hamel E, Moro S, Brun P, Castagliuolo I, et al. MG-2477, a new tubulin inhibitor, induces autophagy through inhibition of the Akt/mTOR pathway and delayed apoptosis in A549 cells. Biochem. Pharmacol. 2012;83:16–26. doi: 10.1016/j.bcp.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li JP, Yang YX, Liu QL, Pan ST, He ZX, Zhang X, et al. The investigational Aurora kinase A inhibitor alisertib (MLN8237) induces cell cycle G2/M arrest, apoptosis, and autophagy via p38 MAPK and Akt/mTOR signaling pathways in human breast cancer cells. Drug Des. Devel. Ther. 2015;9:1627–1652. doi: 10.2147/DDDT.S75378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Si J, Collins SJ. Activated Ca2+/calmodulin-dependent protein kinase IIgamma is a critical regulator of myeloid leukemia cell proliferation. Cancer Res. 2008;68:3733–3742. doi: 10.1158/0008-5472.CAN-07-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu Y, Chen T, Meng Z, Gan Y, Xu X, Lou G, et al. CaMKII gamma, a critical regulator of CML stem/progenitor cells, is a target of the natural product berbamine. Blood. 2012;120:4829–4839. doi: 10.1182/blood-2012-06-434894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chai S, Qian Y, Tang J, Liang Z, Zhang M, Si J, et al. Ca(2+)/calmodulin-dependent protein kinase IIgamma, a critical mediator of the NF-kappaB network, is a novel therapeutic target in non-small cell lung cancer. Cancer Lett. 2014;344:119–128. doi: 10.1016/j.canlet.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 40.Meng Z, Li T, Ma X, Wang X, Van Ness C, Gan Y, et al. Berbamine inhibits the growth of liver cancer cells and cancer-initiating cells by targeting Ca(2)(+)/calmodulin-dependent protein kinase II. Mol. Cancer Ther. 2013;12:2067–2077. doi: 10.1158/1535-7163.MCT-13-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chai S, Xu X, Wang Y, Zhou Y, Zhang C, Yang Y, et al. Ca2+/calmodulin-dependent protein kinase IIgamma enhances stem-like traits and tumorigenicity of lung cancer cells. Oncotarget. 2015;6:16069–16083. doi: 10.18632/oncotarget.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.