We review here the macrophages found in endocrine tissues, placing emphasis on those residing in the islets of Langerhans of the pancreas. The islets represent the endocrine organ where macrophages have been examined in great detail and where our own studies and experience have been directed.
That macrophages normally inhabit endocrine organs became evident from the early studies in mice made in the laboratories of Siamon Gordon and David Hume (1). They used the macrophage surface marker F4/80 to examine tissue sections by immunohistochemistry and found macrophages in all endocrine organs. In most organs the macrophages were found associated with the vessels. To note are several important examples: in the adrenal gland the zona glomerulosa contained abundant macrophages, “…wrapped around capillaries or line vascular sinuses, but membrane processes extend into the surrounding tissue..” In the pituitary they found them distributed differentially in the various areas. In the thyroid many surrounded the follicles. In the testes the macrophages were next to the Leydig cells and not inside the seminiferous tubules. In the ovaries macrophages were abundant, around follicles and usually surrounding the vessels.
In sum they found three distinctive features of endocrine resident macrophages: i] most were associated with blood vessels; ii] some surrounded the endocrine cells or glands, extending filopodia among them; and iii] in some organs such as the pituitary there were differences in the distribution depending on the anatomy.
An understanding of the important role of macrophages in the normal homeostasis of endocrine organs has come from many studies, but particularly from Jeffrey Pollard’s group, many carried out in the Csfmop/op [op/op] mouse strain (2,3,4)]. [Homeostasis is the term used by Walter B. Cannon in discussing the milieu interieur of Claude Bernard: at the cellular level we refer to those cell functions needed to maintain a tissue under steady state (5)]. The op/op mouse strain has a natural null mutation in the Csf1 gene and lacks functional CSF-1 resulting in marked absence of macrophages in most tissues (6). CSF-1 is a protein made in several tissues that regulate the differentiation of the macrophage lineage (7). The op/op mice were first described for their osteopetrosis resulting from a lack of osteoclasts, a differentiated cell of the macrophage lineage required to remove bone matrix. The production of CSF-1 was absent from all tissues examined (7). In the initial evaluation of this strain carried out by Stanley, Pollard and associates, a partial correction was obtaind by infusing mice with CSF-1. The op/op mice also showed a number of abnormalities particularly in endocrine glands (4). The strain is an eloquent example of the role that macrophages play beyond the classical scavenger and phagocytic function accentuated since their classical description by Metchnikoff that resulted in the “phagocytic theory” of immunity.
The islets of Langerhans
Pancreatic islets in all species contain a resident set of macrophages. These were first considered as “passenger leukocytes”, referring to blood leukocytes thought to be trapped in isolated islets. In a series of studies carried out using isolated islets for transplantation to diabetic patients, it was found that a preculture of the islets resulted in the escape of leukocytes from them, hence the conclusion that these cells derived from blood cells. These islets transplanted in certain allogeneic combinations were not rejected. The implication was that the macrophages were the target of the transplantation reaction since they expressed the proteins encoded in the Major Histocompatibility gene Complex (MHC). These findings are extensively reviewed with appropriate references in our refs 8. Studying the phagocytes in islets is important particularly because this mini-organ is the target of diabetic autoimmunity (9). Macrophages have an antigen presenting function that is relevant in the autoimmune process.
Recent analyses have been carried out examining islets obtained from the pancreas using combinations of cell surface markers and gene expression analysis. Islets are harvested by techniques that separate them from the acinar tissue. The islets are then disrupted so individual cells can be examined. These recent studies clearly established that islets under steady state conditions, that is under non-inflammatory situations, harbor a normal set of resident macrophages (10). These macrophages are represented by a single set identified by their display of membrane proteins typical of macrophages as well as by their expression of macrophage specific genes.
Most of the detailed analyses of islet macrophages have been carried out in the mouse. Islets isolated from non-diabetic mice contain a small number of macrophages, usually from 5 to 10. There is an interesting correlation between the size of the islets and their number of macrophages: for example about 5% to 10% of islets are of large size and can harbor as many as thirty macrophages. The macrophages are located next to blood vessels and in very close contact with beta cells; in live image analysis of islets, the macrophages are seen extending long filopodia in between beta cells, some reaching up to the edge of the islet. Moreover, filopodia also extend along the vessel wall and into the blood vessel lumen.
Of great interest are the findings showing that every macrophages takes up secretory granules from beta cells (11). This uptake was substantiated by electron microscopy of islets that showed typical insulin containing dense core granules inside the phagocytic vesicles of the macrophages. Examination of the macrophages using antibodies to insulin or insulin products confirmed the presence of insulin and insulin peptides inside the macrophage phagocytic vesicles (12). Such uptake is a normal feature of the islet macrophage found in all strains examined. The uptake requires live beta cells and it is unrelated to beta cell death.
Pertinent to this finding are the studies made in the NOD mouse, an inbred strain that spontaneously develops autoimmune diabetes. In most mouse colonies the majority of the NOD mice become diabetic within the 18th to the 24th week of life, with islets heavily infiltrated by lymphocytes and inflammatory cells. Diabetes autoimmunity is an autoimmune disease caused by autoreactive T cells that recognize peptides derived from the intracellular processing of beta cell proteins. These peptides associate with the MHC class II molecules (MHC-II) to form the molecular complex recognized by the T cells. The MHC- II genes that confer the propensity for diabetes are very similar structurally between the NOD mouse and humans with type 1 diabetes (13, 14). In both situations their MHC-II molecules lack a critical amino acid, an aspartic acid at the beta chain residues 57. The MHC-II peptidomes of the diabetes propensity molecules from human and mice are very similar. Analysis by mass spectrometry of peptides eluted from the MHC-II molecules showed them to be rich in peptides with acidic residues at their carboxy-end (15). In the NOD mouse that develops spontaneous diabetic autoimmunity the uptake of beta cell granules has biological significance: macrophages degrade the content of the granules incorporating some of the peptides to their MHC-II proteins to be presented to autoreactive T cells (10,11). Beta cells do not express MHC-II proteins but only MHC-I at low levels. Importantly, besides macrophages the islets also contain dendritic cells that infiltrate at a very early stage in the process and that are essential for the development of the autoimmunity presumably in cooperativity with the resident macrophages (16). One autoantigen that is prominent in both NOD and in patients with type 1 diabetes is insulin (9,17,18).
The physiological role of the islet macrophages is to maintain islet homeostasis (2, 10). There is a truly symbiotic relationship between beta cells and macrophages both influencing each other. Examination of the osteopetrotic op/op mouse is to the point: their islets are half the size of the normal and hypoinsulinemic (2, 10). Most islets lack macrophages, at the most, a few may have one or two macrophages per islet. Many of the op/op mice have an impaired glucose tolerance test. On the macrophage side, and in contrast to macrophages from other tissues, the islet macrophages in wild type mice have a gene signature of activation with expression of TNF and IL-1 beta, and very high levels of MHC-II molecules (10). Their gene expression pattern is compatible with an “M1-like” activated macrophage. There is a range of genes expressed in macrophages under conditions of activation and in broad terms can be grouped into an M1 or M2 pattern (19). The M2 gene activation signature was defined after IL-4 treatment (20) and believed to be involved in tissue repair.
The recent report from Calderon et al made a critical analysis of pancreatic macrophages in non-diabetic mice. Islet macrophages were found during embryological development from definitive hematopoiesis (10). (For a review on recent developments in macrophage differentiation see reference 21.) Normally in non-inflammatory conditions islet macrophages showed a low level of replication and were not supplied by blood monocytes. Parabiosis experiments were carried out between members of the pair having a different CD45 allele: there was no exchange between the two partners while monocytes and lymphocytes in the spleen exchanged evenly between the two mice (10). These resident macrophages were maintained by a low level of proliferation.
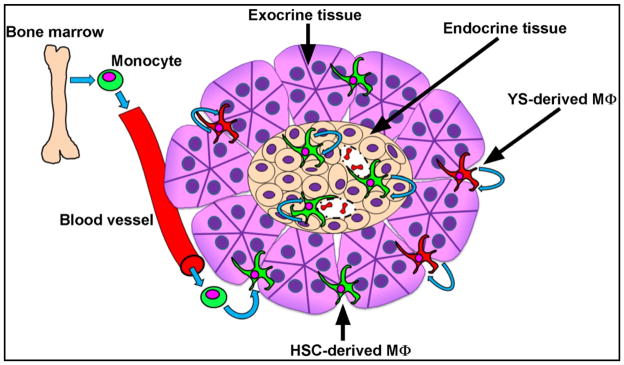
Macrophages are also found in the inter-acinar stroma, about ten times more than in the islets. But these stromal macrophages are very different from those in the islets (Table 1). The stromal macrophages are composed of two distinct sets based on their embryological development, and by their expression of the mannose receptor, CD206, and the protein CLEC10A, CD301. One set expresses low levels of CD206/CD301, high levels of MHC-II molecules, and are constantly maintained from blood monocytes. This set of macrophages are dispersed throughout the stroma. A second set expresses high levels of CD206/CD301 and derive from yolk-sac hematopoiesis. This set is enriched around the pancreatic ducts. Thus both sets of stromal macrophages have a different anatomical localization. Both sets of stromal macrophages express an M2-like pattern of gene transcripts in contrast to the islet macrophages that express an M1-like pattern. The gene expression signatures in islets and stromal macrophages is maintained with time.
Table 1.
Features of Pancreatic Macrophages
| Features | Islet | Stroma-1 | Stroma-2 |
|---|---|---|---|
| Derivation | From embryo Definite hematopoiesis | Yolk-sac Hematopoiesis | Blood monocytes |
| Anatomy | Next to blood vessels | Dispersed | Enriched in peritubular areas |
| Turnover | Resident | Resident | Supplied by blood monocytes |
| Gene Signature | M-1 | M-2 | M-2 |
| Gene Signature after irradiation | M-1 | M-2 | M-2 |
| MHC-II | High | Low | High |
| Role | Homeostatic capture granules | Not known | Not known |
| Pathology | In autoimmune diabetes | Not known Role in pancreatic cancer? |
Not known |
An important experimental manipulation made in the report of Calderon et al was to irradiate heavily the mice in order to deplete the islet macrophages, and to then replace them with bone marrow stem cells. After a period of time, the pancreas was examined. The new macrophages in islets and stroma had the same gene expression pattern as before, M1-like for those of the islets, M2-like for the stroma ones. The differences most likely are explained by the anatomy of the pancreas, its micro-environment. The Calderon et al study concluded that the “pancreas anatomy conditions the origin and properties of the resident macrophages” (10).
Reproductive organs: testes and ovaries
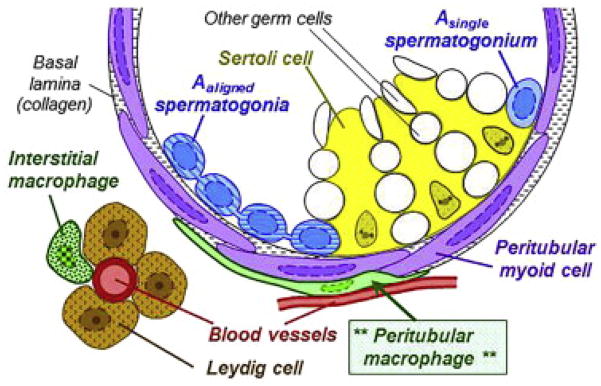
Macrophages are found in the testes where they play a major physiological role (4,22–25). As in the pancreas, macrophages are located in two distinct anatomical sites in the mammalian testes (26). One site is in the interstitium between seminiferous tubules: the macrophages are closely associated with Leydig cells, the testosterone producing cells. The second site is around the border of the seminiferous tubules, forming part of the peritubular capsule (26, see Comment in reference 27). At both sites macrophages profoundly influence testicular function (4, 22–24). In the interstitium the macrophages form tight clusters with Leydig cell (28,29). There is a striking close contact between both cells with cytoplasmic extensions of Leydig cells contacting the macrophages in close membrane to membrane contacts (29).
The macrophages are found during embryonal differentiation of the testes and have a major role in its development and early vascularization. Lineage tracing studies indicate that the macrophages derive from early hematopoietic stem cells in the yolk sac (30). Such macrophages have a gene signature compatible with an M2 features, and express proteins typical of macrophages like Fc receptors. Isolated they can be shown to release cytokines (22). Studies in the op/op mouse showed markedly reduced numbers of macrophages. Accompanying this reduction there was a parallel reduction in the number of Leydig cells and a low level of testosterone (4,25). Leydig cells in the op/op mouse have a dilated endoplasmic reticulum, together with abnormal vesicles. The macrophages produce 25-hydroxycholesterol an intermediate in the biosynthesis of testosterone and may be a substrate for its production by the Leydig cell (28).
As mentioned, macrophages are also found tightly associated with the seminiferous tubules (26). The cells are situated on the outside surface of tubules closed to the stem cells niche that gives rise to spermatogonia (op cit). These macrophages show long filopodia extending along the surface of the tubule. They produce CSF-1 that contributes to the differentiation of the sperm cells. Macrophages from the two sites i.e. the interstitium next to Leyding cell, or on the seminiferous tubules surface can be differentiated by their expression of the receptor for CSF-1 and expression of MHC-II molecules. Those in the intestitium have receptor for CSF-1, the CFSR1, but are MHC-II negative and the reverse was shown for the macrophages of the seminiferous tubules (26). The relationship between these two sets of macrophages is not known but the testes like the pancreas represent another examples of the tissue conditioning their macrophage resident population while being profoundly influenced by it: a true symbiosis.
The testes has been considered an immune privileged tissue and the macrophages may be part of the barrier to the protection of the sperm cells (reviewed in 31, 32). Whether the macrophages have an antigen presenting function in autoimmune orchitis or autoimmune spermatogenesis has not been established. In experimental autoimmune orchitis in which the animal is immunized with testes antigen, an initial reaction is at the surface of the seminiferous tubules (33). The thinking is that the macrophages at that site contain antigens derived from spermatogonia and may have a presenting function for autoreactive T cells.
Macrophages are also found in the ovaries (4,34). They are situated at different sites within the interstitial tissue that surrounds the ovarian follicles but never inside them. CSF1 is made by cells in the granulosa layer of the follicle and has been thought to have a role in maintaining the macrophage population (4). During ovulation the number of macrophages increases in the theca layer outside of the follicle. After the ovum is released, macrophages are prominent in the corpus luteum (35–37). The op/op mouse has defective ovarian function, ascribed in part to the profound reduction in macrophage numbers (4,38). Estrous cycles are much longer and the degree of ovulation is reduced. In brief like in the other endocrine organs, the macrophage modulates the function of the organ (3,4,39)
Other endocrine organs
As reported by Gordon and Hume and collaborators (1), macrophages also are found in adrenal glands, thyroid, and in the pituitary. Analysis of these tissue resident macrophages in terms of their role and embryonic derivation have yet to be done. In the adrenal, the macrophages are found in both cortex and medulla although the number in each layer varies. Macrophages are reported to extend long projections between the endocrine cells and localizing close to blood vessels (1). Addison’s disease is a rare autoimmune disease that results in the destruction of the adrenal cortex. Most patients have autoantibodies to steroid 21-hydroxylase an intracellular endoplasmic reticulum protein enzyme involved in steroid biosynthesis (40). CD4 and CD8 T cells directed to peptides of the enzyme have been reported (41). Although not examined it is likely that the macrophages are responsible for the uptake of the intracellular material and participate in the initiation of the autoimmune process.
Concerning the thyroid few studies have examined the macrophages under steady state conditions. Macrophages have been detected in the pituitary gland. Their distribution varies with the greatest number in the anterior and posterior lobes and practically absent in the intermediate lobe (1,34). The hypothalamus-pituitary-gonadal axis is markedly defective in the op/op mouse (4), but the nature of the macrophage involvement has not been examined.
Final comment
Of the various endocrine organs, the pancreatic islets and the testes are the two in which macrophages have been examined most extensively. There are three important results coming out of their analysis. The first concerns the different gene signatures among the macrophages. The studies in the pancreas where those in the inter-acinar stroma differ from those in the islets, are particularly informative and points to the micro environment controlling the biological response of the macrophage. A second important conclusion is that the endocrine cells require the trophic function of the macrophage. This requirement was shown best through the examination of the op/op mouse. The op/op mice show defective islets and testes function. It is also apparent that macrophages also influences ovarian function and the pituitary-gonadal axis. However not very detailed examination of the macrophages in the latter two sites have been carried out. The conclusion is that there is a striking symbiosis between the endocrine cells and the macrophages, each deeply influencing each other. Finally the macrophages are taking up products of the surrounding endocrine cells. It is likely that these may be the molecules that influence the behavior of the macrophage. As we discussed in the pancreatic islets, the macrophages take up whole dense core granules which not only contain insulin, about 3 × 105 molecules per granule, but other bioactive molecules such as ATP and granins.
Finally to note is that endocrine organs are one of the most frequent targets for autoimmunity and one needs to explain it. Having macrophages expressing high levels of MHC-II plus containing the molecules responsible for the autoimmune reaction places the macrophage as a central player.
Figure 1. Pancreatic macrophages.
The pancreas contains three sets of macrophages. One set resides in the islets-labelled endocrine tissue and have an M1-like transcriptional signature. Two sets are in the stroma between the acinar gland- labelled exocrine tissue. Both have an M2-like transcriptional signature. One set derives from blood monocytes, the second derives from yolk sac hematopoiesis. Taken from Calderon et al, reference 11.
Figure 2. Testicular macrophages.
The macrophages in the testes are found in two locations. One set is in the interstitium forming a cluster with Leydig cells. The second set surrounds the seminiferous tubules- theperitubular macrophages. Taken from Meistrich and Shetty, reference 27
References
- 1.Hume DA, Halpin D, Charlton H, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of endocrine organs. Proc Natl Acad Sci U S A. 1984;81:4174–7. doi: 10.1073/pnas.81.13.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, Castellotti MC, Czernichow P, Pollard JW, Polak M. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukoc Biol. 2004;76:359–367. doi: 10.1189/jlb.1103591. [DOI] [PubMed] [Google Scholar]
- 3.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen PE, Nishimura K, Zhu L, Pollard JW. Macrophages: important accessory cells for reproductive function. J Leukoc Biol. 1999;66(5):765–772. doi: 10.1002/jlb.66.5.765. [DOI] [PubMed] [Google Scholar]
- 5.Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9:399–431. [Google Scholar]
- 6.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–4. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 7.Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Jr, Ahmed-Ansari A, Sell KW, Pollard JW, Stanley ER. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calderon B, Unanue ER. Antigen presentation events in autoimmune diabetes. Curr Opin Immunol. 2011;24:1–10. doi: 10.1016/j.coi.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unanue ER. Antigen presentation in the autoimmune diabetes of the NOD mouse. Annu Rev Immunol. 2014;32:579–608. doi: 10.1146/annurev-immunol-032712-095941. [DOI] [PubMed] [Google Scholar]
- 10.Calderon B, Carrero JA, Ferris ST, Sojka DK, Moore L, Epelman S, Murphy KM, Yokoyam WM, Randolph GJ, Unanue ER. The pancreas anatomy conditions the origin and properties of resident macrophages. J Exp Med. 2015;212:1497–15. doi: 10.1084/jem.20150496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vomund AN, Zinselmeyer BH, Hughes J, Calderon B, Valderrama C, Ferris ST, Wan X, Kanekura K, Carrero JA, Urano F, Unanue ER. Beta cell transfer vesicles containing insulin to phagocytes for presentation to T cells. Proc Natl Acad Sci USA. 2015;112:5496–5502. doi: 10.1073/pnas.1515954112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol. 2010;11:350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Todd JA, Bell JI, McDevitt HO. HLA-DQ β gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329:599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 14.Acha-Orbea H, McDevitt HO. The first external domain of the nonobese diabetic mouse class II I-A beta chain is unique. Proc Natl Acad Sci USA. 1987;84:2435–39. doi: 10.1073/pnas.84.8.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suri A, Walters JJ, Gross M, Unanue ER. Natural peptides selected by diabetogenic DQ8 and murine I-Ag7 molecules show common sequence specificity. J Clin Invest. 2005;115:2268–76. doi: 10.1172/JCI25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferris ST, Carrero JA, Mohan JF, Calderon B, Murphy KM, Unanue ER. A minor subset of Batf3-dependent antigen presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity. 2014;41:657–669. doi: 10.1016/j.immuni.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr Opin Immunol. 2008;20:111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brezar V, Carel JC, Boitard C, Mallone R. Beyond the hormone: insulin as an autoimmune target in type 1 diabetes. Endocr Rev. 2011;32:623–669. doi: 10.1210/er.2011-0010. [DOI] [PubMed] [Google Scholar]
- 19.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon S. Alternative activation of macrophages. Nature Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 21.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol. 2016;17:2–8. doi: 10.1038/ni.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutson JC. Physiologic interactions between macrophages and Leydig cells. Exp Biol Med. 2006;231:1–7. doi: 10.1177/153537020623100101. [DOI] [PubMed] [Google Scholar]
- 23.Hutson JC. Testicular macrophages. Int Rev Cytol. 1994;149:99–143. doi: 10.1016/s0074-7696(08)62087-2. [DOI] [PubMed] [Google Scholar]
- 24.Bhushan S, Meinhardt A. The macrophage in testes function. J Reproduct Immunity. 2016 Jun 30; doi: 10.1016/j.jri.2016.06.008. pii: S0165-0378(16)30083-3. [DOI] [Google Scholar]
- 25.Pollard JW, Dominguez MG, Mocci S, Cohen PE, Stanley ER. Effect of the colony-stimulating factor-1 null mutation, osteopetrotic (csfm(op)), on the distribution of macrophages in the male mouse reproductive tract. Biol Reprod. 1997 May;56(5):1290–300. doi: 10.1095/biolreprod56.5.1290. [DOI] [PubMed] [Google Scholar]
- 26.DeFalco T, Potter SJ, Williams AV, Waller B, Kan MJ, Capel B. Macrophages contribute to the spermatogonial niche in the adult testis. Cell Rep. 2015;12:1107–19. doi: 10.1016/j.celrep.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meistrich ML, Shetty G. The New Director of “the Spermatogonial Niche”: Introducing the Peritubular Macrophage. Cell Rep. 2015;12:1069–70. doi: 10.1016/j.celrep.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 28.Lukyanenko YO, Chen JJ, Hutson JC. Production of 25-hydroxycholesterol by testicular macrophages and its effects on Leydig cells. Biol Reprod. 2001;64:790–6. doi: 10.1095/biolreprod64.3.790. [DOI] [PubMed] [Google Scholar]
- 29.Hutson JC. Development of cytoplasmic digitations between Leydig cells and testicular macrophages of the rat. Cell Tissue Res. 1992;267:385–9. doi: 10.1007/BF00302977. [DOI] [PubMed] [Google Scholar]
- 30.DeFalco T, Bhattacharya I, Williams AV, Sams DM, Capel B. Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc Natl Acad Sci U S A. 2014;111:E2384–93. doi: 10.1073/pnas.1400057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanton PG. Regulation of the blood-testis barrier. Semin Cell Dev Biol. 2016 Jun 25; doi: 10.1016/j.semcdb.06.018. pii: S1084-9521(16)30179-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Mruk DD, Cheng CY. The mammalian blood-testis barrier: its biology and regulation. Endocr Rev. 2015;36:564–591. doi: 10.1210/er.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tung KS, Yule TD, Mahi-Brown CA, Listrom MB. Distribution of histopathology and Ia positive cells in actively induced and passively transferred experimental autoimmune orchitis. J Immunol. 1987;138(3):752–9. [PubMed] [Google Scholar]
- 34.Hoek A, Allaerts W, Leenen PJ, Schoemaker J, Drexhage HA. Dendritic cells and macrophages in the pituitary and the gonads. Evidence for their role in the fine regulation of the reproductive endocrine response. Eur J Endocrinol. 1997;136:8–24. doi: 10.1530/eje.0.1360008. [DOI] [PubMed] [Google Scholar]
- 35.Kirsch TM, Friedman AC, Vogel RL, Flickinger GL. Macrophages in corpora lutea of mice: characterization and effects on steroid secretion. Biol Reprod. 1981;25(3):629–638. doi: 10.1095/biolreprod25.3.629. [DOI] [PubMed] [Google Scholar]
- 36.Brannstrom M, Giesecke L, Moore IC, van den Heuvel CJ, Robertson SA. Leukocyte subpopulations in the rat corpus luteum during pregnancy and pseudopregnancy. Biol Reprod. 1994;50(5):1161–1167. doi: 10.1095/biolreprod50.5.1161. [DOI] [PubMed] [Google Scholar]
- 37.Care AS, Diener KR, Jasper MJ, Brown HM, Ingman WV, Robertson SA. Macrophages regulate corpus luteum development during embryo implantation in mice. J Clin Invest. 2013;123:3472–3487. doi: 10.1172/JCI60561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen PE, Zhu L, Pollard JW. Absence of colony stimulating factor-1 in osteopetrotic (csfmop/csfmop) mice disrupts estrous cycles and ovulation. Biol Reprod. 1997;56:110–117. doi: 10.1095/biolreprod56.1.110. [DOI] [PubMed] [Google Scholar]
- 39.Chua AC, Hodson LJ, Moldenhauer LM, Robertson SA, Ingman WV. Dual roles for macrophages in ovarian cycle-associated development and remodelling of the mammary gland epithelium. Development. 2010;137(24):4229–4238. doi: 10.1242/dev.059261. [DOI] [PubMed] [Google Scholar]
- 40.Winqvist O, Karlsson FA, Kämpe O. 21-Hydroxylase, a major autoantigen in idiopathic Addison’s disease. Lancet. 1992;339:1559–1562. doi: 10.1016/0140-6736(92)91829-w. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell AL, Pearce SH. Autoimmune Addison disease: pathophysiology and genetic complexity. Nat Rev Endocrinol. 2012;8:306–16. doi: 10.1038/nrendo.2011.245. [DOI] [PubMed] [Google Scholar]