Abstract
Acute pancreatitis has several underlying etiologies, and results in consequences ranging from mild to complex multi-organ failure. The wide range of pathology suggests a genetic predisposition for progression. We compared the susceptibility to acute pancreatitis in BALB/c and FVB/N mice, coupled with proteomic analysis, in order to identify potential protein associations with pancreatitis progression.
Methods
Pancreatitis was induced in BALB/c and FVB/N mice by administration of cerulein or feeding a choline-deficient, ethionine-supplemented (CDE) diet. Histology and changes in serum amylase were examined. Proteome profiling in cerulein-treated mice was performed using 2-dimensional difference in-gel electrophoresis (2D-DIGE) followed by mass spectrometry analysis and biochemical validation.
Results
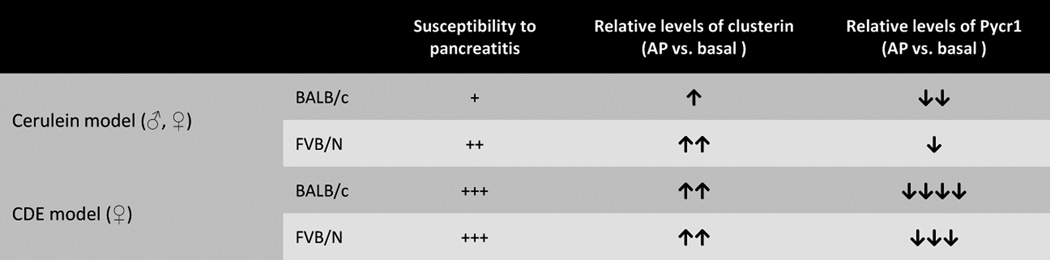
Male and female FVB/N mice manifested more severe cerulein-induced pancreatitis as compared with BALB/c mice, but both strains were similarly susceptible to CDE-induced pancreatitis. Few of the 2D-DIGE alterations were validated by immunoblotting. Clusterin was markedly up-regulated after cerulein-induced pancreatitis in FVB/N but less-so in BALB/c mice. Pyrroline-5-carboxylate reductase (Pycr1), an enzyme involved in proline biosynthesis, had higher basal levels in FVB/N male and female mouse pancreata compared with BALB/c pancreata, and remained relatively more resistant to degradation in FVB/N pancreata. However, serum and pancreas tissue proline levels were similar in the two strains.
Conclusion
FVB/N is more susceptible than BALB/c mice to cerulein-induced but not CDE-induced pancreatitis. Most of the 2D-DIGE alterations in the two strains likely relate to posttranslational modifications rather than protein level differences. Clusterin levels increase dramatically in association with pancreatitis severity, while Pycr1 is higher in FVB/N versus BALB/c pancreata basally and after induction of pancreatitis. Changes in proline metabolism may represent a novel potential genetic modifier in the context of pancreatitis.
Graphical Abstract
1. Introduction
Acute pancreatitis (AP) is an inflammatory disease whose manifestations range from mild forms to complex multi-organ failure. Currently, AP is the most common diagnosis for hospitalization among gastrointestinal diagnosis in the United States, and ranks as the 15th most common cause of gastrointestinal death with 8236 deaths caused by or contributed to by AP in 2012 [1]. Despite decades of research, treatments for pancreatitis remains relegated primarily to supportive care [2,3], thus there is much need to identify early predictors and new molecular targets for therapeutic intervention. Although alcohol continues to be a major cause of AP, epidemiologic data suggests that only a fraction of individuals who consume alcohol develop pancreatitis, and this susceptibility to pancreatitis is markedly dependent on race [4,5]. Earlier study have indicated that the highest frequency of acute alcoholic pancreatitis were noted among Africans, as compared to Caucasian, Hispanic, Asian and American Indian patients [4,6,7]. Differences in susceptibility to acute pancreatitis were also observed in different strains of mice [8]. Thus identifying the genetic modifiers conferring increased susceptibility to pancreatitis in particular strains of mice might help unfold new targets for therapeutic intervention.
One of the well characterized models of AP involves administration of supra-maximal doses of cerulein, a cholecystokinin analogue [9,10]. Cerulein results in mild edematous pancreatitis which recovers within few days [11]. Previously Wang et al. showed the difference in susceptibility to cerulein and choline-deficient, ethionine supplemented (CDE) diet-induced pancreatitis in five different mouse genetic background [8] (JF1, C57BL/6J, BALB/c, CBA/J, C3H/HeJ). In that study they focused on the differences in genetic-driven expression of Spink3 and Prss1 proteins as potential factors manifesting susceptibility to pancreatitis.
An earlier study that examined cytoskeletal alternations in the context of pancreatitis showed that FVB/N mice were more susceptible to cerulein-induced pancreatitis as compared with BALB/c mice [12]. In the present study, an unbiased proteomic approach was used to identify potential genetic modifiers that manifest different pancreatic protein expression profiles in FVB/N and BALB/c mice using two pancreatitis models: cerulein-induced or CDE diet-induced pancreatitis.
2. Materials and Methods
2.1. Animals and induction of pancreatitis
BALB/c and FVB/N mice were obtained from Jackson Laboratory and housed per guidelines of the University Committee on Use and Care of Animals at the University of Michigan. Pancreatitis was induced using two models. For the cerulein model [9,10,11], 8-week old male mice were fasted overnight with free access to water, then administered saline or 50 µg/kg mouse weight cerulein (Sigma) by intraperitoneal injections hourly (seven total injections). Female mice were also tested independently. For CDE diet-induced AP [13], 4-week old female mice were fasted overnight with free access to water, and then fed with normal chow or choline and methionine-deficient diet (Harlan Laboratories) supplemented with 0.5 % DL-ethionine (Sigma). The mice were then euthanized at 12, 24 or 48 hours (from time of cerulein initial administration); or after 2, 3 or 4 days of control or CDE diet feeding followed by collection of the pancreas or serum for subsequent analysis.
2.2. Serum Amylase and histologic analysis
The extent of pancreatic injury was determined by measuring serum amylase level using Phadebas reagent (Magle Life Sciences) as recommended by the supplier. For a histological analysis, pancreata were fixed in 10% neutral buffered formalin and stained with hematoxylin and eosin, then scored using five parameters (edema, necrosis, hemorrhage, inflammation and vacuolization) as described [14] with some modifications wherein vacuolization was scored as the number of vacuolated cells per high power field using the scale: 0 = absent; 0.5 = 1–2; 1.0 = 3–4;1.5 = 5–9; 2.0 = 10–14; 2.5 = 15–19; 3.0 = 20–24; 3.5 = 25–29; 4.0 = 30 or more vacuoles. The analysis was carried out by pathologist (D.M.) in a blinded fashion.
2.3. Two Dimensional-Difference in Gel Electrophoresis (2D-DIGE) and MS/MS analysis
2D-DIGE was performed on total pancreas lysates from BALB/c and FVB/N injected with saline or cerulein, similar to what was described for liver proteomic analysis [15]. Briefly, pancreatic lysates from the saline-injected group were labeled with fluorescent Cy3 dye, while lysates from the cerulein group were labeled with Cy5. The internal control was stained with Cy2. The samples were resolved 2-dimensionally using isoelectric focusing (first dimension) then SDS-PAGE (second dimension). Differentially expressed proteins were analyzed, and approximately 90 spots that had preferential Cy3 or Cy5 dye staining were subjected to mass spectrometric identification.
2.4. Western blotting
Total pancreatic lysates were prepared by homogenizing the pancreata in SDS-sample buffer. The amount of protein was normalized by tissue weight and Coomassie staining. Lysates were resolved on 4–20% gradient SDS-PAGE gels, transferred to polyvinylidene difluoride membrane then probed with antibodies to: Adi1 and Pycr1 (Proteintech); Apoa1, Erp29, Eef2, Reg1 and Prdx6 (Abcam); Clusterin and SerpinA3K (Santacruz); and caspase-7 (Cell Signaling). The binding signal was detected using enhanced chemiluminescence, and densitometric analysis was carried out by measuring the mean band intensity (Adobe Photoshop CS software).
2.5. TUNEL assay
Detection of apoptotic cells were carried out using TUNEL (Terminal-deoxynucleotidyl-transferase-mediated dUTP nick-end labeling) assay kit (Roche) as described [16]. Total apoptotic cells were calculated by counting the number of TUNEL and DAPI (4′,6-diamidino-2-phenylindole) double-positive cells.
2.6. Real time PCR
RNA was extracted using RNAeasy kit (Qiagen), then 2 µg RNA was reverse transcribed to cDNA using TaqMan reverse transcriptase kit (Applied biosystems). Real time PCR was carried out using Brilliant SYBR green master mix and specific primers in MyiQ real time PCR detection system (Bio-Rad Laboratories). Primers used in this study are as follows: Pycr1 (F: 5′-TGCTTTATGGGCTCTGCTTT-3′, R: 5′-TTGGTTGGTTGTTGGTCTCA-3′) and Actb (F: 5′-GGCATAGAGGTCTTTACGGATGT-3’ R: TATTGGCAACGAGCGGTTCC-3′).
2.7. Free Proline measurement
Free proline levels were measured in the pancreas and serum as described [17,18]. Briefly, the tissue was homogenized in Triton X-100 and the lysate was precipitated using sulfosalicyclic acid. The supernatant was then subjected to acid hydrolysis in acidic ninhydrin followed by elution of the red formazone complex with toluene and optical density measurement at 520nm.
2.8. Statistical analysis
Statistical significance was determined by student’s t-test using Graph pad software. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Proteomic differences in BALB/c and FVB/N pancreata under basal conditions and after cerulein-induced pancreatitis
Similar to prior findings which did not specifically test male versus female differences [12], FVB/N male mice are more susceptible to cerulein-induced pancreatitis than BALB/c mice (Fig. 1A–C). Notably and not reported previously, female FVB/N are also more susceptible to cerulein-induced pancreatitis than their BALB/c counterparts (Supplementary Fig. S1A,B). This indicated that FVB/N male and female mice were more susceptible to cerulein induced pancreatitis as compared to BALB/c mice.
Figure 1. Analysis of cerulein-induced pancreatitis in male BALB/c and FVB/N mice.
Eight week-old male BALB/c and FVB/N mice were injected with saline (Control, n=3/condition) or cerulein (n=5/condition) followed by analysis at the indicated time points. (A) Serum amylase at the indicated time points in control and cerulein-administered mice. *p<0.05, **p<0.01. (B) Hematoxylin and eosin staining of pancreata from BALB/c or FVB/N mice (bar=200 µm). Similar findings were noted in two other independent biologic replicates. (C) Total histopathology score derived as described in Materials and Methods; *p<0.05. (D) The protein spots identified by mass spectrometry with an intensity change of more than 1.5.
We then performed 2D-DIGE pancreas profiles to identify potential differential protein expression in saline- or cerulein-treated mice in both FVB/N and BALB/c strains. 2D-DIGE manifested several green and red fluorescent spots, which indicated decreased or increased protein charged isoform species respectively, when the mice were treated with cerulein or under basal conditions. From the 2D-DIGE analysis we selected 94 spots (Supplementary Fig. S2) which were excised and further subjected to mass spectrometry to determine their protein identities. From 94 selected spots, MS/MS analysis identified 48 proteins (Supplementary Table 1) of which 9 proteins had a difference of 1.5-fold or more in intensity between the strains. The identified proteins were related to metabolism, stress response and inflammation pathways (Fig. 1D).
3.2. Validation of the 2D-DIGE analysis
The 9 proteins listed in Fig. 1D were then tested by immunoblotting (Fig. 2A). Some of the 2D-DIGE alterations, such as peroxiredoxin 6 (Prdx6) and endoplasmic reticulum protein 29 (Erp29), which showed maximum basal fold-change by initial 2D-DIGE (15.4 and 10.7 respectively), were not present when tested by immunoblotting (Fig. 2A) which estimates total protein changes. This suggests that the 2D-DIGE alterations are likely due to post-translational modification/isoforms of these proteins [19,20], though validation of potential post-translational modification alterations is beyond the scope of this study. Clusterin and serine protease inhibitor A3K (Serpina3k) were markedly upregulated in both strains during pancreatitis, with clusterin in FVB/N showing a more significant increase compared with BALB/c (Fig. 2A,B). The difference in clusterin induction was also observed in female mice challenged with cerulein (Supplementary Fig. S1C). Since clusterin is known to inhibit apoptosis [21], the levels of caspase-7 activation were analyzed with findings showing that cleaved caspase-7 was increased in BALB/c but not FVB/N pancreata (Fig. 2A). TUNEL staining manifested a nonstatistically significant trend for more TUNEL-positive staining in BALB/c pancreas (Fig. 2C).
Figure 2. Validation of differentially-expressed proteins in pancreata from BALB/c and FVB/N mice treated with cerulein.
(A) Total pancreatic extracts 24h after the first cerulein or saline administration (3 mice/condition) were separated by SDS-PAGE then immunoblotted with antibodies to the indicated proteins. Similar findings were observed in two other independent experiments that included 3 mice/strain/condition/experiment. (B) Mean densiometric intensity of the proteins bands from panel A, with values summarized in the table below. Student’s t-test (*p<0.05) was used to test potential significant differences. N.A., not applicable. (C) TUNEL-positive cells of pancreata from BALB/c and FVB/N (n=3/treatment) mice after saline or cerulein exposure (bar=100 µm).
The only protein that manifested a difference in expression under basal conditions was the proline biosynthesis enzyme, pyrroline-5-carboxylate reductase (Pycr1), which was higher in FVB/N pancreata and remained higher after induction of pancreatitis (Fig. 2A,B; Supplementary Fig. S1C). Pycr1 mRNA was similar in both mouse strains, and decreased similarly in both strains after cerulein (Fig. 3A). Pycr1 is the last enzyme in proline biosynthetic pathway, and high level of proline have been reported to generate superoxide radicals that induce apoptosis [22]. However, proline levels in serum and pancreatic tissues did not vary among the two strains under basal conditions or after induction of pancreatitis (Fig. 3B,C).
Figure 3. Levels of pancreatic Pycr1 mRNA and pancreatic and serum free proline in response to cerulein-induced pancreatitis.
(A) Pancreatic tissue (n=3/condition) was homogenized after saline or cerulein treatment followed by qPCR analysis to determine relative Pycr1 mRNA. (B, C) Free proline in pancreas and serum (n=3 separate specimen/group) from mice given saline or cerulein.
3.3. Proteomic analysis in CDE-diet induced pancreatitis
We then tested whether the protein alterations we observed in the cerulein pancreatitis model also carry over to a second model, namely that the CDE diet-induced pancreatitis. Pancreata were collected from BALB/c and FVB/N female mice fed with normal chow or CDE diet for 48, 72, and 96 hours, followed by analysis of the protein alterations by immunoblotting. Unlike the cerulein model, the extent of damage induced by CDE-diet was more severe but comparable in BALB/c and FVB/N based on serum amylase levels and histology (Fig. 4A; Supplementary Fig. S3A,B), suggesting that susceptibility to pancreatitis depends on the underlying disease mechanism. Similar to the cerulein model, there was up-regulation of clusterin and SerpinA3k and a decrease in Pycr1 during CDE-diet induced pancreatitis, whereas eEf2 levels decreased dramatically which was distinctive in CDE-induced pancreatitis and possibly related to the disease severity (Fig. 4B). Densitometric analysis showed that the differences before and after pancreatitis were similar between the strains for most of the tested proteins (Fig. 4C; Supplementary Fig. S3C). Clusterin protein levels in both strains were comparable, and the extent of apoptosis measured by cleaved caspase-7 (Fig. 4B) and TUNEL staining were not altered significantly in CDE-induced pancreatitis (Supplementary Fig. S3D,E). Pycr1 decreased dramatically in pancreata of both strains but remained present at higher levels in FVB/N versus BALB/c during CDE diet-induced pancreatitis [Fig. 4B,C; as noted in the cerulein model (Fig. 2A,B)]. Taken together, FVB/N mice showed more susceptibility to cerulein-induced pancreatitis than BALB/c but this was not the case for CDE-induced pancreatitis. Changes in clusterin and Pycr1 in the cerulein model, and Pycr1 in the CDE model associated with the FVB/N and BALB/c strains.
Figure 4. Analysis of CDE-mediated pancreatitis severity and protein levels in the context of pancreatitis in BALB/c and FVB/N mice.
BALB/c and FVB/N mice fed normal chow (n=3) or CDE diet (n=3). (A) Hematoxylin and eosin staining of pancreata of BALB/c and FVB/N analyzed after the indicated days of CDE feeding (bar=200 µm). (B) Total pancreatic extracts from the 3-day time-point were separated by SDS-PAGE then immunoblotted using antibodies to the indicated proteins. (C) Mean intensity of the proteins bands from immunoblotting were plotted after densitometric analysis. Similar findings were obtained from independent biologic replica using the same number of mice. N.A., not applicable. (D) A schematic chart showing conclusion.
4. Discussion
We hypothesized that testing the susceptibility to acute pancreatitis in two different strains of mice, coupled with global proteome analysis, could uncover differences in protein levels that reflect genetic modifiers for disease susceptibility. One other study by Wang et al had tested susceptibility to pancreatitis in five mouse strains and focused on the promoter regulation of serine protease inhibitor, Kazal type 3 [8], but FVB/N mice were not included in this study and a proteomic analysis was not part of the study. Findings herein show that BALB/c is less susceptible than FVB/N to cerulein-induced pancreatitis in both male and female mice, but both strains are similarly susceptible to CDE-mediated pancreatitis. For the CDE model, only young female mice were tested since male gender and increasing age are known to protect from pancreatitis in this model [13] which is the reason why male mice are not used in the CDE pancreatitis model.
For the unbiased protein profiling, most proteins that had a change in isoform level were previously known to be involved in pancreatitis. For example, Apoa1 is involved in inflammation and increased during pancreatitis in rats [23]; serpinA3k is reported to have a protective function in pancreatitis since it is a proteinase inhibitor and thus important to limit the activity of proteinases that are released prematurely in the pancreatic tissue [24]; and Prdx6 is associated with elevated oxidative stress [25,26]. None of these three proteins showed significant difference in protein levels between the two strains. However, Prdx6 and Erp29 showed maximum fold-change at the basal levels by 2D-DIGE (15.4 and 10.7 respectively), but not at the total protein level, thereby suggesting differences in post-translational modifications to account for the relative difference in isoform levels [19,20].
Among the notable differences were the decreases in pancreatic Pycr1 levels in BALB/c mice after cerulein-induced pancreatitis despite the observation that the FVB/n strain undergoes more severe pancreatitis thereby indicating that the differences are not simply related to protein degradation per se. As the last enzyme in proline biosynthesis, Pycr1 and proline are implicated to provide anti-oxidant effects [27,28,29,30] although we did not observe differences in serum or pancreatic tissue proline levels. Pycr1 may serve its protective role from oxidative stress and apoptosis possibly through binding with other proteins, such as DJ-1 and ribonucleotide reductase small subunit B [29,31]. Although we did not observed changes in proline levels, other factors such as proline oxidase [32] may be involved. Regardless, the changes reported herein for Pycr1 are novel and the higher Pycr1 levels in FVB/N pancreata correlate with the lower observed caspase-3 activation that we observed.
We also observed a robust increase in clusterin in pancreata of both mouse strains in the two pancreatitis models that were tested. Clusterin exists as three splice isoforms, which show unique functions and localization within cells. The major form detected in pancreas during pancreatitis is ~40-kD (based upon migration on SDS-PAGE) and corresponds to the secreted α- and β-subunit [33]. The increases in clusterin that we observed correlated with the severity of pancreatitis (FVB/N > BALB/c for the cerulein model; FVB/N similar to BALB/c for the CDE model) and suggest that clusterin levels could serve as a stress indicator and marker for the extent of damage. A previous report described induction of clusterin mRNA and protein in pancreas and pancreatic juice from rats with acute pancreatitis [34]. Both mRNA and protein of clusterin are not detectable in healthy pancreata; but become highly induced during acute pancreatitis with cerulein or sodium taurocholate or in chronic pancreatitis in rats and cultured acinar cells [35]. These findings suggest that a possible function of pancreatic clusterin is to protect against apoptosis. The significance of clusterin induction during pancreatitis may relate to its multifunctional role in cell survival, cell cycle, proliferation, differentiation and morphogenesis [36,37].
Taken together, our findings point to the potential importance of protein post-translational modifications under basal conditions in pancreata of two different mouse strains which, in turn, manifest significant differences in susceptibility to cerulein-induced pancreatitis. In addition, clusterin and proline metabolism present potential targets for further study in the context of pancreatitis.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants R01 DK47918 and the Department of Veterans Affairs (M.B.O.); a University of Michigan Postdoctoral Translational Scholars Program Award (M.J.P.); and NIH grant P30 DK34933 to the University of Michigan.
Abbreviations
- AP
acute pancreatitis
- CDE
choline-deficient ethionine supplemented
- DIGE
difference in-gel electrophoresis
- Erp29
endoplasmic reticulum protein 29
- Prdx6
peroxiredoxin 6
- Pycr1
pyrroline-5-carboxylate reductase
- Serpina3k
serine protease inhibitor A3K
- TUNEL
terminal-deoxynucleotidyl-transferase-mediated dUTP nick-end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Peery AF, Crockett SD, Barritt AS, Dellon ES, Eluri S, Gangarosa LM, Jensen ET, Lund JL, Pasricha S, Runge T, Schmidt M, Shaheen NJ, Sandler RS. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology. 2015;149:1731–1741. e1733. doi: 10.1053/j.gastro.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talukdar R, Vege SS. Recent developments in acute pancreatitis. Clin Gastroenterol Hepatol. 2009;7:S3–S9. doi: 10.1016/j.cgh.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg JA, Hsu J, Bawazeer M, Marshall J, Friedrich JO, Nathens A, Coburn N, May GR, Pearsall E, McLeod RS. Clinical practice guideline: management of acute pancreatitis. Can J Surg. 2016;59:128–140. doi: 10.1503/cjs.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168:649–656. doi: 10.1001/archinte.168.6.649. [DOI] [PubMed] [Google Scholar]
- 5.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowenfels AB, Maisonneuve P, Sullivan T. The changing character of acute pancreatitis: epidemiology, etiology, and prognosis. Curr Gastroenterol Rep. 2009;11:97–103. doi: 10.1007/s11894-009-0016-4. [DOI] [PubMed] [Google Scholar]
- 7.Dufour MC, Adamson MD. The epidemiology of alcohol-induced pancreatitis. Pancreas. 2003;27:286–290. doi: 10.1097/00006676-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Ohmuraya M, Suyama K, Hirota M, Ozaki N, Baba H, Nakagata N, Araki K, Yamamura K. Relationship of strain-dependent susceptibility to experimentally induced acute pancreatitis with regulation of Prss1 and Spink3 expression. Lab Invest. 2010;90:654–664. doi: 10.1038/labinvest.2010.44. [DOI] [PubMed] [Google Scholar]
- 9.Niederau C, Ferrell LD, Grendell JH. Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology. 1985;88:1192–1204. doi: 10.1016/s0016-5085(85)80079-2. [DOI] [PubMed] [Google Scholar]
- 10.Silverman M, Ilardi C, Bank S, Kranz V, Lendvai S. Effects of the cholecystokinin receptor antagonist L-364,718 on experimental pancreatitis in mice. Gastroenterology. 1989;96:186–192. doi: 10.1016/0016-5085(89)90779-8. [DOI] [PubMed] [Google Scholar]
- 11.Shorrock K, Austen BM, Hermon-Taylor J. Hyperstimulation pancreatitis in mice induced by cholecystokinin octapeptide, caerulein, and novel analogues: effect of molecular structure on potency. Pancreas. 1991;6:404–406. doi: 10.1097/00006676-199107000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Zhong B, Omary MB. Actin overexpression parallels severity of pancreatic injury. Exp Cell Res. 2004;299:404–414. doi: 10.1016/j.yexcr.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Lombardi B, Estes LW, Longnecker DS. Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by DL-ethionine fed with a choline-deficient diet. Am J Pathol. 1975;79:465–480. [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt J, Lewandrowski K, Fernandez-del Castillo C, Mandavilli U, Compton CC, Warshaw AL, Rattner DW. Histopathologic correlates of serum amylase activity in acute experimental pancreatitis. Dig Dis Sci. 1992;37:1426–1433. doi: 10.1007/BF01296014. [DOI] [PubMed] [Google Scholar]
- 15.Snider NT, Weerasinghe SV, Singla A, Leonard JM, Hanada S, Andrews PC, Lok AS, Omary MB. Energy determinants GAPDH and NDPK act as genetic modifiers for hepatocyte inclusion formation. J Cell Biol. 2011;195:217–229. doi: 10.1083/jcb.201102142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weerasinghe SV, Moons DS, Altshuler PJ, Shah YM, Omary MB. Fibrinogen-gamma proteolysis and solubility dynamics during apoptotic mouse liver injury: heparin prevents and treats liver damage. Hepatology. 2011;53:1323–1332. doi: 10.1002/hep.24203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reversade B, Escande-Beillard N, Dimopoulou A, Fischer B, Chng SC, Li Y, Shboul M, Tham PY, Kayserili H, Al-Gazali L, Shahwan M, Brancati F, Lee H, O'Connor BD, Schmidt-von Kegler M, Merriman B, Nelson SF, Masri A, Alkazaleh F, Guerra D, Ferrari P, Nanda A, Rajab A, Markie D, Gray M, Nelson J, Grix A, Sommer A, Savarirayan R, Janecke AR, Steichen E, Sillence D, Hausser I, Budde B, Nurnberg G, Nurnberg P, Seemann P, Kunkel D, Zambruno G, Dallapiccola B, Schuelke M, Robertson S, Hamamy H, Wollnik B, Van Maldergem L, Mundlos S, Kornak U. Mutations in PYCR1 cause cutis laxa with progeroid features. Nat Genet. 2009;41:1016–1021. doi: 10.1038/ng.413. [DOI] [PubMed] [Google Scholar]
- 18.Bates RPWLS, Teare ID. Rapid determination of free proline for water-stress studies. Plant and Soil. 1973;39:205–207. [Google Scholar]
- 19.Morand JP, Macri J, Adeli K. Proteomic profiling of hepatic endoplasmic reticulum-associated proteins in an animal model of insulin resistance and metabolic dyslipidemia. J Biol Chem. 2005;280:17626–17633. doi: 10.1074/jbc.M413343200. [DOI] [PubMed] [Google Scholar]
- 20.Jeong J, Kim Y, Kyung Seong J, Lee KJ. Comprehensive identification of novel post-translational modifications in cellular peroxiredoxin 6. Proteomics. 2012;12:1452–1462. doi: 10.1002/pmic.201100558. [DOI] [PubMed] [Google Scholar]
- 21.Min BH, Kim BM, Lee SH, Kang SW, Bendayan M, Park IS. Clusterin expression in the early process of pancreas regeneration in the pancreatectomized rat. J Histochem Cytochem. 2003;51:1355–1365. doi: 10.1177/002215540305101012. [DOI] [PubMed] [Google Scholar]
- 22.Phang JM, Pandhare J, Liu Y. The metabolism of proline as microenvironmental stress substrate. J Nutr. 2008;138:2008S–2015S. doi: 10.1093/jn/138.10.2008S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams JA. Proteomics as a systems approach to pancreatitis. Pancreas. 2013;42:905–911. doi: 10.1097/MPA.0b013e31828fddc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan S, Chen R, Stevens T, Bronner MP, May D, Tamura Y, McIntosh MW, Brentnall TA. Proteomics portrait of archival lesions of chronic pancreatitis. PLoS One. 2011;6:e27574. doi: 10.1371/journal.pone.0027574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Phelan SA, Forsman-Semb K, Taylor EF, Petros C, Brown A, Lerner CP, Paigen B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem. 2003;278:25179–25190. doi: 10.1074/jbc.M302706200. [DOI] [PubMed] [Google Scholar]
- 26.Fetaud V, Frossard JL, Farina A, Pastor CM, Buhler L, Dumonceau JM, Hadengue A, Hochstrasser DF, Lescuyer P. Proteomic profiling in an animal model of acute pancreatitis. Proteomics. 2008;8:3621–3631. doi: 10.1002/pmic.200800066. [DOI] [PubMed] [Google Scholar]
- 27.De Ingeniis J, Ratnikov B, Richardson AD, Scott DA, Aza-Blanc P, De SK, Kazanov M, Pellecchia M, Ronai Z, Osterman AL, Smith JW. Functional specialization in proline biosynthesis of melanoma. PLoS One. 2012;7:e45190. doi: 10.1371/journal.pone.0045190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan N, Dickman MB, Becker DF. Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic Biol Med. 2008;44:671–681. doi: 10.1016/j.freeradbiomed.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuda T, Kaji Y, Agatsuma T, Niki T, Arisawa M, Shuto S, Ariga H, Iguchi-Ariga SM. DJ-1 cooperates with PYCR1 in cell protection against oxidative stress. Biochem Biophys Res Commun. 2013;436:289–294. doi: 10.1016/j.bbrc.2013.05.095. [DOI] [PubMed] [Google Scholar]
- 30.Phang JM, Liu W, Zabirnyk O. Proline metabolism and microenvironmental stress. Annu Rev Nutr. 2010;30:441–463. doi: 10.1146/annurev.nutr.012809.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo ML, Lee MB, Tang M, den Besten W, Hu S, Sweredoski MJ, Hess S, Chou CM, Changou CA, Su M, Jia W, Su L, Yen Y. PYCR1 and PYCR2 Interact and Collaborate with RRM2B to Protect Cells from Overt Oxidative Stress. Sci Rep. 2016;6:18846. doi: 10.1038/srep18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Togashi Y, Arao T, Kato H, Matsumoto K, Terashima M, Hayashi H, de Velasco MA, Fujita Y, Kimura H, Yasuda T, Shiozaki H, Nishio K. Frequent amplification of ORAOV1 gene in esophageal squamous cell cancer promotes an aggressive phenotype via proline metabolism and ROS production. Oncotarget. 2014;5:2962–2973. doi: 10.18632/oncotarget.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Silva HV, Harmony JA, Stuart WD, Gil CM, Robbins J. Apolipoprotein J: structure and tissue distribution. Biochemistry. 1990;29:5380–5389. doi: 10.1021/bi00474a025. [DOI] [PubMed] [Google Scholar]
- 34.Calvo EL, Mallo GV, Fiedler F, Malka D, Vaccaro MI, Keim V, Morisset J, Dagorn JC, Iovanna JL. Clusterin overexpression in rat pancreas during the acute phase of pancreatitis and pancreatic development. Eur J Biochem. 1998;254:282–289. doi: 10.1046/j.1432-1327.1998.2540282.x. [DOI] [PubMed] [Google Scholar]
- 35.Xie MJ, Motoo Y, Su SB, Sawabu N. Expression of clusterin in pancreatic acinar cell injuries in vivo and in vitro. Pancreas. 2001;22:126–134. doi: 10.1097/00006676-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 36.French LE, Chonn A, Ducrest D, Baumann B, Belin D, Wohlwend A, Kiss JZ, Sappino AP, Tschopp J, Schifferli JA. Murine clusterin: molecular cloning and mRNA localization of a gene associated with epithelial differentiation processes during embryogenesis. J Cell Biol. 1993;122:1119–1130. doi: 10.1083/jcb.122.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, Wang CY. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol. 2005;7:909–915. doi: 10.1038/ncb1291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.