Abstract
Objective
Operative mortality of patients undergoing symptomatic abdominal aortic aneurysm (Sx-AAA) repair has been reported at 6% to 30% during the past 25 years. We used a multicenter regional database to describe the contemporary outcomes of patients undergoing repair of Sx-AAA.
Methods
All patients undergoing infrarenal AAA repair in 11 hospitals comprising the Vascular Study Group of Northern New England (VSGNNE) between 2003 and 2009 were studied. Sx-AAA was prospectively defined as an AAA accompanied by abdominal or back pain or tenderness, but without rupture. The primary study end point was in-hospital mortality. Secondary end points included in-hospital postoperative major adverse events (MAE) and late survival. These outcomes were compared between symptomatic patients and contemporary VSGNNE cohorts of elective (E-AAA) and ruptured AAAs (R-AAAs) treated within the same study period.
Results
During the study period, 2386 AAA repairs were performed, comprising 1959 (82%) E-AAAs, 156 (7%) Sx-AAAs, and 271 (11%) R-AAAs. Repair was endovascular in 945 (48%) E-AAAs, 60 (38%) Sx-AAAs, and 33 (12%) R-AAAs. Hospital mortality was 1.7% for E-AAA repair and 1.3% for Sx-AAA repair, but was 34.7% for R-AAA repair (P < .001). The MAE rates were 20%, 35%, and 63%, respectively, for E-AAA, Sx-AAA, and R-AAA repairs (P < .001). The mean Glasgow Aneurysm Score (GAS) for Sx-AAA patients who survived was 79 ± 12. Those who died had an average score of 92 ± 7, and 83% of all Sx-AAA and R-AAA patients who died had a GAS >85. Kaplan-Meier analysis demonstrated that at 1 and 4 years, Sx-AAA repair was associated with intermediate survival (83% and 68%) compared with E-AAA repair (89% and 73%) and R-AAA repair (49% and 35%; P < .001).
Conclusion
The operative mortality of patients with Sx-AAA in contemporary practice appears better than that previously reported in the literature. Despite low operative mortality, MAE and late survival are intermediate compared with E-AAA and R-AAA repair. Review of previous series shows a trend for lower operative mortality after Sx-AAA repair in more recent series, which likely reflects improved perioperative care and more use of endovascular aneurysm repair.
The incidence of abdominal aortic aneurysms (AAAs) increases with age and approaches 7% in men by age 70.1 Most AAAs that require repair are done electively, but some are repaired only after rupture. A third subset of AAAs presents with symptoms of abdominal or back pain associated with aneurysm tenderness, suggesting rupture. Often, these patients undergo emergency or urgent AAA repair because of fear that rupture is imminent, even if not demonstrable on abdominal imaging. Such AAAs are termed symptomatic and represent 5.5% to 22% of all AAAs that require operation in previously reported surgical series.2–8
Historically, patients with symptomatic AAAs (Sx-AAAs) have been consistently reported to have worse outcomes than patients undergoing elective AAA (E-AAA) repair, with 30-day or in-hospital mortality rates of 4% to 22%.2–4,9–14 Additionally, major adverse event (MAE) rates after Sx-AAA repair have been found to be much higher than after E-AAA repair.4,5,7,14,15 Although most of the reported experience concerns open repair of Sx-AAAs, more recent series have described successful endovascular aneurysm repair (EVAR).2,6 However, these are small series of patients treated by EVAR, without direct comparison to open repair.
Optimal timing of Sx-AAA treatment is debated. Some have suggested that delay in operative repair might improve outcome by allowing a more complete risk assessment and avoiding off-hours operations by less-experienced surgical and anesthesia teams.4 However, concern about impending rupture argues for rapid repair.3,7,9,11,12 At present, such decision making is difficult because the causes for worse outcomes of Sx-AAAs are not certain, given that these patients do not experience rupture and its physiologic sequelae.
The purpose of this study was to analyze the current outcomes of a large group of patients with Sx-AAAs derived from a multicenter regional database. We sought to compare these patients with those undergoing E-AAA and ruptured AAA (R-AAA) treatment to analyze risk factors for increased morbidity and mortality in patients with Sx-AAAs.
MATERIALS AND METHODS
Patients and database
This is a retrospective analysis of data collected prospectively by the Vascular Study Group of Northern New England (VSGNNE), a regional cooperative quality improvement initiative developed in 2002 to study regional outcomes in vascular surgery. Further details about this registry have been published previously.16 Of note, registry data are compared with hospital claims in regular audits, and missing cases are retrieved to yield a 99% capture rate. Mortality is audited using hospital claims and Social Security Death Index. Not all outcome events are audited, but because reporting is anonymous, there is no benefit to underreporting. The analysis included all patients who underwent AAA repair (elective, symptomatic, or ruptured) by 37 participating surgeons in 11 study hospitals (Appendix A, online only) from January 1, 2003, to March 31, 2009.
Definitions
A Sx-AAA was prospectively defined as an aneurysm that was not ruptured but that required urgent repair because of associated pain or tenderness. R-AAA was defined as an aneurysm with operative, computed tomography, or angiographic evidence of retroperitoneal or intraperitoneal bleeding. Infrarenal AAA was defined as an AAA in which the proximal aortic anastomosis was below both renal arteries, although aortic clamping could be suprarenal or supravisceral (all defined as suprarenal for this analysis).
The database routinely records pre-existing medical comorbidities, including chronic obstructive pulmonary disease (record history), congestive heart failure (record history or documented ejection fraction <50% on preoperative testing), coronary artery disease (CAD; any history of angina, myocardial infarction [MI], prior coronary intervention, or electrocardiographic changes consistent with previous MI), chronic renal insufficiency (CRI; serum creatinine ≥1.8 mg/dL), end-stage renal disease (on dialysis), diabetes mellitus (medical history, designated as diet-controlled, on oral hypoglycemic agents, or on insulin), hypertension (medical history or blood pressure ≥140/90 mm Hg), hyperlipidemia (medical history), and prior aortic surgery. Additionally, preoperative statin, aspirin, and β-blocker use is recorded.
Operative mortality was defined as in-hospital death. Long-term survival was determined from the date of death in the Social Security Death Index Master File. MAEs included MI (based on electrocardiography and troponin elevation), dysrhythmia (requiring medication or cardio-version), respiratory complications (clinical pneumonia or prolonged intubation), worse renal function (rise in creatinine level of 0.5 mg/dL or initiation of dialysis), or bowel ischemia.
The Glasgow Aneurysm Score (GAS) was calculated (age + 7 points for CAD + 10 points for cerebrovascular disease + 14 points for renal disease + 17 points for shock) for all Sx-AAA and R-AAA patients.17 In our database, patients were assigned GAS points based on the following: CAD was assigned by history, cerebrovascular disease-based on prior carotid endarterectomy (vs any symptomatic cerebrovascular disease in the original GAS formula), renal disease was based on creatinine of >1.8 mg/dL (vs 1.7 in the original GAS formula), and shock was based on arrival blood pressure of <80 mm Hg.
Data collection and statistical analysis
Physicians, nurses, or clinical data abstractors entered data prospectively on >70 clinical and demographic variables (www.VSGNNE.org). Research analysts were blinded to patient, surgeon, and hospital identity. Data collection and analysis was approved by the Institutional Review Board of each participating hospital.
Data analysis was performed using STATA software (StataCorp, College Station, Tex). Kaplan-Meier and log-rank test were used to evaluate survival. Demographic and operative characteristics were compared by F-test in analysis of variance for parametric continuous variables. The rank-sum test was used for nonparametric continuous variables, and the χ2 test was used for categoric variables. Missing values were excluded from analysis. Values of P <.05 were considered significant.
RESULTS
Patients and procedures
From January 2003 through March of 2009, 2386 infrarenal AAA repairs were performed in the 11 centers consisting of 1959 (82%) E-AAA, 156 (7%) Sx-AAAA, and 271 (11%) R-AAA repairs. EVAR was performed for 48%, 38%, and 12% of E-AAAs, Sx-AAAs, and R-AAAs, respectively (P <.001).
Demographic data are reported in Table I. Mean age increased significantly with the progression of presentation from elective to symptomatic or rupture. Symptomatic patients were more likely to be women than were patients with R-AAAs or E-AAAs. Acute presentation (symptomatic or rupture) was more likely to occur in patients with a history of congestive heart failure compared with E-AAA patients. Mean AAA diameter increased with acuity of presentation (5.8, 6.6, and 7.1 cm for E-AAA, Sx-AAA, and R-AAA, respectively; P <.001). Patients did not differ by smoking status, hypertension, diabetes, or cerebrovascular disease. Patients with Sx-AAAs and E-AAAs were more often taking preoperative aspirin than patients with R-AAAs. Symptomatic patients were least likely to be taking a preoperative β-blocker, and their statin use was intermediate between patients undergoing elective and rupture repairs.
Table I.
Patient characteristics
| Variablea |
All AAA repairs (N = 2386)
|
||||
|---|---|---|---|---|---|
|
Elective (N = 1959) |
P valueb |
Symptomatic (N = 156) |
P valuec |
Ruptured (N = 271) |
|
| Age, y | 72 ± 8 | .01d | 74 ± 9 | .856 | 74 ± 9 |
| Women, % | 22 | .035d | 31 | .001d | 16 |
| Smoking (prior or current), % | 90 | .213 | 86 | >.99 | 85 |
| Hypertension, % | 82 | .502 | 78 | .860 | 81 |
| Diabetes, % | 18 | >.99 | 17 | >.99 | 15 |
| Congestive heart failure, % | 9 | .019d | 15 | .751 | 12 |
| COPD, % | 37 | .425 | 42 | >.99 | 42 |
| Creatinine ≥1.8 mg/dL, % | 8 | .393 | 10 | .066 | 18 |
| Previous CEA, % | 6 | .461 | 9 | .479 | 6 |
| Max AP AAA diameter, cm | 5.8 ± 0.1 | <.001d | 6.6 ± 1.7 | <.001d | 7.1 ± 2.0 |
| Preop medications, % | |||||
| Aspirin | 71 | .091 | 63 | .001d | 46 |
| Clopidogrel | 5 | >.99 | 6 | .803 | 4 |
| Statin | 62 | .008d | 51 | <.001d | 32 |
| β-blockers | |||||
| None | 16 | .378 | 14 | <.001d | 39 |
| Immediate preop | 25 | <.001d | 43 | <.001d | 24 |
| Chronic | 59 | <.001d | 44 | .165 | 37 |
| EVAR repair, % | 48 | <.001d | 38 | <.001d | 12 |
| Glasgow Aneurysm Score | 79.4 ± 12 | 86.8 ± 15.8 | |||
AAA, Abdominal aortic aneurysm; AP, anteroposterior; CEA, carotid endarterectomy; COPD, chronic obstructive pulmonary disease.
Continuous variables are presented as mean ± standard deviation.
Elective vs symptomatic
symptomatic vs rupture; P value from F-test in analysis of variance for continuous variables and from χ2 test for categoric variables.
These values are significant (significant at level of .05).
Open and EVAR operative details
For open repairs, blood loss, crystalloid infusion, and transfusions were similar between E-AAA and Sx-AAA repairs but were significantly greater for R-AAA repairs (Table II, A). Suprarenal clamping was more likely for R-AAA repairs (P < .001). Operative heart rate and extubation in the operating room were both significantly associated with acuity of surgery (Table II, A).
Table II. A.
Operative characteristics of open abdominal aortic aneurysm repair
| Variable |
Open repair (N = 1348)
|
||||
|---|---|---|---|---|---|
|
Elective (N = 1014) |
P valuea |
Symptomatic (N = 96) |
P valueb |
Ruptured (N = 238) |
|
| Estimated blood loss,c mL | 1100 (700–1700) | .1 | 1200 (800–1850) | <.01 | 2700 (1500–5000) |
| Crystalloid,c mL | 5000 (4000–6500) | .4 | 5500 (4100–7000) | <.01 | 7000 (4500–9700) |
| Intraop pRBC,d U | 0.6 ± 1.29 | .15 | 1.2 ± 2 | <.01 | 6.7 ± 6.1 |
| Heart rate, beats/mind | |||||
| On arrival in OR | 65 ± 13 | .001 | 73 ± 14 | <.001 | 90 ± 22 |
| Highest intraop | 78 ± 15 | .029 | 84 ± 14 | <.0018 | 102 ± 26 |
| Proximal clamp positiond | |||||
| Suprarenal, % | 26 | >.99 | 25 | .001 | 43 |
| Extubated in ORd, % | 77 | <.001 | 43 | <.001 | 4 |
OR, Operating room; pRBC, packed red blood cells.
Elective vs symptomatic
symptomatic vs rupture; significant at level of .05.
Values reported as median (interquartile range) and compared with rank-sum test.
Values reporated as mean ± standard deviation.
For EVAR, operative blood loss and crystalloid infusion for Sx-AAA repairs were similar to E-AAA repairs but were significantly greater for R-AAA repair (P < .001). Despite this, there was a small, but significant increase in intraoperative transfusions during Sx-AAA repair. The initial heart rate on arrival in the operating room was increased based on acuity of surgery (P < .05), and extubation in the operating room was less likely for acute presentations (Table II, B).
Table II. B.
Operative characteristics for endovascular aneurysm repair
| Variable |
Endovascular repair (N = 1038)
|
Ruptured (N = 33) |
|||
|---|---|---|---|---|---|
|
Elective (N = 945) |
P valuea |
Symptomatic (N = 60) |
P valueb | ||
| Estimated blood loss,c mL | 200 (100–400) | <.01 | 300 (150–500) | .4 | 300 (175–650) |
| Crystalloid,c mL | 2400 (2000–3000) | .2 | 2575 (1900–3350) | .1 | 3100 (2000–4900) |
| Intraop pRBC,d U | 0.1 ± 0.7 | .025 | 0.6 ± 1 | <.01 | 3.8 ± 6.4 |
| Heart rate,d beats/min | |||||
| On arrival in OR | 66 ± 12 | .045 | 71 ± 15 | <.001 | 95 ± 21 |
| Highest intraop | 75 ± 15 | .455 | 77 ± 13 | <.001 | 105 ± 27 |
| Extubation in OR,d % | 98 | <.001 | 81 | <.001 | 39 |
OR, Operating room; pRBC, packed red blood cells.
Elective vs symptomatic
symptomatic vs rupture; significant at level of .05.
Values reported as median (interquartile range) and compared with rank-sum test.
Values reported as mean ± standard deviation.
Mortality
Hospital mortality was 1.7% for patients undergoing Sx-AAA repair and 1.3% for those undergoing E-AAA repair (P = 0.71), which was significantly less than the 35% rate for R-AAA repair (P < .01). Among symptomatic patients, there was no difference in mortality between patients undergoing EVAR and open repair. However, patients undergoing EVAR for E-AAA or R-AAA repair had significantly lower mortality than patients undergoing open repair (Fig 1). Only two patients died after undergoing Sx-AAA repair, and both deaths occurred after open repair performed ≤24 hours of admission.
Fig 1.
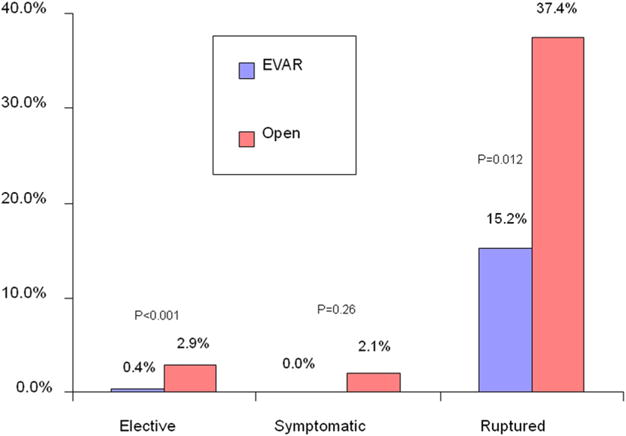
Comparison of in-hospital mortality is shown for all abdominal aortic aneurysm repairs stratified by urgency and endovascular or open method of repair. P value from F-test; <.05 considered significant.
Morbidity
MAEs increased with acuity of AAA repair, from 20% for E-AAAs to 35% for Sx-AAAs to 63% for R-AAA repair (P < .001 between each group). The MAE rate was significantly lower for EVAR than open repair for E-AAAs and R-AAAs but was not significantly different for Sx-AAAs (Fig 2). Sx-AAA was associated with a higher rate of MI, renal function decline, respiratory failure, and dysrhythmia compared with E-AAA. R-AAA was associated with the highest rate of individual MAEs (Table III). There was no significant difference in the relative proportion of different types of MAEs between E-AAA and Sx-AAA repairs. Repair of Sx-AAA was performed ≤24 hours of admission in 75% of patients. Timing of repair of Sx-AAAs (>24 hours or <24 hours) was not associated with the MAE rate (35% vs 34%, respectively; P = .97).
Fig 2.
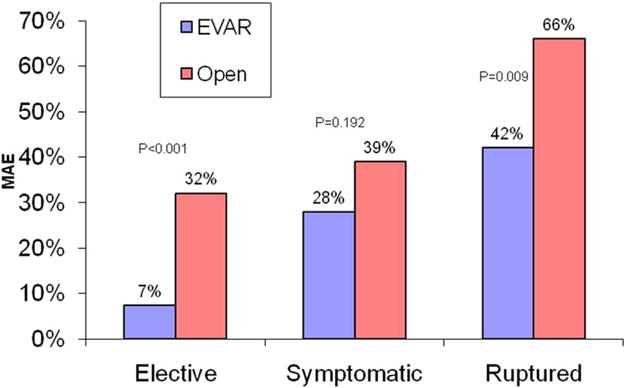
Comparison of major adverse event rates (MAE), defined as myocardial infarction, dysrhythmia, worse renal function, respiratory compromise, or bowel ischemia, is shown stratified by urgency and endovascular or open method of repair. P value from F-test, <.05 considered significant.
Table III.
Major event rates based on total number of events
| Complication |
Elective (N = 579) % |
P valuea |
Symptomatic (N = 82) % |
P valueb |
Ruptured (N = 348) % |
|---|---|---|---|---|---|
| Dysrhythmia | 9 | .01 | 15 | .093 | 22 |
| Renal declinec | 8 | <.01 | 12 | <.01 | 33 |
| Respiratory | 7 | <.01 | 16 | <.01 | 53 |
| Myocardial infarction | 5 | .03 | 8 | <.01 | 24 |
| Bowel | 2 | .6 | 1 | <.01 | 17 |
Elective vs symptomatic
symptomatic vs rupture; P from χ2 test. Significant at level of .05.
Renal decline defined as serum creatinine rise of 0.5 mg/dL or new initiation of dialysis.
GAS
The mean GAS for patients with Sx-AAAs was 80 ± 12 (79 ± 12 for those who survived; 92 ± 7 for the two patients who died). The mean GAS of patients who underwent R-AAA repair was 87 ± 16 (83 ± 14 among survivors; 97 ± 15 among decedents). Among all symptomatic and ruptured patients, 83% of deaths occurred in patients with a GAS > 85 (P < .001).
Long-term survival
Survival of patients undergoing E-AAA was 89% and 73% at 1 and 4 years. Patients undergoing Sx-AAA repair had intermediate survival of 83% and 68% at 1 and 4 years. R-AAA repair had the lowest survival rates, at 49% and 35% at 1 and 4 years. This was due to the high initial mortality, since the subsequent mortality rate was comparable to elective repair. In contrast, long-term survival after Sx-AAA repair decreased more rapidly than E-AAA repair, despite similar in-hospital mortality (Fig 3).
Fig 3.
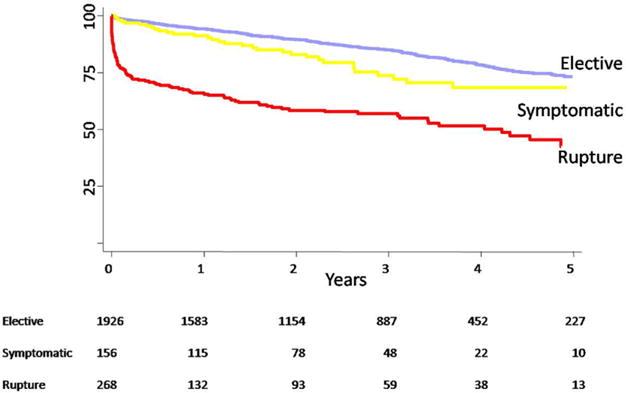
Long-term survival is shown stratified by urgency of repair (P <.001, log-rank between each group; standard error <10% for all time points shown).
DISCUSSION
Patients who present with Sx-AAA present a difficult challenge in clinical practice. Decision making involves a choice between urgent repair in a patient who may not be optimized for major vascular surgery vs postponing repair with risk of interval rupture. This concern is borne out by the fact that Sx-AAA repair has traditionally had an operative mortality rate that is intermediate between E-AAA and R-AAA repair.9,10,14 Our series represents one of the largest reported cohorts of Sx-AAAs and is the largest reported cohort of Sx-AAAs treated by EVAR. Several reports have described results of >150 patients with Sx-AAA but did so in the context of large series of all types of aneurysm repairs with less attention paid to the Sx-AAA cohort.10,13,18 Additionally, apart from one study, most reports are from a single center over a long period of time.18 Our report is unique in that it represents a contemporary evaluation of Sx-AAA using a regional multicenter database including academic and community hospitals.
The 7% proportion of Sx-AAA among our patients requiring AAA repair is within the range of previously reported cohorts (5% to 23%).3–5,10,13,14,18 Our incidence is near the low end of the reported spectrum, similar to the 2008 report of Nevala et al2 who reported a 6% frequency of Sx-AAA. It is unclear if these more contemporary cohorts have a lower frequency of Sx-AAAs based on increased surveillance or elective repair, or if this is related to other, unknown factors. One potential difference in frequency could result from differences in definition. Nearly all of our patients required urgent operation ≤24 hours of presentation, which is consistent with other reports,4,5,11 although few reports have specified this definition.2,3,6,7,9,10,12–15,18–20 Also, our definition of Sx-AAA could include inflammatory aneurysms; however, we did not track this as a separate category, so we cannot comment about treatment specific to inflammatory aneurysms.
Our cohort with Sx-AAAs has similar demographic characteristics as those previously reported, but we observed a higher prevalence of hypertension (78% vs 22% to 48%) and diabetes (17% vs 1.3% to 5%) among symptomatic patients.4,7,10,14 Our study confirms that Sx-AAAs present in older patients with larger aneurysm size compared to E-AAAs. Our finding that symptomatic patients were more often women, compared with patients undergoing E-AAA or R-AAA repair, has also been observed by Cambria et al4 and Sayers et al.14 However, we did not find that women more often presented with R-AAA. The reason for this paradox is not clear and deserves further investigation. Some reports have described women as having a disproportionate number of R-AAA presentations.21 Both these observations may be related to intervention thresholds for AAA diameter or to differences in expansion rates observed in women.22
Lastly, we believe we are the first to report the use of perioperative medications in this patient cohort. Patients undergoing Sx-AAA repair are less likely to be taking aspirin, statins, or β-blockers. This suggests that patients who presented with Sx-AAAs had less rigorous preventive care, which implies more potential for their AAAs to escape detection and elective repair before presenting with symptoms.
One of the most interesting findings in our study was the comparable operative in-hospital mortality rate for E-AAA and Sx-AAA repairs, since most series have reported higher 30-day or in-hospital mortality rates (9.5% to 20%) for Sx-AAAs.3,4,7,9,10,13–15,18 These earlier reports represent almost entirely open repairs compared with only 62% open repairs in the present study (Table IV). Several reports of EVAR to treat Sx-AAA have shown reduced mortality rates compared with historical series using open repair. Nevala et al2 reported a 0% mortality rate in 14 patients with Sx-AAA repaired with EVAR, with only one conversion to open repair. Similarly, Franks et al6 reported 11 Sx-AAA repairs with no operative mortality in those treated with EVAR.6
Table IV.
Mortality and method of repair for prior series reporting symptomatic abdominal aortic aneurysms
| First author | Year | Sx-AAA No. | Reported open % | Reported EVAR % | Mortality % |
|---|---|---|---|---|---|
| Johnson11 | 1980 | 84 | 100 | 0 | 16 |
| McCabe12 | 1981 | 56 | 100 | 0 | 14.3 |
| Sullivan5 | 1990 | 19 | 100 | 0 | 26 |
| Olsen10 | 1991 | 151 | 100 | 0 | 17.2 |
| Cambria4 | 1994 | 36 | 100 | 0 | 11.1 |
| Aune15 | 1995 | 52 | 100 | 0 | 17 |
| Darling20 | 1996 | 103 | 100 | 0 | 12.6 |
| Sayers14 | 1997 | 80 | 100 | 0 | 16 |
| Kantonen18 | 1997 | 156 | 100 | 0 | 13.5 |
| Bradbury13 | 1998 | 156 | 100 | 0 | 14.1 |
| Leo3 | 2005 | 42 | 100 | 0 | 9.5 |
| Antonello7 | 2006 | 42 | 100 | 0 | 11.9 |
| Franks6 | 2006 | 20 | 45 | 55 | 5 |
| Oranen19 | 2006 | 22 | 0 | 100 | 5 |
| Nevala2 | 2008 | 14 | 0 | 100 | 0 |
| Current | 2009 | 156 | 62 | 38 | 1.30 |
EVAR, Endovascular aneurysm repair; Sx-AAA, symptomatic abdominal aortic aneurysm.
Although small in number, these series that included a substantial proportion of Sx-AAAs repaired by EVAR demonstrate a clear trend toward lower operative mortality, as reported in Table IV. Indeed, the only two deaths in our Sx-AAA repairs occurred in patients treated with open repair. However, with very few deaths in our study, it was not possible to determine if EVAR was associated with lower mortality in patients with Sx-AAA. This could be a result of other unaccounted for patient or surgeon variables. A larger series with more events would be needed to accurately assess the effect of EVAR alone on Sx-AAA repair. The trend for lower mortality rates after Sx-AAA repair seen in more recent series is also likely due to overall improvements in perioperative care of this patient population.
Other factors that may affect operative mortality include the timing of the operation. Cambria et al4 noted higher mortality and morbidity for patients operated on ≤4 hours of admission. Outcomes were similar for patients operated on in 4 to 24 hours or in 1 to 7 days after admission. We only categorized operative time as within or exceeding 24 hours, so we cannot clarify this question except to note that the MAE event rate did not differ by the timing of the operation. This observation may result from more thorough preoperative management and optimization of patients where surgery was delayed at least 4 hours. This is consistent with our finding that patients with Sx-AAAs had the highest rate of β-blockers being started preoperatively. Other factors, such as postponing off-hour major aortic surgery until times when there are more experienced personnel available, may affect outcome. Appropriate timing of urgent repair for Sx-AAA is an important question that cannot be answered by our database.
A novel observation in our study was that patients who undergo Sx-AAA repair have a long-term survival rate that decreases significantly faster than patients undergoing E-AAA or R-AAA repair after the initial operative mortality (Fig 3). The reason for this is not clear and contrasts with findings by others who have reported late survival of Sx-AAAs. Olsen et al9,10 observed higher operative mortality for Sx-AAAs but similar survival at 5 years compared with E-AAAs. Oranen et al19 also found that SX-AAA patients who underwent repair had a similar survival slope as E-AAA patients. However, Soisalon-Soininen et al9 observed that Sx-AAA patients who underwent repair had a survival curve similar to R-AAA patients.
The poor longer-term survival after Sx-AAA repair has been attributed to increased cardiac comorbidities in this patient cohort resulting in more cardiac-related deaths.9 It seems plausible that perioperative care has improved to avoid early mortality in patients undergoing Sx-AAA repair, but that these patients are more likely to die from their excess cardiac comorbidities after hospital discharge. It is also possible that the patients undergoing Sx-AAA repair, who are less frequently taking aspirin, statins, and β-blockers preoperatively, are not started on these medications postoperatively, which may affect late survival.23 Determining the cause of death in future studies may help to delineate this.
When we calculated the GAS for each patient, our mean score was 79 ± 12 for Sx-AAA patients who survived and 92 ± 7 for those who died. This is similar to that observed by Leo et al,3 who reported a median GAS of 76 for survivors and 87 for those who died. Their receiver operating characteristic analysis concluded that a score of 85 was the best cutoff score to predict death. In a similar analysis, Antonello et al7 observed a median score of 75 for survivors and 97 for those who died after Sx-AAA repair and found a score of 90 was the best predictor of death. When applied to our patient cohorts, a score of 85 predicted 83% of our Sx-AAA and R-AAA patients’ deaths.
Our estimation of GAS did not capture patients who had a TIA or stroke without carotid endarterectomy, and we used a more strict definition of renal insufficiency (see Methods). Thus, we underestimated the true GAS score; however, our estimate agrees with previous reports and correlated similarly with mortality. Our operative mortality rate for patients with Sx-AAA was too low for a meaningful comparison of this alone, but it is reasonable to assume that a GAS of 85 carries a significant risk for perioperative death and might be used as a guide for more preoperative optimization and consideration of EVAR in patients with Sx-AAAs.
CONCLUSION
Symptomatic AAAs represent <10% of patients who require aneurysm repair. Our contemporary results demonstrate that this is a unique patient population with a low operative mortality but high morbidity and worse long-term survival than patients undergoing E-AAA repair. Although we could not identify a benefit of EVAR because of few overall hospital deaths, we believe that its use in 38% of our patients with Sx-AAA might have improved their outcome.
Appendix (online only)
Participating centers of the Vascular Study Group of Northern New England
Maine
Eastern Maine Medical Center
Central Maine Medical Center
Maine Medical Center
Mercy Hospital
Massachusetts
U Mass Memorial Medical Center
New Hampshire
Catholic Medical Center
Concord Hospital
Cottage Hospital
Dartmouth-Hitchcock Medical Center
Lakes Region Hospital
Vermont
Fletcher Allen Heath Care
Footnotes
Competition of interest: none.
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a competition of interest.
AUTHOR CONTRIBUTIONS
Conception and design: RD, BN, PG, JC
Analysis and interpretation: RD, BN, PG, CC, AS, RC, DB, JC
Data collection: BN, PG, CC, AS, RC, DB
Writing the article: RD, BN, JC
Critical revision of the article: PG, CC, AS, RC, DB
Final approval of the article: RD, BN, PG, CC, AS, RC, DB, JC
Statistical analysis: BN, PG, CC
Obtained funding: Not applicable
Overall responsibility: JC
References
- 1.Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004;329:1259. doi: 10.1136/bmj.38272.478438.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nevala T, Perala J, Aho P, et al. Outcome of symptomatic, unruptured abdominal aortic aneurysms after endovascular repair with the Zenith stent-graft system. Scand Cardiovasc J. 2008;42:178–81. doi: 10.1080/14017430701819105. [DOI] [PubMed] [Google Scholar]
- 3.Leo E, Biancari F, Kechagias A, Ylönen K, Rainio P, Romsi P, et al. Outcome after emergency repair of symptomatic, unruptured abdominal aortic aneurysm: results in 42 patients and review of the literature. Scand Cardiovasc J. 2005;39:91–5. doi: 10.1080/14017430410016422. [DOI] [PubMed] [Google Scholar]
- 4.Cambria RA, Gloviczki P, Stanson AW, Cherry KJ, Jr, Hallett JW, Jr, Bower TC, et al. Symptomatic, nonruptured abdominal aortic aneurysms: are emergent operations necessary? Ann Vasc Surg. 1994;8:121–6. doi: 10.1007/BF02018859. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan CA, Rohrer MJ, Cutler BS. Clinical management of the symptomatic but unruptured abdominal aortic aneurysm. J Vasc Surg. 1990;11:799–803. [PubMed] [Google Scholar]
- 6.Franks S, Lloyd G, Fishwick G, Bown M, Sayers R. Endovascular treatment of ruptured and symptomatic abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2006;31:345–50. doi: 10.1016/j.ejvs.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Antonello M, Lepidi S, Kechagias A, Frigatti P, Tripepi A, Biancari F, et al. Glasgow aneurysm score predicts the outcome after emergency open repair of symptomatic, unruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2007;33:272–6. doi: 10.1016/j.ejvs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Shifrin EG, Pizov R, Perel A, Sidi A, Anner H, Cotev S. Urgent abdominal aortic aneurysm repair in patients over the age 80. J Cardiovasc Surg (Torino) 1987;28:167–70. [PubMed] [Google Scholar]
- 9.Soisalon-Soininen S, Salo JA, Perhoniemi V, Mattila S. Emergency surgery of non-ruptured abdominal aortic aneurysm. Ann Chir Gynaecol. 1999;88:38–43. [PubMed] [Google Scholar]
- 10.Olsen PS, Schroeder T, Agerskov K, Røder O, Sørensen S, Perko M, et al. Surgery for abdominal aortic aneurysms. A survey of 656 patients. J Cardiovasc Surg (Torino) 1991;32:636–42. [PubMed] [Google Scholar]
- 11.Johnson G, Jr, McDevitt NB, Proctor HJ, Mandel SR, Peacock JB. Emergent or elective operation for symptomatic abdominal aortic aneurysm. Arch Surg. 1980;115:51–3. doi: 10.1001/archsurg.1980.01380010043008. [DOI] [PubMed] [Google Scholar]
- 12.McCabe CJ, Coleman WS, Brewster DC. The advantage of early operation for abdominal aortic aneurysm. Arch Surg. 1981;116:1025–9. doi: 10.1001/archsurg.1981.01380200033006. [DOI] [PubMed] [Google Scholar]
- 13.Bradbury AW, Adam DJ, Makhdoomi KR, Stuart WP, Murie JA, Jenkins AM, et al. A 21-year experience of abdominal aortic aneurysm operations in Edinburgh. Br J Surg. 1998;85:645–7. doi: 10.1046/j.1365-2168.1998.00695.x. [DOI] [PubMed] [Google Scholar]
- 14.Sayers RD, Thompson MM, Nasim A, Healey P, Taub N, Bell PR. Surgical management of 671 abdominal aortic aneurysms: a 13 year review from a single centre. Eur J Vasc Endovasc Surg. 1997;13:322–7. doi: 10.1016/s1078-5884(97)80105-0. [DOI] [PubMed] [Google Scholar]
- 15.Aune S, Amundsen SR, Evjensvold J, Trippestad A. The influence of age on operative mortality and long-term relative survival following emergency abdominal aortic aneurysm operations. Eur J Vasc Endovasc Surg. 1995;10:338–41. doi: 10.1016/s1078-5884(05)80053-x. [DOI] [PubMed] [Google Scholar]
- 16.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. discussion 101–2. [DOI] [PubMed] [Google Scholar]
- 17.Samy AK, Murray G, MacBain G. Glasgow aneurysm score. Cardiovasc Surg. 1994;2:41–4. [PubMed] [Google Scholar]
- 18.Kantonen I, Lepantalo M, Salenius JP, Matzke S, Luther M, Ylonen K. Mortality in abdominal aortic aneurysm surgery—the effect of hospital volume, patient mix and surgeon’s case load. Eur J Vasc Endovasc Surg. 1997;14:375–9. doi: 10.1016/s1078-5884(97)80287-0. [DOI] [PubMed] [Google Scholar]
- 19.Oranen BI, Bos WT, Verhoeven EL, Tielliu IF, Zeebregts CJ, Prins TR, et al. Is emergency endovascular aneurysm repair associated with higher secondary intervention risk at mid-term follow-up? J Vasc Surg. 2006;44:1156–61. doi: 10.1016/j.jvs.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 20.Darling C, 3rd, Shah DM, Chang BB, Paty PS, Leather RP. Current status of the use of retroperitoneal approach for reconstructions of the aorta and its branches. Ann Surg. 1996;224:501–6. doi: 10.1097/00000658-199610000-00008. discussion 506–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants Ann Surg. 1999;230:289–96. doi: 10.1097/00000658-199909000-00002. discussion 96–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grootenboer N, Bosch JL, Hendriks JM, van Sambeek MR. Epidemiology, aetiology, risk of rupture and treatment of abdominal aortic aneurysms: does sex matter? Eur J Vasc Endovasc Surg. 2009;38:278–84. doi: 10.1016/j.ejvs.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Barrett TW, Mori M, De Boer D. Association of ambulatory use of statins and beta-blockers with long-term mortality after vascular surgery. J Hosp Med. 2007;2:241–52. doi: 10.1002/jhm.160. [DOI] [PubMed] [Google Scholar]


