Abstract
Objective
Studies of infrainguinal lower extremity bypass for critical limb ischemia (CLI) have traditionally emphasized outcomes of patency, limb salvage, and death. Because functional outcomes are equally important, our objectives were to describe the proportion of CLI patients who did not achieve symptomatic improvement 1 year after bypass, despite having patent grafts, and identify preoperative factors associated with this outcome.
Methods
The prospectively collected Vascular Study Group of Northern New England database was used to identify all patients with elective infrainguinal lower extremity bypass for CLI (2003 to 2007) for whom long-term follow-up data were available. The primary composite study end point was clinical failure at 1 year after bypass, defined as amputation or persistent or worsened ischemic symptoms (rest pain or tissue loss), despite a patent graft. Variables identified on univariate screening (inclusion threshold, P < .20) were included in a multivariable logistic regression model to identify independent predictors.
Results
Long-term follow-up data were available for 1012 patients who underwent infrainguinal bypasses for CLI, of which 788 (78%) remained patent at 1 year. Of these, 79 (10%) met criteria for the composite end point of clinical failure: 21 (2.7%) for major amputations and 58 (7.4%) for persistent rest pain or tissue loss. In multivariable analysis, significant predictors of clinical failure included dialysis dependence (odds ratio [OR], 3.74; 95% confidence interval [CI], 1.84–7.62; P < .001) and preoperative inability to ambulate independently (OR, 2.17; 95% CI, 1.26–3.73; P = .005). A history of coronary artery bypass graft or percutaneous coronary intervention was protective (OR, 0.52; 95% CI, 0.29–0.93; P = .03).
Conclusions
After infrainguinal lower extremity bypass for CLI, 10% of patients with a patent graft did not achieve clinical improvement at 1 year. Preoperative identification of this specific patient subgroup remains challenging. To improve surgical decision making and the overall care of CLI patients, further emphasis needs to be placed on functional outcomes in addition to traditional surgical end points.
The management of patients with critical limb ischemia (CLI) remains complex, with several factors contributing to the treatment decision-making process. Traditional study end points for these patients have included graft patency,1,2 limb salvage,3,4 or death.5,6 More recently, however, alternative end points have emerged that include amputation-free survival,7–9 major adverse cardiovascular events,9 target limb revascularization,10 quality of life,11 and functional outcomes such as the ability to ambulate or to live independently after surgery.6,11,12 Although these findings, interpreted in aggregate, do suggest an association between graft patency and quality of life, most clinicians would acknowledge that patency does not always equate with symptom resolution and functional improvement.
The proportion of patients that does not experience clinical improvement at 1 year postoperatively, despite infrainguinal graft patency, has not been well delineated. Therefore, the objective of the current investigation was to use the prospectively collected Vascular Study Group of Northern New England (VSGNNE) database to address this question. In addition, we sought to identify preoperative factors associated with persistent ischemic symptoms or ipsilateral amputation, despite graft patency, to improve preoperative patient counseling.
METHODS
Cohort assembly
The VSGNNE is a regional cooperative quality improvement initiative that was developed in 2002 to prospectively evaluate outcomes in patients undergoing vascular surgery. Eleven teaching and nonteaching hospitals with 59 vascular surgeons (community and academic) currently participate in this program by reporting data into the registry. All data are self-reported and sent to a central data repository where they are aggregated and reviewed. Research analysts are blinded to patient, surgeon, and hospital identity.
At the time of discharge after the index operation, a perioperative data sheet containing preoperative, intraoperative, and postoperative data is completed and submitted to the VSGNNE. The VSGNNE does not mandate a specific protocol for graft surveillance or medical or wound therapy after lower extremity bypass. These specific management decisions are left to the discretion of the operating surgeon.
The study design for the VSGNNE registry emphasizes the importance of collecting follow-up data at 1 year for all patients with procedures entered into the registry. To facilitate compliance with this requirement, the central site sends an electronically automated follow-up form for each operation to each surgeon in advance of the expected 1-year office visit. Accordingly, at the approximate 1-year follow-up, this data sheet is completed and submitted to the VSGNNE (acceptable window for the current analysis was 6 to 18 months). Data pertaining to ambulation status, symptom status, patency, ankle-brachial index, bypass graft revisions, or amputations are recorded on this form. Since the inception of the study, a claims-based audit system has been used that has demonstrated 99% accuracy in capturing consecutive operations performed at each center. Details relating to the VSGNNE study design have been published previously13 and are available at the VSGNNE Web site (www.vsgnne.org).
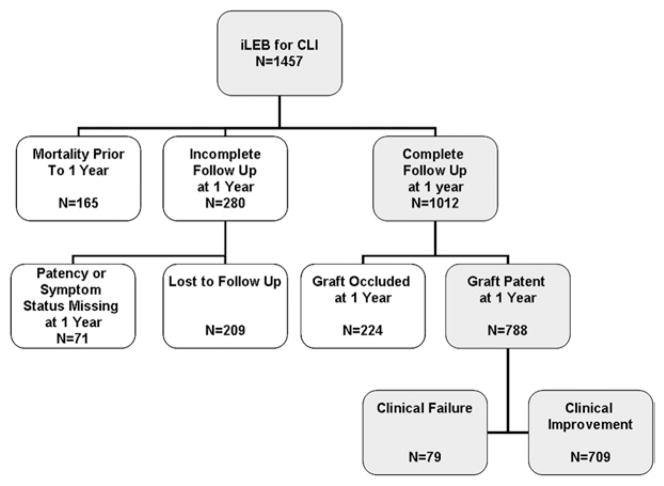
For the purpose of this study, the VSGNNE database was queried for patients undergoing elective and urgent infrainguinal lower extremity bypass (iLEB) performed between January 1, 2003, and December 31, 2007, for an indication of CLI (defined as tissue loss or ischemic rest pain). To assess outcomes at 1 year, patients were excluded if they did not have a completed 1-year follow-up form that included all data pertaining to symptom status and patency, resulting in 445 of the 1457 patients being excluded (Fig 1). All infrainguinal bypass configurations were included for analysis, regardless of the specific inflow site, outflow site, or conduit.
Fig 1.
Flowchart shows cohort selection for primary analysis of clinical failure, defined as persistent rest pain, tissue loss, or ipsilateral major amputation at 1 year, despite a patent graft. CLI, Critical limb ischemia; iLEB, infrainguinal lower extremity bypass.
Covariates examined
Patient information for >70 clinical and demographic variables (available at www.vsgnne.org) was collected. Comorbidities examined included coronary artery disease (history of myocardial infarction or angina), chronic obstructive pulmonary disease (COPD, medication-dependent or home oxygen-dependent), congestive heart failure (by history), diabetes mellitus (insulin-dependent or controlled by oral medication or diet), hypertension (history of hypertension or blood pressure ≥140/90 mm Hg on the preoperative evaluation), and history of tobacco use (never, <1 year prior, or current). Renal disease was categorized in three strata: normal (serum creatinine ≤1.8 mg/dL), renal insufficiency (serum creatinine >1.8 mg/dL), and dialysis-dependent.
Variables related to surgical history included previous coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI), as well as previous carotid, aortic, peripheral bypass or stent, and major extremity amputation. Medication variables included preoperative use of antiplatelet agents, statins, or β-blockers. Functional variables included preoperative living status (home or nursing home) and ambulation status (independent, with assistance, wheelchair-bound, and bed-bound).
Also evaluated were procedural details, such as urgency, bypass conduit, and bypass target vessel. Bypass conduit was considered a preoperatively available variable, based on an assumption that most patients undergoing bypass surgery receive vein mapping, allowing for a preoperative determination of conduit availability. Long-term follow-up data included vital status, patency of the graft (whether primary or secondary by duplex graft surveillance scan), amputation status, and symptoms (asymptomatic, claudication, rest pain, or tissue loss). Vital status was confirmed for all patients using follow-up visit notes and a current version of the Social Security Death Index.
Primary end point
Clinical failure, which was the primary end point, was defined as rest pain, tissue loss, or ipsilateral amputation (major amputation is defined as above- or below-knee [loss of foot] amputation) at long-term follow-up, despite a patent graft. The study excluded four patients who did not undergo amputation but were missing information on their symptoms at follow-up.
Statistical analysis
Baseline characteristics were compared between groups using Pearson χ2 analysis for categoric variables and the t test for continuous variables. Those variables with a value of P < .2 were entered into a logistic regression model for the primary outcome of clinical failure. Significance was accepted at the P < .05 level. All analyses were conducted using SAS 9.1 software (SAS Institute, Cary, NC).
RESULTS
Cohort characteristics
Between 2003 and 2007, 1457 patients underwent elective or urgent iLEB for CLI at 11 participating centers. The perioperative death rate, defined as 30-day mortality, was 2.2%. After excluding 445 patients who did not specifically have patency or symptom status information at the 1-year follow-up, 1012 patients remained in the cohort. Among the 445 patients who were excluded, 165 had died before the 1-year follow-up visit, 71 had incomplete symptom or patency status information, and 209 were lost to follow-up (Fig 1). Of the remaining 1012 patients (69%), 788 (78%) had patent grafts and 224 (22%) had occluded grafts at 1 year after bypass (Fig 1).
Patency at long-term follow-up was judged by duplex examination in 419 patients (55.9%), a palpable graft/ distal pulse in 277 (37.0%), and by ankle-brachial index increase >.15 in 53 (7.1%). The method for judging graft patency was not reported for the remaining 39 patients (4.9%). Mean length of follow-up was 346.9 days (standard deviation, 6.6 days).
Among patients with a patent graft at follow-up, 68% were men, 99% were white, and 82% were current or former smokers. A history of coronary artery disease (CAD) was present in 40%, but only 2% had unstable angina or had sustained a myocardial infarction ≤6 months before surgery. The prevalence of diabetes was 59%, of which 31% were insulin-dependent, 22% were treated with oral medications, and 6% were managed with diet alone. Renal function was normal (creatinine ≤1.8 mg/dL) in 85%, abnormal but without dialysis (creatinine > 1.8 mg/dL) in 9%, and sufficiently poor to necessitate permanent dialysis in 6%. Of the 30% of patients with COPD, 2% required home oxygen therapy. Operations were elective in 78% of patients.
Details of the analysis cohort, stratified according to the end point of clinical failure, are presented in Table I. Salient differences seen in those who experienced clinical failure include a greater proportion of patients who were dialysis-dependent (17.7% vs 5.0%, P < .0001), residing in a nursing home (7.6% vs 3.9%, P = .13), had a history of contralateral major amputation (7.6% vs 4.2%, P = .17), were not ambulating independently preoperatively (45.6% vs 24.7%, P = .0001), and underwent nonelective operation (34% vs 20%, P = .005).
Table I.
Patient characteristics in 788 patients with a patent bypass graft at 1 yeara
| Covariatesb | Improved (n = 709) | Unimproved (n = 79) | P value |
|---|---|---|---|
| Demographics | |||
| Age, y | 69.5 ± 11.1 | 68.0 ± 11.1 | .25 |
| Female gender | 230 (32.4) | 23 (29.1) | .55 |
| Race | .84 | ||
| White | 698 (98.5) | 78 (98.7) | |
| Nonwhite | 11 (1.5) | 1 (1.3) | |
| Preoperative factors | |||
| Smoking status | .90 | ||
| Never | 123 (17.4) | 15 (19.0) | |
| Prior history | 299 (42.2) | 34 (43.0) | |
| Current | 286 (40.4) | 30 (38.0) | |
| Coronary artery disease | 285 (40.2) | 29 (36.7) | .55 |
| History of CABG or PCI | 236 (33.3) | 18 (22.8) | .06 |
| Congestive heart failure | 131 (18.5) | 15 (19.0) | .91 |
| Hypertension | 613 (86.5) | 72 (91.1) | .24 |
| Insulin-dependent diabetes | 210 (29.6) | 36 (45.6) | .004 |
| COPD | 205 (28.9) | 29 (36.7) | .15 |
| Renal function | <.0001 | ||
| Creatinine ≤1.8 mg/dL | 606 (86.7) | 55 (69.6) | |
| Creatinine >1.8 mg/dL | 58 (8.3) | 10 (12.7) | |
| Dialysis-dependent (any creatinine) | 35 (5.0) | 14 (17.7) | |
| Previous | |||
| Arterial bypass (any) | 198 (27.9) | 18 (22.8) | .33 |
| PTA or stent (ipsilateral) | 34 (4.8) | 7 (8.9) | .12 |
| Major amputation (contralateral) | 30 (4.2) | 6 (7.6) | .17 |
| Living | .13 | ||
| Home | 680 (96.1) | 73 (92.4) | |
| Nursing home | 28 (3.9) | 6 (7.6) | |
| Ambulation status | .0001 | ||
| Independent | 534 (75.3) | 43 (54.4) | |
| With assistance | 135 (19.0) | 29 (36.7) | |
| Wheelchair-bound | 34 (4.8) | 4 (5.1) | |
| Bed-bound | 6 (0.9) | 3 (3.8) | |
| Technical factors | |||
| Urgency | .005 | ||
| Elective | 565 (79.7) | 52 (65.8) | |
| Urgent | 144 (20.3) | 27 (34.2) | |
| Prosthetic bypass conduit | 168 (23.7) | 18 (22.8) | .60 |
| Single-segment GSV | 477 (67.4) | 53 (67.1) | .96 |
| Recipient vessel | .58 | ||
| Above-knee popliteal | 162 (22.9) | 14 (17.7) | |
| Below-knee popliteal | 223 (31.5) | 27 (34.2) | |
| Tibial/other | 324 (45.7) | 38 (48.1) | |
| Medications | |||
| Aspirin | 489 (69.0) | 52 (65.8) | .57 |
| Clopidogrel | 72 (10.2) | 6 (7.6) | .47 |
| Statin | 389 (54.9) | 46 (58.2) | .57 |
| β-blockers | 599 (84.5) | 65 (82.3) | .85 |
CABG, Coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; GSV, great saphenous vein; PCI, percutaneous coronary intervention; PTA, percutaneous transluminal angioplasty.
Univariate comparison between those whose symptoms persisted or required a major amputation and those who demonstrated improvement at 1-year follow-up.
All categoric values are presented as number (%); the continuous variable (age) is given as mean ± standard deviation.
At the time of follow-up, 121 major ipsilateral amputations had been performed (Table II). Amputation occurred far more frequently among patients with an occluded graft (44.6% of 224 limbs) than in patients with patent grafts (2.7% of 788 limbs). Of an additional 58 patients (7.4%) with patent bypass grafts, 16 reported persistent rest pain, and 12 reported tissue loss at the time of follow-up. Therefore, 79 patients (10%) reached the composite end point of clinical failure, despite having documented patent bypass grafts.
Table II.
Patient outcomes at 1-year follow-up after infrainguinal lower extremity bypass for critical limb ischemiaa
| Symptom | Bypass graft status, No. (%)
|
P value | |
|---|---|---|---|
| Patent (n = 788) | Occluded (n = 224) | ||
| Asymptomatic | 634 (80.5) | 43 (19.2) | <.0001 |
| Claudication | 75 (9.5) | 38 (17.0) | |
| Rest pain | 16 (2.0) | 14 (6.3) | |
| Tissue loss | 42 (5.3) | 29 (13.0) | |
| Ipsilateral amputation | 21 (2.7) | 100 (44.6) | |
Univariate comparison included.
Analysis by indication
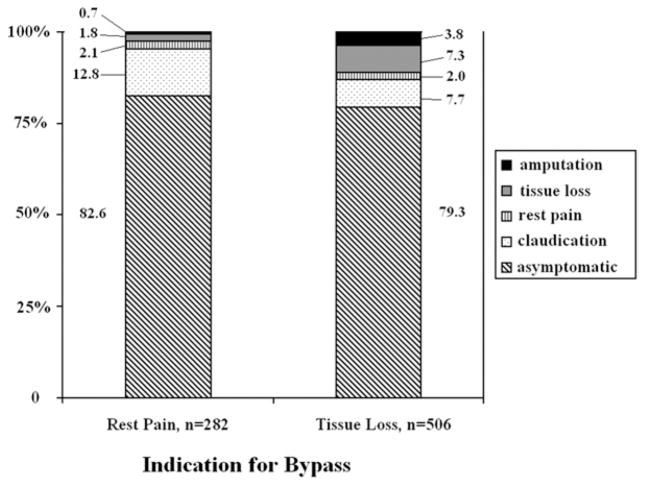
When outcomes were analyzed according to indication for surgery (rest pain or tissue loss), most patients in both groups were asymptomatic at long-term follow up (Fig 2). Among the 282 patients with rest pain preoperatively, 82.6% were asymptomatic, 12.8% reported claudication, 2.1% still had rest pain, 1.8% had progressed to tissue loss, and 0.7% had undergone major amputation. The distribution of outcomes was less favorable among the 506 patients with tissue loss preoperatively: 79.3% were asymptomatic, 7.7% reported claudication, 2.0% had rest pain, 7.3% still had tissue loss, and 3.8% had undergone major amputation.
Fig 2.
Transition of health states are shown at the 1-year follow-up according to indication for lower extremity bypass.
Predictors of clinical failure
Nine covariates demonstrated values of P <.20 on univariate analysis (Table I) and were entered into the multivariable model. On multivariable analysis, two independent predictors of clinical nonimprovement were identified (Table III). Dialysis-dependence was associated with a 3.7-fold increase in the odds of clinical failure compared with patients with normal renal function (odds ratio [OR], 3.74; 95% confidence interval [CI], 1.84–7.62). Preoperative ambulation with assistance was also predictive of clinical failure compared with independent ambulation status (OR, 2.17; 95% CI, 1.26–3.73). Insulin-dependent diabetes demonstrated a nonstatistically significant trend toward increased odds of clinical failure (OR, 1.65; 95% CI, 0.99–2.75, P = .054). History of CABG or PCI was protective against clinical failure (OR, 0.52; 95% CI, 0.29–0.93).
Table III.
Multivariable model for the preoperative predictors of persistent rest pain, tissue loss, or amputation despite graft patency one year after infrainguinal lower extremity bypass
| Covariate | Adjusted OR (95% CI) | P value |
|---|---|---|
| History of CABG or PCI | 0.52 (0.29–0.93) | .027 |
| Insulin-dependent diabetes | 1.65 (0.99–2.75) | .054 |
| COPD | 1.23 (0.74–2.04) | .43 |
| Renal function | ||
| Creatinine ≤1.8 mg/dL | Ref | … |
| Creatinine >1.8 mg/dL | 1.85 (0.85–4.01) | .12 |
| Dialysis-dependent (any creatinine) | 3.74 (1.84–7.62) | .0003 |
| History of ipsilateral PTA or stent | 1.82 (0.74–4.47) | .19 |
| History of contralateral major amputation | 1.42 (0.52–3.85) | .49 |
| Non-nursing home resident | 0.87 (0.30–2.52) | .80 |
| Preoperative ambulation status | ||
| Independent | Ref | … |
| With assistance | 2.17 (1.26–3.73) | .005 |
| Wheelchair-bound | 1.05 (0.31–3.63) | .93 |
| Bed-bound | 3.47 (0.76–15.98) | .11 |
| Urgent procedure (ref = elective) | 1.58 (0.93–2.69) | .094 |
CABG, coronary artery bypass graft; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio; PCI, percutaneous coronary intervention; PTA, percutaneous transluminal angioplasty; Ref, referent.
DISCUSSION
Among patients with CLI, 10% reported symptoms that were not improved at 1 year after lower extremity bypass, despite having patent grafts. These results are consistent with other reports of the incidence of unsatisfactory outcomes after iLEB despite functioning grafts. Dietzek et al14 highlighted this problem in 1990 with a retrospective review of 987 patients, 7.6% of whom experienced limb loss despite patent infrainguinal bypass. A second series demonstrated that 9% of extremities with patent grafts required amputation.15
This analysis also sought to identify risk factors for clinical failure that can be identified preoperatively to optimize clinical decision making for patients with CLI. We identified two primary independent preoperative predictors: dialysis-dependence, which conferred a 3.7-fold increased risk, and ambulation with assistance, which more than doubled the risk. Of note, although ambulation with assistance preoperatively was a risk factor for clinical failure compared with independent ambulation, wheelchair-bound and bed-bound status did not reach statistical significance, possibly reflecting the small numbers of patients in these groups. Alternatively, this may reflect that patients with more significant functional impairments have more assistance with wound care and are less likely to repeatedly traumatize their extremities with impaired ambulation.
An exploratory analysis of the cohort, after the exclusion of patients who were wheelchair-bound or bed-bound, yielded a univariate screen with largely similar results. When entered into the logistic regression model, a similar pattern emerged in terms of significant predictors, with dialysis-dependence conferring a 4.7-fold increase in odds of clinical failure and ambulation with assistance carrying a 2-fold increase in odds of clinical failure. A similar magnitude of effect (OR, 1.8) was also seen for insulin-dependent diabetes, which reached statistical significance. A history of CABG or PCI was not significant in this model.
In addition, the only preoperative variable that this study demonstrated was protective against clinical failure was a history of CABG or PCI. This effect persisted on multivariable analysis, which controlled for potential relevant measured confounders. The mechanism for this risk reduction is not clear. It may be that this represents up-front selection bias. A history of coronary intervention may describe a patient who has survived a major operation and thus is healthier than a patient who has not undergone intervention. Also, this variable may indicate a benefit associated with a comprehensive cardiac rehabilitation program, possibly including better fitness or more attentive wound care, or both. Finally, there may be some other mechanism by which history of coronary intervention preserves functional status or improves wound healing, the precise nature of which cannot be ascertained from these data alone.
The predictors of clinical failure identified in this analysis (dialysis-dependence and ambulation with assistance) differ somewhat from those identified in other reports of clinical failure in the setting of a patent bypass graft, which have included diabetes, extensive pedal necrosis, and advanced infection,14 as well as black race and distal anastomosis to the anterior tibial or dorsalis pedis.15 The identification of dialysis-dependence as a risk factor for clinical failure, however, is consistent with a wide body of literature.
As in our study, Carsten et al15 identified chronic renal failure as an independent predictor of amputation despite graft patency. In another review that focused specifically on end-stage renal disease (ESRD), 59% of patients who underwent infrainguinal bypass for CLI eventually required a major amputation despite having a patent bypass graft.16 Finally, a meta-analysis of 28 reports of iLEB outcomes in patients with ESRD found an overall 10% incidence of amputation despite graft patency,17 equal to that observed in our analysis.
These marginal outcomes in patients with ESRD have prompted some authors to suggest that iLEB should not be offered to patients with ESRD who have extensive infection or tissue loss, because high rates of death and early amputation may outweigh the potential benefits associated with surgical revascularization.17 Others, however, have suggested a more cautious interpretation, concluding that careful risk stratification is imperative, but broad sweeping guidelines are not appropriate.8,9,16,18
We agree that predictors of clinical failure, identified in our study or others, cannot be interpreted in isolation and must be considered in the context of various patient-related and provider-related factors. Rather than offer a definitive set of criteria by which patients should be managed, our findings highlight the need for continued research into nontraditional end points that may have profound effects on patients undergoing treatment for CLI. With a 10% incidence of clinical failure, preoperative counseling should not be limited only to a discussion regarding the risks of graft occlusion, morbidity, and mortality, but patients must also understand that graft patency may not guarantee symptomatic improvement.
It is important to recognize that this analysis is limited only to patients who underwent open iLEB. Many authors have advocated that endovascular techniques may be the treatment of choice in high-risk surgical patients owing to the lower associated morbidity, improved limb salvage, and decreased lengths of stay.19–21 However, the need for major amputation has also been demonstrated to occur despite patent endovascularly treated arterial segments.
Khan et al22 recently reported 236 limbs treated with endovascular therapies for CLI. These authors noted amputation, despite a patent endovascularly treated arterial segment, was the most common means of limb loss, and 80% of amputations occurred in patients with a patent endovascularly treated arterial segment.22 Although predictors of limb salvage failure in this cohort with diabetes, gangrene, and infrapopliteal interventions were similar to those reported with open iLEB, the authors note that because all three significant predictors of limb loss were present in 61% of the endovascularly treated patients who did achieve limb salvage, these risk factors may have limited predictive utility. This again highlights our current deficit in useful information for risk stratification for clinical failure in patients undergoing endovascular as well those undergoing open revascularization.
There are two primary limitations inherent to this study design. First, although all of the data analyzed in this study were prospectively collected, they were reviewed retrospectively. As a result, in the design of the present study, we were limited to those variables that were routinely collected by the VSGNNE. Among the notable covariates not available for analysis were the degree and extent of ulceration or soft tissue infection, the quality of the ipsilateral runoff vessels, and the time interval between symptom onset and the date of surgery. Furthermore, the diagnosis of rest pain at follow-up was self-reported and not corroborated with a validated instrument or physiologic parameters.
Second, the analysis excluded a considerable number of patients who did not have follow-up information. It is possible that the patients without complete long-term follow-up differed from those with follow-up data, thereby introducing selection bias. Univariate comparison of the variables entered into the logistic regression model revealed that the 445 patients excluded from the primary analysis were significantly more likely to have had urgent operations, impaired renal function, and were less likely to ambulate independently and to live independently at baseline. The other five covariates were not significantly different between the two groups. It is also important to recognize that these analyses were conditional on both surviving to long-term follow-up and having a patent graft; other studies7–9 that have addressed risk factors for mortality and loss of graft patency must also be considered in preoperative patient counseling.
Nonetheless, our study has several notable strengths. First, we describe the frequency of a clinically relevant outcome. We made a deliberate decision in this study to model a more inclusive end point than limb salvage or amputation. We believe that the end point of clinical failure, which not only includes amputation but also persistent or worsening symptoms (ie, rest pain or tissue loss), is a clinically relevant condition that negates the benefits of a patent bypass graft.
Second, we describe three preoperatively identifiable patient factors associated with clinical failure. We also highlight the difficulty of capturing all clinically relevant patient characteristics in statistical models like ours. Ultimately, an improved understanding of functional outcomes will help refine clinical decision making and further improve patient and physician expectations for those considering bypass surgery. Just as traditional end points such as patency, limb salvage, and mortality play a large role in the determination of the optimal treatment for a patient with CLI, function and symptom-based outcomes are of significant importance and therefore warrant further study.
CONCLUSIONS
Ten percent of patients with patent bypass grafts do not achieve clinical improvement at 1 year after lower extremity bypass surgery for CLI. Patients who are dialysis-dependent (OR, 3.7) or cannot ambulate independently (OR, 2.2) are at a markedly increased risk for this outcome. Further study is necessary to improve preoperative risk stratification for functional outcomes, such as clinical failure, in order to improve the overall care of patients with CLI.
Footnotes
Competition of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a competition of interest.
AUTHOR CONTRIBUTIONS
Conception and design: AS, JS, PG
Analysis and interpretation: AS, JS, PG
Data collection: AS, PG, BN, JC, LM
Writing the article: AS, PG, JS
Critical revision of the article: JS, PG, BN, JC, AS
Final approval of the article: AS, JS, PG, BN, JC, LM
Statistical analysis: AS, JS, PG
Obtained funding: AS, JC, LM
Overall responsibility: AS
References
- 1.Schanzer A, Hevelone N, Owens CD, Belkin M, Bandyk DF, Clowes AW, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg. 2007;46:1180–90. doi: 10.1016/j.jvs.2007.08.033. discussion 90. [DOI] [PubMed] [Google Scholar]
- 2.Robinson WP, 3rd, Owens CD, Nguyen LL, Chong TT, Conte MS, Belkin M. Inferior outcomes of autogenous infrainguinal bypass in Hispanics: an analysis of ethnicity, graft function, and limb salvage. J Vasc Surg. 2009;49:1416–25. doi: 10.1016/j.jvs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Rossi PJ, Skelly CL, Meyerson SL, Bassiouny HS, Katz D, Schwartz LB, et al. Redo infrainguinal bypass: factors predicting patency and limb salvage. Ann Vasc Surg. 2003;17:492–502. doi: 10.1007/s10016-003-0040-z. [DOI] [PubMed] [Google Scholar]
- 4.Toursarkissian B, D’Ayala M, Stefanidis D, Shireman PK, Harrison A, Schoolfield J, et al. Angiographic scoring of vascular occlusive disease in the diabetic foot: relevance to bypass graft patency and limb salvage. J Vasc Surg. 2002;35:494–500. doi: 10.1067/mva.2002.120046. [DOI] [PubMed] [Google Scholar]
- 5.Owens CD, Ho KJ, Kim S, Schanzer A, Lin J, Matros E, et al. Refinement of survival prediction in patients undergoing lower extremity bypass surgery: stratification by chronic kidney disease classification. J Vasc Surg. 2007;45:944–52. doi: 10.1016/j.jvs.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Taylor SM, Kalbaugh CA, Blackhurst DW, Cass AL, Trent EA, Langan EM, 3rd, et al. Determinants of functional outcome after revascularization for critical limb ischemia: an analysis of 1000 consecutive vascular interventions. J Vasc Surg. 2006;44:747–55. doi: 10.1016/j.jvs.2006.06.015. discussion 55–6. [DOI] [PubMed] [Google Scholar]
- 7.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–34. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 8.Schanzer A, Mega J, Meadows J, Samson RH, Bandyk DF, Conte MS. Risk stratification in critical limb ischemia: derivation and validation of a model to predict amputation-free survival using multicenter surgical outcomes data. J Vasc Surg. 2008;48:1464–71. doi: 10.1016/j.jvs.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schanzer A, Goodney PP, Li Y, Eslami M, Cronenwett J, Messina L, et al. Validation of the PIII CLI risk score for the prediction of amputation-free survival in patients undergoing infrainguinal autogenous vein bypass for critical limb ischemia. J Vasc Surg. 2009;50:769–75. doi: 10.1016/j.jvs.2009.05.055. discussion 775. [DOI] [PubMed] [Google Scholar]
- 10.Das TS, McNamara T, Gray B, Sedillo GJ, Turley BR, Kollmeyer K, et al. Primary cryoplasty therapy provides durable support for limb salvage in critical limb ischemia patients with infrapopliteal lesions: 12-month follow-up results from the BTK Chill Trial. J Endovasc Ther. 2009;16:II19–30. doi: 10.1583/08-2652.1. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen LL, Moneta GL, Conte MS, Bandyk DF, Clowes AW, Seely BL. Prospective multicenter study of quality of life before and after lower extremity vein bypass in 1404 patients with critical limb ischemia. J Vasc Surg. 2006;44:977–83. doi: 10.1016/j.jvs.2006.07.015. discussion 83–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodney PP, Likosky DS, Cronenwett JL. Predicting ambulation status one year after lower extremity bypass. J Vasc Surg. 2009;49:1431–9. e1. doi: 10.1016/j.jvs.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Dietzek AM, Gupta SK, Kram HB, Wengerter KR, Veith FJ. Limb loss with patent infra-inguinal bypasses. Eur J Vasc Surg. 1990;4:413–7. doi: 10.1016/s0950-821x(05)80877-1. [DOI] [PubMed] [Google Scholar]
- 15.Carsten CG, 3rd, Taylor SM, Langan EM, 3rd, Crane MM. Factors associated with limb loss despite a patent infrainguinal bypass graft. Am Surg. 1998;64:33–7. [PubMed] [Google Scholar]
- 16.Johnson BL, Glickman MH, Bandyk DF, Esses GE. Failure of foot salvage in patients with end-stage renal disease after surgical revascularization. J Vasc Surg. 1995;22:280–5. doi: 10.1016/s0741-5214(95)70142-7. [DOI] [PubMed] [Google Scholar]
- 17.Albers M, Romiti M, Braganca Pereira CA, Fonseca RL, da Silva M., Junior A meta-analysis of infrainguinal arterial reconstruction in patients with end-stage renal disease. Eur J Vasc Endovasc Surg. 2001;22:294–300. doi: 10.1053/ejvs.2001.1469. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez LA, Goldsmith J, Rivers SP, Panetta TF, Wengerter KR, Veith FJ. Limb salvage surgery in end stage renal disease: is it worthwhile? J Cardiovasc Surg (Torino) 1992;33:344–8. [PubMed] [Google Scholar]
- 19.Heredero AF, Acin F, March JR, Utrilla F. Impact of endovascular surgery on management of critical lower-limb ischemia in a vascular surgery department. Vasc Endovasc Surg. 2005;39:429–35. doi: 10.1177/153857440503900508. [DOI] [PubMed] [Google Scholar]
- 20.Adam DJ, Raptis S, Fitridge RA. Trends in the presentation and surgical management of the acute diabetic foot. Eur J Vasc Endovasc Surg. 2006;31:151–6. doi: 10.1016/j.ejvs.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 21.Dosluoglu HH, O’Brien-Irr MS, Lukan J, Harris LM, Dryjski ML, Cherr GS. Does preferential use of endovascular interventions by vascular surgeons improve limb salvage, control of symptoms, and survival of patients with critical limb ischemia? Am J Surg. 2006;192:572–6. doi: 10.1016/j.amjsurg.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Khan MU, Lall P, Harris LM, Dryjski ML, Dosluoglu HH. Predictors of limb loss despite a patent endovascular-treated arterial segment. J Vasc Surg. 2009;49:1440–5. doi: 10.1016/j.jvs.2009.02.226. [DOI] [PubMed] [Google Scholar]