ABSTRACT
Treatment of melanoma has significantly advanced over the last decade, with the development of targeted therapies against the MAPK pathway and immunotherapies to reactivate antitumor immunity. Unfortunately, currently more than 50% of patients are in treatment failure. Thus, identification of new common cellular vulnerability among melanoma cells is an urgent need and will help in the discovery of more efficient treatments against melanoma. We have focused our study on protein processing and have identified a new compound, HA15, targeting HSPA5/BiP, the master regulator of the unfolded protein response (UPR). By inhibiting HSPA5 specifically, our molecule increases the UPR and leads to the death of cancer cells by concomitant induction of autophagy and apoptosis, an effect seen both in vitro and in vivo. Our study provides compelling evidence to support the idea that endoplasmic reticulum (ER) stress inducers could be useful as a new therapeutic approach in melanoma treatment.
KEYWORDS: apoptosis, autophagy, BiP, ER stress, melanoma, resistance
Melanoma is the most aggressive form of skin cancer. Recently, significant progress has emerged with the development of new strategies in melanoma treatment. We currently have specific BRAF and MAP3K/MEK inhibitors. However, after a short period of remission, melanomas acquire drug resistance and recurrence of metastases is observed in almost all cases. Second, immunotherapies targeted against CTLA4 and PDCD1/PD1, developed to reactivate the antitumor immune response of the patient, result in an objective and long-lasting response in only approximately 40% of patients. Nevertheless, more than 50% of patients are currently in treatment failure. Clearly, identification of a common cellular vulnerability among melanoma cells is an urgent need and will help us to discover more effective treatments for melanoma.
Neoplastic growth requires synthesis of a significant quantity of new proteins. In this condition, the UPR is hyperactivated to deal with the high flux of proteins processed through the ER and to maintain ER homeostasis. One of the key proteins involved in UPR is HSPA5/GRP78/BiP. Indeed, in addition to fulfilling the function of a protein chaperone, HSPA5 is also considered to be a master regulator of the UPR. Therefore, it is not surprising that HSPA5 positively correlates with an increase of tumor progression, tumor size and poor outcome for patients with melanoma. Hence the UPR, and in particular HSPA5, appear to be interesting alternative targets in melanoma treatment.
To identify new molecules efficient against melanoma, we performed extensive structure-activity relationship screening and identified a new compound family, thiazole benzenesulfonamide, whose HA15 molecule appears as the lead compound. HA15 can significantly decrease melanoma cell viability regardless of the mutation background of the cells. To determine which mechanisms are responsible for the effects of these new molecules, we performed stringent electron microscopy and transcriptomic analyses. Our results highlight that HA15-treated cells present typical dilated ER cisternae, a classical feature of ER stress. In parallel, gene set enrichment analysis (GSEA) revealed a significant increase of genes involved in the UPR leading to ER stress. To identify the molecular target of this molecule responsible for the induction of ER stress and melanoma cell death, we initially exploited the fluorescent properties of HA15 and demonstrated perfect colocalization between HA15 and the ER, indicating that the target indeed is in this organelle. In our next experiments we carried out affinity isolation experiments with HA15 tagged with biotin followed by mass spectrometry analysis, confocal imaging and differential scanning calorimetry to highlight HSPA5 as the specific target responsible for the effects of HA15 on melanoma cells.
Interestingly, HA15 seems to induce moderate ER stress in normal cells without induction of cell death. This differential effect can be explained by a greater sensitivity of cancer cells to ER homeostasis perturbations as a result of elevated ER stress in cancer cells compared to normal cells (Fig. 1).
Figure 1.
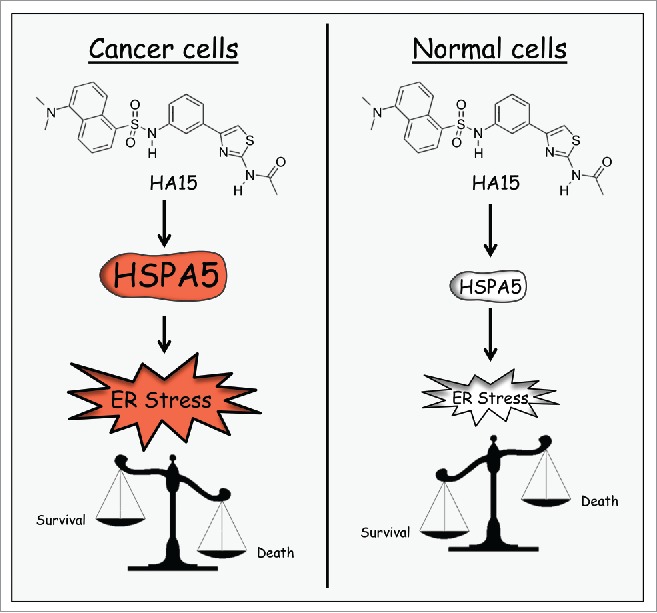
Differential effect of HA15 on cancer cells and normal cells.
Melanoma patients treated with BRAF V600 inhibitors rapidly develop drug resistance; therefore, the development of new treatment strategies is essential. Considering that HSPA5 plays a role in chemoresistance development both inside and outside the ER, we tested the effects of HA15 on different melanoma cell lines resistant to BRAF inhibitors, and melanoma cells freshly isolated from patients who became resistant to BRAF inhibitors. We noted in all cells tested, in vitro and in vivo, a strong induction of resistant cell death by concomitant autophagy and apoptosis after HA15 treatment that was emphasized with the utilization of ER stress inducers in melanoma treatment. Recent reports indicate that BRAF inhibitor-resistant tumors present with increased ER stress and autophagy and that ER stress-induced autophagy could be a potential prosurvival mechanism that contributes to the resistance. Interestingly, our compounds may provide an effective alternative to convert, by the same mechanisms, these protective effects into signals of death to overcome BRAF inhibitor resistance.
Finally, we have tested if targeting HSPA5 to induce ER stress could be a useful strategy in the treatment of other forms of cancer types. To examine this possibility, we used a panel of solid and liquid tumors. Interestingly, we have observed that HA15 treatment induced ER stress, autophagy and apoptosis in all the tested cell lines including ImaR, an acute myeloid leukemia cell line, and MiaPaca, a pancreas cancer cell line, which are resistant to the classical therapies using Imatinib and Gemcitabin, respectively.
To conclude, using structure-activity relationship studies, we have discovered and developed a series of new molecules (thiazole benzenesulfonamides) exhibiting strong lethal effects in melanoma cells. The lead compound HA15 displays antimelanoma effects by targeting the ER stress axis to induce specific cancer cell death by concomitant induction of autophagy and apoptosis. These results highlight the key role of this specific pathway in melanoma malignancy and cancer, strengthening the idea that ER stress inducers could be useful in the future treatment of a various spectrum of cancers.


