Abstract
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia, affecting 1% to 2% of the general population. It is characterized by rapid and disorganized atrial activation leading to impaired atrial function, which can be diagnosed on an EKG by lack of a P-wave and irregular QRS complexes. AF is associated with increased morbidity and mortality and is a risk factor for embolic stroke and worsening heart failure. Current research on AF support and explore the hypothesis that initiation and maintenance of AF require pathophysiological remodeling of the atria, either specifically as in lone AF or secondary to other heart disease as in heart failure-associated AF. Remodeling in AF can be grouped into three categories that include: (i) electrical remodeling, which includes modulation of L-type Ca2+ current, various K+ currents and gap junction function; (ii) structural remodeling, which includes changes in tissues properties, size, and ultrastructure; and (iii) autonomic remodeling, including altered sympathovagal activity and hyperinnervation. Electrical, structural, and autonomic remodeling all contribute to creating an AF-prone substrate which is able to produce AF-associated electrical phenomena including a rapidly firing focus, complex multiple reentrant circuit or rotors. Although various remodeling events occur in AF, current AF therapies focus on ventricular rate and rhythm control strategies using pharmacotherapy and surgical interventions. Recent progress in the field has started to focus on the underlying substrate that drives and maintains AF (termed upstream therapies); however, much work is needed in this area. Here, we review current knowledge of AF mechanisms, therapies, and new areas of investigation.
Introduction
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia, affecting 1% to 2% of the general population (8, 64, 78, 90, 110, 143, 159, 187, 201, 253). It is characterized by rapid and disorganized atrial activation leading to impaired atrial function, which can be diagnosed on an EKG by lack of a P-wave and irregular QRS complexes. AF is associated with increased morbidity and mortality and is a risk factor for embolic stroke and worsening heart failure (26). AF can be defined as paroxysmal (converts to normal sinus rhythm within 7 days), persistent (converts to normal sinus rhythm after 7 days), or permanent (does not spontaneously convert to normal sinus rhythm) (25). As in the case of paroxysmal AF, the intermittent nature of the arrhythmia suggests there may be a higher prevalence than is clinically observed. Numerous risk factors are associated with development of AF, though age and sex are the strongest with 2 times risk per decade and 1.5 times risk for males (8). The lifetime risk for individuals of 40 to 55 years of age is estimated between 22% and 26% (78,90).
Large-scale epidemiological studies have highlighted differences in AF presentation between men and women. Women tend to be older with a higher proportion in the 75 years or older age group and are more symptomatic at first AF presentation than men (76, 100, 145). Thus, presentation of AF in women is associated with a higher risk of stroke (42, 145). There is also evidence for sex-related differences in response to treatment. Rhythm control treatment in women is found to lead to increased morbidity and mortality compared to rate control treatments, which is not observed when treating men (200). In spite of the evidence pointing to significant sex-related differences in AF, the factors underlying these differences are still unknown and require further investigation.
The high prevalence of AF may be attributable to the various mechanisms contributing to development of the arrhythmia. Current research on AF support and explore the hypothesis that initiation and maintenance of AF requires pathophysiological remodeling of the atria, either specifically as in lone AF or secondary to other heart disease as in heart failure-associated AF. Remodeling associated changes in AF can be grouped into three categories that include: (i) electrical remodeling, which includes modulation of L-type Ca2+ current, various K+ currents, and gap junction function; (ii) structural remodeling, which includes changes in tissues properties, size, and ultrastructure; and (iii) autonomic remodeling, including altered sympathovagal activity and hyperinnervation. Electrical, structural, and autonomic remodeling all contribute to creating an AF-prone substrate which is able to produce AF-associated electrical phenomena including a rapidly firing focus, complex multiple reentrant circuit, or rotors (162). The purpose of this review is to summarize current knowledge of the mechanisms contributing to the development and maintenance AF with an emphasis on recent progress, particularly in therapy and diagnosis as well as future directions.
Historical Perspectives on Atrial Fibrillation
Numerous milestones have been achieved in understanding AF etiology and mechanisms. A number of these achievements are of particular relevance to the AF mechanisms presented in this review. A more detailed history of AF research milestones that encompass topics not in this review has been reviewed in detail elsewhere (142, 168). Since the early 20th century, AF has been recognized as the most common cardiac arrhythmia in the general population (168). The electrical conduction abnormalities associated with AF were first described by Garrey in 1924, which include the same electrical patterns currently examined today (72). In the following years, the mechanisms underlying these phenomena in AF have been more directly established. AF electrical modeling methods were vastly improved by work by Moe and colleagues in 1964 who developed the first computer based mathematical model of AF using the multiple-wavelet concept of AF which acted as a fundamental tool for analyzing electrical defects in AF (157). Starting in 1978, Coumel and colleagues established the importance of the autonomic nervous system in AF (41). Renma and colleagues established the role of shortened conduction wavelength in 1988 which is calculated as the refractory period multiplied by the conduction velocity, for reentry in occurrence of AF (199), which serves as the basis for more recent models of reentry including the “leading circle model” and “spiral wave” concept (40). In 1995, Wijffels and colleagues made the observation that AF begets AF, which uncovered the importance of worsening atrial remodeling in AF (252). In the following years, knowledge of the role of atrial remodeling in AF has quickly expanded and is reviewed in subsequent sections.
Various experimental (Table 1) and transgenic (Table 2) animal models have been generated to investigate AF, which have been summarized in this review. Early models have depended on the use of large animal models to induce AF via electrical or surgical intervention, while more recent studies have included use of numerous transgenic mouse models to investigate specific pathways identified in AF. Although transgenic mouse models have paved the way to determining the specific contributions of relevant pathways in AF, limitations imposed by the lack of atrial specific promoters to drive gene ablation and overexpression in the heart have tempered the conclusions within these models.
Table 1.
Experimental Animal Models of Atrial Fibrillation
| Experimental model | Mechanisms of AF explored |
Species | Reference(s) | |
|---|---|---|---|---|
| Stimulation, disease, and injury-based strategies | ||||
| Rapid atrial pacing | Burst pacing or chronic pacing |
Fibrillation-induced remodeling |
Dog, goat, pig, sheep | (9, 73, 192, 252) |
| Cardiovascular disease-induced AF |
Heart failure (rapid ventricular pacing) |
Heart failure-induced remodeling |
Dog, sheep, rabbit | (134, 190, 221) |
| Sterile pericarditis | Postoperative AF | Dog | (179) | |
| Mitral regurgitation | MR-associated AF | Dog | (44) | |
| Volume overload | Chronic atrial stretch | Dog, goat, sheep, rabbit | (53,93,198,223) | |
| Hypertension | Hypertension | Sheep, rat | (37, 122) | |
| Acute atrial insult | Aconitine | Electrical remodeling | Dog, sheep | (216) |
| Direct left atrial dilatation | Acute atrial stretch | Dog rabbit | (195, 227) | |
| MI | MI-induced remodeling | Dog | (224) | |
| Autonomic modulation | Autonomic stimulation | Autonomic remodeling | Dog, sheep | (75, 81, 234) |
Table 2.
Transgenic Mouse Models Targeting Disease Mechanisms Associated with Atrial Fibrillation
| Transgenic mouse model | Mechanism | AF features observed | Reference(s) |
|---|---|---|---|
| Constitutive TGF-beta1 activation |
Elevated profibrotic signaling |
Atrial fibrosis, AF inducibility | (244) |
| ACE overexpression | Atrial enlargement, atrial fibrosis, AF |
(259) | |
| JDP2 overexpression | Atrial dilatation | (115) | |
| Rho-A overexpression | Cardiac disease associated AF |
DCM, atrial dilation, bradycardia, AV block, HF, AF |
(205) |
| Junctin overexpression | DCM, atrial dilation, bradycardia, enlarged left ventricle, AF |
(98) | |
| MURC overexpression | Atrial dilation, AV block, AF | (171) | |
| TNF-a overexpression | DCM, atrial fibrosis, HF, AF | (204, 208) | |
| CREM overexpression | HCM, atrial enlargement, atrial and ventricular hypertrophy; AF |
(158) | |
| Juncate-1 overexpression | HCM, atrial enlargement, ventricular hypertrophy, bradycardia, AF |
(99) | |
| Rac1 overexpression | HCM, AF | (2) | |
| HopX overexpression | HCM, atrial fibrosis, AF inducibility | (146) | |
| Constitutively active Gaq | HCM, left atrial dilatation, and fibrosis; AF under anaesthesia |
(94) | |
| Connexin 40 knockout | Electrical remodeling | AF inducibility | (86) |
| Kir2.1 overexpression | Bradycardia, PVCs, AV block, AF | (136) | |
| Cav1.3 knockout | Bradycardia, AV block, atrial flutter, AF |
(154, 273) | |
| KCNE1 knockout | AF | (236) | |
| NUP155 heterozygous knockout |
AF | (271) | |
| KCNE1-KCNQ1 fusion protein overexpression |
AF inducibility | (206) | |
| FKBP12.6 knockout | AF inducibility | (228) | |
| R176Q mutation of RYR2; RYR2-S2814A knock-in |
Increased AF inducibility with R176Q mutation; decreased AF inducibility with S2814A mutation |
(35) |
Genetics Play a Role in Atrial Fibrillation
Numerous risk factors contribute to AF, including genetics. Lone AF was first documented within a family in 1936 by Orgain and colleagues who described three brothers presenting with AF (178), and in 1943 AF was first documented in a heritable autosomal dominant pattern by Wolff (255). It was not until 1997 until direct evidence of familial AF was established using linkage analysis identifying a locus between D10S1694 and D10S1786 at 10q22-q24 in families with early onset AF, although the causative gene at this locus is still unknown (20). In 2003, Chen and colleagues identified the first mutation in a gene associated with familial AF, as a gain of function mutation in KCNQ1 which codes for the α-subunit of the slowly repolarizing potassium current, IKS (36). Since these initial discoveries, multiple genes associated with development of AF have since been identified.
Numerous potassium channel mutations have now been associated with AF. These include KCNQ1 (14,15,48,88,116,148), KCNA5 (39,176,261,262), KCND3 (174), and KCNJ2 (52,258). In addition to mutations in the channels themselves, mutations in channel accessory proteins are also associated with AF, including KCNE1 (173), KCNE2 (263), KCNE3 (147), and KCNE5 (196). The majority of mutations in these proteins are thought to increase channel activity, which would decrease action potential duration and refractoriness in the atria (250). Mutations that reduce channel activity can prolong atrial action potentials and lead to early afterdepolarizations and AF (131).
Mechanisms Underlying Atrial Fibrillation
AF occurrence is dependent upon complex electrical defects in the atria which include a rapidly firing focus, complex multiple reentrant circuit, or rotors (162). Alterations in after-depolarization, both early and late, can contribute to ectopic atrial foci (185, 247). Reentrant waves can occur due to reduced refractoriness, slow conduction, and conduction barriers (162,212,247,250). Rotors, or localized electrical spiral waves, are a result of complex substrate changes leading to a stable disease wave. These electrical defects are dependent upon remodeling mechanisms, which can be grouped into electrical, structural, and autonomic remodeling that allow for initiation and maintenance of AF (Fig. 1). In the following sections, we describe the prevailing mechanisms leading to AF in relation to the aforementioned electrical defects.
Figure 1.
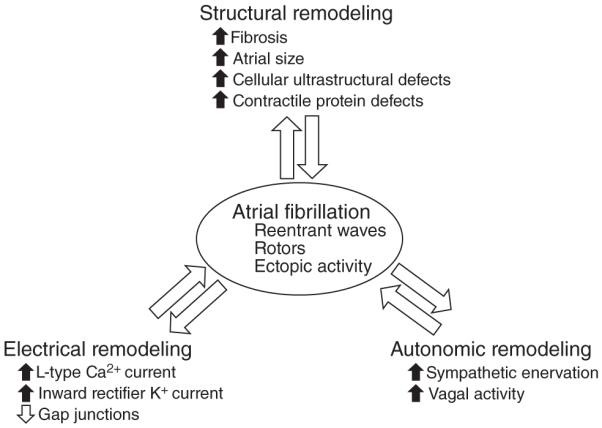
Diagram representing the major types of remodeling (electrical, structural, and autonomic) that lead to AF.
Electrical remodeling
One of the most characterized mechanisms driving AF is the electrical remodeling occurring in atrial cardiomyocytes. Various types of ionic currents have been found to change in AF and, through animal models, to contribute to its development. The ionic currents include the L-type Ca2+ current (35, 56, 163, 164, 214, 245, 250) and inward rectifier K+ currents (55, 111, 132, 183, 246, 248). Gap junction function, specifically connexins 40 and 43, has also been linked to lone AF, though this may have broader effects on conduction (82,102).
Alterations in Ca2+ handling in the atria can contribute to both development and worsening of AF. Numerous studies have shown the connection between altered calcium handling and delayed afterdepolarizations, which contribute to formation of ectopic foci and AF initiation. In cardiomyocytes, intracellular calcium is stored in the sarcoplasmic reticulum (SR) until its release is triggered by specific stimuli. In AF, unwarranted calcium release can be triggered by ryanodine receptor (RyR) hypersensitivity or SR Ca2+ overload. Both RyR hyperphosphorylation and mutations have been shown to increase Ca2+ sensitivity. Data from mouse models also support the role of excessive RyR activation in development of AF (35, 214). This is also reflected in patients harboring activating mutations in RyR that exhibit catecholaminergic polymorphic ventricular tachycardia and AF (114, 232, 269). Mice specifically lacking the RyR stabilizing subunit FKBP12.6 exhibit SR Ca2+ leaks and increased susceptibility to AF (228). AF itself can also promote calcium handling defects, as has been observed in chronic AF patients who display activation of the Ca2+ calmodulin-dependent protein kinase type II (CaMKII) leading to phosphorylation of RyRs (56,164,245,250). Studies in isolated canine atrial cardiomyocytes have revealed detrimental effects of tachypacing on calcium handling (191). As atrial depolarization rates increase, intracellular Ca2+ begins to accumulate, leading to activation of calcineurin/NFAT signaling, which in turn leads to reduced transcription of Cav1.2 L-type calcium channel (CACNA1C), ultimately leading to reduced L-type Ca2+ current (191). Animal models have shown this leads to a reduced action potential duration and atrial effective refractory period, which favors reentrant waves (210, 267). Similar results have also been observed in isolated human atrial myocytes (38, 242), supporting the role for reduced L-type Ca2+ current in human AF.
Increased K+ currents are intimately associated with electrical remodeling in AF. The inward rectifier K+ currents (IK1, and IK,AcH, basal, and acetylcholine dependent, respectively) are increased in AF which alters resting potential and phase 3 activation, leading to reduced atrial refractoriness and wavelength (55, 77, 111, 132, 246, 248). This mechanism has also been supported by in vitro data showing increased magnitude of inward rectifier K+ currents stabilizing reentrant currents (183). The elevated K+ currents observed in AF are likely due to upregulation of the Kir2.1 channel, a major channel protein for IK1 current, which has specifically been shown to be affected in AF (33, 59, 70, 246). It has been hypothesized that regulation of Kir2.1 is controlled by miRNA targeting Kir2.1, specifically miR-1 and miR-26, which are reduced in AF (77, 149). Thus in AF, loss of miR-1 and miR-26 would lead to increased K+ channels and current, leading to reduced atrial refractoriness and wavelength and ultimately allowing for reentrant waves.
Gap junction function is also affected in AF (102). Gap junction function is directly related to conduction velocity, which is a known determinant of AF. Specifically, slower conduction velocity favors reentry, allowing for initiation and maintenance of AF. From clinical studies, GJA5, which codes for connexin 40, has been linked to idiopathic AF (82). Heterogeneous connexin 40 distribution has also been observed in large animal models of AF, specifically in goats undergoing endocardial burst pacing, suggesting that connexin 40 remodeling is involved in maintenance of AF (241). In dogs undergoing atrial tachypacing, connexin 40 has been shown to decrease in the pulmonary vein (270), a region shown to be an important site for reentrant waves. Furthermore, mutations in the GJA5 promoter sequence have been associated with AF vulnerability through human clinical studies (67). Somatic mutations in GJA1, which codes for connexin 43, have been observed specifically in the atria, a phenomenon referred to as genetic mosaicism (237). The mutant connexin 43 contributes to heterogeneous electrical conduction, which favors reentrant waves and ultimately leads to AF. The gap junction inhibitory peptide, rotigaptide, has been used in dog models of AF to varying degrees of success. Rotigaptide was shown to have beneficial effects on AF in the setting of acute ischemia but not when caused by ventricular or atrial tachypacing (222), suggesting that gap junction inhibition may only be necessary for specific stages or etiologies of AF, as reflected in its varying roles in different experimental models of AF.
Electrical changes in the heart, as would be expected based on the principles of excitation-contraction coupling, lead to secondary changes in contractile function in the atria (85, 211). This is broadly demonstrated by the association of chronic AF with atrial contractile dysfunction (18), which has been observed as a reduction in maximum tension as well as in the rates of tension activation and relaxation. These effects have been linked to increased myofilament sensitivity to Ca2+, possibly due to changes in myofilament phosphorylation (251). These effects are also accompanied by a reduction in myofibril passive tension, potentially caused by upregulation of slow beta-myosin heavy chain isoform and the more compliant titin isoform N2BA (251). However, these myofibril alterations may be related to specific mechanisms of AF development, as a dog model of AF induced via atrial tachycardia developed hypocontractile atria (251) while other models of AF have reported no changes in myofibril properties (101). Recent studies have also identified a role for inositol-1,4,5-trisphosphate-receptor (IP3R)-mediated Ca2+ release in AF-related contractile defects which may represent a mechanism independent of myofibril alterations (138, 140).
Structural remodeling
Structural remodeling is perhaps the most obvious change in the atria that occurs in AF. These effects are characterized by changes is tissue properties (most notably fibrosis), atrial size, and cellular ultrastructure. These types of changes predispose the atria to defects in conduction predominantly contributing to reentry and rotor formation.
Various factors contribute to the fibrosis underlying AF, including cell stretch, neurohumoral activity, oxidative stress, and even AF itself can contribute to worsening tissue properties (32, 119, 264). Atrial fibrosis is a salient feature of a majority of animal models of AF, including aging (57), myocardial infarction (MI) (169), volume overload (53), endurance exercise training (84), and tachypacing-induced HF (21,29,134,231). Conversely, numerous animal models of atrial fibrosis exhibit increased susceptibility to AF (160,259). Specific profibrotic signaling molecules are associated with atrial fibrosis and AF including Angiotensin II, aldosterone, and TGF-β1 (60,83,197).
Angiotensin II functions in the renin-angiotensin-aldosterone system (RAAS) and increases in activity have previously been associated with increased cardiac fibrosis (118). Goette and colleagues showed increased levels of angiotensin-converting enzyme (ACE) in AF and corresponding increased levels of activated extracellular signal-regulated kinase 1 and 2 (ERK1/2), consistent with increased RAAS activity (80). Conversely, treatment with candesartan, an angiotensin receptor blocker, reduces the profibrotic effects of rapid atrial pacing induced AF and reduces propensity for AF (125). Similar results were also observed with the ACE inhibitor enalapril. In dogs with ventricular tachypacing-induced congestive heart failure (CHF), atria exhibit conduction slowing, fibrosis, and propensity for atrial burst-pacing induced AF, which occur along with increased atrial concentration of angiotensin II. With enalapril treatment, all of these features are attenuated, supporting the importance of RAAS signaling in developing AF features (29,135,220).
Aldosterone, another important mediator of RAAS signaling that binds to the mineralocorticoid receptor (MR), has also been linked to atrial fibrosis and AF (19, 233, 240). Blockade of aldosterone signaling at the MR via spironolactone improved morbidity and mortality in AF patients (188), suggesting an important role for aldosterone in AF. Another MR blocking drug, eplerenone, has also been successfully used to prevent recurrence of AF after catheter ablation (103). The effects of MR blockade on atrial fibrosis have not been directly examined in patients; however, data from animal and cell-based models of AF have demonstrated reductions in cardiac fibrosis following treatment with MR blockers (120,126,127,274).
TGF-β1 is another profibrotic molecule upregulated in AF, as demonstrated in animal models of AF (30,129) as well as in clinical studies on patients with AF (139,193). TGF-β1 is an established positive regulator of cardiac fibrosis and its specific overexpression in the heart leads to atrial fibrosis and increased susceptibility to AF (54, 58), suggesting that TGF-β1 is sufficient for developing an AF-prone substrate (193). However, the determinants of increased TGF-β1 expression in the heart during AF are still unknown.
Evidence from genetic models of cardiac fibrosis suggest that the atria are particularly sensitive to profibrotic signaling potentially due to increased response of atrial fibroblasts compared to ventricular fibroblasts (22). This may be related to the cases of atrial fibrosis without ventricular fibrosis in patients with lone AF (68). This has been further explored in transgenic mouse studies overexpressing either ACE or a constitutively active TGF-β1 mutant protein in the heart (160, 259). Both of these models lead to fibrosis only in the atria.
MicroRNAs have also been linked to control of atrial fibrosis leading to AF. miR-21 knockdown suppressed the development of an AF substrate in a rat model of post-MI HF (28). This is hypothesized to occur via miR-21’s role in regulating Sprouty-1 levels, which negatively regulate ERK 1/2 activity, which then inhibits fibroblast density (28,238).
The mechanism by which fibrotic tissue serves as a substrate for AF has been examined in detail. Cardiomyocytes in fibrotic atria are more distantly separated than those in nondiseased atria, with the fibroblasts and ECM essentially forming a physical conduction barrier (21). This reduces electrical coupling between cardiomyocytes and provides susceptibility to reentry (21, 134, 230). There is also an increase in fibroblast proliferation in AF and as with other disease states, their proliferation in AF is linked to increases in myofibroblast phenotype (268). Interactions specifically between myofibroblasts and cardiomyocytes have previously been shown in cocultures to negatively affect conduction organization leading to increased propensity to ectopic activity and reentrant arrhythmias (155,277). Fibroblast-myocyte interactions increase, which in turn alter conduction via fibroblast’s function as electric sinks and paracrine activity (268), leading to conduction slowing, depolarization of cardiomyocyte resting potential, variable effects on action potential duration, and the induction of spontaneous phase-4 depolarization all of which predispose to reentry and ectopy (104,150).
From early on in the history of AF research, increased atrial size has been known to favor AF (71, 92). Reentrant circuits form more readily with larger atrial size, potentially due to the additional area available for rotor formation as demonstrated in computer modeling studies (278). Animal models (50, 123, 165), as well as clinical data support this idea (51,95,153). Atrial size may also indirectly affect tissue properties, since it can be a sign of increased atrial stretch, which is generally associated with increased tissue remodeling in the atria (108).
Structural remodeling changes in AF also occur at the ultrastructural level. Numerous defects in cardiomyocyte ultrastructure have been observed in AF including myolysis, glycogen accumulation, as well as changes in nuclear chromatin, mitochondrial disruption and redistribution as well as SR alterations (12,13). Gap junction localization heterogeneity, specifically of connexin 40, is also observed in AF models (241). Interestingly, many of these changes can partially revert back to normal after restoration of sinus rhythm. In a goat model of burst pacing-induced AF, typical ultrastructural defects appear after 4 months of AF, but after restoration of normal sinus rhythm for 2 months myolysis, glycogen accumulation, and mitochondrial defects are improved and nuclear chromatin defects are completely normalized (12).
Autonomic remodeling
The autonomic nervous system exerts significant control of cardiac electrophysiology, and defects in autonomic function have been associated with AF (217). The heart is extensively innervated by the autonomic nervous system by both extrinsic (ganglia outside the heart) and intrinsic (ganglia inside the heart) nervous tissue. The extrinsic nerves include the vagal nerve and nerves arising from the paravertebral ganglion, which includes the thoracic ganglion, cervicothoracic ganglion, middle cervical ganglion, and the superior cervical ganglion (10,106,113).
There is extensive evidence of autonomic dysfunction reflected as increased sympathetic activity in AF observed in various types of large animal AF models. In a dog model of pacing-induced AF, heterogeneous increased sympathetic enervation has been observed in the atria (34,107). In ventricular MI-associated AF, atrial nerve sprouting and sympathetic hyperinnervation have been observed (6,11,166,167,243). In a pacing-induced CHF model of paroxysmal AF, increased autonomic nerve activity was observed (172). Sympathetic innervation of the atria appears to be particularly sensitive to pacing, as observed in an intermittent left atrial tachypacing model which causes sympathetic hyperinnervation, paroxysmal AF, and paroxysmal atrial tachycardia (235). The role of sympathetic innervation is further supported by the observation that simultaneous sympathovagal discharge commonly precedes AF (215, 235). Autonomic changes have also been observed in smaller model systems such as in rats where endurance exercise increased AF susceptibility in the context of autonomic changes, atrial dilation, and fibrosis (84). This effect is also paralleled in humans by the increased prevalence of AF in endurance athletes (229). Though there is much evidence of autonomic remodeling occurring, there is less data directly testing the role of autonomic remodeling on development and progression of AF, though there is suggestion that the increased sympathetic activity leads to heterogeneous changes in atrial refractoriness which in turn favor reentrant waves (117,175).
Ablation of various autonomic innervation sites has revealed the necessity for their function in the maintenance of AF. Cryoablation of atrial sympathetic nerves has been used in a dog model of pacing-induced heart failure as well as in patients with long QT syndrome to moderate success (213, 235). Vagal nerve stimulation has been effective in suppressing induction of AF in an induced model of AF (137, 218, 219, 266). Innervation by nerves beside the vagal nerve has also been explored to similar results. Ablation of the ganglionated plexus can also improve long-term AF symptoms (112,189,209,272,275). This has also been shown with specific denervation of the pulmonary vein (184). Renal sympathetic denervation has also been shown to improve AF features, however this may also affect nonautonomic mechanisms such as RAAS signaling (4, 96, 141). Somatic sensory modulation via low level stimulation to the tragus nerve of the ear has also been shown to improve early stages of AF; however, the mechanism by which this occurs is currently unknown (265). Based on these studies, autonomic innervation appears to function as an exacerbating factor in AF as ablation improves AF severity and delays onset; however, it is unable to prevent or reverse AF, suggesting that the targeted forms of autonomic remodeling are not required for AF.
Therapeutic Approaches for Atrial Fibrillation
Current management for AF includes the use of rate and rhythm control strategies as well as surgical interventions with the goal of controlling symptoms (256). Therapeutic strategies targeted to pathophysiological processes underlying structural changes associated with AF (referred to as “upstream therapies”) are also being exploited as they have potential to prevent the occurrence or recurrence of AF by slowing (and in some cases, preventing) the progression of atrial and left ventricular remodeling (207). Anticoagulants are also prevalently used as blood stasis can develop in the atria as a result of AF, which increases the risk of thromboembolic events such as stroke (7,49). Decisions on the strategies to use are primarily dependent on the age, degree of symptoms, and presence of underlying heart disease exhibited by the patient (256).
Rate control
Rate control strategies are thought to be useful in older AF patients (>65 years of age) in chronic settings that have limited symptoms as they control cardiac ventricular rate by targeting the atrioventricular node (AVN), a key conduction system structure of the heart that transmits electrical signals from the atria to the ventricles (91). These include β-adrenergic receptor blockers, nondihydropyridine calcium channel blockers and digitalis glycosides, which prolong AVN refractoriness (slow conduction velocity) by ultimately decreasing sympathetic tone or circumventing Ca2+ overload to slow ventricular rate at rest and during exercise without converting the heart to a regular rhythm (7,79,180,226,239). Digitalis is not as effective as β-adrenergic receptor blockers and calcium channel blockers as a monotherapy due to its slower onset and weaker potency (63). However, combinatorial uses with β-adrenergic receptor or calcium channel blockers have proven advantageous for rate control (63). For patients exhibiting AF in the setting of CHF, rhythm control drugs that exhibit β-adrenergic receptor blocking properties (e.g., amiodarone and dronedarone) are beneficial in slowing ventricular rate, especially when patients are intolerant to conventional rate control drugs (181). Thus, controlling ventricular rate in AF not only decreases the risk of tachycardia-related symptoms (palpitations) and cardiomyopathy associated with a rapid heart rate but can also alleviate heart failure symptoms by lengthening diastole (61). However, there remain some risks as rate control drugs can slow the heart rate too much, which can then result in complications such as sinus bradycardia and heart block (27). Patients exhibiting and prone to these symptoms, which include elderly patients, may require interventions such as permanent pacemaker implantation and AVN ablation in these cases to regain control of ventricular rate (27). Based on the principles behind rate control strategies, these drugs are also contraindicated (digitalis and calcium channel blockers) or to be used with caution (β-adrenergic receptor blockers) in AF patients exhibiting preexisting cardiac conduction abnormalities and syndromes (e.g., Wolff-Parkinson-White syndrome where an additional abnormal electrical conduction pathway distinct from AVN can cause preexcitation of the ventricle) as they can exacerbate conduction abnormalities and deleterious AF symptoms (7).
Rhythm control
Noninvasive rhythm control strategies seek to convert the heart to sinus rhythm (“cardioversion”) by using antiarrhythmic drugs (“pharmacological cardioversion”) and direct electrical currents (“electrical cardioversion”) (7) (Table 3). They are thought to be useful for patients intolerant to rate control or patients with persistent symptoms in the face of adequate rate control (276). Clinical assessments also favor younger aged patients (<65 years of age), patients with recent onset as well as patients with limited underlying heart disease since AF exacerbates atrial as well as ventricular remodeling (256, 276). The most effective rate control drugs include Class Ic and IIIc antiarrhythmic drugs (Singh and Vaughan-Williams classification) (256). Class Ic drugs include flecainide and propafenone, which are recommended for patients with paroxysmal AF; however, their use is contraindicated for AF patients with underlying structural heart disease due to increased risk of ventricular arrhythmias and atrial flutter (7,144,256). Class IIIc drugs include ibutilide, dofetilide, amiodarone, and sotalol, which are recommended for patients with persistent AF but also found to benefit AF patients with structural heart disease; however, they harbor some proarrhythmia risk to Torsade des pointes and ventricular arrhythmias as well as greater toxicities (7,256,276). Overall, antiarrhythmic drugs work by blocking the sodium, potassium as well as calcium channels and/or adrenergic receptors (Table 3). In general, the mechanisms of action for class Ic largely differ from class IIIc based on the ion channels they target. Class Ic largely exert their effects by blocking sodium channels (“membrane-stabilizing agents”), to reduce the rate of rise of the action potential, thereby reducing excitation of the cardiac tissue (276). Class IIIc antiarrhythmic drugs largely exerted their effects by potassium channel blockade and prolonging action potential duration and refractoriness by lengthening the QT interval and thus, delaying conduction (276). Despite these categorizations, it is evident that many rhythm-control drugs impact AF by targeting ion channels and adrenergic receptors outside of their categorized class (276). For example, the most effective and commonly prescribed drug for AF is the class III drug, amiodarone, which is known to block multiple channels (276). However, caution should be taken when using amiodarone as it can potentiate the effects of anticoagulant drugs based on direct interactions with enzymes (e.g., CYP2C9) involved in drug metabolism and thus, requires surveillance for toxicity effects (66).
Table 3.
Mechanism of Action of Recommended Rhythm Control Strategies for Atrial Fibrillation
| Rhythm control strategies | Singh and Vaughan-Williams classification |
Mechanism of action | Reference(s) |
|---|---|---|---|
| Pharmacological cardioversion | |||
| Flecainide | Class Ic | Blocks fast inward sodium channel to reduce rate of rise of AP depolarization and contractility Selectively effects cells with high rates |
(194) |
| Propafenone | Class Ic | Blocks fast inward sodium current to reduce rate of rise of AP depolarization and contractility β-adrenergic receptor blocking properties |
(69) |
| Ibutilide | Class IIIc | Blocks the delayed rectifier outward potassium current and enhances the slow inward sodium current which both prolong AP duration and conduction |
(128, 260) |
| Dofetilide | Class IIIc | Selectively blocks the delayed rectifier outward potassium current to prolong AP |
(202) |
| Sotalol | Class IIIc | Blocks the delayed rectifier outward potassium current to prolong AP β-adrenergic receptor blocking properties |
(3) |
| Amiodarone | Class IIIc | Multi-channel blocker that effects inward sodium and calcium channels as well as repolarizing outward potassium channels, which ultimately causes prolongation of the repolarization phase of the AP and conduction α- and β-adrenergic receptor and blocking properties |
(124) |
| Electrical cardioversion | |||
| Direct current (monophasic or biphasic waves) |
Not applicable | Block reentrant electrical circuits by depolarizing atrial tissue using high voltage (monphasic waves) or low-voltage (biphasic) electrical currents to make atria refractory |
(133) |
| Ablation therapy | Not applicable | Block reentrant electrical circuits by making lesion/scar lines in and around the pulmonary veins |
(256) |
Direct current cardioversion is also routinely used as a method to restore sinus rhythm and is thought to be particularly useful in AF patients experiencing rapid tachycardia and hemodynamic instability or when there is a relapse in the occurrence of AF (7) (Table 3). The principles stem from disrupting the aberrant atrial electrical impulses and conduction that is associated with AF, which can result in fibrillatory conduction and multiple reentrant circuits (162). AF patients treated with anticoagulant therapies are subjected to electrical currents (monophasic or biphasic waveforms) via metal pads or patches that are synchronized with the R wave of the QRS complex to depolarize the atrial tissue (endocardium) that harbors the reentrant circuits (133). As a result, the circuits no longer propagate or sustain reentry because the atria essentially become refractory (133). Two randomized clinical trials have highlighted the benefits of biphasic (low-energy, current flows in both directions) versus monophasic (high-energy, current flows in one direction) waveform electrical currents in AF treatment (156,182). Predictors thought to sustain normal sinus rhythm following successful electrical cardioversion include patients (i) exhibiting early onset of AF, (ii) exhibiting minimal atrial remodeling (left atrial size of <4.5 cm in diameter and left atrial volume index of <30 mL/m2), (iii) lacking underlying heart disease (e.g., rheumatic disease, LV dysfunction), (iv) using antiarrhythmic drugs, and (iv) harboring minimal electrophysiological defects (P wave durations of <135 ms) (1).
Rhythm control can also be achieved by invasive ablation techniques, which are typically used in symptomatic AF patients that are intolerant to conventional rhythm control strategies or when rhythm control drugs are ineffective or toxic (256). The AF treatment with the highest rate of success is the open-heart surgery ablation technique termed Cox-Maze procedure, which interrupts multiple reentrant circuits and fibrillatory conduction by a series of complex biatrial surgical incisions (“cut-and-sew techniques”) that create barriers at critical locations resembling a maze to prevent sustained AF (43, 45, 46). However, the complexity of the technique, need for prolonged cardiopulmonary bypass as well as lengthy operation times and increased risk for bleeding have prevented it from being widely adopted and is thus, preferentially utilized in patients already undergoing cardiac surgery (69). Modifications of the technique have been implemented to simplify the procedure, which also included exploiting alternative energy sources such as radiofrequency, cryothermal, and microwave energy as a means to create lines of scar (69). Through these efforts, radiofrequency ablation has proven to be efficacious in AF patients (74). Modifications also included the need for less invasive procedures, which gave rise to catheter ablation techniques where no incisions are needed and where catheters are inserted via the groin or neck to target the area of ablation (69). Pioneering studies by Jais and colleagues localized the triggering source of AF to the pulmonary veins (PV) (87,105), which then lead to PV antral isolation (or wide-area circumferential isolation) as the most efficacious AF ablation approach (23, 256). Radiofrequency ablation of this region not only includes the PV but also surrounding regions (left atrial roof and posterior wall and right interatrial septum), which has been shown to lead to a higher success rate and lower complication rate for AF treatment (109). Current approaches do not target the ostia (opening to PV) as their ablation have been previously associated with complications related to pulmonary stenosis (69). Modifications of these catheter ablation procedures have been adapted to include isolated or other ectopic regions in and around the PV, which include procedures such as: (i) PV segmental ostium ablation that includes less of the antrum and incomplete ablation around the vein (ii) linear ablation is used to create ablation lines of scar along the atrial roof and mitral annulus but both are associated with an increased risk of atrial flutter, (iii) complex fractionated electrogram ablation targets specific sites with an unusual electrical pattern that represents macro reentry sites surrounding the PV (iv) ganglionic plexi ablation targets nerves that control autonomic function, and (v) rotor ablation targets electrically mapped and organized reentrant circuits surrounding the PV (256).
Data from several clinical trials have revealed that there are no mortality benefits to rhythm when compared to rate control drugs (31,97,203,257). However, these findings were tempered as they were restricted to patients between the ages of 60 to 80 years (thus excluding the young and elderly populations) and included use of antiarrhythmic drugs with high toxicities as well as patients with excessive stroke risk that had been taken off of anticoagulant therapy, potentially limiting their potential for success (276). Thus, it remains to be determined whether current rhythm control strategies, which now include newer antiarrhythmic drugs and surgical interventions will tip the scale towards favoring these strategies for the treatment of AF in the future.
Upstream therapies
Intense scientific interest has focused on strategies “upstream” of the electrophysiological defects associated with AF as they are thought to target the anatomical substrate in AF, which include the structural alterations associated with atrial and ventricular remodeling (e.g., inflammation, cell death, oxidative stress, hypertrophy, and fibrosis) (207, 256). Major pharmacotherapies that target these pathways include: (i) RAAS inhibitors [angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARBs), and aldosterone inhibitors (e.g., spironolactone)], (ii) statins, and (iii) polyunsaturated fatty acids (207, 256). RAAS inhibitors are thought to have beneficial effects on AF through pleiotropic actions that circumvent the deleterious actions of increased renin, angiotensin II, and aldosterone. Some of these effects include: prevention of left atrial dilation and fibrosis, regression of left ventricular hypertrophy, reduction of oxidative stress, and inflammation as well as modulation of sympathetic nerve activity and ion-channel function; although indirect effects on gap junction coupling and calcium handling have also been noted (69, 256). Data from clinical trials exploiting ACEI and ARBs in AF settings have been mixed especially in terms of the recurrence or secondary prevention of AF (207). However, ACEI and ARBs did exhibit potential for primary prevention of new onset of AF in hypertensive patients with left ventricular hypertrophy and symptomatic heart failure as well as MI following retrospective analyses (89, 152, 177, 186, 249). Use of the aldosterone inhibitor, spironolactone has also been associated with reduction of AF occurrence in AF patients with structural heart disease in a retrospective study; however, larger randomized controlled trials are needed to more robustly determine their effectiveness as a treatment for AF (254). Statins have also been associated with decreasing the incidence and recurrence of AF in patients (62, 225, 256). Although the precise mechanisms underlying these actions are not clear, it is thought that statins exert anti-inflammatory effects, antioxidant effects, plaque stabilizing properties (to reduce atherosclerotic disease which is a risk factor for AF), and antiarrhythmic effects by directly modulating ion channel function (69). Dietary intake of polyunsaturated fats (e.g., fish oil) can also influence the development of postoperative AF following coronary artery bypass surgery (24). It is hypothesized that they may alleviate AF by exerting antiarrhythmic effects by directly modulating ion channels as well as anti-inflammatory effects (24). A better understanding of the underlying mechanisms associated with these strategies is clearly needed. Future avenues focused on disease-specific mechanisms underlying fibrosis (via inhibition of TGF-β1), increased oxidative stress (via calcium/calmodulin-dependent protein kinase III inhibition), and decreasing gap junction uncoupling could provide for better therapeutics as they highlight more targeted approaches to circumvent the pathophysiological processes associated with the AF substrate (256).
New Areas of Investigation in Atrial Fibrillation
One of the newest areas of investigation in the area of AF includes the development of atrio-selective drugs to limit toxicities and proarrhythmia risk to the ventricle, as these are adverse effects encountered with current antiarrhythmic drugs used for rhythm control in AF. Recent studies have identified the selective expression and function of the ultrarapid delayed rectifier channel, IKur, and acetylcholine (ACh)-activated K+(KACh) channel in the atria but not ventricular tissue (69,151). More specifically, blockers for IK,Ach (NTC-801) and IKur (NIP-142, RSD1235, and AVE0118) show promise as they could convert AF to normal sinus without having adverse effects on the ventricles (69,151), highlighting their potential as strong candidates for AF treatment in humans in the future. Gene therapy based approaches have also been exploited to target autonomic (acetylcholine)-based mechanisms to more specifically target autonomic substrate and AVN and provide alternative therapeutic approaches for AF (5,16). More specifically, genetic manipulation of intracellular signals associated with muscarinic cholinergic receptor type 2 (adenoviral-mediated overexpression of constitutively active Gαi in the AV node or nonviral minigene-overexpression of Gαi and Gαo in left atrium) that is targeted by vagally released acetylcholine, were shown to decrease ventricular rate and prevent vagally induced AF, respectively (5, 16). Thus, efforts directed at targeting mechanisms that are atrial-selective and more specifically interrupt or reverse pathophysiology and substrates underlying AF clearly show promise for the future. Recent efforts have also suggested that localized applications of the antiarrhythmic drug, amiodarone, on the atrial epicardium via adhesive hydrogels can be used to reduce risk of AF postoperatively thereby minimizing risks of reported side effects (65), also highlighting the potential importance of exploiting bioengineering approaches to better tailor current therapies for AF.
New areas of research aimed at identifying sensitive ways at imaging the atria and AF are also on the horizon as they have the potential to significantly improve AF diagnosis and treatment strategies. Magnetic resonance imaging is emerging as a noninvasive tool to more precisely map and quantify structural (fibrotic) changes in the left atria as a means to explore left atrial substrate and determine the extent of low voltage tissue, which can help guide ablation therapies as well as decisions on the type of treatment strategies to be used on the patient (17,170). Three-dimensional rotation angiography is also being explored as an alternative technique to gain precise anatomy of the left atria prior to catheterization ablation and may provide advantages over CT scanning based on the low radiation exposure and ability to use contrast medium (130). Noninvasive electrocardiographic body surface imaging and computational modeling-based approaches are also being exploited as novel tools to precisely map atrial conduction and activation patterns in AF to better understand electrophysiological-based mechanisms underlying AF and improve ablation outcomes (47,161). These studies altogether highlight strategies to better diagnose and further stratify AF into subtypes based on anatomical defects to potentially allow for better therapeutic management of AF in patients.
Conclusion
AF is a complex disease that is fueled by the structural alterations (substrate) in the atria that consequently result in complex electrophysiological defects and patterns that render the atria and autonomic system dysfunctional, which then lead to a vicious cycle of exacerbated atrial and ventricular remodeling events (electrical, structural, and autonomic) that promote and maintain AF. Current therapeutic strategies are dedicated to the control of ventricular rate and conversion to sinus rhythm through pharmacotherapies as well as chemical and electrical (direct current and ablation therapy) cardioversion techniques, respectively, to circumvent AF symptoms. However, based on the limited efficiencies and toxic side effects of current strategies as well as some understanding of the substrates propagating AF, research and therapies have now also been directed to identifying and testing strategies that selectively target the atria (atrial selective agents and pathways) and substrates (upstream therapies) underlying AF. Strategies to modify current therapeutics to minimize toxicities (e.g., amiodarone adhesive hydrogels) and complexities of ablation (e.g., hybrid ablation techniques) are also important (65,256). A major focus of future directions for AF is also dedicated to improving technologies to better stratify AF into subtypes through the use of imaging diagnostics (magnetic resonance imagine, three-dimensional rotation angiography, anatomical body surface imaging), genetics and biomarkers (256). Recent studies have also suggested that a new taxonomy may be required based on pathophysiology of the AF subtype to allow for personalized management of AF to be realized in the future (121,256).
Acknowledgements
J.P. is funded by a NIH F31 Ruth L. Kirschstein National Research Service Award Graduate Fellowship (1F31HL120611-01). Funding for F.S. is provided by the National Institute of Health (NIH 1R01HL095780-01) and Saving tiny Heart Society grants.
References
- 1.Abu-El-Haija B, Giudici MC. Predictors of long-term maintenance of normal sinus rhythm after successful electrical cardioversion. Clin Cardiol. 2014;37:381–385. doi: 10.1002/clc.22276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam O, Frost G, Custodis F, Sussman MA, Schafers HJ, Bohm M, Laufs U. Role of Rac1 GTPase activation in atrial fibrillation. J Am Coll Cardiol. 2007;50:359–367. doi: 10.1016/j.jacc.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 3.Advani SV, Singh BN. Pharmacodynamic, pharmacokinetic and antiar-rhythmic properties of d-sotalol, the dextro-isomer of sotalol. Drugs. 1995;49:664–679. doi: 10.2165/00003495-199549050-00003. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed H, Miller MA, Dukkipati SR, Cammack S, Koruth JS, Gangireddy S, Ellsworth BA, D’Avila A, Domanski M, Gelijns AC, Moskowitz A, Reddy VY. Adjunctive renal sympathetic denervation to modify hypertension as upstream therapy in the treatment of atrial fibrillation (H-FIB) study: Clinical background and study design. J Cardiovasc Electrophysiol. 2013;24:503–509. doi: 10.1111/jce.12095. [DOI] [PubMed] [Google Scholar]
- 5.Aistrup GL, Cokic I, Ng J, Gordon D, Koduri H, Browne S, Arapi D, Segon Y, Goldstein J, Angulo A, Wasserstrom JA, Goldberger JJ, Kadish AH, Arora R. Targeted nonviral gene-based inhibition of Galpha(i/o)-mediated vagal signaling in the posterior left atrium decreases vagal-induced atrial fibrillation. Heart Rhythm. 2011;8:1722–1729. doi: 10.1016/j.hrthm.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ajijola OA, Yagishita D, Patel KJ, Vaseghi M, Zhou W, Yamakawa K, So E, Lux RL, Mahajan A, Shivkumar K. Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: Neural remodeling in a spatial context. Am J Physiol Heart Circ Physiol. 2013;305:H1031–H1040. doi: 10.1152/ajpheart.00434.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American College of Cardiology Foundation. American Heart Associtation. European Society of Cardiology. Heart Rhythm Society. Wann LS, Curtis AB, Ellenbogen KA, Estes NA, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey J, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2013;127:1916–1926. doi: 10.1161/CIR.0b013e318290826d. [DOI] [PubMed] [Google Scholar]
- 8.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 9.Anne W, Willems R, Holemans P, Beckers F, Roskams T, Lenaerts I, Ector H, Heidbuchel H. Self-terminating AF depends on electrical remodeling while persistent AF depends on additional structural changes in a rapid atrially paced sheep model. J Mol Cell Cardiol. 2007;43:148–158. doi: 10.1016/j.yjmcc.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 11.Arora R, Ulphani JS, Villuendas R, Ng J, Harvey L, Thordson S, Inderyas F, Lu Y, Gordon D, Denes P, Greene R, Crawford S, Decker R, Morris A, Goldberger J, Kadish AH. Neural substrate for atrial fibrillation: Implications for targeted parasympathetic blockade in the posterior left atrium. Am J Physiol Heart Circ Physiol. 2008;294:H134–H144. doi: 10.1152/ajpheart.00732.2007. [DOI] [PubMed] [Google Scholar]
- 12.Ausma J, van der Velden HM, Lenders MH, van Ankeren EP, Jongsma HJ, Ramaekers FC, Borgers M, Allessie MA. Reverse structural and gap-junctional remodeling after prolonged atrial fibrillation in the goat. Circulation. 2003;107:2051–2058. doi: 10.1161/01.CIR.0000062689.04037.3F. [DOI] [PubMed] [Google Scholar]
- 13.Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–3163. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 14.Bartos DC, Anderson JB, Bastiaenen R, Johnson JN, Gollob MH, Tester DJ, Burgess DE, Homfray T, Behr ER, Ackerman MJ, Guicheney P, Delisle BP. A KCNQ1 mutation causes a high penetrance for familial atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:562–569. doi: 10.1111/jce.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartos DC, Duchatelet S, Burgess DE, Klug D, Denjoy I, Peat R, Lupoglazoff JM, Fressart V, Berthet M, Ackerman MJ, January CT, Guicheney P, Delisle BP. R231C mutation in KCNQ1 causes long QT syndrome type 1 and familial atrial fibrillation. Heart Rhythm. 2011;8:48–55. doi: 10.1016/j.hrthm.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer A, McDonald AD, Nasir K, Peller L, Rade JJ, Miller JM, Held-man AW, Donahue JK. Inhibitory G protein overexpression provides physiologically relevant heart rate control in persistent atrial fibrillation. Circulation. 2004;110:3115–3120. doi: 10.1161/01.CIR.0000147185.31974.BE. [DOI] [PubMed] [Google Scholar]
- 17.Beinart R, Khurram IM, Liu S, Yarmohammadi H, Halperin HR, Bluemke DA, Gai N, van der Geest RJ, Lima JA, Calkins H, Zimmerman SL, Nazarian S. Cardiac magnetic resonance T1 mapping of left atrial myocardium. Heart Rhythm. 2013;10:1325–1331. doi: 10.1016/j.hrthm.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belus A, Piroddi N, Ferrantini C, Tesi C, Cazorla O, Toniolo L, Drost M, Mearini G, Carrier L, Rossi A, Mugelli A, Cerbai E, van der Velden J, Poggesi C. Effects of chronic atrial fibrillation on active and passive force generation in human atrial myofibrils. Circ Res. 2010;107:144–152. doi: 10.1161/CIRCRESAHA.110.220699. [DOI] [PubMed] [Google Scholar]
- 19.Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. 1992;120:893–901. [PubMed] [Google Scholar]
- 20.Brugada R, Tapscott T, Czernuszewicz GZ, Marian AJ, Iglesias A, Mont L, Brugada J, Girona J, Domingo A, Bachinski LL, Roberts R. Identification of a genetic locus for familial atrial fibrillation. N Engl J Med. 1997;336:905–911. doi: 10.1056/NEJM199703273361302. [DOI] [PubMed] [Google Scholar]
- 21.Burstein B, Comtois P, Michael G, Nishida K, Villeneuve L, Yeh YH, Nattel S. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ Res. 2009;105:1213–1222. doi: 10.1161/CIRCRESAHA.108.183400. [DOI] [PubMed] [Google Scholar]
- 22.Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: A potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. 2008;117:1630–1641. doi: 10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]
- 23.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr., Davies DW, DiMarco J, Edgerton J, Ellen-bogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 24.Calo L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, de Ruvo E, Meo A, Pandozi C, Staibano M, Santini M. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: A randomized, controlled trial. J Am Coll Cardiol. 2005;45:1723–1728. doi: 10.1016/j.jacc.2005.02.079. [DOI] [PubMed] [Google Scholar]
- 25.Camm AJ, Al-Khatib SM, Calkins H, Halperin JL, Kirchhof P, Lip GY, Nattel S, Ruskin J, Banerjee A, Blendea D, Guasch E, Needleman M, Savelieva I, Viles-Gonzalez J, Williams ES. A proposal for new clinical concepts in the management of atrial fibrillation. Am Heart J. 2012;164:292–302. e291. doi: 10.1016/j.ahj.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P, Guidelines-CPG ESCCfP, Document R 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation–developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385–1413. doi: 10.1093/europace/eus305. [DOI] [PubMed] [Google Scholar]
- 27.Camm AJ, Savelieva I, Lip GY. Guideline. Development Group for the Ncgftmoaf. Rate control in the medical management of atrial fibrillation. Heart. 2007;93:35–38. doi: 10.1136/hrt.2006.099903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardin S, Guasch E, Luo X, Naud P, Le Quang K, Shi Y, Tardif JC, Comtois P, Nattel S. Role for MicroRNA-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ Arrhythm Electrophysiol. 2012;5:1027–1035. doi: 10.1161/CIRCEP.112.973214. [DOI] [PubMed] [Google Scholar]
- 29.Cardin S, Li D, Thorin-Trescases N, Leung TK, Thorin E, Nattel S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: Angiotensin-dependent and -independent pathways. Cardiovasc Res. 2003;60:315–325. doi: 10.1016/j.cardiores.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Cardin S, Libby E, Pelletier P, Le Bouter S, Shiroshita-Takeshita A, Le Meur N, Leger J, Demolombe S, Ponton A, Glass L, Nattel S. Contrasting gene expression profiles in two canine models of atrial fibrillation. Circ Res. 2007;100:425–433. doi: 10.1161/01.RES.0000258428.09589.1a. [DOI] [PubMed] [Google Scholar]
- 31.Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus S, Walter S, Tebbe U, Investigators S. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: The Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690–1696. doi: 10.1016/s0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 32.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, Kanderian A, Pavia S, Hamlin RL, McCarthy PM, Bauer JA, Van Wagoner DR. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–E38. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 33.Cha TJ, Ehrlich JR, Zhang L, Chartier D, Leung TK, Nattel S. Atrial tachycardia remodeling of pulmonary vein cardiomyocytes: Comparison with left atrium and potential relation to arrhythmogenesis. Circulation. 2005;111:728–735. doi: 10.1161/01.CIR.0000155240.05251.D0. [DOI] [PubMed] [Google Scholar]
- 34.Chang CM, Wu TJ, Zhou S, Doshi RN, Lee MH, Ohara T, Fishbein MC, Karagueuzian HS, Chen PS, Chen LS. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation. 2001;103:22–25. doi: 10.1161/01.cir.103.1.22. [DOI] [PubMed] [Google Scholar]
- 35.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, Jin HW, Sun H, Su XY, Zhuang QN, Yang YQ, Li YB, Liu Y, Xu HJ, Li XF, Ma N, Mou CP, Chen Z, Barhanin J, Huang W. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 37.Choisy SC, Arberry LA, Hancox JC, James AF. Increased susceptibility to atrial tachyarrhythmia in spontaneously hypertensive rat hearts. Hypertension. 2007;49:498–505. doi: 10.1161/01.HYP.0000257123.95372.ab. [DOI] [PubMed] [Google Scholar]
- 38.Christ T, Boknik P, Wohrl S, Wettwer E, Graf EM, Bosch RF, Knaut M, Schmitz W, Ravens U, Dobrev D. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110:2651–2657. doi: 10.1161/01.CIR.0000145659.80212.6A. [DOI] [PubMed] [Google Scholar]
- 39.Christophersen IE, Olesen MS, Liang B, Andersen MN, Larsen AP, Nielsen JB, Haunso S, Olesen SP, Tveit A, Svendsen JH, Schmitt N. Genetic variation in KCNA5: Impact on the atrial-specific potassium current IKur in patients with lone atrial fibrillation. Eur Heart J. 2013;34:1517–1525. doi: 10.1093/eurheartj/ehs442. [DOI] [PubMed] [Google Scholar]
- 40.Comtois P, Kneller J, Nattel S. Of circles and spirals: Bridging the gap between the leading circle and spiral wave concepts of cardiac reentry. Europace. 2005;7(Suppl 2):10–20. doi: 10.1016/j.eupc.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Coumel P, Attuel P, Lavallee J, Flammang D, Leclercq JF, Slama R. The atrial arrhythmia syndrome of vagal origin. Arch Mal Coeur Vaiss. 1978;71:645–656. [PubMed] [Google Scholar]
- 42.Cove CL, Albert CM, Andreotti F, Badimon L, Van Gelder IC, Hylek EM. Female sex as an independent risk factor for stroke in atrial fibrillation: Possible mechanisms. Thromb Haemost. 2014;111:385–391. doi: 10.1160/TH13-04-0347. [DOI] [PubMed] [Google Scholar]
- 43.Cox JL. Cardiac surgery for arrhythmias. Heart Rhythm. 2004;1:85C–101C. doi: 10.1016/j.hrthm.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 44.Cox JL, Canavan TE, Schuessler RB, Cain ME, Lindsay BD, Stone C, Smith PK, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg. 1991;101:406–426. [PubMed] [Google Scholar]
- 45.Cox JL, Schuessler RB, D’Agostino HJ, Jr., Stone CM, Chang BC, Cain ME, Corr PB, Boineau JP. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101:569–583. [PubMed] [Google Scholar]
- 46.Cox JL, Schuessler RB, Lappas DG, Boineau JP. An 8 1/2-year clinical experience with surgery for atrial fibrillation. Ann Surg. 1996;224:267–273. doi: 10.1097/00000658-199609000-00003. discussion 273-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RJ, Jr., Li L, Rudy Y. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das S, Makino S, Melman YF, Shea MA, Goyal SB, Rosenzweig A, Macrae CA, Ellinor PT. Mutation in the S3 segment of KCNQ1 results in familial lone atrial fibrillation. Heart Rhythm. 2009;6:1146–1153. doi: 10.1016/j.hrthm.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis EM, Packard KA, Knezevich JT, Campbell JA. New and emerging anticoagulant therapy for atrial fibrillation and acute coronary syndrome. Pharmacotherapy. 2011;31:975–1016. doi: 10.1592/phco.31.10.975. [DOI] [PubMed] [Google Scholar]
- 50.De Clercq D, Decloedt A, Sys SU, Verheyen T, Van Der Vekens N, van Loon G. Atrial fibrillation cycle length and atrial size in horses with and without recurrence of atrial fibrillation after electrical cardioversion. J Vet Intern Med. 2014;28:624–629. doi: 10.1111/jvim.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DE Sisti A, Leclercq JF, Halimi F, Fiorello P, Bertrand C, Attuel P. Evaluation of time course and predicting factors of progression of paroxysmal or persistent atrial fibrillation to permanent atrial fibrillation. Pacing Clin Electrophysiol. 2014;37:345–355. doi: 10.1111/pace.12264. [DOI] [PubMed] [Google Scholar]
- 52.Deo M, Ruan Y, Pandit SV, Shah K, Berenfeld O, Blaufox A, Cerrone M, Noujaim SF, Denegri M, Jalife J, Priori SG. KCNJ2 mutation in short QT syndrome 3 results in atrial fibrillation and ventricular proarrhythmia. Proc Natl Acad Sci U S A. 2013;110:4291–4296. doi: 10.1073/pnas.1218154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deroubaix E, Folliguet T, Rucker-Martin C, Dinanian S, Boixel C, Validire P, Daniel P, Capderou A, Hatem SN. Moderate and chronic hemodynamic overload of sheep atria induces reversible cellular electrophysiologic abnormalities and atrial vulnerability. J Am Coll Cardiol. 2004;44:1918–1926. doi: 10.1016/j.jacc.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 54.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, Knaut M, Ravens U. The G protein-gated potassium current I(K,ACh) is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–3706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- 56.Dobrev D, Voigt N, Wehrens XH. The ryanodine receptor channel as a molecular motif in atrial fibrillation: Pathophysiological and therapeutic implications. Cardiovasc Res. 2011;89:734–743. doi: 10.1093/cvr/cvq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dun W, Boyden PA. Aged atria: Electrical remodeling conducive to atrial fibrillation. J Interv Card Electrophysiol. 2009;25:9–18. doi: 10.1007/s10840-008-9358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edgley AJ, Krum H, Kelly DJ. Targeting fibrosis for the treatment of heart failure: A role for transforming growth factor-beta. Cardiovasc Ther. 2012;30:e30–e40. doi: 10.1111/j.1755-5922.2010.00228.x. [DOI] [PubMed] [Google Scholar]
- 59.Ehrlich JR, Cha TJ, Zhang L, Chartier D, Villeneuve L, Hebert TE, Nattel S. Characterization of a hyperpolarization-activated time-dependent potassium current in canine cardiomyocytes from pulmonary vein myocardial sleeves and left atrium. J Physiol. 2004;557:583–597. doi: 10.1113/jphysiol.2004.061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: Clinical and experimental evidence. Eur Heart J. 2006;27:512–518. doi: 10.1093/eurheartj/ehi668. [DOI] [PubMed] [Google Scholar]
- 61.Falk RH. Is rate control or rhythm control preferable in patients with atrial fibrillation? Rate control is preferable to rhythm control in the majority of patients with atrial fibrillation. Circulation. 2005;111:3141–3150. doi: 10.1161/CIRCULATIONAHA.104.485565. discussion 3157. [DOI] [PubMed] [Google Scholar]
- 62.Fang WT, Li HJ, Zhang H, Jiang S. The role of statin therapy in the prevention of atrial fibrillation: A meta-analysis of randomized controlled trials. Br J Clinl Pharmacol. 2012;74:744–756. doi: 10.1111/j.1365-2125.2012.04258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farshi R, Kistner D, Sarma JS, Longmate JA, Singh BN. Ventricular rate control in chronic atrial fibrillation during daily activity and programmed exercise: A crossover open-label study of five drug regimens. J Am Coll Cardiol. 1999;33:304–310. doi: 10.1016/s0735-1097(98)00561-0. [DOI] [PubMed] [Google Scholar]
- 64.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 65.Feng XD, Wang XN, Yuan XH, Wang W. Effectiveness of biatrial epicardial application of amiodarone-releasing adhesive hydrogel to prevent postoperative atrial fibrillation. J Thorac Cardiovasc Surg. 2014 doi: 10.1016/j.jtcvs.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 66.Ferreras JM, Iglesias R, Munoz R, Arias FJ, Girbes T. Influence of the structure of alkanols on their inhibition of protein synthesis in Saccharomyces cerevisiae var. ellipsoideus. Cell Mol Biol. 1990;36:337–344. [PubMed] [Google Scholar]
- 67.Firouzi M, Ramanna H, Kok B, Jongsma HJ, Koeleman BP, Doevendans PA, Groenewegen WA, Hauer RN. Association of human connexin40 gene polymorphisms with atrial vulnerability as a risk factor for idiopathic atrial fibrillation. Circ Res. 2004;95:e29–e33. doi: 10.1161/01.RES.0000141134.64811.0a. [DOI] [PubMed] [Google Scholar]
- 68.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 69.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–e198. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 70.Gaborit N, Steenman M, Lamirault G, Le Meur N, Le Bouter S, Lande G, Leger J, Charpentier F, Christ T, Dobrev D, Escande D, Nattel S, Demolombe S. Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation. 2005;112:471–481. doi: 10.1161/CIRCULATIONAHA.104.506857. [DOI] [PubMed] [Google Scholar]
- 71.Garrey WE. The nature of fibrillary contraction of the heart. Its relation to tissue mass and form. Am J Physiol. 1914;33:397–414. [Google Scholar]
- 72.Garrey WE. Auricular fibrillation. Physiol Rev. 1924;4:215–250. [Google Scholar]
- 73.Gaspo R, Bosch RF, Talajic M, Nattel S. Functional mechanisms underlying tachycardia-induced sustained atrial fibrillation in a chronic dog model. Circulation. 1997;96:4027–4035. doi: 10.1161/01.cir.96.11.4027. [DOI] [PubMed] [Google Scholar]
- 74.Gaynor SL, Diodato MD, Prasad SM, Ishii Y, Schuessler RB, Bailey MS, Damiano NR, Bloch JB, Moon MR, Damiano RJ., Jr A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg. 2004;128:535–542. doi: 10.1016/j.jtcvs.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 75.Geddes LA, Hinds M, Babbs CF, Tacker WA, Schoenlein WE, Elabbady T, Saeed M, Bourland JD, Ayers GM. Maintenance of atrial fibrillation in anesthetized and unanesthetized sheep using cholinergic drive. Pacing Clin Electrophysiol. 1996;19:165–175. doi: 10.1111/j.1540-8159.1996.tb03308.x. [DOI] [PubMed] [Google Scholar]
- 76.Gevaert SA, de Bacquer D, Willems AM, Vande Kerckhove B, Weytjens C, van Camp G, de Sutter J. Gender differences in the management and outcome of atrial fibrillation complicating acute heart failure. J Card Fail. 2014;20:431–437. doi: 10.1016/j.cardfail.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Girmatsion Z, Biliczki P, Bonauer A, Wimmer-Greinecker G, Scherer M, Moritz A, Bukowska A, Goette A, Nattel S, Hohnloser SH, Ehrlich JR. Changes in microRNA-1 expression and IK1 up-regulation in human atrial fibrillation. Heart Rhythm. 2009;6:1802–1809. doi: 10.1016/j.hrthm.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 78.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 79.Godfraind T. Calcium channel blockers in cardiovascular pharmacotherapy. J Cardiovasc Pharmacol Ther. 2014 doi: 10.1177/1074248414530508. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 80.Goette A, Staack T, Rocken C, Arndt M, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. 2000;35:1669–1677. doi: 10.1016/s0735-1097(00)00611-2. [DOI] [PubMed] [Google Scholar]
- 81.Goldberger AL, Pavelec RS. Vagally-mediated atrial fibrillation in dogs: Conversion with bretylium tosylate. Int J Cardiol. 1986;13:47–55. doi: 10.1016/0167-5273(86)90078-1. [DOI] [PubMed] [Google Scholar]
- 82.Gollob MH, Jones DL, Krahn AD, Danis L, Gong XQ, Shao Q, Liu X, Veinot JP, Tang AS, Stewart AF, Tesson F, Klein GJ, Yee R, Skanes AC, Guiraudon GM, Ebihara L, Bai D. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med. 2006;354:2677–2688. doi: 10.1056/NEJMoa052800. [DOI] [PubMed] [Google Scholar]
- 83.Gramley F, Lorenzen J, Koellensperger E, Kettering K, Weiss C, Munzel T. Atrial fibrosis and atrial fibrillation: The role of the TGF-beta1 signaling pathway. Int J Cardiol. 2010;143:405–413. doi: 10.1016/j.ijcard.2009.03.110. [DOI] [PubMed] [Google Scholar]
- 84.Guasch E, Benito B, Qi X, Cifelli C, Naud P, Shi Y, Mighiu A, Tardif JC, Tadevosyan A, Chen Y, Gillis MA, Iwasaki YK, Dobrev D, Mont L, Heximer S, Nattel S. Atrial fibrillation promotion by endurance exercise: Demonstration and mechanistic exploration in an animal model. J Am Coll Cardiol. 2013;62:68–77. doi: 10.1016/j.jacc.2013.01.091. [DOI] [PubMed] [Google Scholar]
- 85.Gupta DK, Shah AM, Giugliano RP, Ruff CT, Antman EM, Grip LT, Deenadayalu N, Hoffman E, Patel I, Shi M, Mercuri M, Mitrovic V, Braunwald E, Solomon SD. Effective aNticoaGulation with factor xA next GEneration in AF-Thrombolysis In Myocardial Infarction 48 Echocardiographic Study Investigators. Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. Eur Heart J. 2014;35:1457–1465. doi: 10.1093/eurheartj/eht500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hagendorff A, Schumacher B, Kirchhoff S, Luderitz B, Willecke K. Conduction disturbances and increased atrial vulnerability in Connexin40-deficient mice analyzed by transesophageal stimulation. Circulation. 1999;99:1508–1515. doi: 10.1161/01.cir.99.11.1508. [DOI] [PubMed] [Google Scholar]
- 87.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 88.Hasegawa K, Ohno S, Ashihara T, Itoh H, Ding WG, Toyoda F, Makiyama T, Aoki H, Nakamura Y, Delisle BP, Matsuura H, Horie M. A novel KCNQ1 missense mutation identified in a patient with juvenile-onset atrial fibrillation causes constitutively open IKs channels. Heart Rhythm. 2014;11:67–75. doi: 10.1016/j.hrthm.2013.09.073. [DOI] [PubMed] [Google Scholar]
- 89.Healey JS, Baranchuk A, Crystal E, Morillo CA, Garfinkle M, Yusuf S, Connolly SJ. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: A meta-analysis. J Am Coll Cardiol. 2005;45:1832–1839. doi: 10.1016/j.jacc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 90.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: The Rotterdam study. Eur Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 91.Heist EK, Mansour M, Ruskin JN. Rate control in atrial fibrillation: Targets, methods, resynchronization considerations. Circulation. 2011;124:2746–2755. doi: 10.1161/CIRCULATIONAHA.111.019919. [DOI] [PubMed] [Google Scholar]
- 92.Henry WL, Morganroth J, Pearlman AS, Clark CE, Redwood DR, Itscoitz SB, Epstein SE. Relation between echocardiographically determined left atrial size and atrial fibrillation. Circulation. 1976;53:273–279. doi: 10.1161/01.cir.53.2.273. [DOI] [PubMed] [Google Scholar]
- 93.Hirose M, Takeishi Y, Miyamoto T, Kubota I, Laurita KR, Chiba S. Mechanism for atrial tachyarrhythmia in chronic volume overload-induced dilated atria. J Cardiovasc Electrophysiol. 2005;16:760–769. doi: 10.1046/j.1540-8167.2005.40331.x. [DOI] [PubMed] [Google Scholar]
- 94.Hirose M, Takeishi Y, Niizeki T, Shimojo H, Nakada T, Kubota I, Nakayama J, Mende U, Yamada M. Diacylglycerol kinase zeta inhibits G(alpha)q-induced atrial remodeling in transgenic mice. Heart Rhythm. 2009;6:78–84. doi: 10.1016/j.hrthm.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 95.Hof IE, Vonken EJ, Velthuis BK, Wittkampf FH, van der Heijden JF, Neven KG, Kassenberg W, Meine M, Cramer MJ, Hauer RN, Loh P. Impact of pulmonary vein antrum isolation on left atrial size and function in patients with atrial fibrillation. J Interv Card Electrophysiol. 2014;39:201–209. doi: 10.1007/s10840-013-9860-0. [DOI] [PubMed] [Google Scholar]
- 96.Hogarth AJ, Dobson LE, Tayebjee MH. During ablation for atrial fibrillation, is simultaneous renal artery ablation appropriate? J Human Hypertens. 2013;27:707–714. doi: 10.1038/jhh.2013.75. [DOI] [PubMed] [Google Scholar]
- 97.Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation–Pharmacological Intervention in Atrial Fibrillation (PIAF): A randomised trial. Lancet. 2000;356:1789–1794. doi: 10.1016/s0140-6736(00)03230-x. [DOI] [PubMed] [Google Scholar]
- 98.Hong CS, Cho MC, Kwak YG, Song CH, Lee YH, Lim JS, Kwon YK, Chae SW, Kim DH. Cardiac remodeling and atrial fibrillation in transgenic mice overexpressing junctin. FASEB J. 2002;16:1310–1312. doi: 10.1096/fj.01-0908fje. [DOI] [PubMed] [Google Scholar]
- 99.Hong CS, Kwon SJ, Cho MC, Kwak YG, Ha KC, Hong B, Li H, Chae SW, Chai OH, Song CH, Li Y, Kim JC, Woo SH, Lee SY, Lee CO, Kim do H. Overexpression of junctate induces cardiac hypertrophy and arrhythmia via altered calcium handling. J Mol Cell Cardiol. 2008;44:672–682. doi: 10.1016/j.yjmcc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 100.Humphries KH, Kerr CR, Connolly SJ, Klein G, Boone JA, Green M, Sheldon R, Talajic M, Dorian P, Newman D. New-onset atrial fibrillation: Sex differences in presentation, treatment, and outcome. Circulation. 2001;103:2365–2370. doi: 10.1161/01.cir.103.19.2365. [DOI] [PubMed] [Google Scholar]
- 101.Hunlich M, Tremble SM, Begin KJ, Leavitt BJ, Ittleman FP, VanBuren P. Atrial contractile protein content and function are preserved in patients with coronary artery disease and atrial fibrillation. Coron Artery Dis. 2010;21:357–362. doi: 10.1097/MCA.0b013e32833d5fc9. [DOI] [PubMed] [Google Scholar]
- 102.Igarashi T, Finet JE, Takeuchi A, Fujino Y, Strom M, Greener ID, Rosenbaum DS, Donahue JK. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation. 2012;125:216–225. doi: 10.1161/CIRCULATIONAHA.111.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ito Y, Yamasaki H, Naruse Y, Yoshida K, Kaneshiro T, Murakoshi N, Igarashi M, Kuroki K, Machino T, Xu D, Kunugita F, Sekiguchi Y, Sato A, Tada H, Aonuma K. Effect of eplerenone on maintenance of sinus rhythm after catheter ablation in patients with long-standing persistent atrial fibrillation. Am J Cardiol. 2013;111:1012–1018. doi: 10.1016/j.amjcard.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 104.Jacquemet V, Henriquez CS. Loading effect of fibroblast-myocyte coupling on resting potential, impulse propagation, and repolarization: Insights from a microstructure model. Am J Physiol Heart Circ Physiol. 2008;294:H2040–H2052. doi: 10.1152/ajpheart.01298.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jais P, Haissaguerre M, Shah DC, Chouairi S, Gencel L, Hocini M, Clementy J. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997;95:572–576. doi: 10.1161/01.cir.95.3.572. [DOI] [PubMed] [Google Scholar]
- 106.Janes RD, Brandys JC, Hopkins DA, Johnstone DE, Murphy DA, Armour JA. Anatomy of human extrinsic cardiac nerves and ganglia. Am J Cardiol. 1986;57:299–309. doi: 10.1016/0002-9149(86)90908-2. [DOI] [PubMed] [Google Scholar]
- 107.Jayachandran JV, Sih HJ, Winkle W, Zipes DP, Hutchins GD, Olgin JE. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation. 2000;101:1185–1191. doi: 10.1161/01.cir.101.10.1185. [DOI] [PubMed] [Google Scholar]
- 108.Kalifa J, Jalife J, Zaitsev AV, Bagwe S, Warren M, Moreno J, Berenfeld O, Nattel S. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation. 2003;108:668–671. doi: 10.1161/01.CIR.0000086979.39843.7B. [DOI] [PubMed] [Google Scholar]
- 109.Kanj M, Wazni O, Natale A. Pulmonary vein antrum isolation. Heart Rhythm. 2007;4:S73–S79. doi: 10.1016/j.hrthm.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 110.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: The Framingham study. N Engl J Med. 1982;306:1018–1022. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 111.Karle CA, Zitron E, Zhang W, Wendt-Nordahl G, Kathofer S, Thomas D, Gut B, Scholz E, Vahl CF, Katus HA, Kiehn J. Human cardiac inwardly-rectifying K+ channel Kir(2.1b) is inhibited by direct protein kinase C-dependent regulation in human isolated cardiomyocytes and in an expression system. Circulation. 2002;106:1493–1499. doi: 10.1161/01.cir.0000029747.53262.5c. [DOI] [PubMed] [Google Scholar]
- 112.Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, Camm AJ, Ioannidis JP. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: A randomized clinical trial. J Am Coll Cardiol. 2013;62:2318–2325. doi: 10.1016/j.jacc.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 113.Kawashima T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat Embryol. 2005;209:425–438. doi: 10.1007/s00429-005-0462-1. [DOI] [PubMed] [Google Scholar]
- 114.Kazemian P, Gollob MH, Pantano A, Oudit GY. A novel mutation in the RYR2 gene leading to catecholaminergic polymorphic ventricular tachycardia and paroxysmal atrial fibrillation: Dose-dependent arrhythmia-event suppression by beta-blocker therapy. Can J Cardiol. 2011;27:870, e877–810. doi: 10.1016/j.cjca.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 115.Kehat I, Heinrich R, Ben-Izhak O, Miyazaki H, Gutkind JS, Aronheim A. Inhibition of basic leucine zipper transcription is a major mediator of atrial dilatation. Cardiovasc Res. 2006;70:543–554. doi: 10.1016/j.cardiores.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 116.Ki CS, Jung CL, Kim HJ, Baek KH, Park SJ, On YK, Kim KS, Noh SJ, Youm JB, Kim JS, Cho H. A KCNQ1 mutation causes age-dependant bradycardia and persistent atrial fibrillation. Pflugers Archiv. 2014;466:529–540. doi: 10.1007/s00424-013-1337-6. [DOI] [PubMed] [Google Scholar]
- 117.Kim KB, Rodefeld MD, Schuessler RB, Cox JL, Boineau JP. Relationship between local atrial fibrillation interval and refractory period in the isolated canine atrium. Circulation. 1996;94:2961–2967. doi: 10.1161/01.cir.94.11.2961. [DOI] [PubMed] [Google Scholar]
- 118.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- 119.Kim YM, Guzik TJ, Zhang YH, Zhang MH, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–636. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 120.Kimura S, Ito M, Tomita M, Hoyano M, Obata H, Ding L, Chinushi M, Hanawa H, Kodama M, Aizawa Y. Role of mineralocorticoid receptor on atrial structural remodeling and inducibility of atrial fibrillation in hypertensive rats. Hypertens Res. 2011;34:584–591. doi: 10.1038/hr.2010.277. [DOI] [PubMed] [Google Scholar]
- 121.Kirchhof P, Breithardt G, Aliot E, Al Khatib S, Apostolakis S, Auricchio A, Bailleul C, Bax J, Benninger G, Blomstrom-Lundqvist C, Boersma L, Boriani G, Brandes A, Brown H, Brueckmann M, Calkins H, Casadei B, Clemens A, Crijns H, Derwand R, Dobrev D, Ezekowitz M, Fetsch T, Gerth A, Gillis A, Gulizia M, Hack G, Haegeli L, Hatem S, Georg Hausler K, Heidbuchel H, Hernandez-Brichis J, Jais P, Kappenberger L, Kautzner J, Kim S, Kuck KH, Lane D, Leute A, Lewalter T, Meyer R, Mont L, Moses G, Mueller M, Munzel F, Nabauer M, Nielsen JC, Oeff M, Oto A, Pieske B, Pisters R, Potpara T, Rasmussen L, Ravens U, Reiffel J, Richard-Lordereau I, Schafer H, Schotten U, Stegink W, Stein K, Steinbeck G, Szumowski L, Tavazzi L, Themistoclakis S, Thomitzek K, Van Gelder IC, von Stritzky B, Vincent A, Werring D, Willems S, Lip GY, Camm AJ. Personalized management of atrial fibrillation: Proceedings from the fourth Atrial Fibrillation competence NETwork/European Heart Rhythm Association consensus conference. Europace. 2013;15:1540–1556. doi: 10.1093/europace/eut232. [DOI] [PubMed] [Google Scholar]
- 122.Kistler PM, Sanders P, Dodic M, Spence SJ, Samuel CS, Zhao C, Charles JA, Edwards GA, Kalman JM. Atrial electrical and structural abnormalities in an ovine model of chronic blood pressure elevation after prenatal corticosteroid exposure: Implications for development of atrial fibrillation. Eur Heart J. 2006;27:3045–3056. doi: 10.1093/eurheartj/ehl360. [DOI] [PubMed] [Google Scholar]
- 123.Knackstedt C, Gramley F, Schimpf T, Mischke K, Zarse M, Plisiene J, Schmid M, Lorenzen J, Frechen D, Neef P, Hanrath P, Kelm M, Schauerte P. Association of echocardiographic atrial size and atrial fibrosis in a sequential model of congestive heart failure and atrial fibrillation. Cardiovasc Pathol. 2008;17:318–324. doi: 10.1016/j.carpath.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 124.Kodama I, Kamiya K, Toyama J. Amiodarone: Ionic and cellular mechanisms of action of the most promising class III agent. Am J Cardiol. 1999;84:20R–28R. doi: 10.1016/s0002-9149(99)00698-0. [DOI] [PubMed] [Google Scholar]
- 125.Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. 2003;41:2197–2204. doi: 10.1016/s0735-1097(03)00464-9. [DOI] [PubMed] [Google Scholar]
- 126.Lammers C, Dartsch T, Brandt MC, Rottlander D, Halbach M, Peinkofer G, Ockenpoehler S, Weiergraeber M, Schneider T, Reuter H, Muller-Ehmsen J, Hescheler J, Hoppe UC, Zobel C. Spironolactone prevents aldosterone induced increased duration of atrial fibrillation in rat. Cell Physiol Biochem. 2012;29:833–840. doi: 10.1159/000178483. [DOI] [PubMed] [Google Scholar]
- 127.Lavall D, Selzer C, Schuster P, Lenski M, Adam O, Schafers HJ, Bohm M, Laufs U. The mineralocorticoid receptor promotes fibrotic remodeling in atrial fibrillation. J Biol Chem. 2014;289:6656–6668. doi: 10.1074/jbc.M113.519256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee KS, Lee EW. Ionic mechanism of ibutilide in human atrium: Evidence for a drug-induced Na+ current through a nifedipine inhibited inward channel. J Pharmacol Exp Ther. 1998;286:9–22. [PubMed] [Google Scholar]
- 129.Lee KW, Everett THt, Rahmutula D, Guerra JM, Wilson E, Ding C, Olgin JE. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006;114:1703–1712. doi: 10.1161/CIRCULATIONAHA.106.624320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lehar F, Starek Z, Jez J, Novak M, Wolf J, Stepanova R, Kruzliak P, Kulik T, Zbankova A, Jancar R, Vitovec J. Comparison of clinical outcomes and safety of catheter ablation for atrial fibrillation supported by data from CT scan or three-dimensional rotational angiogram of left atrium and pulmonary veins. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014 doi: 10.5507/bp.2014.040. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 131.Lemoine MD, Duverger JE, Naud P, Chartier D, Qi XY, Comtois P, Fabritz L, Kirchhof P, Nattel S. Arrhythmogenic left atrial cellular electrophysiology in a murine genetic long QT syndrome model. Cardiovasc Res. 2011;92:67–74. doi: 10.1093/cvr/cvr166. [DOI] [PubMed] [Google Scholar]
- 132.Levy S, Beharier O, Etzion Y, Mor M, Buzaglo L, Shaltiel L, Gheber LA, Kahn J, Muslin AJ, Katz A, Gitler D, Moran A. Molecular basis for zinc transporter 1 action as an endogenous inhibitor of L-type calcium channels. J Biol Chem. 2009;284:32434–32443. doi: 10.1074/jbc.M109.058842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Levy S, Ricard P, Gueunoun M, Yapo F, Trigano J, Mansouri C, Paganelli F. Low-energy cardioversion of spontaneous atrial fibrillation. Immediate and long-term results. Circulation. 1997;96:253–259. doi: 10.1161/01.cir.96.1.253. [DOI] [PubMed] [Google Scholar]
- 134.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: Atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 135.Li D, Shinagawa K, Pang L, Leung TK, Cardin S, Wang Z, Nattel S. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104:2608–2614. doi: 10.1161/hc4601.099402. [DOI] [PubMed] [Google Scholar]
- 136.Li J, McLerie M, Lopatin AN. Transgenic upregulation of IK1 in the mouse heart leads to multiple abnormalities of cardiac excitability. Am J Physiol Heart Circ Physiol. 2004;287:H2790–H2802. doi: 10.1152/ajpheart.00114.2004. [DOI] [PubMed] [Google Scholar]
- 137.Li S, Scherlag BJ, Yu L, Sheng X, Zhang Y, Ali R, Dong Y, Ghias M, Po SS. Low-level vagosympathetic stimulation: A paradox and potential new modality for the treatment of focal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:645–651. doi: 10.1161/CIRCEP.109.868331. [DOI] [PubMed] [Google Scholar]
- 138.Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ Res. 2005;96:1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c. [DOI] [PubMed] [Google Scholar]
- 139.Li Y, Jian Z, Yang ZY, Chen L, Wang XF, Ma RY, Xiao YB. Increased expression of connective tissue growth factor and transforming growth factor-beta-1 in atrial myocardium of patients with chronic atrial fibrillation. Cardiology. 2013;124:233–240. doi: 10.1159/000347126. [DOI] [PubMed] [Google Scholar]
- 140.Liang X, Xie H, Zhu PH, Hu J, Zhao Q, Wang CS, Yang C. Enhanced activity of inositol-1,4,5-trisphosphate receptors in atrial myocytes of atrial fibrillation patients. Cardiology. 2009;114:180–191. doi: 10.1159/000228584. [DOI] [PubMed] [Google Scholar]
- 141.Linz D, Hohl M, Nickel A, Mahfoud F, Wagner M, Ewen S, Schotten U, Maack C, Wirth K, Bohm M. Effect of renal denervation on neurohumoral activation triggering atrial fibrillation in obstructive sleep apnea. Hypertension. 2013;62:767–774. doi: 10.1161/HYPERTENSIONAHA.113.01728. [DOI] [PubMed] [Google Scholar]
- 142.Lip GY, Beevers DG. ABC of atrial fibrillation. History, epidemiology, and importance of atrial fibrillation. BMJ. 1995;311:1361–1363. doi: 10.1136/bmj.311.7016.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lip GY, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: A systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142:1489–1498. doi: 10.1378/chest.11-2888. [DOI] [PubMed] [Google Scholar]
- 144.Lip GY, Hee FL. Paroxysmal atrial fibrillation. QJM. 2001;94:665–678. doi: 10.1093/qjmed/94.12.665. [DOI] [PubMed] [Google Scholar]
- 145.Lip GY, Laroche C, Boriani G, Cimaglia P, Dan GA, Santini M, Kalarus Z, Rasmussen LH, Popescu MI, Tica O, Hellum CF, Mortensen B, Tavazzi L, Maggioni AP. Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: A report from the Euro Observational Research Programme Pilot survey on Atrial Fibrillation. Europace. 2014 doi: 10.1093/europace/euu155. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 146.Liu F, Levin MD, Petrenko NB, Lu MM, Wang T, Yuan LJ, Stout AL, Epstein JA, Patel VV. Histone-deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin. J Mol Cell Cardiol. 2008;45:715–723. doi: 10.1016/j.yjmcc.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lundby A, Ravn LS, Svendsen JH, Hauns S, Olesen SP, Schmitt N. KCNE3 mutation V17M identified in a patient with lone atrial fibrillation. Cell Physiol Biochem. 2008;21:47–54. doi: 10.1159/000113746. [DOI] [PubMed] [Google Scholar]
- 148.Lundby A, Ravn LS, Svendsen JH, Olesen SP, Schmitt N. KCNQ1 mutation Q147R is associated with atrial fibrillation and prolonged QT interval. Heart Rhythm. 2007;4:1532–1541. doi: 10.1016/j.hrthm.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 149.Luo X, Pan Z, Shan H, Xiao J, Sun X, Wang N, Lin H, Xiao L, Maguy A, Qi XY, Li Y, Gao X, Dong D, Zhang Y, Bai Y, Ai J, Sun L, Lu H, Luo XY, Wang Z, Lu Y, Yang B, Nattel S. MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest. 2013;123:1939–1951. doi: 10.1172/JCI62185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.MacCannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J. 2007;92:4121–4132. doi: 10.1529/biophysj.106.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Machida T, Hashimoto N, Kuwahara I, Ogino Y, Matsuura J, Yamamoto W, Itano Y, Zamma A, Matsumoto R, Kamon J, Kobayashi T, Ishiwata N, Yamashita T, Ogura T, Nakaya H. Effects of a highly selective acetylcholine-activated K+ channel blocker on experimental atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:94–102. doi: 10.1161/CIRCEP.110.951608. [DOI] [PubMed] [Google Scholar]
- 152.Maggioni AP, Latini R, Carson PE, Singh SN, Barlera S, Glazer R, Masson S, Cere E, Tognoni G, Cohn JN, Val-He FTI. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: Results from the Valsartan Heart Failure Trial (Val-HeFT) Am Heart J. 2005;149:548–557. doi: 10.1016/j.ahj.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 153.Mahabadi AA, Lehmann N, Kalsch H, Bauer M, Dykun I, Kara K, Moebus S, Jockel KH, Erbel R, Mohlenkamp S. Association of epicardial adipose tissue and left atrial size on non-contrast CT with atrial fibrillation: The Heinz Nixdorf Recall Study. Eur Heart J Cardiovasc Imaging. 2014;15:863–869. doi: 10.1093/ehjci/jeu006. [DOI] [PubMed] [Google Scholar]
- 154.Mancarella S, Yue Y, Karnabi E, Qu Y, El-Sherif N, Boutjdir M. Impaired Ca2+ homeostasis is associated with atrial fibrillation in the alpha1D L-type Ca2+ channel KO mouse. Am J Physiol Heart Circ Physiol. 2008;295:H2017–H2024. doi: 10.1152/ajpheart.00537.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–758. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 156.Mittal S, Ayati S, Stein KM, Schwartzman D, Cavlovich D, Tchou PJ, Markowitz SM, Slotwiner DJ, Scheiner MA, Lerman BB. Transthoracic cardioversion of atrial fibrillation: Comparison of rectilinear biphasic versus damped sine wave monophasic shocks. Circulation. 2000;101:1282–1287. doi: 10.1161/01.cir.101.11.1282. [DOI] [PubMed] [Google Scholar]
- 157.Moe GK, Abildskov JA, Rheinboldt WC. Computer model of atrial fibrillation. Am Heart J. 1964;67:200. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- 158.Muller FU, Lewin G, Baba HA, Boknik P, Fabritz L, Kirchhefer U, Kirchhof P, Loser K, Matus M, Neumann J, Riemann B, Schmitz W. Heart-directed expression of a human cardiac isoform of cAMP-response element modulator in transgenic mice. J Biol Chem. 2005;280:6906–6914. doi: 10.1074/jbc.M407864200. [DOI] [PubMed] [Google Scholar]
- 159.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 160.Nakajima H, Nakajima HO, Salcher O, Dittie AS, Dembowsky K, Jing S, Field LJ. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86:571–579. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]
- 161.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ, Miller JM. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 163.Nattel S, Dobrev D. The multidimensional role of calcium in atrial fibrillation pathophysiology: Mechanistic insights and therapeutic opportunities. Eur Heart J. 2012;33:1870–1877. doi: 10.1093/eurheartj/ehs079. [DOI] [PubMed] [Google Scholar]
- 164.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 165.Neuberger HR, Schotten U, Blaauw Y, Vollmann D, Eijsbouts S, van Hunnik A, Allessie M. Chronic atrial dilation, electrical remodeling, and atrial fibrillation in the goat. J Am Coll Cardiol. 2006;47:644–653. doi: 10.1016/j.jacc.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 166.Ng J, Villuendas R, Cokic I, Schliamser JE, Gordon D, Koduri H, Benefield B, Simon J, Murthy SN, Lomasney JW, Wasserstrom JA, Goldberger JJ, Aistrup GL, Arora R. Autonomic remodeling in the left atrium and pulmonary veins in heart failure: Creation of a dynamic substrate for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:388–396. doi: 10.1161/CIRCEP.110.959650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nguyen BL, Fishbein MC, Chen LS, Chen PS, Masroor S. Histopathological substrate for chronic atrial fibrillation in humans. Heart Rhythm. 2009;6:454–460. doi: 10.1016/j.hrthm.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nishida K, Nattel S. Atrial fibrillation compendium: Historical context and detailed translational perspective on an important clinical problem. Circ Res. 2014;114:1447–1452. doi: 10.1161/CIRCRESAHA.114.303466. [DOI] [PubMed] [Google Scholar]
- 169.Nishida K, Qi XY, Wakili R, Comtois P, Chartier D, Harada M, Iwasaki YK, Romeo P, Maguy A, Dobrev D, Michael G, Talajic M, Nattel S. Mechanisms of atrial tachyarrhythmias associated with coronary artery occlusion in a chronic canine model. Circulation. 2011;123:137–146. doi: 10.1161/CIRCULATIONAHA.110.972778. [DOI] [PubMed] [Google Scholar]
- 170.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJ, Rao SN, DiBella EV, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ogata T, Ueyama T, Isodono K, Tagawa M, Takehara N, Kawashima T, Harada K, Takahashi T, Shioi T, Matsubara H, Oh H. MURC, a muscle-restricted coiled-coil protein that modulates the Rho/ROCK pathway, induces cardiac dysfunction and conduction disturbance. Mol Cell Biol. 2008;28:3424–3436. doi: 10.1128/MCB.02186-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ogawa M, Zhou S, Tan AY, Song J, Gholmieh G, Fishbein MC, Luo H, Siegel RJ, Karagueuzian HS, Chen LS, Lin SF, Chen PS. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 173.Olesen MS, Bentzen BH, Nielsen JB, Steffensen AB, David JP, Jabbari J, Jensen HK, Haunso S, Svendsen JH, Schmitt N. Mutations in the potassium channel subunit KCNE1 are associated with early-onset familial atrial fibrillation. BMC Med Genet. 2012;13:24. doi: 10.1186/1471-2350-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Olesen MS, Refsgaard L, Holst AG, Larsen AP, Grubb S, Haunso S, Svendsen JH, Olesen SP, Schmitt N, Calloe K. A novel KCND3 gain-of-function mutation associated with early-onset of persistent lone atrial fibrillation. Cardiovasc Res. 2013;98:488–495. doi: 10.1093/cvr/cvt028. [DOI] [PubMed] [Google Scholar]
- 175.Olgin JE, Sih HJ, Hanish S, Jayachandran JV, Wu J, Zheng QH, Winkle W, Mulholland GK, Zipes DP, Hutchins G. Heterogeneous atrial denervation creates substrate for sustained atrial fibrillation. Circulation. 1998;98:2608–2614. doi: 10.1161/01.cir.98.23.2608. [DOI] [PubMed] [Google Scholar]
- 176.Olson TM, Alekseev AE, Liu XK, Park S, Zingman LV, Bienengraeber M, Sattiraju S, Ballew JD, Jahangir A, Terzic A. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 177.Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, Puu M, Yusuf S, Pfeffer MA, Investigators C. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: Results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47:1997–2004. doi: 10.1016/j.jacc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 178.Orgain ES, Wolff L, White PD. Uncomplicated auricular fibrillation and auricular flutter - frequent occurrence and good prognosis in patients without other evidence of cardiac disease. Arch Intern Med. 1936;57:493–513. [Google Scholar]
- 179.Ortiz J, Niwano S, Abe H, Rudy Y, Johnson NJ, Waldo AL. Mapping the conversion of atrial flutter to atrial fibrillation and atrial fibrillation to atrial flutter. Insights into mechanisms. Circ Res. 1994;74:882–894. doi: 10.1161/01.res.74.5.882. [DOI] [PubMed] [Google Scholar]
- 180.Page RL. beta-blockers for atrial fibrillation: Must we consider asymptomatic arrhythmias? J Am Coll Cardiol. 2000;36:147–150. doi: 10.1016/s0735-1097(00)00676-8. [DOI] [PubMed] [Google Scholar]
- 181.Page RL, Connolly SJ, Crijns HJ, van Eickels M, Gaudin C, Torp-Pedersen C, Hohnloser SH, Investigators A. Rhythm- and rate-controlling effects of dronedarone in patients with atrial fibrillation (from the ATHENA trial) Am J Cardiol. 2011;107:1019–1022. doi: 10.1016/j.amjcard.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 182.Page RL, Kerber RE, Russell JK, Trouton T, Waktare J, Gallik D, Olgin JE, Ricard P, Dalzell GW, Reddy R, Lazzara R, Lee K, Carlson M, Halperin B, Bardy GH, BiCard I. Biphasic versus monophasic shock waveform for conversion of atrial fibrillation: The results of an international randomized, double-blind multicenter trial. J Am Coll Cardiol. 2002;39:1956–1963. doi: 10.1016/s0735-1097(02)01898-3. [DOI] [PubMed] [Google Scholar]
- 183.Pandit SV, Berenfeld O, Anumonwo JM, Zaritski RM, Kneller J, Nattel S, Jalife J. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J. 2005;88:3806–3821. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 185.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 186.Pedersen OD, Bagger H, Kober L, Torp-Pedersen C. The occurrence and prognostic significance of atrial fibrillation/-flutter following acute myocardial infarction. TRACE Study group. TRAndolapril Cardiac Evalution. Eur Heart J. 1999;20:748–754. doi: 10.1053/euhj.1998.1352. [DOI] [PubMed] [Google Scholar]
- 187.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 189.Po SS, Nakagawa H, Jackman WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:1186–1189. doi: 10.1111/j.1540-8167.2009.01515.x. [DOI] [PubMed] [Google Scholar]
- 190.Power JM, Beacom GA, Alferness CA, Raman J, Wijffels M, Farish SJ, Burrell LM, Tonkin AM. Susceptibility to atrial fibrillation: A study in an ovine model of pacing-induced early heart failure. J Cardiovasc Electrophysiol. 1998;9:423–435. doi: 10.1111/j.1540-8167.1998.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 191.Qi XY, Yeh YH, Xiao L, Burstein B, Maguy A, Chartier D, Villeneuve LR, Brundel BJ, Dobrev D, Nattel S. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ Res. 2008;103:845–854. doi: 10.1161/CIRCRESAHA.108.175463. [DOI] [PubMed] [Google Scholar]
- 192.Rahme MM, Cotter B, Leistad E, Subudhayangkul S, Wadhwa M, Ungab G, Feld GK. Persistence of atrial fibrillation after its induction-importance of the duration and dispersion of atrial refractoriness and electrical remodeling. J Cardiovasc Pharmacol Ther. 1999;4:113–120. doi: 10.1177/107424849900400206. [DOI] [PubMed] [Google Scholar]
- 193.Rahmutula D, Marcus GM, Wilson EE, Ding CH, Xiao Y, Paquet AC, Barbeau R, Barczak AJ, Erle DJ, Olgin JE. Molecular basis of selective atrial fibrosis due to overexpression of transforming growth factor-beta1. Cardiovasc Res. 2013;99:769–779. doi: 10.1093/cvr/cvt074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ramos E, O’Leary ME. State-dependent trapping of flecainide in the cardiac sodium channel. J Physiol. 2004;560:37–49. doi: 10.1113/jphysiol.2004.065003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ravelli F, Allessie M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff-perfused rabbit heart. Circulation. 1997;96:1686–1695. doi: 10.1161/01.cir.96.5.1686. [DOI] [PubMed] [Google Scholar]
- 196.Ravn LS, Aizawa Y, Pollevick GD, Hofman-Bang J, Cordeiro JM, Dixen U, Jensen G, Wu Y, Burashnikov E, Haunso S, Guerchicoff A, Hu D, Svendsen JH, Christiansen M, Antzelevitch C. Gain of function in IKs secondary to a mutation in KCNE5 associated with atrial fibrillation. Heart Rhythm. 2008;5:427–435. doi: 10.1016/j.hrthm.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Reil JC, Hohl M, Selejan S, Lipp P, Drautz F, Kazakow A, Munz BM, Muller P, Steendijk P, Reil GH, Allessie MA, Bohm M, Neuberger HR. Aldosterone promotes atrial fibrillation. Eur Heart J. 2012;33:2098–2108. doi: 10.1093/eurheartj/ehr266. [DOI] [PubMed] [Google Scholar]
- 198.Remes J, van Brakel TJ, Bolotin G, Garber C, de Jong MM, van der Veen FH, Maessen JG. Persistent atrial fibrillation in a goat model of chronic left atrial overload. J Thorac Cardiovasc Surg. 2008;136:1005–1011. doi: 10.1016/j.jtcvs.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 199.Rensma PL, Allessie MA, Lammers WJEP, Bonke FIM, Schalij MJ. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res. 1988;62:395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- 200.Rienstra M, Van Veldhuisen DJ, Hagens VE, Ranchor AV, Veeger NJ, Crijns HJ, Van Gelder IC, Investigators R. Gender-related differences in rhythm control treatment in persistent atrial fibrillation: Data of the Rate Control Versus Electrical Cardioversion (RACE) study. J Am Coll Cardiol. 2005;46:1298–1306. doi: 10.1016/j.jacc.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 201.Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: Incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension, Age >75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J. 2008;156:57–64. doi: 10.1016/j.ahj.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 202.Roukoz H, Saliba W. Dofetilide: A new class III antiarrhythmic agent. Expert Rev Cardiovasc Ther. 2007;5:9–19. doi: 10.1586/14779072.5.1.9. [DOI] [PubMed] [Google Scholar]
- 203.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O’Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, Thibault B, Waldo AL, Atrial F, Congestive Heart Failure I Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 204.Saba S, Janczewski AM, Baker LC, Shusterman V, Gursoy EC, Feldman AM, Salama G, McTiernan CF, London B. Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-{alpha} Am J Physiol Heart Circ Physiol. 2005;289:H1456–H1467. doi: 10.1152/ajpheart.00733.2004. [DOI] [PubMed] [Google Scholar]
- 205.Sah VP, Minamisawa S, Tam SP, Wu TH, Dorn GW, II, Ross J, Jr., Chien KR, Brown JH. Cardiac-specific overexpression of RhoA results in sinus and atrioventricular nodal dysfunction and contractile failure. J Clin Invest. 1999;103:1627–1634. doi: 10.1172/JCI6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sampson KJ, Terrenoire C, Cervantes DO, Kaba RA, Peters NS, Kass RS. Adrenergic regulation of a key cardiac potassium channel can contribute to atrial fibrillation: Evidence from an I Ks transgenic mouse. J Physiol. 2008;586:627–637. doi: 10.1113/jphysiol.2007.141333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Savelieva I, Kakouros N, Kourliouros A, Camm AJ. Upstream therapies for management of atrial fibrillation: Review of clinical evidence and implications for European Society of Cardiology guidelines. Part I: Primary prevention. Europace. 2011;13:308–328. doi: 10.1093/europace/eur002. [DOI] [PubMed] [Google Scholar]
- 208.Sawaya SE, Rajawat YS, Rami TG, Szalai G, Price RL, Sivasubramanian N, Mann DL, Khoury DS. Downregulation of connexin40 and increased prevalence of atrial arrhythmias in transgenic mice with cardiac-restricted overexpression of tumor necrosis factor. Am J Physiol Heart Circ Physiol. 2007;292:H1561–H1567. doi: 10.1152/ajpheart.00285.2006. [DOI] [PubMed] [Google Scholar]
- 209.Scherlag BJ, Patterson E, Po SS. The neural basis of atrial fibrillation. J Electrocardiol. 2006;39:S180–S183. doi: 10.1016/j.jelectrocard.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 210.Schotten U, Duytschaever M, Ausma J, Eijsbouts S, Neuberger HR, Allessie M. Electrical and contractile remodeling during the first days of atrial fibrillation go hand in hand. Circulation. 2003;107:1433–1439. doi: 10.1161/01.cir.0000055314.10801.4f. [DOI] [PubMed] [Google Scholar]
- 211.Schotten U, Greiser M, Benke D, Buerkel K, Ehrenteidt B, Stellbrink C, Vazquez-Jimenez JF, Schoendube F, Hanrath P, Allessie M. Atrial fibrillation-induced atrial contractile dysfunction: A tachycardiomyopathy of a different sort. Cardiovasc Res. 2002;53:192–201. doi: 10.1016/s0008-6363(01)00453-9. [DOI] [PubMed] [Google Scholar]
- 212.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 213.Schwartz PJ, Locati EH, Moss AJ, Crampton RS, Trazzi R, Ruberti U. Left cardiac sympathetic denervation in the therapy of congenital long QT syndrome. A worldwide report. Circulation. 1991;84:503–511. doi: 10.1161/01.cir.84.2.503. [DOI] [PubMed] [Google Scholar]
- 214.Shan J, Xie W, Betzenhauser M, Reiken S, Chen BX, Wronska A, Marks AR. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2012;111:708–717. doi: 10.1161/CIRCRESAHA.112.273342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43:483–490. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 216.Sharma PL. Mechanism of atrial flutter and fibrillation induced by aconitine in the dog, with observations on the role of cholinegic factors. Br J Pharmacol Chemother. 1963;21:368–377. doi: 10.1111/j.1476-5381.1963.tb01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shen MJ, Choi EK, Tan AY, Han S, Shinohara T, Maruyama M, Chen LS, Shen C, Hwang C, Lin SF, Chen PS. Patterns of baseline autonomic nerve activity and the development of pacing-induced sustained atrial fibrillation. Heart Rhythm. 2011;8:583–589. doi: 10.1016/j.hrthm.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shen MJ, Hao-Che C, Park HW, George Akingba A, Chang PC, Zheng Z, Lin SF, Shen C, Chen LS, Chen Z, Fishbein MC, Chiamvimonvat N, Chen PS. Low-level vagus nerve stimulation upregulates small conductance calcium-activated potassium channels in the stellate ganglion. Heart Rhythm. 2013;10:910–915. doi: 10.1016/j.hrthm.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shen MJ, Shinohara T, Park HW, Frick K, Ice DS, Choi EK, Han S, Maruyama M, Sharma R, Shen C, Fishbein MC, Chen LS, Lopshire JC, Zipes DP, Lin SF, Chen PS. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation. 2011;123:2204–2212. doi: 10.1161/CIRCULATIONAHA.111.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shi Y, Li D, Tardif JC, Nattel S. Enalapril effects on atrial remodeling and atrial fibrillation in experimental congestive heart failure. Cardiovasc Res. 2002;54:456–461. doi: 10.1016/s0008-6363(02)00243-2. [DOI] [PubMed] [Google Scholar]
- 221.Shimano M, Tsuji Y, Inden Y, Kitamura K, Uchikawa T, Harata S, Nattel S, Murohara T. Pioglitazone, a peroxisome proliferator-activated receptor-gamma activator, attenuates atrial fibrosis and atrial fibrillation promotion in rabbits with congestive heart failure. Heart Rhythm. 2008;5:451–459. doi: 10.1016/j.hrthm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 222.Shiroshita-Takeshita A, Sakabe M, Haugan K, Hennan JK, Nattel S. Model-dependent effects of the gap junction conduction-enhancing antiarrhythmic peptide rotigaptide (ZP123) on experimental atrial fibrillation in dogs. Circulation. 2007;115:310–318. doi: 10.1161/CIRCULATIONAHA.106.665547. [DOI] [PubMed] [Google Scholar]
- 223.Sideris DA, Toumanidis ST, Thodorakis M, Kostopoulos K, Tselepatiotis E, Langoura C, Stringli T, Moulopoulos SD. Some observations on the mechanism of pressure related atrial fibrillation. Eur Heart J. 1994;15:1585–1589. doi: 10.1093/oxfordjournals.eurheartj.a060433. [DOI] [PubMed] [Google Scholar]
- 224.Sinno H, Derakhchan K, Libersan D, Merhi Y, Leung TK, Nattel S. Atrial ischemia promotes atrial fibrillation in dogs. Circulation. 2003;107:1930–1936. doi: 10.1161/01.CIR.0000058743.15215.03. [DOI] [PubMed] [Google Scholar]
- 225.Siu CW, Lau CP, Tse HF. Prevention of atrial fibrillation recurrence by statin therapy in patients with lone atrial fibrillation after successful cardioversion. Am J Cardiol. 2003;92:1343–1345. doi: 10.1016/j.amjcard.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 226.Smith TW. Digitalis. Mechanisms of action and clinical use. N Engl J Med. 1988;318:358–365. doi: 10.1056/NEJM198802113180606. [DOI] [PubMed] [Google Scholar]
- 227.Solti F, Vecsey T, Kekesi V, Juhasz-Nagy A. The effect of atrial dilatation on the genesis of atrial arrhythmias. Cardiovasc Res. 1989;23:882–886. doi: 10.1093/cvr/23.10.882. [DOI] [PubMed] [Google Scholar]
- 228.Sood S, Chelu MG, van Oort RJ, Skapura D, Santonastasi M, Dobrev D, Wehrens XH. Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm. 2008;5:1047–1054. doi: 10.1016/j.hrthm.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sorokin AV, Araujo CG, Zweibel S, Thompson PD. Atrial fibrillation in endurance-trained athletes. Br J Sports Med. 2011;45:185–188. doi: 10.1136/bjsm.2009.057885. [DOI] [PubMed] [Google Scholar]
- 230.Spach MS, Heidlage JF, Barr RC, Dolber PC. Cell size and communication: Role in structural and electrical development and remodeling of the heart. Heart Rhythm. 2004;1:500–515. doi: 10.1016/j.hrthm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 231.Stambler BS, Fenelon G, Shepard RK, Clemo HF, Guiraudon CM. Characterization of sustained atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. J Cardiovasc Electrophysiol. 2003;14:499–507. doi: 10.1046/j.1540-8167.2003.02519.x. [DOI] [PubMed] [Google Scholar]
- 232.Sumitomo N, Sakurada H, Taniguchi K, Matsumura M, Abe O, Miyashita M, Kanamaru H, Karasawa K, Ayusawa M, Fukamizu S, Nagaoka I, Horie M, Harada K, Hiraoka M. Association of atrial arrhythmia and sinus node dysfunction in patients with catecholaminergic polymorphic ventricular tachycardia. Circ J. 2007;71:1606–1609. doi: 10.1253/circj.71.1606. [DOI] [PubMed] [Google Scholar]
- 233.Sun Y, Ramires FJ, Weber KT. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc Res. 1997;35:138–147. doi: 10.1016/s0008-6363(97)00097-7. [DOI] [PubMed] [Google Scholar]
- 234.Swissa M, Zhou S, Paz O, Fishbein MC, Chen LS, Chen PS. Canine model of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia. Am J Physiol Heart Circ Physiol. 2005;289:H1851–H1857. doi: 10.1152/ajpheart.00083.2005. [DOI] [PubMed] [Google Scholar]
- 235.Tan AY, Zhou S, Ogawa M, Song J, Chu M, Li H, Fishbein MC, Lin SF, Chen LS, Chen PS. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Temple J, Frias P, Rottman J, Yang T, Wu Y, Verheijck EE, Zhang W, Siprachanh C, Kanki H, Atkinson JB, King P, Anderson ME, Kupershmidt S, Roden DM. Atrial fibrillation in KCNE1-null mice. Circ Res. 2005;97:62–69. doi: 10.1161/01.RES.0000173047.42236.88. [DOI] [PubMed] [Google Scholar]
- 237.Thibodeau IL, Xu J, Li Q, Liu G, Lam K, Veinot JP, Birnie DH, Jones DL, Krahn AD, Lemery R, Nicholson BJ, Gollob MH. Paradigm of genetic mosaicism and lone atrial fibrillation: Physiological characterization of a connexin 43-deletion mutant identified from atrial tissue. Circulation. 2010;122:236–244. doi: 10.1161/CIRCULATIONAHA.110.961227. [DOI] [PubMed] [Google Scholar]
- 238.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engel-hardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 239.Tieleman RG, Blaauw Y, Van Gelder IC, De Langen CD, de Kam PJ, Grandjean JG, Patberg KW, Bel KJ, Allessie MA, Crijns HJ. Digoxin delays recovery from tachycardia-induced electrical remodeling of the atria. Circulation. 1999;100:1836–1842. doi: 10.1161/01.cir.100.17.1836. [DOI] [PubMed] [Google Scholar]
- 240.Tsai CT, Chiang FT, Tseng CD, Hwang JJ, Kuo KT, Wu CK, Yu CC, Wang YC, Lai LP, Lin JL. Increased expression of mineralocorticoid receptor in human atrial fibrillation and a cellular model of atrial fibrillation. J Am Coll Cardiol. 2010;55:758–770. doi: 10.1016/j.jacc.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 241.van der Velden HM, Ausma J, Rook MB, Hellemons AJ, van Veen TA, Allessie MA, Jongsma HJ. Gap junctional remodeling in relation to stabilization of atrial fibrillation in the goat. Cardiovasc Res. 2000;46:476–486. doi: 10.1016/s0008-6363(00)00026-2. [DOI] [PubMed] [Google Scholar]
- 242.Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 243.Vaseghi M, Lux RL, Mahajan A, Shivkumar K. Sympathetic stimulation increases dispersion of repolarization in humans with myocar-dial infarction. Am J Physiol Heart Circ Physiol. 2012;302:H1838–H1846. doi: 10.1152/ajpheart.01106.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Verheule S, Sato T, Everett Tt, Engle SK, Otten D, Rubart-von der Lohe M, Nakajima HO, Nakajima H, Field LJ, Olgin JE. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94:1458–1465. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR. Defective cardiac ryan-odine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- 246.Voigt N, Friedrich A, Bock M, Wettwer E, Christ T, Knaut M, Strasser RH, Ravens U, Dobrev D. Differential phosphorylation-dependent regulation of constitutively active and muscarinic receptor-activated IK,ACh channels in patients with chronic atrial fibrillation. Cardiovasc Res. 2007;74:426–437. doi: 10.1016/j.cardiores.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 247.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–156. doi: 10.1161/CIRCULATIONAHA.113.006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Voigt N, Maguy A, Yeh YH, Qi X, Ravens U, Dobrev D, Nattel S. Changes in I K, ACh single-channel activity with atrial tachycardia remodelling in canine atrial cardiomyocytes. Cardiovasc Res. 2008;77:35–43. doi: 10.1093/cvr/cvm051. [DOI] [PubMed] [Google Scholar]
- 249.Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlof B, Ibsen H, Julius S, Kjeldsen SE, Lindholm LH, Nieminen MS, Devereux RB. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: The Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:712–719. doi: 10.1016/j.jacc.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 250.Wakili R, Voigt N, Kaab S, Dobrev D, Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest. 2011;121:2955–2968. doi: 10.1172/JCI46315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wakili R, Yeh YH, Yan Qi X, Greiser M, Chartier D, Nishida K, Maguy A, Villeneuve LR, Boknik P, Voigt N, Krysiak J, Kaab S, Ravens U, Linke WA, Stienen GJ, Shi Y, Tardif JC, Schotten U, Dobrev D, Nattel S. Multiple potential molecular contributors to atrial hypocontractility caused by atrial tachycardia remodeling in dogs. Circ Arrhythm Electrophysiol. 2010;3:530–541. doi: 10.1161/CIRCEP.109.933036. [DOI] [PubMed] [Google Scholar]
- 252.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 253.Wilke T, Groth A, Mueller S, Pfannkuche M, Verheyen F, Linder R, Maywald U, Bauersachs R, Breithardt G. Incidence and prevalence of atrial fibrillation: An analysis based on 8.3 million patients. Europace. 2013;15:486–493. doi: 10.1093/europace/eus333. [DOI] [PubMed] [Google Scholar]
- 254.Williams RS, deLemos JA, Dimas V, Reisch J, Hill JA, Naseem RH. Effect of spironolactone on patients with atrial fibrillation and structural heart disease. Clinical cardiology. 2011;34:415–419. doi: 10.1002/clc.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wolff L. Familial auricular fibrillation. New Engl J Med. 1943;229:396–398. [Google Scholar]
- 256.Woods CE, Olgin J. Atrial fibrillation therapy now and in the future: Drugs, biologicals, and ablation. Circ Res. 2014;114:1532–1546. doi: 10.1161/CIRCRESAHA.114.302362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD, Atrial Fibrillation Follow-up Investigation of Rhythm Management I A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 258.Xia M, Jin Q, Bendahhou S, He Y, Larroque MM, Chen Y, Zhou Q, Yang Y, Liu Y, Liu B, Zhu Q, Zhou Y, Lin J, Liang B, Li L, Dong X, Pan Z, Wang R, Wan H, Qiu W, Xu W, Eurlings P, Barhanin J, Chen Y. A Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun. 2005;332:1012–1019. doi: 10.1016/j.bbrc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 259.Xiao HD, Fuchs S, Campbell DJ, Lewis W, Dudley SC, Jr., Kasi VS, Hoit BD, Keshelava G, Zhao H, Capecchi MR, Bernstein KE. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165:1019–1032. doi: 10.1016/S0002-9440(10)63363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yang T, Snyders DJ, Roden DM. Ibutilide, a methanesulfonanilide antiarrhythmic, is a potent blocker of the rapidly activating delayed rectifier K+ current (IKr) in AT-1 cells. Concentration-, time-, voltage-, and use-dependent effects. Circulation. 1995;91:1799–1806. doi: 10.1161/01.cir.91.6.1799. [DOI] [PubMed] [Google Scholar]
- 261.Yang T, Yang P, Roden DM, Darbar D. Novel KCNA5 mutation implicates tyrosine kinase signaling in human atrial fibrillation. Heart Rhythm. 2010;7:1246–1252. doi: 10.1016/j.hrthm.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yang Y, Li J, Lin X, Yang Y, Hong K, Wang L, Liu J, Li L, Yan D, Liang D, Xiao J, Jin H, Wu J, Zhang Y, Chen YH. Novel KCNA5 loss-of-function mutations responsible for atrial fibrillation. J Human Gen. 2009;54:277–283. doi: 10.1038/jhg.2009.26. [DOI] [PubMed] [Google Scholar]
- 263.Yang Y, Xia M, Jin Q, Bendahhou S, Shi J, Chen Y, Liang B, Lin J, Liu Y, Liu B, Zhou Q, Zhang D, Wang R, Ma N, Su X, Niu K, Pei Y, Xu W, Chen Z, Wan H, Cui J, Barhanin J, Chen Y. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am J Hum Genet. 2004;75:899–905. doi: 10.1086/425342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Youn JY, Zhang J, Zhang Y, Chen H, Liu D, Ping P, Weiss JN, Cai H. Oxidative stress in atrial fibrillation: An emerging role of NADPH oxidase. J Mol Cell Cardiol. 2013;62:72–79. doi: 10.1016/j.yjmcc.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yu L, Scherlag BJ, Li S, Fan Y, Dyer J, Male S, Varma V, Sha Y, Stavrakis S, Po SS. Low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve: A noninvasive approach to treat the initial phase of atrial fibrillation. Heart Rhythm. 2013;10:428–435. doi: 10.1016/j.hrthm.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 266.Yu L, Scherlag BJ, Li S, Sheng X, Lu Z, Nakagawa H, Zhang Y, Jackman WM, Lazzara R, Jiang H, Po SS. Low-level vagosympathetic nerve stimulation inhibits atrial fibrillation inducibility: Direct evidence by neural recordings from intrinsic cardiac ganglia. J Cardiovasc Electrophysiol. 2011;22:455–463. doi: 10.1111/j.1540-8167.2010.01908.x. [DOI] [PubMed] [Google Scholar]
- 267.Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- 268.Yue L, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 2011;89:744–753. doi: 10.1093/cvr/cvq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zhabyeyev P, Hiess F, Wang R, Liu Y, Wayne Chen SR, Oudit GY. S4153R is a gain-of-function mutation in the cardiac Ca(2+) release channel ryanodine receptor associated with catecholaminergic poly morphic ventricular tachycardia and paroxysmal atrial fibrillation. Can J Cardiol. 2013;29:993–996. doi: 10.1016/j.cjca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 270.Zhang W, Ma X, Zhong M, Zheng Z, Li L, Wang Z, Zhang Y. Role of the calpain system in pulmonary vein connexin remodeling in dogs with atrial fibrillation. Cardiology. 2009;112:22–30. doi: 10.1159/000137694. [DOI] [PubMed] [Google Scholar]
- 271.Zhang X, Chen S, Yoo S, Chakrabarti S, Zhang T, Ke T, Oberti C, Yong SL, Fang F, Li L, de la Fuente R, Wang L, Chen Q, Wang QK. Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell. 2008;135:1017–1027. doi: 10.1016/j.cell.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 272.Zhang Y, Wang Z, Zhang Y, Wang W, Wang J, Gao M, Hou Y. Efficacy of cardiac autonomic denervation for atrial fibrillation: A meta-analysis. J Cardiovasc Electrophysiol. 2012;23:592–600. doi: 10.1111/j.1540-8167.2011.02270.x. [DOI] [PubMed] [Google Scholar]
- 273.Zhang Z, He Y, Tuteja D, Xu D, Timofeyev V, Zhang Q, Glatter KA, Xu Y, Shin HS, Low R, Chiamvimonvat N. Functional roles of Cav1.3(alpha1D) calcium channels in atria: Insights gained from gene-targeted null mutant mice. Circulation. 2005;112:1936–1944. doi: 10.1161/CIRCULATIONAHA.105.540070. [DOI] [PubMed] [Google Scholar]
- 274.Zhao J, Li J, Li W, Li Y, Shan H, Gong Y, Yang B. Effects of spironolactone on atrial structural remodelling in a canine model of atrial fibrillation produced by prolonged atrial pacing. Br J Pharmacol. 2010;159:1584–1594. doi: 10.1111/j.1476-5381.2009.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zheng S, Li Y, Han J, Zhang H, Zeng W, Xu C, Jia Y, Wang J, Guo K, Jiao Y, Meng X. Long-term results of a minimally invasive surgical pulmonary vein isolation and ganglionic plexi ablation for atrial fibrillation. PLoS One. 2013;8:e79755. doi: 10.1371/journal.pone.0079755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation. 2012;125:381–389. doi: 10.1161/CIRCULATIONAHA.111.019927. [DOI] [PubMed] [Google Scholar]
- 277.Zlochiver S, Munoz V, Vikstrom KL, Taffet SM, Berenfeld O, Jalife J. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophys Jl. 2008;95:4469–4480. doi: 10.1529/biophysj.108.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zou R, Kneller J, Leon LJ, Nattel S. Substrate size as a determinant of fibrillatory activity maintenance in a mathematical model of canine atrium. Am J Physiol Heart Circ Physiol. 2005;289:H1002–H1012. doi: 10.1152/ajpheart.00252.2005. [DOI] [PubMed] [Google Scholar]


