Abstract
The human-adapted organism Neisseria gonorrhoeae is the causative agent of gonorrhea a sexually transmitted infection. It readily colonizes the genital, rectal, and nasalpharyngal mucosa during infection. While it is well-established that N. gonorrhoeae recruits and modulates the functions of polymorphonuclear leukocytes (PMNs) during infection, how N. gonorrhoeae interacts with macrophages present in infected tissue is not fully defined. We studied the interactions of N. gonorrhoeae with two human monocytic cell lines, THP-1 and U937, and primary monocytes, all differentiated into macrophages. Most engulfed bacteria were killed in the phagolysosome, but a subset of bacteria were able to survive and replicate inside the macrophages suggesting that those cells may be an unexplored cellular reservoir for N. gonorrhoeae during infection. N. gonorrhoeae was able to modulate macrophage apoptosis, N. gonorrhoeae induced apoptosis in THP-1 cells whereas it inhibited induced apoptosis in U937 cells and primary human macrophages. Furthermore, N. gonorrhoeae induced expression of inflammatory cytokines in macrophages, suggesting a role for macrophages in recruiting PMNs to the site of infection. These results indicate macrophages may serve as a significant replicative niche for N. gonorrhoeae and play an important role in gonorrheal pathogenesis.
Keywords: Neisseria gonorrhoeae, gonorrhea, macrophages, apoptosis, cytokines
Introduction
The Gram-negative bacterium Neisseria gonorrhoeae is the causative agent of gonorrhea, a sexually transmitted infection that affects more than 100 million people annually (WHO, 2012). This exclusive human pathogen primarily infects the urogenital tract often giving rise to local inflammation. Dissemination from the local site of infection can also occur and lead to pelvic inflammatory disease, dermatitis, endocarditis and arthritis (reviewed in Hook et al., 1985). As an exclusive human pathogen with no known alternative reservoir, N. gonorrhoeae has evolved strategies to promote growth and persistence in the host by modulating the host innate and adaptive immune systems. Symptomatic N. gonorrhoeae infection triggers an inflammatory response driven by large numbers of polymorphonuclear leukocytes (PMNs) and the role of PMNs in N. gonorrhoeae pathogenesis has been extensively investigated. These studies suggest that some N. gonorrhoeae survive and replicate within PMNs, notably by modulating their ability to phagocytose and release antimicrobial components (reviewed by Johnson et al., 2011, Criss et al., 2012).
The genitourinary mucosae also contains significant populations of macrophages, supporting the idea that N. gonorrhoeae encounter these cells during infection (Givan et al., 1997). Moreover acute gonorrhea is characterized by an intense inflammatory exudate that contains macrophages, exfoliated epithelial cells, and numerous PMNs (Hook, 1999). Therefore, it is likely that macrophages have an important role in the pathogenesis of N. gonorrhoeae infections. There is little consensus concerning the survival of N. gonorrhoeae inside the macrophages. Ota et al., (Ota et al., 1975) suggested that N. gonorrhoeae may be capable of intracellular survival within murine macrophages, whereas Cooper and Floyd (Cooper et al., 1982) showed that N. gonorrhoeae phagocytosed by mouse macrophages were completely killed after 30 min of incubation. A more recent study shows that after macrophage internalization the number of N. gonorrhoeae rapidly decreases at early time points but, importantly, a portion of bacteria were able to survive intracellularly over a more prolonged period (Leuzzi et al., 2005). This study was consistent with another report that suggested that some intracellular N. gonorrhoeae can survive inside macrophages (Zughaier et al., 2014).
One common strategy used by microbial pathogens to establish infection in human hosts is the modulation of apoptosis. Apoptotic cells are characterized by distinct morphological and chemical changes that results from signaling cascades initiated through two distinct pathways, the extrinsic and intrinsic pathways (for a review, Elmore, 2007). Engagement of death receptors on the cell surface leads to activation of the extrinsic apoptotic pathway, whereas the intrinsic pathway is activated by intracellular events that cause mitochondrial release of cytochrome c into the cytoplasm. Both pathways result in caspase cleavage and DNA fragmentation. The effect of N. gonorrhoeae infection on apoptosis of different host cells has been studied extensively, but there are conflicting reports. N. gonorrhoeae has been shown to induce apoptosis in epithelial cells (Muller et al., 1999, Muller et al., 2000, Kepp et al., 2007, Kepp et al., 2009), but other studies have shown N. gonorrhoeae to have no effect on apoptosis in the same cell type (Massari et al., 2003). N. gonorrhoeae can inhibit apoptosis induced by staurosporine (STS) in epithelial cells and HL-60 cells (Binnicker et al., 2003, Howie et al., 2008, Follows et al., 2009, Chen et al., 2011). N. gonorrhoeae also inhibits spontaneous apoptosis in primary human PMNs and HL-60 cells (Simons et al., 2006, Chen et al., 2011). The reasons for the divergence in the observed experimental results are unknown, but may be due to the use of different N. gonorrhoeae strains, host cell lines, or infection conditions (Massari et al., 2003). The mechanisms by which N. gonorrhoeae induces and/or inhibits apoptosis have been explored mainly in epithelial cells and PMNs, but nothing is known about the mechanisms used within macrophages, and whether N. gonorrhoeae utilizes similar mechanisms of apoptosis regulation remains to be determined.
In this study, we used the two most common differentiated cell lines, U937 and THP-1 cells as models to study the interaction of N. gonorrhoeae with human macrophages. We demonstrate that N. gonorrhoeae is able to survive in association with these macrophage cell lines and that a subset of intracellular bacteria can survive intracellularly. We also show that during infection N. gonorrhoeae modulates apoptosis in macrophages with the outcome differing in the two cell lines used: N. gonorrhoeae induced apoptosis in THP-1 cells but protected against STS- and TNF-α-induced apoptosis in U937 cells. The phenotypes observed in U937 cells were replicated in primary human macrophages. N. gonorrhoeae also induced the secretion of many cytokines from infected macrophages suggesting that N. gonorrhoeae interactions with macrophages could be an instrumental step in the induction of the inflammatory response characteristic of symptomatic gonorrhea. All of these interactions between N. gonorrhoeae and macrophages strongly support the hypothesis that macrophages are an important innate cell type involved in the pathogenesis of gonorrhea.
Results
N. gonorrhoeae survives in presence of U937 and THP-1 macrophages
The survival of N. gonorrhoeae in association with differentiated THP-1 and U937 cells was examined using viable counts (CFUs). N. gonorrhoeae grew better when associated with the macrophage cell lines than in RPMI medium alone, a medium in which the bacteria can replicate (Fig. 1A). While N. gonorrhoeae viable counts tended to decrease slightly over time in PBSG medium alone since this medium does not support growth, bacteria incubated in the presence of U937 and THP-1 cells in PBSG medium for 7h grew an average of 139- and 40-fold, respectively (Fig. 1A).
Figure 1. N. gonorrhoeae is able to survive and replicate in association with U937 and THP-1 macrophages.
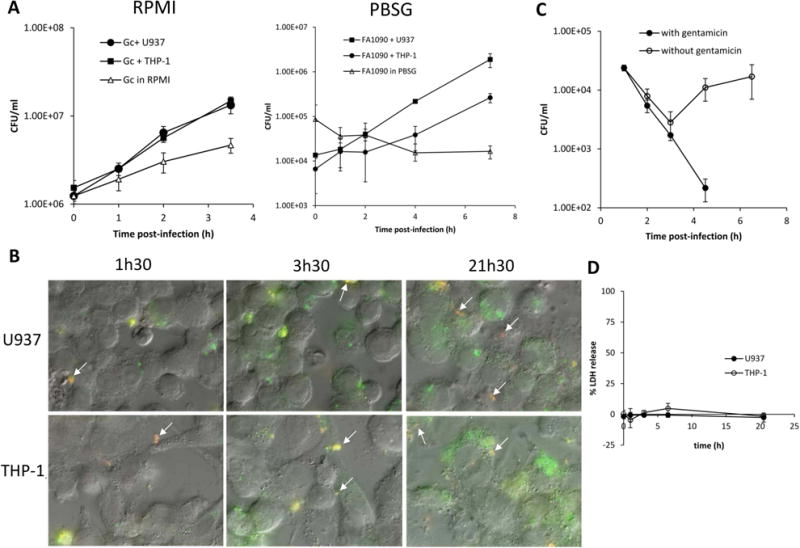
(A) N. gonorrhoeae survives and replicates in association with U937 and THP-1 macrophages. Differentiated U937 and THP-1 cells were challenged with FA1090 at an MOI of 1 and viable CFU/ml was enumerated at various times post-infection. Data are represented as the mean ± SD for 3 independent replicates and are representative of 3 (RPMI) and 2 (PBSG) independent experiments. (B) N. gonorrhoeae associated with U937 and THP-1 cells are both intra- and extracellular. Differentiated U937 and THP-1 cells were challenged at an MOI of 1 and intra-and extracellular bacteria were differentiated using fluorescent microscopy. Extracellular bacteria appear red and yellow (some are indicated with arrows), and intracellular bacteria appear green. (C) N. gonorrhoeae survival in U937 cells. Differentiated U937 cells were challenged for 1.5h with FA1090 at an MOI of 23. After washing, challenged cells were incubated in fresh medium with gentamicin 200 μg/ml for the entire duration of the experiment (with gentamicin, black circle) or 1h before new washing and incubation with fresh medium without gentamicin (open circle). Viable CFU/ml were enumerated at various times post-infection, and the data are represented as the means ± SD for three independent replicates and are representative of two independent experiments. (D) N. gonorrhoeae does not induce LDH release in U937 and THP-1 macrophages. U937 and THP-1 differentiated cell were challenged with FA1090 at a MOI of two and host cell lysis was assessed by measuring release of cytosolic LDH into the supernatant at different times post infection. Data shown are mean ± SD calculated from three replicates and are representative of three independent experiments.
To establish whether the U937 and THP-1 cells bind and phagocytose N. gonorrhoeae, association of bacteria with challenged cells was monitored by differential immunofluorescence of intra- and extra-cellular bacteria (Fig. 1B). After 1.5h, 3.5h and 21.5h of infection, there were both extra- and intra-cellular bacteria associated with the cells. Phagocytosis occurred rapidly as intra-cellular bacteria could be observed as early as 30 min after infection, and when phagocytosis is blocked by pretreatment of the cells with cytochalasin D, no intra-cellular bacteria are detected (data not shown). Furthermore, an increase of macrophage internalized bacteria was observed over the duration of the experiment in both RPMI and PBSG medium (Fig. 1B). Based on the rapid entrance of the bacteria into the macrophages, we conclude that macrophages can phagocytose N. gonorrhoeae without opsonization. We cannot from these analyses determine whether some bacteria can enter using an alternative mechanism from phagocytosis.
To examine bacterial survival within macrophage cell lines, U937 and THP-1 cells were challenged with N. gonorrhoeae at a multiplicity of infection (MOI) of 23 in RPMI medium for 1.5h, and extracellular bacteria were killed by gentamicin treatment. After removal of gentamicin and incubation in fresh media, the number of viable bacteria remaining in association with macrophages was determined at various times (Fig. 1C, data not shown for THP-1). 1% (±0.2) of the total inoculum was internalized in U937 macrophages after 3h of infection. The number of bacteria associated with both cell types decreased initially; however, 3h after the removal of gentamicin, the number of CFUs increased, suggesting that the bacteria were able to replicate in association with the cells. When gentamicin was maintained in the culture medium for the entire duration of the experiment, no viable N. gonorrhoeae were detected after 6h. Two possible explanations for these results are that the macrophages are becoming compromised by the infection to allow gentamicin access to intracellular bacteria, or that N. gonorrhoeae are escaping from the macrophages and were killed extracellularly by the antibiotic.
It was possible that N. gonorrhoeae was being killed by the antibiotic because of disruption of the host cell membrane. To test this hypothesis, cytoplasmic lactate dehydrogenase (LDH) release that occurs upon cell lysis was measured (Fig. 1D). The percentage of LDH release was near zero indicating that N. gonorrhoeae does not cause extensive lysis of U937 or THP-1 cells. The absence of cell lysis was also confirmed with propidium iodide exclusion staining of challenged U937 cells, which showed that less than 1% of challenged cells had disrupted cell membranes (data not shown). These results suggest that N. gonorrhoeae does not induce overt membrane damage during the times studied. Therefore, we reason that the bacteria must be exiting the macrophage cells without lysis. Moreover, treatment of the macrophage cell with the actin polymerization inhibitor cytochalasin D did not inhibit N. gonorrhoeae growth indicating that actin polymerization had no role in bacterial exit from macrophages (data not shown). These results show that N. gonorrhoeae is phagocytosed by U937 and THP-1 differentiated cells and N. gonorrhoeae is able to survive within and potentially escape from these phagocytes.
N. gonorrhoeae can survive and replicate inside macrophages
Although N. gonorrhoeae was unable to expand in PBSG medium alone, it was able to grow in presence of macrophages in PBSG, expand greater in RPMI (Fig 1A) and increase intracellularly (Fig 1B). While these results could reflect bacterial replication within the macrophages, it was possible that continual uptake of bacteria from the medium could contribute to the increased internalized bacteria. We therefore examined the number of intracellular bacteria per macrophage over time by fluorescence microscopy, treating the macrophages with cytochalasin D after infection to block additional phagocytosis. The percentage of macrophages containing more than 10 bacteria increased over time whereas the percentage of macrophages with one to five bacteria decreased (Fig. 2A), indicating that the internalized bacteria were able to survive and replicate inside the macrophage.
Figure 2. A portion of N. gonorrhoeae is able to survive and replicate inside macrophages in nonacidic compartment.
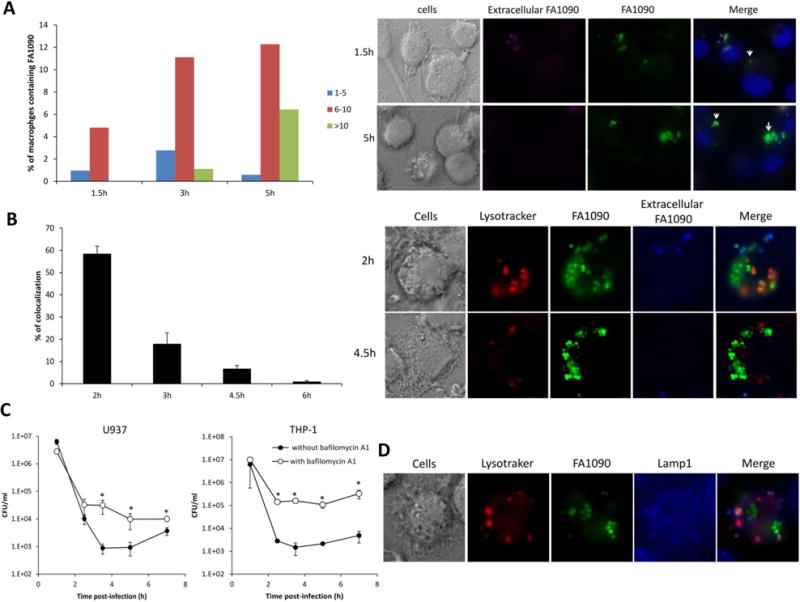
(A) Quantification of bacterial loads in U937 macrophages over time. U937 macrophages incubated with GFP-expressing FA1090 (MOI 5) for 1.5h were washed and further incubated with cytochalasin D to block new phagocytosis of extracellular bacteria. At 1.5h, 3 and 5 h after infection, cells were fixed, extracellular bacteria stained with Alexa Fluor 647-coupled soybean agglutinin, and the number of bacteria per macrophage was analyzed by fluorescence microscopy. Data shown on the left panel are representative of 3 independent experiments. Representative images at time 1.5h and 5h after infection are shown on the right panel, extracellular bacteria appear green and purple, and intracellular bacteria appear green. (B) Time course of N. gonorrhoeae colocalization with the acidotropic dye Lysotracker. GFP-expressing FA1090 were incubated with U937 macrophages (MOI 5 to 15) for 1h. After being washed, challenged macrophages were incubated with cytochalasin D before Lysotracker staining at 2h, 3h, 4.5h and 6h. Data shown on the left panel are means ± SD calculated from 3 independent experiments. Representative images at time 2h and 4.5h after infection are shown on the right panel, cells are stained with Lysotracker red, extracellular bacteria appear green and blue, and intracellular bacteria appear green. (C) N. gonorrhoeae survival in U937 and THP-1 macrophages in presence of bafilomycin A1. Differentiated U937 and THP-1 cells, pretreated with bafilomycin A1 or not, incubated with FA1090 (MOI 20) for 1h were washed and further incubated with cytochalasin D and gentamicin for 1.5h. After those 1.5h, the cells were washed and incubated in fresh medium with cytochalasin D. Viable CFU/ml were enumerated at that times shown. Data shown are means ± SD calculated from three replicates and are representative of three independent experiments. Values that are significantly different (P<0.05) as determined by the Two-tailed, unpaired Student t tests are indicated with an asterisk. (D) Characterization of N. gonorrhoeae containing phagosome. Differentiated U937 cells incubated with GFP-expressing FA1090 (MOI 38) for 1h were washed and further incubated with cyochalasin D before Lysotraker red and LAMP-1 (blue) staining 4h after infection.
To better understand the time course of events involved in intracellular survival and replication of N. gonorrhoeae, we analyzed the bacterium-containing phagosomes. We first evaluated N. gonorrhoeae colocalization with fluorescent microscopy using the acidotropic dye Lysotracker at different time points. After 2h of infection, 58.5% ± 3.4% of the bacteria colocalized with acidic organelles (Fig 2B), demonstrating that bacterial phagosomes fuse with lysosomes at early stages of infection. However, after 3h of infection, coincident with the increase in the number of bacteria inside the macrophage (Fig 2A), most of the bacteria did not colocalize with the acidotropic dye. Furthermore, the decrease of Lysotraker red colocalization with N. gonorrhoeae during the time course of infection suggests that bacteria do not survive within this acidic compartment. In the presence of the acidification inhibitor Bafilomycin A1, the survival of N. gonorrhoeae in macrophages increased compared to no inhibitor (Fig 2C). Interestingly, N. gonorrhoeae that colocalized with acidotropic dye also colocalized with the late endosome marker LAMP-1, and the bacteria that were not colocalized with Lysotracker were also not associated with LAMP-1 (Fig. 2D). The Neisseria IgA1 protease has been described to cleave LAMP-1 and promote survival of bacteria within epithelial cells (Lin et al., 1997). There was no difference in the survival of an igA1 mutant and the parental strain in U937 cells (data not shown). Finally, transmission electron microscopy of U937 cells challenged with N. gonorrhoeae showed a subset of the internalized bacteria had no obvious surrounding membrane suggesting they can escape either from the phagolysosome or from the vacuole after entering the cell by an active process initiated by the bacterium (Fig. 3A). In contrast, phagolysosome encased N. gonorrhoeae were readily observed in Bafilomycin A1 treated cells (Fig 3A). Furthermore, the number of macrophage exhibiting bacteria with no surrounding membrane increased over the time of infection in absence of Bafilomycin A1 (Fig 3B), and the bacteria localized in the cytoplasm retain electron density in contrast to the bacteria localized in vacuole, suggesting that the cytoplasmically localized bacteria retain viability. These results support the hypothesis that a subset of internalized bacteria is able to escape from a phagosome or endosome into the cytoplasm and/or avoid lysosome fusion to prevent killing and it is this population that can replicate within the macrophages.
Figure 3. Localization of N gonorrhoeae within macrophage cell line.
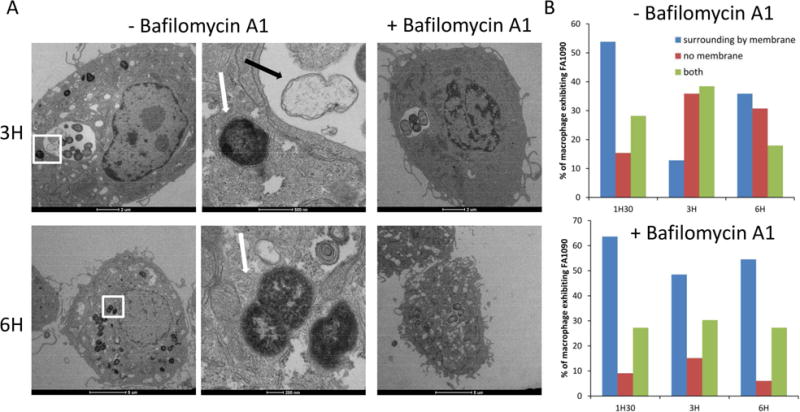
Differentiated U937 cells, were challenged with FA1090 (MOI 19) for 1h30, after washing, challenged cells were incubated in fresh medium with gentamicin 200 μg/ml for 1h before new washing and incubation with fresh medium without gentamicin. At 1.5h, 3h and 6h after infection, cells were fixed and processed for thin section TEM. (A) Left panels show cells without Bafilomycin A1 treatment and the right panels show cells treated to inhibit acidification of the phagolysosome, at 3 and 6h after challenging. The second column of micrograph corresponds of a magnified view of the boxed region of the first micrograph, showing the absence of membrane surrounding the bacteria. Black arrow indicates non-viable intracellular bacteria, while white arrows denote intracellular bacteria that retain electron density, suggesting that they have retained viability. (B) Quantification of U937 macrophages containing FA1090 surrounding by membrane or not, over the time. The localization of bacteria per macrophage was analyzed by TEM.
N. gonorrhoeae differentially influences apoptosis in THP-1 and U937 cells
Since N. gonorrhoeae can survive and replicate within macrophages, we wondered whether N. gonorrhoeae could modulate caspase-dependent apoptosis in macrophages, as has been reported for PMNs (Simons et al., 2006, Chen et al., 2011). Differentiated U937 and THP-1 cells were challenged with N. gonorrhoeae for 3h, and the cells were treated for 3h with either staurosporine (STS) to induce apoptosis or DMSO as a control. Cell lysates were harvested and caspase-3 activity was assessed using the fluorogenic caspase-3 substrate Ac-DEVD-AMC (Fig. 4). Infection of U937 cells with N. gonorrhoeae did not induce significant caspase-3 activity compared to the uninfected control, however, infection of THP-1 cells showed an increase of caspase-3 activity (Fig. 4A and B). Treatment of both cells with STS increased caspase-3 activity over that of control DMSO treated cells. Preincubation of U937 cells with N. gonorrhoeae at an MOI of 100 significantly decreased STS-induced caspase-3 activity, an effect comparable to that of the general caspase-3 inhibitor z-VAD-fmk (Fig. 4A). In contrast, preincubation of THP-1 cells with N. gonorrhoeae did not significantly modify caspase-3 activity levels (Fig. 4B). Studying DNA fragmentation, the last stage of apoptosis, confirmed the absence of apoptosis induction by N. gonorrhoeae in U937 cells and the partial induction of apoptosis in THP-1 cells (35%) (Fig 4C). The effect of N. gonorrhoeae on STS-induced caspase-3 activation of U937 cells was dose dependent, as the inhibition of caspase-3 activity became more pronounced with increasing MOIs (Fig. 4A). These differential results are reminiscent of the contrasting results about the effect of N. gonorrhoeae on apoptosis obtained by different groups using epithelial cell lines and could provide a way to examine the mechanistic basis for these differential responses by comparing the modulation of signal transduction pathways by N. gonorrhoeae.
Figure 4. N. gonorrhoeae inhibits apoptosis induced by STS in U937 cells and induces apoptosis in THP-1 cells.
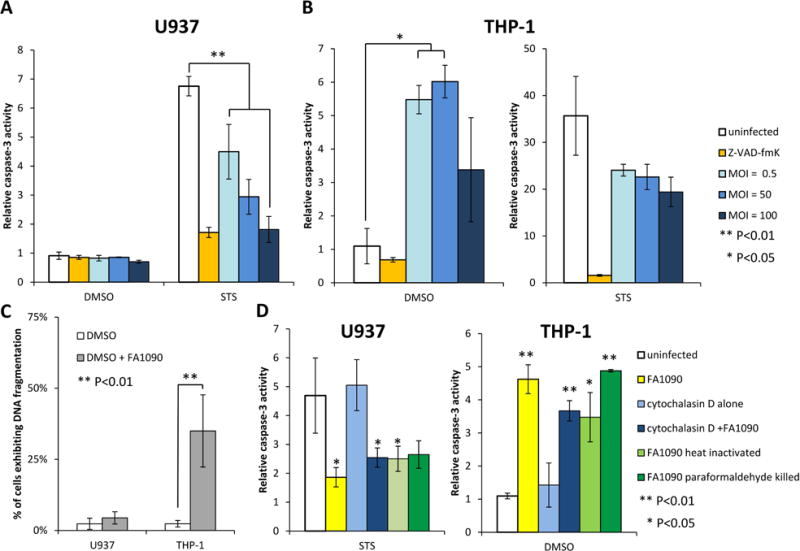
(A) N. gonorrhoeae inhibits caspase-3 activity induced by STS in U937 cells. (B) N. gonorrhoeae induces caspase-3 activity in THP-1 cells. (A, B) Differentiated U937 or THP-1 cells were challenged with FA1090 at different MOI as indicated and then treated with STS or DMSO. Caspase-3 activity was measured using the fluorogenic substrate Ac-DEVD-AMC and data are presented as the caspase-3 activation relative to that for uninfected DMSO-treated controls. Data shown are mean ± SD calculated from three replicates and are representative of three independent experiments. (C) N. gonorrhoeae induces DNA fragmentation in THP-1 cells, not in U937 cells. Differentiated U937 and THP-1 cells were challenged or not with FA1090 during 13h, hypodiploid DNA content was then evaluated by flow cytometry and the average percentage of cells exhibiting DNA fragmentation is indicated. The data are averages ± SD for 5 independent experiments. (D) Neither internalization nor viable N. gonorrhoeae are required to modulate caspase-3 activity. Differentiated U937 and THP-1 cells ± cytochalasin D treated were challenged with live, heat-killed or paraformaldehyde-killed FA1090 at MOI 5 and then treated with STS or DMSO, and caspase 3 activity was measured as described in (A,B). Data shown are mean ± SD calculated from three replicates and are representative of three independent experiments. Values that are significantly different (P<0.05) and (P<0.01) as determined by the Two-tailed, unpaired Student t tests are indicated with one or two asterisks, respectively.
To test whether N. gonorrhoeae internalization is necessary to modulate apoptosis, U937 and THP-1 differentiated cells were pre-treated with cytochalasin D to inhibit phagocytosis, then challenged with bacteria and caspase-3 activity was measured. Cytochalasin D treatment did not alter the effect of N. gonorrhoeae on STS induced caspase-3 activation in U937 cells or on the induction of caspase-3 activity in THP-1 (Fig. 4D). Furthermore, apoptosis regulation did not require viable N. gonorrhoeae, as heat-killed or paraformaldehyde-killed N. gonorrhoeae are still able to fully modulate apoptosis (Fig. 4). These results show that active N. gonorrhoeae processes are not required for apoptosis modulation.
N. gonorrhoeae inhibits both the intrinsic and extrinsic apoptosis pathways in U937 cells and induces the extrinsic apoptosis pathway in THP-1 cells
While we established divergent apoptosis responses to N. gonorrhoeae in U937 and THP-1 macrophages, the identity of the upstream signaling pathways influenced by N. gonorrhoeae remain to be fully defined. In order to define which apoptotic signaling pathways are affected by N. gonorrhoeae infection, the loss of mitochondrial membrane potential (MMP) was studied as a marker of the intrinsic apoptotic pathway. STS induced the loss of MMP in over 90% of both U937 and THP-1 cells (Fig 5). In U937 cells differentiated into macrophages, N. gonorrhoeae inhibited the loss of MMP induced by STS (around 50%), indicating that N. gonorrhoeae is able to inhibit intrinsic apoptosis pathway induced by STS. In THP-1 cells differentiated into macrophages, N. gonorrhoeae was unable to inhibit apoptosis induced by STS (Fig. 5), confirming the caspase-3 results. No induced loss of MMP was observed in THP-1 cells challenged by N. gonorrhoeae suggesting that N. gonorrhoeae does not induce apoptosis via the intrinsic pathway in this cell line. To test whether N. gonorrhoeae modulates the extrinsic apoptosis pathway in THP-1 differentiated cells, Bid cleavage was assayed by western-blot analysis. N. gonorrhoeae induced Bid cleavage in THP-1 cells (Fig. 6), indicating that N. gonorrhoeae induces the extrinsic apoptosis pathway in this cell line. N. gonorrhoeae did not induce Bid cleavage in U937 cells, consistent with our prior results. In contrast, N. gonorrhoeae inhibited Bid cleavage induced by TNF-α + cyclohexymide (CHX) treatment (Fig. 6). Finally, Caspase-8 cleavage, another step of the extrinsic apoptosis pathway, when induced by TNF-α + CHX in U937 cells, was also inhibited by N. gonorrhoeae infection (data not shown). Taken together the results of these assays clearly demonstrate that N. gonorrhoeae inhibits both the intrinsic and extrinsic apoptotic pathways in U937 cells, but only induces extrinsic apoptosis in THP-1 cells.
Figure 5. N. gonorrhoeae inhibits loss of mitochondrial membrane potential (MMP) induced by STS in U937 cells, not in THP-1 cells.
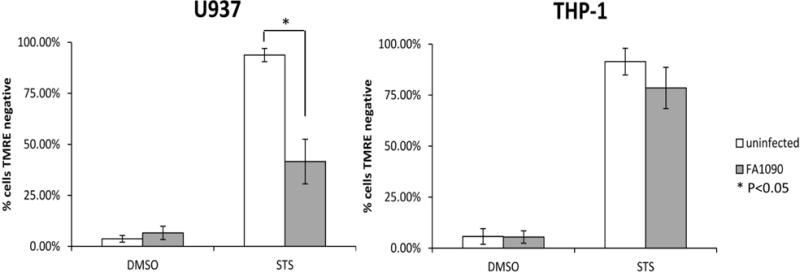
Differentiated U937 and THP-1 cells were challenged or not with FA1090 and then treated with STS or DMSO, the loss of MMP was assessed using the lipophilic cationic dye TMRE and evaluated by flow cytometry and the average percentage of cells TMRE negative is indicated. The data are averages ± SD for three independent experiments. Values that are significantly different (P<0.05) as determined by the Two-tailed, unpaired Student t tests are indicated with an asterisk.
Figure 6. N. gonorrhoeae induces Bid cleavage in THP-1 cells and inhibits Bid cleavage induced by TNF-α in U937 cells.
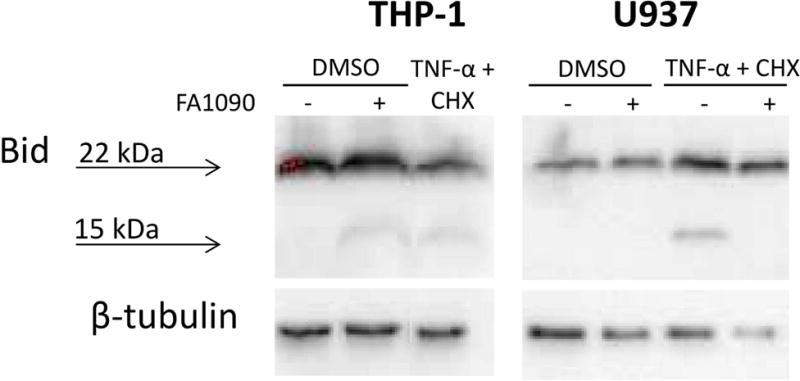
Differentiated U937 and THP-1 cells were challenged or not with FA1090 and then treated with DMSO or TNF-α + CHX. Lysates were harvested and subjected to Western blotting using a Bid antibody. The 22 kDa and 15 kDa products correspond to the full length and cleaved Bid, respectively. β–tubulin was used as a loading control. Data are representative of two independent experiments.
N. gonorrhoeae inhibits apoptosis in Human primary macrophages
The contradictory apoptosis response in two macrophage cell lines, suggested that these standard human monocytic cell lines express distinct phenotypes. In order to determine which macrophage cell line phenotype might be most relevant in vivo, apoptosis modulation was examined in macrophages derived from primary human monocytes (MDM). Similarly to the cell lines, N. gonorrhoeae was internalized without opsonisation by MDM (Fig. 7A) and the percentage of N. gonorrhoeae present within the MDM 2.5h after infection was 1.85% (±0.25). N. gonorrhoeae survived in the presence of MDM, but when gentamicin was present for the entire duration of the assay, no viable N. gonorrhoeae were detected after 6 h of infection (Fig. 7B). Furthermore, no LDH release was observed during MDM infection by N. gonorrhoeae (Fig. 7C). These results were all consistent with those obtained from the macrophage cell lines. Infection of MDM with N. gonorrhoeae alone did not induce significant caspase-3 activity over uninfected controls using cells from six distinct donors (Fig. 7D). While treatment of MDM with STS increased caspase-3 activity over that of control treated cells, preinfection of MDM with N. gonorrhoeae significantly decreased STS-induced caspase-3 activity (Fig 7D). Furthermore, loss of MMP and Bid cleavage are not induced in MDM challenged by N. gonorrhoeae, whereas infection with N. gonorrhoeae protected against loss of MMP induced by STS (data not shown). Therefore, the effect of N. gonorrhoeae on apoptosis of MDM is more similar to the effect in U937 cells than THP-1 cells.
Figure 7. N. gonorrhoeae is able to survive and replicate in association with primary human macrophages (MDM) and inhibits STS induced caspase 3 activity.
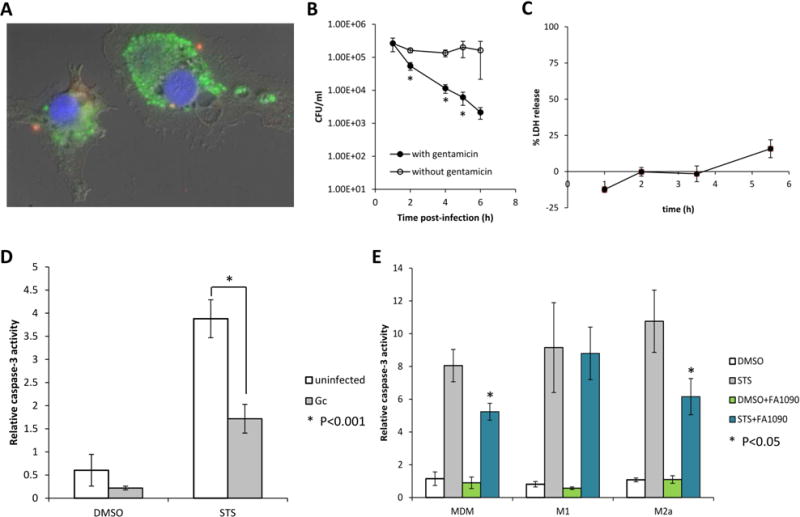
(A) N. gonorrhoeae associated with MDM are both intra- and extracellular. MDM were challenged at an MOI of two for 1.5h, after which the cells were fixed and intra-and extracellular bacteria were differentiated: extracellular bacteria appear red and yellow, and intracellular bacteria appear green. Data are representative of three experiments with MDM from three different donors. (B) MDM were challenged for 1.5h with FA1090 at an MOI of 11. After washing, challenged cells were incubated in fresh medium with gentamicin 200 μg/ml for the entire duration of the experiment (with gentamicin, black circles) or 1h before new washing and incubation with fresh medium without gentamicin (open circles). Viable CFU/ml were enumerated at various times post-infection, and the data are represented as the means ± SD for three independent replicates and are representative of two independent experiments with two different donors. Values that are significantly different (P<0.05) as determined by the Two-tailed, unpaired Student t tests are indicated with an asterisk. (C) N. gonorrhoeae does not induce LDH release in MDM. MDM were challenged with FA1090 at a MOI of 15, host cell lysis was assessed by measuring release of cytosolic LDH into the supernatant at different times postinfection. Data shown are means ± SD calculated from three replicates and are representative of two independent experiments with two different donors. (D) N. gonorrhoeae inhibits STS induced caspase-3 activity in MDM. MDM were challenged with FA1090 at a MOI of 38 and then treated with STS or DMSO. Caspase-3 activity was measured using the fluorogenic substrate Ac-DEVD-AMC and data are presented as the caspase-3 activation relative to that for uninfected DMSO-treated controls. Data shown are means ± SD calculated from three replicates and are representative of six independent experiments with six different donors. (E) N. gonorrhoeae inhibits STS induced caspase-3 activity in MDM polarized in M2a but not in M1 subtype. MDM and MDM polarized in M1 or M2a subtype were challenged with FA1090 at a MOI of 74 and then treated with STS or DMSO and caspase 3 activity was measured. Data shown are means ± SD calculated from three replicates and are representative of three independent experiments with three different donors. Values that are significantly different (P<0.05) from the value for the uninfected sample as determined by the Two-tailed, unpaired Student t tests are indicated with an asterisk.
Macrophages can be polarized to M1 or M2 phenotypes in response to environmental signals, including bacterial infection. It was possible that the macrophage-like cell lines were more closely replicating one of the polarized phenotypes. To determine whether polarized primary macrophages have a similar apoptotic phenotype than non-differentiated primary macrophages, MDM were treated with TNF-α plus INF-γ or IL-4 to polarize them to subtype M1 or M2a phenotypes, respectively, and caspase 3-activity was measured. N. gonorrhoeae did not induce apoptosis in either M1 or M2a MDM (Fig. 7E). N. gonorrhoeae inhibited caspase-3 cleavage induced by STS with M2a macrophages, but not with M1 macrophages (Fig. 7E). Therefore with respect to the regulation of apoptosis during infection, U937 cells, MDM and M1 macrophages were the most similar and THP-1 cells are not a good model.
N. gonorrhoeae induces cytokine production in MDM, U937 and THP-1 cells
Upon recognition of invading microorganisms, macrophages secrete inflammatory mediators including cytokines and chemokines to activate immune defenses that are designed to limit or clear infection. However, as host-adapted organisms it is unclear whether immune responses are effective in limiting infection. To determine whether infected macrophages could be a significant source of cytokines and chemokines during gonococcal infection, primary macrophages and differentiated THP-1 and U937 cells were challenged with N. gonorrhoeae at an MOI of 35. The cell-free supernatants were collected 10h post-infection and the induction of various inflammatory cytokines in the supernatants was assessed by ELISA (Figure 8). Uninfected U937 cells showed higher levels of many of the measured cytokines than THP-1 cells or MDM. None of the cells produced significant amounts of IL2, IL-4, IL-17A and INF-γ in response to N. gonorrhoeae infection. However, N. gonorrhoeae infection induced MDM, THP-1 and U937 cells to secrete IL-1α, IL-1β, IL-10, GM-CSF, and high level of IL-6, IL-8 and TNF-α. The primary macrophages and THP-1 were induced to secrete higher levels of cytokines/chemokines than U937 cells. N. gonorrhoeae infection resulted in the induction of pro-inflammatory cytokines IL1-β, IL-6 and TNF-α from all of the macrophages, and the anti-inflammatory cytokine IL-10 was also induced, but only in large amounts from MDM. IL-12 was only detected from challenged primary MDM. In summary, upon N. gonorrhoeae infection, human macrophages are able to upregulate production of the immunoregulatory cytokines IL-1, IL-6, IL-8, IL-10 and TNF-α. These data indicate that when macrophages interact with N. gonorrhoeae during colonization, the induced cytokines may stimulate both inflammation and immune suppression.
Figure 8. N. gonorrhoeae induces cytokine and chemokine production in macrophages.
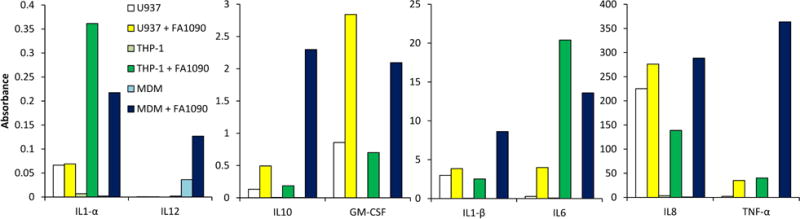
The levels of cytokines and chemokines in U937, THP-1 cells and MDM supernatants were determined by ELISA using the Multi-Analyte ELISArray kit (Qiagen). Data are the corrected absorbance at 450 nm minus 570 nm (correcting for optical background) and the dilution factor.
Discussion
N. gonorrhoeae recruits large number of PMNs during symptomatic infection of both men and women and a proportion of intracellular gonococci survives and multiplies within these cells (Simons et al., 2005). What is less well understood is the results of interaction of N. gonorrhoeae with resident or recruited macrophages and whether macrophages could help recruit the large numbers of PMNs to the genital track. The presence of macrophages in genitourinary mucosae and in gonococcal exudates suggests that macrophages could have an important role in gonococcal pathogenesis (Givan et al., 1997, Hook, 1999). Using two standard monocytic cell lines, differentiated into macrophages, and primary human macrophages; we confirmed that N. gonorrhoeae are phagocytosed without opsonization and are able to survive and grow in association with macrophages. It was previously described that N. gonorrhoeae survives exposure to macrophages but whether survival occurred intracellularly or extracellularly was not established in those studies (Leuzzi et al., 2005, Zughaier et al., 2014). We conclusively show that although many bacteria are killed by macrophages after phagocytosis, a subset of the internalized bacteria are able to survive and replicate.
Macrophages eliminate engulfed microorganisms by delivering them into the lysosomal system. Bacteria not adapted for an intracellular life cycle are effectively digested within the phagolysosome, an acidic environment that contains various activated hydrolytic enzymes, whereas some bacteria are able to survive by escaping into the cytoplasm to avoid lysosomal digestion, or other bacteria inhabit vesicles that do not fuse with lysosomes. Since the subset of N. gonorrhoeae that survives with macrophages does not co-localize with the acidic compartment or LAMP-1, it is possible that N. gonorrhoeae arrests phagosome maturation to survive inside macrophages. This is consistent with the idea that the Neisserial porin (PorB) inhibits phagosome maturation in human macrophages (Mosleh et al., 1998). This alteration in cellular trafficking may also be related to the ability of N. gonorrhoeae containing phagosomes to delay fusion with primary granules inside PMNs (Johnson et al., 2013). The Neisseria IgA1 protease cleaves LAMP-1 and promotes survival of bacteria within epithelial cells (Lin et al., 1997), but does not seem to play a role in the survival of N. gonorrhoeae macrophages as we did not observe any difference of survival between an igA1 mutant and the parental strain in U937 cells. Electron microscopy images indicated the presence of N. gonorrhoeae in the cytoplasm as there was no observable membrane surrounding viable N. gonorrhoeae cells in U937 macrophages (Fig. 3A). To gain entrance to the cytoplasm the bacteria could escape from the vacuole, as has been observed in other pathogens (for a review, Smith et al., 2013), or could use a separate mechanism to enter as they do in epithelial cells (for a review, Sadarangani et al., 2011). In the last hypothesis where N. gonorrhoeae could actively invade the macrophage, actin polymerization will be required, as cytochalasin D addition in the medium before challenging the cells do not allow detection of intracellular bacteria. Free N. gonorrhoeae in the cytoplasm was already observed in urethral epithelial cell (Apicella et al., 1996). While the mechanisms used by the subset of N. gonorrhoeae to avoid macrophage killing, potentially escape the phagolysosome, and survive inside macrophages will need further investigation, it was already suggested that N. gonorrhoeae can subvert the iron-limiting immune defenses to facilitate iron acquisition and intracellular survival (Zughaier et al., 2014).
Many studies have shown both pro- and antiapoptotic effects of both N. gonorrhoeae and Neisseria meningitidis on epithelial cells (Muller et al., 1999, Muller et al., 2000, Binnicker et al., 2003, Kepp et al., 2007, Howie et al., 2008, Follows et al., 2009, Kepp et al., 2009, Chen et al., 2011) and lymphocytes (Massari et al., 2003, Deghmane et al., 2009). Moreover, N. meningitidis has been reported to prevent macrophage apoptosis in U937 macrophages and MDM (Tunbridge et al., 2006), but also has been reported to induce apoptosis in RAW 264.7 cells and THP-1 macrophages (Sjolinder et al., 2012). Our results suggest that these contradictory results could all be correct and show that the effect on apoptosis is not strain or species specific but is most likely due to differences between host cells. While many of the previous studies in different laboratories used the same cell lines, it is well established that immortalized cells can change during propagation and that the characteristics or identity of the cell lines reported are not always correct (Hughes et al., 2007). We found that apoptosis modulation by N. gonorrhoeae in U937 and THP-1 cells does not required live bacteria nor internalization, suggesting gonococcal surface components are sufficient for apoptosis modulation. The induction of the extrinsic apoptosis pathway in THP-1 but not in U937 macrophages by live and killed N. gonorrhoeae suggests there are surface receptor differences between the two cells lines.
During gonococcal infection, TNF-α induction has been observed during experimental human challenge, in vaginal secretions from infected mice, and after challenge of human fallopian tube explants (Ramsey et al., 1995, Maisey et al., 2003, Packiam et al., 2010). Moreover N. gonorrhoeae has been reported to induce TNF-α secretion from epithelial cells and THP-1 monocytes in vitro (Christodoulides et al., 2000, Zughaier et al., 2014), and we confirmed TNF-α secretion from all three types of macrophages infected by N. gonorrhoeae (Fig. 8). While TNF-α secretion can result in accelerated macrophage apoptosis (for a review, Parameswaran et al., 2010), we report that N. gonorrhoeae is able to inhibit apoptosis induced by TNF-α in U937 macrophages. The fact that N. gonorrhoeae is able to inhibit caspase 8 and Bid cleavage induced by TNF-α and loss of MMP induced by STS in U937 macrophages suggests that N. gonorrhoeae acts by blocking an early event in both apoptosis pathways. These mechanisms are supported by the fact that Bcl-2 family proteins have been described to play roles in the anti- and pro-apoptotic phenotypes observed during gonococcal infection in epithelial cells (Binnicker et al., 2003, Binnicker et al., 2004, Kepp et al., 2007, Howie et al., 2008, Follows et al., 2009, Kepp et al., 2009).
The opposite effects of N. gonorrhoeae on apoptosis in U937 and THP-1 macrophages led us to examine these processes in MDM. The difference between U937 and THP-1 cells in our assays may be due to the different origin and maturation stage of cells. In fact, U937 cells are of tissue origin (histocytic lymphoma), thus at a more mature stage, whereas THP-1 cells are of blood leukemic origin, at a less mature stage (Ralph et al., 1976, Tsuchiya et al., 1980). N. gonorrhoeae was able to inhibit STS induced apoptosis in MDM, like in U937 macrophages. Therefore, the U937 cell line is a more relevant model to study the modulation of apoptosis by N. gonorrhoeae. Since N. gonorrhoeae inhibits STS induced apoptosis in M2a polarized macrophages, similarly to undifferentiated MDM and U937 cells, and N. gonorrhoeae induces the secretion of high level of IL-10, but low levels of IL-12 (Fig. 8), we postulate that M2 macrophages may be the more relevant phenotype within the genital tract. Escobar et al. had previously suggested that cytokines induced by N. gonorrhoeae from RAW 264.7 mouse macrophages correspond to an M2 profile (Escobar et al., 2013), and had recently published that N. gonorrhoeae can polarize human macrophages to a M2 profile (Ortiz et al., 2015). The phenotype of macrophages in the genital tract of patients will have to be investigated to determine whether there is polarization during natural gonococcal infection.
Secretion of IL-6 and TNF-α by MDM and U937 macrophages in response to N. gonorrhoeae or purified N. gonorrhoeae LOS was already described (Ellis et al., 2001, Makepeace et al., 2001), and in this study we showed MDM and two human macrophage cells line respond robustly to the presence of N. gonorrhoeae by secreting different cytokines (Fig. 8). The induction of pro-inflammatory cytokines, especially IL-8, by macrophages, in addition to epithelial cells, could contribute to the recruitment of PMN during gonococcal infection. The secretion of TNF-α and GM-CSF can also contribute to prolong the life of PMNs. The secretion of the regulatory cytokine IL-10 can have a potent T-cell suppressive function (Escobar et al., 2013) and it has been previously shown that N. gonorrhoeae suppresses T cell function (Boulton et al., 2002, Lee et al., 2008, Zhu et al., 2012) and may inducing a localized immune suppression (Liu et al., 2014). IL-6 and TNF-α may trigger the influx of lymphocytes. This suggests that macrophages are at least in part responsible for the increased level of TNF-α and IL-6 observed in the urine of men and vaginal secretion of women infected with N. gonorrhoeae (Ramsey et al., 1995, Hedges et al., 1998). Interestingly, IL-1β was also secreted by macrophages in response to N. gonorrhoeae infection, suggesting that the inflammasome would be activated. Inflammasome activation by N. gonorrhoeae was already described in monocytes (Zhou et al., 2014). Gonococcal stimulation of macrophages could have a profound influence on the continued expression of a pro-inflammatory, often damaging, response during natural infection.
Our results suggest that macrophages could be an unexplored cellular reservoir for N. gonorrhoeae during infection. In fact, macrophages may serve as a significant replicative niche for a subset of N. gonorrhoeae bacteria which are able to resist phagocytic killing and the inhibition of apoptosis in MDM may play an important role in facilitating N. gonorrhoeae replication and contribute to gonorrhea pathogenesis. Determining the mechanisms used by N. gonorrhoeae to differentially manipulate macrophage apoptosis and the other relevant host cells will be important to understanding the pathogenesis of this disease.
Experimental procedures
Bacterial strains and culture conditions
N. gonorrhoeae isolate FA1090 piliated and Opa positive was used in this study and was cultured on gonococcal medium base agar (GCB, Difco) plus Kellogg’s supplements (Kellogg et al., 1963). The strain was typically grown at 37°C and 5% CO2 for approximately 15 hours. Prior to cell infections, bacteria were suspended in gonococcal liquid medium containing Kellogg’s supplements and 0.042% Na2HCO3 at an OD550 of 0.16, and grown at 37°C to mid-logarithmic phase. A target number of CFUs was used for each experiment but due to the variability of N. gonorrhoeae growth, for each experiment the measured CFU are reported. For some experiments FA1090 strain harboring a plasmid encoding a green fluorescent protein, pCmGFP, was used (Srikhanta et al., 2009). For experiments with killed bacteria, N. gonorrhoeae was heat-killed at 65°C or treated with 4% paraformaldehyde for 30 minutes.
Cell culture and differentiation
The human monocytic cell lines U937 and THP-1 were obtained from ATCC and maintained at 2.5 × 105 in RPMI 1640 medium supplemented with 10% FBS at 37°C and 5% CO2. U937 and THP-1 cells at 1.106 cells/ml were differentiated to macrophages using 80 nM phorbol 1-myristate 13-acetate (PMA, Sigma-Aldrich) for 2 days in 24 well (1ml/well) or 48 well (0.5ml/well) tissue culture plates, and when necessary on coverslips for microscopy.
Human peripheral blood mononuclear cells (PBMC) were isolated by Ficoll Paque (GE healthcare) density centrifugation from human buffy coats provided by the National Red Cross (PA). Monocytes were negatively isolated from freshly isolated PBMC using Dynabeads untouched human monocytes (Life technologies). Cells at 1.1×106/ml were plated in RPMI, after 1h non-adherent cells were removed, and adherent cells were cultured in RPMI with 20% heat-inactivated FBS (Atlanta) and 10ng/ml M-CSF (Peprotech) during 7 days to allow differentiation of monocytes into monocyte-derived macrophages (MDM), medium was exchanged at 3 days intervals. For macrophage polarization experiments, these MDM were then stimulated for 24h with either 2ng/ml TNF-α and 20ng/ml INF-γ or 20ng/ml IL-4 to obtain M1 or M2a phenotypes, respectively (Peprotech). Flow cytometry on surfaces markers CD80 and CD163 was performed to confirm macrophages polarization in M1 or M2a phenotypes, respectively.
Bacterial survival assays
Host cells (5×105 cells/well in 48 well plates) were challenged with FA1090 in RPMI + 10% FBS or PBSG (Chen et al., 2011) medium and incubated at 37°C in the presence of 5% CO2 after centrifugation for 4 min at 1500 rpm. At different times after infection (as indicated in each Figure legend), the number of bacteria in the supernatant and cells lysed by 1% saponin was determined by serial dilution and plating. In some assay, 1.5 h after infection, non-adherent bacteria were removed by washing three times with PBS, and cells were incubated at 37°C in 5%CO2 in fresh medium containing gentamicin 200 μg/ml (time 0). After 1h, gentamicin was maintained or cells were washed 3 times with PBS and incubated in fresh medium without gentamicin. To study the influence of acidification on bacterial survival, bafilomycin A1 0.5 μM (Sigma) was added in the macrophage well 1h before infection and maintained during each experiment.
Immunofluorescence microscopy
Host cells (1×106 cells/well in 24 well plates, on coverslips) were challenged with FA1090 at 37°C in the presence of 5% CO2. At different times after infection (as indicated in each Figure legend), the cells were washed in PBS and fixed with 4% paraformaldehyde in PBS. In some experiments, one hour after infection cells were washed in PBS and fresh medium with 10 μg/ml cytochalasin D (sigma) was added. When necessary, 100 nM of Lysotraker red (life technologies) were added in the medium 15 min before fixation. After fixation, extracellular bacteria were detected with a polyclonal anti-N. gonorrhoeae antibody (Biodesign), and stained with an Alexa Fluor 647 or 350-conjugated goat anti-rabbit secondary antibody (Jackson and Molecular Probes). Cells were permeabilized with 0.2% saponin, and intracellular bacteria were detected by incubation with the same anti-N. gonorrhoeae antibody and stained with a fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antibody, except when GFP expressing bacteria were used. LAMP-1 was detected after permeabilization of the cells with 0.2% saponin using a rabbit-LAMP-1 antibody (ab24170, Abcam) and Alexa Fluor 350-conjugated goat anti-rabbit secondary antibody. The coverslips were mounted on slides with ProLong gold antifade reagent with or without DAPI staining (Life technologies), and the cells were visualized with a Nikon Eclipse 90i microscope. The number of intracellular bacteria and the percentage of bacteria that colocalized with Lysotraker was calculated by examination of at least 100 macrophages.
Lactate dehydrogenase release assay
Primary human macrophages, U937 and THP-1 differentiated cells (2 × 105 cells/well in 96-well plates) were challenged with FA1090 and centrifuged at 1500 rpm for 4 min to synchronize the infection. At different times after infection, supernatants of the infected macrophages were collected and assayed for lactate dehydrogenase activity (LDH) using the CytoTox 96 kit (Promega) according to the manufacturer’s instructions. The percent cell death was calculated as: 100 × (experimental release – spontaneous release)/(total release – spontaneous release), where spontaneous release is the amount of LDH activity in supernatants of cells incubated in medium alone, and total release is the activity from macrophages lysed with 1% Triton X-100. A control with medium alone (without cells) was also performed.
DNA fragmentation assay
U937 and THP-1 differentiated cells (1×106 cells/well in 24-well plates) were challenged with FA1090 at an MOI of 22 to 107 and the plates were centrifuged at 1500 rpm for 4 min. After 13h of infection, the cells were harvested by trypsinisation, washed with PBS, incubated in ice-cold 70% ethanol for 10 min at 4°C, washed twice more with PBS, and stained with 0.1 ml PBS containing 50 μg/ml propidium iodide (Sigma) and 0.5 mg/ml RNase A at room temperature for 30 min in the dark. Genomic DNA content was then assessed by flow cytometry (BD LSRII, BD Biosciences).
Caspase-3 assay
Differentiated U937 cells, THP-1 cells or primary human macrophages were challenged with FA1090 at various MOIs and the plates were centrifuged at 1500 rpm for 4 min. After 3h of infection, the cells were treated with dimethyl sulfoxide (DMSO) or 1 μM STS (Sigma) in DMSO, and in some cases with 20 μM z-VAD-fmk (BD pharmingen) a general caspase inhibitor, for a further 3h for U937 and THP-1 cells and 6h for MDM. When cytochalasin D was used, 5 μg/ml of cytochalasin D was added to cells 1h before infection and cells were lysed with 70 μl cell lysis buffer (BD Pharmingen). The cells lysates were stored at −80°C before being used for caspase-3 assays. Caspase-3 activity was measured by incubating 25 μl cell lysate with 5 μl reconstituted caspase-3 substrate at 1 mg/ml (Ac-DEVD-AMC, BD Pharmingen) and with 200 μl HEPES buffer (20 mM HEPES (pH 7.5), 10% glycerol, 2 mM dithiothreitol) for 1h at 37°C. 7-amino-4-methylcoumarin fluorescence was then measured using an excitation wavelength of 380 nm and an emission wavelength of 440 nm, using a plate reader (SpectraMax M5, Molecular Devices). Fluorescence levels were normalized to total cellular protein level measured by bicinchoninic acid (BCA) protein assay (Pierce).
Mitochondrial Membrane Potential assay
Differentiated U937 and THP-1 cells (1×106 cells/well in 24-well plates) were challenged with FA1090 at MOIs from 22 to 107 and plates were centrifuged at 1500 rpm for 4 min. After 3h of infection the cells were treated with DMSO or 1 μM STS (Sigma) for an additonal13h. Cells were then harvested by trypsin treatment, washed with fresh medium, stained with 200 μM Mito PT TMRE (TMRE Mitochondrial Membrane Potential Assessment kit, ImmunoChemistry Technologies) for 15 min at 37°C and 5% CO2, and washed one time before staining was assessed by flow cytometry (BD LSRII, BD Biosciences).
Bid Western blotting
Differentiated U937 and THP-1 cells were challenged with FA1090 at an MOI of ~100 and plates were centrifuged at 1500 rpm for 4 min. After 3–4h of infection, the cells were treated with DMSO or TNF-α 10 ng/ml and cycloheximide 200ng/ml for an additional 3h. Cells were washed with PBS and lysed with RIPA buffer for 30 min at 4°C under rotation. Lysates were centrifuged at 12,000 rpm for 10 min at 4°C. The supernatants were transferred to fresh tubes and stored at −80°C. 7 to 23 μg of cellular extracts in SDS sample buffer containing β – mercaptoethanol were separated on 15% SDS-polyacrylmide gel and proteins were transferred to a polyvinylidene fluoride membrane in 10 mM CAPS (N-cyclohexo-3-amino-propanesulfonic acid) buffer with 10% methanol at pH 11 for 1h. After saturation in Tris buffered saline containing 0.1% Tween 20 and 5% dry milk, membranes were incubated overnight with anti-Bid antibody (1:1000, #2002, Cell Signaling) or anti-α-β-tubulin antibody (1:1000, 9F3, Cell Signaling). The membranes were then washed and incubated with secondary goat anti-rabbit antibody conjugated to horseradish peroxidase (1:1000, Jackson ImmunoResearch) for 1h. After washing, blots were developed with ECL Plus Western Blotting detection reagent (Amersham, GE Healthcare) and visualized using a ChemiDox XXRS molecular imager (Bio-Rad).
ELISA for Cytokine Production
Differentiated U937, THP-1 cells and primary human macrophages were either left uninfected or infected with FA1090 strain at an MOI of 35, the plates were centrifuged at 1500 rpm for 4 min and infection were carried out at 37°C with 5% CO2. After 10 h of infection the supernatant were harvested, centrifuged and the resulting supernatants were stored at −80°C before being used. The culture supernatants were examined for 12 cytokines using the Multi-Analyte ELISArray kit for detection of human inflammatory cytokines (Qiagen). IL1-A, IL2, IL4, IL10, IL12, IL17, INF-γ, and GM-CSF were read undiluted. IL1B and IL6 using a 1:10 dilution, and IL8 and TNF-α with a 1:100 dilution. The analyses were performed according to the manufacturer’s instructions.
Thin-section transmission electron microscopy
U937 cells (7×105 cells/well on coverslip) were challenged with FA1090 at a MOI of 19. In some wells, bafilomycin A1 0.5 μM (Sigma) was added 1h before infection and maintained during the experiment. 1.5h after infection, non-adherent bacteria were removed by washing three times with PBS, and cells were incubated at 37°C in 5%CO2 in fresh medium containing 200 μg/ml gentamicin for 1h before new washing and incubation with fresh medium without gentamicin. At 1.5h, 3h and 6h after infection, the cells were washed and fixed at 4°C in 0.1 M sodium cacodylate buffer (pH 7.3) containing 2% paraformaldehyde and 2.5% glutaraldehyde. Samples were treated with 2% osmium tetroxide in 0.1M sodium cacodylate buffer, rinsed with distilled water, dehydrated in ascending grades of ethanol, embedded in resin mixture of Embed 812 kit, and cured in a 60˚C oven. Sample blocks were thin sectioned on a Leica Ultracut UC6 ultramicrotome with 70nm sections collected on 200 mesh Cu grids and then were stained with 3% uranyl acetate and Reynolds lead citrate. Images were captured on a FEI Tecnai Spirit G2 120-kV transmission electron microscope (TEM). The localization of bacteria inside the macrophage was calculated by examination of at least 70 macrophages.
Acknowledgments
Flow cytometry was performed at the Northwestern University Interdepartmental ImmunoBiology Flow Cytometry Core Facility. Thin-section for transmission electron microscopy samples were processed by the Center for Advanced Microscopy at Northwestern University Feinberg School of Medicine. This work was supported by NIH grants R01 AI044239, R56 AI114821 and R37 AI033493.
References
- Apicella MA, Ketterer M, Lee FK, Zhou D, Rice PA, Blake MS. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. The Journal of infectious diseases. 1996;173:636–646. doi: 10.1093/infdis/173.3.636. [DOI] [PubMed] [Google Scholar]
- Binnicker MJ, Williams RD, Apicella MA. Infection of human urethral epithelium with Neisseria gonorrhoeae elicits an upregulation of host anti-apoptotic factors and protects cells from staurosporine-induced apoptosis. Cellular microbiology. 2003;5:549–560. doi: 10.1046/j.1462-5822.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- Binnicker MJ, Williams RD, Apicella MA. Gonococcal porin IB activates NF-kappaB in human urethral epithelium and increases the expression of host antiapoptotic factors. Infection and immunity. 2004;72:6408–6417. doi: 10.1128/IAI.72.11.6408-6417.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nature immunology. 2002;3:229–236. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- Chen A, Seifert HS. Neisseria gonorrhoeae-mediated inhibition of apoptotic signalling in polymorphonuclear leukocytes. Infection and immunity. 2011;79:4447–4458. doi: 10.1128/IAI.01267-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulides M, Everson JS, Liu BL, Lambden PR, Watt PJ, Thomas EJ, Heckels JE. Interaction of primary human endometrial cells with Neisseria gonorrhoeae expressing green fluorescent protein. Molecular microbiology. 2000;35:32–43. doi: 10.1046/j.1365-2958.2000.01694.x. [DOI] [PubMed] [Google Scholar]
- Cooper MD, Floyd SA. In vitro kinetics of phagocytosis and intracellular killing of gonococci by peritoneal macrophages from mice deficient in complement component 5. Infection and immunity. 1982;36:363–370. doi: 10.1128/iai.36.1.363-370.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criss AK, Seifert HS. A bacterial siren song: intimate interactions between Neisseria and neutrophils. Nature reviews. Microbiology. 2012;10:178–190. doi: 10.1038/nrmicro2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deghmane AE, Veckerle C, Giorgini D, Hong E, Ruckly C, Taha MK. Differential modulation of TNF-alpha-induced apoptosis by Neisseria meningitidis. PLoS pathogens. 2009;5:e1000405. doi: 10.1371/journal.ppat.1000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis CD, Lindner B, Anjam Khan CM, Zahringer U, Demarco de Hormaeche R. The Neisseria gonorrhoeae lpxLII gene encodes for a late-functioning lauroyl acyl transferase, and a null mutation within the gene has a significant effect on the induction of acute inflammatory responses. Molecular microbiology. 2001;42:167–181. doi: 10.1046/j.1365-2958.2001.02619.x. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicologic pathology. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar A, Candia E, Reyes-Cerpa S, Villegas-Valdes B, Neira T, Lopez M, et al. Neisseria gonorrhoeae induces a tolerogenic phenotype in macrophages to modulate host immunity. Mediators of inflammation. 2013;2013:127017. doi: 10.1155/2013/127017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follows SA, Murlidharan J, Massari P, Wetzler LM, Genco CA. Neisseria gonorrhoeae infection protects human endocervical epithelial cells from apoptosis via expression of host antiapoptotic proteins. Infection and immunity. 2009;77:3602–3610. doi: 10.1128/IAI.01366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM, Wira CR. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. American journal of reproductive immunology. 1997;38:350–359. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Hedges SR, Sibley DA, Mayo MS, Hook EW, 3rd, Russell MW. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. The Journal of infectious diseases. 1998;178:742–751. doi: 10.1086/515372. [DOI] [PubMed] [Google Scholar]
- Hook EW. Sexually transmitted diseases. 3rd. McGraw-Hill Book Company; New York, NY: 1999. [Google Scholar]
- Hook EW, 3rd, Holmes KK. Gonococcal infections. Annals of internal medicine. 1985;102:229–243. doi: 10.7326/0003-4819-102-2-229. [DOI] [PubMed] [Google Scholar]
- Howie HL, Shiflett SL, So M. Extracellular signal-regulated kinase activation by Neisseria gonorrhoeae downregulates epithelial cell proapoptotic proteins Bad and Bim. Infection and immunity. 2008;76:2715–2721. doi: 10.1128/IAI.00153-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P, Marshall D, Reid Y, Parkes H, Gelber C. The costs of using unauthenticated, over-passaged cell lines: how much more data do we need? BioTechniques. 2007;43:575, 577–578. doi: 10.2144/000112598. 581–572 passim. [DOI] [PubMed] [Google Scholar]
- Johnson MB, Criss AK. Resistance of Neisseria gonorrhoeae to neutrophils. Frontiers in microbiology. 2011;2:77. doi: 10.3389/fmicb.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Criss AK. Neisseria gonorrhoeae phagosomes delay fusion with primary granules to enhance bacterial survival inside human neutrophils. Cellular microbiology. 2013;15:1323–1340. doi: 10.1111/cmi.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DS, Jr, Peacock WL, Jr, Deacon WE, Brown L, Pirkle DI. Neisseria Gonorrhoeae. I. Virulence Genetically Linked to Clonal Variation. Journal of bacteriology. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepp O, Gottschalk K, Churin Y, Rajalingam K, Brinkmann V, Machuy N, et al. Bim and Bmf synergize to induce apoptosis in Neisseria gonorrhoeae infection. PLoS pathogens. 2009;5:e1000348. doi: 10.1371/journal.ppat.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepp O, Rajalingam K, Kimmig S, Rudel T. Bak and Bax are non-redundant during infection- and DNA damage-induced apoptosis. The EMBO journal. 2007;26:825–834. doi: 10.1038/sj.emboj.7601533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Ostrowski MA, Gray-Owen SD. CEACAM1 dynamics during neisseria gonorrhoeae suppression of CD4+ T lymphocyte activation. Journal of immunology. 2008;180:6827–6835. doi: 10.4049/jimmunol.180.10.6827. [DOI] [PubMed] [Google Scholar]
- Leuzzi R, Serino L, Scarselli M, Savino S, Fontana MR, Monaci E, et al. Ng-MIP, a surface-exposed lipoprotein of Neisseria gonorrhoeae, has a peptidyl-prolyl cis/trans isomerase (PPIase) activity and is involved in persistence in macrophages. Molecular microbiology. 2005;58:669–681. doi: 10.1111/j.1365-2958.2005.04859.x. [DOI] [PubMed] [Google Scholar]
- Lin L, Ayala P, Larson J, Mulks M, Fukuda M, Carlsson SR, et al. The Neisseria type 2 IgA1 protease cleaves LAMP1 and promotes survival of bacteria within epithelial cells. Molecular microbiology. 1997;24:1083–1094. doi: 10.1046/j.1365-2958.1997.4191776.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu W, Russell MW. Suppression of host adaptive immune responses by Neisseria gonorrhoeae: role of interleukin 10 and type 1 regulatory T cells. Mucosal immunology. 2014;7:165–176. doi: 10.1038/mi.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisey K, Nardocci G, Imarai M, Cardenas H, Rios M, Croxatto HB, et al. Expression of proinflammatory cytokines and receptors by human fallopian tubes in organ culture following challenge with Neisseria gonorrhoeae. Infection and immunity. 2003;71:527–532. doi: 10.1128/IAI.71.1.527-532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makepeace BL, Watt PJ, Heckels JE, Christodoulides M. Interactions of Neisseria gonorrhoeae with mature human macrophage opacity proteins influence production of proinflammatory cytokines. Infection and immunity. 2001;69:1909–1913. doi: 10.1128/IAI.69.3.1909-1913.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari P, King CA, Ho AY, Wetzler LM. Neisserial PorB is translocated to the mitochondria of HeLa cells infected with Neisseria meningitidis and protects cells from apoptosis. Cellular microbiology. 2003;5:99–109. doi: 10.1046/j.1462-5822.2003.00257.x. [DOI] [PubMed] [Google Scholar]
- Mosleh IM, Huber LA, Steinlein P, Pasquali C, Gunther D, Meyer TF. Neisseria gonorrhoeae porin modulates phagosome maturation. The Journal of biological chemistry. 1998;273:35332–35338. doi: 10.1074/jbc.273.52.35332. [DOI] [PubMed] [Google Scholar]
- Muller A, Gunther D, Brinkmann V, Hurwitz R, Meyer TF, Rudel T. Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. The EMBO journal. 2000;19:5332–5343. doi: 10.1093/emboj/19.20.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Gunther D, Dux F, Naumann M, Meyer TF, Rudel T. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. The EMBO journal. 1999;18:339–352. doi: 10.1093/emboj/18.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz MC, Lefimil C, Rodas PI, Vernal R, Lopez M, Acuna-Castillo C, et al. Neisseria gonorrhoeae Modulates Immunity by Polarizing Human Macrophages to a M2 Profile. PloS one. 2015;10:e0130713. doi: 10.1371/journal.pone.0130713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota F, Morita J, Yoshida N, Ashton F, Diena B. Studies on gonococcal infection. I. Electron microscopic studies on phagocytosis of Neisseria gonorrhoeae by macrophages. Japanese journal of microbiology. 1975;19:149–155. doi: 10.1111/j.1348-0421.1975.tb00861.x. [DOI] [PubMed] [Google Scholar]
- Packiam M, Veit SJ, Anderson DJ, Ingalls RR, Jerse AE. Mouse strain-dependent differences in susceptibility to Neisseria gonorrhoeae infection and induction of innate immune responses. Infection and immunity. 2010;78:433–440. doi: 10.1128/IAI.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran N, Patial S. Tumor necrosis factor-alpha signaling in macrophages. Critical reviews in eukaryotic gene expression. 2010;20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P, Moore MA, Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. The Journal of experimental medicine. 1976;143:1528–1533. doi: 10.1084/jem.143.6.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KH, Schneider H, Cross AS, Boslego JW, Hoover DL, Staley TL, et al. Inflammatory cytokines produced in response to experimental human gonorrhea. The Journal of infectious diseases. 1995;172:186–191. doi: 10.1093/infdis/172.1.186. [DOI] [PubMed] [Google Scholar]
- Sadarangani M, Pollard AJ, Gray-Owen SD. Opa proteins and CEACAMs: pathways of immune engagement for pathogenic Neisseria. FEMS microbiology reviews. 2011;35:498–514. doi: 10.1111/j.1574-6976.2010.00260.x. [DOI] [PubMed] [Google Scholar]
- Simons MP, Nauseef WM, Apicella MA. Interactions of Neisseria gonorrhoeae with adherent polymorphonuclear leukocytes. Infection and immunity. 2005;73:1971–1977. doi: 10.1128/IAI.73.4.1971-1977.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons MP, Nauseef WM, Griffith TS, Apicella MA. Neisseria gonorrhoeae delays the onset of apoptosis in polymorphonuclear leukocytes. Cellular microbiology. 2006;8:1780–1790. doi: 10.1111/j.1462-5822.2006.00748.x. [DOI] [PubMed] [Google Scholar]
- Sjolinder M, Altenbacher G, Hagner M, Sun W, Schedin-Weiss S, Sjolinder H. Meningococcal outer membrane protein NhhA triggers apoptosis in macrophages. PloS one. 2012;7:e29586. doi: 10.1371/journal.pone.0029586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, May RC. Mechanisms of microbial escape from phagocyte killing. Biochemical Society transactions. 2013;41:475–490. doi: 10.1042/BST20130014. [DOI] [PubMed] [Google Scholar]
- Srikhanta YN, Dowideit SJ, Edwards JL, Falsetta ML, Wu HJ, Harrison OB, et al. Phasevarions mediate random switching of gene expression in pathogenic Neisseria. PLoS pathogens. 2009;5:e1000400. doi: 10.1371/journal.ppat.1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) International journal of cancer. Journal international du cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Tunbridge AJ, Stevanin TM, Lee M, Marriott HM, Moir JW, Read RC, Dockrell DH. Inhibition of macrophage apoptosis by Neisseria meningitidis requires nitric oxide detoxification mechanisms. Infection and immunity. 2006;74:729–733. doi: 10.1128/IAI.74.1.729-733.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Global icidence and prevalence of selected curable sexually transmitted infections - 2008. 2012. [PubMed] [Google Scholar]
- Zhou X, Gao X, Broglie PM, Kebaier C, Anderson JE, Thom N, et al. Hexa-acylated lipid A is required for host inflammatory response to Neisseria gonorrhoeae in experimental gonorrhea. Infection and immunity. 2014;82:184–192. doi: 10.1128/IAI.00890-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Ventevogel MS, Knilans KJ, Anderson JE, Oldach LM, McKinnon KP, et al. Neisseria gonorrhoeae suppresses dendritic cell-induced, antigen-dependent CD4 T cell proliferation. PloS one. 2012;7:e41260. doi: 10.1371/journal.pone.0041260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zughaier SM, Kandler JL, Shafer WM. Neisseria gonorrhoeae modulates iron-limiting innate immune defenses in macrophages. PloS one. 2014;9:e87688. doi: 10.1371/journal.pone.0087688. [DOI] [PMC free article] [PubMed] [Google Scholar]


