Abstract
Background
Mild therapeutic hypothermia (TH) is a recommended treatment for comatose patients resuscitated from cardiac arrest. To our knowledge, the prevalence of delirium and its associated risk factors has not been assessed in survivors of cardiac arrest treated with TH.
Objective
To determine the prevalence and risk factors for delirium among survivors of cardiac arrest who were treated with TH.
Methods
We conducted a retrospective observational study of 251 patients following cardiac arrest treated with TH between 2007 and 2014. We obtained baseline demographic data and daily delirium assessments throughout the ICU stay. We assessed for the association between the duration of delirium and various risk factors.
Results
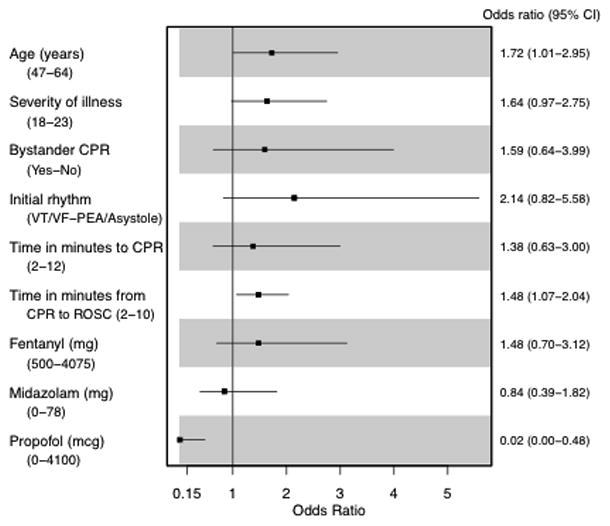
Of 251 patients, 107 (43%) awoke from coma. Of these 107 survivors, all of them had at least one day of delirium during their ICU stay. Median number of delirium days was 4.0 (IQR 2.0 to 7.5). A multivariable analysis revealed that age (OR 1.72, 95% CI 1.01 – 2.95, p=0.05), time from CPR to return of spontaneous circulation (ROSC) (OR 1.52, 95% CI 1.11–2.07 p =0.01), and total dose of pre-warming propofol (OR 0.02, 95% CI 0.00 – 0.48, p= 0.02) were associated with delirium duration.
Conclusions
Among patients treated with TH, delirium lasts a median of 4 days. Duration of delirium was best predicted by age and time from initiation of CPR to ROSC, while pre-warming exposure to total dose of propofol was associated with a shorter duration. These findings are limited to this unique cohort, and may not generalize to broader populations.
Keywords: Therapeutic hypothermia, post-resuscitation care, cardiac arrest, delirium, benzodiazepine, CPR
Introduction
Survival to hospital discharge after sudden cardiac arrest ranges from 5%–40%, with post-resuscitation mortality driven by neurologic injury [1, 2]. Despite recent data that mild therapeutic hypothermia (33 degrees Celsius) is not superior to targeted-temperature management (36 degrees Celsius), mild therapeutic hypothermia (TH) remains the standard of care following post cardiac-arrest with evidence that TH improves neurologic outcomes after ventricular fibrillation (VF) and ventricular tachycardic (VT) arrest [3–7]. However, outcomes remain poor, and, identification of additional interventions and modifiable risk factors may continue to improve neurologic outcomes.
Acute brain dysfunction (delirium) is the most common organ disorder in mechanically ventilated ICU patients [8, 9]. Delirium independently predicts poor outcomes, including long-term cognitive impairment and mortality[10–14]. Importantly, a variety of ICU care processes influence delirium with implications for post cardiac-arrest patients. For example, studies identified the use of benzodiazepines and physical restraints as common modifiable risk factors for the development of delirium [15–17]. Currently, most TH protocols call for high doses of sedatives due to the use of paralytics to prevent shivering, and the majority use midazolam as a sedative agent [18].
This study primarily aims to determine the prevalence of delirium in survivors of cardiac arrest following treatment with mild TH in the cardiovascular intensive care unit (CVICU). In addition, we sought to understand the relationship between potentially modifiable resuscitation and TH protocol exposures and duration of delirium among patients treated with TH to inform the management of post-cardiac arrest patients.
Methods
Study design and population
We conducted a retrospective cohort study performed at the Vanderbilt CVICU, a tertiary level critical care unit managed with twenty-seven beds and an annual volume of 2,800 patients. The study period began on May 15, 2007 and ended on January 1st, 2014. Eligible patients for this study included all patients admitted following cardiac arrest. We included all patients treated with TH in a prospective registry (INTCAR, International Cardiac Arrest Registry). We then collected data retrospectively and input into a Redcap database. We excluded patients if they were persistently comatose or died prior to awakening from coma.
Treating physicians made determinations of the suitability of TH; patients considered for inclusion experienced a cardiac arrest with a primary cardiac etiology, could initiate TH within 12 hours of experiencing ROSC, were 18 years or older, remained unresponsive after experiencing ROSC and had a time to ROSC less than 60 minutes [19]. Physicians cooled patients eligible for TH using an active surface cooling device to maintain a core body temperature of 32–34 degrees Celsius for a total of 24 hours following return of spontaneous circulation (ROSC), after which physicians rewarmed the patients at a rate of 0.25 degrees Celsius per hour. Physicians also administered neuromuscular blockade to all patients, using cisatracurium and a sedative (midazolam or propofol) during active cooling. Vanderbilt’s institutional review board reviewed and approved of the study.
Covariates and Post-Cardiac Arrest Processes of Care
In addition to non-modifiable baseline patient characteristics, we investigated potentially modifiable factors that we a priori hypothesized would have the potential to affect the prevalence and duration of delirium in post-cardiac arrest patients. We included factors related to a.) severity of illness, b.) initial resuscitation, and c.) medication dose prior to a patient reaching a temperature of 36 Celsius. Patient characteristics included age and comorbid illnesses. We obtained all baseline characteristics via retrospective chart review. We characterized severity of illness using the acute physiology score [20]. Initial resuscitation factors included: occurrence of bystander cardiopulmonary resuscitation (CPR), initial rhythm (ventricular fibrillation/tachycardia, pulseless electrical activity/asystole), time in minutes from arrest to CPR by medical personnel, and, time in minutes from CPR by medical personnel to ROSC (determined by review of EMS records). Medication dose exposures during the TH protocol from ICU admission to patient arousal (RASS greater than negative 4) included: Total doses of fentanyl (mcg), midazolam (mg), propofol (mg), and cisatracurium (mcg).
Outcomes
We defined delirium duration as the total number of days of delirium following initial arousal from coma. We assessed delirium status for each 24-hour interval, beginning at the time that the patient first reached an internal core temperature of 36 degrees Celsius until CCU discharge or death.
We assessed for coma and delirium daily using the Richmond Agitation Sedation Scale (RASS) and the Confusion Assessment Method for the ICU (CAM-ICU), respectively [9, 21–23]. The RASS and CAM-ICU are both previously validated tools with excellent interrater-reliability suitable for broad populations of ICU patients to identify the presence and type of delirium [9]. Bedside nurses performed RASS and CAM-ICU measurements at a minimum of twice daily for > 90% of patient days. For those days that we had no usable assessments (6.7%, 52 out of 772 days), we used the patient’s status on the day before, and the day after that missing day to impute for the missing day status. The bedside nurse can reliably use RASS and the CAM-ICU to identify delirious patients, and these mechanisms have been previously validated in the ICUs at Vanderbilt, including the CCU [24].
Our team considered patients with RASS scores of −5 (unresponsive to physical and verbal stimulus) or −4 (responsive only to physical stimulus) for each 24 hours as comatose and therefore not eligible for delirium evaluation. The study classified each such day as “coma”. During a 24-hour period, if a patient’s RASS level reached or exceeded −3, we considered that day delirious (CAM-ICU positive at any time during a 24-hour period) or normal (CAM-ICU negative during all assessments during a 24-hour period). We further categorized delirium, as assessed by the CAM-ICU, into hyper (RASS score +1 to +4) or hypo-active (RASS score 0 to −3). We classified a day as “hypoactive” if all CAM-positive assessments during a 24-hour period ranged from RASS 0 to −3. We used “hyperactive” to classify a 24-hour period of all CAM-positive assessments with RASS +1 to +4. “Mixed” indicated any CAM-positive 24-hour period with both hyperactive and hypoactive RASS scores.
Statistical analysis
We first describe the patient characteristics of the overall population. Mean and standard deviations, medians, and interquartile ranges (IQRs) were reported as appropriate. The primary objective was to characterize the prevalence of delirium among cardiac-arrest patients that were exposed to a TH protocol and successfully warmed and aroused from coma. We report the proportion of patients with at least one day with positive CAM-ICU over the total included cohort.
The second aim was to evaluate the potentially modifiable resuscitation or TH protocol-related risk factors for delirium. In order to achieve this, we constructed a proportional odds logistic regression model that assessed baseline risk factors for delirium duration. A proportional logistic model was used to analyze delirium duration due to the highly skewed distribution. The measure of association between predictors and outcomes in this model type is an odds ratio. We could not include all factors collected in order to avoid model over fitting given the sample size. In addition to non-modifiable baseline patient characteristics, we decided a priori to investigate potentially modifiable factors that were hypothesized to have the potential to affect the prevalence and duration of delirium in post-cardiac arrest patients. We included factors related to a.) severity of illness, b.) initial resuscitation, and c.) medication dose prior to a patient reaching a temperature of 36 Celsius. Specific model variables included: a.) age b.) severity of illness, c.) VT/VF vs. PEA/Asystole, time to CPR, and time from CPR to ROSC, and d.) total dose of each of the following medicines during TH: fentanyl, midazolam, and propofol. All statistical analyses were performed using R statistical software. (R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org).
Sensitivity Analysis
In order to address whether post-warming delirium was driven by sedation alone or not, we divided the population across days between patients who received sedation (propofol, midazolam, or fentanyl) and those who did not. In addition, we analyzed patients on and off sedation excluding fentanyl as a sedative. Finally, we then assessed for the prevalence of and sub-types for each of these subgroups.
Results
We administered TH to 251 consecutive post-cardiac patients. The analysis includes 107 (43%) from the cohort that survived and awoke from coma, and the analysis excluded the other one-hundred forty-four (57%) that died prior to ICU discharge or never had a RASS greater than −3 that allowed for assessment of delirium (Figure 1). Table 1 displays the demographic and baseline characteristics of the study cohort. The median age of survivors to first awakening was 57 (interquartile range 48 to 64) years old with a median APACHE II score of 21.0 (IQR 18.0, 23.5), reflecting a high illness severity. The majority of arrests had shockable rhythms (79%) and were out-of-hospital (77%). We administered fentanyl for pain during TH and midazolam (67%, n=72) for sedation to the majority of patients (84%, N=90).
Figure 1.
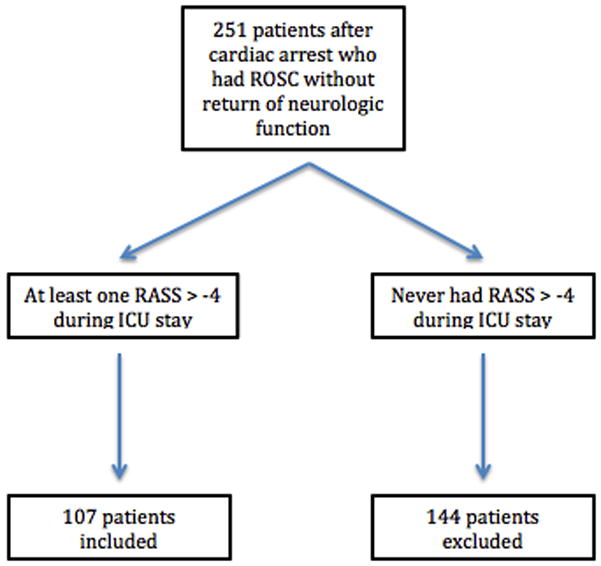
Table 1.
Baseline characteristics of cardiac-arrest survivors that were successfully warmed and awoke from coma. There were 251 patients included in the study.
| Characteristic | Overall |
|---|---|
|
| |
| Age, median (IQR) | 57(47, 64) |
|
| |
| Male gender N (%) | 72 (67) |
|
| |
| Previously healthy N (%) | 21 (20) |
|
| |
| APACHE1 II Score median (IQR) | 21(18,23) |
|
| |
| CPC2 prior to arrest | |
| 1 N (%) | 101 (95) |
| 2 N (%) | 6 (5) |
|
| |
| Cardiac Arrest Site | |
| Out-of-hospital N (%) | 82 (77) |
|
| |
| Witnessed Arrest N (%) | 92 (86) |
|
| |
| Bystander CPR3 N (%) | 73 (63) |
|
| |
| Initial Rhythm | |
| VT4/VF5, N(%) | 85 (79) |
|
| |
| STEMI6, N(%) | 32 (30) |
|
| |
| Intra-Aortic Balloon Pump N(%) | 17 (16) |
|
| |
| Shock N (%) | 46 (44) |
|
| |
| Coronary angiography performed N (%) | 83 (78) |
|
| |
| Percutaneous Coronary Intervention N (%) | (36) |
|
| |
| Temporary Pacemaker | 5 (6) |
|
| |
| Fentanyl, Median total dose in mg during ICU stay( IQR) N7 = 90 | 2700 (1569, 4444) |
|
| |
| Midazolam, Median total dose in mg during ICU stay (IQR) N7 = 72 | 64 (40, 108) |
|
| |
| Propofol, Median total dose in mcg during ICU stay (IQR) N7 = 26 | 1198 (295, 1663) |
Acute Physiology and Chronic Health Evaluation
Cerebral Performance Score
Cardiopulmonary resuscitation
Ventricular tachycardia
Ventricular fibrillation
ST segment elevation myocardial infarction
Median for drug doses is only for those patients that received actual drug
Delirium existed in 100% of survivors from the initiation of rewarming until the end of the ICU stay, for a median number of 4.0 (2.0 – 7.5 IQR) delirium days. Figure 2 shows the distribution of cognitive status each day following patient warming (coma, hypoactive delirium, mixed delirium, hyperactive delirium, and normal). Since the number of patients decreases over time, we display the relative percentage of patients for each patient day. The majority of delirium was hypoactive, with 90% of patients having at least one hypoactive delirium day, 21% of patients having at least one or more hyperactive delirium days, and 64% of patients having at least one mixed delirium day. Normal mental status and coma were less common. In sensitivity analyses, the presence of sedation following warming did not affect the prevalence of delirium (supplemental figure E1).
Figure 2.
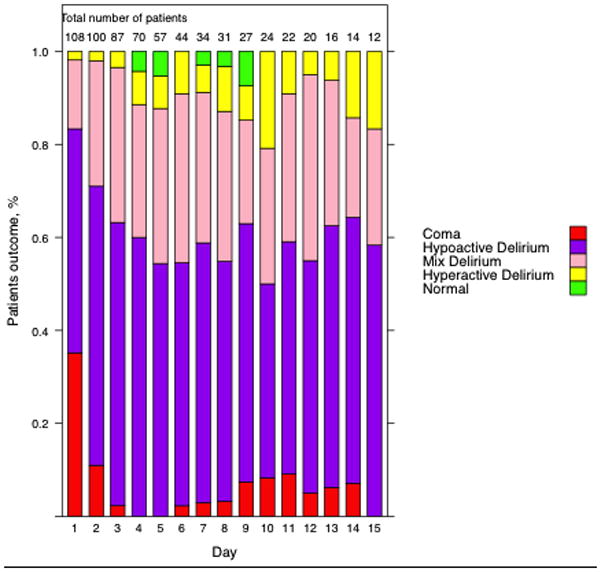
Figure 3 shows the effect of modifiable resuscitation or TH protocol-related risk factors on the duration of delirium. In evaluation of baseline risk factors and number of delirium days, multivariable analysis showed that age (OR 1.72, 95% CI 1.01 – 2.95, p=0.05), and time from initiation of CPR to return of spontaneous circulation (ROSC) (OR 1.52, 95% CI 1.11–2.07, p =0.01) correlated to more days of delirium. In contrast, use of propofol (OR 0.02, 95% CI 0.00 – 0.48, p= 0.02) correlated to shorter duration of delirium.
Figure 3.
Discussion
This study primarily demonstrates that all survivors of cardiac arrest treated with TH who awoke from coma experienced at least one day of delirium between rewarming and then subsequent discharge from the ICU. Although the high prevalence of delirium may seem surprising, this cohort is uniquely different from typical ICU populations; the degree of insult that cardiac arrest induces, the tremendous swings in metabolism from TH, as well as the large doses of psychoactive medications, all likely contribute to the higher prevalence. Independent risk factors for longer duration of delirium inlcuded increased age and longer times from CPR to ROSC. Total propofol dose during TH and prior to warming correlated with shorter days of delirium, whereas total dose of fentanyl and midazolam did not correlate with delirium duration. The high prevalence of delirium in this population calls for a need to understand the relationship between delirium and long-term outcomes in these patients and then, if warranted, interventions that will decrease the prevalence of delirium in the post-resuscitation period.
Delirium in critical-care units varies depending on the specific populations. Reported prevalences vary from 25% in our mixed-population CVICU [25] to 80% in medical-intensive care units [9]. Previous reports have stated that survivors of cardiac arrest who experienced a myocardial infarction have a higher prevalence of delirium [27]. Survivors of cardiac arrest treated with TH have unique characterisitcs; they are older, have multiple comorbidities, a high prevalence of coronary artery disease [26], and experience an acute anoxic insult that typically results in multi-organ failure and a high in-hospital mortality. Although the percentage of subtypes of delirium in our cohort of patients was similar to that reported in prior studies, the overall prevalence of delirium of 100% in this cohort (higher than that reported in other specific ICU populations) may reflect the unique nature of this population. Consistent with prior studies in a general CVICU population [25], the majority of patients experienced hypoactive delirium. Physicians caring for post-resuscitation patients should keep these data in mind, since it may help focus care on decreasing the length of delirium by developing and implementing evidence-based interventions [28].
We examined both pre-and-post resuscitation risk factors that we hypothesized could influence the duration of delirium. Among pre-hospital risk factors we found that age and longer times from initiation of CPR to ROSC correlate with increased number of delirium days. Previous reports include age has as a known risk factor for delirium [16]. Longer times from initiation to CPR to ROSC might reflect the detrimental effect that hypoxia has on brain function after TH. This finding supports the need for continued training of medical personnel in high-quality CPR that includes timely administration of direct cardioversion to re-establish a perfusing rhythm.
Our TH protocol, similar to many other institutions, utilizes high doses of sedatives prior to warming in response to the need for the use of paralytics to prevent shivering. We hypothesized that the use of post-resuscitation sedation prior to rewarming (i.e., sedation used during TH and prior to rewarming) could represent a modifiable risk factor that might raise the prevalence of delirium in this population of patients. Surprisingly, in our investigation of post-resuscitation modifiable risk factors, higher dose exposure to propofol during TH protected patients and correlated with decreasing days of delirium, and total dose of midazolam during TH did not correlate with increasing days of delirium. To our knowledge, no previous study shows an association between total propofol dose and a reduced number of days of delirium. Although previous reports associate benzodiazepines with delirium in multiple different ICU populations [8, 16], this post-resuscitation cohort clearly represents a unique group of patients that responded differently to sedatives than previously reported in other critically ill groups. Type I error may explain these findings related to propofol. Alternatively, our benzodiazepine data may be underpowered to determine truth in this “first look” study. It is worthwhile to consider some additonal hypotheses of why pre-warming total propofol dose and not midazolam correlated with delirium duration. First, we only evaluated the total dose of sedatives used during TH and did not analyze the effect of sedative use after rewarming, therefore, we limited our ability to assess their effect following warming. Second, we provided propofol more frequently to patients in the more recent years of the cohort, and therefore the results may reflect altnerative practice changes that may have occurred in later years. Alternatively, the use of propofol may represent confounding by indication, since we preferentially provided propofol during TH to patients that were at lower risk of delirium.
In order to address whether post-warming sedation drove our high prevalence of delirium, we split the population into patients on and off sedation. Supplemental figure E1 shows that by day 4, the percentage of patients receiving sedation, not receiving sedation, and prevalence of delirium were approximately equivalent. In addition, in the smaller group that received no sedation in the first few days following warming, the prevalence of delirium remained high. This indicates that the high prevalence of delirium, especially at later time points, is not all driven by sedation. Importantly, to our knowledge, there is no data to suggest differences between “sedative-delirium” and “non-sedative delirium” in this population. Further study is needed to understand whether there are any differential effects on outcomes depending if delirium occurs with our without sedation.
This study has several limitations. First, in 2013, Nielsen et al published results that showed that mild therapeutic hypothermia is not superior to targeted temperature management for post-resuscitation care [7]. The overseeing group of that study used a different design and therefore, we cannot directly compare it to the landmark trials from 2002 that established mild TH as the standard of care. Ongoing trials that examine the effect of different doses of TH may better guide the field in the future. Despite the results from the TTM trial, the results of this study still apply to current practice, due to the fact that mild TH remains guideline based therapy for post-resuscitation care. Second, we aimed to examine factors exclusive to patients exposed to TH, and we do not have any data to examine factors among patients not exposed to TH. Third, our institutuion limits us to a specific TH protocol, which may limit the generalizability of these data. Fourth, our model included variables that were determined a priori. In order to prevent over-fitting of our models, the list of confounders did not include all options, and unmeasured variables may exist that could alter our results. Fifth, the prevalence and duration of delirium in our results may exceed those in prior reports because we don’t have differential measurements of CAM-ICU before and after a spontaneous awakening trial, and our results may include patients with rapidly reversible delirium due to sedation [29]. This effect is likely small given the high prevalence of delirium found among patients without exposure to sedation in sensitivity analyses. Finally, we did not structure this study to design nor to assess the role of delirium in adverse outcomes such as, mortality or long-term cognitive impairment; this remains unknown in this specific population of patients and warrants future study.
Conclusion
This retrospective observational study demonstrates that there is a remarkably high prevalence of delirium during the ICU stay in our sample of patients treated with TH following cardiac arrest. Increasing age and longer times from initiation of CPR to ROSC were associated with increasing days of delirium, and higher total propofol dose used during TH was associated with decreasing days of delirium. Given the significant impact of delirium on outcomes shown in numerous other investigations, further prospective research is needed to understand the relationship between sedative and paralytic choice during TH and it’s relation to prevalence and duration of delirium as well as the relationship between delirium and long-term outcomes in this very unique group of patients.
Supplementary Material
Acknowledgments
Dr. Vasilevskis was supported by the National Institute on Aging of the National Institutes of Health under Award Number K23AG040157, Drs. Vasilevskis and Ely are both supported by the Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans’ Affairs
Footnotes
Conflicts of Interest:
All authors state they have no conflict of interest.
References
- 1.Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, Berg RA, Nichol G, Lane-Trultt T. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 2.Stiell IG, Wells GA, Field B, Spaite DW, Nesbitt LP, De Maio VJ, Nichol G, Cousineau D, Blackburn J, Munkley D, et al. Advanced cardiac life support in out-of-hospital cardiac arrest. The New England journal of medicine. 2004;351(7):647–656. doi: 10.1056/NEJMoa040325. [DOI] [PubMed] [Google Scholar]
- 3.Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. The New England journal of medicine. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 4.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. The New England journal of medicine. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 5.Hazinski MF, Nolan JP, Billi JE, Bottiger BW, Bossaert L, de Caen AR, Deakin CD, Drajer S, Eigel B, Hickey RW, et al. Part 1: Executive summary: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122(16 Suppl 2):S250–275. doi: 10.1161/CIRCULATIONAHA.110.970897. [DOI] [PubMed] [Google Scholar]
- 6.Nolan JP, Morley PT, Vanden Hoek TL, Hickey RW, Kloeck WG, Billi J, Bottiger BW, Morley PT, Nolan JP, Okada K, et al. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108(1):118–121. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. The New England journal of medicine. 2013;369(23):2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 8.Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JA, Jr, Dittus R, Ely EW. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. The Journal of trauma. 2008;65(1):34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA: the journal of the American Medical Association. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 10.Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, Truman B, Bernard GR, Dittus RS, Ely EW. Costs associated with delirium in mechanically ventilated patients. Critical care medicine. 2004;32(4):955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 11.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, Gordon SM, Canonico AE, Dittus RS, Bernard GR, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Critical care medicine. 2010;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. American journal of respiratory and critical care medicine. 2009;180(11):1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA: the journal of the American Medical Association. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 14.Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, Truman B, Dittus R, Bernard R, Inouye SK. The impact of delirium in the intensive care unit on hospital length of stay. Intensive care medicine. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pisani MA, Murphy TE, Araujo KL, Van Ness PH. Factors associated with persistent delirium after intensive care unit admission in an older medical patient population. Journal of critical care. 2010;25(3):540e541–547. doi: 10.1016/j.jcrc.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, Bernard GR, Ely EW. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Lat I, McMillian W, Taylor S, Janzen JM, Papadopoulos S, Korth L, Ehtisham A, Nold J, Agarwal S, Azocar R, et al. The impact of delirium on clinical outcomes in mechanically ventilated surgical and trauma patients. Critical care medicine. 2009;37(6):1898–1905. doi: 10.1097/CCM.0b013e31819ffe38. [DOI] [PubMed] [Google Scholar]
- 18.Chamorro C, Borrallo JM, Romera MA, Silva JA, Balandin B. Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: a systematic review. Anesthesia and analgesia. 2010;110(5):1328–1335. doi: 10.1213/ANE.0b013e3181d8cacf. [DOI] [PubMed] [Google Scholar]
- 19.Hollenbeck RD, Wells Q, Pollock J, Kelley MB, Wagner CE, Cash ME, Scott C, Burns K, Jones I, Fredi JL, et al. Implementation of a standardized pathway for the treatment of cardiac arrest patients using therapeutic hypothermia: “CODE ICE”. Critical pathways in cardiology. 2012;11(3):91–98. doi: 10.1097/HPC.0b013e31825b7bc3. [DOI] [PubMed] [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical care medicine. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 21.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Critical care medicine. 2001;29(7):1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. American journal of respiratory and critical care medicine. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 23.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA: the journal of the American Medical Association. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 24.Vasilevskis EE, Morandi A, Boehm L, Pandharipande PP, Girard TD, Jackson JC, Thompson JL, Shintani A, Gordon SM, Pun BT, et al. Delirium and sedation recognition using validated instruments: reliability of bedside intensive care unit nursing assessments from 2007 to 2010. Journal of the American Geriatrics Society. 2011;59(Suppl 2):S249–255. doi: 10.1111/j.1532-5415.2011.03673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McPherson JA, Wagner CE, Boehm LM, Hall JD, Johnson DC, Miller LR, Burns KM, Thompson JL, Shintani AK, Ely EW, et al. Delirium in the cardiovascular ICU: exploring modifiable risk factors. Critical care medicine. 2013;41(2):405–413. doi: 10.1097/CCM.0b013e31826ab49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumas F, Cariou A, Manzo-Silberman S, Grimaldi D, Vivien B, Rosencher J, Empana JP, Carli P, Mira JP, Jouven X, et al. Immediate percutaneous coronary intervention is associated with better survival after out-of-hospital cardiac arrest: insights from the PROCAT (Parisian Region Out of hospital Cardiac ArresT) registry. Circulation Cardiovascular interventions. 2010;3(3):200–207. doi: 10.1161/CIRCINTERVENTIONS.109.913665. [DOI] [PubMed] [Google Scholar]
- 27.Uguz F, Kayrak M, Cicek E, Kayhan F, Ari H, Altunbas G. Delirium following acute myocardial infarction: incidence, clinical profiles, and predictors. Perspect Psychiatr Care. 2010;46(2):135–142. doi: 10.1111/j.1744-6163.2010.00249.x. [DOI] [PubMed] [Google Scholar]
- 28.Balas MC, Vasilevskis EE, Burke WJ, Boehm L, Pun BT, Olsen KM, Peitz GJ, Ely EW. Critical care nurses’ role in implementing the “ABCDE bundle” into practice. Critical care nurse. 2012;32(2):35–38. 40–37. doi: 10.4037/ccn2012229. quiz 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. American journal of respiratory and critical care medicine. 2014;189(6):658–665. doi: 10.1164/rccm.201310-1815OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


