Abstract
Septins are a family of GTP binding proteins that are well conserved in eukaryotic species except plants. Septins contribute to the lateral compartmentalization of membranes, cortical rigidity, and the regulation of membrane trafficking by associating with membrane lipids, actin, and microtubules. The organ of Corti in the cochlea has pivotal roles in auditory perception and includes two kinds of highly polarized cells, hair and supporting cells, both of which are rich in actin and microtubules. To identify the roles of septins in the cochlea, we analyzed the localization of three septin proteins, septin 4 (SEPT4), septin 5 (SEPT5), and septin 7 (SEPT7) that are abundantly expressed in brain tissues, and also examined auditory functions of Sept4 and Sept5 null mice. SEPT4, SEPT5, and SEPT7 were expressed in inner and outer pillar cells and Deiters’ cells but the distribution patterns of each protein in Deiters’ cells were different. SEPT4 and SEPT7 were expressed in the phalangeal process where SEPT5 was not detected. In addition to these cells SEPT5 and SEPT7 were co-localized with presynaptic vesicles of efferent nerve terminals. Only SEPT7 was expressed in the cochlea at embryonic stages. Although expression patterns of septin proteins suggested their important roles in the function of the cochlea, both Sept4 and Sept5 null mice had similar auditory functions to their wild type littermates. Immunohistochemical analysis of Sept4 null mice showed that compensatory expression of SEPT5 in the phalangeal process of Deiters’ cells may have caused functional compensation of hearing ability in Sept4 null mice.
1. Introduction
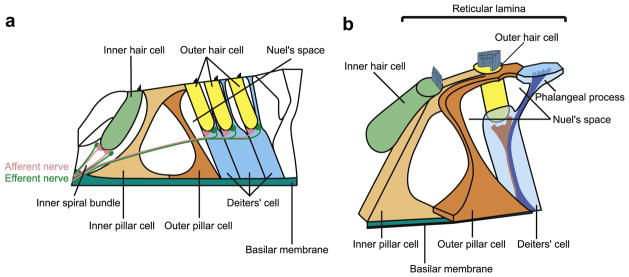
The organ of Corti is the receptor organ of the cochlea that has important roles in transducing the mechanical stimulation of incoming sound waves into electrical signals of the auditory nerve. Its principal cytologic structures are two types of cells; hair cells and supporting cells including inner phalangeal cells, pillar cells, Deiters’ cells, and Hensen’s cells (Fig. 1a). Hair cells have stereocillia on their apical surface and the displacement of stereocillia caused by the tectorial membrane and the vibration of basilar membrane results in excitation of hair cells that is transduced into the auditory nerve. Among the supporting cells, inner phalangeal cells, inner and outer pillar cells, Deiters’ cells and Hensen’s cells rest on the basilar membrane and apically extended to the basolateral surface of the hair cells. Inner phalangeal cells and Deiters’ cells form cups for the support of the hair cells (supporting cups), separating hair cells from the basilar membrane. Deiters’ cells contain two types of bundles within their cell bodies. The bundles originate above the basilar membrane and some extend to the supporting cups and the others to the apical surface of organs of Corti (the reticular lamina) to constitute phalangeal processes (Slepecky and Chamberlain, 1983) (Fig. 1b). In addition at the reticular lamina, hair cells are separated from one another by supporting cells (Slepecky, 1996). Thus, both hair and supporting cells have a highly polarized structure.
Fig. 1.
Schema of organs of Corti. a. Schematic image of section of organs of Corti. b. Schematic three dimensional image of inner and outer hair cells, pillar cells, and Deiter’s cell.
The inner and outer pillar cells and Deiters’ cells form rigid scaffolding with organized bundles of microtubules. These contribute to roles of supporting cells such as withstanding mechanical stress, maintaining the integrity of reticular lamina, transmitting the motion of the basilar membrane to the reticular lamina, and transmitting the motion of hair cells between the reticular lamina and the basilar membrane (Slepecky, 1996). In addition to these roles of supporting cells, recent reports showed that the supporting cells play an important role in recycling endolymphatic potassium via gap junctions including connexin26 (GJB2) during the transduction process (Kikuchi et al., 1995). The importance of the GJB2 gene in the cochlear function is supported by the fact that GJB2 mutations account for up to 50% of congenital deafness (Denoyelle et al., 1997; Estivill et al., 1998; Kelley et al., 1998).
These and other data indicate the importance of the supporting cells in the cochlea. However, little is known about their other functions, their intracellular structural detail, and their pattern of differentiation. In addition, only a few specific markers have been identified for supporting cells (Parker et al., 2011; Rio et al., 2002).
Septins are a family of polymerizing GTP binding proteins that are evolutionally conserved among a variety of eukaryotes but are absent in plants (Pan et al., 2007). Each septin protein contains a conserved GTP binding domain, with or without N-terminal proline-rich and C-terminal coiled coil domains (Kartmann and Roth, 2001). There are 13 and 14 septin genes respectively in the mouse and human genomes (Hall et al., 2005; Tada et al., 2007) and they are divided into four subgroups by structural homologies (Kinoshita, 2003). It was also reported that subtypes of septins can partially compensate for one another functionally (Buser et al., 2009).
Septin proteins can form elaborate networks of coils, rings and gauzes by self-assembly, and constitute an emerging non-canonical cytoskeleton. Septin superstructures can also associate with lipid membranes, actin filaments and microtubules, or can be free-floating in the cytoplasm (Kinoshita, 2006). Septins were originally identified in budding yeast where they localize in the presumptive bud site and form a ring around the bud neck during cytokinesis (Hartwell, 1971). They are postulated to form a scaffold at the bud neck and have a demonstrated role in the localization of other cytokinesis proteins. In addition, septins act as a diffusion barrier to segregate some proteins and mRNAs between mother and daughter cells. Structural septin meshwork interacts directly with membrane lipids and they can contribute to the lateral compartmentalization of membranes (Caudron and Barral, 2009) to provide polarity to cells. Similar scaffolding and diffusion barrier roles have been described for mammalian septins. For example septins have been shown to serve as diffusion barriers at the annulus of spermatozoa (Ihara et al., 2005; Kwitny et al., 2010) and are presumed diffusion barriers in dendritic spines of neurons (Tada et al., 2007; Xie et al., 2007). In addition, they have been shown to regulate exocytosis from platelets (Dent et al., 2002) and the presynaptic compartment of neurons (Amin et al., 2008; Beites et al., 1999).
The organ of Corti has many characteristics that could be contributed by septins. It is rich in microtubules and composed of highly polarized hair and supporting cells. In particular, hair cells, which transduce sound signal to the auditory nerve, are rich in synapses.
Therefore, we examined the expression patterns and functions of septins in the cochlea. As a starting point in the study of septin expression in the cochlea, we picked three subtypes of septins, septin 4 (SEPT4), septin 5 (SEPT5), and septin 7 (SEPT7) that are abundantly expressed in brain (Kinoshita et al., 2000). We reasoned that these proteins would be expressed and have important roles there since the sensory epithelium in the organ of Corti has many characteristics in common with brain especially in their development. It develops from the otic placode that can also give rise to stato-acoustic ganglion neuron cells. In addition, the similar mechanisms, including Notch signaling, are used between the determination of the cell fate from common progenitors to hair and supporting cells in the organ of Corti and the determination of that to neural cells and glia in the neuron. SEPT7 is diffusedly expressed in the brain (Kinoshita et al., 2000) and also it is a core component of multimeric septin complexes in various mammalian cells (Fujishima et al., 2007; Joberty et al., 2001; Nagata et al., 2004; Xie et al., 2007), indicating that the expression pattern of SEPT7 is likely to reflect the sum distribution of most septin proteins. SEPT4 is one of the subtypes which are also abundantly expressed in the adult mouse brain (Kinoshita et al., 2000) and are well characterized with regards to its function in the central nervous system (Ihara et al., 2007). SEPT5 is a subtype which is most similar to SEPT4 in phylogenetic relationship (Hall et al., 2005) and is reported to be associated with presynaptic vesicles of the brain (Beites et al., 1999; Kinoshita et al., 2000), organelles also abundant in the cochlea.
We also examined the auditory function of Sept4 and Sept5 null mice to test their requirement in the cochlea.
2. Materials and methods
2.1. Animals
The Sept4 null mice (Ihara et al., 2005), Sept5 null mice (Peng et al., 2002) and ICR mice (Japan SLC, Hamamatsu, Japan) used in this study were cared for in the Institute of Laboratory Animals of the Kyoto University Graduate School of Medicine. The Animal Research Committee of the Kyoto University Graduate School of Medicine approved all experimental protocols, which were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.2. Reverse transcriptase polymerase chain reaction (RT-PCR)
Postnatal day 7 (P7) ICR mice were used to isolate RNA from brain and cochleae, and adult ICR mice (approximately 12 weeks old) were used for the testis RNA. With regard to a cochlear sample, bony otic capsule was removed under a stereomicroscope and the remaining tissue was collected as a whole cochlear sample. Samples were transferred to ice cold TRIzol (Invitrogen, Carlsbad, CA) and stored at −80 °C prior to RNA isolation. Samples were homogenized and total RNA was isolated from these samples using a standard chloroform/isopropanol extraction and treated with Dnase I (Ambion Inc, Austin, TX). cDNA was synthesized from DNase I-treated total RNA with random hexamer primers using Taq Man reverse transcription reagents (Applied Biosystems, Foster City, CA). Amplification of transcripts of septin gene family (SEPT1–12 and 14) was done by an initial denaturing step of 94 °C for 5 min, followed by 30 cycles of PCR (94 °C for 30 s, 60 °C for 30 s, 72 °C for 1 min) by using ExTaq polymerase (Takara, Shiga, Japan) with primer pairs shown in Table 1.
Table 1.
Nucleotide sequences of primers used for RT-PCR.
| mRNA | Sequences of forward and reverse primers (5′ to 3′) |
|---|---|
| Sept1 | TCTGCTCTACGAGGGCTACC |
| ATCTGGGCTTGCATCTTCTC | |
| Sept2 | CATTGCGAAAGCTGACACTC |
| GCCTTTGGCTTCAATCAACT | |
| Sept3 | AGTCGGAATTCAAGCAGAGG |
| TTGAGGTCCTGGAGGTGAGT | |
| Sept4 | CCAGACTGTGATTCCGATGA |
| CGGGTCACATCCTTTAGGTC | |
| Sept5 | CTGAAACACACCGTCGACAT |
| GATGAGTGGGACGATGTTCA | |
| Sept6 | CTGTAGGCTTTGGGGATCAG |
| GGCAATAACGGGAATGATGT | |
| Sept7 | GAACCTTGAGGGCTATGTGG |
| GTCAGCAGCAACTGAACACC | |
| Sept8 | GAGGATCCATGTTTGCCTCT |
| ATCGGTGGGAAACTGGTAGA | |
| Sept9 | TTTGATGAGGACGCAGAAGA |
| GGTAGGCTTCGAAGTGGATG | |
| Sept10 | GGATCTACGGGAGCAGACAC |
| CTCTCTTTCCGCTTCCTTCA | |
| Sept11 | TGATCCGAGTGAACATGGAG |
| CTTCACCCTCATGACGAACA | |
| Sept12 | TGGGACCCTATCCTGAGCTA |
| TGAAGGCATCTCGTTCTTCA | |
| Sept14 | CAGGACATTCCCTCAAGTCC |
| TTCACTTGAGCACTCGCTTC |
2.3. Immunohistochemistry
Inner ear tissues were harvested from embryonic day 13, 15 (E13, 15), postnatal day 1, 2, 3, 7 (P1, 2, 3, 7) and three month old adult mice. The experimental animals were euthanized using carbon dioxide. In the case of embryonic and neonatal mice, the temporal bones were removed rapidly after the euthanasia. The adult mice were intracardially perfused with 0.01 M phosphate-buffered saline (PBS) at pH 7.4 followed by 4% paraformaldehyde in 0.01 M PBS at pH 7.4 before excision of the temporal bones. Removed temporal bones were immersed in 4% paraformaldehyde (PFA) for 4 h at 4 °C. The adult mice cochleae were decalcified with 10% ethylenediamine-tetraacetic acid (EDTA) for 7 days after fixation. The tissue was washed in several changes of PBS, cryoprotected in 30% sucrose in PBS, embedded in Tissue-Tec OCT Compound (Sakura Finetek, Japan) and sectioned at 10 μm. Sections were collected on SuperFrost micro slide glasses (Matsunami Glass IND., LTD., Osaka, Japan) and air dried for no more than 30 min. The plane of sectioning was oriented to produce mid-modiolar sections through the cochlea.
For immunofluorescence, sections were fixed in 4% PFA for 15 min, washed in PBS and then incubated with 10% normal goat serum (Invitrogen, Carlsbad, CA) in PBS/0.1% TritonX100 for 30 min and then incubated at 4 °C overnight or at room temperature for 1 h (in the case of mouse anti-myosin 7a antibody) with primary antibody, diluted in 10% normal goat serum in PBS/0.1% TritonX100. After that the tissues were rinsed with PBS/0.1% TritonX100, incubated for 1 h with a fluorescent-conjugated secondary antibody and Alexa Fluor 633 phalloidin (Invitrogen), rinsed with PBS three times and coverslipped in Flouromount G (Beckman Coulter, Inc., Fullerton, CA). Primary antibodies used were as follows: rabbit polyclonal anti-SEPT4 antibodies (Ihara et al., 2005) used at 1:100 dilution; rabbit polyclonal anti-SEPT5 antibodies (Kinoshita et al., 2000) used at 1:500 dilution; rabbit polyclonal anti-SEPT7 antibodies (Kinoshita et al., 2000) used at 1:500 dilution; mouse monoclonal anti-β3-tubulin antibody (Tuj-1) (Covance, Princeton, NJ) used at 1:500 dilution; mouse monoclonal anti-acetylated α tubulin antibody (Sigma–Aldrich, St. Louis, MO) used at 1:250 dilution; mouse monoclonal anti-myosin 7a antibody (Developmental studies hybridoma bank) used at 1:10 dilution; Purified mouse anti-synaptophysin monoclonal antibody (BD Biosciences Pharmingen, Franklin Lakes, NJ) used at 1:50 dilution. Secondary antibodies used were Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 568 goat anti-mouse IgG (from Invitrogen, Carlsbad, CA), all of which were used at 1:500. Images of sections were captured on a Leica Laser Scanning Spectral Confocal Microscope (TCS SP2 and TCS SPE).
2.4. Auditory brain stem response (ABR) recording
An ABR recording was used to monitor the auditory function of the experimental animals. Under general anesthesia with mid-azolam (10 mg/kg), medetomidine (37.5 mg/kg) and butorphanol (0.5 mg/kg), ABR measurements were performed as previously described (Shiga et al., 2005). The ABRs of Sept4 or Sept5 null mice and their wild type littermates were recorded at 1 month, 5 months or 9 months after birth. Thresholds were determined for the frequencies of 10, 20, and 40 kHz from a set of responses at various intensities with 5-dB sound pressure level (SPL) intervals. Differences in ABR threshold between Sept4 or Sept5 null mice and wild type mice were analyzed by unpaired t-test. P-values less than 0.05 were considered to be statistically significant.
3. Results
3.1. Results of RT-PCR
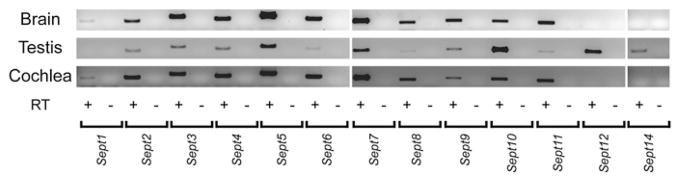
To examine which subtypes of septin gene family are expressed in the cochlea, we performed RT-PCR for all septin genes from the P7 cochlea and brain and the adult testis (Fig. 2). The P7 cochlea expressed all septin genes except Sept12 and Sept14. This expression pattern of septin gene subtype was similar to that of the brain but different from that of the testis.
Fig. 2.
RT-PCR of septin family genes from P7 cochleae. Expression of septin family genes were detected by RT-PCR analysis using P7 brain and cochlea and adult testis. The brain and cochlea showed similar expression patterns.
3.2. Results of immunohistochemistry
Among 13 septin subtypes, we examined the mouse cochlea for the spatial expression of SEPT4, SEPT5, and SEPT7 which are all abundant in the nervous system (Kinoshita et al., 2000) because RT-PCR results suggested that the expression pattern of septin genes were similar between the cochlea and brain.
3.2.1. Expression and distribution of SEPT4, 5, and 7 in the adult mice cochlea
3.2.1.1. Expression and distribution of SEPT4 in the adult mice cochlea
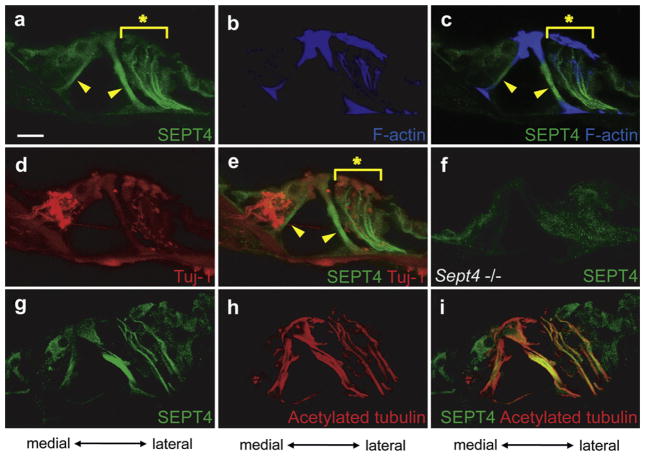
SEPT4 was strongly expressed in the inner and outer pillar cells (Filled arrowheads in Fig. 3a, c and e). SEPT4 was also detected between reticular lamina and basilar membrane in a filament-like pattern (Asterisks in Fig. 3a, c, and e) lateral to outer pillar cells, presumably in the Deiters’ cells. To exclude the possibility that this staining pattern indicates cochlear nerve fibers leading to hair cells, we performed double immunohistochemical staining against SEPT4 and β3-tubulin, a marker of nerve fiber in the cochlea (Fig. 3d and e) and against SEPT4 and acetylated α tubulin, a marker of stabilized microtubules that is abundant in Deiters’ cells (Fig. 3g–i). The results showed that the expression pattern of SEPT4 did not overlap with that of β3-tubulin (Fig. 3a, d, and e) but with that of acetylated α tubulin (Fig. 3g–i). These results indicated that SEPT4 positive filament-like structures were stabilized microtubules that supposedly belonged to Deiters’ cells. SEPT4 was also expressed in hair cells and other supporting cells but weaker in intensity. We confirmed specificity of SEPT4 antibodies using Sept4 null mice (Ihara et al., 2005), where the SEPT4-containing structures were undetectable (Fig. 3f and data not shown).
Fig. 3.
Expression pattern of SEPT4 in the adult mouse cochlea. a–e. Immunohistochemistry of the wild type adult mouse cochlea from a same section. SEPT4 (a), F-actin (b), SEPT4 and F-actin (c), β3-tubulin (Tuj-1) (d), and SEPT4 and β3-tubulin (Tuj-1) (e) were detected. f. Immunohistochemistry of adult Sept4 null mice cochlea for SEPT4. g–i. Immunohistochemistry of the wild type adult mouse cochlea from a same section. SEPT4 (g), acetylated α tubulin (h), and SEPT4 and acetylated α tubulin (i) were detected. SEPT4 (green) was strongly expressed in pillar cells (Arrowheads in a, c, and e). SEPT4 was also strongly expressed in filament-like structures in the lateral side of the organ of Corti from the basilar membrane to the reticular lamina (Asterisks in a, c, and e), presumably in the Deiters’ cells. SEPT4 signal overlapped with that of F-actin (blue) (b and c) and acetylated α tubulin (red) (h and i), but did not overlap with that of Tuj-1 (red) that is expressed in nerve fibers (d and e). These data indicated that SEPT4 was expressed in Deiters’ cells. SEPT4 was also expressed in hair cells and other supporting cells in weaker intensity. Detection of SEPT4 antibodies in Sept4 null mice cochlea was served as negative control of these experiments (f). Scale bar in a indicates 15 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.1.2. Expression and distribution of SEPT5 in the adult mice cochlea
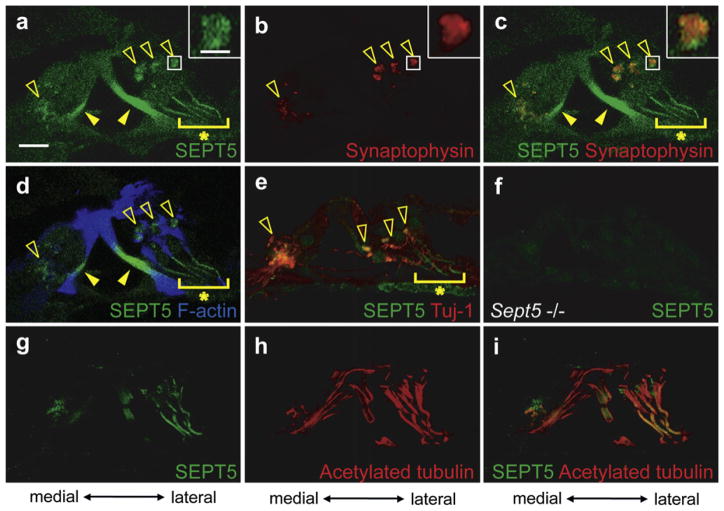
SEPT5 was strongly expressed in inner and outer pillar cells (Filled arrowheads in Fig. 4a, c and d) similar to the expression pattern of SEPT4. SEPT5 was also expressed in filament-like structures arising from the basilar membrane lateral to outer pillar cells, presumably in Deiters’ cells (Asterisk in Fig. 4a). Unlike SEPT4, SEPT5 was detected beneath the inner hair cells and the basal portion of the outer hair cells in a punctate pattern (Open arrowheads in Fig. 4a and c and insets in Fig. 4a and c). Considering that SEPT5 is associated with presynaptic vesicles in the brain (Beites et al., 1999; Kinoshita et al., 2000), we assumed that SEPT5 was co-localized with presynaptic vesicles in the cochlea. To confirm this we performed double immunohistochemical staining against SEPT5 and synaptophysin, a component of the presynaptic vesicles of the efferent nerve terminals around cochlear hair cells, that is, around inner spiral bundle (Fig. 1a) and around the basal portion of outer hair cells (Gil-Loyzaga and Pujol, 1988). The expression of SEPT5 overlapped with that of synaptophysin (Fig. 4a, b, and c). Since the images were the confocal optical sections, these results indicated that SEPT5 co-localized with presynaptic vesicles of efferent nerve terminals in the cochlea. SEPT5 positive regions below inner hair cells and around the basal portion of outer hair cells were also positive for β3-tubulin, suggesting that SEPT5 was expressed in the efferent terminal of the lateral and medial olivocochlear tract. SEPT5 positive filament-like structures did not coexist with β3-tubulin positive fibers (Asterisk in Fig. 4e) but with F-actin positive fibers (Asterisk in Fig. 4d) and acetylated α tubulin (Fig. 4g–i), indicating that these SEPT5 positive fibers existed in Deiters’ cells and were associated with stabilized microtubules. The SEPT5 antibodies did not label the Sept5 null cochlea, indicating the authenticity of the aforementioned data (Fig. 4f).
Fig. 4.
Expression pattern of SEPT5 in the adult mouse cochlea. a–e and g–i. Immunohistochemistry of the wild type adult mouse cochlea for SEPT5 (a and g), synaptophysin (b), SEPT5 and synaptophysin (c), SEPT5 and F-actin (d), and SEPT5 and β3-tubulin (Tuj-1) (e), acetypated α tubulin (h), and SEPT5 and acetylated α tubulin. a–d and g–i are images from the same section, respectively. Images in the insets of a–c are the magnified images of the boxed region in each panel. f. Immunohistochemistry of adult Sept5 null mice cochlea for SEPT5. SEPT5 (green) was expressed in pillar cells (Filled arrowheads of a, c, and d). Moreover SEPT5 was expressed in the punctate structures (open arrowheads in a, c, d, and e and insets in a and c) and filament-like structure arising from the basilar membrane (asterisks in a, c, d, and e). The SEPT5 positive punctate structures were detected below inner hair cells and around the basal portion of outer hair cells (open arrowheads in d) and overlapped with that of synaptophysin (red) (open arrowheads in b and c and insets in b and c), which is a component of the presynaptic vesicles of the efferent nerve terminals in the cochlea. The punctate structure below inner hair cells was also positive for β3-tubulin (Open arrowheads in e). The SEPT5 positive filament-like structure did not express β3-tubulin (Tuj-1, red) (Asterisk in e), but contained F-actin (blue) (Asterisk in d) and acetylated α tubulin (red) (h and i), indicating that this filament-like structure exists in Deiters’ cells. Detection of SEPT5 antibodies in Sept5 null mice cochlea was served as negative control of these experiments (f). Scale bar in a and insets of a indicates 15 μm and 3 μm, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
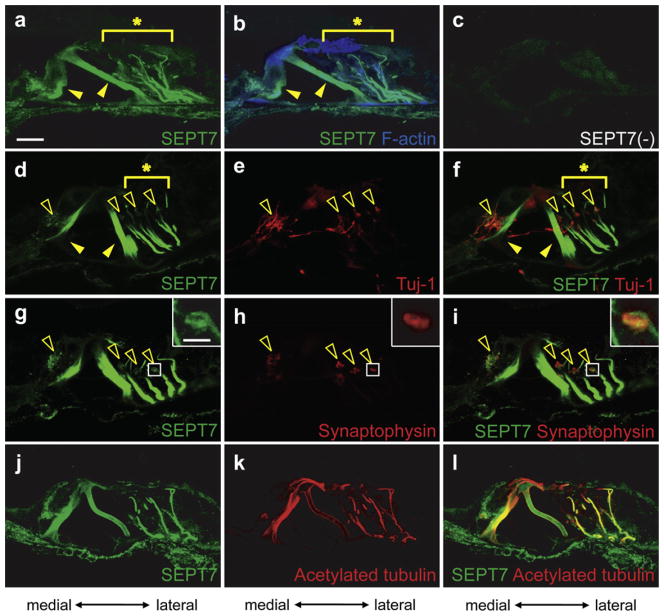
3.2.1.3. Expression and distribution of SEPT7 in the adult mice cochlea
SEPT7 was extensively expressed in the inner and outer pillar cells (Filled arrowheads in Fig. 5a, b, d and f). SEPT7 was also detected between reticular lamina and basilar membrane in a filament-like pattern (Asterisks in Fig. 5a, b, d, and f) lateral to outer pillar cells, presumably in the Deiters’ cells. These two SEPT7 expression patterns were similar to those of SEPT4. The SEPT7 positive filament-like structure did not express β3-tubulin (Asterisk in Fig. 5f) but expressed acetylated α tubulin (Fig. 5j–l), indicating that this structure was a stabilized microtubule that existed in Deiters’ cells as was the case for SEPT4. SEPT7 appeared to present in hair cells and other supporting cells including Hensen’s cells, and inner and outer sulcus cells although weaker in intensity (Fig. 5a and b). In addition, SEPT7 was detected beneath the inner hair cells and the basal portion of the outer hair cells in a punctate pattern like SEPT5 (Open arrowheads in Fig. 5d and g and an inset in Fig. 5g). Double immunohistochemistry of SEPT7 and β3-tubulin (Fig. 5d–f) and SEPT7 and synaptophysin (Fig. 5g–i) showed that this SEPT7 positive punctate structure co-localized with presynaptic vesicles of the efferent nerve terminals. Specificity of anti-SEPT7 antibodies could not be tested because of the embryonic lethality of Sept7 null mice but omission of the anti-SEPT7 primary antibody did not show any signal in the cochlea. SEPT7 was also detected in other parts of the cochlea but its expression level elsewhere was much weaker (data not shown).
Fig. 5.
Expression pattern of SEPT7 in the adult mouse cochlea. Immunohistochemistry of the wild type adult mouse cochlea for SEPT7 (a, d, g, and j), SEPT7 and F-actin (b), no primary antibodies (c), β3-tubulin (Tuj-1) (e), SEPT7 and β3-tubulin (Tuj-1) (f), synaptophysin (h), and SEPT7 and synaptophysin (i), acetylated α tubulin (k), and SEPT7 and acetylated α tubulin (l). a–c, d–f, g–i, and j–l are images from the same sections, respectively. Images in the insets of g–i are the magnified images of the boxed region in each panel. SEPT7 (green) was expressed in pillar cells (Filled arrowheads in a, b, d, and f) and Deiters’ cells (Asterisks in a, b, d, and f), which was similar to the pattern of SEPT4. The filament-like structure did not express β3-tubulin (Tuj-1, red) (e and asterisk in f), but contained F-actin (blue) (b) and acetylated α tubulin (red) (k and l). Moreover SEPT7 was expressed in the punctate structure below inner hair cells and around the basal portion of outer hair cells (open arrowheads in d, f, g, and i and an inset in g), which was a similar pattern to that of SEPT5. This punctate structure contained synaptophysin (red) (open arrowheads in h and i and insets in h and i) which is a component of presynaptic vesicles of the efferent nerve terminals in the cochlea and also expressed β3-tubulin (red) (Open arrowheads in e and f). Scale bar in a and in the inset of g indicates 15 μm and 3 μm, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
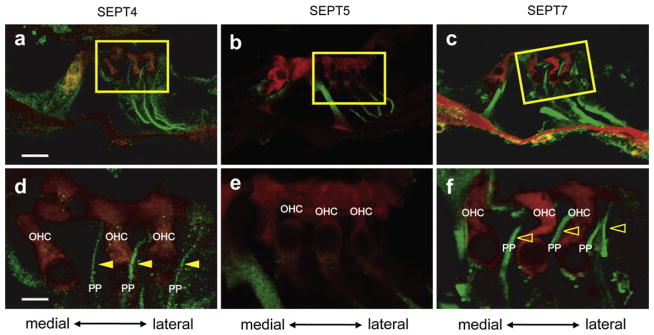
3.2.1.4. Comparison of the expression patterns between SEPT4, SEPT5 and SEPT7 in Deiters’ cells in the adult mice
Although SEPT4 (Fig. 3a and g), SEPT5 (Fig. 4a and g) and SEPT7 (Fig. 5a, d, g, and j) were expressed in the filament-like structures in Deiters’ cells, their expression patterns were different. The filament-like structures of SEPT4 (Fig. 6a and filled arrowheads in Fig. 6d) and SEPT7 (Fig. 6c and open arrowheads in Fig. 6f) extended from the basilar membrane tothe reticular lamina. On the other hand, the filament-like structure of SEPT5 extended from the basilar membrane not to the reticular lamina but only to the supporting cups (basal portion of the outer hair cells) (Fig. 6b and e). These results indicated that SEPT4 and SEPT7 were expressed in both types of bundles in Deiters’ cells (Slepecky and Chamberlain, 1983) but SEPT5 was expressed in only one type of bundles that run from the basilar membrane to supporting cups (Slepecky and Chamberlain, 1983). Double immunohistochemistry of the hair cell marker (myosin 7a) and septinproteins indicated that only SEPT4 and SEPT7 were expressed in the bundles to the reticularlamina, that is, phalangeal process of Deiters’ cells (Fig. 6a, c, d, and f).
Fig. 6.
The difference of the expression patterns of SEPT4, SEPT5, and SEPT7 in Deiters’ cells. Immunohistochemistry of the wild type adult mouse cochlea for myosin 7a and SEPT4 (a and d), SEPT5 (b and e), or SEPT7 (c and f). d, e, and f are the magnified images of the boxes in a, b, and c, respectively. The filament-like structures of SEPT4 (green) (a and d) and SEPT7 (green) (c and f) originated from the basilar membrane and extended to the apices of myosin 7a (Myo7a, red) positive outer hair cells (Filled arrowhead in d for SEPT4 and open arrowhead in f for SEPT7) running outside outer hair cells. On the other hand, the SEPT5 positive filament-like structures (green) originated from the basilar membrane and extended to the supporting cups (basal portion of outer hair cells) but not to the apices of outer hair cells (b and e). These data indicated that SEPT4 and SEPT7 were expressed in the phalangeal process of Deiters’ cells, but SEPT5 was not. OHC: outer hair cell; PP: phalangeal process; Scale bars in a and d indicate 15 μm and 5 μm, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.2. Expression of septin proteins in septin null mice
To examine the status of other septins in the absence of SEPT4 or SEPT5, we performed immunohistochemistry on the cochleae of Sept4 or Sept5 knockout mice.
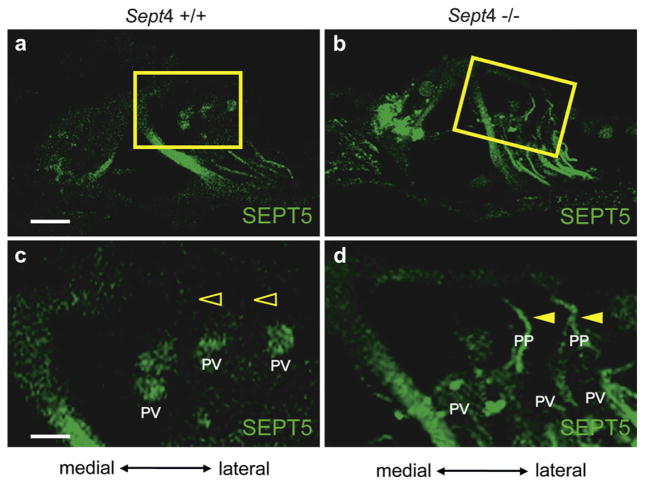
3.2.2.1. Expression and distribution of SEPT5 in adult Sept4 null mice cochlea
SEPT5 was expressed in pillar cells, Deiters’ cells and the presynaptic vesicles in Sept4 null mice cochlea (Fig. 7b and d) as in the wild type mice cochlea (Fig. 7a and c). However, the SEPT5 positive filament-like structure in Deiters’ cells extended from the basilar membrane to the apices of outer hair cells in Sept4 null mice (Fig. 7b and filled arrowheads in d). With regard to Deiters’ cells, SEPT5 protein was present in the bundles to the reticular lamina (phalangeal processes) in the Sept4 null cochlea (Filled arrowheads in Fig. 7d). These results indicate the compensatory expression and the distribution of SEPT5 in phalangeal processes that lack SEPT4.
Fig. 7.
Expression of SEPT5 protein in Sept4 null mouse cochlea. Immunohistochemistry of SEPT5 in the wild type adult mouse cochlea (a and c) and in the Sept4 knockout mouse cochlea (b and d). c and d are magnified images of a and b, respectively. SEPT5 (green) was not expressed in phalangeal processes of Deiters’ cells in the wild type mouse (a and open arrowheads in c). But the SEPT5 positive filament-like structure in Deiters’ cells of Sept4 null mouse extended to the apices of the outer hair cells (b and filled arrowheads in d), indicating that SEPT5 was expressed in the phalangeal process in Sept4 null mice. PV: presynaptic vesicle; PP: phalangeal process; Scale bars in a and c indicate 15 μm and 5 μm, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
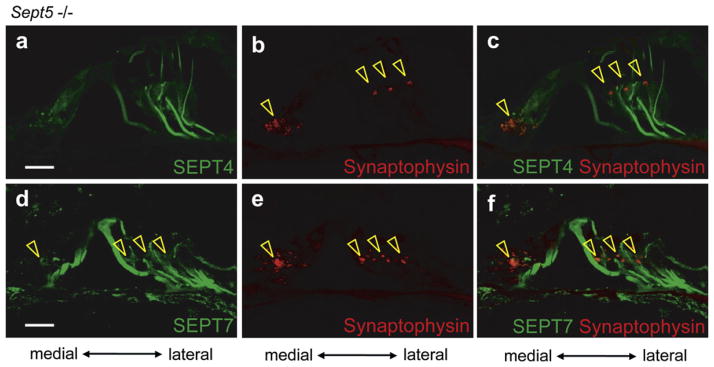
3.2.2.2. Expression and distribution of SEPT4 and SEPT7 in adult Sept5 null mice cochlea
The expression patterns of SEPT4 in Sept5 null mice cochleae (Fig. 8a and c) were indistinguishable from those of the wild type control (Fig. 3a). Neither compensatory redistribution nor delocalization of SEPT4 and SEPT7 was detected in Sept5 null mice (Open arrowheads in Fig. 8b–f indicate synaptophysin positive presynaptic puncta).
Fig. 8.
The expression pattern of SEPT4 and SEPT7 in Sept5 null mouse cochlea. Immunohistochemistry for various proteins in the Sept5 knockout mice cochlea. a–c and d–f are images from the same sections, respectively. (a–c) Immunohistochemistry for SEPT4 (a), synaptophysin (b), and SEPT4 and synaptophysin (c). (d–f) Immunohistochemistry for SEPT7 (d), synaptophysin (e), and SEPT7 and synaptophysin (f). The expression pattern of SEPT4 in Sept5 null mice cochleae (green) (a and c) was similar to that in wild type mice cochleae. The expression pattern of SEPT7 (green) in Sept5 null mouse cochlea was also similar to that in the wild type mouse cochlea in pillar cells, Deiters’ cells, and presynaptic vesicles (d and f). The expression patterns of SEPT4 did not overlap with that of synaptophysin (red) (Open arrowheads in b and c) and that of SEPT7 positive punctate structure did as in the wild type mice (Open arrowheads in d–f). These data indicated that compensatory expression of SEPT4 for Sept5 deficiency among presynaptic vesicles of the efferent nerves was not observed in Sept5 null mice. Scale bars in a and d indicate 15 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.3. Expression and distribution of SEPT4, SEPT5, and SEPT7 in the cochlea of developing and neonatal mice
To gain insight into the differential behaviors of SEPT4, SEPT5, and SEPT7, we examined their expression pattern in the embryonic and neonatal cochleae.
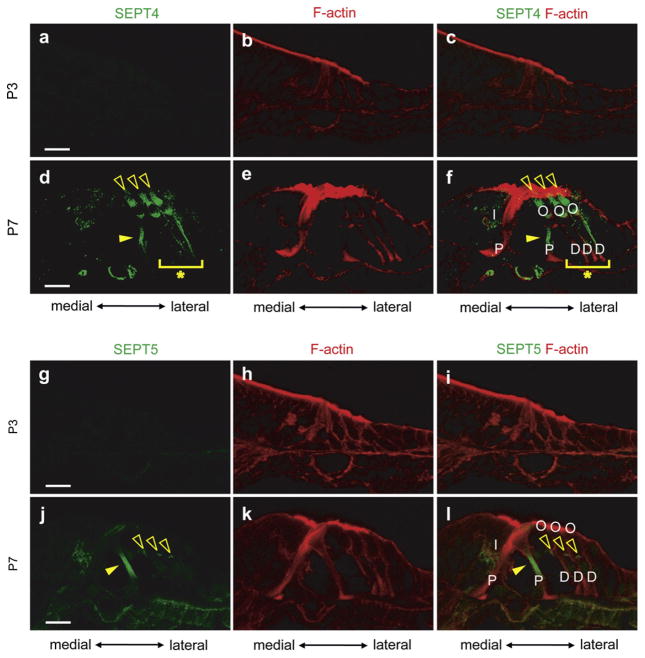
3.2.3.1. Expression and distribution of SEPT4 in the cochleae of developing and neonatal mice
We performed immunohistochemical staining on the cochleae from embryonic days 13 (E13, data not shown), 15 (E15, data not shown), postnatal days 1 (P1, data not shown), 2 (P2, data not shown), 3 (P3, Fig. 9a–c) and 7 (P7, Fig. 9d–f) mice using antibodies against SEPT4 protein. SEPT4 was undetectable in the cochlea at E13, E15, P1, P2, and P3. SEPT4 expression was first seen in outer pillar cells (filled arrowheads in Fig. 9d and f) and Deiters’ cells (Asterisks in Fig. 9d and f) at P7. It was also weakly expressed in inner hair cells (Fig. 9f). Moreover SEPT4 was strongly expressed in outer hair cells at P7 (Open arrowheads in Fig. 9d and f) and inner pillar cells did not express SEPT4 at this stage, which were different patterns from those seen in adult cochlea. The expression of SEPT4 in outer hair cells weakened as the cochlea matured (Fig. 3a–c), suggesting that SEPT4 might have some roles in outer hair cell maturation during the neonatal stage.
Fig. 9.
The expression pattern of SEPT4 and SEPT5 in developing mouse cochlea. Immunohistochemistry of P3 (a–c and g–i) and P7 (d–f and j–l) mouse cochleae for SEPT4 (a and d), SEPT5 (g and j), F-actin (b, e, h, and k), SEPT4 and F-actin (c and f), and SEPT5 and F-actin (i and l). a–c, d–f, g–i and j–l are images from the same sections, respectively. SEPT4 was not expressed in the cochlea till P3 (a). SEPT4 started its expression in outer pillar cells (Filled arrowheads in d and f) and Deiters’ cells (Asterisks in d and f) at P7. It was also weakly expressed in inner hair cells (d). These expression patterns of SEPT4 in the P7 mouse cochlea were similar to that in the adult mouse cochlea except that inner pillar cells did not express SEPT4. Interestingly SEPT4 was strongly expressed in the outer hair cells at this stage (Open arrowheads in d and f). SEPT5 was also not expressed in the cochlea till P3 (g). SEPT5 started its expression in outer pillar cells (Filled arrowheads in j and l) and presynaptic vesicles (Open arrowheads in j and l) at P7. We did not observe any SEPT5 signals in inner pillar cells and Deiters’ cells where the signals were detected in adult cochleae. D: Deiters’ cell; I: inner hair cell; O: outer hair cell; P: pillar cell; Scale bars indicate 15 μm in a, d, g, and j.
3.2.3.2. Expression and distribution of SEPT5 in the cochleae of developing and neonatal mice
SEPT5 was undetectable in the cochlea at E13 (data not shown), E15 (data not shown), P1 (data not shown), P2 (data not shown), and P3 (Fig. 9g–i). SEPT5 expression was first detected in the outer pillar cells (Filled arrowheads in Fig. 9j and l) and with presynaptic vesicles (Open arrowheads in Fig. 9j and l) at P7. We did not observe any SEPT5 signals in inner pillar cells and Deiters’ cells where the signals were detected in adult cochleae (Fig. 4). These results suggest that SEPT5 may contribute to the development of outer pillar cells and presynaptic vesicles in the cochlea but it likely contributes only to the maturation or maintenance of inner pillar cells and Deiters’ cells rather than their development.
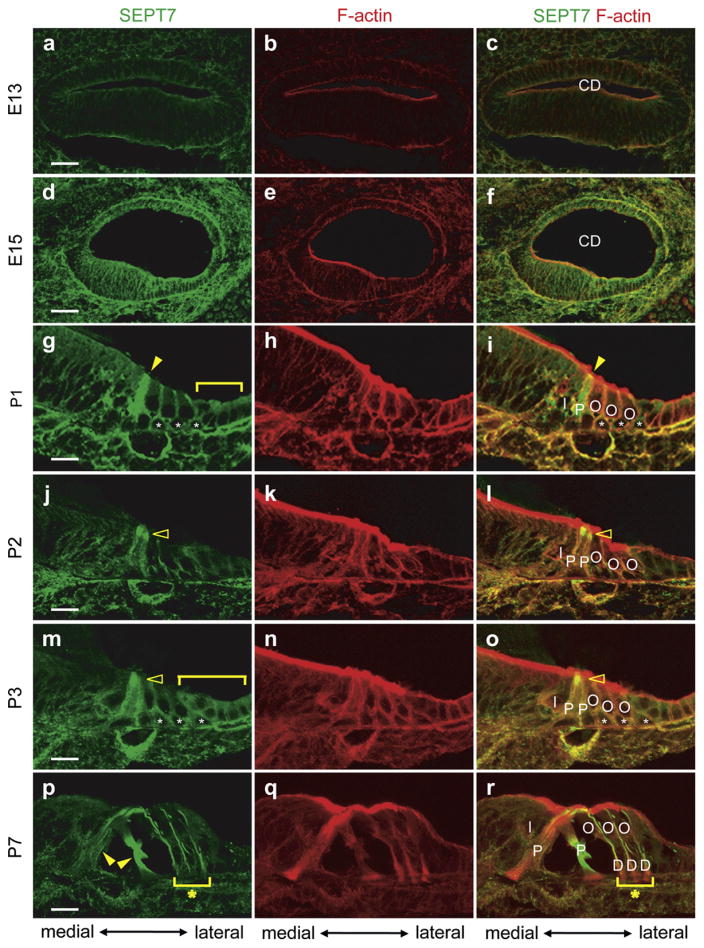
3.2.3.3. Expression and distribution of SEPT7 in the cochleae of developing and neonatal mice
SEPT7 was weakly expressed at the plasma membrane of most epithelial cells in the cochlear duct at E13 (Fig. 10a–c). Its expression pattern at E15 was similar to that at E13 but became much stronger (Fig. 10d–f). The expression of SEPT7 in cochlear epithelial cells grew weaker in most parts of the cochlear duct except inner and outer pillar cells (Filled arrowheads in Fig. 10g and i), Deiters’ cells (Asterisks in Fig. 10g and i), Hensen’s cells, and Claudius cells (Bracket in Fig. 10g) from E15 to P1. SEPT7 was expressed throughout the whole cytoplasm of the pillar cells and the plasma membrane of the Deiters’ cells, Hensen’s cells, and Claudius cells at P1 (Fig. 10g–i). The expression of SEPT7 became more intensive at the apical portion of the inner and outer pillar cells at P2 (Open arrowheads in Fig. 10j) and P3 (Open arrowheads in Fig. 10m). SEPT7 expression levels became stronger in pillar cells, Deiters’ cells (Asterisks in Fig. 10m and o), Hensen’s cells (Bracket in Fig. 10m), and Claudius cells (Bracket in Fig. 10m) compared with other epithelial cells at P3. SEPT7 was strongly expressed in inner and outer pillar cells and Deiters’ cells in a similar pattern as observed in the adult mice cochlea by P7 (Filled arrowheads and asterisks in Fig. 10p and r). However, we did not see any SEPT7 co-localization with presynaptic vesicles in the cochlea at this stage. These data suggest that SEPT7 may have important roles in developing pillar cells, Deiters’ cells, Hensen’s cells, and Claudius cells but is not co-localized with presynaptic vesicles in the developing cochlea.
Fig. 10.
The expression pattern of SEPT7 in developing mouse cochlea. Immunohistochemistry of E13 (a–c), E15 (d–f), P1 (g–i), P2 (j–l), P3 (m–o) and P7 (p–r) mouse cochleae for SEPT7 (a, d, g, j, m, and p), F-actin (b, e, h, k, n, and q), and SEPT7 and F-actin (c, f, i, l, o, and r). a–c, d–f, g–i, j–l, m–o, and p–r are images from the same sections, respectively. SEPT7 was expressed in most epithelial cells of the cochlea duct at E13 as (a–c) and the similar expression pattern was maintained until E15 with more intensive SEPT7 expression level (d–f). SEPT7 was intensely expressed in the cytoplasm of pillar cells at P1 (filled arrowhead in g and i) and also expressed in Deiters’ cells (Asterisks in g and i) and Hensen’s and Claudius cells (Bracket in g). The intensity of SEPT7 expression grew at the apical portion of the pillar cells through P2 (Open arrowheads in j and l) and P3 (Open arrowheads in m and o). At P3 SEPT7 expression level was stronger than other epithelial cells in pillar cells, Deiters’ cells (Asterisks in m and o), Hensen’s cells (Bracket in m), and Claudius cells (Bracket in m). The expression pattern of SEPT7 in the P7 mouse cochlea was similar to that in the adult mouse cochlea (Filled arrow in p and asterisks in p and r) except for its absence among presynaptic vesicles. CD: cochlear duct; D: Deiters’ cell; I: inner hair cell; O: outer hair cell; P: pillar cell; Scale bars indicate 30 μm in a and d and 15 μm in g, j, m, and p.
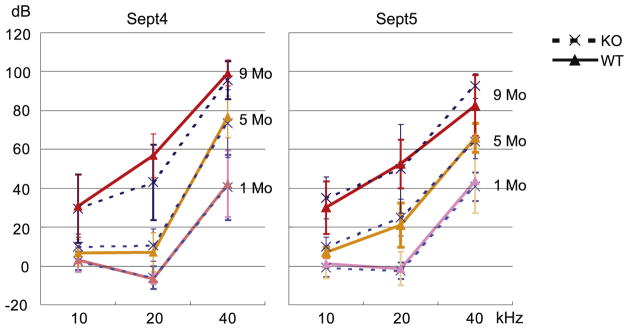
3.3. Evaluation of ABR threshold in Sept4 and Sept5 null mice
The results of immunohistochemical study strongly suggested that septin proteins might have important roles in the cochlea especially after birth. To examine this possibility we measured the hearing ability of Sept4 or Sept5 null mice using ABR. We used 1, 5 and 9 months old Sept4 or Sept5 null mice and their wild type littermates in ABR measurements. The auditory function became worse as they got older, because the background of the mice was C57BL/6, a strain that is known to have progressive hearing loss. However, no statistically significant differences were seen in the auditory function between Sept4 or Sept5 null mice and their wild type littermate controls at any ages we examined (Fig. 11).
Fig. 11.
The auditory function of Sept4 and Sept5 null mice. ABR threshold was measured in Sept4 or Sept5 null mice (broken lines) and their littermate wild type mice (solid lines) at 1, 5 and 9 month of age respectively. No statistically significant difference was seen in the auditory function between the knockout mice and wild type mice at any age examined. KO: knockout mice; WT: wild type mice. Error bars represent standard deviation.
4. Discussion
In this study we elucidated the expression patterns of three different septin proteins in the cochlea where septin expression had not previously been examined. All septins tested (SEPT4, SEPT5, and SEPT7) were strongly expressed in pillar cells and Deiters’ cells (Figs. 3–5) that contain organized bundles of microtubules forming rigid scaffold and were not detected in inner pharyngeal cells, another type of supporting cells that lack microtubules (Slepecky, 1996). Actually, SEPT4, SEPT5, and SEPT7 positive filament-like structures were positive for acetylated α tubulin (Fig. 3g–i, Fig. 4g–i, and Fig. 5j–l) that is a marker of stabilized microtubules. These results raise the possibility that these septin proteins may contribute to functions dependent on microtubules in pillar and Deiters’ cells including withstanding mechanical stress, maintaining integrity of the reticular lamina, transmitting motion of the basilar membrane to the reticular lamina, and transmitting motion of the hair cells between the reticular lamina and the basilar membrane (Slepecky, 1996). This is supported by the fact that one of the cell components that septin protein superstructures interact with is the microtubule (Nagata et al., 2003; Surka et al., 2002).
We also noted that distribution patterns of SEPT4, SEPT5, and SEPT7 in Deiters’ cells were different by carefully observing the results of immunohistochemistry (Fig. 6). There are two kinds of microtubule bundles in Deiters’ cells; both originate above the basilar membrane and one extends to the base of outer hair cells to constitute supporting cups and another to the reticular lamina to constitute phalangeal processes (Slepecky and Chamberlain, 1983). SEPT4 and SEPT7 were detected in both of these structures (Fig. 6a, c, d and f) but, in contrast, SEPT5 was detected only in the bundles extending to the supporting cups (Fig. 6b and e). These results suggest that, at least in Deiters’ cells, SEPT4, SEPT5, and SEPT7 may contribute to the transmission of the hair cell motion to the basilar membrane through the structure of supporting cups, while only SEPT4 and SEPT7 would contribute to the maintenance of the integrity of reticular lamina and the transmission of the basilar membrane motion to the reticular lamina through the structure of phalangeal processes.
SEPT5 and SEPT7 were also associated with presynaptic vesicles in the efferent nerves in the cochlea (Figs. 4 and 5). SEPT5 was originally cloned as the protein that was co-immunofractionated with synaptophysin on presynaptic membranes in human brain (Caltagarone et al., 1998) and SEPT5 is indirectly linked to in the vesicle machinery (Beites et al., 1999). SEPT5 is mostly expressed in GABAergic neuron in the brain (Kinoshita et al., 2000) and GABAergic neuron is rich in cochlear efferent nerves (Fex et al., 1986; Schrott-Fischer et al., 2002). Our results suggest that SEPT5 is also involved in synaptic vesicle machinery in the mouse cochlear efferent nerve terminals. SEPT7 is also expressed and co-exists with SEPT5 in presynaptic vesicle of the brain (Tsang et al., 2011), which is similar to the situation in the cochlear efferent nerve terminals.
In the adult mouse cochlea, SEPT7 was detected where SEPT4 or/and SEPT5 were found. Considering that SEPT7 is a core component of many multimeric septin complexes in mammals (Fujishima et al., 2007; Joberty et al., 2001; Nagata et al., 2004; Xie et al., 2007), our results suggest that SEPT7 is also a core component of septin complexes in the cochlea.
We also evaluated the expression pattern of SEPT4, 5 and 7 in the developing and neonatal mouse cochlea. Among three septin proteins examined, only SEPT7 was expressed in embryonic cochleae. Its expression was diffuse in the cochlear epithelia at E13 and E15 (Fig. 10a–f) but it became more intensive in Deiters’ cells and pillar cells at P7 than in other cells in the cochlea duct as the maturation of the cochlea proceeded (Fig. 10p–r). This suggests that SEPT7 may participate in the development of cochlear epithelia probably with septin other than SEPT4 and SEPT5. The timing of initiation of SEPT7 expression in the cochlea is consistent with that in the brain (Tsang et al., 2011). SEPT7 expression was temporarily strong in the apical portion of pillar cells at limited stages, P2 (Fig. 10j–l) and P3 (Fig. 10m–o) when Corti’s tunnel, which exists between inner and outer pillar cells, begins to develop. These results suggest that SEPT7 may also participate in the formation of Corti’s tunnel.
In contrast to SEPT7, SEPT4 and SEPT5 (Fig. 9) start their expression only around P7, indicating that these two septin proteins have roles in the later development or maturation of the cochlea rather than its early development. At P7 both SEPT4 and SEPT5 have different expression patterns from those at adult stage (Figs. 3 and 4). At P7 both SEPT4 and SEPT5 signals were not detected in inner pillar cells where SEPT7 was detected at this stage, suggesting that inner pillar cell maturation is dependent on SEPT7 interaction with septins other than SEPT4 and SEPT5, similar to the development of cochlear epithelia. As for SEPT5, its expression was not detected in Deiters’ cells at P7 (Fig. 9j–l). The main roles of SEPT5 in the cochlea at this stage may be maturation of outer pillar cells and synaptic vesicles and in the maintenance of Deiters’ cells. The expression of SEPT4 in outer hair cells at this stage was strong (Fig. 9d–f) although it was very weak in the adult cochlea (Fig. 3a–c). SEPT4 may have some specific roles in the maturation of outer hair cells at neonatal stages.
The expression of SEPT4 and SEPT5 in the cochlea started around P7 when its morphological (Kikuchi and Hilding, 1965) and functional (Sadanaga and Morimitsu, 1995) maturation begins. Morphological maturation includes the formation of Corti’s tunnel that appears between inner and outer pillar cells and of Nuel’s space, an extracellular space between outer pillar cells and outer hair cells or Deiters’ cells (Fig. 1a). This morphological maturation contributes to the functional maturation of the cochlea including the formation of an electric gradient (endocochlear potential) (Mistrik and Ashmore, 2009). Considering that SEPT4, SEPT5, and SEPT7 were expressed in pillar cells and Deiters’ cells at this stage and that septin proteins are involved in the control of cortical rigidity of the cells by associating with membrane lipid and other molecules beneath the cell membrane (Gilden and Krummel, 2010), septin proteins may play roles in morphological change in Deiters’ cells and pillar cells during neonatal periods to form the Corti tunnel and Nuel’s space and, as a result, in functional maturation of the cochlea.
Immunohistochemical studies on SEPT4, SEPT5, and SEPT7 indicated that these septin proteins are likely to have some important roles in the development or maintenance of the cochlear function, that is, the ability to hear. Surprisingly, however, we did not observe any deterioration of the hearing ability in either Sept4 or Sept5 knockout mice compared with wild type littermate mice even at nine month of age (Fig. 11). These phenotypes were probably due to the functional compensation by other septin proteins. Septin genes in the mammalian genome are divided into four subgroups based on structural similarity, and septins that belong to the same group can partially compensate for one another functionally (Buser et al., 2009). SEPT4 and SEPT5 belong to the same group with SEPT1 and SEPT2. In Sept4 null mice we found that SEPT5 protein was expressed in phalangeal process of Deiters’ cells (Fig. 7) where SEPT5 protein was not detected in wild type mice (Fig. 4), suggesting that SEPT5 can functionally compensate for SEPT4 in Sept4 null mice cochleae. In contrast, SEPT4 was not associated with presynaptic vesicles of wild type or Sept5 null mice, indicating that it did not compensate for loss of SEPT5. It remains possible that other septin proteins belonging to the same group (SEPT1 or SEPT2) or that alternative multimeric complexes (SEPT2, SEPT7, and SEPT8) (Bläser et al., 2002, 2003; Peng et al., 2002) may compensate for the lack of SEPT5 on presynaptic vesicles of cochlear efferent nerve terminals. It is difficult to determine whether SEPT4 or SEPT7 compensates for the defect of SEPT5 in pillar cells and Deiters’ cells because SEPT4 and SEPT7 are originally expressed in these cells.
5. Conclusion
SEPT4, SEPT5, and SEPT7 proteins were expressed in pillar cells and Deiters’ cells of the postnatal mouse cochlea. SEPT5 and SEPT7 were expressed also in the presynaptic vesicles of efferent nerve terminals of the postnatal mouse cochlea and SEPT7 was expressed in the epithelia of the embryonic mouse cochlea. From these expression patterns SEPT4, SEPT5, and SEPT7 were considered to have important roles in the auditory function although Sept4 and Sept5 null mice have normal auditory function presumably due to compensation by other septin family proteins.
Acknowledgments
This project was supported by a Grant-in-Aid for Young Scientists (B) (22791595) to NY, a Grant-in-Aid for Scientific Research (S) (23229009) to JI from the Ministry of Education, Culture, Sports, Science and Technology in Japan and Japan Society for Promotion of Science, and the National Institutes of Health (HD05311), National Alliance for Research on Schizophrenia and Depression Independent Investigator Award, and the Maltz Foundation to N.H.
References
- Amin ND, Zheng YL, Kesavapany S, Kanungo J, Guszczynski T, Sihag RK, Rudrabhatla P, Albers W, Grant P, Pant HC. Cyclin-dependent kinase 5 phosphorylation of human septin SEPT5 (hCDCrel-1) modulates exocytosis. J Neurosci. 2008;28:3631–3643. doi: 10.1523/JNEUROSCI.0453-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beites CL, Xie H, Bowser R, Trimble WS. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat Neurosci. 1999;2:434–439. doi: 10.1038/8100. [DOI] [PubMed] [Google Scholar]
- Bläser S, Jersch K, Hainmann I, Wunderle D, Zgaga-Griesz A, Busse A, Zieger B. Human septin–septin interaction: CDCrel-1 partners with KIAA0202. FEBS Lett. 2002;519:169–172. doi: 10.1016/s0014-5793(02)02749-7. [DOI] [PubMed] [Google Scholar]
- Bläser S, Jersch K, Hainmann I, Zieger W, Wunderle D, Busse A, Zieger B. Isolation of new splice isoforms, characterization and expression analysis of the human septin SEPT8 (KIAA0202) Gene. 2003;312:313–320. doi: 10.1016/s0378-1119(03)00635-8. [DOI] [PubMed] [Google Scholar]
- Buser AM, Erne B, Werner HB, Nave KA, Schaeren-Wiemers N. The septin cytoskeleton in myelinating glia. Mol Cell Neurosci. 2009;40:156–166. doi: 10.1016/j.mcn.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Caltagarone J, Rhodes J, Honer WG, Bowser R. Localization of a novel septin protein, hCDCrel-1, in neurons of human brain. Neuroreport. 1998;9:2907–2912. doi: 10.1097/00001756-199808240-00042. [DOI] [PubMed] [Google Scholar]
- Caudron F, Barral Y. Septins and the lateral compartmentalization of eukaryotic membranes. Dev Cell. 2009;16:493–506. doi: 10.1016/j.devcel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Denoyelle F, Weil D, Maw MA, Wilcox SA, Lench NJ, Allen-Powell DR, Osborn AH, Dahl HH, Middleton A, Houseman MJ, Dodé C, Marlin S, Boulila-ElGaï A, Grati M, Ayadi H, BenArab S, Bitoun P, Lina-Granade G, Godet J, Mustapha M, Loiselet J, El-Zir E, Aubois A, Joannard A, Petit C. Prelingual deafness: high prevalence of a 30delG mutation in the connexin 26 gene. Hum Mol Genet. 1997;6:2173–2177. doi: 10.1093/hmg/6.12.2173. [DOI] [PubMed] [Google Scholar]
- Dent J, Kato K, Peng XR, Martinez C, Cattaneo M, Poujol C, Nurden P, Nurden A, Trimble WS, Ware J. A prototypic platelet septin and its participation in secretion. Proc Natl Acad Sci U S A. 2002;99:3064–3069. doi: 10.1073/pnas.052715199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estivill X, Fortina P, Surrey S, Rabionet R, Melchionda S, D’Agruma L, Mansfield E, Rappaport E, Govea N, Milà M, Zelante L, Gasparini P. Connexin-26 mutations in sporadic and inherited sensorineural deafness. Lancet. 1998;351:394–398. doi: 10.1016/S0140-6736(97)11124-2. [DOI] [PubMed] [Google Scholar]
- Fex J, Altschuler RA, Kachar B, Wenthold RJ, Zempel JM. GABA visualized by immunocytochemistry in the guinea pig cochlea in axons and endings of efferent neurons. Brain Res. 1986;366:106–117. doi: 10.1016/0006-8993(86)91285-0. [DOI] [PubMed] [Google Scholar]
- Fujishima K, Kiyonari H, Kurisu J, Hirano T, Kengaku M. Targeted disruption of Sept3, a heteromeric assembly partner of Sept5 and Sept7 in axons, has no effect on developing CNS neurons. J Neurochem. 2007;102:77–92. doi: 10.1111/j.1471-4159.2007.04478.x. [DOI] [PubMed] [Google Scholar]
- Gil-Loyzaga P, Pujol R. Synaptophysin in the developing cochlea. Int J Dev Neurosci. 1988;6:155–160. doi: 10.1016/0736-5748(88)90040-8. [DOI] [PubMed] [Google Scholar]
- Gilden J, Krummel MF. Control of cortical rigidity by the cytoskeleton: emerging roles for septins. Cytoskeleton (Hoboken) 2010;67:477–486. doi: 10.1002/cm.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PA, Jung K, Hillan KJ, Russell SE. Expression profiling the human septin gene family. J Pathol. 2005;206:269–278. doi: 10.1002/path.1789. [DOI] [PubMed] [Google Scholar]
- Hartwell LH. Genetic control of the cell division cycle in yeast. IV Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971;69:265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, Goto M, Okubo K, Nishiyama H, Ogawa O, Takahashi C, Itohara S, Nishimune Y, Noda M, Kinoshita M. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell. 2005;8:343–352. doi: 10.1016/j.devcel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ihara M, Yamasaki N, Hagiwara A, Tanigaki A, Kitano A, Hikawa R, Tomimoto H, Noda M, Takanashi M, Mori H, Hattori N, Miyakawa T, Kinoshita M. Sept4, a component of presynaptic scaffold and Lewy bodies, is required for the suppression of alpha-synuclein neurotoxicity. Neuron. 2007;53:519–533. doi: 10.1016/j.neuron.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Joberty G, Perlungher RR, Sheffield PJ, Kinoshita M, Noda M, Haystead T, Macara IG. Borg proteins control septin organization and are negatively regulated by Cdc42. Nat Cell Biol. 2001;3:861–866. doi: 10.1038/ncb1001-861. [DOI] [PubMed] [Google Scholar]
- Kartmann B, Roth D. Novel roles for mammalian septins: from vesicle trafficking to oncogenesis. J Cell Sci. 2001;114:839–844. doi: 10.1242/jcs.114.5.839. [DOI] [PubMed] [Google Scholar]
- Kelley P, Harris D, Comer B, Askew J, Fowler T, Smith S, Kimberling W. Novel mutations in the connexin 26 gene (GJB2) that cause autosomal recessive (DFNB1) hearing loss. Am J Hum Genet. 1998;62:792–799. doi: 10.1086/301807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Hilding D. The development of the organ of Corti in the mouse. Acta Otolaryngol. 1965;60:207–222. doi: 10.3109/00016486509127003. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Kimura RS, Paul DL, Adams JC. Gap junctions in the rat cochlea: immunohistochemicalandultrastructuralanalysis. Anat Embryol (Berl) 1995;191:101–118. doi: 10.1007/BF00186783. [DOI] [PubMed] [Google Scholar]
- Kinoshita M. Assembly of mammalian septins. J Biochem. 2003;134:491–496. doi: 10.1093/jb/mvg182. [DOI] [PubMed] [Google Scholar]
- Kinoshita M. Diversity of septin scaffolds. Curr Opin Cell Biol. 2006;18:54–60. doi: 10.1016/j.ceb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Noda M, Kinoshita M. Differential localization of septins in the mouse brain. J Comp Neurol. 2000;428:223–239. doi: 10.1002/1096-9861(20001211)428:2<223::aid-cne3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Kwitny S, Klaus AV, Hunnicutt GR. The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during the late steps of spermiogenesis. Biol Reprod. 2010;82:669–678. doi: 10.1095/biolreprod.109.079566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistrik P, Ashmore J. The role of potassium recirculation in cochlear amplification. Curr Opin Otolaryngol Head Neck Surg. 2009;17:394–399. doi: 10.1097/MOO.0b013e328330366f. [DOI] [PubMed] [Google Scholar]
- Nagata K, Kawajiri A, Matsui S, Takagishi M, Shiromizu T, Saitoh N, Izawa I, Kiyono T, Itoh TJ, Hotani H, Inagaki M. Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J Biol Chem. 2003;278:18538–18543. doi: 10.1074/jbc.M205246200. [DOI] [PubMed] [Google Scholar]
- Nagata K, Asano T, Nozawa Y, Inagaki M. Biochemical and cell biological analyses of a mammalian septin complex, Sept7/9b/11. J Biol Chem. 2004;279:55895–55904. doi: 10.1074/jbc.M406153200. [DOI] [PubMed] [Google Scholar]
- Pan F, Malmberg RL, Momany M. Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol Biol. 2007;7:103. doi: 10.1186/1471-2148-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MA, Jiang K, Kempfle JS, Mizutari K, Simmons CL, Bieber R, Adams J, Edge AS. TAK1 expression in the cochlea: a specific marker for adult supporting cells. J Assoc Res Otolaryngol. 2011;12:471–483. doi: 10.1007/s10162-011-0265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng XR, Jia Z, Zhang Y, Ware J, Trimble WS. The septin CDCrel-1 is dispensable for normal development and neurotransmitter release. Mol Cell Biol. 2002;22:378–387. doi: 10.1128/MCB.22.1.378-387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio C, Dikkes P, Liberman MC, Corfas G. Glial fibrillary acidic protein expression and promoter activity in the inner ear of developing and adult mice. J Comp Neurol. 2002;442:156–162. doi: 10.1002/cne.10085. [DOI] [PubMed] [Google Scholar]
- Sadanaga M, Morimitsu T. Development of endocochlear potential and its negative component in mouse cochlea. Hear Res. 1995;89:155–161. doi: 10.1016/0378-5955(95)00133-x. [DOI] [PubMed] [Google Scholar]
- Schrott-Fischer A, Kammen-Jolly K, Scholtz AW, Glückert R, Eybalin M. Patterns of GABA-like immunoreactivity in efferent fibers of the human cochlea. Hear Res. 2002;174:75–85. doi: 10.1016/s0378-5955(02)00640-8. [DOI] [PubMed] [Google Scholar]
- Shiga A, Nakagawa T, Nakayama M, Endo T, Iguchi F, Kim T, Naito Y, Ito J. Aging effects on vestibuloocular responses in C57BL/6 mice: comparison with alteration in auditory function. Audiol Neurootol. 2005;10:97–104. doi: 10.1159/000083365. [DOI] [PubMed] [Google Scholar]
- Slepecky NB. Structure of the mammalian cochlea. In: Dallos P, Popper A, Fay R, editors. The Cochlea. Springer-Verlag; New York: 1996. pp. 44–129. [Google Scholar]
- Slepecky N, Chamberlain SC. Distribution and polarity of actin in inner ear supporting cells. Hear Res. 1983;10:359–370. doi: 10.1016/0378-5955(83)90098-9. [DOI] [PubMed] [Google Scholar]
- Surka MC, Tsang CW, Trimble WS. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol Biol Cell. 2002;13:3532–3545. doi: 10.1091/mbc.E02-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M. Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr Biol. 2007;17:1752–1758. doi: 10.1016/j.cub.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang CW, Estey MP, Diciccio JE, Xie H, Patterson D, Trimble WS. Characterization of presynaptic septin complexes in mammalian hippocampal neurons. Biol Chem. 2011;392:739–749. doi: 10.1515/BC.2011.077. [DOI] [PubMed] [Google Scholar]
- Xie Y, Vessey JP, Konecna A, Dahm R, Macchi P, Kiebler MA. The GTP-binding protein Septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr Biol. 2007;17:1746–1751. doi: 10.1016/j.cub.2007.08.042. [DOI] [PubMed] [Google Scholar]