Abstract
Background
The link between DNA methylation, obesity, and adiposity-related diseases in the general population remains uncertain.
Methods and Findings
We conducted an association study of body mass index (BMI) and differential methylation for over 400,000 CpGs assayed by microarray in whole-blood-derived DNA from 3,743 participants in the Framingham Heart Study and the Lothian Birth Cohorts, with independent replication in three external cohorts of 4,055 participants. We examined variations in whole blood gene expression and conducted Mendelian randomization analyses to investigate the functional and clinical relevance of the findings. We identified novel and previously reported BMI-related differential methylation at 83 CpGs that replicated across cohorts; BMI-related differential methylation was associated with concurrent changes in the expression of genes in lipid metabolism pathways. Genetic instrumental variable analysis of alterations in methylation at one of the 83 replicated CpGs, cg11024682 (intronic to sterol regulatory element binding transcription factor 1 [SREBF1]), demonstrated links to BMI, adiposity-related traits, and coronary artery disease. Independent genetic instruments for expression of SREBF1 supported the findings linking methylation to adiposity and cardiometabolic disease. Methylation at a substantial proportion (16 of 83) of the identified loci was found to be secondary to differences in BMI. However, the cross-sectional nature of the data limits definitive causal determination.
Conclusions
We present robust associations of BMI with differential DNA methylation at numerous loci in blood cells. BMI-related DNA methylation and gene expression provide mechanistic insights into the relationship between DNA methylation, obesity, and adiposity-related diseases.
Daniel Levy and colleagues examine mechanisms that could link body mass index to cardiovascular diseases via gene expression.
Author Summary
Why Was This Study Done?
Genetic sequence variants explain only a modest proportion of the variation in body mass index (BMI) and cardiometabolic disease in the general population.
There is limited understanding of the link of DNA methylation—a well-characterized epigenetic modification—with BMI and cardiometabolic disease in the general population.
What Did the Researchers Do and Find?
We conducted a cross-sectional analysis of the association of BMI with leukocyte DNA methylation at over 400,000 sites in the genome among 7,798 community-dwelling adults.
We identified associations between BMI and methylation at 83 replicated sites (including 50 novel sites) and concurrent differences in expression in whole blood of genes overrepresented in lipid metabolism pathways.
Using genetic sequence variants to model exposure to differential DNA methylation and tissue-specific gene expression, we found differential methylation and expression of SREBF1 to be implicated in BMI, adiposity-related traits, and coronary artery disease.
Using genetic sequence variants to model exposure to differences in BMI, we found a substantial proportion of the differentially methylated sites (16 of 83) to be downstream of BMI.
What Do These Findings Mean?
Evidence is accumulating that epigenetic modifications, such as DNA methylation, are related to obesity-related diseases in the general population.
We provide support for a role of genomic regulation of a lipid metabolism transcription factor, SREBF1, in adiposity and coronary artery disease.
Mendelian randomization approaches can help prioritize relevant loci for future functional studies, but the cross-sectional observational nature of our study limits definitive causal inference.
Introduction
Obesity is highly prevalent in developed nations [1] and contributes to a substantial burden of morbidity and mortality [2,3]. Despite advances in the understanding of genetic variants, lifestyle factors, and gene–environment interactions associated with obesity [4–7], much of the interindividual variation in body weight remains unexplained by measurable lifestyle and genetic factors. DNA methylation, one of the most frequent and well-characterized epigenetic modifications, reflects at the molecular level a wide range of environmental exposures and genetic influences [8]. By stabilizing chromatin structure and altering gene expression, DNA methylation has the potential to affect an individual’s susceptibility to obesity (see review in [9]). Further, changes in the methylation of DNA may occur secondarily to obesity and may consequently influence the development of adiposity-related diseases such as diabetes, dyslipidemia, hypertension, and cardiovascular disease. Large gaps in knowledge remain as to how human epigenetic modifications relate to obesity and its sequelae.
Epigenetic biomarkers represent a largely untapped precision medicine resource to guide therapy decisions using an individual’s epigenetic profile obtained from blood samples [10]. Identification of clinically relevant epigenetic loci in blood holds the potential to create a foundation upon which to base future functional studies and trials to test epigenetically guided clinical decision making for cardiometabolic diseases. In addition, we may gain novel insights into the molecular underpinnings of obesity and adiposity-related diseases through the study of differentially methylated DNA loci in blood. Doing so may lead to the identification of biologically relevant therapeutic targets.
The present study provides results of an epigenome-wide association study (EWAS) of body mass index (BMI) in over 3,700 participants from the Framingham Heart Study (FHS) and the Lothian Birth Cohorts (LBCs) of 1921 and 1936 (LBC1921 and LBC1936). We conducted independent external replication in over 4,000 individuals from the Atherosclerosis Risk in Communities (ARIC), Genetics of Lipid Lowering Drugs and Diet Network (GOLDN), and Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) cohort studies. We examined the functional relevance of the identified loci by interrogating the known trans-tissue regulatory functions and concomitant changes in gene expression in blood. In addition, we explored the clinical relevance of the findings for adiposity-related diseases with genetic instrumental variable (IV) analyses using bidirectional and two-step trans-tissue Mendelian randomization (MR) approaches [11–13].
Methods
Study Design
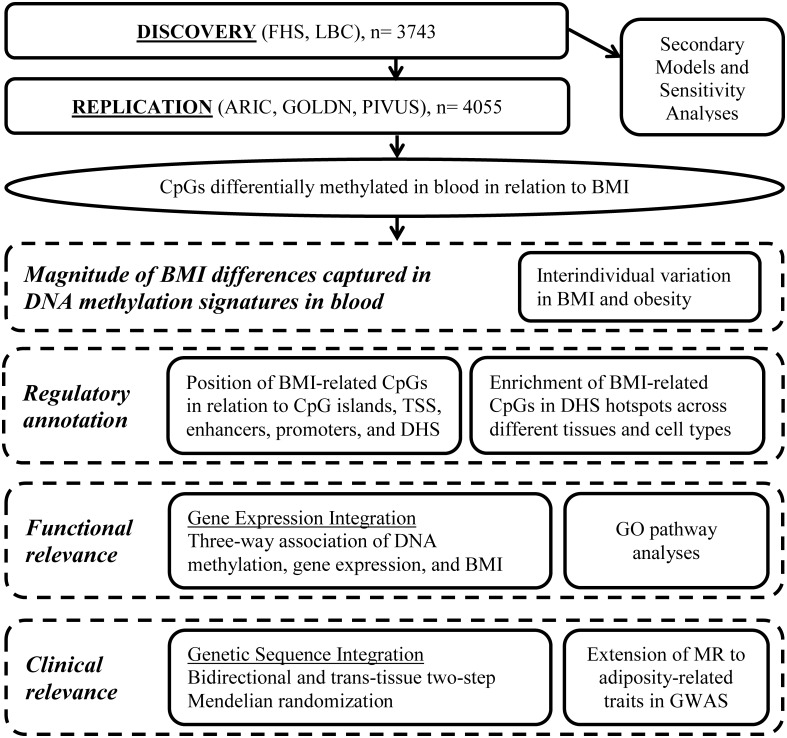
The study includes two major components. First, we conducted an EWAS of BMI. Second, BMI-related differentially methylated loci were taken forward for further analyses to better understand the magnitude of association, regulatory annotation, functional implications, and clinical relevance (Fig 1). The discovery/replication design and secondary models for the BMI EWAS were defined a priori (S1 Text). Downstream analyses to characterize the discovered loci were outlined a priori, but the final approach was primarily driven by the findings and concurrent advancements in the field.
Fig 1. Series of analyses conducted for the epigenome-wide association study of body mass index.
ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; DHS, DNase I hypersensitive site; FHS, Framingham Heart Study; GO, Gene Ontology; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; GWAS, genome-wide association study; LBC, Lothian Birth Cohorts; MR, Mendelian randomization; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors; TSS, transcription start site.
Ethics
The FHS protocols and participant consent forms were approved by the institutional review board of Boston University School of Medicine. Ethics permission for the LBC1921 was obtained from the Lothian Research Ethics Committee (Wave 1: LREC/1998/4/183). Ethics permission for the LBC1936 was obtained from the Multi-Centre Research Ethics Committee for Scotland (Wave 1: MREC/01/0/56) and the Lothian Research Ethics Committee (Wave 1: LREC/2003/2/29). Written informed consent was obtained from all discovery cohort (FHS and LBC) and replication cohort (ARIC, GOLDN, and PIVUS) participants.
Study Participants
Data for the discovery phase of this investigation were drawn from the FHS offspring cohort [14] and the LBCs of 1921 and 1936 [15–17]. As previously described [14], the FHS offspring cohort was initially recruited in 1971 and included 5,124 offspring (and their spouses) from the FHS original cohort [18]. The eligible sample for this investigation was from the 3,021 participants in the FHS offspring cohort who attended the eighth examination cycle from 2005 to 2008. The LBC1921 and LBC1936 samples derive from the Scottish Mental Surveys of 1932 and 1947, respectively, when nearly all 11-y-old children in Scotland completed an IQ-type test in school. The LBC studies provided follow-up of surviving participants, most of whom were living in the Lothian region (Edinburgh city and outskirts) of Scotland. The current study draws upon the older-age baseline examinations of 551 participants in LBC1921 recruited in 1999–2001 and 1,091 participants in LBC1936 recruited in 2004–2007.
Anthropometric Measurements
Height and weight were measured in each study using established protocols as described in detail in the S1 Methods. BMI was calculated as weight (in kilograms) divided by height (in meters) squared.
Molecular Genomics
DNA from whole blood samples was collected at the same examination assessment as the anthropometric and covariate measurements in both studies. DNA methylation, assayed with the Infinium HumanMethylation450 BeadChip [19] (Illumina), was available for 2,846 FHS participants and 1,518 LBC participants (514 from LBC1921 and 1,004 from LBC1936). Details of rigorous quality control, normalization procedures, and exclusions of non-autosomal probes, cross-hybridizing probes, and probes with underlying single nucleotide polymorphisms (SNPs) are described in S1 Methods. Each discovery and replication cohort conducted cohort-specific preprocessing pipelines that allowed each cohort to address study-specific technical and batch effects. This design allowed for the selection of true biological signals independent of bias introduced from uniform processing methods. After quality control in the discovery cohorts, there were 402,358 shared CpG (cytosine-phosphate-guanine) methylation probes available for analyses in 2,377 FHS and 1,366 LBC participants (446 from LBC1921 and 920 from LBC1936). Final sample size was determined by the number of community-based participants in the discovery cohorts who consented to genomic studies and who had available DNA and methylation assays passing quality control measures. In the FHS, SNP data were obtained from the Affymetrix 550K Array imputed to the 1000 Genomes Project reference panel, as previously reported [20]. The LBC samples were genotyped using the Illumina Human610-Quad v1.0 genotyping platform and imputed to the 1000 Genomes Project reference panel as well. Gene expression in blood was available in the FHS and was measured using the Affymetrix Human Exon 1.0 ST GeneChip as described in S1 Methods.
Epigenome-Wide Association Study of BMI
In the FHS, linear mixed effects regression models were conducted to test the association between site-specific DNA methylation and BMI. The primary model was adjusted for age, sex, family relatedness (random effect), and surrogate variables (to account for differential cell proportions and technical effects) [21], with BMI as the independent variable of interest and DNA methylation (inverse-normal transformed) as the dependent variable. In the LBC, linear regression models were conducted adjusting for age, sex, and white blood cell counts, with each DNA methylation probe (residual taken forward from a generalized linear model with a logistic link function adjusting for technical and batch effects) as the dependent variable and BMI as the independent variable of interest. Further analytical details for the discovery cohorts are described in S1 Methods. In both cohorts, secondary models were conducted: (1) additionally adjusting for smoking status, (2) restricted to participants with BMI 18–35 kg/m2 in order to avoid confounding due to frailty or morbid obesity and obesity-related diseases, and (3) testing for age and sex interactions. Results from the FHS and LBCs were meta-analyzed using methods that weighted the p-value by sample size [22]. Directional consistency of statistically significant cohort-specific effects was confirmed for all methylome-wide significant findings from the discovery meta-analysis. We focused our analyses on the resultant test statistic and direction of effect from the independent variable of interest (BMI) as the cohort-specific linear regression coefficients were not directly comparable due to the differences in the preprocessing approach between cohorts. The threshold for statistical significance in the discovery phase was defined by Bonferroni correction for multiple testing to be 0.05/405,000 (p-value < 1.2 × 10−7). A flowchart of analyses conducted is presented in Fig 1.
External Replication of EWAS Findings
The methylome-wide significant CpGs from the FHS and LBC meta-analysis were taken forward to external replication in three independent cohorts that used the same methylation microarray: the ARIC study, using whole-blood-derived DNA from 2,096 participants of African ancestry; the GOLDN study, using DNA derived from CD4+ cells from 992 participants of European ancestry; and the PIVUS study, using whole blood-derived DNA from 967 participants of Swedish ancestry. Description and analytical methods of the replication cohorts are supplied in S1 Methods. Replication cohorts also conducted cohort-specific preprocessing. Replication was examined within each cohort individually and then in a meta-analysis of all three replication cohorts (using p-value-weighted methods and ensuring directional consistency as described above). The threshold for statistically significant replication was determined by Bonferroni correction to be 0.05 divided by the number of CpGs taken forward from discovery.
Sensitivity Models Adjusting EWAS Findings for Potential Confounding by Genetic Variation
In order to demonstrate whether the DNA methylation and BMI association results were independent of genetic variants influencing methylation (methylation quantitative trait loci [meQTLs]), we conducted sensitivity models in the FHS for the replicated BMI-related CpGs conditional on the top cis-meQTL (selected by lowest p-value; ±500 kb from the CpG) for each replicated CpG. The approach to identify cis-meQTLs for the BMI-related CpGs is described in S1 Methods.
Interindividual Variation in BMI and Distribution of Obesity in Relation to EWAS Findings
In order to determine the magnitude of variation in BMI contained within the studied epigenetic signatures in blood, we examined the variation captured in three ways. First, we examined the increase in model R2 starting from the baseline covariate-only linear regression model, with BMI as the dependent variable, when adding nonredundant (|r| < 0.7) replicated CpGs as independent variables in order of decreasing statistical significance. We conducted this analysis in two discovery test sets: (1) methylome-wide significant CpGs in the FHS only were tested in the LBCs and (2) replicated nonredundant CpGs from the BMI EWAS were tested in one of the replication cohorts, PIVUS. Due to differences from the discovery cohorts in ethnicity (African ancestry in ARIC) and cell line (CD4+ cells in GOLDN), we conducted the variation analyses only in PIVUS. Second, we created an additive composite measure of the same nonredundant statistically significant replicated CpGs weighted by effect size. The composite methylation measure was generated for each individual by summing the product of the methylation beta-value and the cohort-specific effect size (including direction of effect) for each of the nonredundant replicated CpGs. The distribution of BMI and prevalence of obesity (BMI ≥ 30 kg/m2) was assessed across deciles of the additive weighted composite measure in the PIVUS cohort. Third, the change in BMI and odds of overweight (BMI 25–29.9 kg/m2) and obesity were tested in age- and sex-adjusted linear and logistic regression models for each standard deviation (SD) change in the additive weighted composite measure in the PIVUS cohort. The weighted summation of the composite methylation measure was converted to SD units (mean = 0, SD = 1) to enhance interpretability of results. As some of the cross-sectional differential methylation changes were expected to be secondary to BMI differences, the purpose of these analyses was not to develop a biomarker or risk predictor for cross-sectional BMI measures but to determine if a large proportion of variation in BMI and obesity, and hence obesity-related cardiometabolic risk, is reflected in the blood DNA methylation patterns. Further analyses examine the molecular pathways that are affected and attempt to infer which methylation changes are causally influencing BMI, which are secondary to BMI differences, and which have relevance for clinical disease outcomes.
Gene Expression Analyses
We analyzed whole blood gene expression data in the FHS to identify which BMI-related differentially methylated CpGs demonstrated association with altered gene expression. The replicated CpGs were tested using linear mixed effects models for association, with the expression level of the corresponding gene in whole blood (based on annotation by the manufacturer) as the dependent variable and DNA methylation as the independent variable, adjusted for age, sex, and technical and batch effects (further details in S1 Methods).
Functional and Regulatory Annotation
We studied the Gene Ontology (GO) biological process, molecular function, and cellular component pathways (release 2016-08-22) of the genes identified in the BMI EWAS using the PANTHER (protein annotation through evolutionary relationship) overrepresentation test [23]. Secondarily, we restricted analysis to the higher certainty genes shown to have altered whole blood gene expression in association with BMI-related differential methylation, as described in the previous section. If multiple probes were annotated to the same gene, then the gene was included only once (unweighted). As the methylation array covers 99% of RefSeq genes, the background universe of genes tested was not restricted. Results were corrected for multiple testing within each category.
In addition, we used eFORGE v1.2 (http://eforge.cs.ucl.ac.uk/) [24] to identify if the replicated CpGs were enriched in DNase I hypersensitive sites (DHSs) (markers of active regulatory regions) and loci with overlapping histone modifications (H3Kme1, H3Kme4, H3K9me3, H3K27me3, and H3K36me3) across available cell lines and tissues from Roadmap Epigenomics Project, BLUEPRINT Epigenome, and ENCODE (Encyclopedia of DNA Elements) consortia data [25–27].
Bidirectional and Two-Step Trans-tissue Mendelian Randomization
IV analyses using SNPs as IVs for (1) DNA methylation, (2) gene expression, and (3) BMI were conducted in order to infer potential causal relationships between EWAS findings, BMI, and adiposity-related diseases (the series of analyses conducted is outlined in Table 1). The detailed approach is provided in S1 Methods. In brief, differences in methylation and expression were modeled using quantitative trait loci (QTLs), thus leveraging the contribution of genetic variation to epigenetic traits to infer causal relations. Blood QTL IVs were selected as the single top SNP methylation or expression association (by lowest p-value) in the FHS with replication in the external cohorts or public datasets. As QTLs vary in effect in different tissue types, we selected tissue-specific methylation and expression QTLs to examine tissue-specific effects (details in S1 Methods). To model the effect of BMI on methylation (reverse causation), the IV for BMI was assembled as an additive weighted genetic risk score from the 97 genome-wide significant SNPs from the Genetic Investigation of ANthropometric Traits (GIANT) consortium 2015 genome-wide association study (GWAS) results [7]. A sensitivity analysis utilizing a single SNP in the FTO (fat mass and obesity associated) locus as the IV for BMI was conducted to examine an IV less prone to pleiotropy bias but also less powerful to detect potential causal relations.
Table 1. Schema of instrumental variable analyses conducted in order to infer the potential causal relations between DNA methylation, gene expression, BMI, and adiposity-related disease.
| Method | Exposure | IV | Source of IV | Selection | Outcome | Setting |
|---|---|---|---|---|---|---|
| Forward MR | DNA methylation | meQTL | FHS/replication cohorts | All replicated CpGs (as the exposure) | BMI | FHS/GIANT consortium |
| Two-step MR—first step | DNA methylation | meQTL | FHS/replication cohorts | Significant in forward MR | Gene expression in multiple tissues | FHS/external eQTL datasets from blood, liver, and adipose tissue |
| Two-step MR—second step | Gene expression in multiple tissues | eQTL | FHS/GTEx/external eQTL datasets | Significant in forward MR | BMI | FHS/GIANT consortium |
| Extension of causal relations to adiposity-related traits | DNA methylation | meQTL | FHS/replication cohorts | Significant in forward MR | Adiposity-related traits | GWAS results |
| Gene expression in multiple tissues | eQTL | FHS/GTEx/external eQTL datasets | Significant in two-step MR | Adiposity-related traits | GWAS results | |
| Reverse MR | BMI | BMI GRS | GWAS results | All replicated CpGs (as the outcome) | DNA methylation | FHS |
BMI, body mass index; eQTL, expression quantitative trait locus; FHS, Framingham Heart Study; GIANT, Genetic Investigation of ANthropometric Traits; GRS, genetic risk score; GTEx, Genotype-Tissue Expression Project; GWAS, genome-wide association study; IV, instrumental variable; meQTL, methylation quantitative trait locus; MR, Mendelian randomization.
Forward MR, using the two-stage least squares method, tests the causal relation of differential methylation with BMI. SNP IVs that implicated a causal effect of differential methylation on BMI from the forward MR (Bonferroni-corrected and, secondarily, nominal causal p-value < 0.05) were tested in the trans-tissue two-step MR. The trans-tissue two-step MR was implemented to further break down the relationship between DNA methylation and BMI and to infer whether the hypothesized mediator (gene expression in multiple tissues) is influenced by the exposure (DNA methylation) and, second, whether the mediator (gene expression in multiple tissues) affects the outcome (BMI). SNP IVs that implicated a causal effect of differential methylation and expression on BMI were tested for associations with adiposity-related phenotypes from published GWAS results. Finally, the reverse MR was conducted to test the causal relation of BMI with downstream changes in DNA methylation.
Results
Discovery Cohort Characteristics
The discovery sample included 3,743 individuals: 2,377 from the FHS and 1,366 from the LBCs (n = 446 from LBC1921 and n = 920 from LBC1936). The FHS, LBC1921, and LBC1936 cohorts were older adults (mean [SD] age 67 [9], 79 [1], and 70 [1] y, respectively) and had similar sex distribution (50%–60% female) and proportion of current smokers (8%–11%) (Table 2).
Table 2. Study characteristics of the Framingham Heart Study and Lothian Birth Cohort participants (discovery cohorts) at the time of DNA methylation assays.
| Characteristic | FHS | LBC1936 | LBC1921 |
|---|---|---|---|
| N | 2,377 | 920 | 446 |
| Age (years) | 67 ± 9 | 70 ± 1 | 79 ± 1 |
| Female | 55% | 40% | 61% |
| BMI (kg/m2) | 28.3 ± 5.4 | 27.8 ± 4.4 | 26.2 ± 4.0 |
| Current smoking | 8% | 11% | 7% |
Data are counts, means ± standard deviation, and proportions as appropriate.
BMI, body mass index; FHS, Framingham Heart Study; LBC, Lothian Birth Cohort.
Epigenome-Wide Association Study of BMI
Discovery
In the FHS-LBC EWAS meta-analysis, 135 CpGs were significantly associated with BMI after correction for multiple testing in the primary age- and sex-adjusted model (p < 1.2 × 10−7; full list and regression coefficients are provided in S1 Table; Q-Q plots in S1 and S2 Figs; Manhattan plot in S3 Fig; genomic inflation factor of discovery meta-analysis, λ = 1.14). Similar results were observed following additional adjustment for smoking status and after excluding 313 individuals with BMI outside of 18–35 kg/m2 (Models 2–3 in S2 Table; S4 Fig).
External replication
The 135 statistically significant CpGs from the discovery BMI EWAS meta-analysis (primary model) were tested for external replication in the ARIC (n = 2,096), GOLDN (n = 992), and PIVUS (n = 967) cohorts. There was external replication of 83 of 135 CpGs in at least one cohort (73 in ARIC, 22 in GOLDN, and 19 in PIVUS; S5 Fig) at p-value < 3.7 × 10−4 (Bonferroni-corrected p-value for 135 tests), and 83 of 135 CpGs replicated in the meta-analyses of the three replication cohorts and were taken forward for subsequent analyses (S3 Table). Greater methylation was associated with higher BMI at 49 (59%) of the 83 replicated CpGs. The majority of BMI-related CpGs (65%–85% of CpGs depending on the cohort) had mean sample CpG methylation levels between 20% and 80% (S4 Table). Fifty of the 83 replicated differentially methylated CpGs have not been previously reported in microarray-based EWASs of BMI [28–36] (Table 3).
Table 3. Fifty novel replicated differentially methylated CpGs associated with BMI sorted by p-value in the discovery cohorts.
| CpG | Gene | Discovery | Replication | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FHS-LBC (n = 3,743) | ARIC (n = 2,096) | GOLDN (n = 992) | PIVUS (n = 967) | Meta-analysis | ||||||
| p-Value | Dir | p-Value | Dir | p-Value | Dir | p-Value | Dir | p-Value | ||
| cg17501210 | RPS6KA2 | 4.14E−21 | − − − − | 1.72E−04 | − | 1.36E−06 | − | 4.15E−02 | − | 1.07E−06 |
| cg14870271 | LGALS3BP | 4.87E−16 | + + + + | 2.28E−04 | + | 3.62E−03 | + | 4.80E−02 | + | 1.51E−05 |
| cg11202345 | LGALS3BP | 6.22E−15 | + + + + | 7.70E−05 | + | 1.36E−02 | + | 1.83E−01 | + | 4.20E−05 |
| cg19750657 | UFM1 | 2.21E−13 | + + + + | 5.55E−06 | + | 6.92E−07 | + | 2.27E−08 | + | 5.60E−13 |
| cg17901584 | DHCR24 | 2.87E−12 | − − − − | 6.26E−07 | − | 1.23E−04 | − | 7.99E−07 | − | 4.14E−12 |
| cg10179300 | TRIO | 5.71E−12 | + + + + | 7.37E−04 | + | 1.16E−02 | + | 8.82E−02 | + | 1.06E−04 |
| cg10508317 | SOCS3 | 2.90E−11 | − − − − | 1.31E−03 | − | 3.49E−05 | − | 2.85E−02 | − | 1.12E−05 |
| cg17836612 | LGALS3BP | 1.05E−10 | + + + + | 4.73E−06 | + | 1.99E−03 | + | 7.40E−04 | + | 1.24E−08 |
| cg26950531 | DPF1 | 1.13E−10 | − − − − | 6.28E−04 | − | 6.49E−03 | − | 9.90E−04 | − | 2.22E−06 |
| cg21429551 | GARS | 1.31E−10 | − − − − | 3.95E−03 | − | 1.13E−05 | − | 8.58E−02 | − | 6.76E−05 |
| cg01881899 | ABCG1 | 1.37E−10 | + + + + | 4.90E−03 | + | 4.99E−04 | + | 8.25E−02 | + | 1.98E−04 |
| cg17782974 | TRIM8 | 1.79E−10 | + + + + | 7.54E−06 | + | 5.71E−02 | + | 1.39E−01 | + | 1.05E−05 |
| cg16611584 | AKAP10 | 2.40E−10 | + + + + | 2.08E−04 | + | 9.92E−01 | + | 1.98E−02 | + | 1.40E−04 |
| cg07730360 | 3.32E−10 | + + + + | 1.37E−06 | + | 3.31E−02 | + | 5.38E−02 | + | 8.00E−07 | |
| cg17738521 | HIVEP2 | 3.51E−10 | − − − − | 2.36E−04 | − | 1.37E−01 | − | 7.56E−02 | − | 1.07E−04 |
| cg18098839 | GOLIM4 | 1.21E−09 | − − − + | 1.48E−03 | − | 1.12E−03 | − | 3.66E−04 | − | 1.42E−06 |
| cg00108715 | NT5DC2 | 1.71E−09 | + + + + | 1.98E−07 | + | 4.60E−01 | + | 4.00E−04 | + | 1.61E−08 |
| cg24531955 | LOXL2 | 2.26E−09 | − − − − | 3.85E−05 | − | 1.67E−02 | − | 9.20E−03 | − | 1.46E−06 |
| cg17058475 | CPT1A | 2.28E−09 | − − − − | 3.18E−04 | − | 1.64E−05 | − | 1.29E−01 | − | 1.17E−05 |
| cg24678869 | DENND4B | 2.56E−09 | + + + + | 4.72E−08 | + | 6.92E−01 | − | 4.46E−01 | + | 2.64E−05 |
| cg13134297 | 3.21E−09 | − − − − | 1.10E−04 | − | 5.62E−03 | − | 2.06E−01 | − | 4.59E−05 | |
| cg10474597 | SERPINE3 | 3.82E−09 | + + + + | 3.51E−04 | + | 2.13E−01 | + | 1.39E−04 | + | 2.19E−06 |
| cg03725309 | SARS | 3.93E−09 | − − − − | 2.67E−04 | − | 2.75E−04 | − | 2.43E−03 | − | 6.00E−07 |
| cg26894079 | ASAM | 4.06E−09 | − − − − | 2.31E−06 | − | 4.60E−05 | − | 1.96E−03 | − | 3.52E−09 |
| cg22012981 | ACOX2 | 4.65E−09 | + + + + | 2.64E−04 | + | 2.27E−02 | + | 1.97E−03 | + | 2.71E−06 |
| cg26361535 | ZC3H3 | 5.46E−09 | − + + + | 1.56E−07 | + | 2.65E−02 | + | 1.37E−02 | + | 3.43E−08 |
| cg04286697 | B3GNT7 | 5.96E−09 | + + + + | 8.70E−06 | + | 4.51E−02 | + | 7.53E−01 | + | 1.22E−04 |
| cg26651978 | 6.24E−09 | − − − − | 6.42E−03 | − | 3.07E−04 | − | 2.76E−03 | − | 1.68E−05 | |
| cg19017142 | 7.86E−09 | − − − − | 1.48E−04 | − | 1.92E−02 | − | 1.80E−02 | − | 8.35E−06 | |
| cg10919522 | C14orf43 | 9.71E−09 | − − − − | 3.98E−06 | − | 2.78E−04 | − | 8.58E−04 | − | 5.44E−09 |
| cg25649826 | USP22 | 1.05E−08 | + + + + | 9.69E−06 | + | 1.41E−03 | + | 6.62E−02 | + | 1.18E−06 |
| cg07037944 | DAPK2 | 1.19E−08 | − − − − | 2.47E−04 | − | 1.36E−01 | − | 4.12E−09 | − | 4.32E−09 |
| cg24145109 | 1.23E−08 | + + + + | 2.69E−05 | + | 8.59E−02 | + | 2.28E−04 | + | 1.62E−07 | |
| cg01751802 | KANK2 | 1.32E−08 | + + + + | 6.33E−07 | + | 8.89E−01 | + | 3.31E−01 | + | 4.30E−05 |
| cg13274938 | RARA | 1.43E−08 | + + + + | 5.48E−07 | + | 3.47E−03 | + | 3.70E−02 | + | 9.57E−08 |
| cg11673687 | SLC9A1 | 2.52E−08 | + + + + | 7.80E−04 | + | 8.37E−01 | − | 7.96E−03 | + | 2.51E−04 |
| cg26800893 | ATPGD1 | 2.87E−08 | − − − − | 5.04E−04 | − | 8.10E−04 | − | 3.80E−04 | − | 4.29E−07 |
| cg01368219 | CACNA2D3 | 3.20E−08 | + + + + | 3.90E−06 | + | 2.21E−02 | + | 2.62E−02 | + | 6.87E−07 |
| cg01130991 | 4.00E−08 | + + + + | 7.46E−06 | + | 2.49E−04 | + | 2.91E−02 | + | 2.19E−07 | |
| cg27470213 | LGALS3BP | 4.63E−08 | − − − − | 3.88E−05 | − | 1.23E−03 | − | 4.18E−03 | − | 2.64E−07 |
| cg26955383 | CALHM1 | 6.04E−08 | + + + + | 2.02E−04 | + | 3.52E−02 | + | 1.70E−01 | + | 1.15E−04 |
| cg03500056 | ABAT | 6.19E−08 | + + + + | 4.21E−06 | + | 1.53E−02 | + | 8.29E−03 | + | 2.10E−07 |
| cg09182678 | 6.22E−08 | − − − − | 1.03E−05 | − | 1.32E−03 | − | 5.88E−07 | − | 1.59E−10 | |
| cg02286155 | 6.39E−08 | + + + + | 2.99E−03 | + | 6.56E−03 | + | 7.40E−02 | + | 2.41E−04 | |
| cg12593793 | 7.12E−08 | − − − − | 1.59E−04 | − | 8.31E−03 | − | 2.04E−08 | − | 1.06E−09 | |
| cg23172671 | 7.47E−08 | + + + + | 1.22E−04 | + | 7.70E−02 | + | 1.25E−01 | + | 8.01E−05 | |
| cg13139542 | 7.93E−08 | − + + + | 1.23E−05 | + | 1.05E−02 | + | 3.78E−01 | + | 2.67E−05 | |
| cg02571142 | DKK4 | 9.91E−08 | + + + + | 6.33E−04 | + | 4.34E−03 | + | 6.97E−02 | + | 5.34E−05 |
| cg21766592 | SLC1A5 | 1.07E−07 | − − − − | 7.69E−03 | − | 5.41E−01 | − | 3.33E−04 | − | 1.34E−04 |
| cg01526748 | FGF12 | 1.18E−07 | + + + + | 5.32E−04 | + | 4.17E−04 | + | 3.32E−02 | + | 1.11E−05 |
The full list of the 83 replicated BMI-related differentially methylated CpGs is presented in S3 Table. The four directions of association with BMI for the FHS-LBC cohorts indicate, in order, the two lab batches in the FHS and the LBC1936 and LBC1921 cohorts individually.
ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; Dir, direction of association with body mass index; FHS, Framingham Heart Study; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network; LBC, Lothian Birth Cohort; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors.
Age and sex interactions among the BMI EWAS findings
Among the 135 discovery CpGs, a significant sex interaction was demonstrated in the discovery cohorts for one unannotated CpG (cg26651978 on Chromosome 17q25.3; <3 kbp from the 3′ end of LGALS3BP [lectin galactoside-binding soluble 3-binding protein]), and a significant age interaction for one CpG (cg24678869; DENND4B [DENN domain 4B Rab GDP-GTP exchange factor]) at p-value < 3.7 × 10−4 (Bonferroni-corrected p-value for 135 tests) (S4 Table). The sex interaction identified at cg26651978 (LGALS3BP) modestly replicated in the external cohorts (replication meta-analyses p = 0.02), with larger regression coefficients and lower p-values in stratified models among men than among women (replication meta-analyses p = 1.73 × 10−6 and 0.002 in men and women, respectively; overall and sex-stratified regression coefficients for each cohort in S5 Table). The age interaction at cg24678869 (DENND4B) did not replicate in the external cohorts (replication meta-analyses p = 0.9). Due to the narrow age range in PIVUS, however, this interaction was tested only in ARIC and GOLDN (n = 3,079).
HIF3A locus methylation
Examining a previously identified BMI-related differential methylation at the HIF3A locus [28], we demonstrated modest associations with BMI in the FHS-LBC discovery cohorts for the three reported CpGs (p = 0.02 for cg22891070, p = 0.03 for cg16672562, and p = 0.04 for cg27146050; no significant sex interactions). Stratifying models at the median age of 66 y in the FHS (age range too narrow in LBC for stratification) revealed stronger associations in the younger subset and null associations in the older subset (for cg22891070, cg16672562, and cg27146050, p = 0.003, p = 0.008, and p = 0.046, respectively, among participants ≤66 y of age, and p = 0.9, p = 0.6, and p = 0.4, respectively, among participants >66 y of age).
Sensitivity models conditioning on cis methylation quantitative trait loci
Sensitivity models conditioning on the top cis-meQTL (selected by lowest p-value; ±500 kb from the CpG) in the FHS demonstrated minimal attenuation of the test statistic for the association of BMI, with differential methylation at the majority of CpGs (81/83 [98%]) attenuated by less than 20% (S6 Table).
Interindividual Variation in BMI and Distribution of Obesity
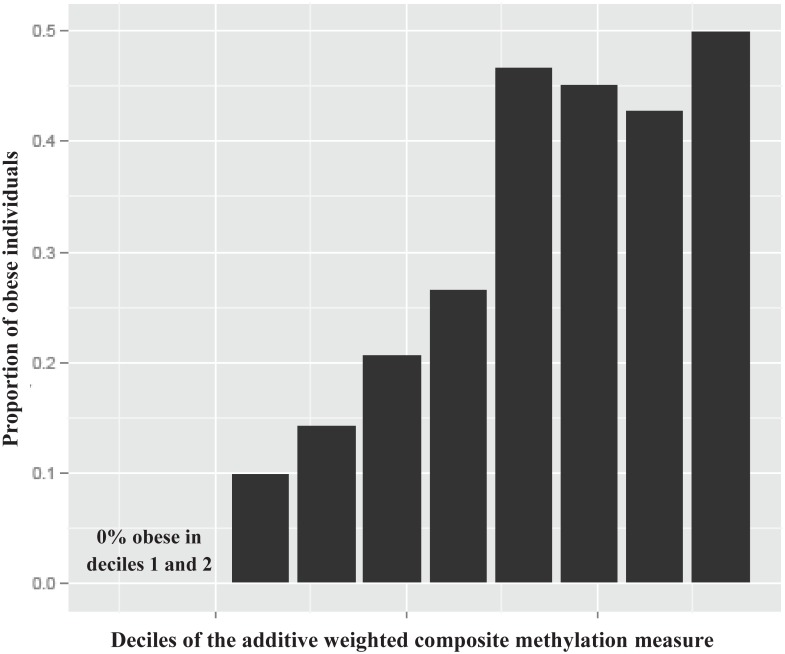
The interindividual variation in BMI and distribution of obesity captured in the BMI EWAS findings was evaluated. Regressing BMI on the 77 nonredundant (inter-probe correlation |r| < 0.7) CpGs from the 83 replicated CpGs identified in the BMI EWAS revealed that 18% of the interindividual variation (adjusted R2) in BMI is captured by differential methylation beyond age and sex in the external replication cohort PIVUS (S6 Fig). This proportion is similar to that observed when examining a completely independent discovery test set using the 75 CpGs that were methylome-wide significant in the FHS discovery cohort (no replication), which accounted for 17.5% of the interindividual variation in BMI (adjusted R2) beyond age and sex in the LBCs. Creating an additive weighted composite measure of the 77 nonredundant replicated CpGs and examining the distribution of BMI and obesity (BMI ≥ 30 kg/m2) across deciles of the measure demonstrated that the median BMI increased in a graded manner from 22 to 34 kg/m2 and the prevalence of obesity rose from 0% to 50% (Figs 2 and S7). For each SD increase in the composite DNA methylation measure in the PIVUS replication cohort, BMI increased by 1.63 (standard error 0.13) kg/m2 (p = 3.7 × 10−34). The odds ratios for obesity (BMI ≥ 30 kg/m2) and overweight (BMI 25–29.9 kg/m2) compared to the reference group (BMI < 25 kg/m2) were 2.8 (95% CI 2.3–3.5; p = 1.6 × 10−25) and 1.9 (95% CI 1.6–2.2; p = 2.5 × 10−18), respectively, for each SD increase in methylation measure in age- and sex-adjusted models.
Fig 2. Histogram of the proportion of obese individuals (BMI ≥ 30 kg/m2) in the PIVUS cohort across deciles of the additive weighted composite methylation measure of the 77 nonredundant replicated CpGs (|r| < 0.7) from the BMI epigenome-wide association study.
BMI, body mass index; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors.
Three-Way Association of DNA Methylation, Gene Expression, and BMI
We examined the association of DNA methylation at the 83 replicated BMI-related CpGs with gene expression among 2,246 FHS participants, in order to determine which genes in blood may be influenced by differential methylation of the BMI EWAS CpGs. Of the 83 replicated CpGs, annotated gene expression from whole blood was available for 62 CpG–gene expression pairs (three transcript results were unavailable on the microarray, and 18 CpGs were intergenic). There were significant associations (p-value < 8 × 10−4; 0.05/62) between differential DNA methylation and gene expression in whole blood for 19 CpG–gene expression pairs, representing ten unique gene transcripts (ABCG1, CPT1A, SREBF1, LGALS3BP, DHCR24, PHGDH, SARS, NOD2, CACNA2D3, and SLC1A5), with almost all of the CpG–gene expression pairs (18/19; 95%) demonstrating an inverse association of methylation with expression (S7 Table). There were significant three-way associations (CpG versus BMI; CpG versus gene expression; gene expression versus BMI) for 11 CpGs with seven unique annotated genes (Table 4). Five of the seven genes (71%) with significant three-way associations between CpG–gene expression–BMI are known to exhibit cardiometabolic phenotypes in murine gene knockout models [37–44].
Table 4. Association results from 11 replicated CpGs with significant three-way associations in whole blood between CpG methylation and BMI, CpG methylation and gene expression, and gene expression and BMI.
| Gene (GEx Probe Number) | CpG | CpG versus BMI | CpG versus GEx | GEx versus BMI | Protein Function and Transgenic Mouse Cardiometabolic Phenotype | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dir | p-Value | Dir | R2 | p-Value | Dir | R2 | p-Value | |||
| ABCG1 (#3922444) | cg06500161 | + | 7.6 × 10−43 | − | 0.112 | 1.2 × 10−59 | − | 0.023 | 6.2 × 10−73 | Function: membrane transporter for cholesterol and phospholipids; transgenic mouse: decreased susceptibility to diet-induced obesity, smaller adipocytes, lower weight [37] |
| cg27243685 | + | 2.3 × 10−15 | − | 0.054 | 4.4 × 10−29 | − | 0.023 | 6.2 × 10−73 | ||
| cg01881899 | + | 1.4 × 10−10 | − | 0.035 | 3.8 × 10−19 | − | 0.023 | 6.2 × 10−73 | ||
| cg10192877 | + | 1.7 × 10−08 | − | 0.014 | 1.7 × 10−08 | − | 0.023 | 6.2 × 10−73 | ||
| CACNA2D3 (#2624639) | cg01368219 | − | 3.2 × 10−08 | − | 0.014 | 2.9 × 10−08 | − | 0.006 | 1.1 × 10−08 | Function: voltage-dependent calcium channel complex; transgenic mouse: decreased serum free fatty acids [38] |
| CPT1A (#3379644) | cg00574958 | − | 2.2 × 10−29 | − | 0.025 | 7.7 × 10−14 | + | 0.017 | 4.5 × 10−24 | Function: transporter across the mitochondrial inner membrane for fatty acid beta oxidation; transgenic mouse: decreased serum glucose and increased serum free fatty acid levels after fasting [39] |
| cg17058475 | − | 2.3 × 10−09 | − | 0.014 | 2.2 × 10−08 | + | 0.017 | 4.5 × 10−24 | ||
| DHCR24 (#2413907) | cg17901584 | − | 2.9 × 10−12 | − | 0.017 | 8.9 × 10−10 | + | 0.003 | 1.4 × 10−07 | Function: catalyzes the reduction of sterol intermediates during cholesterol biosynthesis; transgenic mouse: decreased subcutaneous and mesenteric adipose stores, decreased body size, decreased circulating cholesterol [40] |
| SARS (#2350551) | cg03725309 | − | 3.9 × 10−09 | − | 0.011 | 6.6 × 10−07 | + | 0.011 | 4.7 × 10−10 | Function: catalyzes the transfer of L-serine to tRNA; transgenic mouse: no cardiometabolic phenotypes reported |
| SLC1A5 (#3866276) | cg02711608 | + | 6.3 × 10−08 | − | 0.013 | 5.1 × 10−08 | + | 0.023 | 1.7 × 10−69 | Function: amino acid transporter; transgenic mouse: no cardiometabolic phenotypes reported |
| SREBF1 (#3747966) | cg11024682 | + | 4.8 × 10−22 | − | 0.009 | 8.0 × 10−06 | − | 0.003 | 5.2 × 10−05 | Function: transcription factor for sterol biosynthesis; transgenic mouse: abnormal fat cell and fat pad morphology, abnormal lipid homeostasis, insulin resistance, enlarged liver [41–44] |
Complete list of results for all methylome-wide significant CpGs is available in S8 Table.
BMI, body mass index; Dir, direction of correlation; GEx, gene expression.
Functional and Regulatory Annotation of the BMI EWAS Findings
Gene Ontology pathway analyses
GO analyses of biological process, molecular function, and cellular component pathways of the 55 unique genes annotated to the 83 replicated CpGs (ten CpGs were annotated to genes annotated to other replicated CpGs, and 18 CpGs were intergenic) did not identify any statistically significant pathways after adjustment for multiple testing. Secondarily, in order to further refine gene selection for GO analyses to the genes that demonstrated altered expression, we restricted the GO analyses to the ten unique genes for which variation in expression was associated with differential methylation, as described in the previous section. We identified significant overrepresentation of a biological process pathway in the positive regulation of lipid metabolic processes (GO:0045834; adjusted p-value = 0.002; 64-fold enrichment; four overlapping genes [ABCG1, SREBF1, CPT1A and NOD2] of 130 total genes in pathway) and two related processes (positive regulation of the cholesterol biosynthetic [GO:0045542] and cholesterol metabolic [GO:0090205] processes; adjusted p-value = 0.02–0.03).
Regulatory annotation of CpGs associated with gene expression in blood
Most BMI-related CpGs associated with altered gene expression were located within 50 kb of the transcription start site and were within known enhancer or DHSs (S8 and S9 Figs). CpGs associated with BMI were more likely to be in enhancers and DHSs (enrichment p-value = 4.5 × 10−7 and 9.4 × 10−4, respectively) and less likely to reside in CpG islands (depletion p-value = 3.2 × 10−11) compared to the full set of measured CpGs on the microarray (S8 Table).
DNase I hypersensitive site testing of all identified CpGs
Tissue- and cell-type-specific DHS enrichment testing using the eFORGE v1.2 tool demonstrated that the BMI-related CpGs are enriched in DHSs across almost every tissue and cell type assayed in the included ENCODE, BLUEPRINT Epigenome, and Roadmap Epigenomics Project datasets (S10 and S11 Figs), thus supporting the notion that the CpGs identified in blood are also situated in known active regulatory regions in not only blood, but also other metabolically active tissues. Further stratification by whether BMI-related CpGs had overlapping H3 histone methylation revealed that the BMI-related CpGs predominately overlapped regions with mono-methylation and, to a lesser extent, tri-methylation of lysine 4 on histone H3K4 (H3K4me1 and H3K4me3) across numerous tissues from the consolidated Roadmap Epigenomics Project data (S12–S14 Figs). H3K4me1 marks are indicative of enhancers, H3K4me3 marks are indicative of promoters, and both are known markers of transcriptional activation.
Genetic Instrumental Variable Analyses (Mendelian Randomization)
Successive genetic IV analyses were conducted to infer causal relations between differential methylation, gene expression, and BMI, followed by evaluation of the modeled epigenetic changes on adiposity-related traits using GWAS results (Table 1).
Forward Mendelian randomization
Testing the causal association of DNA methylation with BMI revealed that differential methylation at two CpGs had nominally significant causal associations (p-value < 0.05) with BMI: (1) cg11024682 (SREBF1; cis-meQTL SNP IV rs752579) and (2) cg07730360 (a non-annotated CpG on Chromosome 3q21.3; trans-meQTL SNP IV rs13437553), with causal p-value = 0.02 and 0.04, respectively (S15 Fig; S9 Table). Taking forward the two causal CpGs in discovery for external validation, we found that modeled differential methylation at one of the two CpGs (cis-meQTL SNP IV rs752579 for differential methylation at cg11024682 [SREBF1]) was associated with BMI in the 2015 GIANT consortium results (p = 0.0003; all ancestries).
Two-step Mendelian randomization (first step)
In the first step (DNA methylation affecting the mediator, gene expression), the SNP IV (rs752579) utilized in the forward MR analyses to model differential methylation of the SREBF1 locus (cg11024682) was also found to be strongly associated with altered SREBF1 gene expression in blood in the FHS (p = 3 × 10−12; decreased expression in relation to the C allele), a published [45] blood expression quantitative trait locus (eQTL) dataset (p = 3.2 × 10−6; direction of effect in blood consistent with that seen in the FHS), and liver (p = 1 × 10−15; in the same direction as observed in blood in a reanalysis of 958 samples [46,47]).
Two-step Mendelian randomization (second step)
In the second step (gene expression in blood and alternate tissues affecting BMI), we identified adequate eQTLs for SREBF1 expression in whole blood (rs1889018; p = 1.7 × 10−15) from the FHS; in adrenal gland (rs4925138; p = 1.1 × 10−6) and liver (rs11078366; p = 1.8 × 10−6) from the Genotype-Tissue Expression (GTEx) Project; and in adipose tissue (rs4985779; p = 8.4 × 10−4) from the larger MuTHER dataset [48]. The multi-tissue SREBF1 eQTLs were selected to be largely independent from the SREBF1 methylation locus SNP IV (details in S1 Methods). We identified significant associations with BMI (adjusted for the four tests, p < 0.013) in the GIANT consortium results for two of the four tissue types; specifically, BMI was associated with the SNP IV for SREBF1 expression in whole blood (rs1889018, p = 0.002) and adrenal gland (rs4925138, p = 0.0098), but not liver (rs11078366, p = 0.89) or adipose tissue (rs4985779, p = 0.80).
Adiposity-related traits in GWASs
Assessing other cardiometabolic disease associations from published GWASs, the SNP IV (rs752579) for exposure to differential methylation at the SREBF1 locus (cg11024682) was also found to be associated with (1) adiposity-related traits [49–51] (waist-hip ratio adjusted for BMI [p = 2.0 × 10−4], adiponectin [p = 0.007], birthweight [p = 0.046]), (2) diabetes traits [52–55] (type 2 diabetes [p = 0.002], fasting insulin adjusted for BMI [p = 0.001], HbA1C [p = 0.003], HOMA-B [p = 0.007]), (3) lipid levels [56] (triglycerides [p = 0.001], high-density lipoprotein cholesterol [p = 0.03]), and (4) coronary artery disease [57] (p = 1.7 × 10−6). Additionally, the SNP IV for increased SREBF1 expression in whole blood (rs1889018) was also associated with waist-hip ratio (p = 0.0002), adiponectin (p = 0.003), and triglycerides (p = 0.02) based on GWAS results [49,50,56]. The SNP IV for increased SREBF1 expression in the adrenal gland (rs4925138) was also nominally associated with adiponectin (p = 0.02), triglycerides (p = 0.022), and low-density lipoprotein change in response to statin treatment (p = 0.04) [50,56,58].
Causal effect estimates
Each SD increase in DNA methylation at the SREBF1 locus (cg11024682) was predicted to result in a 2.8-kg/m2 decrease in BMI in the FHS (modeling the effect of allele C for rs752579). In contrast, the observed relationship between methylation in blood and BMI in the FHS was in the opposite direction: a 1.0-kg/m2 increase in BMI per SD increase in DNA methylation at cg11024682. The predicted direction of effect between methylation and BMI is partly derived from the observed direction of effect between the SNP IV and methylation in blood. Previous literature has reported cell-type-dependent QTLs with opposite directions of effect between a SNP and methylation or expression depending on the cell or tissue type examined [59]. As extensive databases of trans-tissue methylation are unavailable, we examined trans-tissue eQTLs for SREBF1 from the GTEx Portal [60]. A series of eQTLs for SREBF1 (false discovery rate ≤ 0.05) demonstrate opposite direction of effect between blood versus adrenal gland (p-value < 10−6) and additional tissues (at p-value < 10−5) such as skeletal muscle, esophagus, aorta tissue, and tibial nerve (http://www.gtexportal.org/home/bubbleHeatmapPage/SREBF1). Strong eQTLs for SREBF1 are likely present in adrenal tissue as SREBF1 is highly expressed in the adrenal gland compared to other tissues (http://www.proteinatlas.org/ENSG00000072310-SREBF1/tissue). For example, rs854764 is a strong eQTL for SREBF1 in both blood and adrenal tissue but in opposite directions (p = 3.8 × 10−12 and p = 4 × 10−6, respectively, in the GTEx catalog) and is associated with BMI in GIANT (p = 0.001) and waist-hip ratio (p = 9.2 × 10−4), adiponectin (p = 0.02), HbA1C (p = 0.02), type 2 diabetes (p = 0.03), triglycerides (p = 0.04), and coronary artery disease (p = 1.1 × 10−5) in GWAS results [4,7,50,52,54,57,61]. This SNP, rs854764, is also a meQTL for SREBF1 locus methylation at cg11024682 in the FHS (p = 2.8 × 10−18), but the association with SREBF1 locus methylation in adrenal gland, the potential tissue of effect, is unknown. See S10 Table for causal effect estimates and confidence intervals for the second step of the two-step MR analyses.
Reverse Mendelian randomization
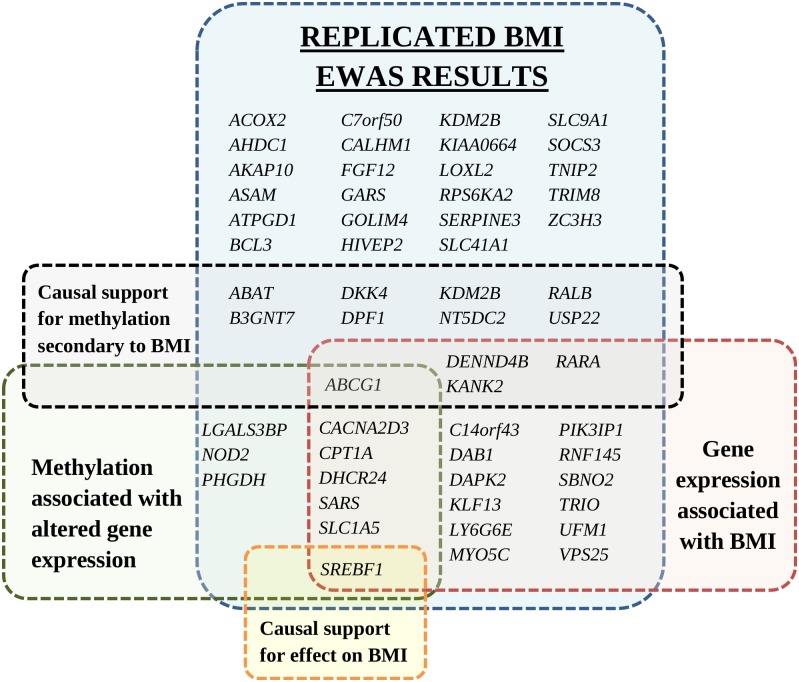
To test whether BMI affects methylation at the identified CpGs, the additive weighted genetic risk score of 97 known BMI SNPs [7] was used as an IV for BMI (F-test statistic = 26). Sixteen CpGs were found to be differentially methylated as a consequence of BMI using a nominal causal p-value < 0.05 cutoff (full list in S11 Table). The 16 downstream CpGs were annotated to 12 genes (ABCG1, USP22, DPF1, RARA, KDM2B, KANK2, RALB, NT5DC2, DENND4B, B3GNT7, DKK4, and ABAT). A sensitivity analysis using a single SNP in the FTO locus as a BMI IV (S12 Table) further supported causal associations downstream of BMI at two of the 16 CpGs (nominal causal p-value < 0.05 for cg06500161 and cg04286697, at the ABCG1 and B3GNT7 loci, respectively). The annotated genes with BMI-related differential methylation are characterized in Fig 3.
Fig 3. Annotated genes of replicated differentially methylated CpGs identified in the BMI epigenome-wide association study.
Genes are grouped by association with gene expression, association of gene expression with BMI, and Mendelian randomization analyses for causal support. Duplicate gene names within the same group are not shown. Figure does not include 18 intergenic CpGs without a gene annotation. BMI, body mass index; EWAS, epigenome-wide association study.
Discussion
In this analysis of the association of BMI with differential methylation of blood-derived DNA, we provide robust evidence of a connection between replicable epigenetic signaling at 83 CpGs and BMI. We also demonstrate the correlation of BMI-related differential methylation with the altered expression of ten genes in whole blood that are overrepresented in lipid metabolism pathways. Among the 83 replicated BMI-related CpGs, one differentially methylated locus (cg11024682) at the lipid metabolism transcription factor SREBF1 demonstrated evidence of a causal effect on BMI; genetically predicted exposure to differential methylation and expression of SREBF1 was found to be associated with BMI and other adiposity traits, glycemic traits, dyslipidemia, and coronary artery disease. In contrast, we found that a substantial proportion (16 out of 83 [19%]) of the BMI-related differentially methylated CpGs identified in this EWAS are likely a consequence of BMI (i.e., downstream signals).
BMI Variation Is Reflected in DNA Methylation Signatures in Blood
A substantial proportion (~18%) of interindividual variation in BMI is captured by the replicated differentially methylated CpGs in blood. The magnitude of BMI difference (~12 kg/m2 between the highest and lowest deciles) equates to substantial health risks; for example, each 5-kg/m2 increase in BMI in the general population is associated with a 30% increase in mortality [62].
Our results suggest that epigenetic biomarkers hold the potential to improve risk prediction and help tailor therapy choices to prevent or treat cardiometabolic diseases. For example, at the population level, BMI is an effective measure of average future cardiometabolic disease risk [63], but it is insufficiently predictive at the individual level. Regardless of causality, blood-based biomarkers can be useful for prognostic or diagnostic purposes. Further research is required to determine whether refining BMI-related risk by incorporating epigenetic biomarkers can improve risk prediction and help guide treatment decisions.
Differential Methylation Is Identified in Loci Known to Be Involved in Adiposity
Lipid metabolism
Previously conducted experiments support a causal role of SREBF1 in adiposity [64]. SREBF1 (also known as SREBP1) plays a central role in energy homeostasis by promoting glycolysis, lipogenesis, and adipogenesis via induction of the conversion of acetyl-CoA to triglycerides (S16 Fig). SREBF1 promotes the conversion of free fatty acids to triglycerides in the liver and to triglyceride-rich lipoproteins in the bloodstream. In situations of caloric excess, SREBF1 is a key mediator of the induction of lipogenesis in humans [64]. In mice with diet-induced insulin resistance, inhibition of SREBF1 attenuates accelerated atherosclerosis, supporting a link to atherosclerosis and coronary artery disease [65]. The causal connection between increased triglyceride-rich lipoproteins and coronary disease is supported by human genetic studies [66]. We highlight the potential role of SREBF1 expression in the adrenal gland in weight regulation and adiposity-related diseases based on results from the MR analyses. Diseases of the adrenal gland are known to be linked to severe obesity, and adrenalectomy in murine models can reverse genetically induced obesity [67,68]. Our results suggest that altered genomic regulation of SREBF1 is causally related to BMI; however, the lack of large datasets of meQTLs in numerous tissues and under various conditions, in combination with the inability to conduct tissue-targeted epigenetic editing in relevant experimental models, limits our ability to make a definitive causal inference. Regulation of SREBF1 is an underexplored target for the prevention of coronary artery disease.
Another of our top genes, CPT1A (carnitine palmitoyltransferase 1A), is an outer mitochondrial membrane enzyme involved in the utilization of acetyl-CoA, functioning as a key enzyme in the beta-oxidation of long-chain fatty acids in mitochondrial energy metabolism. Acetyl-CoA has recently been identified as a central link between altered lipolysis due to adiposity or inflammation and resultant changes in hepatic insulin resistance with cross-communication between liver and adipose tissue [69]. ABCG1 (ATP binding cassette G1), a cell-membrane lipid transporter, has an established role in reverse cholesterol transport; its role in obesity is supported in previous animal [37] and human studies [70]. DHCR24 (24-dehydrocholesterol reductase) catalyzes the reduction of sterol intermediates during cholesterol synthesis. Differential methylation of SREBF1, CPT1A, ABCG1, and DHCR24 has been reported in previous EWASs of adiposity, glycemic traits, and lipids [29–31,71–76]. We add to the published literature and provide evidence that differential methylation at the ABCG1 locus is likely a downstream effect of BMI. From these findings taken together, epigenetic dysregulation is emerging as a common link between obesity and obesity-related comorbidities. Although further functional research is required, we hypothesize that obesity and adiposity-related diseases are partly driven by changes in DNA methylation, with resultant dysregulation of energy balance via effects on expression of lipid metabolism pathway genes. Regulatory mechanisms involved in energy homeostasis have been proposed as attractive targets for the treatment of obesity, metabolic syndrome, and heart disease [77,78]. Our results demonstrate that these connections are evident in humans, adding to previous evidence from animal models [78].
Inflammatory pathways
Aside from the lipid metabolism genes, a number of loci involved in inflammatory pathways were identified by our EWAS. Enlarged adipocytes in obese individuals are known to promote inflammation. One BMI-related differentially methylated CpG was identified at the NOD2 (nucleotide binding oligomerization domain 2) locus. NOD2, an innate immune receptor, is involved in the immune response to bacterial lipopolysaccharides (LPSs) by activating NF-κB signaling. Uptake of LPS from gut microbiota has been demonstrated to result in increased internalization of LPS-rich lipoproteins into adipocytes and promote macrophage conversion from the M2 form to the inflammatory M1 form [79]. NOD2 is also included in the GO pathway for regulation of lipid metabolism (0045834) as it is a positive regulator of phosphatidylinositol 3-kinase activity and has been demonstrated to promote vascular inflammation and formation of lipid-rich atherosclerotic lesions in hypercholesterolemic LDLR−/− mice [80,81]. NOD2 interacts with another BMI-related differentially methylated inflammatory gene locus at SOCS3 (suppressor of cytokine signaling 3), a negative regulator of cytokine signaling. In addition, LGALS3BP (lectin, galactoside binding soluble 3 binding protein), also known as MAC2BP (Mac-2-binding protein), is involved in the immune response associated with lymphokine-activated killer cell cytotoxicity and platelet activation, signaling, and aggregation. LGALS3BP has been found to stimulate host defenses and is elevated in individuals with various types of cancer such as breast, lung, colorectal, ovary, and endometrial cancers, many of which are obesity-related. In addition, LGALS3BP was recently identified as a promising biomarker for non-alcoholic steatohepatitis and pancreatitis [82,83], known obesity-related diseases. Methylation at the LGALS3BP locus demonstrated a significant sex interaction, with a stronger effect in men. This may be related to environmental factors more common in men (such as specific dietary patterns) or male-specific physiology.
Differential Methylation Is Identified in Loci Not Previously Linked to Adiposity
Serine metabolism
Two of the ten genes differentially expressed in association with BMI-related methylation (PHGDH and SARS) are involved in L-serine metabolism. PHGDH (phosphoglycerate dehydrogenase) is involved in the early steps of the synthesis of the amino acid L-serine, which plays a role in oxidoreductase as a NADP acceptor in the tricarboxylic acid cycle. SARS (seryl-TRNA synthetase) catalyzes the transfer of L-serine to tRNA. In addition, RPS6KA2 (ribosomal protein S6 kinase A2), a locus not previously reported as being BMI-related, is a serine/threonine kinase that acts downstream of MAPK signaling and is involved in cell proliferation. L-serine is necessary for specific functions in the central nervous system; however, the link between adiposity and functional health consequences via effects on serine metabolism is currently unknown.
Cell-membrane transporters
In addition to the cell-membrane transporters discussed, two additional membrane transporters were identified among the ten genes associated with differential methylation. SLC1A5 (solute carrier family 1 member 5), which was found to have significant three way associations with altered gene expression in blood and BMI, is a sodium-dependent transporter of amino acids. It is activated by insulin concentration, which is often elevated in individuals with obesity. BMI-related differential methylation was also identified at the CACNA2D3 (calcium channel, voltage-dependent, alpha 2/delta subunit 3) locus. CACNA2D3 is involved in nerve signal transmission and cardiac conduction.
BMI EWAS Findings in the Context of Published Epigenetic Epidemiology Studies
Previous in silico methods of identifying putative epigenetically regulated obesity genes highlighted SOCS3 (suppressor of cytokine signaling 3) and RARA (retinoic acid receptor alpha) [84], both of which were identified in the FHS-LBC meta-analysis (p = 2.7 × 10−11 for cg27637521 in SOCS3 and p = 1.3 × 10−8 for cg13274938 in RARA). An association study of DNA methylation and BMI in 459 individuals from the Cardiogenics Consortium identified an association of methylation at three CpGs intronic to HIF3A (hypoxia inducible factor 3A) in blood and adipose cells with BMI [28]. We found modest associations of differential methylation and expression at the HIF3A locus with BMI in our study. However, the associations were stronger in younger individuals in the FHS, suggesting that the connection may be less apparent at older ages.
At a nominal causal p-value < 0.05, we found that many (16 [19%]) of the replicated CpGs are downstream of BMI. This is consistent with recent findings from longitudinal methylation data and bidirectional MR in the Avon Longitudinal Study of Parents and Children [85] that BMI-related HIF3A methylation is likely secondary to differences in BMI.
There is substantial overlap between the identified BMI-related CpGs and reported CpG–metabolite associations in blood from 1,814 participants in the KORA cohort (Kooperative Gesundheitsforschung in der Region Augsburg) [86] (S13 Table). Notably, ceramides and sphingolipids—known to have altered levels among obese individuals and implicated in the development of the metabolic syndrome [87–89]—were identified. In addition, the BMI-related differentially methylated CpG (cg03725309) at the SARS locus, as discussed above in the serine metabolism section, was found to be associated with blood levels of serine.
BMI EWAS Findings in the Context of BMI GWAS Results and Nearby Genetic Variants
Of note, none of the CpGs associated with BMI was near genes previously identified in GWASs of obesity-related traits, such as FTO (fat mass and obesity associated) or MC4R (melanocortin 4 receptor). We hypothesize that many of the replicated differentially methylated loci reflect novel pathways involved in the regulation of adiposity or adiposity-related diseases. Long-range interactions of DNA methylation with known obesity-related loci, however, may exist [90]. Further work to understand the role of the novel loci in relation to adiposity is also required. In addition, combining information from DNA methylation with genetic markers identified from DNA sequence variation may allow for improvements in risk prediction previously not possible with sequence variants alone [91].
Many of the significant loci from the discovery phase (73 of 135) were replicated in African-Americans from the ARIC study [30]. Similarly, many of the BMI-related differentially methylated CpGs identified in this study were also reported in relation to BMI in people of Arabic ancestry [34]. In GWASs, failure to replicate across racial/ethnic groups may be due to differences in allele frequencies and linkage disequilibrium patterns. In contrast, the high rate of replication of DNA methylation results for BMI in individuals of European and African and other ancestries suggests that shared environmental exposures or changes secondary to differences in BMI, and not genetic variation, may underlie many of the associations. Further work is needed to identify environmental factors that promote or mitigate disease-relevant obesity-related epigenetic dysregulation. Our analyses that conditioned on top meQTLs showed minimal attenuation, suggesting that the association between differential methylation and BMI is largely independent of genetic variants near the reported CpGs.
Study Limitations
Our study has several limitations. Results from MR analyses utilizing genetically predicted methylation and expression levels do not prove causation but provide supportive evidence. The results of the MR analyses are based on numerous assumptions, for example, that there are not alternative pathways through which the SNP IV may act on BMI (i.e., pleiotropic effects). The MR assumptions cannot be tested directly and may bias the results. The forward MR results did not reach Bonferroni-adjusted significance thresholds for multiple testing; however, validation of the nominally significant results in the larger GIANT consortium supports our findings. We avoided the use of multi-SNP score IVs as we had already identified adequate single SNP meQTLs and using multi-SNP score IVs would have further risked introducing bias due to pleiotropy. The meQTLs for the MR analyses were derived in the FHS and the outcome was tested within the same cohort, which can potentially result in bias toward significance. The MR analyses, using the blood meQTL IV, suggest an inverse relationship between the predicted methylation of the SREBF1 locus and BMI, the reverse of the observed relationship, which can be interpreted as a null result. This finding is potentially explained by different directions of effect of QTLs in alternate tissues, which was supported by examining the association of genetic variants in blood versus other metabolically active tissues in the GTEx Project resource. Unfortunately, there are limited datasets of meQTLs in various tissues to explore this further. The observation of associations of BMI with methylation at the same CpG in different directions of effect in blood versus adipose-derived DNA has been previously reported at BMI-related CpG sites [30]. For SREBF1, we presume that the metabolic consequences of altered methylation and the effect on BMI occur in tissues other than blood, such as the adrenal gland, with the methylation changes in blood that we were able to detect representing a biomarker of trans-tissue differential methylation [92]. In addition, it is possible that positive and negative feedback loops can result in regulation of the same gene to be both a causal and a downstream effect of adiposity. We would not be able to discern this scenario from the observational cross-sectional data in this study.
An alternate methylation assay would be required for clinical purposes as the current microarrays are unsuitable in a clinical setting. Future research would be required for technical validation for clinical purposes. Our study supports blood cells as a useful accessible tissue for epigenetic biomarker discovery in large population studies. However, our study would not be able to detect tissue-specific methylation changes occurring in non-blood cell lines (e.g., neuron-specific epigenetic modifications in relation to BMI). Many of our top CpGs replicated in the GOLDN study, which assessed DNA methylation in a single blood cell type (CD4+), suggesting that the associations we detected are not likely to be due to confounding by blood cell heterogeneity. Many of the genes associated with BMI-related differential methylation were known to have a role in adiposity and cardiometabolic traits from murine knockout models; however, the universe of knockout models is likely enriched for the study of adiposity and cardiometabolic traits, and we could not directly test whether our results identified more than expected. Our study was conducted among older-age adults, and the findings may not be generalizable to younger ages.
Conclusions
We provide the results of a large EWAS of BMI in almost 8,000 individuals that identified 83 replicable DNA methylation loci and evidence of complementary transcriptomic differences that were enriched for gene products involved in lipid metabolism. The genetic IV analyses prioritize the SREBF1 locus for future functional studies to further define the causal relation with adiposity, insulin resistance, obesity-related dyslipidemia, and coronary artery disease. Our findings provide a foundation for further research to determine if individualized epigenetic profiles can be used to guide clinical decision making and improve health outcomes. Our findings may have additional clinical and therapeutic relevance if other loci that are differentially methylated in relation to BMI represent attractive targets for the treatment or prevention of obesity and adiposity-related diseases.
Supporting Information
Models: (A) age- and sex-adjusted, (B) additionally smoking-adjusted, and (C) additionally excluding frailty/morbid obesity (BMI < 18 kg/m2 and > 35 kg/m2).
(EPS)
Using surrogate variables to adjust for cell count proportion and technical effects (A) compared to the alternate approach of imputed cell counts and measured technical effects (B). Genomic inflation factor lambda is lower in the surrogate variable analysis approach compared to the approach of imputed cell counts and measured technical effects (1.04 versus 1.25), suggesting fewer potential false positives and a more conservative approach.
(EPS)
The dotted line indicates the Bonferroni cutoff for significance of p-value < 1.2 × 10−7. The top six CpGs with the lowest p-values are shown, annotated to their closest gene transcript.
(EPS)
(A) Model 1 (age- and sex-adjusted) + Model 2 (additionally smoking-adjusted). (B) Model 1 + Model 3 (excluding BMI < 18 and > 35 kg/m2).
(EPS)
Replication significance defined as Bonferroni-adjusted p-value < 3.7 × 10−4 (0.05/135). CpGs significant in one, two, and all three replication cohorts are depicted in green, yellow, and red, respectively. Annotated genes are labeled for CpGs replicated in all three cohorts. Full list of replication results is available in S2 Table.
(EPS)
CpGs are added in decreasing order of significance and are adjusted for age, sex, and preceding CpGs.
(EPS)
(EPS)
CpGs located in known DHS or enhancer regions are depicted in red. bp, base pairs; DHS, DNase I hypersensitive site; TSS, transcription start site.
(EPS)
Shores are defined as up to 2 kb from the CpG island, and shelves are defined as up to 2 kb from the CpG shore. TSS, transcription start site.
(EPS)
(A) ENCODE and (B) 2012 Roadmap Epigenomics Project.
(EPS)
(C) consolidated Roadmap Epigenomics Project and (D) BLUEPRINT Epigenome.
(EPS)
Presented is enrichment of BMI EWAS CpGs in regions overlapping (A) H3K4me1 and (B) H3K4me3 histone modifications.
(EPS)
Presented is enrichment of BMI EWAS CpGs in regions overlapping (C) H3K9me3 and (D) H3K27me3 histone modifications.
(EPS)
Presented is enrichment of BMI EWAS CpGs in regions overlapping (E) H3K36me3 histone modifications.
(EPS)
Example shown illustrates the bidirectional relationship of cg11024682 intronic to SREBF1 and BMI using a meQTL to model the exposure of differential methylation at that locus and an additive weighted genetic risk score using known BMI-related SNPs to model the exposure of elevated BMI.
(EPS)
(EPS)
(DOCX)
(DOC)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
Acknowledgments
Data on adiposity traits have been contributed by GIANT investigators and have been downloaded from https://www.broadinstitute.org/collaboration/giant/index.php/. Data on glycemic traits have been contributed by MAGIC investigators and have been downloaded from https://www.magicinvestigators.org. Data on diabetes traits have been contributed by the DIAGRAM consortium and have been downloaded from http://diagram-consortium.org/. Data on coronary artery disease/myocardial infarction have been contributed by CARDIoGRAMplusC4D investigators and have been downloaded from http://www.cardiogramplusc4d.org/.
The views expressed in this paper are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Health and Medical Research Council or the Australian Research Council.
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- DHS
DNase I hypersensitive site
- eQTL
expression quantitative trait locus
- EWAS
epigenome-wide association study
- FHS
Framingham Heart Study
- GIANT
Genetic Investigation of ANthropometric Traits
- GO
Gene Ontology
- GOLDN
Genetics of Lipid Lowering Drugs and Diet Network
- GTEx
Genotype-Tissue Expression
- GWAS
genome-wide association study
- IV
instrumental variable
- LBC
Lothian Birth Cohort
- meQTL
methylation quantitative trait locus
- MR
Mendelian randomization
- PIVUS
Prospective Investigation of the Vasculature in Uppsala Seniors
- QTL
quantitative trait locus
- SD
standard deviation
- SNP
single nucleotide polymorphism
Data Availability
Participant-level phenotype and genotype data from the Framingham Heart Study are accessible from the U.S. National Center for Biotechnology Information (NCBI) database of Genotypes and Phenotypes (dbGaP) at https://dbgap.ncbi.nlm.nih.gov/ to approved scientific investigators pursuing research questions that are consistent with the informed consent agreements provided by individual research participants. The FHS methylation data are available at dbGaP under the accession number phs000724.v2.p9 and gene expression data at accession number phs000363.v3.p6. Lothian Birth Cohorts' methylation data have been submitted to the European Genome-phenome Archive under accession number EGAS00001000910; phenotypic data are available at dbGaP under the accession number phs000821.v1.p1.
Funding Statement
The Framingham Heart Study is funded by National Institutes of Health contract N01-HC-25195 and HHSN268201500001I. The Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) epigenetics study is funded by the NIH National Heart, Lung, and Blood Institute grant R01 HL 104135-01. The Atherosclerosis Risk in Communities (ARIC) study is carried out as a collaborative study supported by the National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN2682011000010C, HHSN2682011000011C, HHSN2682011000012C). Funding support for “Building on GWAS for NHLBI-diseases: the U.S. CHARGE consortium” was provided by the NIH through the American Recovery and Reinvestment Act of 2009 (ARRA) (5RC2HL102419). The laboratory work for this investigation was funded by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, and by a Director’s Challenge Award, National Institutes of Health (DL, PI). The analytical component of this project was funded by the Division of Intramural Research, National Heart, Lung, and Blood Institute, and the Center for Information Technology, National Institutes of Health, Bethesda, MD. This study utilized the computational resources of the Biowulf system at the National Institutes of Health, Bethesda, MD (http://biowulf.nih.gov). MMM is partly supported by the Tommy Kaplan Fund, Boston Children’s Hospital. LL is partially supported by NIH grant (P30 DK46200). Phenotype collection in the Lothian Birth Cohort 1921 was supported by the UK’s Biotechnology and Biological Sciences Research Council (BBSRC), The Royal Society and The Chief Scientist Office of the Scottish Government. Phenotype collection in the Lothian Birth Cohort 1936 was supported by Age UK (The Disconnected Mind project). Methylation typing was supported by Centre for Cognitive Ageing and Cognitive Epidemiology (Pilot Fund award), Age UK, The Wellcome Trust Institutional Strategic Support Fund, The University of Edinburgh, and The University of Queensland. REM, SEH, PMV, JMS, and IJD are members of the University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology (CCACE). CCACE is supported by funding from the BBSRC, the Economic and Social Research Council (ESRC), the Medical Research Council (MRC), and the University of Edinburgh as part of the cross-council Lifelong Health and Wellbeing initiative (MR/K026992/1). Research reported in this publication was supported by National Health and Medical Research Council (NHMRC) project grants 613608, APP496667, APP1010374 and APP1046880. NHMRC Fellowships to PMV, and NRW (613602) and Australian Research Council (ARC) Future Fellowship to NRW (FT0991360). AFM is supported by the NHMRC fellowship scheme (1083656). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. 10.1001/jama.2014.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masters RK, Reither EN, Powers DA, Yang YC, Burger AE, Link BG. The impact of obesity on US mortality levels: the importance of age and cohort factors in population estimates. Am J Public Health. 2013;103(10):1895–901. 10.2105/AJPH.2013.301379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet. 2014;383(9921):970–83. 10.1016/S0140-6736(13)61836-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berndt SI, Gustafsson S, Magi R, Ganna A, Wheeler E, Feitosa MF, et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet. 2013;45(5):501–12. 10.1038/ng.2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42(11):937–48. 10.1038/ng.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Vliet-Ostaptchouk JV, Snieder H, Lagou V. Gene-lifestyle interactions in obesity. Curr Nutr Rep. 2012;1:184–96. 10.1007/s13668-012-0022-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. 10.1038/nature14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Relton CL, Davey Smith G. Epigenetic epidemiology of common complex disease: prospects for prediction, prevention, and treatment. PLoS Med. 2010;7(10):e1000356 10.1371/journal.pmed.1000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS. Epigenetics and human obesity. Int J Obes (Lond). 2014;39(1):85–97. 10.1038/ijo.2014.34 [DOI] [PubMed] [Google Scholar]
- 10.Bray MS, Loos RJ, McCaffery JM, Ling C, Franks PW, Weinstock GM, et al. NIH working group report-using genomic information to guide weight management: From universal to precision treatment. Obesity (Silver Spring). 2016;24(1):14–22. 10.1002/oby.21381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Relton CL, Davey Smith G. Mendelian randomization: applications and limitations in epigenetic studies. Epigenomics. 2015;7(8):1239–43. 10.2217/epi.15.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Relton CL, Davey Smith G. Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol. 2012;41(1):161–76. 10.1093/ije/dyr233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. 2015. August 17 10.1177/0962280215597579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–90. [DOI] [PubMed] [Google Scholar]
- 15.Deary IJ, Whiteman MC, Starr JM, Whalley LJ, Fox HC. The impact of childhood intelligence on later life: following up the Scottish mental surveys of 1932 and 1947. J Pers Soc Psychol. 2004;86(1):130–47. 10.1037/0022-3514.86.1.130 [DOI] [PubMed] [Google Scholar]
- 16.Deary IJ, Gow AJ, Taylor MD, Corley J, Brett C, Wilson V, et al. The Lothian Birth Cohort 1936: a study to examine influences on cognitive ageing from age 11 to age 70 and beyond. BMC Geriatr. 2007;7:28 10.1186/1471-2318-7-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deary IJ, Gow AJ, Pattie A, Starr JM. Cohort profile: the Lothian Birth Cohorts of 1921 and 1936. Int J Epidemiol. 2012;41(6):1576–84. 10.1093/ije/dyr197 [DOI] [PubMed] [Google Scholar]
- 18.Dawber TR, Meadors GF, Moore FE Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bibikova M, Barnes B, Tsan C, Ho V, Klotzle B, Le JM, et al. High density DNA methylation array with single CpG site resolution. Genomics. 2011;98(4):288–95. 10.1016/j.ygeno.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Johnson AD, Hendricks AE, Hwang SJ, Tanriverdi K, Ganesh SK, et al. Genetic associations with expression for genes implicated in GWAS studies for atherosclerotic cardiovascular disease and blood phenotypes. Hum Mol Genet. 2014;23(3):782–95. 10.1093/hmg/ddt461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28(6):882–3. 10.1093/bioinformatics/bts034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaykin DV. Optimally weighted Z-test is a powerful method for combining probabilities in meta-analysis. J Evol Biol. 2011;24(8):1836–41. 10.1111/j.1420-9101.2011.02297.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8(8):1551–66. 10.1038/nprot.2013.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breeze CE, Paul DS, van Dongen J, Butcher LM, Ambrose JC, Barrett JE, et al. eFORGE: a tool for identifying cell type-specific signal in epigenomic data. Cell Rep. 2016;17(8):2137–50. 10.1016/j.celrep.2016.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roadmap Epigenomics Consortium, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518(7539):317–30. 10.1038/nature14248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams D, Altucci L, Antonarakis SE, Ballesteros J, Beck S, Bird A, et al. BLUEPRINT to decode the epigenetic signature written in blood. Nat Biotechnol. 2012;30(3):224–6. 10.1038/nbt.2153 [DOI] [PubMed] [Google Scholar]
- 27.ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dick KJ, Nelson CP, Tsaprouni L, Sandling JK, Aissi D, Wahl S, et al. DNA methylation and body-mass index: a genome-wide analysis. Lancet. 2014;383(9933):1990–8. 10.1016/S0140-6736(13)62674-4 [DOI] [PubMed] [Google Scholar]
- 29.Aslibekyan S, Demerath EW, Mendelson M, Zhi D, Guan W, Liang L, et al. Epigenome-wide study identifies novel methylation loci associated with body mass index and waist circumference. Obesity (Silver Spring). 2015;23(7):1493–501. 10.1002/oby.21111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demerath EW, Guan W, Grove ML, Aslibekyan S, Mendelson M, Zhou YH, et al. Epigenome-wide association study (EWAS) of BMI, BMI change and waist circumference in African American adults identifies multiple replicated loci. Hum Mol Genet. 2015;24(15):4464–79. 10.1093/hmg/ddv161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding J, Reynolds LM, Zeller T, Muller C, Lohman K, Nicklas BJ, et al. Alterations of a cellular cholesterol metabolism network are a molecular feature of obesity-related type 2 diabetes and cardiovascular disease. Diabetes. 2015;64(10):3464–74. 10.2337/db14-1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang RC, Garratt ES, Pan H, Wu Y, Davis EA, Barton SJ, et al. Genome-wide methylation analysis identifies differentially methylated CpG loci associated with severe obesity in childhood. Epigenetics. 2015;10(11):995–1005. 10.1080/15592294.2015.1080411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronn T, Volkov P, Gillberg L, Kokosar M, Perfilyev A, Jacobsen AL, et al. Impact of age, BMI and HbA1c levels on the genome-wide DNA methylation and mRNA expression patterns in human adipose tissue and identification of epigenetic biomarkers in blood. Hum Mol Genet. 2015;24(13):3792–813. 10.1093/hmg/ddv124 [DOI] [PubMed] [Google Scholar]
- 34.Al Muftah WA, Al-Shafai M, Zaghlool SB, Visconti A, Tsai PC, Kumar P, et al. Epigenetic associations of type 2 diabetes and BMI in an Arab population. Clin Epigenetics. 2016;8:13 10.1186/s13148-016-0177-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansego ML, Milagro FI, Zulet MA, Moreno-Aliaga MJ, Martinez JA. Differential DNA methylation in relation to age and health risks of obesity. Int J Mol Sci. 2015;16(8):16816–32. 10.3390/ijms160816816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali O, Cerjak D, Kent JW Jr, James R, Blangero J, Carless MA, et al. Methylation of SOCS3 is inversely associated with metabolic syndrome in an epigenome-wide association study of obesity. Epigenetics. 2016. August 26 10.1080/15592294.2016.1216284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchmann J, Meyer C, Neschen S, Augustin R, Schmolz K, Kluge R, et al. Ablation of the cholesterol transporter adenosine triphosphate-binding cassette transporter G1 reduces adipose cell size and protects against diet-induced obesity. Endocrinology. 2007;148(4):1561–73. 10.1210/en.2006-1244 [DOI] [PubMed] [Google Scholar]
- 38.Skarnes WC, Rosen B, West AP, Koutsourakis M, Bushell W, Iyer V, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474(7351):337–42. 10.1038/nature10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nyman LR, Cox KB, Hoppel CL, Kerner J, Barnoski BL, Hamm DA, et al. Homozygous carnitine palmitoyltransferase 1a (liver isoform) deficiency is lethal in the mouse. Mol Genet Metab. 2005;86(1–2):179–87. 10.1016/j.ymgme.2005.07.021 [DOI] [PubMed] [Google Scholar]
- 40.Wechsler A, Brafman A, Shafir M, Heverin M, Gottlieb H, Damari G, et al. Generation of viable cholesterol-free mice. Science. 2003;302(5653):2087 10.1126/science.1090776 [DOI] [PubMed] [Google Scholar]
- 41.Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest. 1996;98(7):1575–84. 10.1172/JCI118951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S, Huang LW, Snow KJ, Ablamunits V, Hasham MG, Young TH, et al. A mouse model of conditional lipodystrophy. Proc Natl Acad Sci U S A. 2007;104(42):16627–32. 10.1073/pnas.0707797104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, et al. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12(20):3182–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi A, Shimano H, Nakagawa Y, Yamamoto T, Motomura K, Matsuzaka T, et al. Transgenic mice overexpressing SREBP-1a under the control of the PEPCK promoter exhibit insulin resistance, but not diabetes. Biochim Biophys Acta. 2005;1740(3):427–33. 10.1016/j.bbadis.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 45.Fehrmann RS, Jansen RC, Veldink JH, Westra HJ, Arends D, Bonder MJ, et al. Trans-eQTLs reveal that independent genetic variants associated with a complex phenotype converge on intermediate genes, with a major role for the HLA. PLoS Genet. 2011;7(8):e1002197 10.1371/journal.pgen.1002197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schadt EE, Molony C, Chudin E, Hao K, Yang X, Lum PY, et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 2008;6(5):e107 10.1371/journal.pbio.0060107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greenawalt DM, Dobrin R, Chudin E, Hatoum IJ, Suver C, Beaulaurier J, et al. A survey of the genetics of stomach, liver, and adipose gene expression from a morbidly obese cohort. Genome Res. 2011;21(7):1008–16. 10.1101/gr.112821.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grundberg E, Meduri E, Sandling JK, Hedman AK, Keildson S, Buil A, et al. Global analysis of DNA methylation variation in adipose tissue from twins reveals links to disease-associated variants in distal regulatory elements. Am J Hum Genet. 2013;93(5):876–90. 10.1016/j.ajhg.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heid IM, Jackson AU, Randall JC, Winkler TW, Qi L, Steinthorsdottir V, et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet. 2010;42(11):949–60. 10.1038/ng.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dastani Z, Hivert MF, Timpson N, Perry JR, Yuan X, Scott RA, et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8(3):e1002607 10.1371/journal.pgen.1002607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horikoshi M, Yaghootkar H, Mook-Kanamori DO, Sovio U, Taal HR, Hennig BJ, et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat Genet. 2013;45(1):76–82. 10.1038/ng.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris AP, Voight BF, Teslovich TM, Ferreira T, Segre AV, Steinthorsdottir V, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44(9):981–90. 10.1038/ng.2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manning AK, Hivert MF, Scott RA, Grimsby JL, Bouatia-Naji N, Chen H, et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44(6):659–69. 10.1038/ng.2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soranzo N, Sanna S, Wheeler E, Gieger C, Radke D, Dupuis J, et al. Common variants at 10 genomic loci influence hemoglobin A(1)(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59(12):3229–39. 10.2337/db10-0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105–16. 10.1038/ng.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–13. 10.1038/nature09270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.CARDIoGRAMplusC4D Consortium, Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45(1):25–33. 10.1038/ng.2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barber MJ, Mangravite LM, Hyde CL, Chasman DI, Smith JD, McCarty CA, et al. Genome-wide association of lipid-lowering response to statins in combined study populations. PLoS ONE. 2010;5(3):e9763 10.1371/journal.pone.0009763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dimas AS, Deutsch S, Stranger BE, Montgomery SB, Borel C, Attar-Cohen H, et al. Common regulatory variation impacts gene expression in a cell type-dependent manner. Science. 2009;325(5945):1246–50. 10.1126/science.1174148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Consortium GTEx. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648–60. 10.1126/science.1262110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–83. 10.1038/ng.2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mongraw-Chaffin ML, Peters SA, Huxley RR, Woodward M. The sex-specific association between BMI and coronary heart disease: a systematic review and meta-analysis of 95 cohorts with 1.2 million participants. Lancet Diabetes Endocrinol. 2015;3(6):437–49. 10.1016/S2213-8587(15)00086-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010;45(3):199–214. 10.3109/10409231003667500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13(4):376–88. 10.1016/j.cmet.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45(11):1345–52. 10.1038/ng.2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feldkircher KM, Mistry AM, Romsos DR. Adrenalectomy reverses pre-existing obesity in adult genetically obese (ob/ob) mice. Int J Obes Relat Metab Disord. 1996;20(3):232–5. [PubMed] [Google Scholar]
- 68.Solomon J, Mayer J. The effect of adrenalectomy on the development of the obese-hyperglycemic syndrome in ob-ob mice. Endocrinology. 1973;93(2):510–2. 10.1210/endo-93-2-510 [DOI] [PubMed] [Google Scholar]
- 69.Perry RJ, Camporez JP, Kursawe R, Titchenell PM, Zhang D, Perry CJ, et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160(4):745–58. 10.1016/j.cell.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frisdal E, Le Lay S, Hooton H, Poupel L, Olivier M, Alili R, et al. Adipocyte Atp-binding cassette G1 promotes triglyceride storage, fat mass growth and human obesity. Diabetes. 2015;64(3):840–55. 10.2337/db14-0245 [DOI] [PubMed] [Google Scholar]
- 71.Hidalgo B, Irvin MR, Sha J, Zhi D, Aslibekyan S, Absher D, et al. Epigenome-wide association study of fasting measures of glucose, insulin, and HOMA-IR in the Genetics of Lipid Lowering Drugs and Diet Network Study. Diabetes. 2014;63(2):801–7. 10.2337/db13-1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Irvin MR, Zhi D, Joehanes R, Mendelson M, Aslibekyan S, Claas SA, et al. Epigenome-wide association study of fasting blood lipids in the Genetics of Lipid-lowering Drugs and Diet Network study. Circulation. 2014;130(7):565–72. 10.1161/CIRCULATIONAHA.114.009158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfeiffer L, Wahl S, Pilling LC, Reischl E, Sandling JK, Kunze S, et al. DNA methylation of lipid-related genes affects blood lipid levels. Circ Cardiovasc Genet. 2015;8(2):334–42. 10.1161/CIRCGENETICS.114.000804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chambers JC, Loh M, Lehne B, Drong A, Kriebel J, Motta V, et al. Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: a nested case-control study. Lancet Diabetes Endocrinol. 2015;3(7):526–34. 10.1016/S2213-8587(15)00127-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sayols-Baixeras S, Subirana I, Lluis-Ganella C, Civeira F, Roquer J, Do AN, et al. Identification and validation of seven new loci showing differential DNA methylation related to serum lipid profile: an epigenome-wide approach. The REGICOR study. Hum Mol Genet. 2016. September 15 10.1093/hmg/ddw285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dekkers KF, van Iterson M, Slieker RC, Moed MH, Bonder MJ, van Galen M, et al. Blood lipids influence DNA methylation in circulating cells. Genome Biol. 2016;17(1):138 10.1186/s13059-016-1000-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Folmes CD, Lopaschuk GD. Role of malonyl-CoA in heart disease and the hypothalamic control of obesity. Cardiovasc Res. 2007;73(2):278–87. 10.1016/j.cardiores.2006.10.008 [DOI] [PubMed] [Google Scholar]
- 78.Schreurs M, Kuipers F, van der Leij FR. Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obes Rev. 2010;11(5):380–8. 10.1111/j.1467-789X.2009.00642.x [DOI] [PubMed] [Google Scholar]
- 79.Hersoug LG, Moller P, Loft S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: implications for inflammation and obesity. Obes Rev. 2016;17(4):297–312. 10.1111/obr.12370 [DOI] [PubMed] [Google Scholar]
- 80.Bansal K, Balaji KN. Intracellular pathogen sensor NOD2 programs macrophages to trigger Notch1 activation. J Biol Chem. 2011;286(7):5823–35. 10.1074/jbc.M110.192393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan H, Zelkha S, Burkatovskaya M, Gupte R, Leeman SE, Amar S. Pivotal role of NOD2 in inflammatory processes affecting atherosclerosis and periodontal bone loss. Proc Natl Acad Sci U S A. 2013;110(52):E5059–68. 10.1073/pnas.1320862110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kamada Y, Ono M, Hyogo H, Fujii H, Sumida Y, Mori K, et al. A novel noninvasive diagnostic method for nonalcoholic steatohepatitis using two glycobiomarkers. Hepatology. 2015;62(5):1433–43. 10.1002/hep.28002 [DOI] [PubMed] [Google Scholar]
- 83.Maekawa T, Kamada Y, Ebisutani Y, Ueda M, Hata T, Kawamoto K, et al. Serum Mac-2 binding protein is a novel biomarker for chronic pancreatitis. World J Gastroenterol. 2016;22(17):4403–10. 10.3748/wjg.v22.i17.4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campion J, Milagro FI, Martinez JA. Individuality and epigenetics in obesity. Obes Rev. 2009;10(4):383–92. 10.1111/j.1467-789X.2009.00595.x [DOI] [PubMed] [Google Scholar]
- 85.Richmond RC, Sharp GC, Ward ME, Fraser A, Lyttleton O, McArdle WL, et al. DNA methylation and body mass index: investigating identified methylation sites at HIF3A in a causal framework. Diabetes. 2016;65(5):1231–44. 10.2337/db15-0996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Petersen AK, Zeilinger S, Kastenmuller G, Romisch-Margl W, Brugger M, Peters A, et al. Epigenetics meets metabolomics: an epigenome-wide association study with blood serum metabolic traits. Hum Mol Genet. 2014;23(2):534–45. 10.1093/hmg/ddt430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest. 2011;121(11):4222–30. 10.1172/JCI57144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Borodzicz S, Czarzasta K, Kuch M, Cudnoch-Jedrzejewska A. Sphingolipids in cardiovascular diseases and metabolic disorders. Lipids Health Dis. 2015;14:55 10.1186/s12944-015-0053-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bellini L, Campana M, Mahfouz R, Carlier A, Veret J, Magnan C, et al. Targeting sphingolipid metabolism in the treatment of obesity/type 2 diabetes. Expert Opin Ther Targets. 2015;19(8):1037–50. 10.1517/14728222.2015.1028359 [DOI] [PubMed] [Google Scholar]
- 90.Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gomez-Marin C, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507(7492):371–5. 10.1038/nature13138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shah S, Bonder MJ, Marioni RE, Zhu Z, McRae AF, Zhernakova A, et al. Improving phenotypic prediction by combining genetic and epigenetic associations. Am J Hum Genet. 2015;97(1):75–85. 10.1016/j.ajhg.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma B, Wilker EH, Willis-Owen SA, Byun HM, Wong KC, Motta V, et al. Predicting DNA methylation level across human tissues. Nucleic Acids Res. 2014;42(6):3515–28. 10.1093/nar/gkt1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Models: (A) age- and sex-adjusted, (B) additionally smoking-adjusted, and (C) additionally excluding frailty/morbid obesity (BMI < 18 kg/m2 and > 35 kg/m2).
(EPS)
Using surrogate variables to adjust for cell count proportion and technical effects (A) compared to the alternate approach of imputed cell counts and measured technical effects (B). Genomic inflation factor lambda is lower in the surrogate variable analysis approach compared to the approach of imputed cell counts and measured technical effects (1.04 versus 1.25), suggesting fewer potential false positives and a more conservative approach.
(EPS)
The dotted line indicates the Bonferroni cutoff for significance of p-value < 1.2 × 10−7. The top six CpGs with the lowest p-values are shown, annotated to their closest gene transcript.
(EPS)
(A) Model 1 (age- and sex-adjusted) + Model 2 (additionally smoking-adjusted). (B) Model 1 + Model 3 (excluding BMI < 18 and > 35 kg/m2).
(EPS)
Replication significance defined as Bonferroni-adjusted p-value < 3.7 × 10−4 (0.05/135). CpGs significant in one, two, and all three replication cohorts are depicted in green, yellow, and red, respectively. Annotated genes are labeled for CpGs replicated in all three cohorts. Full list of replication results is available in S2 Table.
(EPS)
CpGs are added in decreasing order of significance and are adjusted for age, sex, and preceding CpGs.
(EPS)
(EPS)
CpGs located in known DHS or enhancer regions are depicted in red. bp, base pairs; DHS, DNase I hypersensitive site; TSS, transcription start site.
(EPS)
Shores are defined as up to 2 kb from the CpG island, and shelves are defined as up to 2 kb from the CpG shore. TSS, transcription start site.
(EPS)
(A) ENCODE and (B) 2012 Roadmap Epigenomics Project.
(EPS)
(C) consolidated Roadmap Epigenomics Project and (D) BLUEPRINT Epigenome.
(EPS)
Presented is enrichment of BMI EWAS CpGs in regions overlapping (A) H3K4me1 and (B) H3K4me3 histone modifications.
(EPS)
Presented is enrichment of BMI EWAS CpGs in regions overlapping (C) H3K9me3 and (D) H3K27me3 histone modifications.
(EPS)
Presented is enrichment of BMI EWAS CpGs in regions overlapping (E) H3K36me3 histone modifications.
(EPS)
Example shown illustrates the bidirectional relationship of cg11024682 intronic to SREBF1 and BMI using a meQTL to model the exposure of differential methylation at that locus and an additive weighted genetic risk score using known BMI-related SNPs to model the exposure of elevated BMI.
(EPS)
(EPS)
(DOCX)
(DOC)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
Data Availability Statement
Participant-level phenotype and genotype data from the Framingham Heart Study are accessible from the U.S. National Center for Biotechnology Information (NCBI) database of Genotypes and Phenotypes (dbGaP) at https://dbgap.ncbi.nlm.nih.gov/ to approved scientific investigators pursuing research questions that are consistent with the informed consent agreements provided by individual research participants. The FHS methylation data are available at dbGaP under the accession number phs000724.v2.p9 and gene expression data at accession number phs000363.v3.p6. Lothian Birth Cohorts' methylation data have been submitted to the European Genome-phenome Archive under accession number EGAS00001000910; phenotypic data are available at dbGaP under the accession number phs000821.v1.p1.