Abstract
Tree mortality due to warming and drought is a critical aspect of forest ecosystem in responding to climate change. Spatial patterns of tree mortality induced by drought and its influencing factors, however, have yet to be documented at the global scale. We collected observations from 248 sites globally where trees have died due to drought and then assessed the effects of climatic and forest factors on the rate of tree mortality. The global mean annual mortality rate was 5.5%. The rate of tree mortality was significantly and negatively correlated with mean annual precipitation (P < 0.01). Tree mortality was lowest in tropical rainforests with mean annual precipitation >2000 mm and was severe in regions with mean annual precipitation <1000 mm. Mortality rates varied amongst species. The global annual rate of mortality was much higher for gymnosperms (7.1%) than angiosperms (4.8%) but did not differ significantly between evergreen (6.2%) and deciduous (6.1%) species. Stand age and wood density affected the mortality rate. Saplings (4.6%) had a higher mortality rate than mature trees (3.2%), and mortality rates significantly decreased with increasing wood density for all species (P < 0.01). We therefore concluded that the tree mortality around the globe varied with climatic and forest factors. The differences between tree species, wood density, stand density, and stand age should be considered when evaluating tree mortality at a large spatial scale during future climatic extremes.
Introduction
Warm droughts, one of the most important global climate changes, have recently occurred in North America, Africa, Europe, Amazonia, and Australia, with major effects on terrestrial ecosystems, carbon balances, and food security [1, 2]. Warm droughts can alter ecosystemic structure, composition, and services, such as carbon sequestration, biological conservation, and water regulation [3–6]. Prolonged droughts or heat can kill trees [7]. Recent studies have indicated that forest mortality induced by rising temperatures and increased drought have rapidly increased around the world during the past decade [6–9]. For example, severe drought has led to massive mortality of aspen along the southern edge of the Canadian boreal forest [10]. van Mantgem et al. [3] showed that increases in the rates of tree mortality in the western United States could not be attributed solely to endogenous increases in competition or to the aging of large trees. Regional warming and the consequent increases in water deficits are likely contributors to the increases in mortality rates. Pervasive tree growth has declined in the semi-arid forests of central Asia since 1994 due to warming-induced increases in the demand for atmospheric moisture [11]. Continued warming in central Asia would likely further increase forest stress and tree mortality, potentially driving the eventual loss of regional semi-arid forests. Drought stress can also decrease the self-organisation of forest ecosystems and consequently increase vulnerability to climate change [12]. Drought- and heat-induced tree mortality have occurred globally, and the effects of extreme climatic events, such as heat waves, drought, and flooding, on plant growth and mortality have been reviewed [6,7], but the spatial patterns of tree mortality induced by drought and heat around the world have yet to be determined.
Drought-induced tree mortality can be influenced by various factors, such as climatic and biotic factors [13–15]. Campos et al. [1] showed that the patterns of response to drought are strongly associated with annual precipitation across biomes, indicating an intrinsic systemic sensitivity to water availability across annual precipitation regimes. Clifford et al. [13] found thresholds of annual precipitation and vapour-pressure deficit (VPD) for tree mortality in southwestern North America. They showed that the patterns of Pinus edulis die-off had threshold responses to annual precipitation and VPD, with little to no mortality (<10%) above 600 mm and below a warm-season VPD of ca. 1.7 kPa. Tree mortality always accompanies warmer temperatures triggered by increasingly severe drought. Adams et al. [14] showed that an increase in temperature of 4.3°C shortened the time to drought-induced mortality in P. edulis and that this effect of temperature also predicted a 5-fold increase in the frequency of tree deaths in the southwestern United States. Therefore, we hypothesized that tree mortality decreased with increasing annual precipitation but increased with increasing annual temperature.
Regional-scale die-off of trees may also be controlled by biotic factors such as tree species, wood density, stand density, and size class [15–19]. Demographic changes, such as decreasing stand density, would greatly favour reductions in the rate of tree mortality compared to denser stands [16]. Larger trees were confronted with a stronger mortality than smaller trees in tropical rainforests [15], contrasting with the drought-related mortality in North America, where smaller trees encountered more risk than larger trees [3, 20]. Kraft et al. [18] found that mortality rates declined with increasing wood density at half of their study sites, but the relationship varied amongst families, plots, and even census intervals within sites. These biotic factors may therefore affect the response of tree mortality to drought at a large spatial scale, which needs further examination. Thus, we hypothesized that response of tree mortality to warming and drought shifted with these biotic factors.
The objective of this study was to investigate the relationships of mean annual precipitation (MAP), mean annual temperature (MAT), elevation, the standardized precipitation-evapotranspiration index (SPEI), elevation, species, tree density, wood density and stand age to heat- drought induced mortality. We collected observations from 62 peer-reviewed publications to create a global data set of 248 sites where tree deaths have been reported.
Materials and Methods
Data preparation
We performed a three-pronged search of the literature on drought and tree mortality. First, we searched Google Scholar and Web of Science using the keywords drought, forest, tree, vegetation, mortality, and dieback. The literature conformed to our study published up to 2016. We then drew on references cited in several extensive peer-reviewed syntheses that comprehensively documented studies reporting drought-induced tree mortality at a regional scale [7, 8, 15]. Finally, we paid close attention to the IPCC 2014 Working Group II Bureau report that discussed the globally increasing forest mortality due to drought and heat stress but without specifying rates of tree mortality [21]. All data for mortality rates were either obtained from tables or extracted by digitising graphs using Get Data Graph Digitizer (ver. 2.25.0.32, Russia).
To be included in the meta-analysis, the study had to follow the criteria below:
specific tree mortality rates, with drought attributed as a prominent or the dominant driver;
indicate that no other disturbance such as fire or harvesting had occurred that could induce tree mortality;
drought episodes must be associated with natural droughts rather than drought experiments.
Attributing mortality to drought is not always straightforward, so we included only studies that stated or demonstrated that drought had driven elevated mortality rates for at least some species or region in the study. We restricted this meta-analysis to droughts shorter than five years [15]. We were limited to 62 of 64 studies that focused on specific short-term (≤5 years) droughts, suggesting a higher confidence that drought was the dominant signal in these studies. Sheil and May [22] provided a mathematical proof that estimates of mortality rates for heterogeneous populations decreased as census interval increased, so we removed the two studies that reported specific mortality rates for long-term droughts. The remaining 62 studies included 248 sites in 30 countries around the world for 1985–2016 and hundreds of species across a range of biomes, including tropical rainforests, temperate deciduous and evergreen forests, boreal forests, and savannah woodlands (S1 File).
Calculation of mortality rate
Tree mortality was generally reported in two ways, depending on the publication. The first was the annual change in mortality (% y-1). For a population experiencing mortality as a constant fraction my (0 ≤ m ≤ 1) each year, the mortality rate after t years compounds as [22]:
| (1) |
where my is an approximation of the annual mortality rate (% y-1), and N0 and Nt are population counts at the beginning and end of a census interval or drought t, respectively.
The second was the total mortality rate during droughts (%). For a population experiencing mortality at a constant fraction m (0 ≤ m ≤ 1), the mortality rate after t years compounds as [22]:
| (2) |
where m is an approximation of the total mortality rate during droughts (%), and N0 and Nt are as above.
Estimates of mortality rate are potentially sensitive to the census interval or drought over which they are calculated because of the non-standard turnover rates. To allow comparison of the tree mortality data, we transformed the data between total mortality rate during drought (%) and annual mortality rate (% y-1) based on the tree mortality of droughts as [22]:
| (3) |
| (4) |
Mortality analyses
We collected a variety of data for each site, which are important for estimating mortality at a regional scale [3, 13, 23]. Specifically, we collected data for mean annual precipitation (MAP), mean annual temperature (MAT), the standardized precipitation-evapotranspiration index (SPEI), elevation, angiosperm or gymnosperm, evergreen or deciduous leaf habit, wood density, stand age, and stand density. The MAP and MAT data were obtained from the publications and http://www.worldweather.cn/zh/home.html, and the SPEI data were obtained from SPEIbase (http://sac.csic.es/spei/database.html), at a spatial resolution of 0.5° for each site. We further determined the relationship between tree mortality rate and SPEI in the drought periods based on the meteorological data in each study. The effects of droughts can be quantified using the SPEI at different time scales [24]. The SPEI is derived using an original Standardized Precipitation Index calculation procedure (SPI) [25], based on a precipitation probabilistic approach. This parameter represents a simple climatic water balance obtained at different time scales. In this study, the values of SPEI during drought periods was used by calculating at monthly scale.
The hundreds of tree species in the data set were generally divided into two groups, angiosperms and gymnosperms, for comparisons of hydraulic traits and mechanisms for mortality prediction [23]. The differences in phenological leaf habit play an important role in the response of plant growth to drought [26, 27], so we also assessed the relationship between mortality rate and phenological leaf habit (evergreen and deciduous).
Calculations of mortality rates are often limited by stand age. For example, some studies rated stand age as adults and saplings, using diameter at breast height (DBH), or directly provided the age of stand establishment. We examined the effects of stand age on drought-induced tree mortality by dividing the species with specific mortality rates into two main sizes: saplings (reported in the publications as saplings, seedings, young stands, or DBHs <20 cm) and adults (reported as adults, old stands, or DBHs ≥20 cm). Twenty of the studies reported correlations between mortality rate and stand age.
We also analysed the relationship between stand density and mortality rate despite limited data for 10 of the publications. Wood density was defined as the dry weight per unit volume of wet wood. The data for wood density was obtained from the Global Wood Density Database [28]. Two studies reported mortality rates only at the genus level, so we calculated generic averages for hundreds of species from the Global Wood Density Database. Eight studies that reported average mortality rates for hundreds of species have no data of specific wood density. Using global values for traits for a given species at specific sites or regions presents challenges, so we used the study-specific values for species, when reported, to improve accuracy [23]. The agreement between our findings for the average role of wood density in predicting cross-species mortality patterns and those of detailed studies across multiple biomes that directly measured tree traits indicated that our approach was reasonable [29, 30].
We also divided the data into nine climatic types to compare the patterns of tree mortality across different climatic regions: tropical rainforest, tropical monsoon, tropical savannah, subtropical monsoon, Mediterranean, temperate maritime, temperate continental, temperate monsoon, and alpine. Only climatic types with >10 sites with specific mortality rates were analysed.
The effect of drought on biomass followed an approximately linear relationship with drought intensity [15], however, we had no priori reason to expect such a linear relationship to be universally true, and the current data sets may include sites that subjected to drought more extensively. Mortality rate could increase rapidly once a certain drought stress threshold is passed [13], inconsistent with our expectation that forests might be resilient to modest drought. We therefore adopted a curve-fitting procedure, examining best fits for linear, log, exponential, and three-factor polynomial relationships.
Statistical analysis
The various analytical combinations required that we develop multiple models. We initially considered 48 statistical models based on 3 groups (angiosperms, gymnosperms, and all data) × 2 mortality metrics (annual mortality rate (% y-1) and total mortality rate during droughts (%)) × 2 drought metrics (MAP and SPEI) × 4 linear and various nonlinear relationships). We then selected the best models for each of the group⁄mortality⁄drought combinations on the basis of R2 and Akaike’s information criterion (AIC) [15] and calculated 95% confidence intervals for the lines of best fit. For polynomial models, we fitted all possible two- and three-factor models and only selected a model with cubic terms when it had an AIC lower than all other models (except the exponential model).
All values in this study represent the mean ± SE. Multi-way analyses of variance (ANOVAs) tested the effects of the differences amongst MAP, MAT, and elevation and between forest type and wood density. Stepwise regression analysis was used to assess the relationship between tree mortality rate and elevation, MAP, MAT, SPEI, and wood density. All statistical analyses were performed using SPSS 18.0 (SPSS for windows, Chicago, IL, USA).
Results
Tree mortality rates around the globe
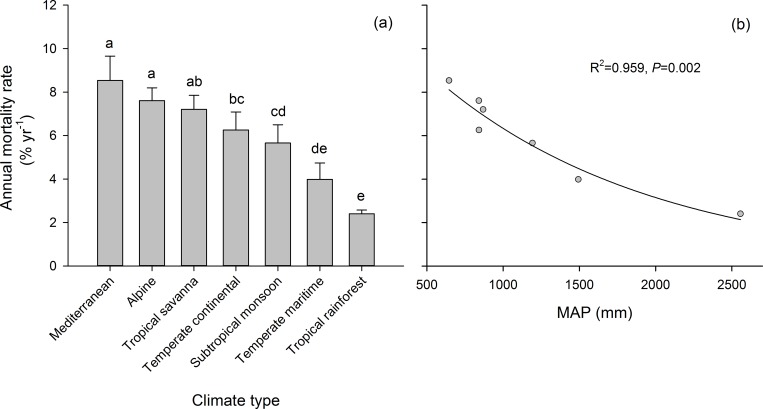
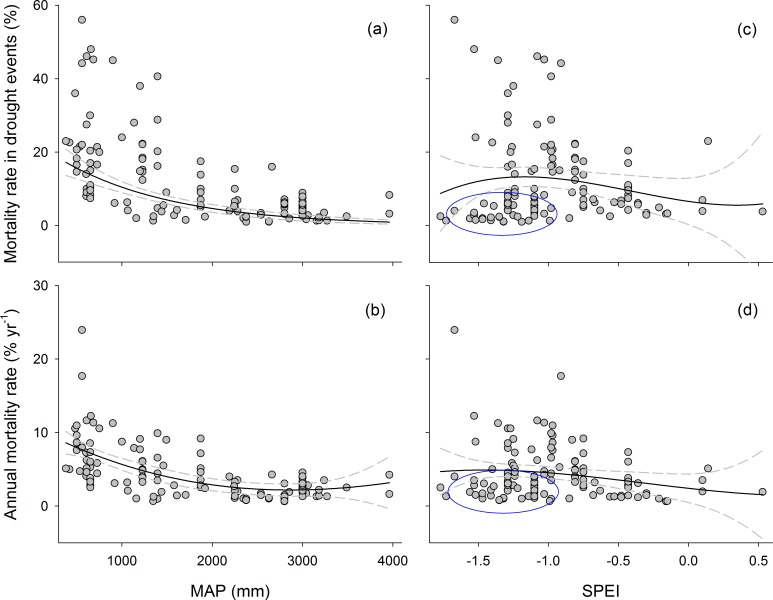
The average annual rate of drought-induced tree mortality was 5.5 ± 0.3% from 248 sites around the world. Only seven of the nine climatic types met our requirement of >10 sites. The annual mortality rate (8.5 ± 1.1%) was highest in the Mediterranean zones of southern Europe, followed by alpine (7.6 ± 0.6%), tropical savannah (7.2 ± 0.6%), temperate continental (6.3 ± 0.8%), subtropical monsoon (5.7 ± 0.8%), and temperate maritime (4.0 ± 0.8%) zones, and the rate was lowest in the tropical rainforest zones of the Amazon Basin (2.4 ± 0.2%) (Fig 1A). The annual mortality rate exponentially decreased with annual precipitation across the climatic zones (P < 0.001, Fig 1B).
Fig 1.
(a) Annual mortality rates for the climatic types. Significant differences between the annual mortality rates of the climatic types are labelled with different lowercase letters (P < 0.05). Values represent the mean ± SE. (b) Relationship between annual mortality rate and mean annual precipitation (MAP).
Effects of biotic and climatic factors
The annual mortality rate was significantly higher for gymnosperms (7.1 ± 0.5%) than angiosperms (4.8 ± 0.4%) (Table 1). Mortality rate, however, did not differ significantly between evergreen and deciduous forests. The rate was higher for saplings (4.6 ± 0.5%) than adults (3.2 ± 0.6%), but not significantly.
Table 1. Mean tree mortality rates and wood densities by tree group, forest type, and stand age.
Significant differences between biotic factors (angiosperm or gymnosperm, deciduous or evergreen, sapling or adult) are labelled with different lowercase letters (P < 0.05).
| Biotic factor | Number of plots | Wood density (g cm-3) | Annual mortalityrate (% y-1) | Mortality rate during droughts (%) |
|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | ||
| Angiosperm | 106 | 0.562 ± 0.14a | 4.8 ± 0.4b | 13.9 ± 1.1b |
| Gymnosperm | 104 | 0.396 ± 0.07b | 7.1 ± 0.5a | 21.4 ± 1.1a |
| Deciduous | 40 | 0.492 ± 0.11a | 6.1 ± 0.7a | 17.4 ± 1.9a |
| Evergreen | 160 | 0.466 ± 0.14a | 6.2 ± 0.4a | 18.8 ± 1.0a |
| Sapling | 34 | 0.449 ± 0.11a | 4.6 ± 0.5a | 18.2 ± 1.9a |
| Adult | 45 | 0.459 ± 0.10a | 3.2 ± 0.6b | 13.5 ± 1.9b |
| All | 248 | 0.479 ± 0.14 | 5.5 ± 0.3 | 15.9 ± 0.8 |
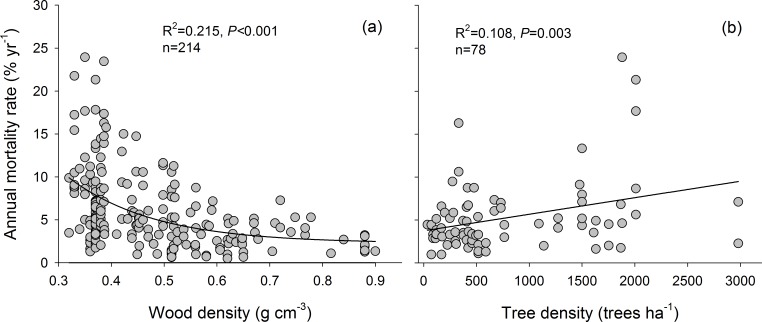
The annual mortality rate for all species decreased significantly with increasing wood density (Fig 2A) and increased significantly with stand density (Fig 2B). Group and forest type both had significant interactive effects with wood density, but the interactive effects between group and forest type and between group, forest type, and wood density were not significant (Table 2).
Fig 2.
Relationships between annual mortality rate and (a) wood density and (b) tree density.
Table 2. Multi-way analysis of variance of tree mortality rate for mean annual precipitation (MAP), mean annual temperature (MAT), elevation, group, forest type, and wood density.
Group includes angiosperm and gymnosperm species; forest type includes evergreen and deciduous species.
| Parameter | df | F | P |
|---|---|---|---|
| MAP | 4 | 8.936 | 0.000 |
| MAT | 3 | 0.440 | 0.724 |
| Elevation | 4 | 2.132 | 0.078 |
| MAP × MAT | 6 | 1.866 | 0.088 |
| MAP × Elevation | 8 | 1.828 | 0.073 |
| MAT × Elevation | 7 | 6.596 | 0.000 |
| MAP × MAT × Elevation | 7 | 3.058 | 0.004 |
| Group | 1 | 2.048 | 0.045 |
| Forest type | 1 | 0.074 | 0.650 |
| Wood density | 3 | 5.166 | 0.002 |
| Group × Forest type | 1 | 0.560 | 0.349 |
| Group × Wood density | 3 | 3.889 | 0.010 |
| Forest type × Wood density | 3 | 4.860 | 0.003 |
| Group × Forest type × Wood density | 1 | 2.163 | 0.143 |
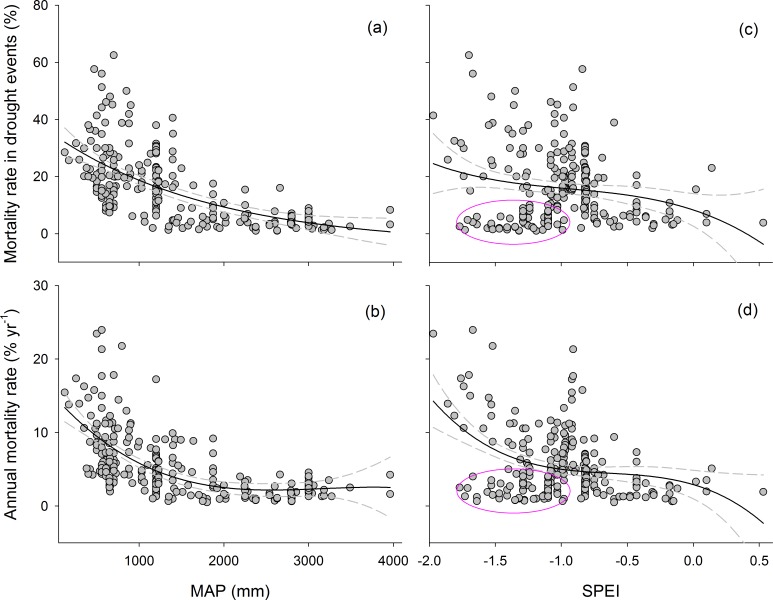
The tree mortality rate was significantly and negatively correlated with MAP around the world (P < 0.01) (Fig 3A and 3B). The mortality rate was higher (8.6 ± 0.5%) in regions with MAPs <1000 mm and lower (2.1 ± 0.1%) in tropical rainforests with MAPs >2000 mm.
Fig 3. Sensitivity of mortality to drought for all sites with available data.
(a) and (b) showed relationships between tree mortality metrics and MAP. (c) and (d) showed relationships between tree mortality metrics and SPEI. Dark-grey dots indicate droughts. The best-fit models for each drought index and mortality-rate metric are shown, with the 95% bootstrapped confidence intervals. MAP, mean annual precipitation; SPEI, standardized precipitation-evapotranspiration index. The pink ovals indicate the lower tree mortality rate for tropical rainforests.
Annual mortality rate was not significantly correlated with MAT (P = 0.724) or elevation (P = 0.078) (Table 2) but was significantly affected by their interaction (P < 0.01). The effect of changes in SPEI on the rate varied amongst the regions. For example, the rate was low in Amazonia where SPEI was low (pink ovals in Fig 3C and 3D).
Variation of tree mortality rate with multiple factors
The multifactorial statistical model explained ~47% of the variance in annual mortality rates for all data sets (Table 3). Some of the unexplained variation may have been caused by our inability to include topoedaphic and plant community effects or those of other potentially useful traits because of the lack of data. The main factors that contribute to heat and drought induced mortality varied with tree group or forest type. Elevation, MAP, SPEI, and wood density were the main factors for angiosperms and evergreen forests, but elevation was the only main factor for deciduous forests. Elevation, MAP, SPEI, MAT, and wood density has the most effect on gymnosperm mortality. Elevation, MAP, SPEI, and wood density were the main factors for all species combined.
Table 3. Stepwise regression to identify factors (elevation, mean annual precipitation, mean annual temperature, standardized precipitation-evapotranspiration index, and wood density) determining the annual mortality rate during droughts.
M, annual mortality rate during droughts; E, elevation; P, mean annual precipitation; T, mean annual temperature; S, standardized precipitation-evapotranspiration index; W, wood density.
| Tree group/type | Equation | R2 | P | n |
|---|---|---|---|---|
| Angiosperm | M = 0.002E-0.001P-2.455S-6.233W+7.5 | 0.608 | 0.000 | 106 |
| Gymnosperm | M = -0.002E-0.003P-6.994S-0.109T-28.097W+18.4 | 0.592 | 0.000 | 104 |
| Evergreen | M = -0.001E-0.003P-4.436S-10.052W+11.3 | 0.508 | 0.000 | 160 |
| Deciduous | M = 0.003E+3.6 | 0.452 | 0.000 | 40 |
| All | M = -0.001E-0.002P-4.773S-8.575W+9.1 | 0.471 | 0.000 | 214 |
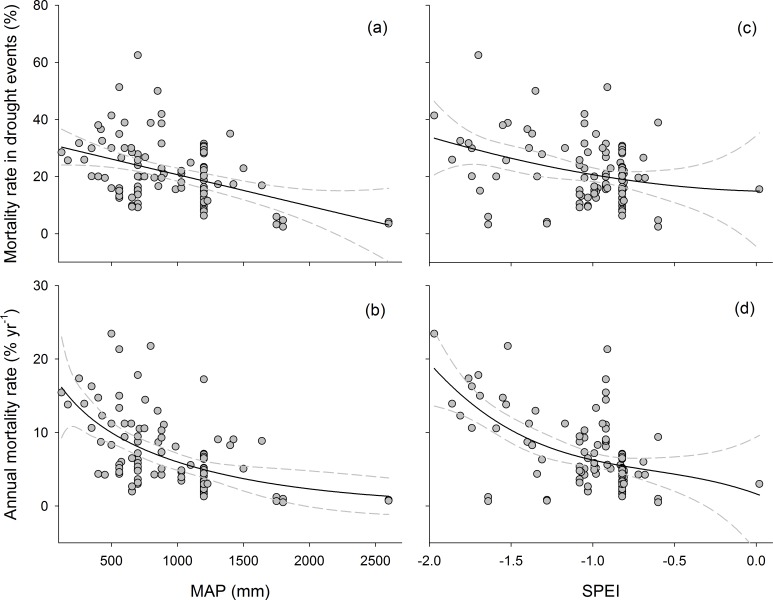
As expected, mortality rate decreased with increasing MAP and SPEI (Table 4 and Figs 3–5). The relationship for the entire data set was nonlinear: the rates tended to decrease disproportionately. These results indicated biome-wide sensitivity to drought, but mortality was lowest for Amazonia and Borneo (pink and blue ovals in Figs 3C and 3D and 4C and 4D). Nonlinearity produced better fits than linearity–AICs are optimal for models with a cubic term. The tropical forests in Amazonia and Borneo were less drought-sensitive than other forests, which was suggested by the displacement of the best fit across the range of drought (Fig 3C and 3D). MAP resulted in a slightly improved fit compared with SPEI for most models (Table 4). R2 tended to be higher, and lower AIC tended to be lower, for comparable models for all data sets.
Table 4. Model fits for the global response of tree mortality to drought.
Data sets varied with tree group (angiosperm and gymnosperm), tree mortality metric (annual mortality rate (% y-1) and mortality rate during droughts (%)), and drought metric (MAP and SPEI). Best-fit models are highlighted in bold and are displayed graphically in Figs 3, 4 and 5. For polynomial models, we fitted all possible two- and three-factor models and only selected a model with cubic terms when it had an AIC lower than all other models and an R2 higher than all other models. MAP, mean annual precipitation; SPEI, standardized precipitation-evapotranspiration index; AIC, Akaike’s information criterion.
| All data (MAP) | R2 | AIC | Angiosperms (MAP) | R2 | AIC | Gymnosperms (MAP) | R2 | AIC |
| Mortality rate during droughts (%) | Mortality rate during droughts (%) | Mortality rate during droughts (%) | ||||||
| Linear | 0.385 | 2498 | Linear | 0.362 | 1191 | Linear | 0.197 | 960 |
| Log | 0.389 | 2496 | Log | 0.385 | 1186 | Log | 0.153 | 968 |
| Exponential (Fig 3A) | 0.514 | 1156 | Exponential (Fig 4A) | 0.432 | 540 | Exponential (Fig 5A) | 0.328 | 339 |
| Polynomial | 0.410 | 2487 | Polynomial | 0.392 | 1185 | Polynomial | 0.192 | 963 |
| Annual mortality rate (% y-1) | Annual mortality rate (% y-1) | Annual mortality rate (% y-1) | ||||||
| Linear | 0.314 | 2018 | Linear | 0.321 | 899 | Linear | 0.279 | 787 |
| Log | 0.392 | 1988 | Log | 0.362 | 891 | Log | 0.312 | 782 |
| Exponential | 0.397 | 1150 | Exponential | 0.352 | 502 | Exponential (Fig 5B) | 0.427 | 382 |
| Polynomial (Fig 3B) | 0.401 | 1984 | Polynomial (Fig 4B) | 0.372 | 889 | Polynomial | 0.315 | 779 |
| All data (SPEI) | R2 | AIC | Angiosperms (SPEI) | R2 | AIC | Gymnosperms (SPEI) | R2 | AIC |
| Mortality rate during droughts (%) | Mortality rate during droughts (%) | Mortality rate during droughts (%) | ||||||
| Linear | 0.044 | 2607 | Linear | 0.013 | 1247 | Linear | 0.107 | 974 |
| Log | — | — | Log | — | — | Log | — | — |
| Exponential | 0.006 | 1332 | Exponential | 0.003 | 612 | Exponential | 0.050 | 374 |
| Polynomial (Fig 3C) | 0.048 | 2606 | Polynomial (Fig 4C) | 0.025 | 1246 | Polynomial (Fig 5C) | 0.111 | 973 |
| Annual mortality rate (% y-1) | Annual mortality rate (% y-1) | Annual mortality rate (% y-1) | ||||||
| Linear | 0.116 | 2080 | Linear | 0.034 | 944 | Linear | 0.297 | 785 |
| Log | — | — | Log | — | — | Log | — | — |
| Exponential | 0.065 | 1259 | Exponential | 0.026 | 557 | Exponential | 0.153 | 424 |
| Polynomial (Fig 3D) | 0.147 | 2071 | Polynomial (Fig 4D) | 0.038 | 944 | Polynomial (Fig 5D) | 0.322 | 781 |
Fig 5. Sensitivity of mortality to drought for gymnosperms.
(a) and (b) showed relationships between tree mortality metrics and MAP. (c) and (d) showed relationships between tree mortality metrics and SPEI. Dark-grey dots indicate droughts. Dark-grey dots indicate droughts. The best-fit models for each drought index and mortality-rate metric are shown, with the 95% bootstrapped confidence intervals. MAP, mean annual precipitation; SPEI, standardized precipitation-evapotranspiration index.
Fig 4. Sensitivity of mortality to drought for angiosperms.
(a) and (b) showed relationships between tree mortality metrics and MAP. (c) and (d) showed relationships between tree mortality metrics and SPEI. Dark-grey dots indicate droughts. Dark-grey dots indicate droughts. The best-fit models for each drought index and mortality-rate metric are shown, with the 95% bootstrapped confidence intervals. MAP, mean annual precipitation; SPEI, standardized precipitation-evapotranspiration index. The blue ovals indicate the lower tree mortality rate for tropical rainforests.
Discussion
Drought-induced tree mortality varied globally. The spatial variation in tree mortality can be ascribed to the variations in climate and properties of the vegetation [3, 4]. The mortality rate was significantly and negatively correlated with MAP globally (Fig 3). Mortality was generally severe in regions with MAPs <1000 mm and lower in regions with MAPs >2000 mm. A higher MAP in tropical rainforests can buffer the negative effects of short-term severe drought, decreasing mortality rates in these regions [15]. SPEI was also low but with lower rates in tropical rainforests (the pink ovals in Fig 3C and 3D), suggesting that trees in these forests that are normally wettest may be less vulnerable to higher dry-season deficits than normal [31]. Conversely, the drier the long-term climate, the greater the impact of a given increase on tree mortality rate. The high mortality rates in the regions with MAPs <1000 mm may also be ascribed to uneven seasonal distributions of rainfall, highlighted by the occasional variation in the spatial extent of tree mortality (29%) during the drought in the late 1990s in northeastern Australia [32].
Mortality rate was negatively correlated with drought regardless of the mortality and drought metrics used (Table 4 and Figs 3–5). A three-factor polynomial or exponential relationship clearly provided the best fits in all four modelled fits for the entire data set, suggesting nonlinear responses of forests to drought and a possible threshold zone beyond which mortality is high, but the current data set is not yet sufficiently representative across all regions to state this proposal with confidence. Clifford et al. [13], however, reported that the patterns of pinyon die-off indicated threshold responses to annual precipitation, with little to no tree mortality (<10%), above 600 mm, in southwestern North America. A threshold for survival associated with annual precipitation could be explained by the survival of many tree species at the limit of tolerable potential water deficits [2].
Mortality rate was not significantly correlated with elevation at the global scale, consistent with the findings by Ganey and Vojta [33]. The rate, however, increased with elevation at a regional scale [3, 4]. For example, the loss of tree cover across an elevation gradient of a pinyon-juniper woodland in southwestern United States was greater at higher elevations [34]. Water stress created by regional droughts may be a dominant contributor to widespread increases in mortality rates, which increased with elevation [4].
Mortality rate was not significantly correlated with MAT, which is inconsistent with the findings for tropical forests in the Amazon Basin [15, 35] and for temperate forests in the western United States [3]. We also did not find a temperature threshold for tree mortality at the global scale. Adams et al. [14], however, reported that experimentally induced warmer temperatures (≈4°C) shortened the time to drought-induced mortality in P. edulis trees by nearly a third. Also, a 5-fold increase in the frequency of events inducing P. edulis mortality accompanied a 4.3°C increase in temperature in a 103-year record of regional drought duration [14]. The different scopes of the study areas and high temperature sensitivity in drought-induced mortality may have contributed to this discrepancy [36]. Temperature patterns within a region can also influence tree die-off, contributing to its uncertainty when other factors, such as stand demography and landscape heterogeneity, come into play [13].
Stand density, a predictor of drought mortality risk [33], has important implications for forest management [34]. Powers et al. [19] showed that thinning significantly influenced mortality as a function of density. Stand density in our study had a significantly positive effect on mortality rate globally, as expected. Forests begin to self-thin to maintain tree densities around 60% for survival [19]. Greenwood and Weisberg [37] found that mortality rate across multiple spatial scales was higher at sites with higher stand densities. Santos and Whitham [38] also showed by predictive modelling that high density would lead to higher tree mortality. The effects of annual precipitation on tree mortality may thus be regulated by stand density; forests with lower densities may be more resistant to drought stress. Water stress may explain the effect of self-thinning, but the mechanisms underlying density-dependent mortality may be uncertain due to the direct intraspecific competition. Tree mortality is expected to be high in dense stands, because high stand density typically increases moisture stress and competition amongst trees [39] and predisposes trees to attack by bark beetles [40]. Conifer mortality, however, increased slightly with decreasing density or basal area in southwestern United States during climatic stress, across both space [41] and time [3, 42]. The latter finding is potentially important, because it may indicate that thinning high-density stands would not significantly reduce tree mortality during severe drought [33]. The level of drought-induced mortality due to the climatic drivers in these studies was great enough to mask the effects of density-dependence that might have been apparent at lower levels of mortality [3, 43]. The results of our and other studies have thus highlighted the complexities of density-dependent mortality resulting in temporal and spatial variation amongst stands [33].
Low wood density was a significant predictor of the risk of drought mortality in angiosperms but not gymnosperms, perhaps because of their fundamentally different wood anatomies, with gymnosperms having more negative P50 values (the water potentials at which 50% of hydraulic conductivity is lost because of embolism), more conservative stomatal responses, and larger hydraulic safety margins (between the typical minimum xylem water potential and 50%) [2, 43]. Wood density may also be a valuable proxy for mortality risk amongst angiosperms in particular, as has been observed previously by a meta-analysis across 475 species [23] and in tropical biomes [18]. Measures of wood density were lacking in our study, so we used species-level means to estimate wood density, which may have affected the relationship with vulnerability to moisture deficits, where individual-level wood structural properties may diverge significantly from species means [44]. The functions in angiosperms of resprouting from branch nodes below dead segmented tissues and the higher amount of parenchyma in stem wood linked to their higher storage capacity (for water and non-structural carbohydrates) likely increase the ability to recover from drought [23]. Parenchyma, however, would provide little tolerance to stresses in gymnosperms, likely because of correlations with other traits not identified here, such as low specific hydraulic conductivity or the ability to regrow xylem after embolism [45]. Gymnosperms also tend to have less parenchyma or non-structural carbohydrates in their wood than angiosperms [43]. The ability to rapidly repair embolisms may rely on the presence of nearby parenchymal cells [46], which could explain the need for greater safety margins in gymnosperm than angiosperm wood [43]. In addition, annual mortality rates did not differ significantly across all studies between evergreen (6.2%) and deciduous (6.1%) species (Table 1), demonstrated for example by a meta-analysis of mortality rates across 475 species [23]. The variation of non-structural carbohydrates may be extremely similar between deciduous and evergreen species and not differ significantly seasonally [27].
Stand age tightly correlated with growth and reproduction under normal conditions with no severe drought stress or biotic agents [47]. Mortality rates in our study differed significantly between saplings and adults (Table 1). However, the annual mortality rate of both saplings and mature trees is lower than the global mean rate of 5.5%. This lower rate might be ascribed to the low amount sites of saplings and mature trees, which cannot be compared with the global mean mortality rates. Saplings responded differently from adults to extreme drought in the field and sometimes suffered higher mortality rates [48, 49]. Vulnerability to drought-related mortality and associated carbon and water fluxes varies with stand age due to the physiological and environmental (e.g. microclimate and nutrients) differences [50]. Mortality rate was higher for saplings than adults for the combination of elevation and stand age [51]. The greater sensitivity of saplings is presumably a factor driving the biomass-drought relationship [3]. Saplings or small trees often have limited root networks and so are more vulnerable to acute water stress than mature or large trees with deeper or more extensive root networks [52]. Small trees or saplings also have much smaller reserves of stored non-structural carbohydrates [53], rendering them also potentially more vulnerable to carbon stress. In addition, foliar dieback is generally controlled by hydraulic factors in response to drought [54]. For example, potted coniferous saplings experienced extreme hydraulic damage [48], but adult juniper trees during either natural [55] or experimental [56] drought experienced little hydraulic damage. Floyd et al. [57] reported that larger or older trees were often more prone to drought-induced mortality, inconsistent with our results, but this relationship was species-dependent. Phillips et al. [15] also showed that large size conferred a strong penalty in Amazonia under drought conditions but also in Borneo where drought was more severe. The mechanism may set an ultimate limit on the stand-level biomass of tropical rainforests [58]. Adults should also be at risk, especially from stronger droughts, consistent with theoretical predictions that invoke hydraulic limitations as the dominant limiter of tree height [59]. The lower gas exchange in taller trees has been proposed to explain age-related declines in tree growth [60].
Much effort is currently focused on resolving the mechanistic details associated with mortality, including the interrelationships between hydraulic failure, carbon starvation, and biotic agents [2, 9, 61]. Furthermore, studies of annual precipitation, soil moisture, and plant water potential and conductance associated with plant hydraulics and carbon metabolism are emerging [14, 56, 62]. Annual precipitation and related metrics such as SPEI or Palmer Drought Severity Index (PDSI), in association with forest data, are nonetheless the most widely accessible data for identifying the key aspects of the thresholds of drought mortality [63]. Our meta-analysis indicates that the study of multiple factors is a promising avenue for modelling mortality but also highlights some of the gaps in our understanding. Firstly, estimates of mortality rate are potentially sensitive to the census interval because of the different turnover rates of subpopulations. Mortality rates should be adjusted to a standard interval of one year by applying a generic census-interval adjustment [64]. Secondly, many experimental droughts have been attempted in some regions. However, the results from these studies suggest that the experimental forests are susceptible to drought [15]. Thus, tree mortality from experimental droughts should not be compared with the episodes associated with natural droughts. Finally, comparisons amongst sites and regions or trees and species are also complicated by biogeographic and biotic factors that can produce inconclusive results.
Supporting Information
(XLSX)
Acknowledgments
We would like to thank Chencheng Zhang, Changkun Ma and Xiangdong Li for the help in data analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (41390461, 41501233) to Xiaoxu Jia (http://www.nsfc.gov.cn/); the National Natural Science Foundation of China (41530854) (http://www.nsfc.gov.cn/); the National Key Project for Research and Development (2016YFC0501605) to Mingan Shao (http://www.most.gov.cn/); the Program for Bingwei Excellent Talents from Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences (2015RC204) to Xiaoxu Jia (http://www.cas.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Campos GEP, Moran MS, Huete A, Zhang YG, Bresloff C, Huxman TE, et al. Ecosystem resilience despite large-scale altered hydroclimatic conditions. Nature. 2013; 494: 349–352. 10.1038/nature11836 [DOI] [PubMed] [Google Scholar]
- 2.Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R, et al. Global convergence in the vulnerability of forests to drought. Nature. 2012; 491: 752–755. 10.1038/nature11688 [DOI] [PubMed] [Google Scholar]
- 3.van Mantgem PJ, Stephenson NL, Byrne JC, Daniels LD, Franklin JF, Fulé PZ, et al. Widespread Increase of Tree Mortality Rates in the Western United States. Science. 2009; 323: 521–524. 10.1126/science.1165000 [DOI] [PubMed] [Google Scholar]
- 4.Peng CH, Ma ZH, Lei XD, Zhu Q, Chen H, Wang WF, et al. A drought-induced pervasive increase in tree mortality across Canada's boreal forests. Nat Clim Change. 2011; 1: 467–471. [Google Scholar]
- 5.Reichstein M, Bahn M, Ciais P, Frank D, Mahecha MD, Seneviratne SI, et al. Climate extremes and the carbon cycle. Nature. 2013; 500: 287–295. 10.1038/nature12350 [DOI] [PubMed] [Google Scholar]
- 6.Niu SL Luo YQ, Li DJ, Cao SH, Xia JY, Li JW, et al. Plant growth and mortality under climatic extremes: An overview. Environ Exp Bot. 2014; 98: 13–19. [Google Scholar]
- 7.Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecol Manag. 2010; 259: 660–684. [Google Scholar]
- 8.Allen CD, Breshears DD, McDowell NG. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere. 2015; 6: 129. [Google Scholar]
- 9.McDowell NG, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol. 2008; 178: 719–739. 10.1111/j.1469-8137.2008.02436.x [DOI] [PubMed] [Google Scholar]
- 10.Michaelian M, Hogg EH, Hall RJ, Arsenault E. Massive mortality of aspen following severe drought along the southern edge of the Canadian boreal forest. Global Change Biol. 2011; 17: 2084–2094. [Google Scholar]
- 11.Liu HY, Williams AP, Allen CD, Guo DL, Wu XC, Anenkhonov OA, et al. Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Global Change Biol. 2013; 19: 2500–2510. [DOI] [PubMed] [Google Scholar]
- 12.Song QH, Lin H, Zhang YP, Tan ZH, Zhao JF, Zhao JB, et al. The effect of drought stress on self-organisation in a seasonal tropical rainforest. Ecol Model. 2013; 265: 136–139. [Google Scholar]
- 13.Clifford MJ, Royer PD, Cobb NS, Breshears DD, Ford PL. Precipitation thresholds and drought-induced tree die-off: insights from patterns of Pinus edulis mortality along an environmental stress gradient. New Phytol. 2013; 200: 413–421. 10.1111/nph.12362 [DOI] [PubMed] [Google Scholar]
- 14.Adams HD, Guardiola-Claramonte M, Barron-Gafford GA, Villegas JC, Breshears DD, Zou CB, et al. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. P Natl Acad Sci USA. 2009; 106: 7063–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips OL, van der Heijden G, Lewis SL, Lopez-Gonzalez G, Aragao LEOC, Lloyd J, et al. Drought-mortality relationships for tropical forests. New Phytol. 2010; 187: 631–646. 10.1111/j.1469-8137.2010.03359.x [DOI] [PubMed] [Google Scholar]
- 16.Fraver S, Jonsson BG, Jonsson M, Esseen PA. Demographics and disturbance history of a boreal old-growth Picea abies forest. J Veg Sci. 2008; 19: 789–798. [Google Scholar]
- 17.Laarmann D, Korjus H, Sims A, Stanturf JA, Kiviste A, Koster K. Analysis of forest naturalness and tree mortality patterns in Estonia. Forest Ecol Manag. 2009; 258: 187–195. [Google Scholar]
- 18.Kraft NJB, Metz MR, Condit RS, Chave J. The relationship between wood density and mortality in a global tropical forest data set. New Phytol. 2010; 188: 1124–1136. 10.1111/j.1469-8137.2010.03444.x [DOI] [PubMed] [Google Scholar]
- 19.Powers MD, Palik BJ, Bradford JB, Fraver S, Webster CR. Thinning method and intensity influence long-term mortality trends in a red pine forest. Forest Ecol Manag. 2010; 260: 1138–1148. [Google Scholar]
- 20.van Gunst KJ, Weisberg PJ, Yang J, Fan YC. Do denser forests have greater risk of tree mortality: A remote sensing analysis of density-dependent forest mortality. Forest Ecol Manag. 2016; 359: 19–32. [Google Scholar]
- 21.IPCC, 2014: Climate Change 2014: Impacts, Adaptation, and Vulnerability Part A: Global and Sectoral Aspects Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, et al. (eds)] Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 1132 pp.
- 22.Sheil D and May RM. Mortality and recruitment rate evaluations in heterogeneous tropical forests. J Ecol. 1996; 84: 91–100. [Google Scholar]
- 23.Anderegg WRL, Klein T, Bartlett M, Sack L, Pellegrini AFA, Choat B, et al. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. P Natl Acad Sci USA. 2016; 113: 5024–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vicente-Serrano SM, Gouveia C, Camarero JJ, Beguería S, Trigo R, López-Moreno JI, et al. Response of vegetation to drought time-scales across global land biomes. P Natl Acad Sci USA. 2013; 110: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKee TB, Doesken NJ, Kleist J. The relationship of drought frequency and duration to time scales. Eight Conf. On Applied Climatology. Anaheim, CA, Amer. Meteor. Soc. 1993; 179–184. [Google Scholar]
- 26.Brodribb TJ, Holbrook NM, Edward EJ. Relations between stomatal closure and, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ. 2003: 26: 443–450. [Google Scholar]
- 27.Hoch G, Richter A, Korner C. Non-structural carbon compounds in temperate forest trees. Plant Cell Environ. 2003; 26: 1067–1081. [Google Scholar]
- 28.Zanne AE, Lopez-Gonzalez G, Coomes DA, Ilic J, Jansen S, Lewis SL, et al. Data from: Towards a worldwide wood economics spectrum. 2009; [DOI] [PubMed]
- 29.Hoffmann WA, Marchin RM, Abit P, Lau OL. Hydraulic failure and tree dieback are associated with high wood density in a temperate forest under extreme drought. Global Change Biol. 2011; 17: 2731–2742. [Google Scholar]
- 30.Nardini A, Battistuzzo M, Savi T. Shoot desiccation and hydraulic failure in temperate woody angiosperms during an extreme summer drought. New Phytol. 2013; 200: 322–329. 10.1111/nph.12288 [DOI] [PubMed] [Google Scholar]
- 31.Engelbrecht BMJ, Comita LS, Condit R, Kursar TA, Tyree MT, Turner B, et al. Drought sensitivity shapes species distribution patterns in tropical forests. Nature. 2007; 447: 80–82. 10.1038/nature05747 [DOI] [PubMed] [Google Scholar]
- 32.Fensham RJ, Fairfax RJ, Ward DP. Drought-induced tree death in savanna. Global Change Biol. 2009; 15: 380–387. [Google Scholar]
- 33.Ganey JL and Vojta SC. Tree mortality in drought-stressed mixed-conifer and ponderosa pine forests, Arizona, USA. Forest Ecol Manag. 2011; 261: 162–168. [Google Scholar]
- 34.Clifford MJ, Cobb NS, Buenemann M. Long-term tree cover dynamics in a pinyon-juniper woodland: climate-change-type drought resets successional clock. Ecosystems. 2011; 14: 949–962. [Google Scholar]
- 35.Phillips OL Aragao LEOC, Lewis SL, Fisher JB, Lloyd J. Drought Sensitivity of the Amazon Rainforest. Science. 2009; 323: 1344–1347. 10.1126/science.1164033 [DOI] [PubMed] [Google Scholar]
- 36.Smith JB Schneider SH, Oppenheimer M, Yohe GW, Hare W, Mastrandrea MD, et al. Assessing dangerous climate change through an update of the Intergovernmental Panel on Climate Change (IPCC) "reasons for concern''. P Natl Acad Sci USA. 2009; 106: 4133–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenwood DL and Weisberg PJ. Density-dependent tree mortality in pinyon-juniper woodlands. Forest Ecol Manag. 2008; 255: 2129–2137. [Google Scholar]
- 38.Santos MJ and Whitham TG. Predictors of Ips confusus Outbreaks During a Record Drought in Southwestern USA: Implications for Monitoring and Management. Environ Manag. 2010; 45: 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guarin A and Taylor AH. Drought triggered tree mortality in mixed conifer forests in Yosemite National Park, California, USA. Forest Ecol Manag. 2005; 218: 229–244. [Google Scholar]
- 40.Negron JF and Wilson JW. Attributes associated with probability of infestation by the piñon Ips confusus (Coleoptera: Scolytidae), in pinon pine, Pinus edulis. West N Am Nat. 2003; 63: 440–51. [Google Scholar]
- 41.Floyd ML Clifford M, Cobb NS, Hanna D, Delph R, Ford P, et al. Relationship of stand characteristics to drought-induced mortality in three Southwestern pinon-juniper woodlands. Ecol Appl. 2009; 19: 1223–1230. [DOI] [PubMed] [Google Scholar]
- 42.van Mantgem PJ and Stephenson NL. Apparent climatically induced increase of tree mortality rates in a temperate forest. Ecol Lett. 2007; 10: 909–916. 10.1111/j.1461-0248.2007.01080.x [DOI] [PubMed] [Google Scholar]
- 43.Johnson DM, McCulloh KA, Woodruff DR, Meinzer FC. Hydraulic safety margins and embolism reversal in stems and leaves: Why are conifers and angiosperms so different? Plant Sci. 2012; 195: 48–53. 10.1016/j.plantsci.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 44.Patino S, Lloyd J, Paiva R, Quesada CA, Baker TR, Mercado L, et al. Branch xylem density variation across the Amazon Basin. Biogeosciences. 2009; 6: 545–568. [Google Scholar]
- 45.Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE. Towards a worldwide wood economics spectrum. Ecol Lett. 2009: 12: 351–366. 10.1111/j.1461-0248.2009.01285.x [DOI] [PubMed] [Google Scholar]
- 46.Kim YX, Steudle E. Light and turgor affect the water permeability (aquaporins) of parenchyma cells in the midrib of leaves of Zea mays. J Exp Bot. 2007; 58: 4119–4129. 10.1093/jxb/erm270 [DOI] [PubMed] [Google Scholar]
- 47.Keeling HC, Baker T, Martinez R, Monteagudo A, Phillips O. Contrasting patterns of diameter and biomass increment across tree functional groups in Amazonian forests. Oecologia. 2008; 158: 521–534. 10.1007/s00442-008-1161-4 [DOI] [PubMed] [Google Scholar]
- 48.Anderegg WRL and Anderegg LDL. Hydraulic and carbohydrate changes in experimental drought-induced mortality of saplings in two conifer species. Tree Physiol. 2013; 33: 252–260. 10.1093/treephys/tpt016 [DOI] [PubMed] [Google Scholar]
- 49.He JS, Zhang QB, Bazzaz FA. Differential drought responses between saplings and adult trees in four co-occurring species of New England. Trees-Struct Funct. 2005; 19: 442–450. [Google Scholar]
- 50.Law BE. Regional analysis of drought and heat impacts on forests: current and future science directions. Global Change Biol. 2014; 20: 3595–3599. [DOI] [PubMed] [Google Scholar]
- 51.Acker SA, Boetsch JR, Bivin M, Whiteaker L, Cole C, Philippi T. Recent tree mortality and recruitment in mature and old-growth forests in western Washington. Forest Ecol Manag. 2015; 336: 109–118. [Google Scholar]
- 52.Mueller RC Scudder CM, Porter ME, Trotter RT, Gehring CA, Whitham TG. Differential tree mortality in response to severe drought: evidence for long-term vegetation shifts. J Ecol. 2005; 93: 1085–1093. [Google Scholar]
- 53.Niinemets U. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. Forest Ecol Manag. 2010; 260: 1623–1639. [Google Scholar]
- 54.Sperry JS, Hacke UG, Oren R, Comstock JP. Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ. 2002; 25: 251–263. [DOI] [PubMed] [Google Scholar]
- 55.West AG, Hultine KR, Sperry JS, Bush SE, Ehleringer JR. Transpiration and hydraulic strategies in a pinon-juniper woodland. Ecol Appl. 2008; 18: 911–927. [DOI] [PubMed] [Google Scholar]
- 56.Plaut JA Yepez EA, Hill J, Pangle R, Sperry JS, Pockman WT, et al. Hydraulic limits preceding mortality in a pinon-juniper woodland under experimental drought. Plant Cell Environ. 2012; 35: 1601–1617. 10.1111/j.1365-3040.2012.02512.x [DOI] [PubMed] [Google Scholar]
- 57.Floyd ML, Romme WH, Rocca ME, Hanna DP, Hanna DD. Structural and regenerative changes in old-growth pinon-juniper woodlands following drought-induced mortality. Forest Ecol Manag. 2015; 341: 18–29. [Google Scholar]
- 58.Stegen JC Swenson NG, Enquist BJ, White EP, Phillips OL, Jorgensen PM, et al. Variation in above-ground forest biomass across broad climatic gradients. Global Ecol Biogeogr. 2011; 20: 744–754. [Google Scholar]
- 59.Niklas KJ and Spatz HC. Growth and hydraulic (not mechanical) constraints govern the scaling of tree height and mass. P Natl Acad Sci USA. 2004; 101: 15661–15663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sala A and Hoch G. Height-related growth declines in ponderosa pine are not due to carbon limitation. Plant Cell Environ. 2009; 32: 22–30. 10.1111/j.1365-3040.2008.01896.x [DOI] [PubMed] [Google Scholar]
- 61.McDowell NG. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiology. 2011; 155: 1051–1059. 10.1104/pp.110.170704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adams HD, Germino MJ, Breshears DD, Barron-Gafford GA, Claramonte M, Zou CB, et al. Nonstructural leaf carbohydrate dynamics of Pinus edulis during drought-induced tree mortality reveal role for carbon metabolism in mortality mechanism. New Phytol. 2013; 197: 1142–1151. 10.1111/nph.12102 [DOI] [PubMed] [Google Scholar]
- 63.Peterman W, Waring RH, Seager T, Pollock WL. Soil properties affect pinyon pine-juniper response to drought. Ecohydrology. 2012; [Google Scholar]
- 64.Lewis SL, Phillips OL, Sheil D, Vinceti B, Baker TR, Brown S, et al. Tropical forest tree mortality, recruitment and turnover rates: calculation, interpretation, and comparison when census intervals vary. J Ecol. 2004; 92: 929–944. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.