Abstract
B. parapertussis is a whooping cough etiological agent with the ability to evade the immune response induced by pertussis vaccines. We previously demonstrated that in the absence of opsonic antibodies B. parapertussis hampers phagocytosis by neutrophils and macrophages and, when phagocytosed, blocks intracellular killing by interfering with phagolysosomal fusion. But neutrophils can kill and/or immobilize extracellular bacteria through non-phagocytic mechanisms such as degranulation and neutrophil extracellular traps (NETs). In this study we demonstrated that B. parapertussis also has the ability to circumvent these two neutrophil extracellular bactericidal activities. The lack of neutrophil degranulation was found dependent on the O antigen that targets the bacteria to cell lipid rafts, eventually avoiding the fusion of nascent phagosomes with specific and azurophilic granules. IgG opsonization overcame this inhibition of neutrophil degranulation. We further observed that B. parapertussis did not induce NETs release in resting neutrophils and inhibited NETs formation in response to phorbol myristate acetate (PMA) stimulation by a mechanism dependent on adenylate cyclase toxin (CyaA)-mediated inhibition of reactive oxygen species (ROS) generation. Thus, B. parapertussis modulates neutrophil bactericidal activity through two different mechanisms, one related to the lack of proper NETs-inducer stimuli and the other one related to an active inhibitory mechanism. Together with previous results these data suggest that B. parapertussis has the ability to subvert the main neutrophil bactericidal functions, inhibiting efficient clearance in non-immune hosts.
Introduction
Bordetella pertussis and Bordetella parapertussis are human pathogens that cause whooping cough. Epidemiological studies revealed that B. parapertussis infections are globally more common than expected, affecting mainly vaccinated populations [1]. The incidence in vaccinated populations increased after introduction of acellular pertussis vaccines [2]. Current acellular pertussis vaccines, formulated with B. pertussis components, fail to protect against B. parapertussis infections [3–5]. Although cross-immunity could be expected between bacteria that share the majority of the vaccine antigens, the O antigen molecule on B. parapertussis surface blocks antibody access to the vaccine antigens common to both species [5]. Thus B. parapertussis largely avoids opsonophagocytosis and other activities mediated by vaccine-induced antibodies [5–7], surviving and growing primarily extracellularly within the respiratory tract. Avoiding antibody-mediated effects does not prevent exposure to the many other anti-microbial activities unleashed upon invading bacteria. Neutrophils are rapidly recruited to the site of bacterial infection. These cells are equipped with extracellular bactericidal mechanisms, such as degranulation and NETs [8, 9]. Degranulation occurs when specific membrane receptors are activated and neutrophil cytoplasmic granules translocate to the plasma membrane [10], or when during bacterial phagocytosis, nascent unclosed phagosomes fuse with granules releasing their content into the extracellular medium [11, 12]. On the other hand, NETs have been observed in vivo during infection with bacteria [9, 13, 14], fungi [15, 16], protozoa [17, 18], and viruses [19–21]. The microbicidal activity of NETs is mediated by the same components released via degranulation, including neutrophil elastase (NE), myeloperoxidase, cathepsin G, proteinase 3, bacterial permeability-increasing protein, lactoferrin, gelatinase, the cathelicidin LL-37, and tryptase with the addition of nuclear antimicrobial factors as DNA and histones [22, 23]. Therefore, the key difference between NETs release and degranulation is that the NETs, through their network structure, act as a physical container of microbes that reduces their dissemination, allowing the microbicidal factors to exert their action more efficiently, and limiting the damage to the surrounding tissues by confining antimicrobial agents to a restricted area [9, 24]. NETs released may be induced by several stimuli including bacterial components, proinflammatory cytokines or PMA [9, 23]. Most of the NETs inducer stimuli initiate NETs release through ROS generated by the activation of the NADPH oxidase (NOX) system. ROS lead to the translocation of NE from azhurophilic granules to the nucleus where it initiates several chromatin structure rearrangements leading to chromatin decondensation. Next, the nuclear envelope is dismantled and chromatin is mixed with bactericidal compounds of cytoplasmic, granular, and nuclear origin. Finally, the cell membrane is broken and NETs are released into the extracellular medium trapping and/or killing bacteria [25, 26]. Importantly, NETs were found released in the respiratory tract in response to several pathogen infections [16, 19, 27–29], although their role in whooping cough is still uncertain.
In the present study, we investigated neutrophils extracellular antimicrobial functions against B. parapertussis and explored how this pathogen is able to resist these functions. Our results indicate that B. parapertussis is able to evade neutrophil extracellular killing mechanisms if specific antibodies are not present at the site of interaction.
Materials and Methods
Bacterial strains and growth
The B. parapertussis strain CN2591 and the isogenic B. parapertussis mutant strain lacking the O antigen, CN2591Δwbm [30, 31], were used in this study. Bacteria were stored at -70°C and recovered by growth on Bordet-Gengou agar (BGA) (BD Difco) plates supplemented with 15% defibrinated sheep blood (Laboratorio Argentino, Caseros, Argentina) (bBGA) for 24 h at 36°C. Animal handling and all procedures were in compliance with the Argentinean animal protection Law 14346. Virulent (Bvg+) bacteria were subsequently seeded on Stainer-Scholte (SS) liquid medium, cultured for 20 h at 36°C, and used in the experiments. Avirulent (Bvg-) B. parapertussis was obtained by growing the bacteria on bBGA plates supplemented with magnesium sulfate at a final concentration of 50 mM for 24 h at 36°C, subsequently seeded on SS liquid medium containing 50 mM magnesium sulfate, cultured for 20 h at 36°C, and used in the experiments. Transition from Bvg+ to Bvg- phase was confirmed by the loss of expression of the CyaA and the filamentous hemagglutinin by western blot analysis of whole-cell lysates as previously described [32] (data not shown). Inactivation of B. parapertussis was obtained by treating bacterial cells with 4% (w/v) paraformaldehyde for 1 h, washed once with phosphate-buffered saline (PBS), incubated for 10 min at room temperature with PBS containing 50 mM NH4Cl, washed, and suspended in the appropriate medium. Pseudomona aeruginosa strain PA01 was grown for 12 h at 37°C in Luria–Bertani medium (Gibco, Rockville, MD).
Cells
Human neutrophils were isolated from heparinized venous blood using Ficoll-Histopaque (Sigma, St Louis, MO) gradient centrifugation. Neutrophils were harvested and the remaining erythrocytes were removed by hypotonic lysis. Cell viability was 99% as determined by Trypan blue exclusion. Prior to functional assays, neutrophils were washed twice with Dulbecco’s modified Eagle medium (DMEM) (Gibco) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Gibco), suspended, and used immediately (the presence of heat-stable nucleases in FCS was discarded as in [33]). All experiments were carried out with freshly isolated neutrophils lacking FcγRI expression, as monitored by FACS analysis using a fluorescence-activated cell sorter FACScalibur flow cytometer with anti-hFcγRI mAb 22 (mIgG1) [34]. Data were processed using the CellQuest software (BD Biosciences, San Jose, CA). All procedures involving human samples were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments, and approved by the Institutional Review Board. Peripheral blood was collected from healthy donors. All individuals provided written informed consent for the collection of samples and subsequent analysis.
Antibodies
The following antibodies were used: mouse anti-human LAMP-1 monoclonal antibodies (Pharmingen, San Diego, CA), rabbit anti-human flotillin-1 (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-human NE polyclonal antibodies (Calbiochem-Merk Millipore; Darmstad, Germany), mouse anti-hFcγRI (CD64) MAb 22 (mIgG1) (Medarex, Annandale, NJ). Cy3-conjugated goat F(ab´)2 fragments of anti-rabbit IgG and fluorescein isothiocyanate (FITC)-conjugated goat F(ab´)2 fragments of anti-rabbit IgG (both from Jackson ImmunoResearch, West Grove, PA), Cy3-conjugated goat F(ab´)2 fragments of anti-mouse IgG, phycoerythrin (PE)-conjugated goat F(ab´)2 fragments of anti-mouse IgG (both from Molecular Probes, Eugene, OR), and FITC-conjugated goat F(ab´)2 fragments of anti-mouse IgG (from Southern Biotechnology, Birmingham, AL). Immunoglobulin G (IgG) fractions from pooled sera of whooping cough patients with high titers of anti-B. parapertussis antibodies, as measured by enzyme-linked immunosorbent assay [35], were isolated as described previously [36]. Polyclonal rabbit anti-B. parapertussis antiserum and polyclonal mouse anti-B. parapertussis antiserum were obtained as described elsewhere [37].
Respiratory burst determination
ROS production by neutrophils was determined as previously described [7], with minor modifications. Briefly, tubes containing 2x107 bacteria were transferred to a Luminometer (Luminoskan TL Plus, Thermo LabSystems, Finland), in which chemoluminescence response of 2x105 neutrophils was measure every minute for 120 min at 37°C after injection of 120 μM luminol (Sigma). Neutrophils incubated with PMA were used as positive control. Neutrophils incubated with diphenyleneiodonium (DPI), a NOX inhibitor, 30 min before PMA treatment were used as a control of inhibition of PMA-induced ROS.
Neutrophil degranulation studies
Exocytosis of the lysosomal enzyme β-glucuronidase was determined as previously described [38], with minor modifications. Briefly, 2x105 neutrophils were incubated with bacteria at a multiplicity of infection (MOI) of 300 for 10 min at 37°C and centrifuged at 300 x g. Supernatants were further centrifuged at 10,000 x g for 10 min to eliminate bacteria. β-glucuronidase activity was measured at 405 nm in cell lysed with 0.2% (v/v) Triton X-100 (Sigma), and in the cell-free supernatants. Controls were performed by determination of β-glucuronidase activity from non-infected 2x105 neutrophils lysed by Triton X-100 treatment, assumed as a 100% of release. Cell-free supernatant of uninfected neutrophils was used as a negative control.
Confocal microscopy studies were performed as previously described [7], with minor modifications. Briefly, neutrophils were incubated with B. parapertussis at a MOI of 300 at 37°C. Ten minutes post infection neutrophils were incubated with or without 200 nM LysoTracker DND-99 (Molecular Probes) (5 min at 37°C) prior to fixation with 4% (w/v) paraformaldehyde. Samples incubated with LysoTracker stain were then washed twice with PBS and further incubated 10 min at room temperature with PBS containing 50 mM NH4Cl. After two washing steps, the cells were incubated for 30 min at 25°C with PBS containing 0.1% (w/v) saponin (Sigma) and 0.2% (w/v) bovine serum albumin (BSA) (Sigma), followed by incubation with polyclonal rabbit anti-B. parapertussis antiserum (30 min at 25°C) in the presence of 0.1% (w/v) saponin and 0.2% (w/v) BSA. After three washing steps, neutrophils were incubated with FITC-conjugated goat F(ab´)2 fragments of anti-rabbit immunoglobulin (30 min at 25°C). Samples of infected cells that were not incubated with LysoTracker stain were washed twice with PBS and incubated for 10 min at room temperature with PBS containing 50 mM NH4Cl. After two washing steps, the cells were further incubated for 30 min at 25°C with PBS containing 0.1% (w/v) saponin and 0.2% (w/v) BSA. Next, the cells were incubated for 30 min at 25°C with either mouse anti-human LAMP-1 monoclonal antibodies plus polyclonal rabbit anti-B. parapertussis antiserum or rabbit anti-human flotillin-1 plus polyclonal mouse anti-B. parapertussis antiserum antibodies (30 min at 25°C) in the presence of 0.1% (w/v) saponin and 0.2% (w/v) BSA. After three washing steps, the cells were incubated with either Cy3-conjugated F(ab´)2 fragments of goat anti-mouse antibodies plus FITC-conjugated goat F(ab´)2 fragments of anti-rabbit immunoglobulin or Cy3-conjugated F(ab´)2 fragments of goat anti-rabbit antibodies plus FITC-conjugated goat F(ab´)2 fragments of anti-mouse immunoglobulin for another 30 min at 25°C. To avoid cytophilic binding of antibodies to the FcR, all incubations were done in the presence of 25% (v/v) heat-inactivated human serum. Additionally, isotype controls were run in parallel. Microscopic analyses were performed using a confocal laser scanning microscope (model TCS SP5; Leica, Mannheim, Germany). The percentage of phagosomes containing the bacterium that colocalized with a given marker was calculated from the number of total intracellular bacteria by analyzing at least 50 phagosomes per donor.
In control studies neutrophils were incubated with 10 mg/ml of β-methyl cyclodextrin (Sigma) (15 min at 37°C) in serum-free DMEM plus bovine serum albumin (BSA) (0.2%) and lovastatin (5 μg/ml) (Sigma) (DMEM-BSA-L), as previously described [7]. Cells were then washed, suspended in DMEM-BSA-L, and used immediately in infection studies. No decrease in neutrophils viability was detected after treatment.
NETs formation studies
NETs formation studies were performed as previously described [9], with minor modifications. Briefly, 2x105 neutrophils were seeded in 24 well-plates containing 12 mm coverslip and incubated with bacteria at different MOI (10, 50 or 100), centrifuged for 5 min at 300 × g, and further incubated at 37°C for different time periods. Neutrophils incubated with or without PMA (Sigma) (20 nM) were used as positive and negative control, respectively. In some experiments, neutrophils were incubated with either bacteria or conditioned medium (CM) (obtained as described below) for 30 min at 37°C before incubation with PMA (20 nM). Neutrophils incubated with DPI 30 min before PMA treatment were used as a control of inhibition of PMA-induced NETs release. The NETs were visualized by immunofluorescence microscopy as described below.
Fluorescence microscopy was used to visualize NETting cells. Immunostaining of cells was performed as described before [7], with minor modifications. At different time points samples were fixed with 4% (w/v) paraformaldehyde, washed twice with PBS, and incubated for 10 min at room temperature with PBS containing 50 mM NH4Cl. After two washing steps, the cells were incubated for 30 min at 25°C with PBS containing 0.1% (w/v) saponin and 0.2% (w/v) BSA. Next, the cells were incubated with rabbit anti-human NE polyclonal antibodies (30 min at 25°C) in the presence of 0.1% (w/v) saponin and 0.2% (w/v) BSA. After two washing steps, neutrophils were incubated with FITC-conjugated goat F(ab´)2 fragments of anti-rabbit immunoglobulin in the presence of 0.1% (w/v) saponin and 0.2% BSA (w/v) for another 30 min at 25°C, and washed twice. Finally, the samples were incubated with propidium iodide (Sigma) for DNA stain at a concentration of 2 mg/ml in PBS for 5 min. After washing with distillated water, coverslips were mounted on microscope slides and analyzed by fluorescence microscopy using a DMLB microscope coupled to a DC100 camera (Leica Microscopy Systems Ltd., Heerbrugg, Switzerland).
In order to evaluate if NETs can immobilize B. parapertussis, 2x105 neutrophils were seeded in 24 well-plates containing 12 mm coverslip and then stimulated with PMA 100 nM for 1 h to induce NETs release. Neutrophils were incubated with bacteria for 3 h, washed and fixed with 4% (w/v) paraformaldehyde. NE and DNA were stained as above described. After washing with distillated water, coverslips were mounted on microscope slides and analyzed by confocal laser scanning microscope (model TCS SP5; Leica).
NETosis quantification
Quantification of neutrophils that underwent NETosis was performed using the image processing software, ImageJ (National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/), as previously described [39] with minor modifications. Briefly, fluorescence microscopy images captured with a 40X objective were used to measure individual nuclear area of neutrophils stained with propidium iodide by adjusting a threshold above background. The average value of nuclear area of resting neutrophils was chosen as a cutoff value. Cell nucleus with areas larger than this cutoff value were considered NETting cells. This correlation was confirmed by checking whether randomly chosen cells which nuclear area exceeding the cutoff value showed chromatin colocalizing with the major NETs-associated granular protein NE. Results were obtained from three independent experiments run in duplicate using neutrophils from three different donors. At least 500 cells from each condition were examined.
Conditioned medium
CM was obtained as follows. Bacteria were grown in SS medium, washed, and further incubated in DMEM plus 10% FCS at a concentration of 2×108 bacteria/ml for 2 h at 37°C. Next, the medium was filtered with a 0.22 μm membrane filter to remove the bacteria and further used in experiments. In selected experiments CM was treated with a monoclonal antibody against both activities of CyaA (2A12), a monoclonal antibody that prevents translocation of the enzyme catalytic domain into the cell (5D1), or a monoclonal antibody against the hemolytic activity (6E1) of the CyaA [40], all kindly supplied by Erik L. Hewlett, Division of Infectious Diseases and International Health, Department of Medicine, University of Virginia, Charlottesville, Virginia, USA. In other experiments CM was treated with proteinase K (PK) 100 μg/mL for 60 min at 37°C before used.
DNA degradation studies
DNase activity of B. parapertussis was determined as previously described [41], with minor modifications. Briefly, purified neutrophil DNA (300 ng) was incubated at 37°C for 60 min with PBS (negative control), 500 mU/ml of Micrococcal nuclease I (MNase I) (Roche Diagnostics; Mannheim, Germany) (positive control), B. parapertussis (6x109 colony forming units (CFU)/ml), or bacterial-free culture supernatant (obtained by filtration of a 20 h bacterial culture in SS medium). Next, samples were mixed with sample buffer and loaded onto a 1% agarose gel and run in TBE buffer with 1% ethidium bromide at 100 volts.
Bacterial killing by NETs
The ability of NETs to kill B. parapertussis was evaluated as previously described [42] with minor modifications. Briefly, 2×105 neutrophils were seeded in 24 well-plates and then incubated with 100 nM PMA for 1 h at 37°C, to induce NETs. Next, neutrophils were treated or not with 100 μg/ml Cytochalasin D (CytD) (Sigma) to inhibit phagocytosis or with 500 mU/ml MNase I, to breakdown NETs, for 30 min. In control experiments a combination of CytD and MNase I was used. Neutrophil were then incubated with bacteria (MOI:100) for 3 h and further treated with MNase I for 20 min to release the bacteria eventually immobilized by NETs. Neutrophils were further subjected to hypotonic lysis by using 0.1% saponin in sterile deionized water and serial dilutions of lysates were rapidly plated onto bBGA to enumerate the CFU. To confirm that NETs can kill B. parapertussis a microscopic analysis using a LIVE/DEAD Cell Viability Assays Kit (Molecular Probes) was performed according to the manufacturer’s instructions.
Statistical analysis
Student’s t test (95% confidence level) or analysis of variance (ANOVA) was used for statistical data evaluation. The significance of the differences between the mean values of the data evaluated by ANOVA was determined with the least-significant-difference (LSD) test at a 95% confidence level. Results are shown as means and standard deviations (SD).
Results
B. parapertussis inhibits neutrophil degranulation
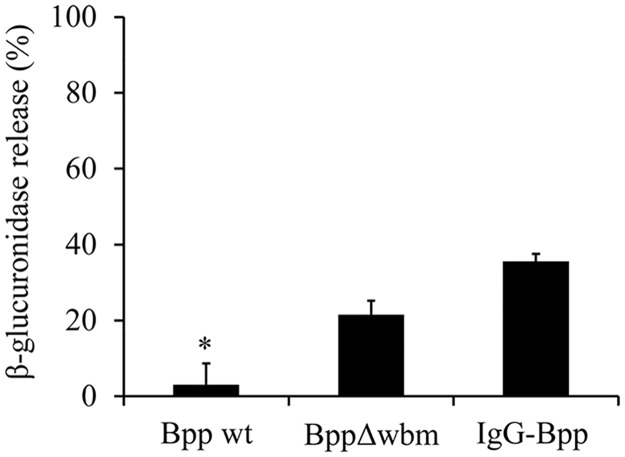
We previously demonstrated that in the absence of opsonic antibodies, B. parapertussis avoids neutrophil intracellular killing by inhibiting phagolysosomal fusion through an O antigen dependent mechanism [7]. In this study, we investigated whether these interactions modulate neutrophil degranulation. To this end, human neutrophils were incubated with non-opsonized B. parapertussis, non-opsonized O antigen deficient B. parapertussis or IgG-opsonized B. parapertussis at a MOI of 300. Ten minutes after exposure the azurophil granule enzymeβ-glucuronidase activity in the cell-free supernatants was measured. Supernatant of uninfected neutrophils was used as negative control. Total β-glucuronidase activity of uninfected neutrophils was determined and set as 100%. As can be seen in Fig 1, non-opsonized B. parapertussis failed to trigger significant neutrophil degranulation. In contrast, the B. parapertussis mutant lacking the expression of the O antigen induced strong neutrophil degranulation, suggesting that O antigen blocks degranulation. The ability of B. parapertussis to impair phagosome-lysosome maturation was previously found abrogated by IgG opsonization of the bacteria [7]. Accordingly, Fig 1 (S1 Data) shows that IgG-opsonized B. parapertussis also induced neutrophil degranulation.
Fig 1. Non-opsonized B parapertussis fails to trigger neutrophil degranulation.
Neutrophils were incubated with non-opsonized B. parapertussis (Bpp), non-opsonized B. parapertussis Δwbm (BppΔwbm), or IgG-opsonized B. parapertussis (IgG-Bpp) at a MOI of 300. The release of β-glucuronidase 10 min post infection was expressed as a percentage of the total β-glucuronidase cell content. The data represent the means ± SD of three independent experiments. The β-glucuronidase activity in the cell free supernatant of neutrophils infected with non-opsonized B. parapertussis was significantly different from the β-glucuronidase activity in the cell free supernatant of neutrophils infected with opsonized B. parapertussis or the O antigen deficient B. parapertussis (*P <0.05).
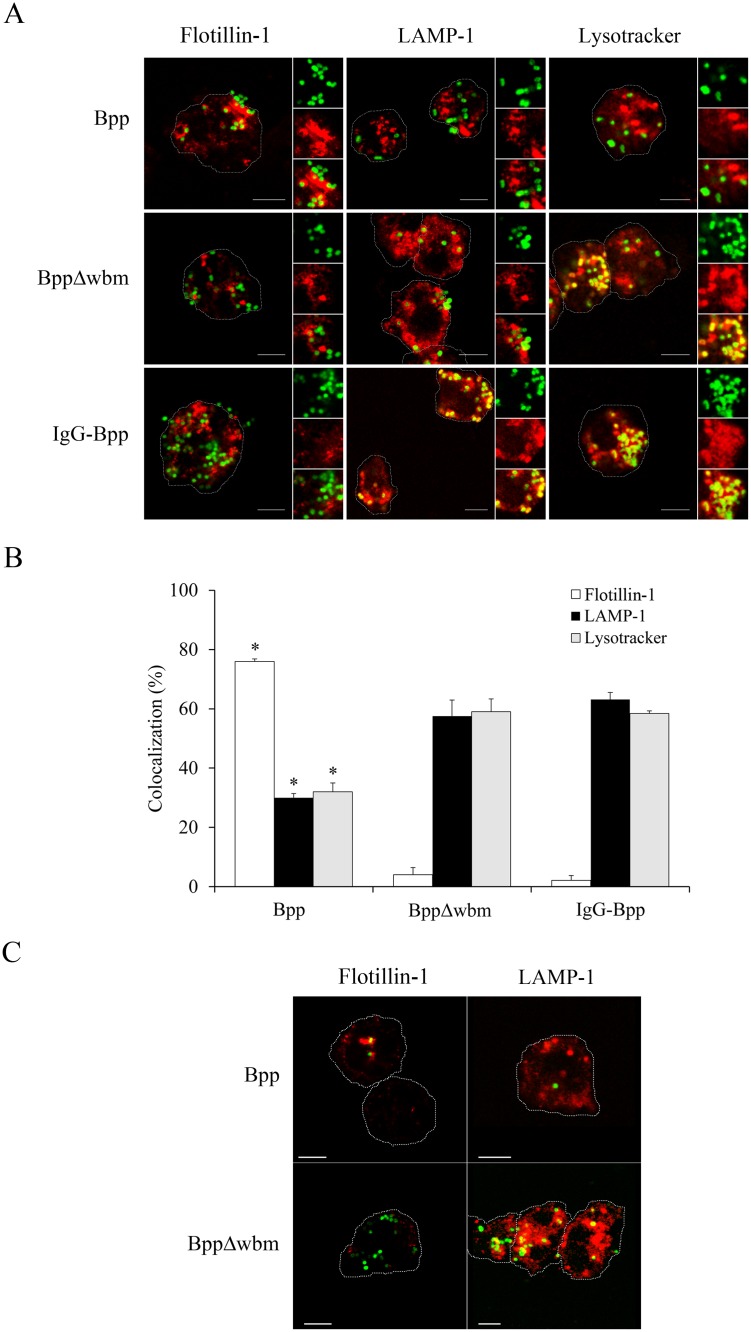
We previously found that the interaction of B. parapertussis with lipid rafts through the O antigen was involved in inhibition of phagosome-lysosome fusion [7]. We examined this interaction in relation to neutrophil degranulation by confocal microscopy using flotillin-1 as lipid raft marker and Lysotracker and LAMP-1 as markers of azurophil [43] and specific granules [44], respectively. Fig 2 (S2 Data) shows that as early as 10 min post infection, a high number of wild type bacteria were mainly colocalizing with flotillin-1, and only a small percentage of bacteria were found colocalizing with LAMP-1 or Lysotracker. On the other hand, at the same time point post infection, neither the O antigen deficient mutant nor the IgG-opsonized bacteria were found colocalizing with flotillin-1, whereas they did colocalize with the LAMP-1 or Lysotracker markers (Fig 2). In control experiments we further confirmed that, as previously found [7], the treatment of neutrophils with β-methyl cyclodextrin, a compound that disrupts cholesterol-rich domains by extracting cholesterol, did not affect the attachment of the O antigen deficient mutant but did induce a significant decrease in the number of attached wild type B. parapertussis. Neutrophils treatment with β-methyl cyclodextrin, on the other hand, did not affect the colocalization of LAMP-1 positive compartments with the O antigen deficient mutant strain. Neither was affected the level of colocalization of the wild type strain of B. parapertussis with LAMP-1 positive compartments in β-methyl cyclodextrin treated neutrophils, which is in agreement with the finding that the few bacteria found in these neutrophils were colocalizing with the residual flotillin-1 positive spots (Fig 2C). Accordingly, β-glucuronidase release was not modified by treatment of neutrophils with β-methyl cyclodextrin prior to incubation with either the wild type strain or the O antigen deficient mutant (data not show). All together, these results suggest that B. parapertussis inhibits neutrophil degranulation via an O antigen- lipid raft- dependent mechanism.
Fig 2. B. parapertussis entry through lipid rafts prevents fusion of nascent phagosomes with specific and azhurophil granules.
Neutrophils were incubated with non-opsonized B. parapertussis (Bpp), non-opsonized B. parapertussis Δwbm (BppΔwbm), or IgG-opsonized B. parapertussis (IgG-Bpp) at a MOI of 300 for 10 min at 37°C. After washing, samples were incubated with or without Lysotracker (azhurophil granule marker), fixed and permeabilized prior to incubation with antibodies against B. parapertussis to FITC stained the bacteria. Samples that were not incubated with LysoTracker were fixed and permeabilized prior to incubation with antibodies against either flotillin-1 (red) (lipid raft marker) or LAMP-1 (red) (specific granule marker). B. parapertussis was FITC stained by using antibodies against bacteria. Finally samples were analyzed by confocal microscopy. (A) Colocalization of bacteria with lipid rafts is reflected by green-fluorescent B. parapertussis surrounded by red labeled flotillin-1. Bacterial interaction with either specific or azhurophilic granules is reflected by green-fluorescent B. parapertussis surrounded by red labeled LAMP-1 or Lysotracker positive compartments, respectively. Representative panels of one of three independent experiments are shown. Scale bar: 5 μm. (B) The bars indicate the respective percentages of bacteria colocalizing with a given marker. The data represent the means ± SD of three independent experiments. At least 50 phagosomes were counted per sample. The percentage of colocalization of non-opsonized B. parapertussis with a given marker was significantly different from the percentage of colocalization with the respective marker in neutrophils infected with either the non-opsonized B. parapertussis Δwbm or the IgG-opsonized B. parapertussis (*P <0.05). (C) Neutrophils were treated with a cholesterol-depleting drug (β-methyl cyclodextrin, 10 mg/ml) prior to incubation with Bpp or BppΔwbm (both at an MOI of 300) for 10 min at 37°C. After washing, cells was fixed and permeabilized prior to incubation with antibodies against B. parapertussis to FITC stain the bacteria and antibodies against either flotillin-1 (red) or LAMP-1 (red). Representative panels of one of three independent experiments are shown. Scale bar: 5 μm.
B. parapertussis does not induce NETs in resting neutrophils
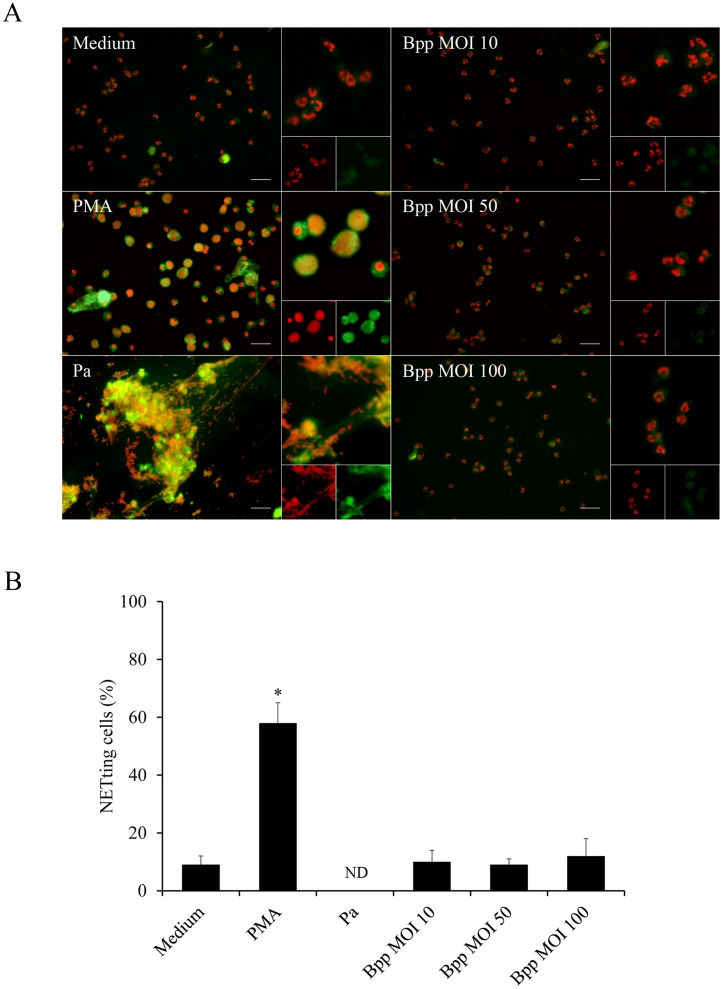
We next investigated whether the interaction of B. parapertussis with neutrophils induces NETosis, the other neutrophil extracellular bactericidal mechanisms. NETs induction was evaluated by fluorescence microscopy using a DNA intercalating dye and specific antibodies against NET-associated proteins. Fig 3 (S3 Data) shows that neutrophils that were incubated with medium alone did not undergo NETosis and exhibit a lobulated nucleus, NE is not colocalizing with the cell DNA, and no extracellular DNA structures can be observed. In contrast, neutrophils that underwent NETosis by PMA treatment or exposure to P. aeruginosa at an MOI of 10 showed a decondensed nucleus in which the chromatin colocalizes with NE and extracellular structures in which chromatin and NE are colocalizing, respectively. In contrast, B. parapertussis failed to induce NETosis at any MOI assayed, indicating that B. parapertussis does not induce NETosis in resting neutrophils.
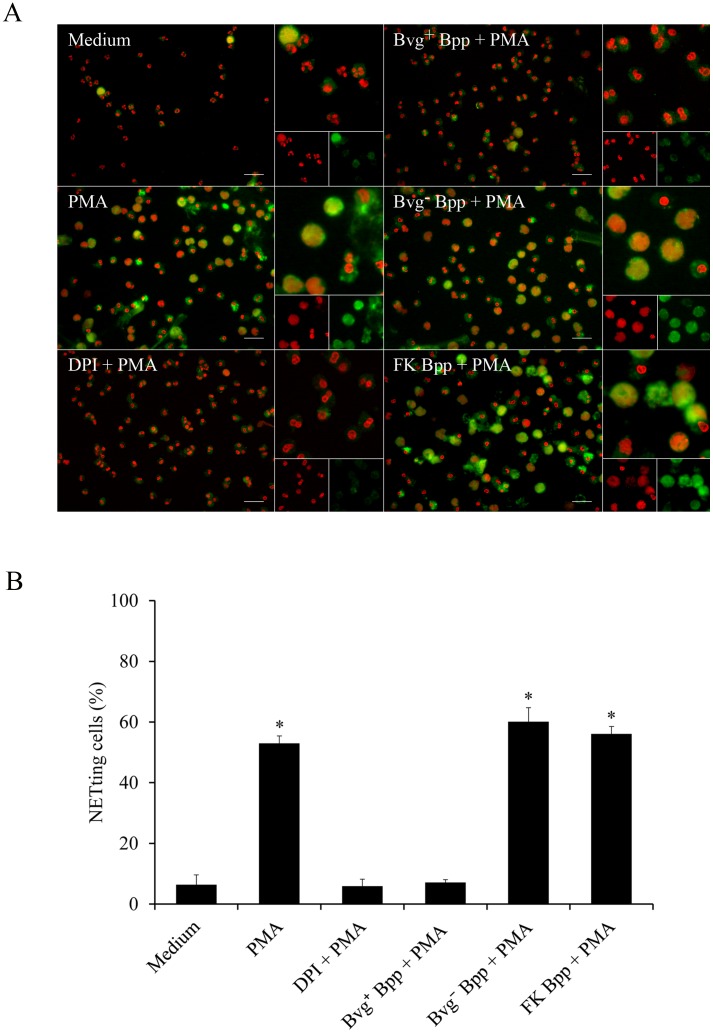
Fig 3. B. parapertussis precludes NETs release in resting neutrophils.
Neutrophils were infected with non-opsonized B. parapertussis (Bpp) (MOI: 10, 50 or 100) or P. aeruginosa (Pa) (MOI: 10) for 4 h at 37°C. Samples were fixed and permeabilized prior to labeling the NE in green and neutrophil DNA with a red fluorescent dye. Neutrophils incubated with PMA or medium alone were used as a positive and negative control, respectively. Samples were analyzed by fluorescence microscopy and the number of NETting cells was determined by the ImageJ softaware. At least 500 cells were counted per sample. (A) Representative microscopy images of neutrophils 4 h post infection are shown. Scale bar: 20 μm. (B) The bars represent the percentage of neutrophils that underwent NETosis. The data represent the mean ± SD of three experiments with neutrophils from different donors. ND: non-determinable (NETotic cells are no longer observed; only big clusters of NETs are seen). The percentage of NETting cells in PMA treated neutrophils was significantly different from the percentage of NETting cells observed in the other samples (*P <0.05).
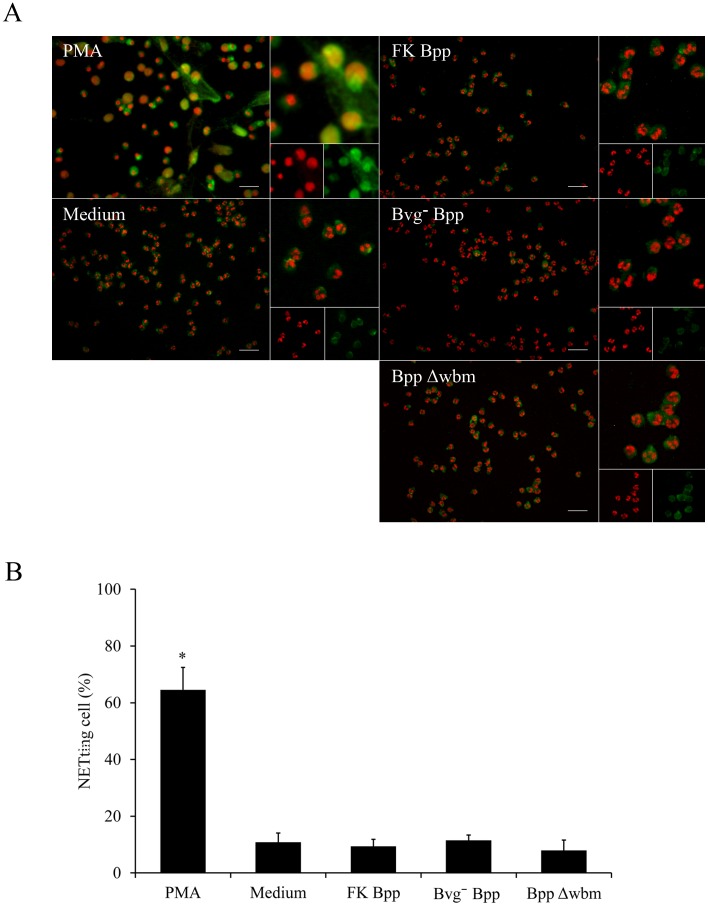
To evaluate whether the inhibitory effect of B. parapertussis on NETs formation was dependent on any of the Bvg-regulated virulence factors or bacterial viability, neutrophils were incubated with avirulent B. parapertussis or inactivated bacteria, respectively. As can been seen in Fig 4 (S4 Data), neither avirulent nor inactivated B. parapertussis induced NETosis. These results show that the lack of induction of NETs release in resting neutrophils by B. parapertussis is not dependent on a Bvg-regulated virulence factor or bacterial viability.
Fig 4. Preclusion of NETs release by B. parapertussis does not depend on Bvg-regulated factors, bacterial viability, or O antigen expression.
Neutrophils were incubated with formalin inactivated B. parapertussis (FK Bpp), avirulent B. parapertussis (Bvg- Bpp), or B. parapertussis Δwbm (BppΔwbm) (MOI: 100) for 4 h at 37°C. Neutrophils incubated with either medium alone or PMA were used as negative and positive controls, respectively. After incubation, samples were fixed and permeabilized prior to labeling the NE in green and neutrophil DNA with a red fluorescent dye. Samples were analyzed by fluorescence microscopy and the number of NETting cells was determined by the ImageJ softaware. At least 500 cells were counted per sample. (A) Representative microscopy images of each condition are shown. Scale bar: 20 μm. (B) The bars represent the percentage of neutrophils that underwent NETosis. The data represent the mean ± SD of three experiments with neutrophils from different donors. The percentage of NETting cells in PMA treated samples was significantly different from the percentage of NETting cells observed in the other samples (*P <0.05).
Since the O antigen has been found responsible for preclusion of neutrophil activation during the interaction with B. parapertussis [7], we investigated the relationship between the lack of NETs induction in resting neutrophils infected with B. parapertussis and the expression of the O antigen. As can been seen in Fig 4, neutrophils that were infected with an O antigen deficient mutant strain did not induce NETs formation, indicating that the O antigen is not involved in the preclusion of NET release.
The absence of NETosis upon neutrophil infection with the wild type B. parapertussis is in agreement with the lack of oxidative burst response observed in previous studies [7] and confirmed here for the whole period of time of the experiment (Fig 5). Since incubation of neutrophils with PMA is known to induce NETs formation in a ROS-dependent way [26], PMA-treated neutrophils were used as positive control. The ROS production was evaluated during 4 h after infection. Fig 5 (S5 Data) shows that PMA treatment induced an effective ROS production in neutrophils while B. parapertussis or medium alone did not. The O antigen defective mutant of B. parapertussis, however, induced a low but significant level of oxidative burst response (Fig 5, S5 Data) but did not promote NETs release. These results might indicate that ROS induction or, at least, this level of ROS, is not sufficient to induce NETosis.
Fig 5. B. parapertussis does not induce ROS in resting neutrophils.
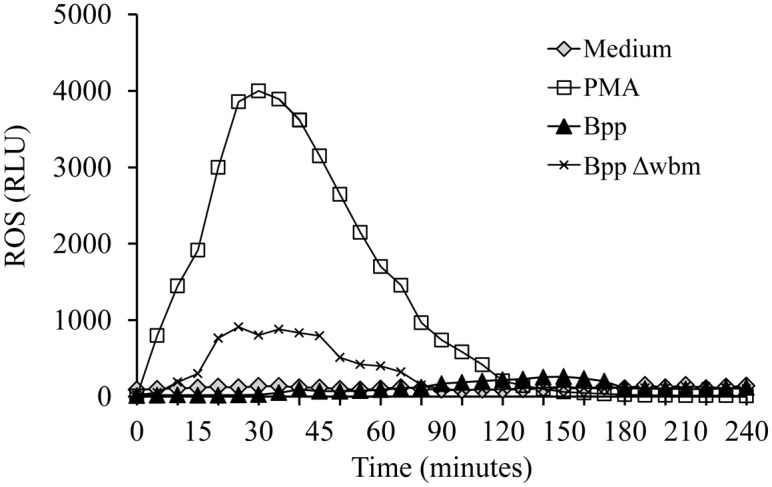
B. parapertussis (Bpp) or B. parapertussis Δwbm (BppΔwbm) were incubated with neutrophils (MOI: 100) and luminol at 37°C. Neutrophils incubated with medium alone and luminol served as a negative control. Neutrophils incubated with PMA were used as positive controls. Chemiluminescence response was measured every 5 min. The data are representative of three independent experiments. RLU, relative light units.
B. parapertussis inhibits NETs release in PMA stimulated neutrophils
NETosis is induced in vivo by a wide range of stimuli that includes different cytokines, bacterial components or a combination of these and other signals [21]. To examine whether B. parapertussis is able to actively inhibit NETs formation neutrophils infected with B. parapertussis (MOI: 10) for 30 min were treated with PMA and 4 h later NETs formation was evaluated by fluorescence microscopy. Neutrophils treated with or without DPI for 30 min before PMA treatment were used as a control of PMA-induced NETs inhibition and positive control, respectively. Fig 6 (S6 Data) shows that B. parapertussis efficiently inhibits PMA-induced NETs. In order to determine whether this inhibition depends on bacterial virulence factors or bacterial viability we investigated the effect of avirulent B. parapertussis or inactivated bacteria on PMA induction of NETs. As can be seen in Fig 6 (S6 Data), PMA treatment induced NETosis in neutrophils previously incubated with either inactivated or avirulent B. parapertussis, suggesting that the active form of a Bvg-regulated factor is involved in the observed inhibition. To further elucidate whether the inhibitory factor was released into the surrounding medium, a CM was prepared by incubating virulent bacteria (Bvg+ CM) or avirulent bacteria (Bvg¯ CM) in the medium for 2 h. Each CM was then filtered to remove the bacteria and further used to treat the neutrophils 30 min before PMA addition. Fig 7 shows that treatment of neutrophils with Bvg+ CM prior to PMA stimulation inhibited PMA induction of NETs. On the other hand, incubation of neutrophils with the Bvg¯ CM did not inhibit PMA-induced NETs release (Fig 7, S7 Data). Finally, both Bvg+ CM and Bvg¯ CM were treated with proteinase K prior to incubation with neutrophils and further treated with PMA. Proteinase K treatment abolished the capacity of the Bvg+ CM to inhibit NETs formation in response to PMA, but had no effect on Bvgˉ CM (Fig 7, S7 Data), indicating that the factor blocking NET formation is a protein sensitive to proteinase K.
Fig 6. B. parapertussis inhibits PMA-induced NETs through a Bvg-regulated factor.
Neutrophils were incubated with virulent B. parapertussis (Bvg+ Bpp), formalin inactivated B. parapertussis (FK Bpp), avirulent B. parapertussis (Bvg- Bpp) (MOI 10), or DPI for 30 min at 37°C prior to PMA treatment. Neutrophils incubated with medium alone were used as negative control. Neutrophils incubated with PMA alone were used as positive control. Neutrophils treated with DPI 30 min before PMA treatment were used as control of PMA-induced NETs inhibition. Four hours post infection samples were fixed and permeabilized prior to labeling the NE in green and neutrophil DNA in red. Samples were analyzed by fluorescence microscopy and the number of NETting cells was determined by the ImageJ software. At least 500 cells were counted per sample. (A) Representative microscopy images of each condition are shown. Scale bar: 20 μm. (B) The bars represent the percentage of neutrophils that underwent NETosis. The data represent the mean ± SD of three experiments with neutrophils from different donors. * indicates a P value <0.05 for comparison to results for neutrophils incubated with medium alone.
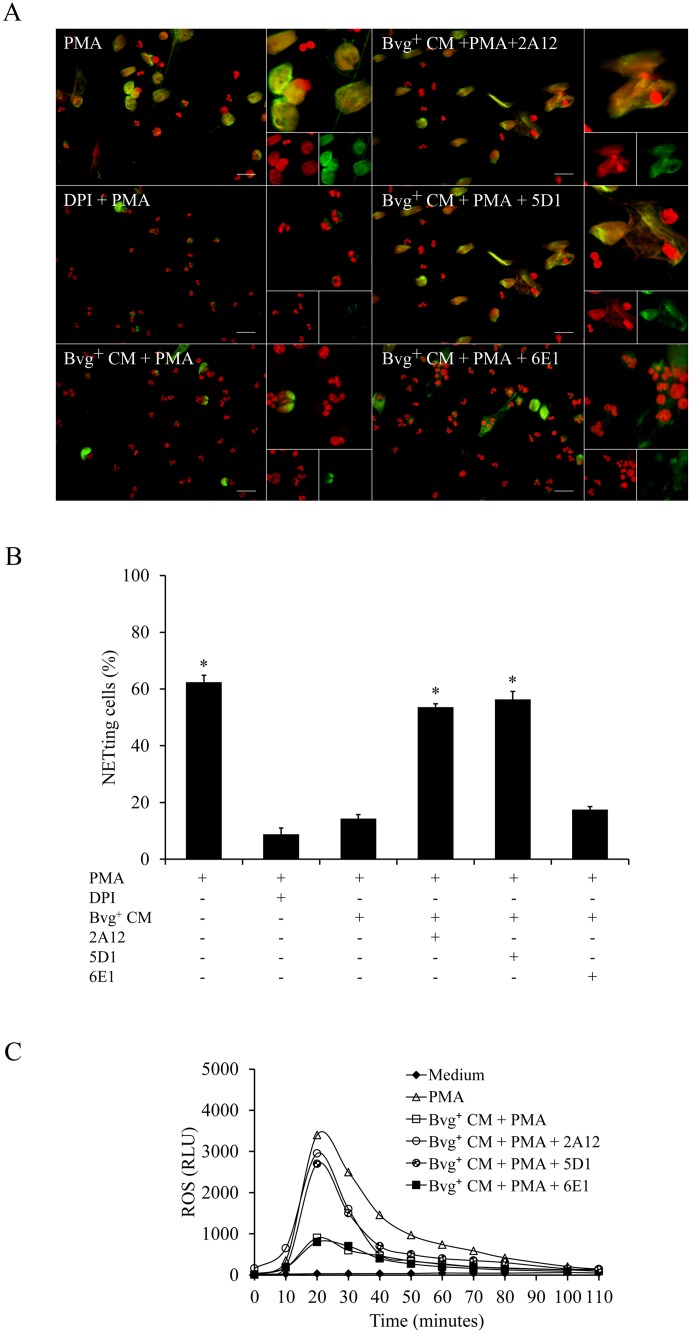
Fig 7. A B. parapertussis secreted Bvg-regulated virulence factor is involved in PMA-induced NETs inhibition.
CM was prepared by incubating 2x108 CFU/ml of virulent B. parapertussis (Bvg+ CM) or avirulent B. parapertussis (Bvg- CM) in medium for 2 h. In some samples CM was treated with proteinase K (PK) (100 μg/mL) for 60 min at 37°C prior use. Neutrophils were incubated with each CM 30 min before treatment with PMA. Neutrophils incubated with PMA alone were used as positive control. Four hours after PMA treatment samples were fixed and permeabilized prior to labeling the NE in green and neutrophil DNA in red. Samples were analyzed by fluorescence microscopy and the number of NETting cells was determined by the ImageJ softaware. At least 500 cells were counted per sample. (A) Representative microscopy images of each condition are shown. Scale bar: 20 μm. (B) The bars represent the percentage of neutrophils that underwent NETosis. The data represent the mean ± SD of three experiments with neutrophils from different donors. The percentage of NETting cells in neutrophils incubated with Bvg+ CM prior to PMA treatment was significantly different from the percentage of NETting cells observed in the other samples (*P <0.05).
Having in mind that PMA induces NOX/ROS-dependent NETs formation [26], we next evaluated whether Bvg+ CM inhibits ROS production in PMA treated neutrophils. To this end, neutrophils were treated with either Bvg+ CM or Bvg- CM for 30 min prior to PMA addition. Generation of ROS was measured for at least 2 h post treatment. Neutrophils stimulated with PMA were used as a positive control for ROS production. Neutrophils incubated with the NOX inhibitor DPI prior to PMA addition were used as a control for inhibition of PMA-induced ROS. The results presented in Fig 8 (S8 Data) showed that Bvg+ CM inhibited PMA induced ROS in neutrophil, whereas the Bvg- CM did not. Again, proteinase K treatment of Bvg+ CM abrogated the inhibition of PMA-induced ROS. Altogether these results suggest that Bvg-regulated proteins released into the extracellular medium prevent PMA induction of NETs release by NOX/ROS inhibition.
Fig 8. A Bvg-regulated virulence factor secreted into extracellular medium is involved in the preclusion of ROS generation in PMA treated neutrophils.
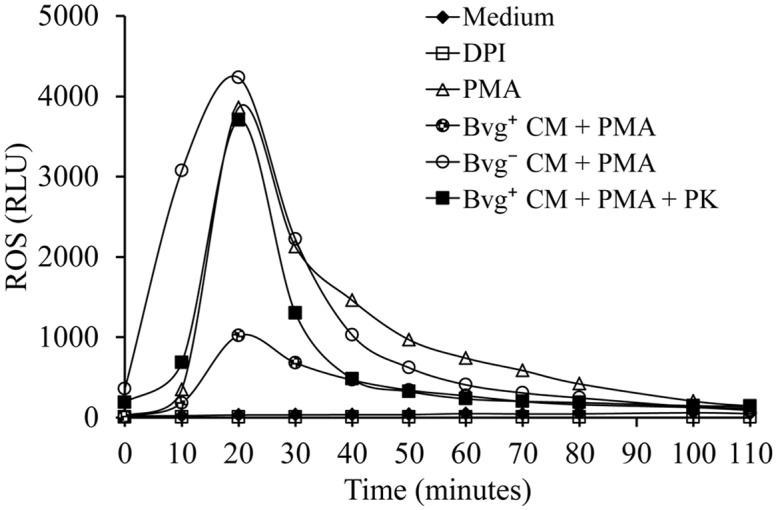
CM prepared by incubating 2x108 CFU/ml of virulent B. parapertussis (Bvg+ CM) or avirulent B. parapertussis (Bvg- CM) in medium for 2 h were used to incubate neutrophils 30 min before treatment with PMA. In some cases the CM was treated with proteinase K (PK) (100 μg/mL) for 60 min at 37°C, and further used to incubate neutrophils 30 min before treatment with PMA. Neutrophils incubated with medium alone and luminol served as a negative control. Neutrophils treated with DPI 30 min before PMA treatment were used as a control of PMA-induced NETs inhibition (DPI). Neutrophils incubated with PMA alone were used as positive control. Chemiluminescence responses were measured every 10 min in the presence of luminol. Data are representative of three independent experiments. RLU, relative light units.
B. parapertussis CyaA inhibits ROS-dependent NETs generation
Previous studies have shown that B. pertussis CyaA inhibits PMA-generated NETs by ROS inhibition [45]. B. parapertussis also expresses CyaA in a Bvg-regulated manner. This protein toxin is released into the extracellular medium and could be responsible for the observed inhibition of PMA stimulated NETs by Bvg+ CM. To investigate the role of CyaA, the Bvg+ CM was incubated with a monoclonal antibody against CyaA (2A12), which partially blocks both the adenylate cyclase and hemolytic activities of this toxin. Neutrophils were incubated with this 2A12-treated Bvg+ CM 30 min before PMA treatment and NETs formation was determined by fluorescence microscopy as described above. As can be seen in Fig 9A and 9B, the 2A12 antibody abrogated the inhibitory effect of the Bvg+ CM on PMA-induced NETs. To evaluate which of the toxin activities is responsible for this inhibition, Bvg+ CM was incubated with either a monoclonal antibody against adenylate cyclase activity (5D1), which prevents translocation of the enzyme catalytic domain into the cell without affecting the binding of the toxin to cells, or with a monoclonal antibody against the hemolytic activity of this toxin (6E1), which binds to the hemolytic domain of the enzyme without impairing intracellular toxin-induced cAMP accumulation. Fig 9A and 9B (S9 Data) shows that 2A12 and 5D1 antibody abolished PMA-induced NETs inhibitory activity of Bvg+ CM. On the other hand, treatment with 6E1 had no effect on the inhibitory activity of Bvg+ CM. These results indicate that the inhibition of PMA-induced NETs by B. parapertussis depends on adenylate cyclase activity of CyaA. In agreement with these results, incubation with 2A12 or 5D1 suppressed the inhibitory activity of Bvg+ CM on the PMA-induced ROS (Fig 9C, S10 Data), while incubation with 6E1 had no effect. These results demonstrate that CyaA, through its adenylate cyclase activity, prevents PMA induction of NETs release through NOX/ROS inhibition.
Fig 9. B. parapertussis adenylate cyclase inhibits NETs and ROS generation in PMA treated neutrophils.
CM prepared by incubating 2x108 CFU/ml of virulent B. parapertussis (Bvg+ CM) in medium for 2 h was used to incubate neutrophils 30 min before treatment with PMA. In some cases CM was treated with antibodies against both activities of adenylate cyclase toxin (2A12), or antibodies against either adenylate cyclase (5D1) or the haemolytic (6E1) activity. Four hours post incubation samples were fixed and permeabilized prior to labeling the NE in green and neutrophil DNA in red. Neutrophils incubated with DPI 30 min before PMA treatment were used as a control of inhibition of PMA-induced NETs (DPI). Neutrophils incubated with PMA alone were used as a positive control. The percentage NETing cell was determined using the ImageJ softaware. (A) Representative microscopy images of each condition are shown. Scale bar: 20 μm. (B) The bars represent the percentage of neutrophils that underwent NETosis. The data represent the mean ± SD of three experiments with neutrophils from different donors. * indicates a P value <0.05 for comparison to results for control of PMA-induced NETs inhibition (DPI). (C) Chemiluminescence responses of neutrophils incubated with 2A12, 5D1, or 6E1-treated Bvg+ CM prior to the incubation with PMA. Neutrophils incubated with medium alone and luminol served as a negative control. Neutrophils incubated with PMA alone were used as positive control. Chemiluminescence was measured every 10 min during the whole time of the experiment. Data are representative of three independent experiments. RLU, relative light units.
NETs trap and kill B. parapertussis
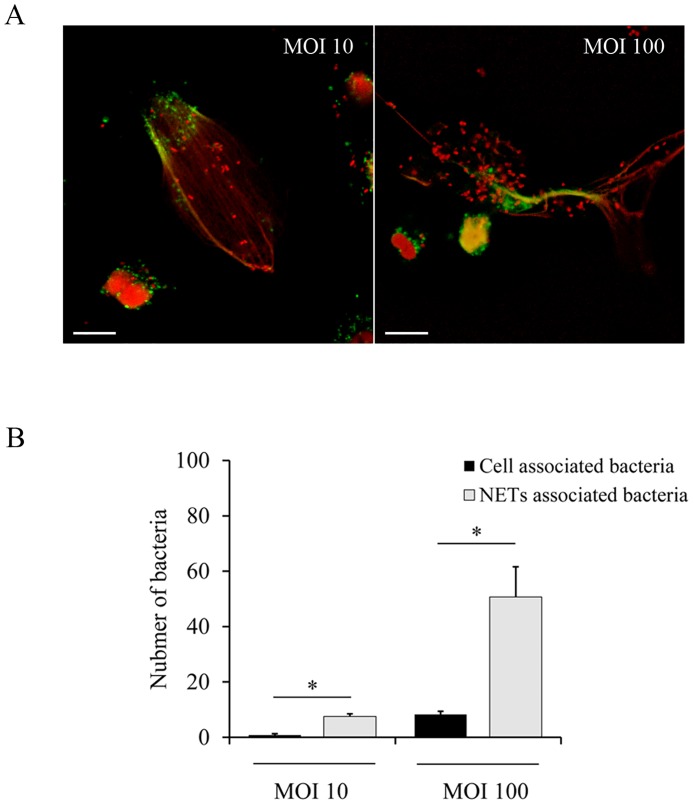
Although B. parapertussis per se does not seem to induce NETs, NETs can be induced by many different stimuli in vivo at the site of infection [21]. We investigated whether these extracellular structures can trap and kill B. parapertussis. To this end, NETs were induced by PMA (100 nM) treatment before adding B. parapertussis. The capacity of NETs to immobilize B. parapertussis was examined by confocal microscopy. As can been seen in Fig 10 (S11 Data), NET structures stained for DNA (red) and NE (green) are surrounding B. parapertussis bacteria (red; stained for DNA), indicating that NETs can trap B. parapertussis. Fig 10 shows the number of bacteria attached to NETs as compared with the number of bacteria taken up by non-NETting neutrophils at two different multiplicities of infection, 10 and 100. In both cases NETs captured more than 80% of the total number of bacteria (90.07% ± 11.37 and 85.96% ± 18.46, respectively).
Fig 10. NETs can trap and immobilize B. parapertussis.
Neutrophils were incubated with PMA (100 nM) for 1 h prior to infection with B. parapertussis (MOI 10 or 100) for 4 h at 37°C. Next, neutrophils were fixed and permeabilized prior to labeling the NE in green and both the neutrophil and bacterial DNA in red. (A) Representative confocal microscopy images of three independent experiments are shown. Scale bar: 10 μm. (B) The number of associated bacteria to both NETs- and non-NETting neutrophils was determined by analyzing at least 20 fields per sample. The bars represent the number of associated bacteria to either NETs- or non-NETting neutrophils. The data represent the mean ± SD of at least three experiments with neutrophils from different donors. The number of associated bacteria to NETs was significantly different to the number of associated bacteria to non-NETting cells at both MOI assayed (*P <0.05).
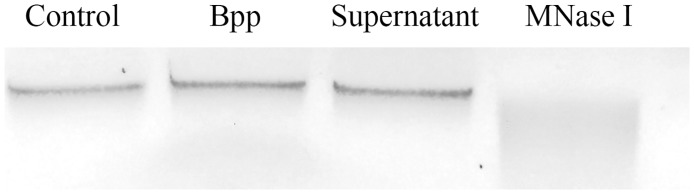
Several pathogens may escape from NETs by breaking these structures by a self-DNase activity [46–48]. We investigated whether B. parapertussis can breakdown extracellular DNA by determining bacterial DNase activity both in the whole cell bacteria and in bacterial-free culture supernatant by incubation with purified neutrophil DNA for 60 min. DNA integrity was evaluated by agarose gel electrophoresis. As can be seen in the Fig 11 we found neither B. parapertussis cell-associated nor secreted DNAase activity, suggesting that this bacterium does not have this mechanism for NETs evasion.
Fig 11. B. parapertussis does not have DNAse activity.
Gel electrophoresis analysis of DNA (300 ng) incubated 1 h a 37°C with PBS (PBS), B. parapertussis (Bpp), culture supernatant of B. parapertussis (supernatant), and MNase I (500 mU/ml). Representative images are shown.
Since some microorganisms can be trapped but not killed by NETs [20], we investigated B. parapertussis survival after NETs encounter. Again, NETs were induced by PMA treatment before infection with B. parapertussis. Bacterial survival was evaluated 3 h after infection. CFU counts of bacterial inoculum was determined and set as 100%. As can be seen in Fig 12 (S12 Data), PMA-induced NETs significantly reduced the number of viable bacteria. In order to discriminate between bacteria killed by NETs or phagocytosis, neutrophils were either incubated with CytD or MNase I, prior to incubation with bacteria. CytD treatment inhibits phagocytosis, while MNase I treatment breaks down NETs structure. A combination of MNase I and CytD was also used to exclude both killing effects. Fig 12 show that the reduction in the number of viable B. parapertussis in PMA treated neutrophils was due to the bactericidal activity of NETs.
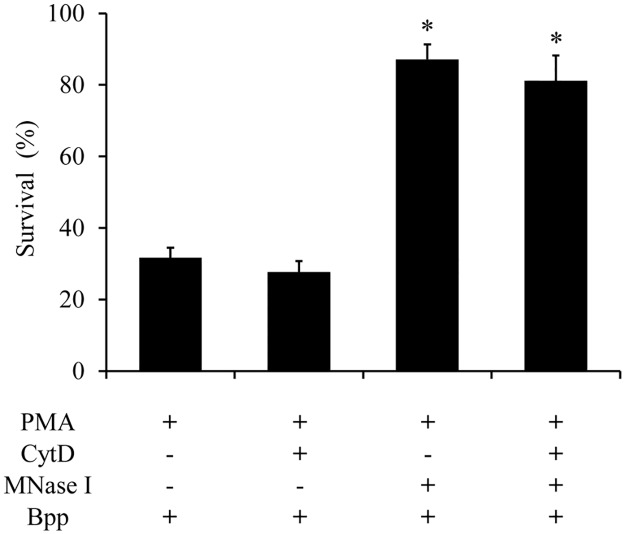
Fig 12. B. parapertussis is killed by NETs.
Neutrophils were incubated with PMA (100 nM) for 1 h at 37°C to induce NETs release. Next, neutrophils were treated with cytochalasinD (100 μg/ml; CytD), MNase I (500 mU/ml), or a combination of both for 30 min at 37°C. Neutrophils were then incubated with B. parapertussis (Bpp) (MOI 100) for 3 h at 37°C. Next, all samples were treated with MNase for 20 min to release entrapped bacteria from NETs and serial dilutions of cell lysates were rapidly plated onto bBGA to enumerate CFU. The percentage of viable bacteria was referred to bacterial inoculums. The number of viable B. parapertussis in samples treated with MNase I prior bacterial infection was significantly different from that found in samples that were not treated with MNase I. * indicates a P value <0.05 for comparison to results for PMA-treated neutrophils infected with Bpp.
Discussion
Neutrophils are rapidly recruited by microbial infection, and have a variety of anti-bacterial mechanisms. To survive this onslaught, many microbial pathogens have evolved strategies to evade or counteract these mechanisms to successfully infect the host. We have previously found that in the absence of opsonic antibodies B. parapertussis avoids neutrophil intracellular bactericidal mechanism by multiple mechanisms [7]. According to those results, the great majority of bacteria remain extracellularly, where they might be subject to neutrophil degranulation and NETs release. In this study we investigated the relevance of these neutrophil extracellular bactericidal mechanisms in the control of B. parapertussis.
Degranulation usually takes place once phagocytosis is initiated and neutrophil cytoplasmic granules are fused with nascent unclosed phagosomes leading to phagolysosome formation [11, 12]. This process eventually leads to the release of bactericidal granular components into the surrounding medium ultimately killing extracellular bacteria. The results of this study showed that at a multiplicity of infection as high as 300 bacteria per neutrophil there was not significant degranulation, as determined by the lack of β-glucuronidase activity in the cell-free supernatants of infected cells. A mutant lacking O antigen stimulated degranulation, indicating both that B. parapertussis can stimulate this neutrophil response and that the O antigen molecule blocks it. We had previously shown that the O antigen targets the bacteria to the lipid raft domains on the host cell membrane [7]. Bacterial entry through lipid raft platforms is involved in the evasion of intracellular signaling pathways linked to the activation of neutrophil bactericidal mechanisms [49, 50]. Soon after bacteria-neutrophil contact confocal studies showed the wild type strain of B. parapertussis in close contact with lipid rafts at the plasma membrane of neutrophils, and the absence of specific and azurophilic granules in the bacterial surroundings at the site of entry during phagocytosis. Conversely, the B. parapertussis O antigen-deficient mutant that did not colocalize with lipid rafts domains was found surrounded by a great number of specific and azurophilic granules. The fusion of these cytoplasmic granules with unclosed phagosomes containing B. parapertussis O antigen mutant probably contributed to the observed robust neutrophil degranulation. This key role of the O antigen in the inhibition of neutrophils degranulation adds to the already long list of the bacterial protective characteristics of this molecule.
IgG opsonization of B. parapertussis overcomes O antigen-mediated inhibition of degranulation, as determined by the high release of β-glucuronidase in the cell supernatant of neutrophils infected with IgG opsonized B. parapertussis. This result is in agreement with the finding that IgG-opsonized bacteria do not enter through lipid rafts domains, are trafficked to lysosomes, and strongly activate neutrophils [7]. These results stress the need for opsonic antibodies at the site of infection not only to promote efficient phagocytosis and intracellular bacterial inactivation [7] but also to induce extracellular bacterial killing through neutrophil degranulation.
NETs represent a complementary approach to contain and kill extracellular bacteria. Several stimuli can activate specific signaling pathways leading to NETotic cell death and the release of a mixture of granular content associated with DNA fibers in the extracellular medium [9]. This is a potent antimicrobial mechanism since the NETs immobilize and eventually kill microbes that are in the surrounding of the neutrophil. Bacterial components are strong inductors of NETs [51]. However, we observed that B. parapertussis interactions with resting neutrophils do not induce NETs production. According to our results B. parapertussis does not induce NETosis even at high multiplicities of infections at which fungi, virus, protozoa, and several bacterial pathogens induce a strong NETosis [21]. Some pathogens use active mechanisms to circumvent this neutrophil extracellular killing mechanism, like the secretion of nucleases that break down NETs [46–48]. But B. parapertussis did not express detectable cell-associated nor secreted DNase activity. It does expresses a potent set of Bvg-regulated virulence factors that mediates several immune evasion mechanisms, but none of these virulence factors were found implicated in the lack of NETs induction in resting neutrophils. The lack of induction of NETs in resting neutrophils by B. parapertussis was not even dependent on the viability of the bacteria as was proved by infection with inactivated B. parapertussis.
Bacterial LPS is a strong NETs inductor [28, 52]. However, bacterial pathogens have coevolved with their host to hide themselves from recognition by the immune system. In some pathogens adaptation to humans has provided selective pressure to modify surface structures like LPS. Previous studies have shown that B. parapertussis LPS has specific modifications that determine its low stimulatory activity of the immune system [7, 53, 54]. Particularly, the LPS O antigen decoration has been found responsible for the prevention of neutrophil oxidative burst by B. parapertussis [7]. Since ROS production is required by almost all stimuli that lead to NETs release [25, 26], we hypothesized that the O antigen was involved in the lack of NETs induction. However, the O antigen deficient B. parapertussis, although able to induce a small but significant oxidative burst, did not promote NETs release. These results seem to indicate that either ROS stimulation induced by the O antigen mutant strain is not strong enough to promote NETs release (like observed for B. pertussis [45]) or other required mechanisms are not triggered during this bacterial-cell interaction, i.e. autophagy activity [26]. In summary, the lack of NETs induction by B. parapertussis seems associated to the low stimulatory potential of its LPS as previously found for other pathogens like Brucella abortus [55].
Neutrophil activation and NETs release can be initiated by several stimuli such as a combination of cytokines, or bacterial components (LPS) plus proinflamatory cytokines [21], among others. In this study, we challenged the ability of B. parapertussis to inhibit NETs release in the presence of the most potent NETs inducer, PMA [26]. We observed that B. parapertussis is able to inhibit PMA-induced NETosis by preventing PMA-induced oxidative burst. This inhibition proved dependent on the CyaA released into the extracellular medium indicating that NETs inhibition does not require the close proximity of bacteria with neutrophils. CyaA has two different biological activities, a hemolysin activity implicated in cell lysis, and the adenylate cyclase activity that intoxicates target cell by increasing the intracellular level of cAMP. By using specific monoclonal antibodies against the different CyaA activities, we found that the adenylate cyclase activity is responsible for both ROS and NETosis inhibition in PMA-stimulated neutrophils. Our findings are in agreements with those of Eby, et al. who demonstrated that CyaA inhibits PMA-induced NOX activity [45]. Inhibition of NOX prevents ROS generation and chromatin decondensation which is essential for NET formation [26]. In this previous work, Eby, et al. [45] suggested that CyaA inhibits NOX activity through the inhibition of the Raf-MEK-ERK signaling pathway, which is involved in the assembly and activation of the NOX. Accordingly, we observed that CyaA arrests PMA treated neutrophils in a delobulated nuclei stage (Fig 6), which is typical in the inhibition of Raf-MEK-ERK signaling pathway [56]. Therefore, like B. pertussis, B. parapertussis inhibition of PMA-induced NETs probably depends on an active inhibition of Raf-MEK-ERK signaling pathway mediated by CyaA.
While many human pathogens have been shown to induce NETs formation, and some have mechanisms to escape from NETs, B. parapertussis is the first reported human pathogen that does not induce NETs and had the ability to inhibit NET formation. Through two different mechanisms, one related to the lack of proper NET-inducer stimuli and the other related to a virulence factor-mediated inhibition, B. parapertussis control an essential neutrophil mechanism against microbial infections. This dual strategy to avoid NETs induction has only been described in the Gram-positive bacterium Lactobacillus rhamnosus [57], but to our knowledge there is no precedent in Gram-negative bacterium. Interestingly, unlike other pathogens that avoid neutrophil extracellular killing by escaping from NETs, the capacity of B. parapertussis to block NETs induction can prevent an exacerbated inflammatory response originated by the release of tissue-damaging molecules after neutrophil lysis during NETs release. Furthermore, NETs inhibition mediated by an excreted virulence factor might be useful for B. parapertussis at the site of infection where several potential soluble NETs inducer stimulus may be present. By being able to control NETs release without intimate contact with the cell B. parapertussis generates a relatively large environment free of NETs. This NETs release control might be quite relevant for B. parapertussis survival since NETs can efficiently trap and kill this bacterium. On the other hand, the inhibition of neutrophil death mechanisms like NETosis, might be advantageous for a bacterium like B. parapertussis that is able to remain alive inside the cell as a transient intracellular stage.
We have previously shown that opsonic antibodies are required for neutrophils and macrophages to efficiently phagocytose and kill B. parapertussis [6, 7]. The results obtained in this study demonstrate that without opsonizing antibodies this pathogen might also avoid extracellular bactericidal mechanisms of neutrophils. These results are consistent with previous studies performed in a murine model of infection that demonstrated that B. parapertussis clearance requires both the presence of neutrophils and specific antibodies against this bacterium [58]. The lack of either precludes bacterial clearance.
Adenylate cyclase has been proposed as a pertussis vaccine candidate. In this work, we showed that antibodies against B. pertussis adenylate cylcase toxin neutralize B. parapertussis CyaA released into the extracellular medium allowing NETs induction by stimulated neutrophils. Therefore, in the absence of proper opsonic antibodies to induce a strong neutrophil bactericidal activity, antibodies against this toxin might be valuable for the control of B. parapertussis.
Supporting Information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability
All relevant data are included in the manuscript and its Supporting Information files.
Funding Statement
This work was partially supported by the National Agency for Scientific and Technological Promotion (ANPCyTMINCyT) PICT 2013 1937 (to MER) [http://www.agencia.mincyt.gob.ar/]. MER and RMG are members of the Scientific Career of CONICET. JG and ES are postdoctoral fellows of CONICET. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mastrantonio P, Giuliano M, Stefanelli P, Sofia T, De Marzi L, Tarabini G, et al. Bordetella parapertussis infections. Dev Biol Stand. 1997;89:255–9. [PubMed] [Google Scholar]
- 2.Liese JG, Renner C, Stojanov S, Belohradsky BH. Clinical and epidemiological picture of B pertussis and B parapertussis infections after introduction of acellular pertussis vaccines. Arch Dis Child. 2003;88(8):684–7. 10.1136/adc.88.8.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David S, van Furth R, Mooi FR. Efficacies of whole cell and acellular pertussis vaccines against Bordetella parapertussis in a mouse model. Vaccine. 2004;22(15–16):1892–8. 10.1016/j.vaccine.2003.11.005 [DOI] [PubMed] [Google Scholar]
- 4.Heininger U, Stehr K, Christenson P, Cherry JD. Evidence of efficacy of the Lederle/Takeda acellular pertussis component diphtheria and tetanus toxoids and pertussis vaccine but not the Lederle whole-cell component diphtheria and tetanus toxoids and pertussis vaccine against Bordetella parapertussis infection. Clin Infect Dis. 1999;28(3):602–4. 10.1086/515154 [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Rodriguez ME, Harvill ET. O antigen allows B. parapertussis to evade B. pertussis vaccine-induced immunity by blocking binding and functions of cross-reactive antibodies. PLoS One. 2009;4(9):e6989 10.1371/journal.pone.0006989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorgojo J, Harvill ET, Rodriguez ME. Bordetella parapertussis Survives inside Human Macrophages in Lipid Raft-Enriched Phagosomes. Infect Immun. 2014;82(12):5175–84. 10.1128/IAI.02553-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorgojo J, Lamberti Y, Valdez H, Harvill ET, Rodriguez ME. Bordetella parapertussis survives the innate interaction with human neutrophils by impairing bactericidal trafficking inside the cell through a lipid raft-dependent mechanism mediated by the lipopolysaccharide O antigen. Infect Immun. 2012;80(12):4309–16. 10.1128/IAI.00662-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. 10.1111/j.1600-065X.2007.00550.x [DOI] [PubMed] [Google Scholar]
- 9.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 10.Lacy P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol. 2006;2(3):98–108. 10.1186/1710-1492-2-3-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzaki E, Kobayashi H, Kodama Y, Masujima T, Terakawa S. Video-rate dynamics of exocytotic events associated with phagocytosis in neutrophils. Cell Motil Cytoskeleton. 1997;38(3):215–28. [DOI] [PubMed] [Google Scholar]
- 12.Tapper H, Grinstein S. Fc receptor-triggered insertion of secretory granules into the plasma membrane of human neutrophils: selective retrieval during phagocytosis. J Immunol. 1997;159(1):409–18. [PubMed] [Google Scholar]
- 13.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18(9):1386–93. 10.1038/nm.2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12(3):324–33. 10.1016/j.chom.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 15.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5(10):e1000639 10.1371/journal.ppat.1000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, Thywissen A, et al. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 2010;6(4):e1000873 10.1371/journal.ppat.1000873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abi Abdallah DS, Denkers EY. Neutrophils cast extracellular traps in response to protozoan parasites. Front Immunol. 2012;3:382 10.3389/fimmu.2012.00382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guimaraes-Costa AB, Nascimento MT, Froment GS, Soares RP, Morgado FN, Conceicao-Silva F, et al. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc Natl Acad Sci U S A. 2009;106(16):6748–53. 10.1073/pnas.0900226106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179(1):199–210. 10.1016/j.ajpath.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu T, Kobayashi SD, Quinn MT, Deleo FR. A NET Outcome. Front Immunol. 2012;3:365 10.3389/fimmu.2012.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng OZ, Palaniyar N. NET balancing: a problem in inflammatory lung diseases. Front Immunol. 2013;4:1 10.3389/fimmu.2013.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30(11):513–21. 10.1016/j.it.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 23.Guimaraes-Costa AB, Nascimento MT, Wardini AB, Pinto-da-Silva LH, Saraiva EM. ETosis: A Microbicidal Mechanism beyond Cell Death. J Parasitol Res. 2012;2012:929743 10.1155/2012/929743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5(8):577–82. 10.1038/nrmicro1710 [DOI] [PubMed] [Google Scholar]
- 25.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677–91. 10.1083/jcb.201006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E, De Rycke R, et al. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011;21(2):290–304. 10.1038/cr.2010.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirose T, Hamaguchi S, Matsumoto N, Irisawa T, Seki M, Tasaki O, et al. Dynamic changes in the expression of neutrophil extracellular traps in acute respiratory infections. Am J Respir Crit Care Med. 2012;185(10):1130–1. 10.1164/ajrccm.185.10.1130 [DOI] [PubMed] [Google Scholar]
- 28.Douda DN, Jackson R, Grasemann H, Palaniyar N. Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. J Immunol. 2011;187(4):1856–65. 10.4049/jimmunol.1004201 [DOI] [PubMed] [Google Scholar]
- 29.Ng HH, Narasaraju T, Phoon MC, Sim MK, Seet JE, Chow VT. Doxycycline treatment attenuates acute lung injury in mice infected with virulent influenza H3N2 virus: involvement of matrix metalloproteinases. Exp Mol Pathol. 2012;92(3):287–95. 10.1016/j.yexmp.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 30.Allen A, Maskell D. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol Microbiol. 1996;19(1):37–52. [DOI] [PubMed] [Google Scholar]
- 31.Preston A, Allen AG, Cadisch J, Thomas R, Stevens K, Churcher CM, et al. Genetic basis for lipopolysaccharide O antigen biosynthesis in bordetellae. Infect Immun. 1999;67(8):3763–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez Vidakovics ML, Lamberti Y, van der Pol WL, Yantorno O, Rodriguez ME. Adenylate cyclase influences filamentous haemagglutinin-mediated attachment of Bordetella pertussis to epithelial alveolar cells. FEMS Immunol Med Microbiol. 2006;48(1):140–7. 10.1111/j.1574-695X.2006.00136.x [DOI] [PubMed] [Google Scholar]
- 33.von Kockritz-Blickwede M, Chow OA, Nizet V. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood. 2009;114(25):5245–6. 10.1182/blood-2009-08-240713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Repp R, Valerius T, Sendler A, Gramatzki M, Iro H, Kalden JR, et al. Neutrophils express the high affinity receptor for IgG (Fc gamma RI, CD64) after in vivo application of recombinant human granulocyte colony-stimulating factor. Blood. 1991;78(4):885–9. [PubMed] [Google Scholar]
- 35.Wolfe DN, Goebel EM, Bjornstad ON, Restif O, Harvill ET. The O antigen enables Bordetella parapertussis to avoid Bordetella pertussis-induced immunity. Infect Immun. 2007;75(10):4972–9. 10.1128/IAI.00763-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez ME, Hellwig SM, Hozbor DF, Leusen J, van der Pol WL, van de Winkel JG. Fc receptor-mediated immunity against Bordetella pertussis. J Immunol. 2001;167(11):6545–51. [DOI] [PubMed] [Google Scholar]
- 37.Hellwig SM, van Oirschot HF, Hazenbos WL, van Spriel AB, Mooi FR, van De Winkel JG. Targeting to Fcgamma receptors, but not CR3 (CD11b/CD18), increases clearance of Bordetella pertussis. J Infect Dis. 2001;183(6):871–9. 10.1086/319266 [DOI] [PubMed] [Google Scholar]
- 38.Lamberti Y, Alvarez Hayes J, Perez Vidakovics ML, Rodriguez ME. Cholesterol-dependent attachment of human respiratory cells by Bordetella pertussis. FEMS Immunol Med Microbiol. 2009;56(2):143–50. 10.1111/j.1574-695X.2009.00557.x [DOI] [PubMed] [Google Scholar]
- 39.Hosseinzadeh A, Messer PK, Urban CF. Stable Redox-Cycling Nitroxide Tempol Inhibits NET Formation. Front Immunol. 2012;3:391 10.3389/fimmu.2012.00391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SJ, Gray MC, Guo L, Sebo P, Hewlett EL. Epitope mapping of monoclonal antibodies against Bordetella pertussis adenylate cyclase toxin. Infect Immun. 1999;67(5):2090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16(4):401–7. 10.1016/j.cub.2006.01.056 [DOI] [PubMed] [Google Scholar]
- 42.Jayaprakash K, Demirel I, Khalaf H, Bengtsson T. The role of phagocytosis, oxidative burst and neutrophil extracellular traps in the interaction between neutrophils and the periodontal pathogen Porphyromonas gingivalis. Mol Oral Microbiol. 2015;30(5):361–75. 10.1111/omi.12099 [DOI] [PubMed] [Google Scholar]
- 43.Bassoe CF, Li N, Ragheb K, Lawler G, Sturgis J, Robinson JP. Investigations of phagosomes, mitochondria, and acidic granules in human neutrophils using fluorescent probes. Cytometry B Clin Cytom. 2003;51(1):21–9. 10.1002/cyto.b.10003 [DOI] [PubMed] [Google Scholar]
- 44.Dahlgren C, Carlsson SR, Karlsson A, Lundqvist H, Sjolin C. The lysosomal membrane glycoproteins Lamp-1 and Lamp-2 are present in mobilizable organelles, but are absent from the azurophil granules of human neutrophils. Biochem J. 1995;311 (Pt 2):667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eby JC, Gray MC, Hewlett EL. Cyclic AMP-mediated suppression of neutrophil extracellular trap formation and apoptosis by the Bordetella pertussis adenylate cyclase toxin. Infect Immun. 2014;82(12):5256–69. 10.1128/IAI.02487-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Buhr N, Neumann A, Jerjomiceva N, von Kockritz-Blickwede M, Baums CG. Streptococcus suis DNase SsnA contributes to degradation of neutrophil extracellular traps (NETs) and evasion of NET-mediated antimicrobial activity. Microbiology. 2014;160(Pt 2):385–95. 10.1099/mic.0.072199-0 [DOI] [PubMed] [Google Scholar]
- 47.Berends ET, Horswill AR, Haste NM, Monestier M, Nizet V, von Kockritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010;2(6):576–86. 10.1159/000319909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Kockritz-Blickwede M, Blodkamp S, Nizet V. Interaction of Bacterial Exotoxins with Neutrophil Extracellular Traps: Impact for the Infected Host. Front Microbiol. 2016;7:402 10.3389/fmicb.2016.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1(1):31–9. 10.1038/35036052 [DOI] [PubMed] [Google Scholar]
- 50.Fantini J, Garmy N, Mahfoud R, Yahi N. Lipid rafts: structure, function and role in HIV, Alzheimer's and prion diseases. Expert Rev Mol Med. 2002;4(27):1–22. 10.1017/S1462399402005392 [DOI] [PubMed] [Google Scholar]
- 51.White PC, Chicca IJ, Cooper PR, Milward MR, Chapple IL. Neutrophil Extracellular Traps in Periodontitis: A Web of Intrigue. J Dent Res. 2016;95(1):26–34. 10.1177/0022034515609097 [DOI] [PubMed] [Google Scholar]
- 52.Guimaraes-Costa AB, Nascimento MT, Wardini AB, Pinto-da-Silva LH, Saraiva EM. ETosis: A Microbicidal Mechanism beyond Cell Death. J Parasitol Res. 2012;2012:929743 10.1155/2012/929743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolfe DN, Buboltz AM, Harvill ET. Inefficient Toll-like receptor-4 stimulation enables Bordetella parapertussis to avoid host immunity. PLoS One. 2009;4(1):e4280 10.1371/journal.pone.0004280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann PB, Wolfe D, Latz E, Golenbock D, Preston A, Harvill ET. Comparative toll-like receptor 4-mediated innate host defense to Bordetella infection. Infect Immun. 2005;73(12):8144–52. 10.1128/IAI.73.12.8144-8152.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barquero-Calvo E, Mora-Cartin R, Arce-Gorvel V, de Diego JL, Chacon-Diaz C, Chaves-Olarte E, et al. Brucella abortus Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide. PLoS Pathog. 2015;11(5):e1004853 10.1371/journal.ppat.1004853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7(2):75–7. 10.1038/nchembio.496 [DOI] [PubMed] [Google Scholar]
- 57.Vong L, Lorentz RJ, Assa A, Glogauer M, Sherman PM. Probiotic Lactobacillus rhamnosus inhibits the formation of neutrophil extracellular traps. J Immunol. 2014;192(4):1870–7. 10.4049/jimmunol.1302286 [DOI] [PubMed] [Google Scholar]
- 58.Wolfe DN, Kirimanjeswara GS, Harvill ET. Clearance of Bordetella parapertussis from the lower respiratory tract requires humoral and cellular immunity. Infect Immun. 2005;73(10):6508–13. 10.1128/IAI.73.10.6508-6513.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are included in the manuscript and its Supporting Information files.