STRUCTURED ABSTRACT
PURPOSE
Although executive dysfunction and depression are common among ICU survivors, their relationship has not been evaluated in this population. We sought to determine 1) if executive dysfunction is independently associated with severity of depressive symptoms or worse mental health-related quality of life (HRQOL) in ICU survivors, and 2) if age modifies these associations.
METHODS
In a prospective cohort (n=136), we measured executive dysfunction by the Behavior Rating Inventory of Executive Function-Adult, depression by the Beck Depression Inventory-II, and mental HRQOL by the Short-Form 36 (SF-36). We used multiple linear regression models, adjusting for potential confounders. We included age as an interaction term to test for effect modification.
RESULTS
Executive dysfunction 3 months post-ICU was independently associated with more depressive symptoms and worse mental HRQOL 12 months post-ICU [25th vs 75th percentile of executive functioning scored 4.3 points worse on the depression scale (95% CI =1.3–7.4, p=0.015) and 5 points worse on the SF-36 (95% CI=1.7–8.3, p=0.006)]. Age did not modify these associations (depression p=0.12; mental HRQOL p=0.80).
CONCLUSION
Regardless of age, executive dysfunction was independently associated with subsequent worse severity of depressive symptoms and worse mental HRQOL. Executive dysfunction may have a key role in the development of depression.
Keywords: cognitive impairment, executive dysfunction, depression, quality of life, critical illness
1. INTRODUCTION
With improving mortality rates in critical care, over 1.4 million older adults survive critical illness in the U.S. annually [1]. Survivors face burdensome sequelae of critical illness even after acute hospitalization, suffering from physical, functional, psychiatric, and cognitive impairment [2, 3]. Cognitive impairment, which affects over half of intensive care unit (ICU) survivors during the first year after critical illness, is newly acquired, occurring in the young and old alike, and persisting for years [4–6]. Although multiple cognitive domains are affected, executive function is one of the most commonly impaired domains [7, 8]. Executive dysfunction affects 20–48% of survivors within the first year after critical illness and may persist to at least two years after the ICU [6, 9, 10]. Executive function plays a crucial role in planning, sequencing, problem-solving, and organizing and is essential for higher order cognitive tasks, such as medication adherence and informed consent [11].
Executive dysfunction in other neurodegenerative pathologies has been associated with poor outcomes—impaired instrumental activities of daily living (IADL) [12, 13], unemployment [14, 15], medication noncompliance [16], and poor social function [17, 18]. Additionally, among older adults with late-life depression, executive dysfunction is a predictor of more severe depression and is associated with poor response to antidepressants, higher relapse rates, and suicidality [19–21]. In addition to executive dysfunction, depression is also common after the ICU, occurring in over 1 in 3 survivors within one year after critical illness [22–24]. Despite the high prevalence of executive dysfunction in survivors of critical illness and its known detrimental impact in other diseases, no investigations in survivors of critical illness have evaluated executive dysfunction comprehensively and examined its impact on mental health outcomes.
Additionally, there is conflicting evidence on the role of age in the course of depression after the ICU. While younger age has been associated with anxiety and PTSD after the ICU, its association with depression after the ICU has been less consistently identified [25–27]. Among over 400 medical and surgical ICU survivors, we found older age to be independently associated with worse depression and mental health-related quality of life (HRQOL) one year after the ICU [23]. Furthermore, whether older age might differentially affect the relationship between executive dysfunction and depression or mental HRQOL has not yet been studied.
Applying knowledge from the fields of neurodegenerative disease and late-life depression, we first hypothesized that executive dysfunction occurring after critical illness would be independently associated with worse depression and mental HRQOL. Second, we hypothesized that older age would augment these associations, reasoning that older adults may have less cognitive reserve and are more vulnerable to the negative effects of cognitive impairment. Thus, the objectives of this study are 1) to determine if executive dysfunction at 3 months after the ICU is independently associated with severity of depressive symptoms or worse mental HRQOL 12 months after the ICU in survivors of critical illness, and 2) to determine if age modifies the association of executive function and severity of depressive symptoms or mental HRQOL.
2. MATERIAL AND METHODS
2.1. Study Population and Setting
The BRAIN-ICU study is a multicenter, prospective cohort study conducted at Vanderbilt University Medical Center and St. Thomas Hospital in Nashville, Tennessee.
The BRAIN-ICU study enrolled patients upon admission to the surgical or medical ICU for respiratory failure, cardiogenic shock, or septic shock. Detailed exclusion criteria for the BRAIN-ICU study have been reported [4]. Most pertinently, patients at high risk for preexisting cognitive impairment due to neurodegenerative disease, recent cardiac surgery, anoxic brain injury, or severe dementia were excluded. Screening for cognitive impairment was completed with the Short Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE; on a scale from 1.0 to 5.0, with 5.0 indicating severe cognitive impairment) [28]. Patients with IQCODE scores of 3.3 or more were assessed by certified evaluators with the Clinical Dementia Rating (CDR) scale (with scores ranging from 0 to 3.0, and higher scores indicating more severe dementia) [29]. Patients with a CDR score of 3.0 were excluded.
Patients were followed up at 3 and 12 months with neuropsychological battery and mental health assessment. Part-way through the BRAIN-ICU study, executive function testing was added to the neuropsychological battery. For our study, we included only patients with executive function testing at 3 months. We excluded patients who were missing depression or HRQOL assessment at 3 months, as we adjusted for this variable in the multivariable regression analysis.
2.2. Covariates
We collected baseline patient characteristics upon ICU admission: age, race, gender, marital status, years of education, employment status, baseline IQCODE, history of depression and other mental health illnesses as reported by a proxy, Charlson comorbidity score [30], and activities of daily living (ADL) and IADL using the Katz Activities of Daily Living [31] and Pfeffer Functional Activities Questionnaire [32]. In-hospital characteristics collected included admission diagnosis, ICU type, severity of illness according to the Sequential Organ Failure Assessment (SOFA) score [33], duration of mechanical ventilation, days of delirium, days of coma, days of sepsis, stroke, ICU length of stay, hospital length of stay, and discharge destination. On our follow-up questionnaire, we also collected self-reported information on whether psychiatric treatment (either medications or therapy) was received since the last follow-up.
2.3. Executive Function
At 3 and 12 months after the ICU, we performed executive function and mental health assessments. Executive function was assessed by two measures: the Behavior Rating Inventory of Executive Function-Adult (BRIEF-A) [34], a subjective, self-reported measure, and the Trail Making Test B (Trails B) [35], an objective, observed measure. The BRIEF-A is a reliable and valid standardized rating of executive functioning in everyday life for adults that measures a broad range of executive function subdomains (inhibition, set shifting, emotional control, self-monitoring, initiation, working memory, planning, task monitoring, and organizing). The BRIEF-A has been shown to detect subtle executive function problems in patients with mild cognitive impairment and pre-clinical Alzheimer disease when neuropsychiatric testing has been normal [36]. It has a self-report version completed by the patient and an informant-report version completed by a family member. Both versions include the same 75 items that provide an overall score reported as an age- and gender-adjusted T-score (range 0–100, mean = 50, SD = 10, higher score is worse), with impairment defined as T-score ≥65. We administered the BRIEF-A self-report version to the patient.
Additionally, we administered the BRIEF-A informant-report version to a family member. We included the informant version to address the concern that a patient with cognitive impairment may not provide a reliable report of executive function, as the BRIEF-A is a subjective measure of executive function. We determined the correlation between executive function scores from the self-report and informant-report versions, as this has not been studied in survivors of critical illness.
The Trails B is a valid and reliable neuropsychological test administered by research personnel that quantifies a narrower spectrum of executive function (divided attention and set shifting) as well as visual processing speed [35]. Scores are reported as age-, sex-, and education-adjusted T scores (range 0–100, mean=50, SD=10, lower score is worse), and impairment is defined as T-score <35. Trails B has robust psychometric properties and is effective in identifying cognitive impairment and in distinguishing those with and without this condition, with sensitivities and specificities of this reported to be in the 70s and 80s, respectively [37].
2.4. Depression
Depression was assessed by the Beck Depression Inventory-II (BDI), a self-reported measure with 21 items providing a score from 0 to 63. Scores of 14–19 correlate with mild depression, 20–28 with moderate depression, and 29–63 with severe depression. The BDI is a valid and reliable tool with good internal consistency, test-retest stability, and sensitivity and specificity for major depressive disorder [38, 39]. The minimally important clinical difference is 3 [40].
2.5. Mental HRQOL
Mental HRQOL was assessed at 3 and 12 months by the Mental Component Score of the Short-Form 36 (SF-36) [41]. The SF-36 provides an age- and gender-adjusted T-score (mean=50, SD=10) with higher scores indicating better HRQOL. We also examined the SF-36 mental subscales (vitality, role-emotional, social function, mental health) as narrower measures of domains within mental HRQOL. The subscales together comprise the Mental Component Score. The minimally important clinical difference is 5 [42].
2.6. Statistical Analysis
We used descriptive statistics to characterize the study population and to determine outcome measures. Measures of central tendency and dispersion are reported as medians and interquartile ranges (IQR). We used proportions to characterize categorical variables. We define executive dysfunction as 1.5 standard deviations worse than the mean on either the BRIEF-A (≥ 65) or the Trails B (≤ 35). We categorized the BDI score into minimal (0–13), mild (14–19), moderate (20–28), or severe (29–63) depression.
To test whether executive dysfunction at 3 months was independently associated with severity of depressive symptoms (BDI score) at 12 months, we used a multiple linear regression model with the BRIEF-A score as the independent variable. We adjusted for potential confounders selected a priori, including age, gender, marital status, comorbidity, severity of illness, days of delirium, and whether psychiatric treatment was received between hospital discharge and 3-month follow-up. Since depression at baseline may influence depression score at 12 months, we included BDI score at 3 months as a covariate. In sensitivity analyses, we used two separate models, one using the Trails-B score as a continuous variable and a second that dichotomized executive dysfunction as present or absent (present if either BRIEF-A ≥ 65 or Trails B ≤ 35). For each model using a continuous independent variable, we report the difference in BDI score at 12 months between those at the 25th vs 75th percentile of executive functioning at 3 months. For the model that dichotomizes executive dysfunction, we report the difference in BDI between those with vs without executive dysfunction.
Similarly, to test whether executive dysfunction at 3 months was independently associated with worse mental HRQOL at 12 months, we used a multiple linear regression model for each outcome: SF-36 Mental Component Score and each subscale (vitality, role-emotional, social function, and mental health). We performed sensitivity analyses using separate models for the Trails B score and executive function as a dichotomous variable based on the BRIEF and Trails B, as above. We adjusted for potential confounders selected a priori, including age, gender, marital status, comorbidity, severity of illness, days of delirium, whether psychiatric treatment was received between hospital discharge and 3-month follow-up, and for each respective mental HRQOL score or subscale at 3 months in each model.
For each outcome, distribution was examined for normality, and proportional odds model was used if normal assumption was not met.
To address the concern that a patient with cognitive impairment may not provide a reliable report of executive function, we determined the correlation between the respondent BRIEF-A (patient) and the informant BRIEF-A (family) scores using Spearman’s correlation. We also stratified this analysis by an objective measure of executive function (Trails B ≤ 35 or >35).
The BRAIN-ICU study was approved by the Vanderbilt University IRB.
3. RESULTS
Of the 826 patients enrolled upon admission to the ICU, 151 died in the hospital and 39 withdrew from the study in the hospital. Detailed enrollment is available in Figure S1. A total of 356 were discharged from the hospital before 2009 when executive function testing was added to the follow-up testing. Of the 280 patients eligible for this study, 38 died before 3-month follow-up. At 3 months after the ICU, 178 of 217 (82%) underwent cognitive testing that included executive function testing. At 12 months after the ICU, 153 of 177 (86%) underwent follow-up. Seventeen patients were excluded for missing either dependent variable (BDI or SF-36 at 12 months), resulting in a final study population of 136.
Table 1 shows characteristics of the study population at ICU admission and at 3 months after the ICU. Median age was 59 years. Seven percent had preexisting cognitive impairment based on the IQCODE. Ten percent had impairments in IADL and none had impairments in ADL. Thirty-seven percent had a history of ever having depression, and 12% had a history of non-depressive psychiatric illness.
Table 1.
Baseline Characteristics of Study Participants
| n=136 | 3 month follow-up |
|---|---|
| Demographics | |
| Age—years [median(IQR)] | 60 (50–66) |
| Race—no. (%) | |
| White | 123 (90) |
| Black | 13 (10) |
| Male sex—no. (%) | 72 (53) |
| Married—no. (%)a | 81 (61) |
| Employed at ICU admission—no. (%) | 37 (27) |
| Years of education [median(IQR)] | 12 (12–14) |
| Baseline clinical status | |
| Disabilityb | |
| ADL—no. (%) | 0 |
| IADL—no. (%) | 14 (10) |
| Preexisting cognitive impairment—no. (%)c | 9 (7) |
| History of depression—no. (%) | 50 (37) |
| History of non-depression psychiatric illness—no. (%) | 17 (12) |
| Charlson comorbidity index [median(IQR)]d | 2 (1–4) |
| In-hospital characteristics | |
| Admission diagnosis—no. (%) | |
| Sepsis, ARDS due to infection or septic shock | 46 (34) |
| CHF/cardiogenic shock | 11 (8) |
| Airway protection | 11 (8) |
| COPD/asthma | 9 (7) |
| Hepatobiliary/pancreatic surgery | 9 (7) |
| ARDS without infection | 7 (5) |
| Other surgery | 23 (17) |
| Other medical diagnosis | 20 (15) |
| ICU type—no. (%) | |
| Medical | 76 (56) |
| Surgical | 60 (44) |
| Modified SOFA—median(IQR)e | 4.8 (3.6 – 7.0) |
| Mechanical ventilation | |
| No. of patients—(%) | 123 (90) |
| No. of days—median(IQR) | 1.8 (0.3 – 4.8) |
| Delirium | |
| No. of patients—(%) | 94 (68) |
| No. of days –median(IQR) | 2 (0–5) |
| Ever septic—no (%) | 88 (65) |
| Stroke in hospital—no. (%) | 4 (3) |
| ICU length of stay in days—median(IQR) | 4.4 (2.0–10.0) |
| Discharge Destination—no. (%) | |
| Home | 84 (62) |
| Inpatient rehab | 34 (25) |
| Nursing Home | 8 (6) |
| Long-term acute care | 9 (7) |
| Other hospital | 1 (1) |
| Cognitive state at 3 months | |
| BRIEF-Af | |
| Score—median(IQR) | 56 (47–65) |
| Impaired—no. (%) | 33 (26) |
| Trails Bg | |
| Score—median(IQR) | 42 (35–49) |
| Impaired—no. (%) | 36 (27) |
| Psychological state at 3 months | |
| BDI score—median(IQR)h | 10 (5–15) |
| Mild depression (BDI 14–19)—no. (%) | 18 (14) |
| Moderate depression (BDI 20–28) —no. (%) | 14(11) |
| Severe depression (BDI ≥29) —no. (%) | 11 (8) |
| New mental health medications received—no. (%) | 22 (16) |
| New mental health therapy received—no. (%) | 14 (10) |
| Mental HRQOL 3 monthsi | |
| SF-36 score—median(IQR) | |
| Mental Component Score | 55 (41–60) |
| Social Function | 51 (40–57) |
| Vitality | 43 (33–50) |
| Mental Health | 53 (44–60) |
| Role-emotional | 55 (24–55) |
Marital status was assessed by questionnaire at 3 month follow-up.
Disability in activities of daily living (ADL) was determined by the Katz. Disability in instrumental activities of daily living (IADL) was determined by the Pfeffer Functional Activities Questionnaire (FAQ) score using a validated cutoff score of ≥ 9.
The Short Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) ranges from 1 to 5, with a score of 3 indicating no change in cognition over the past 10 years, a score lower than 3 indicating improvement, and a score higher than 3 indicating decline in cognition, as compared with 10 years before. A score ≥ 3.6 indicates preexisting cognitive impairment.
Scores on the Charlson comorbidity index range from 0 to 33, with higher scores indicating a greater burden of illness; a score of 1 or 2 is associated with mortality of approximately 25% at 10 years.
Scores on the Sequential Organ Failure Assessment (SOFA) range from 0 to 24 (from 0 to 4 for each of six organ systems), with higher scores indicating more severe organ dysfunction. We used a modified SOFA score, which excluded the Glasgow Coma Scale components, since coma was included separately in our models.
The BRIEF-A is reported as age- and gender-adjusted T-scores; impairment is T-score ≥65.
The Trails B is reported as age-, sex-, and education-adjusted T-scores; impairment is T-score≤35.
The Beck Depression Inventory-II (BDI) ranges from 0–63. Scores of 14–19 correlate with mild depression, 20–28 moderate depression, and 29–63 with severe depression.
Mental health-related quality of life was measured using the Short Form-36 (SF-36), which provides age- and gender-adjusted T-scores with lower scores indicating worse health-related quality of life.
3.1. Executive Dysfunction and Depression
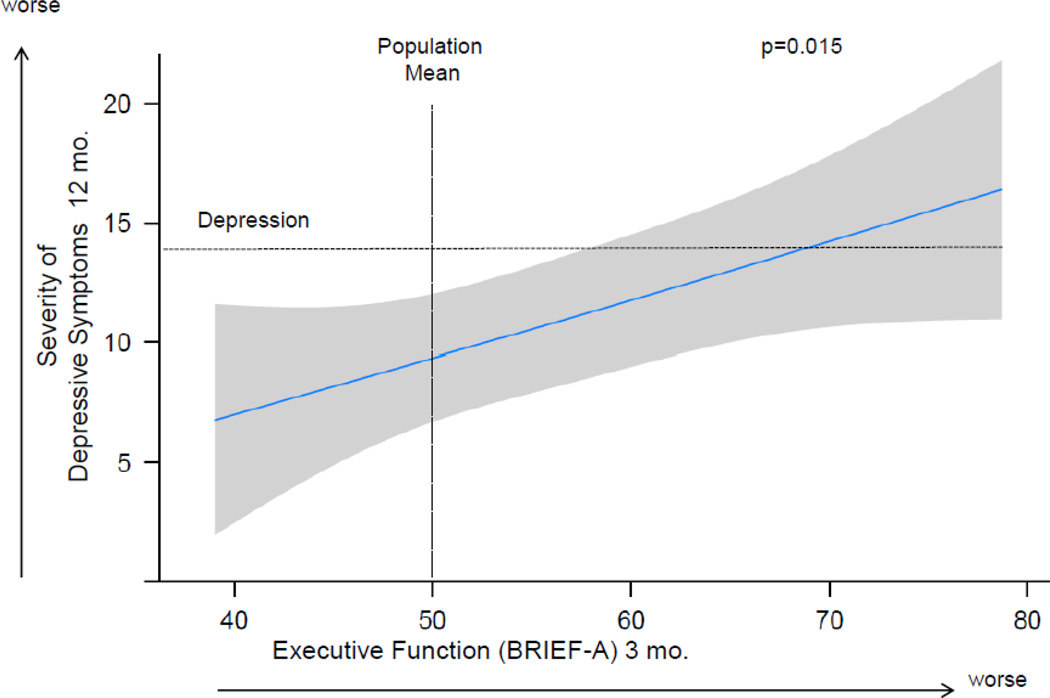
At 3 months after the ICU, 33 (26%) patients had executive dysfunction according to the BRIEF-A and 36 (27%) according to Trails B. Median (IQR) BRIEF-A T-score was 56 (47–65), or half a standard deviation worse than population norms, and median (IQR) Trails B T-score was 42 (35–49), almost one standard deviation worse than population norms. At 12 months after the ICU, median BDI score was 9, and 39 (31%) survivors met criteria for depression (BDI≥14). The multiple linear regression model showed that executive dysfunction at 3 months after the ICU, as measured by the BRIEF-A, was independently associated with worse severity of depressive symptoms at 12 months after controlling for age, gender, marital status, comorbidity, severity of illness, days of delirium, whether mental health treatment was received by 3 months, and depression at 3 months (Figure 1). The 25th compared to the 75th percentile of executive functioning at 3 months was associated with 4.3 worse points on the BDI at 12 months [95% CI (1.3, 7.4), p=0.015] (Table 2).
Figure 1. The Association of Executive Function at 3 Months and Depressive Symptoms at 12 Months.
Worse executive function at 3 months was independently associated with worse severity of depressive symptoms at 12 months, after adjusting for age, gender, marital status, years of education, Charlson comorbidity score, severity of illness, days of delirium, whether psychiatric treatment was received between hospital discharge and 3-month follow-up, and severity of depressive symptoms at 3 months.
Point estimates and the 95% confidence interval for this relationship are shown by the solid line and the gray band, respectively. Executive function was determined using the Behavior Rating Inventory of Executive Function-Adult (BRIEF-A), a subjective, self-reported measure of executive functioning in everyday life, and is reported as an age- and gender-adjusted T-score with mean (±SD) of 50±10. The vertical dashed line delineates the age- and gender-adjusted population mean score of 50. Severity of depressive symptoms was determined using the Beck Depression Inventory-II (BDI), a self-report measure of depression. The BDI provides a score ranging from 0 to 63. The horizontal dashed line indicates the cutoff score for depression. Scores of 14–19 correlate with mild depression, 20–28 correlate with moderate depression, and 29–63 correlate with severe depression.
Table 2.
Association of Executive Dysfunction at 3 Months With Severity of Depressive Symptoms and Mental Health-Related Quality of Life at 12 Months
| n=136 | Difference in scorea (95% CI) |
Odds of worse scoreb (95% CI) |
p |
|---|---|---|---|
| Depressive Symptomsc | 4.3 (1.3, 7.4) | - | 0.02 |
| Mental HRQOLd | |||
| Mental Component Score | −5.0 (−8.3, −1.7) | - | 0.006 |
| Vitality | −2.6 (−5.5, 0.2) | - | 0.07 |
| Mental Health | −2.7 (−4.7, −0.10) | - | 0.004 |
| Social Function | - | OR 0.33 (0.17, 0.64) | 0.005 |
| Role-emotional | - | OR 0.26 (0.10, 0.65) | 0.01 |
Difference in score reported is from comparing the median of those scoring in the 25th percentile on the BRIEF-A (T-score=47) versus the median of those scoring in the 75th percentile (T-score=65).
Proportional odds models were used for social function and role-emotional subscales due to their non-normal distribution.
Depressive symptoms were measured by the Beck Depression Inventory-II (BDI). Higher BDI score indicates more severe depressive symptoms.
Mental health-related quality of life is measured using the SF-36. Lower SF-36 score indicates worse HRQOL.
The sensitivity analysis that defined executive dysfunction in two alternative ways did not show a statistically significant association between executive dysfunction and severity of depressive symptoms (see Table S1).
3.2. Executive Dysfunction and Mental-HRQOL
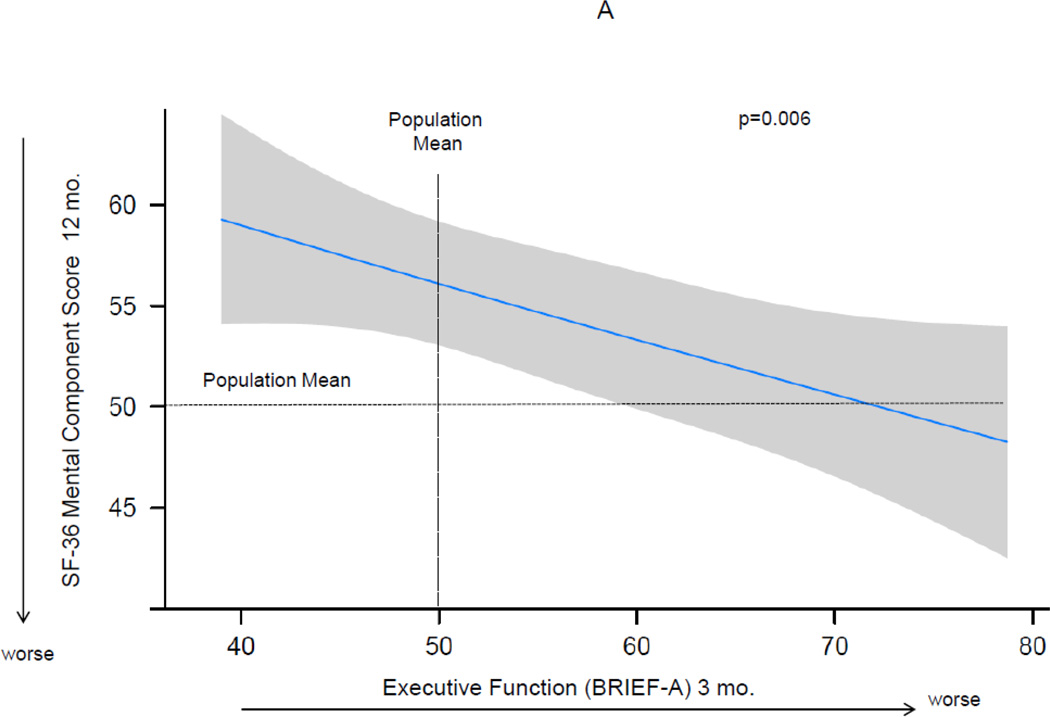
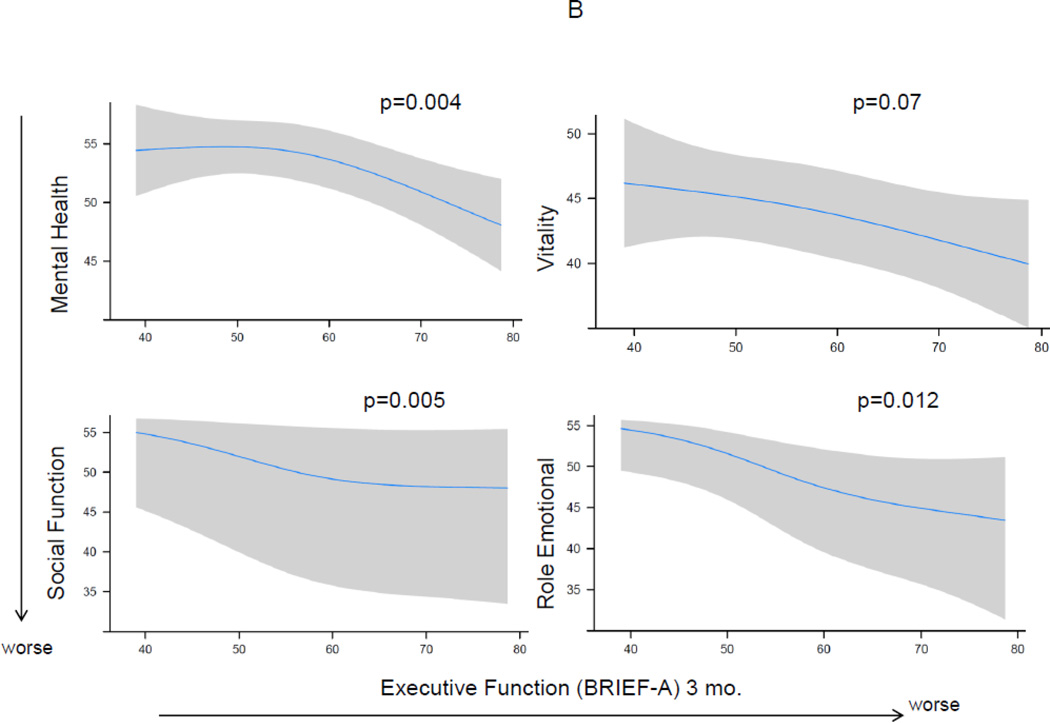
At 12 months after the ICU, median (IQR) mental HRQOL scores on the SF-36 were as follows—Mental Component Score 55 (41–60), social function 51 (40–57), vitality 43 (33–50), mental health 53 (44–60), and role-emotional 55 (24–55). Executive dysfunction at 3 months after the ICU as measured by the BRIEF-A was associated with worse mental HRQOL at 12 months for the Mental Component Score and each subscale, after adjusting for age, gender, marital status, comorbidity, severity of illness, days of delirium, whether psychiatric treatment was received between hospital discharge and 3-month follow-up, and mental HRQOL at 3 months (Figure 2). The 25th compared to the 75th percentile of executive functioning at 3 months was associated with worse mental HRQOL for the Mental Component Score and each subscale (Table 2).
Figure 2. The Association of Executive Function at 3 Months and Mental Health-Related Quality of Life at 12 Months.
Worse executive function at 3 months was independently associated with worse mental-health related quality of life at 12 months, after adjusting for age, gender, marital status, years of education, Charlson comorbidity score, severity of illness, days of delirium, whether psychiatric treatment was received between hospital discharge and 3-month follow-up, and mental-health related quality of life at 3 months. Point estimates and the 95% confidence interval for this relationship are shown by the solid line and the gray band, respectively. The horizontal dashed lines show the general population mean on the Short-Form 36 (SF-36). Executive function was determined using the Behavior Rating Inventory of Executive Function-Adult, a subjective, self-reported measure of executive functioning in everyday life, and is reported as an age- and gender-adjusted T-score with mean (±SD) of 50±10 and higher score indicating worse executive function. The vertical dashed line shows the BRIEF-A score of the population mean. Panel A shows the SF-36 Mental Component Score, reported as an age- and gender-adjusted T score with mean (±SD) of 50±10 and lower score indicating worse mental health-related quality of life. Panel B shows the individual models using each of the SF-36 mental subscales at 12 months, which together comprise the Mental Component Score.
Results from the sensitivity analysis that defined executive dysfunction by the Trails B score found no significant association between executive dysfunction at 3 months and mental HRQOL scores (Mental Component Sore or any of the subscales) at 12 months (see Table S1). Similarly, the sensitivity analysis that defined executive dysfunction as either present or absent did not show an association between executive dysfunction and any of the mental HRQOL scores at 12 months.
3.3. Correlation between Self-reported BRIEF-A and Informant BRIEF-A
The correlation between the respondent BRIEF-A (patient) and the informant BRIEF-A (family) scores was found to be fair (Spearman rho=0.48). The correlation for those who scored poorer on an objective test of executive function (Trails B score ≤ 35) was better (rho=0.57) than for those who scored > 35 (rho = 0.32) at 3 months.
3.4. The Role of Age
Age was not found to modify the association of executive function and severity of depression symptoms or mental HRQOL when we included age as an interaction term (Table 3).
Table 3.
Effects of Executive Dysfunction at 3 Months on Severity of Depressive Symptoms and Mental Health-Related Quality of Life at 12 Months Modified by Age
| n=136 | Difference in scorea (95% CI) | p | |
|---|---|---|---|
| 50 years-old | 67 years-old | ||
| Depressive Symptomsb | 5.6 (2.2, 9.1) | 2.7 (−0.9, 6.3) | 0.12 |
| Mental HRQOLc | |||
| Mental Component Score | −4.6 (−8.3, −0.8) | −4.8 (−9.0, −0.6) | 0.80 |
| Vitality | −2.3 (−5.5, 0.9) | −3.8 (−7.4, −0.1) | 0.76 |
| Mental Health | −1.4 (−4.1, 1.3) | −2.9 (−5.9, 0.1) | 0.55 |
| Social Functiond | 0.27 (0.13, 0.56) | 0.40 (0.16, 1.00) | 0.58 |
| Role-emotionald | 0.24 (0.09, 0.64) | 0.22 (0.06, 0.86) | 0.92 |
Difference in score is reported comparing the median of those scoring in the 25th percentile on the BRIEF-A (T-score=47) versus the median of those scoring in the 75th percentile (T-score=65). Multiple linear regression was used, except where outcomes were not normally distributed (social function and role-emotional), in which case proportional odds models were used. All models included age as an interaction term.
Depressive symptoms were measured by the Beck Depression Inventory-II (BDI). Higher BDI score indicates more severe depressive symptoms.
Mental health-related quality of life is measured using the SF-36. Lower SF-36 score indicates worse HRQOL.
Proportional odds models were used for social function and role-emotional subscales due to their non-normal distribution. Odds ratios are reported.
4. DISCUSSION
This multicenter, prospective cohort study showed that executive dysfunction at 3 months after the ICU is independently associated with both worse severity of depressive symptoms and worse mental HRQOL at 12 months after the ICU. To our knowledge, this is the first prospective study conducted examining this association. Moreover, among studies examining cognitive impairment more broadly, this is the only one to account for depression status at baseline, which can potentially confound the relationship between cognitive impairment and depressive symptoms. We suspect the magnitude of these associations between executive dysfunction and depression and mental HRQOL is not only statistically significant, but also clinically relevant. The lowest 25th percentile compared to the 75th percentile of executive function had depression scores that were 4.3 points worse and mental HRQOL scores that were 5 points worse, both of which meet the threshold for the minimally important clinical difference of 3 and 5, respectively [40, 42].
In this cohort, over one in four survivors of critical illness had executive dysfunction at 3 months. It is likely that these deficits were new as a result of critical illness, as we found only 7% had preexisting cognitive impairment and 10% were impaired in IADLs prior to admission. Executive dysfunction is profoundly disruptive to functioning and is associated with poor outcomes in patients with primary psychiatric and neurodegenerative disease [12–18], but the impacts of executive dysfunction after critical illness have not yet been studied. A recent randomized clinical trial of a primary care management intervention in ICU sepsis survivors proved ineffective at improving mental HRQOL [43]. In light of our findings that patients with executive dysfunction are at increased risk for worsening mental health outcomes, we suspect targeting executive dysfunction may make such an intervention more effective. We propose that survivors of critical illness undergo routine screening for executive dysfunction, and that those with executive dysfunction be closely monitored for mental health problems, as they appear to be at high risk for these. Additionally, in survivors of critical illness with depression, it may be beneficial to target executive dysfunction therapeutically with cognitive rehabilitation, cognitive stimulation, or goal management training.
These findings both confirm and extend previous studies that have identified associations between cognitive impairment and poor mental health outcomes. In a cross-sectional study of 46 ARDS survivors, Rothenhausler and colleagues performed cognitive testing in the domains of memory and attention at a median of 6 years after the ICU; cognitive impairment was associated with both disability and impaired HRQOL in the role-physical and social function subscales [44]. In another cross-sectional study of 79 survivors of ARDS, Christie and colleagues performed a neuropsychiatric battery of 9 tests across a broader range of domains and found an association between cognitive impairment and both impaired mental HRQOL and worse anxiety [7]. While a higher prevalence of depression occurred in the group with cognitive impairment (41%) vs those without (31%), this difference was not statistically significant. The cross-sectional designs of these studies preclude one from making inferences about causality or mechanism of the relationship between cognitive impairment and HRQOL, disability, or anxiety.
Only one study to our knowledge has used a prospective design to examine whether cognitive impairment predicts depression. Hopkins and colleagues enrolled 74 ARDS survivors and found that alcohol dependence, female gender, and younger age predicted depression at 1 year after the ICU [25]. The only variables that continued to predict depression at 2 years were depression at 1 year and cognitive impairment at 1 year. However, cognitive impairment may be a symptom of depression, and this study was unable to distinguish whether cognitive impairment was merely a marker or a cause of worse depression. In contrast, we were able to adjust for depressive symptoms at the time of cognitive assessment at 3 months, and we still found an independent association of executive dysfunction with severity of depressive symptoms. This supports our conclusion that executive dysfunction may be more than a mere marker for worse depression.
Although we cannot conclude that executive dysfunction is the cause of worse mental health outcomes, it seems plausible that executive dysfunction may play a key role in the development and/or progression of depressive symptoms and mental HRQOL. Three potential mechanisms are feasible. First, a direct effect of executive dysfunction via decreased cognitive reserve is possible. Recovery from stressful events, such as critical illness, requires cognitive flexibility and higher order thinking to navigate the “new normal” that patients often experience in the form of Post-Intensive Care Syndrome, defined as new or worsening impairments in physical, cognitive, or mental health status arising after critical illness and persisting beyond acute hospitalization [2]. Executive dysfunction may leave one’s brain more vulnerable to the stresses of life that follow critical illness with less capacity to heal itself and cope with stressors. Second, an indirect effect of executive dysfunction via mediator deficits is possible. As has been shown in other non-critically ill populations, executive dysfunction may result in impairments—functional decline [12, 13], unemployment [14, 15], medication noncompliance [16], and poor social function [17, 18]—each of which may independently contribute to depressive symptoms. Third, executive dysfunction and depression may be a result of a common hit. Critical illness may cause a direct injury to the brain that predisposes to executive dysfunction, depression, and other unmeasured confounders. The prefrontal cortex has been implicated in the pathogenesis of both executive dysfunction and depression [45], and animal studies support the hypothesis that inflammation and/or hypoxemia may play an important role [46].
These findings also add to the knowledge on the role of age in the course of depression after the ICU. There is conflicting evidence, with some studies reporting that younger age [25] is associated with worse depression after critical illness, whereas evidence from BRAIN-ICU found older age [23] to be associated with worse depression after critical illness. We had hypothesized that in older adults, executive dysfunction would have more severe consequences than in younger adults because of a higher vulnerability of the aged brain. Yet, among these patients, we did not find that age modified the association between executive dysfunction and either depression or mental HRQOL. We do believe that the role of age in the relationship between executive dysfunction and mental health should be further explored in other populations and settings.
Our sensitivity analysis that measured executive function with Trails B instead of BRIEF-A found no association between executive dysfunction and mental health outcomes. Three potential explanations arise. First, although the Trails B has been shown to be sensitive in detecting executive dysfunction [37], the BRIEF-A may be an even more sensitive marker of milder executive dysfunction. In MCI and preclinical Alzheimer disease, the BRIEF-A has detected executive dysfunction when neuropsychiatric testing has been normal [36]. The BRIEF-A also measures more subdomains of executive functioning, whereas Trails B measures fewer. Second, the BRIEF-A may identify more clinically significant executive dysfunction since it ascertains real-world difficulties with functioning. It is possible that the more clinically significant executive dysfunction (detected by the BRIEF-A) increases the risk for depression and impaired mental HRQOL. Third, the BRIEF-A may be identifying patients with depression who have negative cognitive biases and as a result report more difficulty with functioning than a non-depressed patient. This is possible given over one in three of our population reported a history of ever having depression. However, the BRIEF-A has been validated to detect executive functioning and not depression [35].
The strengths of this study include being a longitudinal prospective study, having a broad study population of general medical and surgical ICU survivors which increases generalizability, and having detailed baseline neuropsychological status. Yet, this study has several potential limitations. First, although our follow-up rate was above 85%, we may be underestimating the prevalence of executive dysfunction and depression since these patients may be less likely to follow up. Second, we measured depression and executive dysfunction with self-reported tools, which may differ from what an objective evaluator determines. However, these self-reported measures are valid as well as important because they are patient-centered, allowing us to better understand the patient’s experience. Additionally, the self-reported measures are also limited in that cognitively impaired patients may lack awareness of their deficits and report normal executive function. However, we found that the informant’s responses to the BRIEF-A correlated with the patient’s responses, and in those who were impaired by the objective measure of executive function, this correlation was even stronger. Third, our small sample size precluded us from excluding patients already depressed at 3 months, making it impossible to determine if executive function is a true risk factor for incident depression. However, we included depression or mental HRQOL score at 3 months as a covariate for each respective linear regression model, thus modeling the impact of executive dysfunction on depressive symptoms or mental HRQOL irrespective of baseline status.
5. CONCLUSION
Executive dysfunction and depression are common after critical illness, affecting up to half and one-third of survivors, respectively. We found that executive dysfunction at 3 months after the ICU is independently associated with more severe depressive symptoms and with worse mental HRQOL at 12 months after the ICU. We did not find that age modifies the associations between executive dysfunction and depression or mental HRQOL, although there was a non-statistically significant trend showing younger patients with executive dysfunction had worse depression scores at 12 months compared to older patients. Future research is needed to fully elucidate the relationship between executive function and mental health outcomes and the impact of age in this relationship. Studies evaluating the effectiveness of cognitive therapy or rehabilitation should evaluate depression and mental HRQOL as secondary outcomes, since executive function may impact these outcomes. Additionally, a better understanding of the neurobiological mechanisms underlying executive dysfunction and depression could guide effective management strategies that could potentially apply to other patient populations where both executive dysfunction and depression co-occur, such as in those with dementia, traumatic brain injury, and depression.
Supplementary Material
Acknowledgments
Funding:
This work was supported by the National Center for Advancing Translational Sciences of the National Institute of Health [award number UL1 TR000445-06].
Financial disclosures:
Dr. Dittus receives grant funding from the NIH and the VA. None of his board memberships, consulting relationships or honoraria are related to this work.
Dr. Ely receives honoraria from Abbott, Hospira and Orion pharmaceuticals, which are unrelated to this work. His grants and funding come from the VA and NIH.
Dr. Jackson receives grant funding from the NIH and the VA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble W. Three-Year Outcomes for Medicare Beneficiaries Who Survive Intensive Care. Jama. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 2.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Critical care medicine. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 3.Milbrandt EB, Eldadah B, Nayfield S, Hadley E, Angus DC. Toward an integrated research agenda for critical illness in aging. American journal of respiratory and critical care medicine. 2010;182:995–1003. doi: 10.1164/rccm.200904-0630CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-term cognitive impairment after critical illness. The New England journal of medicine. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adhikari NK, Tansey CM, McAndrews MP, Matte A, Pinto R, Cheung AM, et al. Self-reported depressive symptoms and memory complaints in survivors five years after ARDS. Chest. 2011;140:1484–1493. doi: 10.1378/chest.11-1667. [DOI] [PubMed] [Google Scholar]
- 6.Jackson JC, Hart RP, Gordon SM, Shintani A, Truman B, May L, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Critical care medicine. 2003;31:1226–1234. doi: 10.1097/01.CCM.0000059996.30263.94. [DOI] [PubMed] [Google Scholar]
- 7.Christie JD, Biester RC, Taichman DB, Shull WH, Jr, Hansen-Flaschen J, Shea JA, et al. Formation and validation of a telephone battery to assess cognitive function in acute respiratory distress syndrome survivors. Journal of critical care. 2006;21:125–132. doi: 10.1016/j.jcrc.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Jones C, Griffiths RD, Slater T, Benjamin KS, Wilson S. Significant cognitive dysfunction in non-delirious patients identified during and persisting following critical illness. Intensive care medicine. 2006;32:923–926. doi: 10.1007/s00134-006-0112-y. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson LV. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 1999;160:50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 10.Sukantarat KT, Burgess PW, Williamson RC, Brett SJ. Prolonged cognitive dysfunction in survivors of critical illness. Anaesthesia. 2005;60:847–853. doi: 10.1111/j.1365-2044.2005.04148.x. [DOI] [PubMed] [Google Scholar]
- 11.Lezak MD. Neuropsychological Assessment. New York: Oxford University Press; 1995. [Google Scholar]
- 12.Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl Neuropsychol. 2002;9:187–191. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- 13.Boyle PA, Paul RH, Moser DJ, Cohen RA. Executive impairments predict functional declines in vascular dementia. The Clinical neuropsychologist. 2004;18:75–82. doi: 10.1080/13854040490507172. [DOI] [PubMed] [Google Scholar]
- 14.Ownsworth T, McKenna K. Investigation of factors related to employment outcome following traumatic brain injury: a critical review and conceptual model. Disabil Rehabil. 2004;26:765–783. doi: 10.1080/09638280410001696700. [DOI] [PubMed] [Google Scholar]
- 15.McGurk SR, Mueser KT. Cognitive and clinical predictors of work outcomes in clients with schizophrenia receeiving supportedemploment servcies: 4 year follow-up. Adm Policy Ment Health. 2006;33:598–606. doi: 10.1007/s10488-006-0070-2. [DOI] [PubMed] [Google Scholar]
- 16.Insel K, Morrow D, Brewer B, Figueredo A. Executive function, working memory, and medication adherence among older adults. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 2006;61:P102–P107. doi: 10.1093/geronb/61.2.p102. [DOI] [PubMed] [Google Scholar]
- 17.Struchen MA, Clark AN, Sander AM, Mills MR, Evans G, Kurtz D. Relation of executive functioning and social communication measures to functional outcomes following traumatic brain injury. NeuroRehabilitation. 2008;23:185–198. [PubMed] [Google Scholar]
- 18.Washburn A, Sands L. Social cognition in nursing home residents with and without cognitive impairment. The journals of gerontology Series B, Psychological sciences and social sciences. 2006;61:174–179. doi: 10.1093/geronb/61.3.p174. [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, et al. Executive dysfunction and long-term outcomes of geriatric depression. Archives of general psychiatry. 2000;57:285–290. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- 20.Murphy CF, Alexopoulos GS. Longitudinal association of initiation/perseveration and severity of geriatric depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2004;12:50–56. [PubMed] [Google Scholar]
- 21.Potter GG, Kittinger JD, Wagner HR, Steffens DC, Krishnan KR. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:2266–2271. doi: 10.1038/sj.npp.1300551. [DOI] [PubMed] [Google Scholar]
- 22.Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive care medicine. 2009;35:796–809. doi: 10.1007/s00134-009-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson JC, Pandharipande PP, Girard TD, Brummel NE, Thompson JL, Hughes CG, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2:369–379. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70:512–519. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopkins RO, Key CW, Suchyta MR, Weaver LK, Orme JF., Jr Risk factors for depression and anxiety in survivors of acute respiratory distress syndrome. General hospital psychiatry. 2010;32:147–155. doi: 10.1016/j.genhosppsych.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Rattray JE, Johnston M, Wildsmith JA. Predictors of emotional outcomes of intensive care. Anaesthesia. 2005;60:1085–1092. doi: 10.1111/j.1365-2044.2005.04336.x. [DOI] [PubMed] [Google Scholar]
- 27.Huang M, Parker AM, Bienvenu OJ, Dinglas VD, Colantuoni E, Hopkins RO, et al. Psychiatric Symptoms in Acute Respiratory Distress Syndrome Survivors: A 1-Year National Multicenter Study. Critical care medicine. 2016;44:954–965. doi: 10.1097/CCM.0000000000001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. International psychogeriatrics / IPA. 2004;16:275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- 29.Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24:637–639. [PubMed] [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 31.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. Jama. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 32.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. Jama. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 34.Roth R, Isquith PK, Gioia GA. The Behavior Rating Inventory of Executive Function - Adult Version (BRIEF-A) Manual. Lutz, Florida: Psychological Assessment Resources; 2005. [Google Scholar]
- 35.Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- 36.Rabin LA, Roth RM, Isquith PK, Wishart HA, Nutter-Upham KE, Pare N, et al. Self- and informant reports of executive function on the BRIEF-A in MCI and older adults with cognitive complaints. Arch Clin Neuropsychol. 2006;21:721–732. doi: 10.1016/j.acn.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Demery JA, Larson MJ, Dixit NK, Bauer RM, Perlstein WM. Operating characteristics of executive functioning tests following traumatic brain injury. The Clinical neuropsychologist. 2010;24:1292–1308. doi: 10.1080/13854046.2010.528452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beck AT. BDI-II depression inventory manual. New York: Harcourt Brace; 1996. [Google Scholar]
- 39.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of personality assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 40.NCCMH. Depression: Management of Depression in Primary and Secondary Care, British Psychological Society and Royal College of Psychiatrists: Leicester and London [full guideline] 2004 [Google Scholar]
- 41.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36).I. Conceptual framework and item selection. Medical care. 1992;30:473–483. [PubMed] [Google Scholar]
- 42.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Medical care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt K, Worrack S, Von Korff M, Davydow D, Brunkhorst F, Ehlert U, et al. Effect of a Primary Care Management Intervention on Mental Health-Related Quality of Life Among Survivors of Sepsis: A Randomized Clinical Trial. Jama. 2016;315:2703–2711. doi: 10.1001/jama.2016.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothenhausler HB, Ehrentraut S, Stoll C, Schelling G, Kapfhammer HP. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. General hospital psychiatry. 2001;23:88–94. doi: 10.1016/s0163-8343(01)00123-2. [DOI] [PubMed] [Google Scholar]
- 45.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural brain research. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semmler A, Okulla T, Sastre M, Dumitrescu-Ozimek L, Heneka MT. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat. 2005;30:144–157. doi: 10.1016/j.jchemneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.