Abstract
Sudden death syndrome (SDS) is caused by the fungal pathogen, Fusarium virguliforme, and is a major threat to soybean production in North America. There are two major components of this disease: (i) root necrosis and (ii) foliar SDS. Root symptoms consist of root necrosis with vascular discoloration. Foliar SDS is characterized by interveinal chlorosis and leaf necrosis, and in severe cases by flower and pod abscission. A major toxin involved in initiating foliar SDS has been identified. Nothing is known about how root necrosis develops. In order to unravel the mechanisms used by the pathogen to cause root necrosis, the transcriptome of the pathogen in infected soybean root tissues of a susceptible cultivar, ‘Essex’, was investigated. The transcriptomes of the germinating conidia and mycelia were also examined. Of the 14,845 predicted F. virguliforme genes, we observed that 12,017 (81%) were expressed in germinating conidia and 12,208 (82%) in mycelia and 10,626 (72%) in infected soybean roots. Of the 10,626 genes induced in infected roots, 224 were transcribed only following infection. Expression of several infection-induced genes encoding enzymes with oxidation-reduction properties suggests that degradation of antimicrobial compounds such as the phytoalexin, glyceollin, could be important in early stages of the root tissue infection. Enzymes with hydrolytic and catalytic activities could play an important role in establishing the necrotrophic phase. The expression of a large number of genes encoding enzymes with catalytic and hydrolytic activities during the late infection stages suggests that cell wall degradation could be involved in root necrosis and the establishment of the necrotrophic phase in this pathogen.
Introduction
Sudden death syndrome (SDS) is a serious, emerging soybean disease. It is caused by Fusarium virguliforme, which prefers cold and wet climates for developing the disease [1]. In the United States, the disease was first reported in Arkansas in 1971 [2]. Subsequently, the disease spread northward and now it has been reported in soybean growing areas in the United States and Canada [2–4]. In 2010, the estimated soybean yield suppression from SDS was estimated at 2.1% of total yield valued at $0.82 billion dollars [5]. SDS can be divided into two components: (i) root necrosis and (ii) foliar SDS. Root symptoms consist of root necrosis with vascular discoloration spreading to a few nodes and internodes of the stem. At the initial stage, foliar SDS is characterized by interveinal chlorosis and necrosis of leaves, and in severe cases by flower and pod abscission [6].
F. virguliforme (formerly known as F. solani f. sp. glycines) is a hemibiotrophic fungal pathogen with an initial biotrophic phase [7–10]. Although symptoms appear on soybean leaves, the pathogen has never been isolated from the diseased foliar tissues. The pathogen remains in infected roots and produces toxins that are involved in foliar SDS development [11–14]. F. virguliforme secretes several proteins, including phytotoxins, into the culture medium [12]. Treatment of cut soybean seedlings with cell-free F. virguliforme culture filtrates results in degradation of the Rubisco large subunit in the presence of light [15]. A major F. virguliforme proteinacious toxin, FvTox1, has recently been shown to cause foliar SDS [11]. It is an acidic 13.5 kDa protein, which is secreted to the infected soybean roots and F. virguliforme culture media [11]. Expression of a single-chain variable fragment (scFv) antibody against this toxin enhanced foliar SDS resistance in transgenic soybean plants [16]. Knockout mutants created by homologous recombination established that FvTox1 is a major virulence factor for foliar SDS development in soybean [17]. How the toxin causes foliar SDS is not yet known and there may be additional toxins that contribute to development of foliar SDS [14].
SDS management options are limited. Genetic resistance to F. virguliforme is partial and encoded by a large number of quantitative trait loci (QTL), each contributing only a small amount of resistance [18–23]. No single genes conditioning SDS resistance have been identified and most unlikely are available. Since F. virguliforme is a root pathogen and remains in soil, little can be done once the toxins start to induce foliar SDS.
Significant changes in expression patterns of 2,467 soybean genes were observed during the soybean—F. virguliforme interaction [24]. In addition to the soybean genes, 93 small RNA and 42 micro RNA with putative target sites in the soybean genome were identified [24]. Toxins of the F. virguliforme culture filtrates induce defense pathways in leaves [25]. ESTs upregulated during root responses of recombinant inbred lines (RILs) obtained from a cross of ‘Essex' x 'Forrest’ showed the involvement of resistance gene analogs, genes governing signal transduction, plant defense, cell wall synthesis and transport of metabolites [8].
Sequencing of the F. virguliforme genome revealed 14,845 predicted genes [26]. However, the expression of F. virguliforme genes during infection in soybean and their possible involvement in infection of soybean roots has not yet been investigated. In this study, deep sequencing of transcripts collected from F. virguliforme infected root tissues of a highly susceptible soybean cultivar ‘Essex’ was conducted to identify candidate virulence genes involved in root necrosis. Comparison of the transcriptome of F. virguliforme infected root tissues with that of germinating conidia and mycelia identified 1,886 F. virguliforme genes that are induced at least two fold during infection. Gene ontology analyses of genes with ≥10-fold increase in expression levels in infected root tissues vs. germinating conidia and mycelia were classified into three major functional categories: cell-wall modification, oxidative stress, and membrane transport. A large number of the infection-induced genes are predicted to have hydrolytic activities and may play a major role in establishing the necrotrophic phase of the pathogenic fungus. The candidate virulence genes identified in this study lay the foundation for identification of F. virguliforme virulence mechanisms and the molecular basis of the root necrosis caused by this soybean pathogen.
Materials and Methods
Plant materials and inoculation
Soybean [Glycine max (L.) Merrill] cultivar Essex, highly susceptible to F. virguliforme, was sown in vermiculite under dark conditions at 22°C. The 7-day old seedlings were uprooted and used for root infection. F. virguliforme Mont-1 isolate was maintained on Bilay agar plates [(0.1% KH2PO4 (w/v), 0.1% KNO3 (w/v), 0.05% MgSO4 (w/v), 0.05% KCl (w/v), 0.02% starch (w/v), 0.02% glucose (w/v), 0.02% sucrose (w/v) and 2% agar(w/v)] and sub-cultured on 1/3 PDA agar plates [0.04% potato starch (w/v), 0.2% glucose (w/v), 2% agar (w/v)]. On 1/3 PDA plates the F. virguliforme Mont-1 isolate produced the characteristic blue mass containing conidia after two weeks of growth. The roots of 10 etiolated 7 day old Essex seedlings, grown in coarse vermiculites, were either infected with F. virguliforme Mont-1 conidia suspensions (107 spores/mL) or treated with sterile water for specified periods under dark conditions prior to harvesting for RNA isolation [11]. Harvested roots infected with conidia were thoroughly washed prior to freezing in liquid nitrogen for RNA preparation.
RNA extraction from germinating conidia, mycelia and root samples
F. virguliforme infected root samples were collected at 3, 5, 10 and 24 days post inoculation (dpi) from three independent experiments. The overview of the experimental strategy is shown in Fig 1. Necrotic symptoms were visible at 10 dpi with gradual spreading of root rot symptoms until 24 dpi. RNA samples of infected roots from three experiments, collected 3 and 5 dpi, were bulked and named as “early infection” whereas the pooled RNA sample isolated from the infected roots, collected 10 and 24 dpi, were termed as “late infection”. Early time points (3 and 5 dpi) were selected to study the putative biotrophic phase with very little root necrosis; while late time points (10 and 24 dpi) were selected for investigating the necrotrophic phase (S1 Fig).
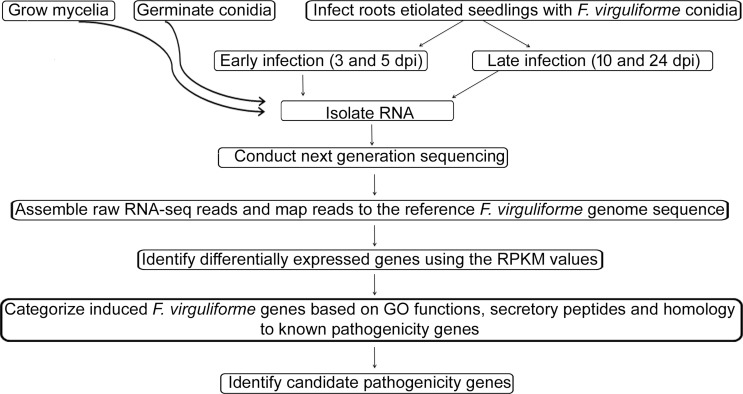
Fig 1. Flow diagram of the steps followed in conducting the transcriptomic analysis.
(i) RNA samples were isolated from germinating spore, mycelia, early stage of infection and late stage of infection (details in materials and methods). (ii) RNA samples were reverse transcribed into cDNA and sequenced using Solexa sequencing platform. (iii) Assembling of the RNA sequence datasets and mapping to reference sequence (http://fvgbrowse.agron.iastate.edu/) was conducted using Bowtie. (iv) Determined the expression levels of individual genes (RPKM values). (v) Validation of the RNA sequence data by PCR analyses (iv) Conducted functional categorization of F. virguliforme genes by BLAST2GO analyses and validated expression levels of a few selected genes from different functional categories. (v) Identification of putative F. virguliforme virulence genes. dpi, day post inoculation.
For semi-quantitative RT-PCR and quantitative (qRT-PCR) analyses, root samples were harvested at 1, 3, 5 and 10 dpi either following F. virguliforme infection or treatment with water. In RT-PCR, we included 1 dpi in RT-PCR to determine the early changes in gene expression while 10 dpi was considered to represent the late time point ignoring 24 dpi treatment. In each experiment, roots of five plants were pooled for each treatment and frozen in liquid nitrogen and stored at -80°C until further use.
Germinating conidia were grown for 12 h in liquid modified Septoria medium (MSM) [13]. Mycelia were harvested from spores grown in MSM liquid media for two weeks. Total RNAs were isolated from the germinating conidia, mycelia and infected root tissues using TRIzol Reagent (Invitrogen, Carlsbad, CA, U.S.A.). The quality of RNA samples was determined by running RNAs on a denaturing agarose gel.
cDNA library preparation for sequencing transcripts
10 ug total RNAs from early and late time points of F. virguliforme infected soybean root tissues, germinating conidia, and mycelia were used to purify poly (A)+ RNAs using oligo (dT) attached to magnetic beads (Promega, Madison, WI). Poly (A)+ RNAs were fragmented into short sequences in the presence of divalent cations at 94°C for 5 min. RNA samples were reverse transcribed using a cDNA synthesis kit from Illumina (Illumina, Inc. San Diego, CA, U.S.A.).
Sequencing of the F. virguliforme transcripts isolated from infected soybean roots, germinating conidia and mycelia
cDNAs of an individual RNA sample were sequenced in a single lane of the Illumina NGS platform GAII (Illumina, Inc. San Diego, CA, U.S.A.) at the DNA Facility, Iowa State University. The raw sequence reads were generated using the Solexa GA pipeline 1.6 (deposited in GEO; accession GSE86201). The draft F. virguliforme genome sequence (available at http://fvgbrowse.agron.iastate.edu/gb2/gbrowse/fvirguli/ [26]) has been shown to contain 14,845 predicted genes [26]. Reads with quality scores more than Phred 33 were used to map transcripts to the cDNA sequences of predicted F. virguliforme genes using the Bowtie program [27] with the default parameters. The generated SAM (Sequence Alignment/Map) output for each condition was used to extract mapped reads (S1 Table) for corresponding genes using a custom script [28]. The reads per kb per million reads (RPKM) for each gene was calculated according to the formula R = 109C/NL [29], where C is the number of mappable reads aligned onto the exonic sequence of a gene, N is the total number of mappable reads in the sample, and L is the length of the gene. CIMminer was used to generate color-coded Clustered Image Maps (CIMs) ("heat maps") representing "high-dimensional" data sets such as gene expression profiles (http://discover.nci.nih.gov/cimminer/). A heat map was generated in one matrix CIM for the normalized values for each gene after RPKM analysis [30]. TargetP1.1 (http://www.cbs.dtu.dk/services/TargetP/) set for non-plant as organism with no cutoffs, winner-takes-all was used to identify the candidate secreted proteins.
Identification of the candidate F. virguliforme virulence genes
We looked for infection-induced F. virguliforme genes that showed high identity to functionally characterized virulence genes by running the NCBI BlastX program against the non-redundant protein sequences (nr) database (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Semi-quantitative RT-PCR and quantitative real-time PCR (qRT-PCR) analyses
Semi-quantitative PCR of several induced F. virguliforme genes was conducted to validate the expression profiles deciphered from deep sequencing of single RNA samples pooled from three biological experiments. Genes were selected randomly from individual functional categories. Total RNA samples were isolated from the F. virguliforme-infected or water treated etiolated root samples of soybean cv. Essex at 1, 3, 5 and 10 days post inoculation or water treatment by using TRIzol (Invitrogen, Carlsbad, CA, U.S.A.). E. coli DNase I treatment was performed in order to remove any contaminating genomic DNA from the total RNA samples (Invitrogen, Carlsbad, CA, U.S.A.). cDNA was prepared from individual RNA samples using random primers (Invitrogen, Carlsbad, CA, U.S.A.). cDNA samples were used to conduct semi-quantitative RT-PCR at 94°C for 2 min, and then 35 cycles of 94°C for 30 s, 50°C or 55°C for 30 s and 72°C for 30 s then 72°C for 10 min. Primers for RT-PCR are listed in S2 Table. The amplified products were resolved on a 2% (w/v) agarose gel through electrophoresis at 8 V/cm. Normalization of the gene expression for F. virguliforme genes in qRT-PCR was carried out using the FvTox1 (g6924) transcript levels because the transcript levels of FvTox1 do not change during the time-course of infection (S2 Fig). qRT-PCR for a few selected genes were conducted on an iCycler sequence detection system (Bio-Rad; Hercules, CA, U.S.A) using SYBR Green fast qPCR master mix (Bio-RAD; Hercules, CA, U.S.A).
Results
The goal of this study was to identify candidate pathogenicity genes for understanding the mechanisms used by F. virguliforme to cause root necrosis in soybean. Seven day-old etiolated seedlings were inoculated with the F. virguliforme Mont-1 isolate showed symptoms on the 10th day after inoculation (S1 Fig). Infected seedlings showed very mild symptoms until five day post inoculation (dpi). Severe root necrosis was observed by 10 dpi. Therefore, we classified the infected seedlings to two groups: (i) infected roots harvested 3-d and 5-d post inoculation as early infection phase (early infection) and (ii) 10-d and 24-d post inoculation as the late infection phase (late infection) (Fig 1; S1 Fig). We consider that during the early infection phase the pathogen is most likely in the biotrophic phase and turns to the necrotrophic phase at least by 10-d post inoculation. In order to identify most of the F. virguliforme genes induced during the infection process, deep sequencing of individual RNA samples was conducted. Since only a small proportion of the RNA transcripts of infected soybean root tissues is encoded by F. virguliforme genes, deep sequencing of an individual RNA sample collected from three biological replications was conducted in a single lane to detect transcripts of most transcribed genes including those that are expressed at low levels. Since the deep-transcript sequencing was conducted only once, we could not conduct any statistical analysis. We considered only those genes as expressed genes that have shown to contain at least three sequence reads. We validated the transcriptomic data by conducting semi-quantitative RT-PCR and qRT-PCR analyses of 34 and eight F. virguliforme genes, respectively. Furthermore, only F. virguliforme genes showing at least 10-fold or more changes in transcript levels between infected roots and average expression levels of germinating conidia and mycelia were considered as differentially expressed genes (DEGs) for GO term enrichment analyses.
F. virguliforme gene expression patterns among germinating conidia, mycelia and infected soybean roots
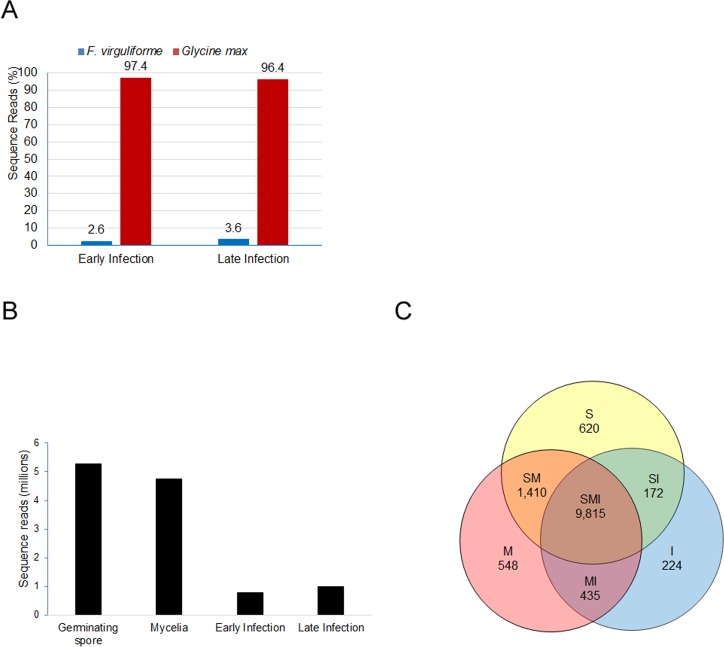
The numbers of F. virguliforme and soybean sequence reads were calculated for early and late infection stages. F. virguligforme transcripts comprised less than 4% of the total transcripts in infected soybean roots (Fig 2A). An increased proportion of F. virguliforme transcripts was detected in late infection as compared to the early infection stage (Fig 2A). The sequence reads of four tissue samples: (i) germinating spores, (ii) mycelia; (iii) early infection, and (iv) late infection are presented in Fig 2B. Of the 14,845 predicted genes, 13,224 F. virguliforme genes were expressed among the three-tissue samples. Of the 13,224 F. virguliforme expressed genes, 9,815 genes were expressed in all samples. Eighty-one percent of F. virguliforme (12,017 genes) were expressed in germinating spores (Table 1). Among these genes, 620 genes were specifically expressed in germinating conidia (Table 1; Fig 2C). In mycelia, 82% of the predicted F. virguliforme genes (12,208 genes) were expressed, of which 548 (Fig 2C) are unique to mycelia (Table 1; Fig 2C). Of the 14,845 predicted F. virguliforme genes, 10,626 (72%) were expressed in infected tissues, of which 224 were expressed only in infected roots. The number of expressed genes was increased during infection from 64% of the predicted F. virguliforme genes during early infection to 70% in the late infection stage (Table 1). Among the 14,845 predicted F. virguliforme genes, 2,578 were expressed only in germinating spores and mycelia and are most likely involved in fungal growth and development (Fig 2C).
Fig 2. Transcript profiles of the F. virguliforme genes in various tissues.
(A). Percentage sequence reads of F. virguliforme and soybean genes during infection. (B). Sequence reads of F. virguliforme genes among various tissue samples. (C). Venn diagram showing unique and common F. virguliforme genes among germinating spore, mycelia and infected soybean roots. S, genes expressed only in germinating conidia; M, genes expressed only in mycelia; I, genes expressed in infected roots; SM, genes expressed in both spores and mycelia; SI, genes expressed in both spores and infected roots; MI, genes expressed in mycelia and infected roots; SMI, genes expressed in spores, mycelia and infected roots. The genes with a minimum of three reads were considered in developing the Venn diagram. The 984 genes having sequence reads below three were not considered for the Venn diagram.
Table 1. F. virguliforme genes expressed in germinating conidia, mycelia and infected soybean root tissues.
| Sample | Number of genes | % of genes expressed | Number of unique genes |
|---|---|---|---|
| Germinating conidia | 12,017 | 81 | 620 |
| Mycelia | 12,208 | 82 | 548 |
| Infection | 10,626 | 72 | 224 |
| (a) Early infection | 9,545 | 64 | 18 |
| (b) Late infection | 10,344 | 70 | 79 |
Identification of differentially expressed F. virguliforme genes during infection
To quantify the expression patterns of F. virguliforme genes, a digital measure of relative gene expression was applied [31]. Typically, large genes have more reads than small genes even if they have the same transcript levels. To avoid this gene size-associated read number bias, the RPKM value for each gene was calculated as described in materials and methods. Fold changes in gene expression during early and late infection stages was calculated by comparing RPKM values of individual genes in infected roots with the average RPKM values of the corresponding genes in spores and mycelia. Of the 10,626 genes expressed in infected roots, 1,886 genes showed at least a 2-fold increase in expression levels when compared to germinating conidia and mycelia (Table 2). Of these 1,886 genes, 80 showed over 50-fold, and 33 showed over 100-fold induction in infected roots as compared to their corresponding average transcript levels in germinating conidia and mycelia (Table 2). In infected soybean roots, expression of 4,204 genes, transcribed in germinating spores and mycelia, were suppressed during infection (Table 2).
Table 2. Up or down regulated F. virguliforme genes during infection compared to germinating conidia and mycelia.
| Fold | Number of genes up regulated | Number of genes down regulated |
|---|---|---|
| 2 | 1,886 | 4,204 |
| 5 | 665 | 1,062 |
| 10 | 369 | 490 |
| 50 | 80 | 123 |
| 100 | 33 | 79 |
| 250 | 8 | 44 |
| >500 | 0 | 20 |
Semi quantitative RT-PCR and qRT-PCR validation
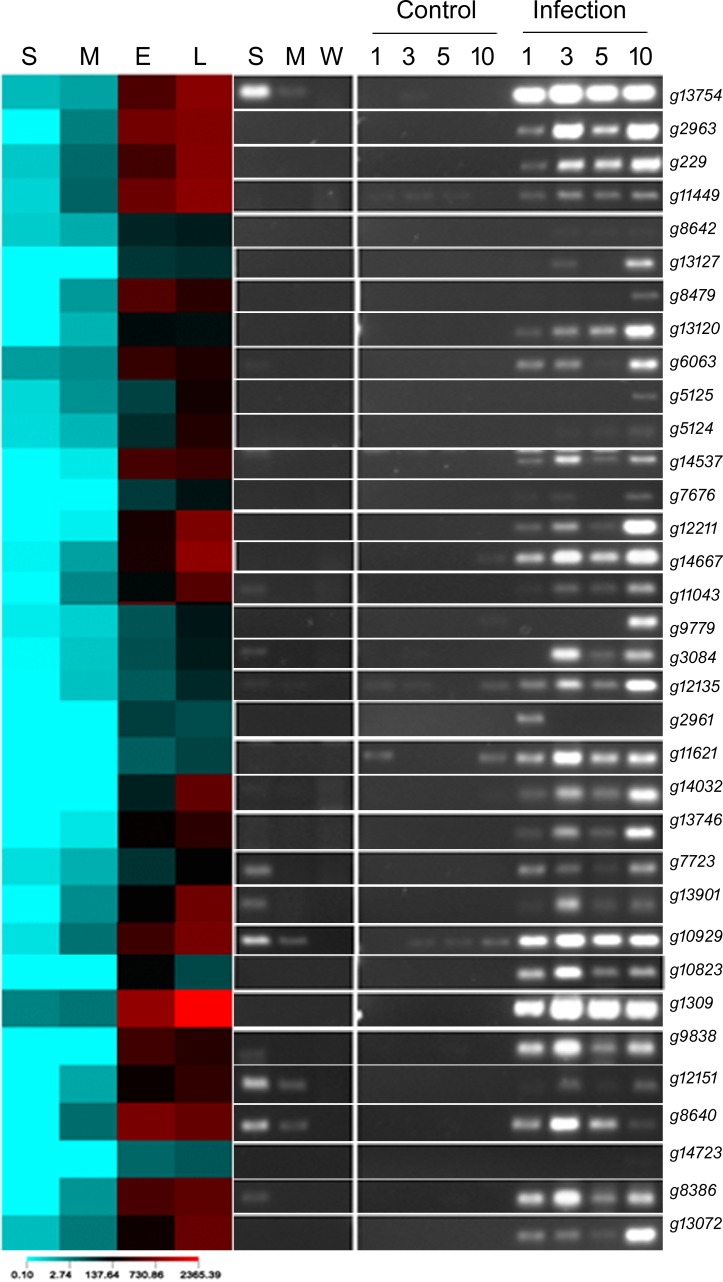
To validate the expression data gathered by deep-sequencing of transcripts, 34 genes with ≥10-fold average induction in infected roots as compared to the corresponding averages in germinating spores and mycelia were selected to conduct semi-quantitative RT-PCR (S2 Table). Expression patterns revealed by RT-PCR analyses were consistent with the digital expression data of 30 of the 34 selected genes (Fig 3). Four failed to show expression in RT-PCR, which could be due to primer- or PCR-condition-related issues. For 11 F. virguliforme genes, we observed amplification of transcripts collected from germinating spores in RT-PCR. The RPKM values of these 11 genes were very low in germinating spores (Fig 3).
Fig 3. Semi-quantitative RT-PCR of selected genes from various functional categories of genes.
On the left, heat map of transcript levels of 34 randomly selected genes in RPKM values is shown. A few genes with unknown functions were also included. On the right, semi-quantitative RT-PCR data of the selected genes are presented. S; germinating conidia, M; mycelia, E; early stage of infection, L; late stage of infection, W; negative control; Control, roots of etiolated soybean (Essex) seedlings were treated with water for 1, 3, 5, and 10 days prior to harvesting; Infection, roots of etiolated soybean (Essex) seedlings were infected with F. virguliforme (107 spores/mL) for 1, 3, 5, and 10 days prior to harvesting.
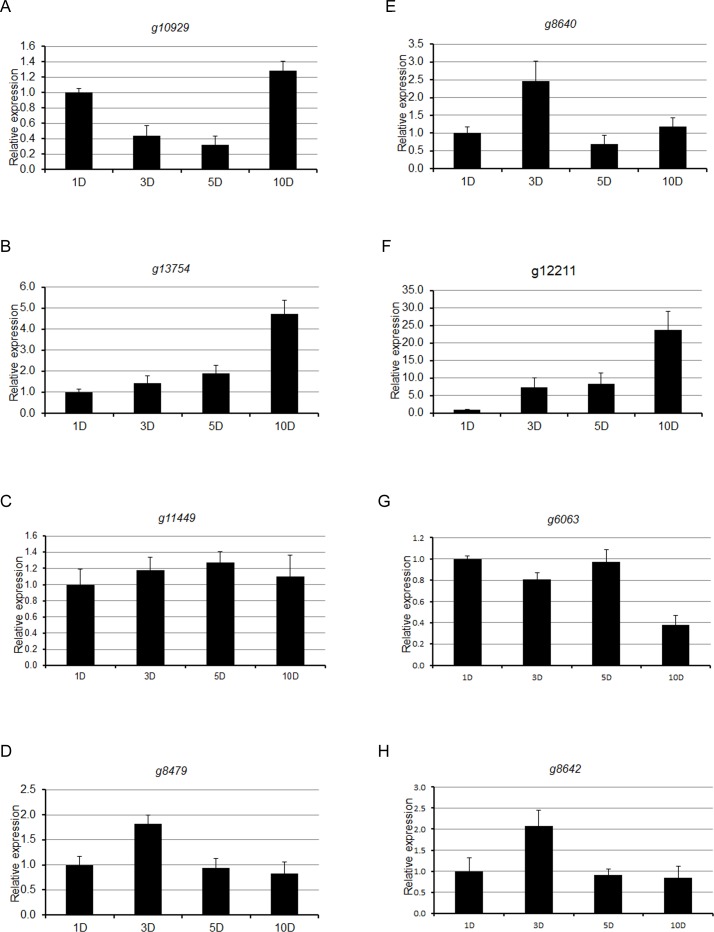
To validate the semi-quantitative expression data gathered by RT-PCR, qRT-PCR was conducted on a few randomly selected expressed genes (Fig 4), RT-PCR data of which are shown in Fig 3. Overall, expression pattern of F. virguliforme genes determined by RT-PCR was in agreement with the expression pattern of the selected genes in qRT-PCR analysis. Our RT-PCR and qPCR data validated the transcriptomic data gathered from single RNA samples, pooled from three biological replications (Figs 3 and 4).
Fig 4. Quantitative RT-PCR of a few selected genes, evaluated in RT-PCR analyses.
Quantitative RT-PCR (qRT-PCR) was performed for a few selected virulence genes using gene specific primers to validate semi-quantitative RT-PCR data. (A) g10929, (B) g13754, (C) g11449, (D) g8479, (E) g8640, (F) g12211, (G) g6063 and (H) g8642. The Fusarium GAPDH gene was used as an internal control. Relative gene expression levels were determined in proportions to levels of corresponding levels of 1 d F. virguliforme-infected samples, which are defined as 1. Error bars represent standard deviation (SD) of two independent biological replicates (n = 6).
Blast2GO Functional categorization of induced F. virguliforme genes
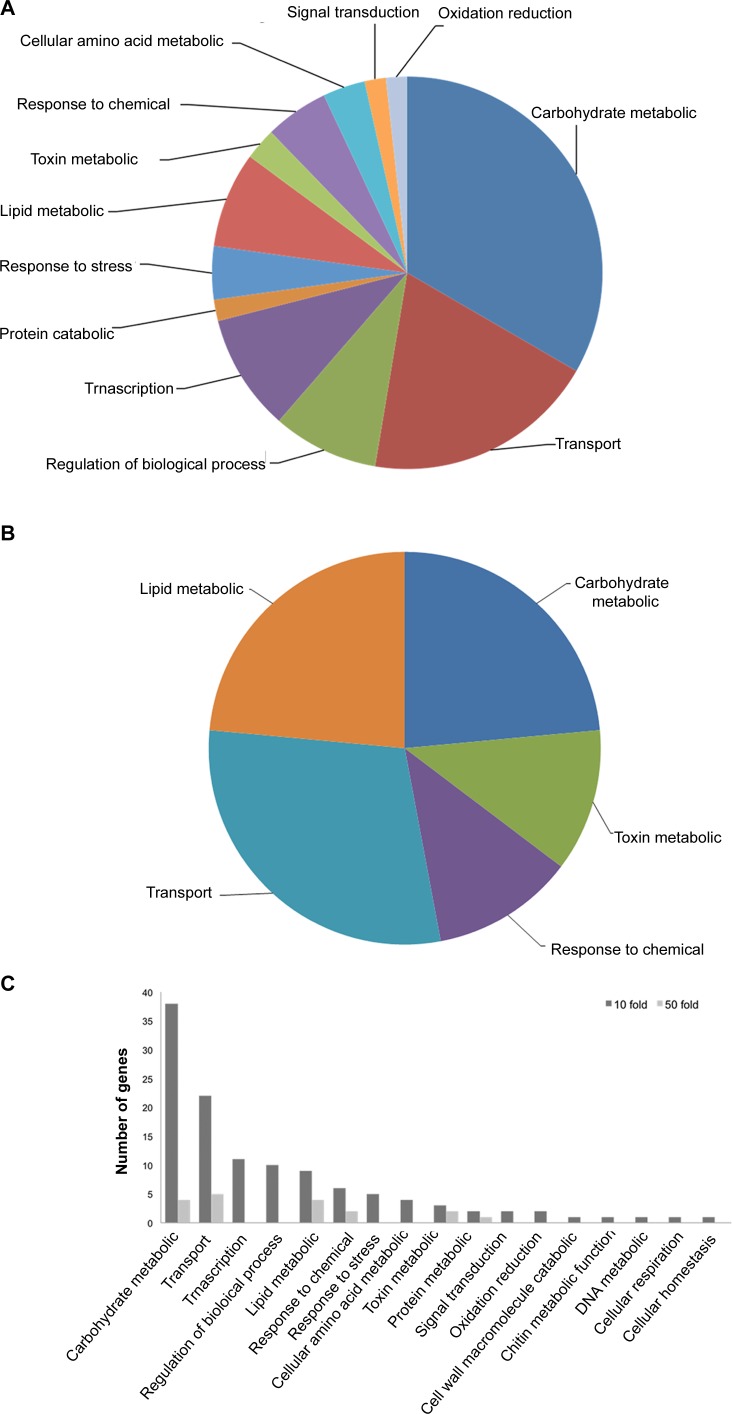
Gene ontology associates molecular functions, cellular components and biological processes to a gene if it shows high sequence identity to previously characterized genes. We used Blast2GO to classify the F. virguliforme infection-induced genes based on their biological processes and molecular function [32]. F. virguliforme genes with ≥10 and ≥50 fold increase in expression in infected roots as compared to germinating conidia and mycelia were classified for biological processes and molecular functions to understand the overall basic mechanisms of virulence for this fungal pathogen. Considering the availability of transcript sequences for only a single pooled sample of RNAs, genes with lower than 10 fold changes in expression were not considered in this study. Among the 369 genes with ≥10-fold up-regulation, 273 genes (79%) could be classified by Blast2GO analysis into 17 biological process groups. The most prominent categories of genes are involved in: (i) carbohydrate metabolic processes, (ii) transport, (iii) transcription, (iv) regulation of biological processes, and (v) lipid metabolic processes (Fig 5A and 5C).
Fig 5. Categories of F. virguliforme genes based on BLAST2GO analyses for biological processes.
A, Categories of F. virguliforme genes with 10-fold induction. B, Categories of F. virguliforme genes with 50-fold induction. C, Percentage of genes distributed under different functional categories for biological process are presented as bar diagram for F. virguliforme genes with 10 and 50 fold induction over mycelia and germinating conidia.
Of the 80 genes with ≥50-fold induction, 56 genes (79%) could be classified. These genes were classified into five GO categories: (i) carbohydrate metabolism, (ii) transport, (iii) lipid metabolism processes, (iv) toxin metabolism, and (v) response to chemicals (Fig 5B and 5C).
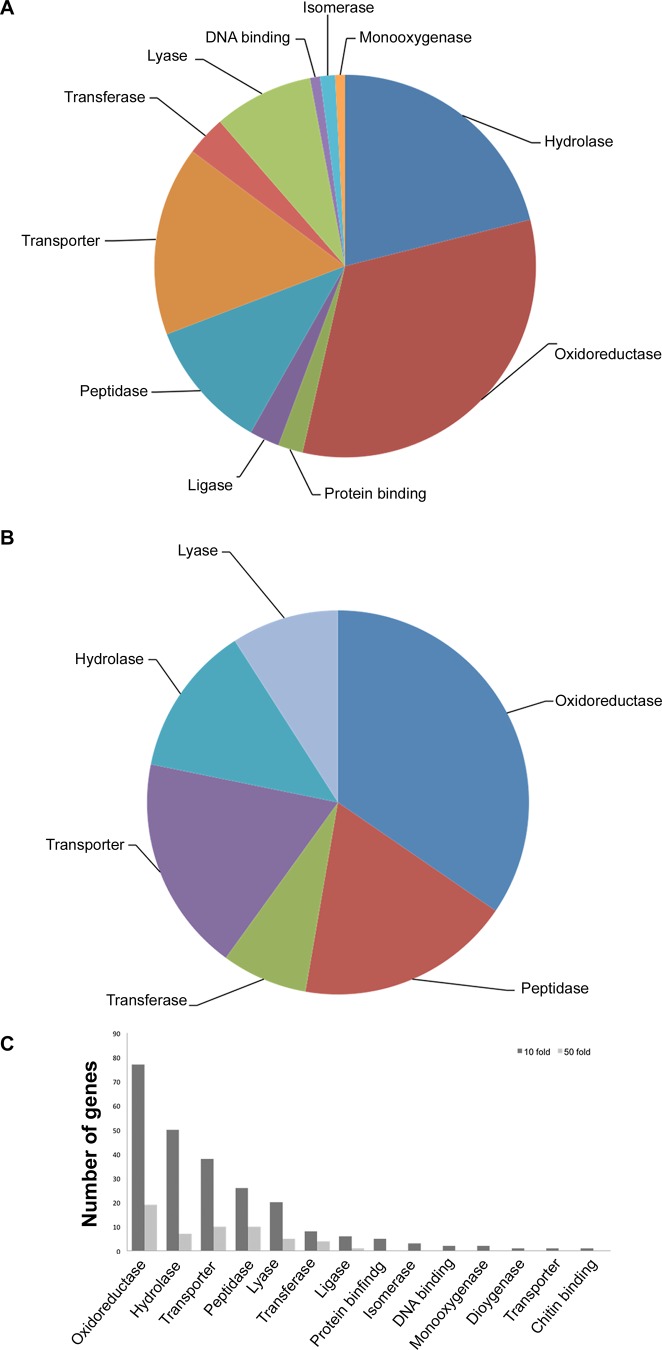
GO molecular function term enrichment analysis was also conducted for the 369 F. virguliforme genes with ≥10-fold up-regulation. This led to the classification of 273 genes into 11 categories. The largest GO category includes genes encoding enzymes with oxidoreductase and hydrolase activities (Fig 6A and 6C). On the other hand, GO annotation for molecular functions of F. virguliforme genes with ≥50-fold induction in infected tissues revealed five classes of genes, with most genes encoding enzymes with oxidoreductase, peptidases and hydrolase activities, and transporters (Fig 6B and 6C).
Fig 6. Categories of F. virguliforme genes based on BLAST2GO analyses for molecular functions.
A, Categories of F. virguliforme genes with 10-fold induction. B, Categories of F. virguliforme genes with 50-fold induction. C, Percentage of genes distributed under different functional categories for molecular functions are presented as bar diagram for F. virguliforme genes with 10 and 50 fold induction over mycelia and germinating conidia.
Genes with unknown function induced during infection
Ninety-six F. virguliforme genes with ≥10-fold increased expression could not be assigned to a functional category by Blast2GO analysis (S3 Table). Of these 96 genes, 64 were annotated by conducting BLASTX search. One of the genes (g9838), showed high identity to a stress responsive a/b barrel domain containing protein identified in Aspergillus niger (XP_003188860). Three polyketide synthetic genes including: snoal-like polyketide cyclase protein (g13126), snoal-like polyketide cyclase family protein (g14688) and lovastatin-like diketide synthase gene (g14617) were identified. Polyketides such as the T-toxin produced by Cochliobolus heterostrophus [33, 34] have been shown to be virulence factors and host specific toxins.
We also identified four genes (g12373, g12372, g14510, and g5504) encoding CFEM domain containing proteins. CFEM domains are unique to fungi and are found in large numbers in pathogenic ascomycetes relative to non-pathogenic ascomycetes [35]. We failed to find homologous functionally characterized genes for 32 F. virguliforme genes that showed ≥10-fold induction during infection. These genes could encode species-specific factors, some of which could be involved in virulence.
Identification of candidate secretory proteins
The secretory peptides including virulence factors and digestive enzymes are used by fungi to attack plants. Therefore, secreted proteins are considered as candidate virulence factors involved in suppression of defense mechanisms (e.g. effector proteins), degradation of host proteins (e.g. peptidases), degradation of antifungal compounds (e.g. glyceollin detoxification enzymes) or degradation of host tissues (e.g. hydrolytic enzymes). Seventy-three of the 369 F. virguliforme genes with ≥10-fold induction in infected roots were shown to encode putative secretory proteins (S4 Table). Blast2Go analysis of these proteins for cellular components classified these genes mainly into extracellular and membrane-associated proteins. We investigated the early and late infection-specific genes encoding candidate secretory peptides/proteins (Table 3). Secretory genes induced during early infection stage (S1 Fig) encode proteins that are unlikely have hydrolytic activities; whereas, in the late infection stage many induced genes encode enzymes that have hydrolytic activities (Table 3).
Table 3. Differentially induced F. virguliforme genes that are predicted to encode secretory proteins during early and late infection stages.
| Gene name | Sequence description | Fold Change | |||
| 1Early | 1Late | 2Early/Late | 3R/C values | ||
| g11354 | Hypothetical protein AFLA_059330 | 82.7 | 7.7 | 10.7 | 2 |
| g13527 | Extracellular matrix protein precursor | 5.2 | 1 | 5.2 | 1 |
| g10823 | Unknown | 115.3 | 33.8 | 3.4 | 2 |
| g9652 | Triacylglycerol lipase | 16.7 | 5.5 | 3 | 1 |
| g35 | Unknown | 24.2 | 8.3 | 2.9 | 1 |
| g13651 | Acetolactate synthas | 5 | 1.8 | 2.9 | 3 |
| Gene name | Sequence description | Fold Change | 3R/C values | ||
| 1Early | 1Late | 2Late/Early | |||
| g4306 | Pectate lyase c | 0.9 | 33.2 | 38.1 | 1 |
| g14206 | Glycoside hydrolase family 43 protein | 0.8 | 12.7 | 15.1 | 1 |
| g13672 | Cell wall glycosyl hydrolase | 0.9 | 11.6 | 12.5 | 1 |
| g13332 | Lipolytic protein g-d-s-l family | 1 | 12.4 | 12.4 | 3 |
| g10242 | Endoglucanase iv precursor | 0.9 | 10.7 | 12.1 | 2 |
| g10829 | Expansin-like protein | 1 | 11.7 | 11.7 | 1 |
| g14123 | Pectate lyase | 1 | 11.1 | 11.1 | 1 |
| g11732 | Glycoside hydrolase family 10 protein | 0.9 | 6.3 | 6.9 | 1 |
| g12142 | Glycoside hydrolase family 5 protein | 2.1 | 11.6 | 5.4 | 4 |
| g2034 | Hypothetical protein NECHADRAFT_99942 | 1 | 5.4 | 5.4 | 5 |
| g10874 | Sugar transporter stl1 | 1.7 | 7.3 | 4.4 | 4 |
| g3939 | Para-nitrobenzyl esterase | 1 | 3.5 | 3.5 | 1 |
| g13138 | Glycoside hydrolase family 115 protein | 1.3 | 3.9 | 3 | 1 |
| g12597 | Cytochrome p450 monooxygenase | 1 | 2.9 | 2.9 | 2 |
| g225 | Xylosidase: arabinofuranosidase | 2.4 | 6.5 | 2.8 | 1 |
| g3691 | Nitrate reductase | 2.3 | 6 | 2.6 | 2 |
| g218 | Exopolygalacturonase | 2.1 | 4.4 | 2.1 | 1 |
| g14347 | Saponin hydrolase precursor | 2.4 | 4.8 | 2 | 1 |
| g10894 | Hexose carrier protein | 1.7 | 3.4 | 2 | 1 |
| g228 | Hypothetical protein FOXB_14459 | 1.7 | 3.4 | 2 | 1 |
1Fold changes in expression levels during early and late stages are calculated against the mean RPKM values of individual genes in germinating conidia and mycelia.
2Minimum of two-fold differences in expression levels between early and late infection stages are presented. Rest of the genes encoding putative secretory peptides or proteins can be found in S4 Table.
3RC values are confidence scores for secretory protein prediction. RC value 1 indicates highest confidence; while 5 the lowest.
Identification of candidate virulence genes
Virulence genes are involved in disease development, but are not essential for completing the pathogen's life cycle [36]. In order to identify virulence genes, the sequences of the 369 genes with ≥10-fold induction during root infection were investigated to determine if any of them show high identity to any genes with known virulence functions. Twenty-seven candidate virulence genes were identified from this search (S5 Table).
Genes expressed during infection only may also be candidate virulence genes. Two-hundred and twenty-four F. virguliforme genes were expressed only in the infected soybean roots. Some of these genes showed very low RPKM values. However, 83 of the 224 genes showed high transcript levels (more than 20 reads in infected tissues) and could be considered candidate virulence genes. Blast2GO analysis for possible molecular functions of these genes showed that a majority of the 83 induced genes encode the pectate lyases and glucoside hydrolyases involved in cell wall degradation (S6 Table).
Discussion
To better understand how F. virguliforme causes root necrosis in soybean, we conducted a comparative transcriptomic analysis to identify genes that are induced during infection of soybean roots. F. virguliforme is considered to be a hemi-biotrophic fungal pathogen with an initial biotrophic phase [8–10, 37]. To provide molecular evidence supporting this hypothesis, we developed a model system with 7-day old etiolated seedlings (Fig 1; S1 Fig; [11]). This system provides a uniform infection of root tissue by the pathogen. We observed that over 10,000 (72%) F. virguliforme genes are induced during infection as opposed to only few hundreds in our initial study of the infected roots of light-grown soybean seedlings (B.B. Sahu and M.K. Bhattacharyya, unpublished). Less than 4% of the transcripts from infected roots of etiolated soybean seedlings are F. virguliforme-specific (Fig 2). Our deep sequencing approach identified 10,626 (72%) of the 14,845 predicted F. virguliforme genes that were expressed during infection. Of these genes, 224 were expressed only in infected roots based on their absence in germinating conidia and mycelia. In addition to classifying the F. virguliforme genes based on their identities with annotated genes, we identified genes encoding putative secretory peptides/protein that may play a role in virulence. We identified 27 candidate virulence genes based on their homologies to other functionally characterized virulence genes (S5 Table).
Genes induced during early infection stage
Over all, relatively few of the infection-inducible genes showed higher expression levels in the early infection stage as compared to that in the late infection stage. These genes may be important in establishment of the infection process. Of these genes, g9652 (Table 3) showed high identity to a triacylglycerol lipase gene, a virulence factor gene in Mycobacteria tuberculosis [38, 39]. In pathogenic fungi, it has been suggested that lipases in general are involved in the penetration of the waxy cuticle [40]. It has been shown that a lipase (FGL1) determines the virulence of the wheat pathogen, F. gramminerum [41].
F. virguliforme g13527 protein (Table 3) shows high identity to an extracellular matrix protein with GPI anchored domain and a transmembrane domain found in epidermal growth factors. Extracellular matrix proteins may be involved in cell adhesion and are secreted by fungi. GPIs are membrane or cell wall proteins. GPI7 in F. graminearium governs the virulence function [42].
Phytopathogenic fungi relay signals upon sensing the cuticle. CHIP2 and CHIP3 encoding hard surface inducible proteins are induced in Colletotrichum gloeosporioide following contact with the cuticle [43]. Expression of the F. virguliforme homologue (g12834) of CHIP2 during early stage infection may suggest its role in surface sensing and signaling for tissue entry (S3 Table).
Fungal pathogens use secreted extracellular enzymes to degrade structural barriers to penetrate host cells [44, 45]. Upon successful entry into the host plant, the fungi must resist plant produced antimicrobial compounds [46]. Fungi employ enzymes with oxidative-reductive properties to degrade antimicrobial compounds such as phytoalexins. In response to the pathogenic fungal attack, plants secrete polyamines [47] and phytoalexins [48] to defend against invading pathogens. Consistent with this, F. virguliforme genes encoding enzymes involved in detoxification of plant defense molecules show high expression during the early infection stages with declining transcript levels as infection progresses. Glyceollin is a soybean phytoalexin produced in response to microbial invaders [49]. The expression of the Fusarium oxysporum f. sp. pisi pisatin demethylase has been documented to detoxify pisatin, a pea phytoalexin, for establishing compatible interaction [50]. The F. virguliforme g13127 gene showing high identity to pisatin demethylase was induced to a high level during infection (S5 Table). Most likely g13127 encodes glyceollin demethylase and is involved in glyceollin metabolism. Along with phytoalexins, plants produce aromatic defense compounds that are toxic to fungi. The F. virguliforme g8644 gene encodes dienelactone hydrolase (S5 Table). The enzyme is involved in the β-ketoadipate pathway that catabolizes aromatic compounds into acetyl co-A and succinyl co-A [51–53].
Expression of toxin genes for foliar SDS development
As the fungus switches to the necrotrophic phase it produces toxins and necrosis inducing factors and starts killing plant cells for rapid nutrient uptake and growth. Trichothecene is a mycotoxin that severely impacts protein synthesis by inhibiting either the initiation or the elongation process of translation by interfering with peptidyl transferase activity [54]. Hydroxylation at carbon-2 (C-2) is the first committed intermediate of the trichodiene pathway and is mediated by the cytochrome P450 monooxygenase [55]. Expression of multiple cytochrome p450 related genes (g5125, g9837, g9839, g9830) increased in this pathogen during the different stages of infection (S1 and S5 Tables). In addition to the role in the trichodiene pathway, cytochrome P450 enzymes are considered to be involved in fungal adaptations [56, 57]. The related cytochrome P450 genes are arranged in a group in the F. virguliforme genome. This grouped arrangement suggests that these genes may be coordinately expressed and operate as a cassette.
To date at least 12 potential toxins have been identified in F. virguliforme including proteinacious FvTox1, non-ribosomally produced peptides, polyketides and effector proteins [11, 12, 14]. We observed increased expression of necrosis inducing peptide 2 (FvNIP2, g12151) during infection of F. virguliforme, thus identifying it as a potential F. virguliforme toxin (S1 and S5 Tables; [14]). We did not find increased expression of FvTox1 (g6924) in infected root. FvTox1 has been shown to be important for foliar SDS symptom development [11, 17]. This gene is however constitutively expressed in the fugal mycelia and germinating spores (S1 Table).
Induction of genes for root necrosis during late infection phase
The necrotrophic phase in infected soybean roots presumably begins with the accumulation of hydrolytic and glycosyl hydrolases to cause root necrosis. In our model system, roots of etiolated seedlings showed very little visible signs of root necrosis at early stage of infection; however, in the late infection stage root rotting due to necrosis was observed (S1 Fig). Among the genes encoding candidate virulence peptides/proteins with secretory signals (S3 Table), only a few are strongly induced in the early infection stage; whereas, a large number of genes encoding secretory proteins are induced during late infection stage (Table 3). The pattern of gene expression completely changed as F. virguliforme entered the necrotrophic phase. A large number of pectate lyases with secretary signals involved in degradation of the cell wall component pectin are induced (Table 3). Among the infection-induced genes, a majority showed increasing levels of expression during the late stage of infection. The genes with enhanced expression during late stage infection include functional categories such as localization, cell hydrolysis, oxidation-reduction, membrane transport, many degradative enzymes such as pectate lyases, glucoside hydrolases, and elastinolytic metalloproteinase (Figs 5A, 6A and 6B; S4 and S6 Tables).
Investigation of the infection-induced (i) genes encoding putative secretory peptides/proteins (Table 3; S4 Table), (ii) genes specifically induced during infection (S6 Table) or (iii) infection-induced genes with homology to functionally characterized virulence genes (S5 Table), strongly suggest that during the late infection stage a large number of genes encoding cell wall degrading enzymes are induced. Expression of genes encoding degradative enzymes is common in other necrotrophic fungi. Necrotrophic fungi have also been shown to use a wide array of cell wall degrading enzymes to breakdown host tissues for penetration. Taken together, this suggests that degradative enzymes may play a major role in changing the fungus from its biotrophic phase to the necrotrophic phase. In the case of the pea pathogen Nectria haematococca, pathogenesis associated protein PEP2 has been shown to play a role in infection [58]. PEP2 is possibly involved in degrading the extracellular matrix proteins, and cleavage of the cell surface receptors. The F. virguliforme gene g8640, with high identity to PEP2 and induced during infection of soybean roots, may be involved in degradation of soybean root tissues (Fig 6; S1 and S5 Tables).
Plants cell walls are composed of complex carbohydrate and degradation of these complex walls requires specialized carbohydrate degrading enzymes. Hemicellulose constitutes a large fraction of the cell wall and contains xyloglucan, glucuronoarabinoxylan, mannan, galactan, arabinan, mixed-linked glucan, and glucuronoarabinoxylan [59]. During infection, necrotrophic pathogens degrade these building blocks by increasing the secretion of cell wall degrading enzymes such as pectate lyase and polygalacturonases [60–62].
Many cell wall degrading enzymes were found to be induced during infection with a dramatic increase in expression during the late infection phase, presumably to degrade the plant cells when the fungus becomes necrotrophic. In F. virguliforme, two pectate lyase genes (g7676 and g11622), one exopolygalacturonase gene (g4533), one endo-beta-xylanase (g11621), and one cellobiohydrolase ii (g14515) gene were induced during late infection stage (S1, S4 and S6 Tables). Endo-β-1,4-xylanase has been shown to be important for infection by Fusarium oxysporum f. sp. lycopersici [63].
F. virguliforme gene g14032 encoding a putative elastinolytic metalloproteinase Mep is highly induced during late infection (S5 Table). Mep proteins belong to a family of proteins likely involved in degradation of plant tissues [64]. Increased accumulation of g14032 transcripts during late stage infection suggest that it may be involved in transitioning the pathogen from its biotrophic to the necrotrophic phase.
As the carbohydrates of the cell walls are degraded, the fungi encounter cell wall associated peptides that could be toxic. Following infection of soybean, F. virguliforme, genes g8474, and g10809 encoding carboxypeptidases, and g12211 and g12135 encoding leucyl aminopeptidases were induced (S1 and S5 Tables). Carboxypeptidases are specific proteases which hydrolyze the peptide bond of an amino acid at the C-terminus [65], whereas the leucyl aminopeptidases catalyze the hydrolysis of the leucine residues at the N-terminus. These infection-induced F. virguliforme genes may be involved in digesting the cell-wall associated peptides.
After successful entry the fungal pathogens travel through the intra and intercellular passages and come in contact with the structural proteins and enzymes important for host defense. Enzymes such as subtilases and alkaline proteinases have been shown to degrade defensive enzymes [66]. Increased expression of F. virguliforme gene g14667 encoding subtilase in the infected tissues especially during late infection suggests its possible involvement in nullifying host defense-related enzymes (S5 Table).
Conclusions
In this comparative transcriptomic study, we were able to identify putative virulence factors by the following approaches: (i) investigation of the functions of infection-induced genes based on sequence homology using Blast2GO analyses; (ii) identification of secretory proteins and their GO annotation for possible functions; (iii) search for candidate virulence genes through sequence homology search with functionally characterized virulence genes; and (iv) studying the genes that are only induced in infected roots. Expression of several infection-induced genes encoding enzymes with oxidation-reduction properties for degradation of antimicrobial compounds such as the phytoalexin glyceollin could be an important virulence mechanism in this pathogen during early biotrophic phase. Induced expression hydrolytic and cell wall degrading enzyme genes (pectate lyase, glycoside hydrolase, polygalacturonases) in F. virguliforme during soybean root infection parallels the similar observations made recently in the transition of multiple Zymoseptoria tritici genotypes [67]. Expression of a large number of genes encoding enzymes with catalytic and hydrolytic activities during late infection stage suggests that cell wall degradation is involved in establishing the necrotrophic phase in this pathogen. This study suggests that enzymes with hydrolytic and catalytic activities play an important role in the transitioning the pathogen from biotrophic to necrotrophic phase.
Supporting Information
(TIF)
Relative expression of FvTox1 was determined by conducting qRT-PCR at 1-d, 3-d, 5-d and 10-d post inoculation with F. virguliforme Mont-1 conidia. Relative gene expression levels was compared with the expression level at 1-d post F. virguliforme inoculation. Constitutively expressed F. virguliforme GAPDH (g2019) (S2 Table) was used for normalization of the FvTox1 expression levels. Data are means and standard deviations (SD) of two independent biological replications with three technical replications (n = 6).
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(XLSX)
Acknowledgments
We thank the DNA Facility, Iowa State University for sequencing the RNA samples using Illumina sequencing platform (GAII). This work was supported by Iowa Soybean Association and the National Institute of Food and Agriculture (NIFA), United States Department of Agriculture (Grant no. 2013-68004-20374). We thank David Grant for kindly reviewing this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Iowa Soybean Association and the National Institute of Food and Agriculture (NIFA), United States Department of Agriculture (Grant no. 2013-68004-20374).
References
- 1.Leandro LFS, Robertson AE, Mueller DS, Yang XB. Climatic and environmental trends observed during epidemic and non-epidemic years of soybean sudden death syndrome in Iowa. Plant Health Progress. 2013 [Google Scholar]
- 2.Hirrel MC. Sudden death syndrome of soybean: New insight into its development. Pages 95–104 in Proceedings of Soybean Seed Conference, 16th Soybean Research Conference, Chicago, December 9–10, 1986. American Seed Trade Association, Alexandria, VA, U.S.A.
- 3.Anderson TR, Tenuta AU. First report of Fusarium solani f. sp. glycines causing sudden death syndrome of soybean in Canada. Plant Disease. 1998; 82: 448. [DOI] [PubMed] [Google Scholar]
- 4.Hartman GL, Chang HX, Leandro LF. Research advances and management of soybean sudden death syndrome. Crop Prot. 2015; 73: 60–66. [Google Scholar]
- 5.Wrather J KS. Soybean disease loss estimates for the United States, 1996–2010 Delta Research Center: agriculture experiment station. University of Missouri, College of Agriculture, Food and Natural Resources; 2011. [Google Scholar]
- 6.Rupe JC. Frequency and pathogenicity of Fusarium solani recovered from soybean with sudden death syndrome. Plant Dis. 1989; 73: 581–584. [Google Scholar]
- 7.Aoki T, O'Donnell K, Homma Y, Lattanzi AR. Sudden-death syndrome of soybean is caused by two morphologically and phylogenetically distinct species within the Fusarium solani species complex—F. virguliforme in North America and F. tucumaniae in South America. Mycologia. 2003; 95: 660–684. [PubMed] [Google Scholar]
- 8.Iqbal M, Yaegashi S, Ahsan R, Shopinski K, Lightfoot D. Root response to Fusarium solani f. sp. glycines: temporal accumulation of transcripts in partially resistant and susceptible soybean. Theor Appl Genet. 2005; 110: 1429–1438. 10.1007/s00122-005-1969-9 [DOI] [PubMed] [Google Scholar]
- 9.Yuan J, Zhu M, Lightfoot D, Iqbal MJ, Yang J, Meksem K. In silico comparison of transcript abundances during Arabidopsis thaliana and Glycine max resistance to Fusarium virguliforme. BMC Genomics. 2008; 9 (Suppl 2): S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perfect SE, Green JR. Infection structures of biotrophic and hemibiotrophic fungal plant pathogens. Mol Plant Pathol. 2001; 2: 101–108. [DOI] [PubMed] [Google Scholar]
- 11.Brar HK, Swaminathan S, Bhattacharyya MK. The Fusarium virguliforme toxin FvTox1 causes foliar sudden death syndrome-like symptoms in soybean. Mol Plant-Microbe Interact. 2011; 24: 1179–1188. 10.1094/MPMI-12-10-0285 [DOI] [PubMed] [Google Scholar]
- 12.Abeysekara NS, Bhattacharyya MK. Analyses of the xylem sap proteomes identified candidate Fusarium virguliforme proteinacious toxins. PLoS ONE. 2014; 9: e93667 10.1371/journal.pone.0093667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin H, Hartman GL, Nickell CD, JM W. Characterization and purification of a phytotoxin produced by Fusarium solani, the causal agent of soybean sudden death syndrome. Phytopathol. 1996; 86: 277–282. [Google Scholar]
- 14.Chang H-X, Domier LL, Radwan O, Yendrek CR, Hudson ME, Hartman GL. Identification of multiple phytotoxins produced by Fusarium virguliforme including a phytotoxic effector (FvNIS1) associated with sudden death syndrome foliar symptoms. Mol Plant-Microbe Interact. 2016; 29: 96–108. 10.1094/MPMI-09-15-0219-R [DOI] [PubMed] [Google Scholar]
- 15.Ji J, Scott MP, Bhattacharyya MK. Light is essential for degradation of ribulose-1,5-bisphosphate carboxylase-oxygenase large subunit during sudden death syndrome development in soybean. Plant Biol. 2006; 8: 597–605. 10.1055/s-2006-924175 [DOI] [PubMed] [Google Scholar]
- 16.Brar HK, Bhattacharyya MK. Expression of a single-chain variable-fragment antibody against a Fusarium virguliforme toxin peptide enhances tolerance to sudden death syndrome in transgenic soybean plants. Mol Plant-Microbe Interact. 2012; 25: 817–824. 10.1094/MPMI-12-11-0317 [DOI] [PubMed] [Google Scholar]
- 17.Pudake R, Swaminathan S, Sahu B, Leandro L, Bhattacharyya M. Investigation of the Fusarium virguliforme fvtox1 mutants revealed that the FvTox1 toxin is involved in foliar sudden death syndrome development in soybean. Curr Genet. 2013; 59: 107–117. 10.1007/s00294-013-0392-z [DOI] [PubMed] [Google Scholar]
- 18.Iqbal MJ, Meksem K, Njiti VN, Kassem MA, Lightfoot DA. Microsatellite markers identify three additional quantitative trait loci for resistance to soybean sudden-death syndrome (SDS) in Essex × Forrest RILs. Theor Appl Genet. 2001; 102: 187–192. [Google Scholar]
- 19.Luckew A, Leandro LF, Bhattacharyya MK, Nordman DJ, Lightfoot DA, Cianzio SR. Usefulness of 10 genomic regions in soyben associated with sudden death syndrome resistance. Theor Appl Genet. 2013; 126: 2391–2403. 10.1007/s00122-013-2143-4 [DOI] [PubMed] [Google Scholar]
- 20.Wen Z, Tan R, Yuan J, Bales C, Du W, Zhang S, et al. Genome-wide association mapping of quantitative resistance to sudden death syndrome in soybean. BMC Genomics. 2014; 15: 809 10.1186/1471-2164-15-809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson J, Akond M, Kassem MA, Meksem K, Kantartzi SK. Quantitative trait loci underlying resistance to sudden death syndrome (SDS) in MD96-5722 by ‘Spencer’ recombinant inbred line population of soybean. 3 Biotech. 2015; 5: 203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Farias Neto AL, Hartman GL, Pedersen WL, Li S, Bollero GA, Diers BW. Irrigation and inoculation treatments that increase the severity of soybean sudden death syndrome in the field. Crop Sci. 2006; 46: 2547–2554. [Google Scholar]
- 23.Navi SS, and Yang XB. Foliar symptom expression in association with early infection and xylem colonization by Fusarium virguliforme (formerly F. solani f. sp. glycines), the causal agent of soybean sudden death syndrome. Plant Health Progress 2008 [Google Scholar]
- 24.Radwan O, Liu Y, Clough SJ. Transcriptional analysis of soybean root response to Fusarium virguliforme, the causal agent of sudden death syndrome. Mol Plant-Microbe Interact. 2011; 24: 958–972. 10.1094/MPMI-11-10-0271 [DOI] [PubMed] [Google Scholar]
- 25.Radwan O, Li M, Calla B, Li S, Hartman GL, Clough SJ. Effect of Fusarium virguliforme phytotoxin on soybean gene expression suggests a role in multidimensional defence. Mol Plant Pathol. 2013; 14: 293–307. 10.1111/mpp.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivastava SK, Huang X, Brar HK, Fakhoury AM., Bluhm BH, Bhattacharyya MK. The genome sequence of the fungal pathogen Fusarium virguliforme that causes sudden death syndrome in soybean. PLoS ONE. 2014; 9: e81832 10.1371/journal.pone.0081832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langmead B, Trapnell C, Pop M, Salzberg S. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009; 10: R25 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/Map format and SAMtools. Bioinformatics. 2009; 25: 2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Meth. 2008; 5: 621–628. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein JN, Myers TG, O'Connor PM, Friend SH, Fornace AJ Jr., Kohn KW, et al. An information-intensive approach to the molecular pharmacology of cancer. Science. 1997; 275: 343–349. [DOI] [PubMed] [Google Scholar]
- 31.Ji Y, Xu Y, Zhang Q, Tsui K-W, Yuan Y, Norris C Jr, et al. BM-Map: Bayesian mapping of multireads for next-generation sequencing data. Biometrics. 2011; 67: 1215–1224. 10.1111/j.1541-0420.2011.01605.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005; 21: 3674–3676. 10.1093/bioinformatics/bti610 [DOI] [PubMed] [Google Scholar]
- 33.Baker SE, Kroken S, Inderbitzin P, Asvarak T, Li B-Y, Shi L, et al. Two polyketide synthase-encoding genes are required for biosynthesis of the polyketide virulence factor, T-toxin, by Cochliobolus heterostrophus. Mol Plant-Microbe Interact. 2006; 19: 139–149. 10.1094/MPMI-19-0139 [DOI] [PubMed] [Google Scholar]
- 34.Yang G, Rose MS, Turgeon BG, Yoder OC. A polyketide synthase is required for fungal virulence and production of the polyketide T-toxin. Plant Cell. 1996; 8: 2139–2150. 10.1105/tpc.8.11.2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z-N, Wu Q-Y, Zhang G-Z, Zhu Y-Y, Murphy RW, Liu Z, et al. Systematic analyses reveal uniqueness and origin of the CFEM domain in fungi. Scientific Reports. 2015; 5: 13032 10.1038/srep13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayanasamy P. Molecular biology in plant pathogenesis and disease management: microbial plant pathogens: 1st ed.; Springer: New York, NY, USA; 2008. [Google Scholar]
- 37.Roy KW, Hershman DE, Rupe JC, Abney TS. Sudden death syndrome of soybean. Plant Dis. 1997; 81: 1100–1111. [DOI] [PubMed] [Google Scholar]
- 38.Lun S, Bishai WR. Characterization of a novel cell wall-anchored protein with carboxylesterase activity required for virulence in Mycobacterium tuberculosis. J Biol Chem. 2007; 282: 18348–18356. 10.1074/jbc.M700035200 [DOI] [PubMed] [Google Scholar]
- 39.Guo J, Zheng X, Xu L, Liu Z, Xu K, Li S, et al. Characterization of a novel esterase Rv0045c from Mycobacterium tuberculosis. PLoS ONE. 2010; 5: e13143 10.1371/journal.pone.0013143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramoni S, Suárez-Moreno ZR, Venturi V. Lipases as Pathogenicity Factors of Plant Pathogens In Handbook of hydrocarbon and lipid microbiology. pp 3269–3277. Timmis KN, McGenity TJ, Meer JR, de Lorenzo V, Editors: Springer-Verlag; Berlin Heidelberg; 2010. [Google Scholar]
- 41.Voigt CA, Schafer W, Salomon S. A secreted lipase of Fusarium graminearum is a virulence factor required for infection of cereals. Plant J. 2005; 42: 364–75. 10.1111/j.1365-313X.2005.02377.x [DOI] [PubMed] [Google Scholar]
- 42.Rittenour WR, Harris SD. Glycosylphosphatidylinositol-anchored proteins in Fusarium graminearum: Inventory, variability, and virulence. PLoS ONE. 2013; 8: e81603 10.1371/journal.pone.0081603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim Y-K, Liu Z-M, Li D, Kolattukudy PE. Two novel genes induced by hard-surface contact of Colletotrichum gloeosporioides conidia. J Bacteriol. 2000; 182: 4688–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolattukudy PE. Enzymatic penetration of the plant cuticle by fungal pathogens. Annu Rev Phytopathol. 1985; 23: 223–250. [Google Scholar]
- 45.Kolattukudy PE. Biopolyester membranes of plants: Cutin and suberin. Science. 1980; 208: 990–1000. 10.1126/science.208.4447.990 [DOI] [PubMed] [Google Scholar]
- 46.Morrissey JP, Osbourn AE. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol Mol Biol Rev. 1999; 63: 708–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chibucos MC, Morris PF. Levels of polyamines and kinetic characterization of their uptake in the soybean pathogen Phytophthora sojae. Appl Environ Microbiol. 2006; 72: 3350–3356. 10.1128/AEM.72.5.3350-3356.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snyder BA, Nicholson RL. Synthesis of phytoalexins in sorghum as a site-specific response to fungal ingress. Science. 1990; 248: 1637–1639. 10.1126/science.248.4963.1637 [DOI] [PubMed] [Google Scholar]
- 49.Hann MG, Bonhoff A, Grisebach H. Quantitative localization of the phytoalexin glyceollin I in relation to fungal hyphae in soybean roots infected with Phytophthora megasperma f. sp. glycinea. Plant Physiol. 1985; 77: 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coleman JJ, Wasmann CC, Usami T, White GJ, Temporini ED, McCluskey K, et al. Characterization of the gene encoding pisatin demethylase (FoPDA1) in Fusarium oxysporum. Mol Plant-Microbe Interact. 2011; 24: 1482–1491. 10.1094/MPMI-05-11-0119 [DOI] [PubMed] [Google Scholar]
- 51.Cheah E, Ashley GW, Gary J, Oilis D. Catalysis by dienelactone hydrolase: A variation on the protease mechanism. Proteins: Structure, Function, and Bioinformatics. 1993; 16: 64–78. [DOI] [PubMed] [Google Scholar]
- 52.Pathak D, Ashley G, Ollis D. Thiol protease-like active site found in the enzyme dienelactone hydrolase: localization using biochemical, genetic, and structural tools. Proteins: Structure, Function, and Bioinformatics. 1991; 9: 267–279. [DOI] [PubMed] [Google Scholar]
- 53.Timothy DHB. Diverse catalytic activities in the αβ-hydrolase family of enzymes: activation of H2O, HCN, H2O2, and O2. Bioorg Chem. 2004; 32: 367–375. 10.1016/j.bioorg.2004.05.005 [DOI] [PubMed] [Google Scholar]
- 54.Cundliffe E, Davies JE. Inhibition of initiation, elongation, and termination of eukaryotic protein synthesis by trichothecene fungal toxins. Antimicrob Agents Chemother. 1977; 11: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tokai T, Koshino H, Takahashi-Ando N, Sato M, Fujimura M, Kimura M. Fusarium Tri4 encodes a key multifunctional cytochrome P450 monooxygenase for four consecutive oxygenation steps in trichothecene biosynthesis. Biochem Biophys Res Commun. 2007; 353: 412–417. 10.1016/j.bbrc.2006.12.033 [DOI] [PubMed] [Google Scholar]
- 56.van den Brink HM, van Gorcom RFM, van den Hondel CAMJJ, Punt PJ. Cytochrome P450 enzyme systems in fungi. Fungal Genet Biol. 1998; 23: 1–17. [DOI] [PubMed] [Google Scholar]
- 57.Maloney A, VanEtten H. A gene from the fungal plant pathogen Nectria haematococca that encodes the phytoalexin-detoxifying enzyme pisatin demethylase defines a new cytochrome P450 family. Molec Gen Genet. 1994; 243: 506–514. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Inlow M, VanEtten H. Expression profiles of pea pathogenicity genes in vivo and in vitro, characterization of the flanking regions of the cluster and evidence that the cluster region resulted from horizontal gene transfer in the fungal pathogen Nectria haematococca. Curr Genet. 2003; 44: 95–103. 10.1007/s00294-003-0428-x [DOI] [PubMed] [Google Scholar]
- 59.Scott-Craig JS, Borrusch MS, Banerjee G, Harvey CM, Walton JD. Biochemical and molecular characterization of secreted α-xylosidase from Aspergillus niger. J Biol Chem. 2011; 286: 42848–42854. 10.1074/jbc.M111.307397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ben-Daniel B-H, Bar-Zvi D, Tsror L. Pectate lyase affects pathogenicity in natural isolates of Colletotrichum coccodes and in pelA gene-disrupted and gene-overexpressing mutant lines. Mol Plant Pathol. 2011; 13:187–197. 10.1111/j.1364-3703.2011.00740.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong Z, Wang Z. Isolation and characterization of an exopolygalacturonase from Fusarium oxysporum f.sp. cubense race 1 and race 4. BMC Biochemistry. 2011; 12: 51 10.1186/1471-2091-12-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garcıća Maceira FI, Di Pietro A, Roncero MIG. Purification and characterization of a novel exopolygalacturonase from Fusarium oxysporum f. sp. lycopersici. FEMS Microbiol Lett. 1997; 154: 37–43. [Google Scholar]
- 63.Gómez-Gómez E, RuıÏz-Rold n MC, Di Pietro A, Roncero MIG, Hera C. Role in pathogenesis of two endo-β-1,4-xylanase genes from the vascular wilt fungus Fusarium oxysporum. Fungal Genet Biol. 2002; 35: 213–222. 10.1006/fgbi.2001.1318 [DOI] [PubMed] [Google Scholar]
- 64.Sirakova TD, Markaryan A, Kolattukudy PE. Molecular cloning and sequencing of the cDNA and gene for a novel elastinolytic metalloproteinase from Aspergillus fumigatus and its expression in Escherichia coli. Infect Immun. 1994; 62: 4208–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takahashi Y, Shirai T, Ishii S-i. Characterizations of acylagmatine amidohydrolase and carboxypeptidase from Fusarium anguioides. J Biochem. 1975; 77: 823–830. [DOI] [PubMed] [Google Scholar]
- 66.Pietro AD, Huertas-González MD, Gutierrez-Corona JF, Martínez-Cadena G, Méglecz E, Roncero MIG. Molecular characterization of a subtilase from the vascular wilt fungus Fusarium oxysporum. Mol Plant-Microbe Interact. 2001; 14: 653–662. 10.1094/MPMI.2001.14.5.653 [DOI] [PubMed] [Google Scholar]
- 67.Palma-Guerrero J, Torriani SF, Zala M, Carter D, Courbot M, Rudd JJ, McDonald BA, Croll D. Comparative transcriptomic analyses of Zymoseptoria tritici strains show complex lifestyle transitions and intraspecific variability in transcription profiles. Mol Plant Pathol. 2016; 17: 845–859. 10.1111/mpp.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Relative expression of FvTox1 was determined by conducting qRT-PCR at 1-d, 3-d, 5-d and 10-d post inoculation with F. virguliforme Mont-1 conidia. Relative gene expression levels was compared with the expression level at 1-d post F. virguliforme inoculation. Constitutively expressed F. virguliforme GAPDH (g2019) (S2 Table) was used for normalization of the FvTox1 expression levels. Data are means and standard deviations (SD) of two independent biological replications with three technical replications (n = 6).
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.