Abstract
Objective
To investigate association between restless legs syndrome (RLS) and depression and to what extent sleep disturbance, periodic limb movements during sleep (PLMS), and antidepressant medication mediate this relationship.
Methods
Conducted was cross-sectional analysis of Osteoporotic Fractures in Older Men (MrOS) Study data in 982 men assessed for RLS (International RLS Study Group scale [IRLSS]) and depression (Geriatric Depression Scale [GDS]), who underwent actigraphy (for sleep latency/efficiency) and polysomnography (for PLMS). Men were split into three groups: no RLS (n=815), mild RLS (IRLSS≤12, n=85), moderate-to-severe RLS (IRLSS>12, n=82). Depression was defined as GDS≥6. Logistic and linear regression assessed associations of RLS and depression or number depressive symptoms, respectively. Models were adjusted for age, site, race, education, BMI, personal habits, benzodiazepine/dopaminergic medication, physical activity, cardiovascular risk factors, and apnea-hypopnea index.
Results
Of 982 men, 167 (17.0%) had RLS. Depression was significantly associated with moderate-to-severe RLS after adjustment (vs. no RLS: OR [95% CI] 2.85 [1.23,6.64]). Further adjustment for potential mediators attenuated effect size modestly, most for sleep efficiency (OR 2.85 to 2.55). Compared to no RLS, moderate-to-severe RLS was associated with number of depressive symptoms after adjustment (adjusted means [95% CI]; no RLS: 1.14 [1.05,1.24] vs. IRLSS>12: 1.69 [1.32,2.11]). Further adjustment for potential mediators didn’t alter effect size. For men with PLMS index≥median, number of depressive symptoms significantly increased as RLS category became more severe.
Conclusions
Depression is more common as RLS severity worsens. The RLS-depression relationship is modestly explained by sleep disturbance and PLMS.
Keywords: restless legs syndrome, depression, periodic limb movement during sleep, sleep
INTRODUCTION
Restless legs syndrome (RLS) is a prevalent sensorimotor condition, which consists of a distressing urge to move at night, when rest is most desired. RLS occurs in 5%–10% of adult populations and its prevalence increases with age up to the eighth decade.(1) There is a well-known association between RLS and depression in all age groups including elderly.(2–4) Lifetime prevalence of depression is 19%–35% in persons with RLS, representing a greater than two-fold increased odds of depression compared to those without RLS.(5–7) Furthermore, in persons with RLS, the negative impact associated with depression on quality of life may exceed associations with sleep disturbance or RLS severity.(8) This is especially significant since reduction in life quality in RLS rivals that for patients with diseases such as osteoarthritis and diabetes.(1) Despite the clinical importance of the relationship between RLS and depression, mechanisms underlying this association are not known and relatively understudied.
Sleep disturbances including the inability to initiate or maintain sleep occur in up to half of RLS sufferers.(1, 4) It has been proposed (but not studied) that disturbed sleep may be a mediator of depression when co-occurring with RLS.(9, 10) Periodic limb movements during sleep (PLMS), which occur in up to 90% of RLS sufferers, may also disturb sleep and (11) modify the RLS-depression relationship, as those with RLS and PLMS may differ fundamentally from those with RLS but no PLMS. This notion is supported by observations that certain genetic polymorphisms are more tightly associated with PLMS than RLS,(12) and persons with RLS and PLMS compared to those without PLMS are more likely to respond therapeutically to certain medications.(13) An additional factor that may confound the RLS-depression relationship is antidepressant medications, which are known to provoke RLS, increase PLMS, and mitigate depression.(14–16)
With this background in mind, the purpose of this study is to evaluate the relationship between RLS and depressive symptoms in a cross-sectional analysis of an older male cohort, and determine which factors may mediate (sleep latency or sleep fragmentation), moderate (PLMS), or confound (antidepressant medication) this association. We first evaluated the association between RLS and depressive symptoms, then examined if sleep latency and efficiency, PLMS, and antidepressant use were potential mediators or modifiers of this RLS-depression association. Insight into which factors affect the RLS-depression relationship might help to identify targets for treatment of this therapeutically complex, yet common neuropsychiatric overlap syndrome.
METHODS
Participants
Baseline examination for the Osteoporotic Fractures in Men (MrOS) Study in 2000–2002 included 5,994 community-dwelling men ≥65 years at six clinical centers: University of Alabama (Birmingham, Alabama); University of Minnesota (Minneapolis, Minnesota); Stanford University (Palo Alto, California); University of Pittsburgh (Monongahela Valley, Pennsylvania); Oregon Health and Science University (Portland, Oregon); and University of California, San Diego (San Diego, California).(17, 18) Participants needed to be able to walk without assistance, and must not have had either a bilateral hip replacement, or a medical condition that (in the judgment of investigators) might cause imminent death. These inclusion criteria were designed to yield a cohort that reasonably represented a broad population of older men.
The ancillary MrOS Sleep Study recruited 3,135 participants from the parent cohort in 12/2003–3/2005 (Sleep Visit 1) for comprehensive sleep assessment. All active MrOS Sleep Study participants with available Sleep Visit 1 polysomnography (PSG) and actigraphy data were eligible to participate in Sleep Visit 2 (11/2009-3/2012, mean follow-up 6.5±0.7 years) (Figure 1). For this analysis, data was collected at Sleep Visit 2 (RLS, depression, sleep variables, covariates), except PLMS, which was measured at Sleep Visit 1.
Figure 1.
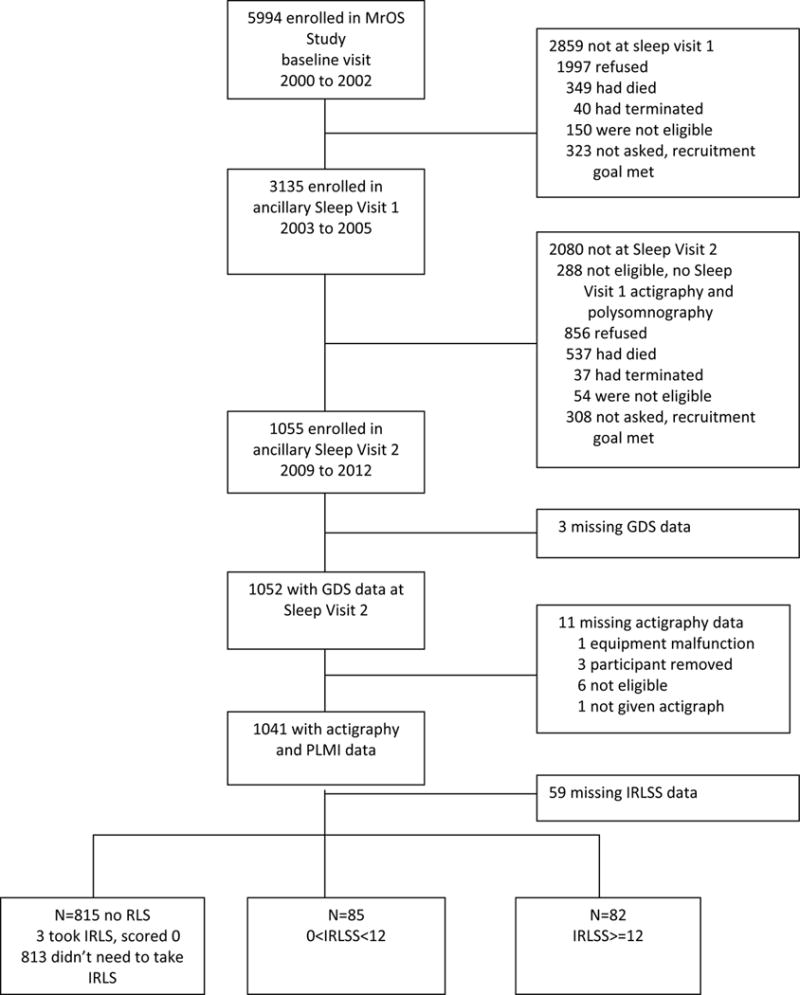
Progression of Participants through the MrOS and MrOS Sleep Studies
Of 1,055 Sleep Visit 2 participants, missing data occurred in 73 persons (3 incomplete GDS, 11 incomplete actigraphy, 59 incomplete RLS data), leaving 982 participants, comprising this study’s analytic sample. Compared to the analysis subset, the 73 excluded men were more likely minority (23% vs. 12%, p=0.007, χ2 [1,N=1055], χ2 =7.19), had lower cognitive function (Modified Mini-Mental State examination (3MS) score 90.5 vs. 92.6, Wilcoxon two-sample test N=1051, Z=−2.38, p=0.02), more depressive symptoms (2.5 vs. 1.8, Wilcoxon two-sample test N=1052, Z=2.94, p=0.003), and had higher stroke prevalence (10% vs. 4%, p=0.03, χ2 [1,N1054], χ2=4.75). Protocols were approved by Institutional Review Boards at each site. All participants provided written informed consent.
RLS Assessment
RLS was defined by 2003 NIH/International RLS Study Group diagnostic criteria.(19) Participants were asked: 1) Do you ever experience a desire to move your legs or arms because of discomfort or disagreeable sensations? If so: 2) Do you sometimes feel the need to move to relieve discomfort, for example by walking, or to relieve the discomfort by rubbing your legs? 3) Are these symptoms worse when you are at rest, with at least temporary relief by activity? 4) Are these symptoms worse later in the day or at night, than in the morning? Men who did not answer yes to these four questions were considered not to have RLS. Those that answered yes to all four questions or reported prior RLS diagnosis completed the International RLS Study Group Severity Scale (IRLSS), comprised of 10 questions concerning RLS symptom severity or frequency.(20) Questions are rated 0–4, corresponding to none to very severe (or frequent) symptoms. Summed responses created an IRLSS score (range 0–40) and category: (1) no RLS (did not answer ‘yes’ to 4 questions or IRLSS=0, n=815); (2) mild RLS (IRLSS≤12, n=85); (3) moderate-to-severe RLS (IRLSS>12, n=82).
Polysomnography
Unattended in-home PSG (Safiro, Compumedics, Inc., Melborne, Australia) occurred on Sleep Visits 1 and 2, with the following montage: C3/A2; C4/A1 electroencephalography, bilateral electrooculography, submental electromyography, thoracic/abdominal respiratory inductance plethysmography, naso-oral thermistry, nasal-pressure transduction, finger-pulse oximetry, and EKG. Piezoelectric sensors on bilateral anterior tibialis muscles were included in Sleep Visit 1 PSG. Home visits were performed by centrally-trained staff using procedures previously described.(21)
Data were scored by certified research polysomnologists at the central Sleep Reading Center using standardized criteria.(21) For this analysis, only apneas and hypopneas causing ≥3% oxygen desaturation were included in the apnea-hypopnea index (AHI; i.e., total number of apneas and hypopneas per hour of sleep). Participants received a letter with sleep study results indicating if there was sleep apnea (AHI included). Severe sleep apnea results (AHI ≥ 50; approved by MrOS Steering Committee and Observational Study Monitoring Board) were referred for review by a local physician within 10 days of PSG. There were 61 (6.2%) men with AHI ≥50; of these,, 5 were using CPAP therapy and 19 began CPAP treatment sometime during follow-up of 3.6±0.9 years.
PLMS were scored to be consistent with American Academy of Sleep Medicine guidelines active at the time of scoring.(22) Individual leg movements were scored if there was a clear amplitude increase from baseline and movement duration was between 0.5 and 5.0 seconds. To be considered periodic, a minimum of four movements needed to occur in succession no less and no more than 5 and 90 seconds apart. Leg movements following respiratory events were excluded unless part of a ≥4 movement cluster with ≥2 movements independent of respiratory events. Periodic limb movement index (PLMI) was computed as the total number of periodic leg movements per hour of sleep. Previously, we conducted a blinded in-laboratory validation study in 51 subjects to compare piezoelectric and electromyographic leg sensors in PLMS detection and found high correlation, r=0.81.(23)
Actigraphy Data
The Actiwatch® 2 actigraph, (Philips Respironics, Murrysville, PA) was used to estimate sleep/wake Actigraphs were worn on the non-dominant wrist for ≥5 consecutive 24-hour periods (mean 5.1±0.7). Actigraphs digitally recorded an integrated measure of gross motor activity using a solid-state piezoelectric accelerometer with 0.025 G sensitivity and 23 Hz sampling rate. Activity measures were stored in one-minute epochs.
Actigraphy data were scored and processed at the San Francisco Coordinating Center. The sleep scoring algorithm in Actiware software (version 5.54, Philips Respironics)(24) calculates an constituted sleep onset. Sleep offset was the last minute scored as sleep. Sleep latency was the average time in minutes to sleep onset over all nights. Sleep efficiency was the average percent time sleeping over all nights.
Depression Assessment
The Geriatric Depression Scale (GDS) assessed number of depressive symptoms and presence of depression.(27, 28) Depression was defined as GDS≥6, yielding 90.9% sensitivity and 64.5% specificity, compared to gold standard clinical diagnosis using Diagnostic and Statistical of Mental Disorders-IV criteria.(27)
Covariate Data
Questionnaires included information about demographic characteristics, education, medical history, physical activity, caffeine, smoking and alcohol use. Cognition was assessed using 3MS, with higher scores representing better cognitive functioning.(29) Probable cognitive impairment was defined as 3MS score<80.(30) Insomnia was assessed with the Insomnia Severity Index.(31) Anxiety was assessed using the Goldberg Anxiety Scale, with a score≥5 defining clinically relevant anxiety.(32) Medications were verified by examining pill bottles and matched to ingredient(s) using Iowa Drug Information Service Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).(33) Dopaminergic medications included levodopa, ropinirole, and pramipexole. Dopamine antagonists included s antipsychotics, domperidone, prochlorperazine, perphenazine, chlorpromazine, or metoclopramide. Physical Activity Scale for the Elderly measured physical activity.(34) Body weight (kilograms) and height (meters) were measured and used to calculate body mass index (BMI; kg/m2).
Statistical Analysis
Participant characteristics were compared across RLS category using chi-square tests for categorical variables, analysis of variance for normally distributed continuous variables, and Kruskal-Wallis tests for non-normal continuous variables. Tests for linear trend across categories were performed using Chochran-Armitage or Jonckheere-Terpstra tests for categorical data, or linear regression model for continuous data.
Logistic regression was used to assess association of RLS category and depression. Model results are presented as odds ratios (OR) with [95% confidence intervals (CI)]. Linear regression models were used to assess association of RLS category and number of depressive symptoms. Results are presented as adjusted means with [95% CIs]. A test for linear trend across categories was performed. Number of depressive symptoms was log-transformed to meet model normality assumptions, then back-transformed to display results. Models were minimally adjusted for age and clinic site, then further adjusted for race (white vs. nonwhite), education, BMI, alcohol, caffeine, smoking status, physical activity, 3MS, stroke, diabetes, myocardial infarction or congestive heart failure history, benzodiazepine or dopaminergic medication usage, and AHI.
To examine if associations of RLS and depression were partially explained by antidepressant use, sleep efficiency, or sleep latency, models were further adjusted by these covariates, added separately. Because no clear cut-point has been established, we considered a reduction in beta coefficient for the association of RLS and depression or number of depressive symptoms of ≥10% to support a hypothesis of mediation, similar to other publications.(35–38) Effect modification of PLMS was examined by testing for interaction of RLS category and PLMI. Data was stratified (by median PLMI), since p-interaction for this potential modifier and RLS was <0.10.
All significance levels were two-sided. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
Of 982 elderly men, 167 met our definition of RLS (prevalence 17.0%). Table 1 shows participant characteristics, in the cohort overall and by RLS category. Individuals with compared to without RLS were younger, more likely taking dopaminergic medications, anticonvulsants and prescription sleep medication, had higher rates of anxiety, and were more likely to report a Parkinson’s disease history. Other demographic characteristics, including race and BMI were similar across RLS categories. There was no difference in dopamine antagonists or antihistamine usage and smoking, alcohol, or caffeine usage among groups. Use of benzodiazepine and antidepressant medication was highest in the moderate-to-severe RLS group. Antidepressant medication usage is shown in Table 2. The most commonly used antidepressants were trazadone (2.3%), citalopram (2.0%), and fluoxetine (1.5%). Depression (GDS≥6) was most frequent and mean number of depressive symptoms was greatest in the moderate-to-severe RLS group. The observed 17.0% RLS prevalence is higher than observations among other groups, (1, 39) likely the result of the cohort being elderly (RLS prevalence increases with age),(40) and the use of questionnaire to diagnose RLS (less specific than in-person screening).(41)
Table 1.
Baseline Characteristics by Restless Legs Syndrome Category
| Characteristic | All (N= 982) |
No RLS (N= 815) |
0<IRLSS≤12 (N= 85) |
IRLSS>12 (N= 82) |
P-value, test statistic |
P-trend, test statistic |
|---|---|---|---|---|---|---|
| Age,y | 81.04 ± 4.58 | 81.20 ± 4.67 | 80.38 ± 3.70 | 80.11 ± 4.31 | p=0.04, F=3.12 | p=0.01, t(980)=− 2.45 |
| Not of Caucasian race | 121 (12.32) | 98 (12.02) | 9 (10.59) | 14 (17.07) | p=0.36, χ2=2.02 | p=0.30, Z=−1.03 |
| Education | p=0.36, χ2=4.36 | p=0.15, Z=1.45 | ||||
| Less than high school | 32 (3.26) | 30 (3.68) | 2 (2.44) | |||
| High school | 139 (14.15) | 118 (14.48) | 12 (14.12) | 9 (10.98) | ||
| Some college or graduate school | 811 (82.59) | 667 (81.84) | 73 (85.88) | 71 (86.59) | ||
| Body mass index, kg/m2 | 26.95 ± 3.83 | 26.97 ± 3.88 | 26.64 ± 3.37 | 27.13 ± 3.83 | p=0.69, F=0.38 | p=0.97, t(979)=0.04 |
| Apnea-hypopnea index, median (IQR) | 14.13 (6.53, 26.15) | 14.36 (6.58, 26.3) | 14.75 (6.16, 22.4) | 11.13 (6.61, 29.31) | p=0.76, χ2=0.56 | p=0.61, t(953)=−0.50 |
| Smoking | p=0.24, χ2=5.54 | p=0.53, Z=−0.62 | ||||
| Never | 464 (47.25) | 380 (46.63) | 44 (51.76) | 40 (48.78) | ||
| Past | 506 (51.53) | 427 (52.39) | 40 (47.06) | 39 (47.56) | ||
| Current | 12 (1.22) | 8 (0.98) | 1 (1.18) | 3 (3.66) | ||
| Alcohol intake, drinks/week | p=0.88, χ2=1.22 | p=0.71, Z=0.37 | ||||
| <1 | 464 (47.44) | 388 (47.84) | 36 (42.35) | 40 (48.78) | ||
| 1–13 | 463 (47.34) | 380 (46.86) | 45 (52.94) | 38 (46.34) | ||
| ≥14 | 51 (5.21) | 43 (5.30) | 4 (4.71) | 4 (4.88) | ||
| Caffeine use, mg/day, median (IQR) | 200 (36, 356) | 192 (36, 356) | 220 (36, 408) | 188 (36, 364) | p=0.87, χ2=0.27 | p=0.77, t(978)=0.30 |
| PASE physical activity score | 124.86 ± 68.68 | 124.96 ± 69.38 | 134.86 ± 63.75 | 113.53 ± 65.53 | p=0.13, F=2.02 | p=0.43, t(979)=−0.78 |
| 3MS score (range 0 to 100), median (IQR) | 94 (90, 97) | 94 (90, 97) | 95 (92, 97) | 94 (90, 97) | p=0.03, χ2=6.88 | p=0.16, t(977)=1.40 |
| Probable cognitive impairment (3MS<80) | 40 (4.09) | 37 (4.56) | 1 (1.18) | 2 (2.44) | p=0.24, χ2=2.86 | p=0.16,Z=1.40 |
| Current medication use: | ||||||
| Benzodiazepine use | 38 (3.87) | 28 (3.44) | 2 (2.35) | 8 (9.76) | p=0.01, χ2=8.56 | p=0.02, Z=−2.32 |
| Antidepressant use | 99 (10.09) | 74 (9.09) | 8 (9.41) | 17 (20.73) | p=0.004, χ2=11.17 | p=0.003, Z=−2.99 |
| Dopaminergic medication | 13 (1.33) | 6 (0.74) | 0 | 7 (8.54) | p<.0001, χ2=35.91 | p<.0001, Z=−5.00 |
| Dopamine antagonists | 10 (1.02) | 8 (0.98) | 0 | 2 (2.44) | p=0.28, χ2=2.52 | p=<.0001, Z=−5.00 |
| Anticonvulsants | 39 (3.98) | 32 (3.93) | 0 | 7 (8.54) | p=0.02, χ2=7.99 | p=0.26, Z=−1.12 |
| Prescription sleep medication | 34 (3.47) | 25 (3.07) | 2 (2.35) | 7 (8.54) | p=0.03, χ2=7.00 | p=0.03, Z=−2.15 |
| Antihistamines | 129 (13.15) | 107 (13.14) | 9 (10.59) | 13 (15.85) | p=0.60, χ2=1.01 | p=0.72, Z=−0.36 |
| Lithium | 2 (0.20) | 2 (0.25) | 0 | 0 | ||
| Number of depressive symptoms, GDS score (range 0 to 15) |
1.77 ± 2.14 | 1.70 ± 2.05 | 1.55 ± 1.82 | 2.70 ± 2.90 | p=0.003, χ2=11.40 | p=0.002, t(980)=3.09 |
| GDS score≥6 | 49 (4.99) | 36 (4.42) | 3 (3.53) | 10 (12.20) | p=0.007, χ2=9.93 | p=0.009, Z=−2.60 |
| Goldberg Anxiety Scale (GAS) Score (range 0 to 9) | 0.72 ± 1.58 | 0.61 ± 1.40 | 0.88 ± 1.82 | 1.63 ± 2.49 | p<.0001, χ2=24.25 | p<.0001, t(979)=5.29 |
| Clinically important anxiety disturbance (GAS≥5) | 52 (5.30) | 31 (3.81) | 6 (7.06) | 15 (18.29) | p<.0001 χ2=31.71 | p<.0001, Z=−5.44 |
| History of: | ||||||
| Diabetes | 161 (16.40) | 138 (16.93) | 11 (12.94) | 12 (14.63) | p=0.58, χ2=1.10 | p=0.40, Z=0.84 |
| Stroke | 41 (4.18) | 32 (3.93) | 3 (3.53) | 6 (7.32) | p=0.33, χ2=2.24 | p=0.22, Z=−1.23 |
| Myocardial infarction or congestive heart failure | 207 (21.08) | 164 (20.12) | 19 (22.35) | 24 (29.27) | p=0.15, χ2=3.84 | p=0.06, Z=−1.90 |
| Parkinson’s disease | 17 (1.73) | 10 (1.23) | 2 (2.35) | 5 (6.10) | p=0.005, χ2=10.60 | p=0.002, Z=−3.15 |
| Renal disease | 27 (2.75) | 23 (2.82) | 1 (1.18) | 3 (3.66) | p=0.59, χ2=1.06 | p=0.96, Z=−0.05 |
| Periodic limb movement index, median (IQR) | 21.33 (2.6, 52.46) | 19.14 (2.26, 50.4) | 30.72 (4.1, 56.41) | 31.73 (5.39, 62.58) | p=0.01, χ2=8.74 | p=0.008, t(980)=2.66 |
| Total sleep time, min | 394.53 ± 64.02 | 395.94 ± 64.61 | 386.96 ± 56.14 | 388.33 ± 65.58 | p=0.31, F=1.18 | p=0.16, t(980)=−1.39 |
| Sleep latency, min, median (IQR) | 28.9 (16, 51) | 28.4 (15.4, 49.4) | 32.6 (17.6, 50.75) | 36.6 (22.6, 74.4) | p=0.007, χ2=9.94 | p=0.001, t(980)=3.25 |
| Sleep efficiency, % | 79.18 ± 8.11 | 79.43 ± 7.95 | 78.39 ± 9.26 | 77.53 ± 8.29 | p=0.08, F=2.50 | p=0.03, t(980)=−2.24 |
| Insomnia Severity Index score (range 0 to 28), median (IQR) | 4 (1, 7) | 3 (1, 7) | 5 (3, 7) | 9 (5, 13) | p<.0001, χ2=69.67 | p<.0001, t(977)=8.49 |
| Insomnia Severity Index category | p<.0001, χ2=119.76 | p<.0001, Z=6.68 | ||||
| No clinically significant insomnia | 744 (76.00) | 646 (79.56) | 65 (76.47) | 33 (40.24) | ||
| Subthreshold insomnia | 203 (20.74) | 152 (18.72) | 19 (22.35) | 32 (39.02) | ||
| Clinical insomnia (moderate) | 30 (3.06) | 14 (1.72) | 1 (1.18) | 15 (18.29) | ||
| Clinical insomnia (severe) | 2 (0.20) | 0 | 0 | 2 (2.44) |
Data are shown as mean ± SD or n (%), unless otherwise noted.
N=982 for all variables except: N=981 for body mass index, physical activity, medication use and anxiety; N=980 for caffeine use; N=979 for 3MS and Insomnia Severity Index; N=978 for alcohol intake; N=955 for apnea-hypopnea index.
P-values for continuous variables from a ANOVA for normally distributed variables, a Kruskal Wallis chi-square test with 2 degrees of freedom for skewed data (apnea-hypopnea index, caffeine use, 3MS score, number of depressive symptoms, anxiety score, periodic leg movement index, sleep latency, Insomnia Severity Index score).
P-values for categorical variables from a chi-square test with 2 degrees of freedom for all but education, smoking and alcohol intake (4 degrees of freedom) and Insomnia Severity Index category (6 degrees of freedom).
P-trend are from a Cochran-Armitage test for linear trend or a Jonckheere-Terpstra test for monotonic trend for categorical variables, a linear regression testing trend over the IRLS categories for continuous variables.
Abbreviations: SD, standard deviation; IQR, interquartile range; PASE, Physical Activity Scale for the Elderly; 3MS, Modified mini-mental state exam; GDS, geriatric depression scale; GAS, Goldberg anxiety scale; IRLSS, International Restless Legs Syndrome Rating Scale score.
Table 2.
Type of Antidepressant use by Restless Legs Syndrome Category, n (%)
| Medication | All (N= 981) |
No RLS (N= 815) |
0<IRLSS≤12 (N= 85) |
IRLSS>12 (N= 82) |
P-value, χ2 [2, N=981] |
P-trend, Z |
|---|---|---|---|---|---|---|
| Antidepressant use | 99 (10.09) | 74 (9.09) | 8 (9.41) | 17 (20.73) | p=0.004, χ2=11.17 | p=0.003, Z=−2.99 |
| Trazodone | 23 (2.34) | 17 (2.09) | 0 | 6 (7.32) | p=0.004, χ2=11.13 | p=0.03, Z=−2.18 |
| Bupropion | 14 (1.43) | 13 (1.60) | 0 | 1 (1.22) | p=0.49, χ2=1.42 | p=0.48, Z=0.70 |
| Venlafaxine | 2 (0.20) | 1 (0.12) | 0 | 1 (1.22) | p=0.10, χ2=4.59 | p=0.08, Z=−1.77 |
| Nefazodone | 1 (0.10) | 1 (0.12) | 0 | 0 | p=0.90, χ2=0.21 | p=0.67, Z=0.43 |
| Selegiline | 1 (0.10) | 1 (0.12) | 0 | 0 | p=0.90, χ2=0.21 | p=0.67, Z=0.43 |
| Rasagiline | 2 (0.20) | 0 | 0 | 2 (2.44) | p<.0001, χ2=21.97 | p<.0001, Z=−4.14 |
| Amitriptyline | 9 (0.92) | 6 (0.74) | 1 (1.18) | 2 (2.44) | p=0.29, χ2=2.44 | p=0.13, Z=−1.52 |
| Imipramine | 1 (0.10) | 0 | 0 | 1 (1.22) | p=0.004, χ2=10.97 | p=0.003, Z=−2.93 |
| Mirtazapine | 10 (1.02) | 9 (1.11) | 1 (1.18) | 0 | p=0.63, χ2=0.93 | p=0.42, Z=0.82 |
| Doxepin | 1 (0.10) | 1 (0.12) | 0 | 0 | p=0.90, χ2=0.21 | p=0.67, Z=0.43 |
| Nortriptyline | 1 (0.10) | 1 (0.12) | 0 | 0 | p=0.90, χ2=0.21 | p=0.67, Z=0.43 |
| Fluoxetine | 15 (1.53) | 13 (1.60) | 1 (1.18) | 1 (1.22) | p=0.93, χ2=0.15 | p=0.73, Z=0.35 |
| Paroxetine | 7 (0.71) | 5 (0.61) | 2 (2.35) | 0 | p=0.14, χ2=3.93 | p=0.89, Z=−0.14 |
| Sertraline | 12 (1.22) | 8 (0.98) | 1 (1.18) | 3 (3.66) | p=0.11, χ2=4.42 | p=0.05, Z=−1.92 |
| Citalopram | 20 (2.04) | 16 (1.97) | 2 (2.35) | 2 (2.44) | p=0.94, χ2=0.13 | p=0.73, Z=−0.35 |
| Duloxetine | 2 (0.20) | 2 (0.25) | 0 | 0 | p=0.81, χ2=0.41 | p=0.55, Z=0.60 |
| Escitalopram | 6 (0.61) | 5 (0.61) | 0 | 1 (1.22) | p=0.60, χ2=1.02 | p=0.74, Z=−0.33 |
P-values from a chi-square test or from a Cochran-Armitage test for trend.
Abbreviations: IRLSS, International Restless Legs Syndrome Rating Scale score.
Sleep Characteristics
Sleep latency was significantly highest and sleep efficiency tended to be poorest in the moderate-to-severe RLS group (Table 1). This was reflected in insomnia scale measures, where insomnia was most severe in those with moderate-to-severe RLS. PLMI was highest, intermediate, and lowest in the moderate-to-severe, mild, and no RLS groups, respectively.
Association of RLS and Depression
Table 3 shows results of regression models examining the association of RLS and depression. Table 4 shows the association of RLS and the number of depressive symptoms. For the depression outcome, there was significant association for the moderate-to-severe RLS group in the minimally adjusted model, where there was more than a three-fold increase in the likelihood of depression (IRLSS>12 vs. no RLS: OR [95% CI]: 3.06 [1.42,6.58]). This association remained after further adjustment (2.85 [CI 1.23,6.64]). There was significant association of RLS with number of depressive symptoms. Compared to those without RLS, moderate-to-severe RLS was associated with number of depressive symptoms after multivariate adjustment (adjusted means [95% CI]: no RLS, 1.14 [1.05,1.24] vs. IRLSS>12, 1.69 [1.32,2.11]).
Table 3.
Adjusted Associations of Restless Legs Syndrome and Depression, Odds Ratio (95% Confidence Interval), Wald χ2 test statistic.
| Model | No RLS (n=815) |
0<IRLSS≤12 (n=85) |
IRLSS>12 (n=82) |
p-trend χ2 value |
|---|---|---|---|---|
| Minimally adjusteda | 1.00 (reference) | 0.81 (0.24, 2.71), χ2=0.12 | 3.06 (1.42, 6.58), χ2=8.15 | p=0.01, χ2=6.24 |
| Multivariate adjustedb | 1.00 (reference) | 0.93 (0.27, 3.17), χ2=0.02 | 2.85 (1.23, 6.64), χ2=5.92 | p=0.03, χ2=4.78 |
| Multivariateb + antidepressant use | 1.00 (reference) | 0.92 (0.27, 3.17), χ2=0.02 | 2.60 (1.10, 6.13), χ2=4.75 | p=0.05, χ2=3.82 |
| Multivariateb + sleep efficiency | 1.00 (reference) | 0.88 (0.25, 3.06), χ2=0.04 | 2.55 (1.08, 6.03), χ2=4.54 | p=0.06, χ2=3.57 |
| Multivariateb + sleep latency | 1.00 (reference) | 0.91 (0.26, 3.11), χ2=0.03 | 2.61 (1.11, 6.11), χ2=4.86 | p=0.05, χ2=3.89 |
adjusted for age and site, Wald χ2 [1, N=982].
adjusted for age, site, race (white vs. nonwhite), body mass index, alcohol use, caffeine intake, smoking status, physical activity, cognitive function, history of stroke, history of diabetes, history of myocardial infarction or congestive heart failure, use of benzodiazepines, use of dopamenergics, education, apnea-hypopnea index, Wald χ2 [1, N=945].
Bolded odds ratios and 95% confidence intervals are p<0.05 compared to the reference.
Abbreviations, IRSS=International RLS Study Group Severity Scale.
Table 4.
Associations of Restless Legs Syndrome and Number of Depressive Symptoms Adjusted Means (95% Confidence Interval), t-value
| Model | No RLS (n=815) |
0<IRLSS≤12 (n=85) |
IRLSS>12 (n=82) |
p-trend, t-value |
|---|---|---|---|---|
| Minimally adjusteda | 1.15 (1.05, 1.24) | 1.15 (0.87, 1.46), t(973)= −0.006 |
1.90 (1.52, 2.34), t(973)=3.99 |
p=0.0004, t(974)=3.53 |
| Multivariate adjustedb | 1.14 (1.05, 1.24) | 1.22 (0.94, 1.53), t(919)=0.47 |
1.69 (1.32, 2.11), t(919)=2.90 |
p=0.007, t(920)=2.72 |
| Multivariateb + antidepressant use | 1.15 (1.06, 1.24) | 1.21 (0.94, 1.52), t(918)=0.42 |
1.65 (1.29, 2.06), t(918)=2.71 |
p=0.01, t(919)=2.54 |
| Multivariateb + sleep efficiency | 1.14 (1.05, 1.24) | 1.21 (0.93, 1.52), t(918)=0.42 |
1.67 (1.31, 2.09), t(918)=2.83 |
p=0.008, t(919)=2.64 |
| Multivariateb + sleep latency | 1.14 (1.05, 1.24) | 1.21 (0.94, 1.52), t(918)=0.43 |
1.67 (1.31, 2.09), t(918)=2.81 |
p=0.009, t(919)=2.62 |
adjusted for age and site. N=982
adjusted for age, site, race (white vs. nonwhite), body mass index, alcohol use, caffeine intake, smoking status, physical activity, cognitive function, history of stroke, history of diabetes, history of myocardial infarction or congestive heart failure, use of benzodiazepines, use of dopamenergics, education, apnea-hypopnea index. N=945. Bolded adjusted means ratios and 95% confidence intervals are p<0.05 compared to the reference (no RLS).
Abbreviations, IRSS=International RLS Study Group Severity Scale
Effect of Potential Mediators
After further adjustment of multivariate models by potential mediators/confounders (sleep efficiency, sleep latency, antidepressant use), the association of RLS severity and depression remained significant, although effect size was attenuated (Table 3). Addition of sleep efficiency had the greatest effect, decreasing the odds ratio by 10.5% (percent difference in beta coefficients 100*(1.05−0.94)/1.05=10.5%).
Further adjustment for potential mediators in models with the outcome, number of depressive symptoms, did not substantially alter effect size of RLS or statistical significance. Adjusted means for those with IRLSS>12 were slightly attenuated from 1.69 to 1.67 (addition of sleep efficiency or sleep latency, percent difference in beta coefficients 100*(0.2530.247)/0.253=2.4%, 100*(0.253–0.245)/0.253=3.2%, respectively), or 1.65 (antidepressant use, 100*(0.253–0.234)/0.253=7.5%). So association of RLS category with depression and number of depressive symptoms was only partially modified by antidepressant use, sleep latency, or sleep efficiency.
Stratification Models and Exploratory Analyses
Analysis for the outcome, number of depressive symptoms, was stratified by PLMI as there was significant interaction between RLS category and PLMI (p=0.04, F[2,979]=3.13, Figure 2). For men with PLMI≥median, there was a significant increase in number of depressive symptoms as category of RLS increased (p-trend=0.002, t[d.f=447]=3.06). No significant associations were seen among men with below median PLMI.
Figure 2. Multivariable Adjusted Associations of Restless Legs Syndrome and Number of Depressive Symptoms Stratified by Periodic Limb Movement Index, Adjusted Means (95% Confidence Interval).
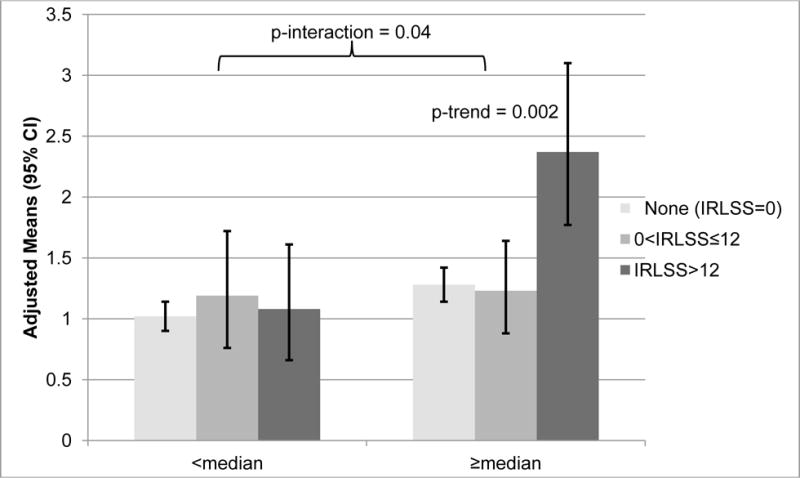
Adjusted for age, site, race (white vs. nonwhite), body mass index, alcohol use, caffeine intake, smoking status, physical activity, cognitive function, history of stroke, history of diabetes, history of myocardial infarction or congestive heart failure, use of benzodiazepines, use of dopaminergics, education, apnea-hypopnea index. Test for interaction, F[2,979]=3.13. Test for trend from a linear regression model, t[N=447]=3.06.
Abbreviations, IRSS=International RLS Study Group Severity Scale; PLMI=periodic limb movement index.
In an exploratory analysis, among persons with moderate-to-severe RLS, those taking antidepressant medication had significantly fewer depressive symptoms compared to those not taking these medicines (4.3±3.8 vs. 2.3±2.5; p for interaction= 0.02, F[1,978]=5.13).
DISCUSSION
In this study of community-dwelling older men, about one in eight men with moderate-to-severe RLS had depression, compared to just one in twenty-five men with no or mild RLS. Men with moderate-to-severe RLS were more likely to be taking antidepressant medication and had higher PLMI, lower sleep efficiency, and more prolonged sleep latency, each of these clinical entities being potential mediators or modifiers of the RLS-depression association. The extent to which these factors mediated the relationship between RLS and depression was explored using regression modeling. For the relationship between depression and RLS severity, effect size was attenuated most when sleep efficiency was added to multivariate models, suggesting that poor sleep was a stronger mediator of this relationship than other clinic factors. Sleep efficiency was the only potential mediator that met criteria of reducing the association or RLS and depression by ≥10%. PLMS was a potential modifier of the RLS-depression relationship, meaning that those with RLS and PLMS behaved differently than RLS sufferers without PLMS. Only in those with high PLMI and moderate-to-severe RLS was there an association with number of depressive symptoms.
These findings argue for effect mediation and moderation of RLS-depression by poor sleep efficiency and PLMS, respectively. Persons with RLS are well known to have disrupted sleep, (1, 42, 43) which is among the most troublesome of symptoms to those with RLS. The most common sleep abnormalities in RLS are prolonged sleep latency and reduced sleep efficiency;(11, 42, 44) both of these sleeping abnormalities is commonly seen in depression.(45) In our study, both sleep latency and sleep efficiency were significantly associated with RLS. The inclusion of sleep efficiency in particular lessened the effect size in models of depression by RLS category.
PLMS showed potential to moderate the RLS–depression relationship. There is reason to believe that persons with RLS and PLMS differ from RLS sufferers without PLMS, thus PLMS could be a moderating factor of RLS-depression. It has been suggested by some that PLMS is an endophenotype for RLS.(46) Genetically, variants in the BTBD9 gene are more strongly associated with RLS when combined with PLMS.(12) Furthermore, RLS sufferers without PLMS may be less likely to respond therapeutically to dopaminergic medications.(13) Finally, persons with frequent PLMS are known to have RLS that is more severe.(47)
Historically, antidepressant medication has a controversial association to RLS and PLMS with numerous reports that they aggravate both RLS and PLMS.(14) Our current analysis did not support the idea that antidepressant medications mediate the association between RLS and depression. In our study, persons with moderate-to-severe RLS were significantly more likely to be taking antidepressant medication, most commonly trazodone, sertraline, fluoxetine, and citalopram, with the first two agents being used at higher rates in those with moderate-to-severe RLS. Among persons with moderate-to-severe RLS, those taking an antidepressant medication had significantly fewer depressive symptoms compared to those not taking these medicines. Because of the cross-sectional nature of study, directionality and causality cannot be shown. So the question remains: Are antidepressants associated with worsening of RLS symptoms? Or are patients with RLS more likely to take antidepressants because RLS itself is associated with depression? This study cannot sort out this important question, but it is reassuring that for many RLS sufferers on antidepressant medication (compared to not on medication), depressive symptoms were less numerous.
Trazodone in particular was being used more commonly in persons with severe RLS. Again, it is not clear whether this medicine was associated with RLS worsening or was prescribed to treat sleep difficulty present with severe RLS. Given the pharmacology of trazodone includes serotonin reuptake inhibition, it is possible that it would aggravate RLS symptoms, and thus should be used with caution in individuals with RLS.
Our study has several strengths. This is a large community-based cohort of elderly men in which sleep and PLMS have been carefully measured. Actigraphy conducted over five days is more likely to reflect an individual’s true sleep pattern than polysomnography done on one night. The use of GDS is also a strength as this scale is validated and has excellent sensitivity and specificity in detecting depression.(48) Nevertheless, the use of a scale cannot replace a clinical diagnosis by a trained psychiatrist. There are limitations to consider. PLMS was measured on one night during polysomnography, several years before the measurement of RLS and GDS. PLMS represents the only data that was not measured in sleep visit 2, but in this analysis, the data as a whole were analyzed as if cross-sectional. PLMS does vary night-to-night even within individuals, so this is an important limitation to consider.(49) Furthermore, limb movements were detected by piezo-electric sensors, not the more standardly-used electromyographic sensors. The cross-sectional nature of the study does not allow testing of directionality or causality, yet only that RLS and depression are associated. The criteria for effect modification were arbitrarily chosen. Although these criteria have been used in other past studies, for these data it is within the confidence intervals of the statistics and so, may have been a chance finding. And the inclusion of older men only does not allow generalizability to women and younger persons. Among the cohort, included were persons with neurologic disease including stroke and Parkinson disease, which may have introduced confounding, as RLS and depression are more prevalent in both conditions; and in Parkinson disease, an issue of dopaminergic treatment, which is used to treat RLS.
Our definition of hypopnea included only breathing events associated with ≥3% oxygen desaturation. Hypopneas associated with arousal were not scored, so we cannot rule out the possibility that PLMS were over-scored to include limb movements produced by disordered breathing. To our knowledge, this is the first study that attempts to determine what factors mediate, modify, or confound the common association between RLS and depression. In large part, we expected that measures of sleep disruption would mediate and antidepressant usage would confound this important clinical association. None of the candidate measures were very strong mediators of RLS-depression, but sleep efficiency did explain a modest amount of the RLS-depression relationship in regression models. Effect modification was further suggested for PLMI in stratification models, as only among men with frequent PLMS was there significant association of RLS and number of depressive symptoms. This study provides a rationale to study what factors may affect the RLS-depression relationship. Similar studies are needed to examine this association among women and other groups. It will be important in future studies to perform formal mediation analyses in a prospective cohort, where a depression outcome is measured at a later date. Such studies would help determine the sequence of events when considering the co-existing entities of RLS and depression.
Acknowledgments
FUNDING
The authors would like to thank all of the participating MrOS centers and the centralized sleep reading center.
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128.
The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers:, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
FINANCIAL DISCLOSURES
Brian Koo serves on the medical advisory board for the Restless Legs Syndrome Foundation. Terri Blackwell reports no disclosures.
Hochang B. Lee is funded by NIH: R01 MH085740.
Katie Stone is funded by NIH: R01 AG026720, R01 HL071194.
Elan Louis is funded by NIH: R01 NS073872, R01 NS039422.
Susan Redline received a grant from the Resmed Foundation; her institution has received equipment for use in NIH funded studies and is supported by multiple NIH grants: R01 HL098433, R01 HL113338.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURE
Dr. Koo has no conflicts of interest to disclose.
Dr. Blackwell reports no disclosures.
Dr. Lee reports no disclosures.
Dr. Stone is funded by NIH: RO1 2803205.
Dr. Louis reports no disclosures.
Dr. Redline has received a grant from the Resmed Foundation; her institution has received equipment for use in NIH funded studies and is supported by multiple NIH grants.
References
- 1.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–1292. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 2.Kim WH, Kim BS, Kim SK, et al. Restless legs syndrome in older people: a community-based study on its prevalence and association with major depressive disorder in older Korean adults. Int J Geriatr Psychiatry. 2012;27:565–572. doi: 10.1002/gps.2754. [DOI] [PubMed] [Google Scholar]
- 3.Aguera-Ortiz L, Perez MI, Osorio RS, et al. Prevalence and clinical correlates of restless legs syndrome among psychogeriatric patients. Int J Geriatr Psychiatry. 2011;26:1252–1259. doi: 10.1002/gps.2674. [DOI] [PubMed] [Google Scholar]
- 4.Winkelman JW, Redline S, Baldwin CM, et al. Polysomnographic and health-related quality of life correlates of restless legs syndrome in the Sleep Heart Health Study. Sleep. 2009;32:772–778. doi: 10.1093/sleep/32.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee HB, Hening WA, Allen RP, et al. Restless legs syndrome is associated with DSM-IV major depressive disorder and panic disorder in the community. J Neuropsychiatry Clin Neurosci. 2008;20:101–105. doi: 10.1176/jnp.2008.20.1.101. [DOI] [PubMed] [Google Scholar]
- 6.Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006;7:545–552. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Rye DB, Trotti LM. Restless legs syndrome and periodic leg movements of sleep. Neurol Clin. 2012;30:1137–1166. doi: 10.1016/j.ncl.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Happe S, Reese JP, Stiasny-Kolster K, et al. Assessing health-related quality of life in patients with restless legs syndrome. Sleep Med. 2009;10:295–305. doi: 10.1016/j.sleep.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76:255–259. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 10.Winkelmann J, Prager M, Lieb R, et al. “Anxietas tibiarum”. Depression and anxiety disorders in patients with restless legs syndrome. J Neurol. 2005;252:67–71. doi: 10.1007/s00415-005-0604-7. [DOI] [PubMed] [Google Scholar]
- 11.Montplaisir J, Boucher S, Poirier G, et al. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–65. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 12.Stefansson H, Rye DB, Hicks A, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357:639–647. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 13.Baumann CR, Marti I, Bassetti CL. Restless legs symptoms without periodic limb movements in sleep and without response to dopaminergic agents: a restless legs-like syndrome? Eur J Neurol. 2007;14:1369–1372. doi: 10.1111/j.1468-1331.2007.01981.x. [DOI] [PubMed] [Google Scholar]
- 14.Page RLn, Ruscin JM, Bainbridge JL, et al. Restless legs syndrome induced by escitalopram: case report and review of the literature. Pharmacotherapy. 2008;28:271–280. doi: 10.1592/phco.28.2.271. [DOI] [PubMed] [Google Scholar]
- 15.Prospero-Garcia KA, Torres-Ruiz A, Ramirez-Bermudez J, et al. Fluoxetine-mirtazapine interaction may induce restless legs syndrome: report of 3 cases from a clinical trial. J Clin Psychiatry. 2006;67:1820. doi: 10.4088/jcp.v67n1122d. [DOI] [PubMed] [Google Scholar]
- 16.Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58:510–514. doi: 10.1016/j.biopsych.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 20.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–132. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 21.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 22.Recording and scoring leg movements. The Atlas Task Force. Sleep. 1993;16:748–759. [PubMed] [Google Scholar]
- 23.Iber C, Ancoli-Israel S, Chesson A. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1st. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24.Chae KY, Kripke DF, Poceta JS, et al. Evaluation of immobility time for sleep latency in actigraphy. Sleep Med. 2009;10:621–625. doi: 10.1016/j.sleep.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Oakley N. Validation with polysomnography of the Sleepwatch sleep/wake scoring algorithm used by the Actiwatch activity monitoring system. 1997 [Google Scholar]
- 26.Kushida CA, Chang A, Gadkary C, et al. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 27.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Sheikh J, Yesavage J. Geriatric Depression Scale: recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 29.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 30.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16:2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 31.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg D, Bridges K, Duncan-Jones P, et al. Detecting anxiety and depression in general medical settings. Bmj. 1988;297:897–899. doi: 10.1136/bmj.297.6653.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pahor M, Chrischilles EA, Guralnik JM, et al. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 34.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 35.Hall MH, Smagula SF, Boudreau RM, et al. Association between sleep duration and mortality is mediated by markers of inflammation and health in older adults: the Health, Aging and Body Composition Study. Sleep. 2015;38:189–195. doi: 10.5665/sleep.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmbeck GN. Toward terminological, conceptual, and statistical clarity in the study of mediators and moderators: examples from the child-clinical and pediatric psychology literatures. J Consult Clin Psychol. 1997;65:599–610. doi: 10.1037//0022-006x.65.4.599. [DOI] [PubMed] [Google Scholar]
- 37.Barbour KE, Lui LY, Ensrud KE, et al. Inflammatory markers and risk of hip fracture in older white women: the study of osteoporotic fractures. J Bone Miner Res. 2014;29:2057–2064. doi: 10.1002/jbmr.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visser M, Simonsick EM, Colbert LH, et al. Type and intensity of activity and risk of mobility limitation: the mediating role of muscle parameters. J Am Geriatr Soc. 2005;53:762–770. doi: 10.1111/j.1532-5415.2005.53257.x. [DOI] [PubMed] [Google Scholar]
- 39.Hanewinckel R, Maksimovic A, Verlinden VJ, et al. The impact of restless legs syndrome on physical functioning in a community-dwelling population of middle-aged and elderly people. Sleep Med. 2015;16:399–405. doi: 10.1016/j.sleep.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Tison F, Crochard A, Leger D, et al. Epidemiology of restless legs syndrome in French adults: a nationwide survey: the INSTANT Study. Neurology. 2005;65:239–246. doi: 10.1212/01.wnl.0000168910.48309.4a. [DOI] [PubMed] [Google Scholar]
- 41.Walters AS, Frauscher B, Allen R, et al. Review of diagnostic instruments for the restless legs syndrome/Willis-Ekbom Disease (RLS/WED): critique and recommendations. J Clin Sleep Med. 2014;10:1343–1349. doi: 10.5664/jcsm.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hornyak M, Feige B, Voderholzer U, et al. Polysomnography findings in patients with restless legs syndrome and in healthy controls: a comparative observational study. Sleep. 2007;30:861–865. doi: 10.1093/sleep/30.7.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hening W, Walters AS, Allen RP, et al. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. 2004;5:237–246. doi: 10.1016/j.sleep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Wetter TC, Stiasny K, Kohnen R, et al. Polysomnographic sleep measures in patients with uremic and idiopathic restless legs syndrome. Mov Disord. 1998;13:820–824. doi: 10.1002/mds.870130511. [DOI] [PubMed] [Google Scholar]
- 45.Sandor P, Shapiro CM. Sleep patterns in depression and anxiety: theory and pharmacological effects. J Psychosom Res. 1994;38(Suppl 1):125–139. doi: 10.1016/0022-3999(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 46.Winkelman JW. Periodic limb movements in sleep–endophenotype for restless legs syndrome? N Engl J Med. 2007;357:703–705. doi: 10.1056/NEJMe078129. [DOI] [PubMed] [Google Scholar]
- 47.Aksu M, Demirci S, Bara-Jimenez W. Correlation between putative indicators of primary restless legs syndrome severity. Sleep Med. 2007;8:84–89. doi: 10.1016/j.sleep.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 49.Ferri R, Fulda S, Manconi M, et al. Night-to-night variability of periodic leg movements during sleep in restless legs syndrome and periodic limb movement disorder: comparison between the periodicity index and the PLMS index. Sleep Med. 2013;14:293–296. doi: 10.1016/j.sleep.2012.08.014. [DOI] [PubMed] [Google Scholar]


