Abstract
Objectives To assess the effectiveness of five gastroprotective strategies for people taking non-steroidal anti-inflammatory drugs (NSAIDs)—H2 receptor antagonists plus non-selective (or cyclo-oxygenase-1) NSAIDs; proton pump inhibitors plus non-selective NSAIDs; misoprostol plus non-selective NSAIDs; COX-2 selective NSAIDs; or COX-2 specific NSAIDs—in reducing serious gastrointestinal complications, symptomatic ulcers, serious cardiovascular or renal disease, and deaths, and improving quality of life.
Data sources The Cochrane Library, Medline, Embase, Current Controlled Trials, and System for Information on Grey Literature in Europe (SIGLE) were searched to May 2002. Bibliographies and author contacts were used to identify further studies; non-English articles were included.
Review methods Trial selection, data extraction, and quality assessment were performed independently, in duplicate. Articles were rejected only if the study was not a randomised controlled trial; did not assess a gastroprotective strategy versus placebo; included exclusively children or healthy volunteers; lasted less than 21 days; or no review outcomes were measured. Quality assessment included allocation concealment and baseline similarity.
Random effects meta-analysis, meta-regression and subgrouping were used to pool effects and analyse associations with length of follow up, mean age, and baseline gastrointestinal status. Heterogeneity was examined and sensitivity analyses performed.
Results Of 112 included randomised controlled trials (74 666 participants), five were judged to be at low risk of bias, and 138 deaths and 248 serious gastrointestinal events were reported overall. On comparing gastroprotective strategies versus placebo we found no evidence of effectiveness of H2 receptor antagonists for any primary outcomes (few events reported); proton pump inhibitors may reduce the risk of symptomatic ulcers (relative risk 0.09, 95% confidence interval 0.02 to 0.47); misoprostol reduces the risk of serious gastrointestinal complications (0.57, 0.36 to 0.91) and symptomatic ulcers (0.36, 0.20 to 0.67); COX-2 selectives reduce the risk of symptomatic ulcers (0.41, 0.26 to 0.65) and COX-2 specifics reduce the risk of symptomatic ulcers (0.49, 0.38 to 0.62) and possibly serious gastrointestinal complications (0.55, 0.38 to 0.80). All strategies except COX-2 selectives reduce the risk of endoscopic ulcers (at least 3 mm in diameter).
Conclusions Misoprostol, COX-2 specific and selective NSAIDs, and probably proton pump inhibitors significantly reduce the risk of symptomatic ulcers, and misoprostol and probably COX-2 specifics significantly reduce the risk of serious gastrointestinal complications, but data quality is low. More data on H2 receptor antagonists and proton pump inhibitors are needed, as is better reporting of rare but important outcomes.
Introduction
In 1999, more than 18.5 million courses of non-steroidal anti-inflammatory drugs (NSAIDs) were prescribed in England and Wales1 for musculoskeletal conditions such as rheumatoid arthritis, osteoarthritis, and back pain. NSAIDs cause gastrointestinal side effects, ranging in severity from mild dyspepsia to gastric haemorrhage and perforation, potentially resulting in admission to hospital, surgery, and death. In the United Kingdom they result in 10 000 admissions to hospital and 2000 deaths annually.2
Standard protection against gastrointestinal toxicity induced by NSAIDs has entailed co-prescription of gastroprotective agents such as H2 receptor antagonists, proton pump inhibitors, or prostaglandin analogues (primarily misoprostol), but prescription of COX-2 NSAIDs alone is now an alternative. Some older NSAIDs exhibit substantial COX-2 activity (COX-2 selectives, which include etodolac, meloxicam, nabumetone, and nimesulide). Newer COX-2 NSAIDs have been developed for their COX-2 activity (COX-2 specifics include celecoxib and rofecoxib). Guidelines from the National Institute of Clinical Excellence (NICE) say that COX-2 NSAIDs and non-selective (COX-1 or conventional) NSAIDs without gastroprotection are equivalent in reducing pain and improving physical functioning in people with arthritis, and fewer gastrointestinal events are associated with COX-2 NSAIDs.1 Lister3,4 found that several NSAIDs all had equivalent efficacy at equivalent dosage (at population level), therefore all NSAIDs were assumed to be of equal efficacy.
This review aimed to assess effectiveness of five protective strategies—non-selective NSAID plus H2 receptor antagonists; non-selective NSAID plus proton pump inhibitors; non-selective NSAID plus misoprostol; COX-2 selective NSAID only; or COX-2 specific NSAID only—in reducing the incidence of gastrointestinal adverse effects. We also assessed the effects of length of follow up, age of participants, baseline gastrointestinal status, number of risk factors, and initial NSAID dose.
Methods
Searching
We searched the Cochrane Library (Issue 2, 2002), Medline (Ovid, 1966 to 2002), Embase (Ovid, 1980 to 2002), Current Controlled Trials, and System for Information on Grey Literature in Europe (SIGLE) by using structured electronic search strategies (text and MeSH terms in the format (((“NSAID” and “PPI or H2RA or misoprostol”) or “COX-2”) and “prospective study design”) in May 2002.
We checked bibliographies of included studies and identified systematic reviews.5 We attempted to contact authors of all included randomised controlled trials to provide information about unidentified studies, our primary outcomes, and study quality criteria. We had all potentially relevant non-English articles translated.
Selection
We assessed titles or abstracts and full text articles for inclusion, independently and in duplicate. We resolved differences by discussion. When neither assessor could reject a title or abstract with certainty we obtained the full text article. We rejected articles only if the reviewers could determine that the article was not a randomised controlled trial; the trial did not address any of the five treatment strategies compared with non-selective NSAIDs alone; the trial included exclusively children or healthy volunteers; the study period was less than 21 days; or none of our outcomes were measured (this was based only on assessment of the full publication). We had originally planned that all COX-2 NSAIDs would constitute one gastroprotective strategy. However, subgrouping into specifics and selectives showed that combining the two groups was inappropriate, so we separated the two strategies.
Primary outcomes were serious gastrointestinal complications (including haemorrhage, haemorrhagic erosions, recurrent upper gastrointestinal bleeds, perforation, pyloric obstruction, melaena, and death from any of these); symptomatic ulcers; serious cardiovascular or renal illness; health related quality of life (not measures of arthritis pain or disability); and mortality.
Secondary outcomes included total gastrointestinal symptoms, endoscopic ulcers (at least 3 mm in diameter), anaemia, occult bleeding, total dropouts, and dropouts owing to gastrointestinal symptoms.
We assessed outcomes to the latest point available in each study and assessed them as numbers of people with events for each outcome, for each arm. We sought ulcer healing studies for information on deaths, quality of life, cardiovascular and renal events; and we sought cohort studies for information on deaths but identified no suitable studies.
Validity assessment and data extraction
Quality assessment of randomised controlled trials included information on randomisation procedures, allocation concealment, similarity at baseline, blinding of participants, providers of care and assessors of outcomes, and losses to follow up.6,7 We used Cohen's κ to assess agreement for allocation concealment.8 Two reviewers independently extracted study data and quality assessed included studies; we resolved differences between reviewers' results by discussion and, when necessary, through consultation.
We based the summary risk of bias on assessment of allocation concealment and baseline comparability (see table 1).
Table 1.
Study characteristics and summary risk of bias of the studies included in the five comparisons
|
Baseline gastrointestinal status*
|
Studies with participants' mean age >65 years
|
Study arms at too high dosage
|
Summary risk of bias†
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparison | No of studies (participants) | Publication dates | % of women | 1 | 2 | 3 | 4 | 5 | 6 | Low | Medium | High | ||
| H2 receptor antagonist v placebo | 15 (2621) | 1987-1997 | 66‡ | 4 | 7 | 0 | 2 | 1 | 1 | 0§ | 7 | 0 | 13 | 2 |
| Proton pump inhibitor v placebo | 6 (1358) | 1996-2002 | 69 | 1 | 3 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 3 | 2 |
| Misoprostol v placebo | 23 (16 945) | 1988-2002 | 68 | 1 | 12 | 1 | 3 | 3 | 3 | 3¶ | 0 | 1 | 18 | 4 |
| COX-2 selective v non-selective | 51 (28 178) | 1989-2002 | 65 | 1 | 4 | 0 | 0 | 46 | 0 | 8** | 5 | 0 | 34 | 17 |
| COX-2 specific v non-selective | 17 (25 564) | 1999-2002 | 73 | 0 | 6 | 0 | 0 | 11 | 0 | 0 | 5 | 3 | 13 | 1 |
1=normal gut on endoscopy for all participants; 2=some participants have a normal gut on endoscopy, others have some erosions or haemorrhages, but no frank ulcers; 3=all participants have some abnormal symptoms on baseline endoscopy (no ulcers or up to 50% recently healed ulcers); 4=all participants have recently healed ulcers on baseline endoscopy (at least 50% recently healed ulcers); 5=no baseline endoscopy, or no gut status reported; 6=mix, from normal gut on endoscopy to frank ulcers.
Based on assessment of allocation concealment and baseline comparability. If either or both criteria were classed as “inadequate” the summary risk of bias was judged “high,” if either or both criteria were “unclear” then summary risk of bias was “moderate,” and if both criteria were “adequate” the summary risk of bias was “low.”
Not stated in three studies.
Not stated in two studies.
Not stated in two studies.
Not stated in four studies.
Quantitative data synthesis
We tabulated data on included studies. Where appropriate we used relative risks in random effects meta-analysis9 on RevMan, version 4.2, software to combine numbers of people with outcomes. We examined heterogeneity visually and by using Cochran's test (significant at P < 0.1).
We performed random effects meta-regression (Stata, version 7.010) to analyse associations between treatment effect and duration of follow up; participants' mean age; baseline gastrointestinal status (quantified as percentage of participants with a history of ulcers or bleeds); and number of initial risk factors for gastrointestinal toxicity. The outcome was symptomatic ulcers (but where insufficient studies provided data, we used endoscopic ulcers). Numbers needed to treat were the inverse of risk differences for studies where participants had a normal gut or some erosions or haemorrhages or both (no frank ulcers) on endoscopy, and reported symptomatic ulcers.
Sensitivity analyses assessed robustness of results to removal of studies that had a “high” summary risk of bias, arms with higher than recommended doses of NSAID or gastroprotective agent,11 and naproxen arms (for deaths and serious cardiovascular and renal events).
We used funnel plots and related inferential methods to assess for evidence of small study effects, including publication bias,12 using Egger's13 and Begg's14 tests (on StatsDirect, version 2.2.8).
Results
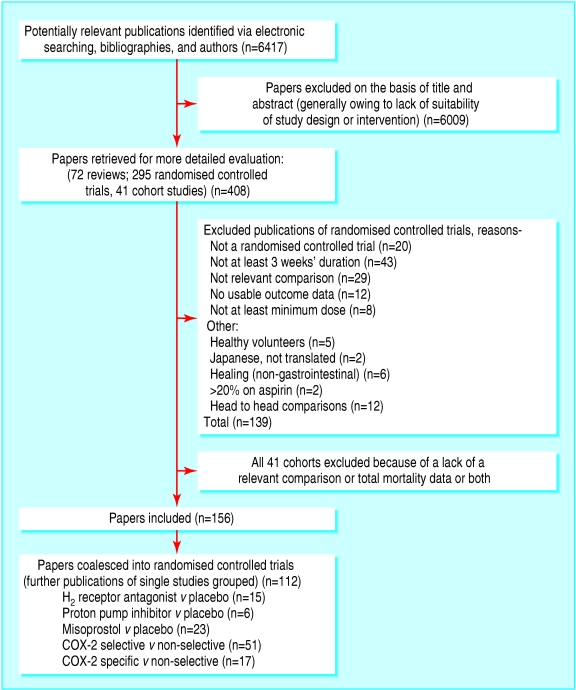
Figure 1 shows the flow of participants through the trial15; table 1 shows characteristics and validity of included studies; table 2 shows results from the meta-analysis; and figures 2, 3, 4 and 5 show forest plots of the primary outcomes.
Fig 1.
Flow of studies through the trial
Table 2.
Results of a meta-analysis for the five gastroprotective strategies on primary and secondary health outcomes. Values are numbers of events (relative risks, with 95% confidence intervals) unless otherwise indicated
| H2 receptor antagonist v placebo | Proton pump inhibitor v placebo | Misoprostol v placebo | COX-2 selectives v non-selectives | COX-2 specifics v non-selectives | |
|---|---|---|---|---|---|
| Primary outcomes | |||||
| Total No of trial participants | 2621 | 1358 | 16 945 | 28 178 | 25 564 |
| Serious gastrointestinal complications | 1 (0.33, 0.0 to 8.1) | 3 (0.46, 0.1 to 2.9) | 75 (0.57, 0.4 to 0.9)* | 43 (0.61, 0.3 to 1.1) | 114 (0.55, 0.4 to 0.8)† |
| Symptomatic ulcers | 1 (1.46, 0.1 to 35.5) | 18 (0.09, 0.0 to 0.5)† | 54 (0.36, 0.2 to 0.7)* | 82 (0.41, 0.3 to 0.7)* | 281 (0.49, 0.4 to 0.6)* |
| Serious cardiovascular or renal events | 5 (0.53, 0.1 to 3.5) | 3 (0.78, 0.1 to 6.3) | 4 (1.78, 0.3 to 12.1) | 48 (0.95, 0.6 to 1.7) | 241 (1.19, 0.8 to 1.8) |
| Mortality | 1 (3.00, 0.1 to 68.3) | 1 (0.17, 0.0 to 4.1) | 35 (0.89, 0.5 to 1.7) | 19 (0.68, 0.3 to 1.6) | 78 (1.02, 0.6 to 1.9) |
| Health related quality of life | N/A | N/A | N/A | Weighted mean difference −0.10 (−1.0 to 0.8) | N/A |
| Secondary outcomes | |||||
| Gastrointestinal symptoms | 201 (0.72, 0.6 to 0.9)† | 45 (0.43, 0.2 to 0.8)* | 1218 (0.97, 0.7 to 1.4) | 3894 (0.73, 0.7 to 0.8)* | 5184 (0.81, 0.7 to 0.9)* |
| Endoscopic ulcers | 250 (0.55, 0.4 to 0.7)* | 281 (0.37, 0.3 to 0.5)* | 658 (0.33, 0.3 to 0.4)* | 24 (0.41, 0.2 to 1.1) | 522 (0.25, 0.2 to 0.3)* |
| Anaemia | 1 (3.00, 0.1 to 73.3) | N/A | 1 (2.66, 0.1 to 63.8) | 6 (0.30, 0.1 to 1.3) | 464 (0.62, 0.5 to 0.7)† |
| Occult bleeding | N/A | N/A | 16 (0.46, 0.2 to 1.3) | 17 (0.86, 0.3 to 2.2) | N/A |
| Total dropouts | 362 (0.97, 0.8 to 1.1) | 116 (0.98, 0.6 to 1.5) | 4772 (1.11, 1.0 to 1.2)‡ | 4274 (0.93, 0.9 to 1.0)* | 9510 (0.82, 0.7 to 0.9)† |
| Dropouts due to gastrointestinal symptoms | 57 (0.71, 0.4 to 1.2) | 48 (0.45, 0.3 to 0.8)* | 2332 (1.36, 1.3 to 1.5)§ | 1174 (0.63, 0.6 to 0.7)* | 2171 (0.69, 0.6 to 0.8)† |
N/A=not available.
Significance level 5%.
Significant protective relation, no heterogeneity and significance not lost on sensitivity analysis.
Significant protective relationship, but significant heterogeneity or significance lost on sensitivity analysis.
Significant harmful relationship, but significant heterogeneity or significance lost on sensitivity analysis.
Significant harmful relationship, no heterogeneity and significance not lost on sensitivity analysis
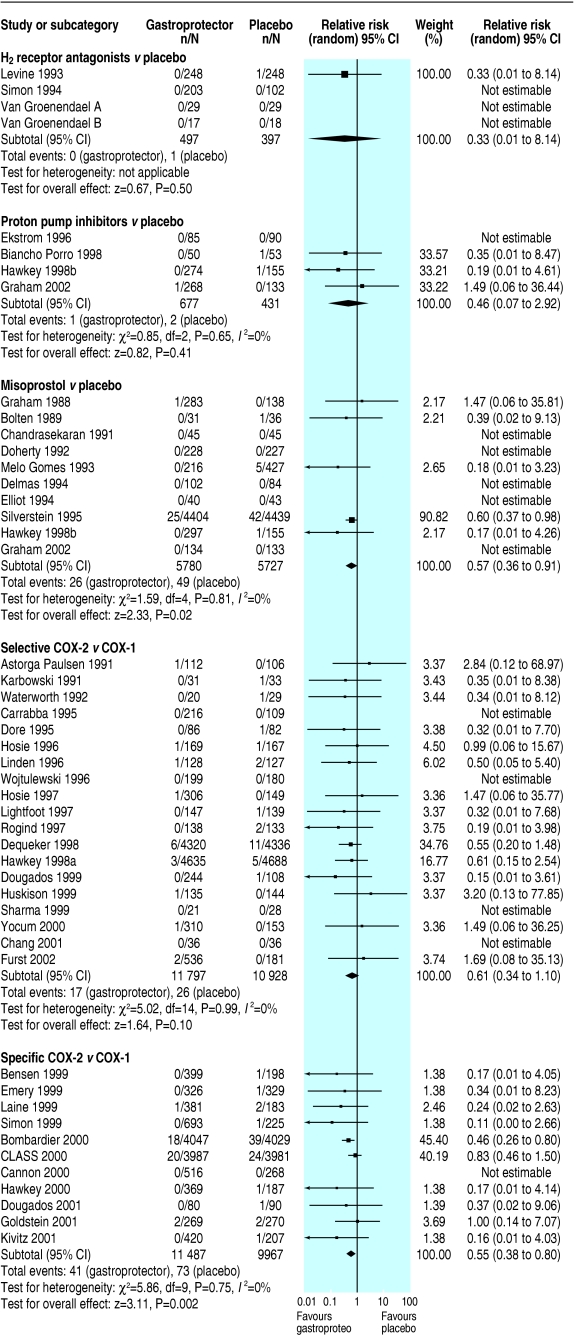
Fig 2.
Effects of gastroprotective strategies on serious gastrointestinal complications
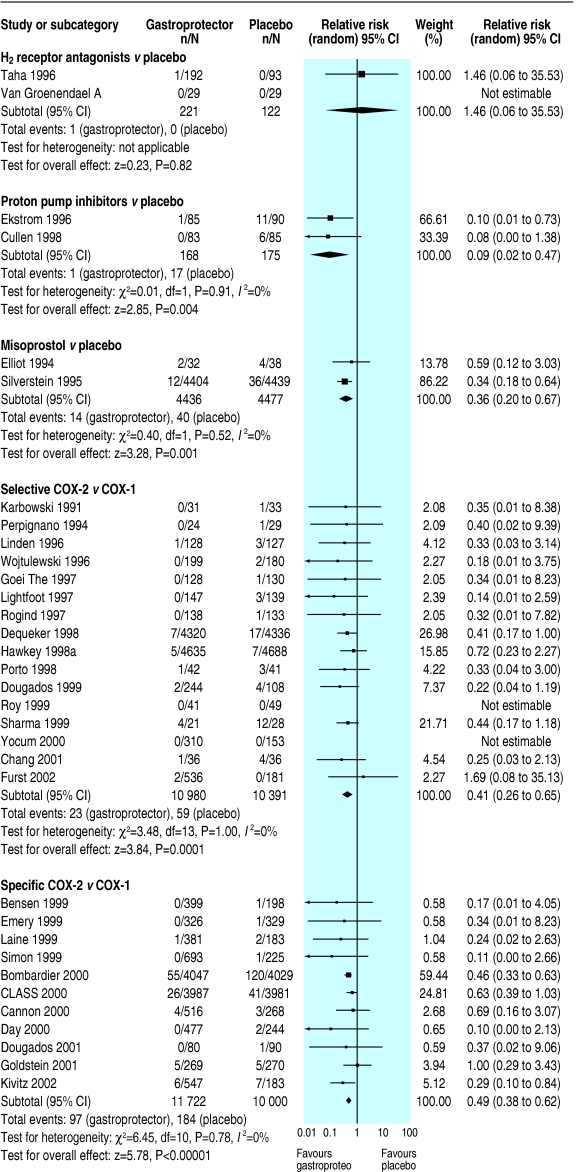
Fig 3.
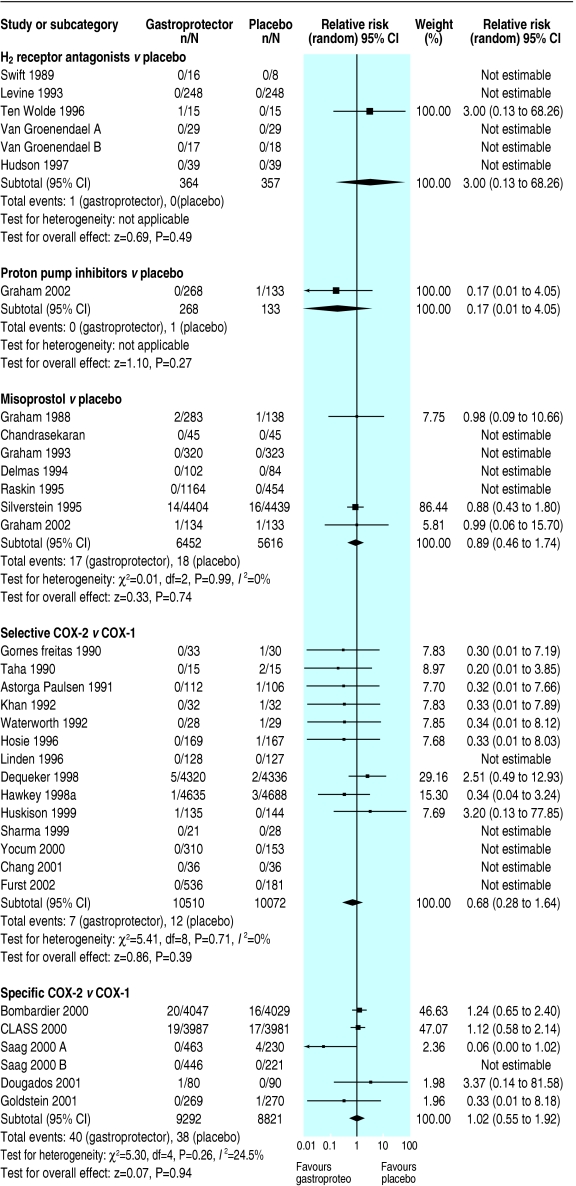
Effects of gastroprotective strategies on symptomatic ulcers
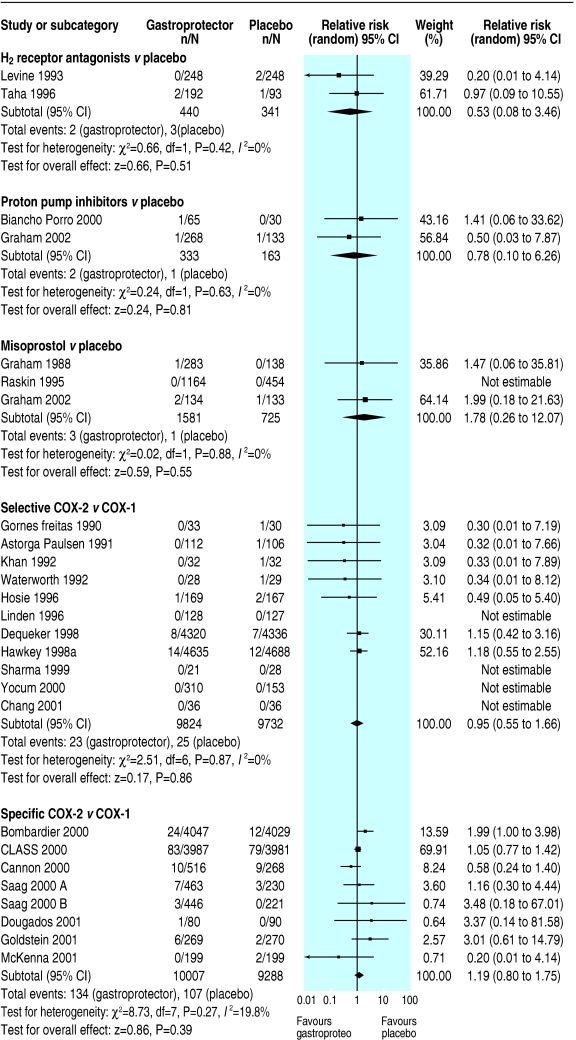
Fig 4.
Effects of gastroprotective strategies on serious cardiovascular or renal illness
Fig 5.
Effects of gastroprotective strategies on deaths
We found no evidence of publication bias in any of the five comparisons. The κ coefficient for agreement on allocation concealment was 0.36 (95% confidence interval 0.18 to 0.53), observed agreement 91.3%. Extra data from authors on outcomes and study design arrived for eight included studies.
H2 receptor antagonists plus non-selective NSAID versus placebo plus non-selective NSAID
Fifteen randomised controlled trials (including 2621 participants) studied this comparison; we assessed risk of bias as low in none of them.
Only one serious gastrointestinal event, one symptomatic ulcer, one death, and four serious cardiovascular events were reported, with no data on health related quality of life measures. Data were insufficient to draw conclusions on effect of H2 receptor antagonists compared with placebo on any primary outcomes. More data related to endoscopic ulcers, which were significantly reduced in participants on H2 receptor antagonists compared with placebo (relative risk 0.55, 95% confidence interval 0.4 to 0.7). Subgrouping was not possible owing to paucity of primary outcomes.
Proton pump inhibitors plus non-selective NSAID versus placebo plus non-selective NSAID
Six randomised controlled trials (1358 participants) compared proton pump inhibitors with placebo; we assessed one as having a low risk of bias.
Few studies reported this review's primary outcomes. It was not possible to assess the effect of proton pump inhibitors compared with placebo on serious gastrointestinal complications, serious cardiovascular or renal illness, quality of life, or death. The apparently significant reduction of symptomatic ulcers in patients taking proton pump inhibitors (0.09, 0.0 to 0.5) compared with placebo was lost on sensitivity analysis. Endoscopic ulcers seemed significantly reduced in participants taking proton pump inhibitors compared with placebo; this finding was stable to sensitivity analysis (0.37, 0.3 to 0.5).
Misoprostol plus non-selective NSAID versus placebo plus non-selective NSAID
Twenty three randomised controlled trials compared misoprostol with placebo (16 945 participants); we assessed one as having a low risk of bias.
Misoprostol significantly reduced serious gastrointestinal complications (0.57, 0.4 to 0.9), symptomatic ulcers (0.36, 0.2 to 0.7), and endoscopic ulcers (0.33, 0.3 to 0.4); all findings were stable to sensitivity analysis, with no apparent heterogeneity. No significant effects of misoprostol on serious cardiovascular or renal illness, deaths, anaemia or occult bleeding occurred, but few events had been recorded.
COX-2 selective NSAID versus non-selective NSAID
Fifty one randomised controlled trials compared COX-2 selective NSAIDs with non-selective NSAIDs (28 178 participants); we assessed the risk of bias as low in none of them.
Symptomatic ulcers were less likely in patients taking COX-2 selectives than in patients taking non-selectives (0.41, 0.3 to 0.7, result robust to sensitivity analysis, without apparent heterogeneity). Fewer than 50 events were reported for other primary outcomes, none reached significance. Few endoscopic ulcers occurred, with no significant differences from non-selectives.
COX-2 specific NSAID versus non-selective NSAID
Seventeen randomised controlled trials compared COX-2 specific NSAIDs with non-selective NSAIDs (25 564 participants). We assessed summary risk of bias as low in three studies.
Serious gastrointestinal complications (0.55, 0.4 to 0.8) and symptomatic ulcers (0.49, 0.4 to 0.6) seemed significantly reduced in participants randomised to COX-2 specifics compared with non-selectives (symptomatic ulcers, but not serious gastrointestinal complications, were robust to sensitivity analysis, both without apparent heterogeneity). Serious cardiovascular or renal illness and total deaths were not significantly different, and data were insufficient to draw conclusions regarding quality of life. Endoscopic ulcers were significantly less common in patients taking COX-2 specifics (0.25, 0.2 to 0.3, results stable to sensitivity analysis).
Associations between treatment effects and potential effect modifiers
Meta-regression found no significant relations between relative risk of symptomatic ulcers (for COX-2 selectives and specifics) or endoscopic ulcers (for H2 receptor antagonists, proton pump inhibitors, misoprostol) and duration of follow up, mean age, or baseline gastrointestinal status. The exception was between endoscopic ulcers and study duration for misoprostol, showing reduced protection from endoscopic ulcers in longer trials. Subgrouping trials by mean age (over 65 years or not) did not show any clear pattern in reductions of absolute risk for serious gastrointestinal events, symptomatic ulcers, or endoscopic ulcers, but data were sparse (not shown).
Numbers needed to treat to prevent one symptomatic ulcer
For misoprostol we could not calculate the number needed to treat to prevent one additional symptomatic ulcer for groups of people with normal gastrointestinal tracts, some erosions, or submucosal haemorrhages (but no frank ulcers) on endoscopy. It was infinite for H2 receptor antagonists and COX-2 specifics (risk differences zero), 14 (8 to 100) for proton pump inhibitors, and 17 (number needed to treat to harm 100 to ∞ to number needed to treat to benefit 8) for COX-2 selectives.
Discussion
Summary
Comparing gastroprotective strategies with placebo, we found no evidence of effectiveness of H2 receptor antagonists for any primary outcomes (few events reported); proton pump inhibitors may reduce the risk of symptomatic ulcers (relative risk 0.09, 95% confidence interval 0.02 to 0.47); misoprostol reduces the risk of serious gastrointestinal complications (0.57, 0.36 to 0.91) and symptomatic ulcers (0.36, 0.20 to 0.67); COX-2 selectives reduce the risk of symptomatic ulcers (0.41, 0.26 to 0.65); and COX-2 specifics reduce the risk of symptomatic ulcers (0.49, 0.38 to 0.62) and possibly serious gastrointestinal complications (0.55, 0.38 to 0.80). All strategies except COX-2 selectives reduce the risk of endoscopic ulcers.
We judged five of 112 included randomised controlled trials (74 666 participants) as having a low risk of bias, and overall 138 deaths and 248 serious gastrointestinal events were reported.
Limitations and strengths of the study
Sparse reporting of our primary outcomes was a weakness of the review. Many publications did not report important outcomes, or they mentioned events in an ad hoc manner. In collecting these few events their real level of occurrence may not have been accurately reflected, which may have led to bias. Some outcomes overlapped—for example, a patient who died from a serious gastrointestinal bleed was recorded in both the “mortality” and the “serious gastrointestinal complications” outcomes. However, an individual was recorded only once within any outcome category.
We hoped to overcome this lack of data partly through contact with study authors, but we received few replies. A common response from contact authors was that the relevant data were held by the sponsoring pharmaceutical company. When additional information arrived it invariably improved the study's quality ratings. Few studies clearly reported allocation concealment or blinding of outcome assessors; some trials are likely to be of higher methodological quality than our analysis indicates. The recent review of celecoxib studies by Deeks et al accessed manufacturer reports (which provide greater detail than published studies) and rated all nine studies highly.16
Few studies listed funding sources that did not include a pharmaceutical company. This may increase bias where few hard outcomes occur or are reported.17,18
The lack of significant results in the meta-regressions does not necessarily imply a lack of a relation between risk of ulcers and study duration, baseline gastrointestinal status, or age in people taking these gastroprotectors. Our use of summary data (rather than data at the level of the patient) weakened our power to see small effects. Data were too sparse to interpret whether the absolute risk of events was reduced in elderly people, and no reductions in relative risks in older people were apparent, although observational studies show that the older the people taking NSAIDs are, the greater their risk of hospital admission for a gastrointestinal event.19
These issues limit the conclusions that can be drawn from this review, and need to be borne in mind when making clinical or policy decisions based on this evidence.
The strength of this systematic review was in pooling all the relevant studies to answer a question about relatively rare events that few trials will be powered to assess individually. It allows assessment of the consistency of this information across studies and settings. Primary outcomes were events that clearly affect health, longevity, and quality of life of people taking NSAIDs; we collected other outcomes with less direct links to health and wellbeing only as secondary outcomes. However, this process is useful only when information about rare events is available.
The large body of evidence comparing COX-2 NSAIDs with non-selectives is not matched by studies of the other gastroprotectors. Fewer than 2000 people participated in the proton pump inhibitor trials, but more than 25 000 participated in studies of both selective and COX-2 specifics. We may pick up small effects of COX-2 NSAIDs as significant but miss larger effects of other gastroprotectors, because of the relative volumes of data.
What is already known on this topic
Non-steroidal anti-inflammatory drugs (NSAIDs) are known to have gastrointestinal side effects
These range from mild dyspepsia to death from gastric haemorrhage and perforation
Gastroprotective agents are commonly co-prescribed with NSAIDs to protect against these side effects
What this study adds
Misoprostol, COX-2 specific and selective NSAIDs, and probably proton pump inhibitors significantly reduce the risk of symptomatic ulcers
Misoprostol and probably COX-2 specific NSAIDs reduce the risk of serious gastrointestinal complications, but the quality of data is low
Comparisons with other systematic reviews
A Cochrane review by Rostom et al assessed the effectiveness of H2 receptor antagonists, proton pump inhibitors, and misoprostol against endoscopic ulcers, ulcer complications, symptoms, and dropouts.5 They found that all three prevented endoscopic ulcers and only misoprostol prevented ulcer complications (such as perforation, haemorrhage, or obstruction). Our review added assessment of COX-2 selectives and specifics and downplayed the importance of endoscopic ulcers as being of little relevance to patients or usual clinical practice.
Recommendations for health care
Implications for practice need to come from head to head studies of the five gastroprotective strategies and from health economic work on the overall implications. However, all of the strategies except H2 receptor antagonists (where only one event was reported) are apparently protective of symptomatic ulcers, and all of the strategies except COX-2 selectives (where only 24 events were reported) are protective against endoscopic ulcers. Misoprostol (and probably COX-2 specifics) is protective against serious gastrointestinal complications, but misoprostol seems to increase trial dropouts.
Implications for further research
A need exists for rare but important events (such as deaths, cardiovascular events, or serious gastrointestinal bleeds) to be recorded in trials (even where such trials are not powered to analyse the events), published in papers, and so become available for independent meta-analysis. At the very least, named contact authors should have access to these data.
A case is made for more independently funded research into gastroprotective agents. A very large, independent, multicentre trial measuring important outcomes for at least a year and with H2 receptor antagonists, proton pump inhibitors, misoprostol, COX-2 selective, COX-2 specific, and placebo arms would be ideal.
Many thanks to our kind and expert project steering group: Linda Davies (University of Manchester), Andrew Herxheimer (Cochrane Collaboration), Qasim Aziz (Hope Hospital, Salford). Thank you too to those researchers who generously replied to our emails and letters with information and suggestions: Kate Adams (GlaxoSmithKline), John Borrill (Pharmacia), DM Chang (Tri-Service General Hospital, Taiwan), Julian Cole (Merck Sharp & Dohme), Cyndy Collis (TAP Pharmaceuticals), Frank Degner (Boehringer Ingelheim), Lisa DeTora (Merck), Jay L Goldstein (University of Illinois at Chicago), Susan Jick (Boston Collaborative Drug Surveillance Program), Ronald Jubb (University of Birmingham), Loren Laine (University of Southern California), Louise Levine (Lilly Research Laboratories), Muhammad Mamdani (University of Toronto), David Neustadt (University of Louisville), Jeffrey B Raskin (University of Miami), Sanford H Roth (Arizona Research and Education), and Sangeeta Sharma (Institute of Human Behaviour and Allied Sciences, Delhi). Our gratitude also extends to Karen Schafheutle (University of Manchester) for German translations, Mary Ingram (University of Manchester) for help in locating papers, Roger Webb (University of Manchester) for initiating the study, and Alaa Rostom (University of Ottawa) for comments and support during the review.
Contributors: CR was involved in designing, securing funding, and providing statistical support for the review, and in editing the paper. DS was involved in the conception and design of, and securing funding for, the review, coordinating the work, abstracting data from papers, interpreting data, providing a clinical perspective, providing general advice on the review, and editing the paper. KP was involved in the design of the review, editing the paper, and providing an economic perspective. LH was involved in designing, and securing funding for, the review, designing the electronic search strategies, screening search results, screening retrieved papers against inclusion criteria, appraising quality of papers, abstracting data, writing to authors of review papers for additional information, performing meta-regressions and meta-analysis, calculations on reliability and numbers needed to treat, interpretation of the data, providing a methodological perspective, writing the paper, incorporating the edits of others, and final editing. RE was involved in designing, coordinating, and securing funding for the review; designing electronic search strategies; commenting on the review; and providing an economic perspective. TJB was involved in screening search results, checking bibliographies for further studies, organising retrieval of papers, screening retrieved papers against inclusion criteria, appraising quality of papers, abstracting data from papers, writing to authors of papers for additional information, data management for the review, performing meta-analysis and sensitivity analyses of relative risks, organising project team meetings and telephone meetings with external advisers, keeping minutes of meetings, and editing the paper. LH is guarantor.
Funding: NHS Executive, UK (NHS HTA project number 01/40/02).
Competing interests: DS has been reimbursed by Pharmacia for attending a conference.
Ethical approval: As this work consisted entirely of secondary research, ethical approval was not required.
References
- 1.National Institute for Clinical Excellence. Guidance on the use of cyclo-oxygenase (COX) II selective inhibitors, celecoxib, rofecoxib, meloxicam, and etodolac for osteoarthritis and rheumatoid arthritis. London, NICE: 2001. (Technology Appraisal Guidance, 27.)
- 2.Blower AL, Brooks A, Fenn GC, Hill A, Pearce MY, Morant S, et al. Emergency admissions for upper gastrointestinal disease and their relation to NSAID use. Aliment Pharmacol Ther 1997;11: 283-91. [DOI] [PubMed] [Google Scholar]
- 3.Eversmeyer W, Poland M, DeLapp RE, Jensen CP. Safety experience with nabumetone versus diclofenac, naproxen, ibuprofen, and piroxicam in osteoarthritis and rheumatoid arthritis. Am J Med 1993;95: 10S-8S. [DOI] [PubMed] [Google Scholar]
- 4.Lister BJ, Poland M, DeLapp RE. Efficacy of nabumetone versus diclofenac, naproxen, ibuprofen, and piroxicam in osteoarthritis and rheumatoid arthritis. Am J Med 1993;95: 2S-9S. [DOI] [PubMed] [Google Scholar]
- 5.Rostom A, Dube C, Wells G, Tugwell P, Welch V, Jolicoeur E, et al. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst Rev 2002;(4): CD002296. [DOI] [PubMed]
- 6.Cochrane Reviewers Handbook 4.1 [updated June 2000]. Oxford, England (www.cochrane.org/cochrane/hbook.htm): Cochrane Collaboration, 2000.
- 7.Juni P, Altman DG, Egger M. Assessing the quality of randomised controlled trials. In Egger M, Davey Smith G, Altman DG, eds. Systematic reviews in health care: meta-analysis in context. London: BMJ Publishing Group, 2001: 87-108.
- 8.Altman DG. Practical statistics for medical research. London: Chapman & Hall, 1991.
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials 1986;7: 177-88. [DOI] [PubMed] [Google Scholar]
- 10.Sterne JAC, Bradburn MJ, Egger M. Meta-analysis in Stata. In: Egger M, Davey Smith G, Altman DG, eds. Systematic reviews in health care: meta-analysis in context. London: BMJ Publishing Group, 2001: 347-69.
- 11.British Medical Association, Royal Pharmaceutical Society of Great Britain. British national formulary. London: BMA, RPS, 2003. (No 45.)
- 12.Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG, eds. Systematic reviews in health care: meta-analysis in context. London: BMJ Publishing Group, 2001: 189-208.
- 13.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315: 629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Begg CB. Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50: 1088-101. [PubMed] [Google Scholar]
- 15.Moher D, Eastwood S, Olkin I, Rennie D, Stroup D. Improving the quality of reports on meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 1999;354: 1896-900. [DOI] [PubMed] [Google Scholar]
- 16.Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ 2002;325: 619-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326: 1167-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rochon PA, Gurwitz JH, Simms RW, Fortin PR, Felson DT, Minaker KL, et al. A study of manufacturer-supported trials of nonsteroidal anti-inflammatory drugs in the treatment of arthritis. Arch Intern Med 1994;154: 157-63. [PubMed] [Google Scholar]
- 19.Dieppe P, Bartlett C, Davey P, Doyal L, Ebrahim S. Balancing benefits and harms: the example of non-steroidal anti-inflammatory drugs. BMJ 2004;329: 31-4. [DOI] [PMC free article] [PubMed] [Google Scholar]