Abstract
Compulsive tanning despite awareness of ultraviolet radiation (UVR) carcinogenicity may represent an “addictive” behavior. Many addictive disorders are associated with alterations in dopamine (D2/D3) receptor binding and dopamine reactivity in the brain’s reward pathway. To determine if compulsive tanners exhibited neurobiologic responses similar to other addictive disorders, this study assessed basal striatal D2/D3 binding and UVR-induced striatal dopamine efflux in ten addicted and ten infrequent tanners. In a double-blind crossover trial, UVR or sham UVR was administered in separate sessions during brain imaging with single photon emission computerized tomography (SPECT). Basal D2/D3 receptor density and UVR-induced dopamine efflux in the caudate were assessed using 123I-iodobenzamide (123I-IBZM) binding potential non-displaceable (BPnd). Basal BPnd did not significantly differ between addicted and infrequent tanners. Whereas neither UVR nor sham UVR induced significant changes in bilateral caudate BPnd in either group, post-hoc analyses revealed left caudate BPnd significantly decreased (reflecting increased dopamine efflux) in the addicted tanners – but not the infrequent tanners –during the UVR session only. Bilateral ΔBPnd correlated with tanning severity only in the addicted tanners. These preliminary findings are consistent with a stronger neural rewarding response to UVR in addicted tanners, supporting a cutaneous-neural connection driving excessive sunbed use.
Keywords: Tanning, Addiction, Dopamine, Mesostriatal reward system, SPECT, Imaging
1. Introduction
Almost 30 million Americans visit indoor tanning salons each year (Kwon et al., 2002) including over 40% of college students and 10% of teens (Wehner et al., 2014). This younger age group is particularly vulnerable to development of melanoma (Bleyer et al., 2006), an often fatal and increasingly common disease in adolescents and young adults. In recognition of these risks, ultraviolet radiation (UVR) has recently been classified as a known human carcinogen by the United States Department of Health and Human Services and the World Health Organization International Agency has elevated the UVA/UVB rays utilized in tanning devices to Group 1 (i.e. “carcinogenic to humans”) (El Ghissassi et al., 2009).
Persistent tanning despite perceived and experienced consequences suggests tanning has “addictive” properties (Nolan and Feldman, 2009). Approximately 40% of frequently sunbathers (Harrington et al., 2011; Mosher and Danoff-Burg, 2010; Poorsattar and Hornung, 2007; Warthan et al., 2005) report behaviors consistent with an addictive disorder, including an inability to decrease tanning frequency and continued tanning despite adverse consequences. Awareness of UVR toxicity, including warning labels on tanning beds, has not altered tanning activity (Knight et al., 2002; Monfrecola et al., 2000; Zeller et al., 2006). UVR may therefore have physiologically reinforcing properties distinct from any psychosocial benefits of having a tan (Feldman et al., 2004; Harrington et al., 2011). A neurocutaneous pathway mediated by β-endorphin has been posited to produce physiologic dependence to UVR and potentially affect reward and addiction-related neurobiological systems (Fell et al., 2014; Kaur et al., 2005).
The mesostriatal dopamine pathway plays a key role in both reward and uncontrolled compulsive behaviors defining the addicted state (Adinoff, 2004; Koob and Volkow, 2010). Increases in dopamine efflux follow the administration of cocaine (Mach et al., 1997), amphetamine (Drevets et al., 2001; Martinez et al., 2003), alcohol (Boileau et al., 2003), and nicotine (Fehr et al., 2008) and are associated with substance-induced euphoria (Barrett et al., 2004; Brody et al., 2004; Drevets et al., 2001; Yoder et al., 2005). Basal striatal post-synaptic D2/D3 receptors are decreased in a number of substance use disorders, presumably due to either pre-morbid risk and/or down-regulation due to persistent substance-induced dopaminergic stimulation (Fehr et al., 2008; Martinez et al., 2007; Volkow et al., 2002). Additionally, in cocaine-addicted subjects, a blunted dopaminergic efflux in response to a rewarding substance has been shown to predict greater drug craving (Martinez et al., 2007).
Our group previously explored the central nervous system (CNS) effects of UVR by exposing addicted tanners to UVR in a commercial tanning bed with one of two filters in place (Feldman et al., 2004). One filter removed UVR (“sham UVR”) whereas the other filter did not (“active UVR”). Using single photon emission computerized tomography (SPECT) to measure brain perfusion, addicted indoor tanners exposed to UVR, relative to sham UVR, showed increased regional cerebral blood flow (rCBF) in the striatum (Harrington et al., 2012). UVR may therefore have centrally active properties driving tanning over and above cosmetic benefit.
The goal of this study was to determine if UVR induces striatal dopaminergic efflux and if basal D2/D3 receptors and UVR-induced dopamine efflux was altered in addicted sunbed tanners relative to infrequent tanners. Basal D2/D3 receptors and UVR-induced dopamine efflux were assessed using 123I-iodobenzamide (123I-IBZM) striatal binding potential (BPnd) and SPECT. We hypothesized (1) striatal D2/D3 would be lower in addicted relative to infrequent tanners, (2) striatal dopamine efflux, as reflected by decreases in 123I-IBZM BPnd, would increase in response to active UVR but not sham UVR, and (3) striatal dopamine efflux would be blunted in the addicted relative to infrequent tanners. Region of interest was limited to the dorsal striatum (i.e., bilateral caudate) given the previously observed increased in rCBF (Harrington et al., 2012). Secondary aims included exploring the relationship between striatal D2/D3 BPnd and dopaminergic efflux with measures of tanning severity.
2. Methods
2.1. Study population
The study was approved by the University of Texas Southwestern Institutional Review Board (clinicaltrials.gov identifier NCT01761032). Participants were recruited through flyers and Internet advertisements. Initial screening information was collected using Research Electronic Data Capture (REDCap), a biomedical informatics tool (Harris et al., 2009). Subjects were 18–45 years old Caucasian or Hispanic men and women with Fitzpatrick skin phototype II–IV. Addicted sunbed users must have reported using a sunbed at least two times weekly over the previous year and met previously validated criteria for “tanning dependence” (Hillhouse et al., 2012), including an inability to cut down or stop tanning. Sex-, age-, ethnicity-, and skin phototype-matched infrequent tanners were included as a comparison group Inclusion criteria included a minimum of 10 lifetime episodes of sunbed use, no more than 4 indoor tanning episodes in the previous 90 days, and failure to meet criteria for tanning abuse or dependence. Familiarity with salon tanning without the addictive behaviors offered a more appropriate comparison group than a tanning-naïve group due to their lack of having formed an addiction to tanning despite adequate sunbed exposure. All subjects were right-handed. Exclusion criteria for all participants included pregnancy, use of medications with CNS properties (e.g., psychotropic medication), medical disorders that might interfere with normal brain functioning, any lifetime history of Diagnostic Statistical Manual (DSM)-IV Substance Dependence, Seasonal Affective, or Body Dysmorphic Disorder, or any active mood, psychotic or anxiety disorder.
2.2. Assessments
Assessments included the Structured Interview for Tanning Abuse and Dependence (SITAD) (Hillhouse et al., 2012), Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 2002), Body Dysmorphic Disorder modification of the Yale-Brown Obsessive-Compulsive Scale (Phillips et al., 1997), Beck Depression Scale (Beck et al., 1979), Speilberger State Anxiety Inventory (Speilberger, 1971), Fitzpatrick Skin Phototype (Fitzpatrick, 1988), routine laboratory chemistry and complete blood count, and urine drug screen. To quantitate tanning addiction severity, lifetime history of sunbed tanning episodes was obtained using the Time Line Follow Back (TLFB) (Fals-Stewart et al., 2000). The TLFB uses significant life events as chronological anchor points to accurately recall temporal patterns of tanning episodes.
A high-resolution T1-weighted magnetic resonance imaging (MRI) [3-T Phillips Achieva MR scanner; 3D magnetization prepared rapid gradient-echo (MPRAGE) sequence] was acquired in all subjects to assure the absence of cerebral anatomic pathology.
2.3. Scanning procedure
UVR was administered using a Sunquest 3000S canopy while participants were undergoing active SPECT imaging. One of two visually identical plastic/acrylic filters (Polycast UF3, Sterling Industries, Shawnee, Kansas) (Kucenic et al., 2002) was placed under the canopy (Feldman et al., 2004; Harrington et al., 2012). One filter was transparent to UVR (irradiance for UVA and UVB was 0.1 W/cm2 and 0.047 W/ cm2); the other blocked UVR (0.001 W/cm2 and 0.0 W/cm2). Both filters were transparent to visible light. The estimated dose delivered for the UVR transparent filter was 6 J/cm2 UVA and 0.282 J/cm2 UVB; for the UVR blocked filter 0.06 J/cm2 UVA and 0 J/cm2 UVB. The tanning bed canopy was placed 8 in. above the participants’ abdomen. On two separate visits, participants were imaged via SPECT while exposed to either UVR or sham UVR. Sessions were approximately seven days apart (addicted tanners: 8.4±3.6 days, range 5–17; infrequent tanners: 7.9±3.7, 4–17 days). Scan order (active or sham) was balanced across groups. Exposure to active UVR and sham UVR was presented in a double-blind design; all study staff having contact with the participant were blinded to filter placement. Prior to each session, participants were instructed to refrain from tanning for at least 48 hours so that tanners were in an unsatiated state. Upon arrival for the session, TLFB was obtained from participants since their last visit to confirm tanning had not occurred in the previous 48 h. One hour following iodoral administration (to limit thyroid exposure) a 10 mCi bolus of 123I-IBZM was administered (Anazao Health, Tampa, Florida, IND 115555). Just prior to imaging, participants changed into their tanning attire (typically a bathing suit, with torso exposed) and placed in the scanning bed. Participants were asked to rate “How much do you feel like tanning right now?” from 1 (“Not at all”) to 10 (“More than I ever have.”). To avoid overexposure in infrequent tanners, UVR exposure was determined based upon the Sunquest 3000 manufacturer recommendation ranging from 4 min (skin type II) to 8 min (skin type IV) (Table 1). To provide a physiologically relevant UVR dose, addicted tanners received 10 min of UVR exposure [consistent with Feldman et al. (2004), Harrington et al. (2012) and Kaur et al. (2006)]. The first three addicted tanners, however, received between 4 and 6 min of UVR exposure. Fifteen minutes after the initiation of UVR exposure, participants rated their enjoyment and expectations of UVR administration [“How much did you enjoy the tanning session?” from “not at all” (1) to “the most ever” (5); “How good of a tan do you expect to get from this session?” from “no tan” (1) to “the perfect tan” (5); “Do you think you received active or non-active tanning light?”]. In the second session, participants were also asked, “Did you prefer the first or second tanning session or have no preference?” To maintain blinding, upon completion of the first scan participants covered exposed body areas with a sunless tanning lotion prior to leaving the sunbed. A thermometer at the participant’s side recorded temperature during UVR. As the scanning room was chilly, blankets were placed on the participants prior to and following light exposure.
Table 1.
Demographic, clinical and UVR-response characteristics of study population.
| Addicted tanners | Infrequent tanners | |
|---|---|---|
| Demographics | ||
| Age (yrs) | 31.6±6.6 | 31±7.8 |
| Sex (female) | 7 | 8 |
| Ethnicity | ||
| Caucasian | 7 | 8 |
| Hispanic | 3 | 2 |
| Sunbed Use Measures | ||
| Age at first use (yrs) | 19.7±4.4 | 17.3±2.1 |
| # of dependence criteria (SITAD+) | 5.7±1.9** | 0.5±0.5 |
| Days sunbed use – previous 90 days | 36.5±15.2** | 4.2±3.2 |
| Days sunbed use – lifetime | 1852±1420* | 542±710 |
| Years sunbed use | 11.9±7.8 | 13.7±9.5 |
| Lifetime indoor days/yrs tanning | 165.9±64.8** | 35.2±40.3 |
| UVR Exposure During Scanning | ||
| Tanning time (min) | 8.6±2.3 | 5.0±1.1 |
| Change in temperature (°C) | ||
| Active UVR | 1.3±0.5 °F | 1.0±0.8 °F |
| Sham UVR | 1.0±0.8 °F | 0.6±0.3 °F |
| Craving | ||
| Pre-active UVR | 7.1±3.4 | 4.0±3.6 |
| Post-active UVR | 7.9±3.4 | 4.3±3.2 |
| Pre-sham UVR | 8.1±1.6 | 3.7±3.2 |
| Post-sham UVR | 7.4±2.8 | 5.1±3.6 |
Structured Interview for Tanning Abuse and Dependence (SITAD) (Hillhouse et al., 2012).
p<0.05.
p<0.005.
2.4. Image acquisition
SPECT scans were obtained at Zale Lipshy University Hospital Nuclear Medicine department. To limit thyroid exposure to 123I-iodobenzamide (123I-IBZM), 100 mg iodoral (IOD-50) was administered one hour prior to 123I-IBZM administration. One hour following iodoral, a 10 mCi bolus of 123I-IBZM was administered (Anazao Health, Tampa, Florida, IND 114748). Upon session completion, participants were provided with iodoral and instructed to take two tablets every 12 h for the next 48 h (4 doses). Scanning began 120 min after 123I-IBZM.
SPECT images were acquired on a dual-headed Siemens Ecam SPECT camera using ultra high-resolution fan-beam collimators (reconstructed resolution of 10–15 mm) in a 128 × 128 matrix into a 15% symmetric energy window centered on 159 keV. Reconstructed data from SPECT scans were filtered with a 3D Butterworth filter with order of 8 and cutoff of 0.42 and attenuation corrected using the Chang method. As a modest signal was anticipated, region of interest (ROI) analyses was limited to the caudate head as it is relatively resistant to partial volume sampling error due its large size and spheroidal shape. Caudate and occipital regions (the latter for background reference) were determined using a previously generated template using the average from 34 scans of healthy controls administered the SPECT dopamine transporter ligand 123I-ioflupane (Fig. 1, left panel). Target:back-ground ratio −1 approximated the binding potential, BPnd, reflecting the available D2/D3 receptor density. For demonstration purposes, a basal SPECT image from a single participant is provided in Fig. 1 (right panel).
Fig. 1.
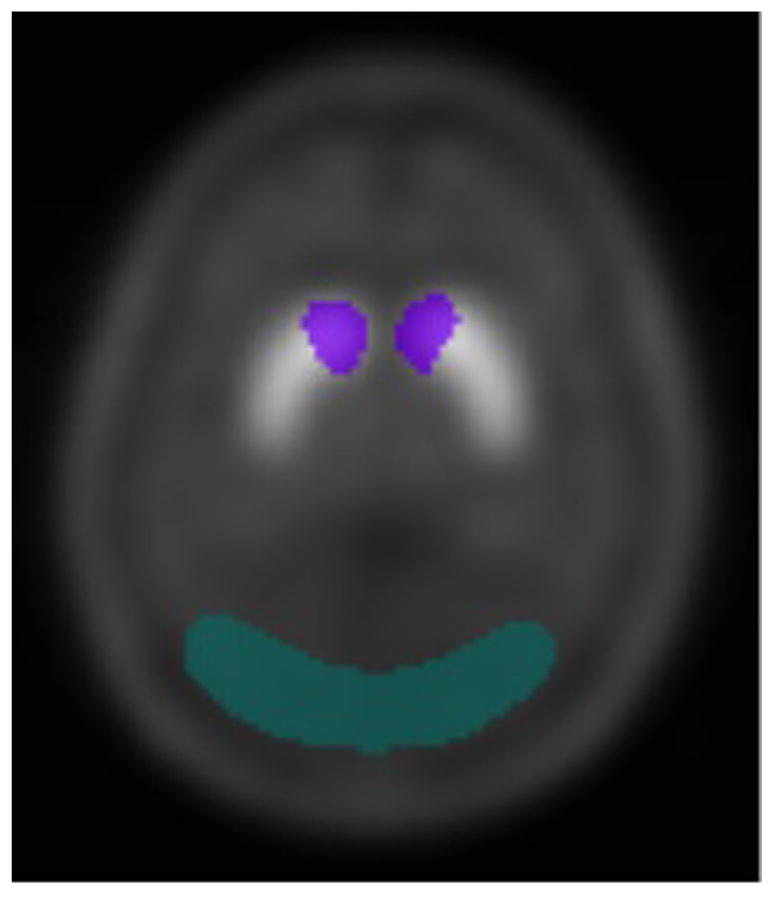
Caudate and occipital brain regions. Basal D2/D3 receptor density and UVR-induced dopamine efflux in the caudate head were assessed every 5 min using 123I-iodobenzamide (123I-IBZM) to calculate striatal binding potential (BPnd). Caudate (purple) and occipital regions (in green), the latter for background reference, were determined using a previously generated template using the average from 34 scans of healthy controls administered the SPECT dopamine transporter ligand 123I-io-flupane. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The optimal scanning sequence for assessing dopaminergic efflux following UVR had not previously been explored. Thus, two infrequent tanners were first assessed with active UVR to determine the optimal timing sequence using a single-blind design (their data was not included in the final analysis). These pilot scans were acquired with a temporal resolution of 5-minute frames to investigate the dynamic characteristics of 123I-IBZM receptor binding. Nine scan frames (total 45 min) were collected prior to UVR for baseline BPnd, followed by UVR activation. During and following UVR exposure, nine additional scan frames were continuously acquired. Basal dopamine BPnd was determined to achieve a secular equilibrium at 90 min and up to 3.5 h following 123I-IBZM administration. This window of relative BPnd stability was, therefore, chosen to assess basal and UVR-induced changes in BPnd. A decrease in BPnd was noted during the first 15 min following UVR onset. Subsequent participants were, therefore, scanned for 60 min: 30 min (beginning 120 min after 123I-IBZM administration) to determine basal BPnd and 30 min during and after UVR light. These two time periods allowed for secular equilibrium to be achieved during basal and UVR-induced measures of BPnd.
Six five-minute basal scans were merged and averaged into one 30-min image to determine basal D2/D3 BPnd. Given the unknown time course and effect size of UVR-induced dopaminergic efflux, coupled with the low striatal counts anticipated during each 5 min SPECT scan, three sequential 5-minute blocks were merged in a rolling average fashion for noise reduction. Resulting scans represented minutes 0–15 (T1), 5–20 (T2), 10–25 (T3), and 15–30 (T4) following the onset of UVR. BPnds during each 15-minute block were normalized to baseline BPnd (nBPnd), providing a participant-independent measurement of changes in BPnd during and after UVR exposure.
2.5. Statistical analysis
2.5.1. Demographic and clinical data
Intragroup and intergroup comparisons were conducted with paired and unpaired t-tests, respectively. Pearson correlations assessed relationships between changes in D2/D3 BPnd and clinical variables. Lifetime number of total salon tanning episodes/years tanning was used to describe tanning severity.
2.5.2. Image analysis
SPECT images were co-registered to Montreal Neurologic Institute (MNI) space using a DAT template in MNI space, re-sliced to 2 −2 −4 mm3 isotropic voxels. Attenuation correction was performed using a Chang zero-order method. The accuracy of spatial normalization of SPECT is limited by the spatial resolution of the original data (10–15 mm for these data), by partial volume effects and by the limits of the normalization algorithm used. Thus, accuracy of normalization and anatomic designations assigned to 123I-IBZM effects are constrained by these limitations. Region of Interest (ROI) analyses comparing UVR versus sham-UVR effects were conducted using Statistical Parametric Mapping (SPM8; University College, London, England).
3. Results
3.1. Study demographics
359 responders to study advertisements were screened by telephone interview. 38 participants underwent an in-person interview; 27 qualified for a history and physical examination. Five participants were excluded due to results of the history and physical examination or to scheduling conflicts. Two participated in the pilot scan only; 10 addicted and 10 infrequent tanners participated in both sessions. The addicted and infrequent tanners were similar in sex, age, ethnicity, and skin-phototype but, as expected, differed on measures of tanning frequency (Table 1).
3.2. UVR sessions
UVR exposure was 8.6±2.3 (range 4–10) min in addicted tanners and 5.0±1.1 (range 4–6) min in the infrequent tanners. Temperatures prior to active and sham UVR were similar (addicted, p=0.35; infrequent, p=0.92). Following both sham and UVR exposure there was a slight, albeit significant, increase in temperature (Table 1). There was no significant temperature difference (pre- vs. post-exposure) between the two sessions (addicted, p=0.31; infrequent, p=0.2) (Table 1).
3.3. Basal and UVR-induced 123I-IBZM BPnd
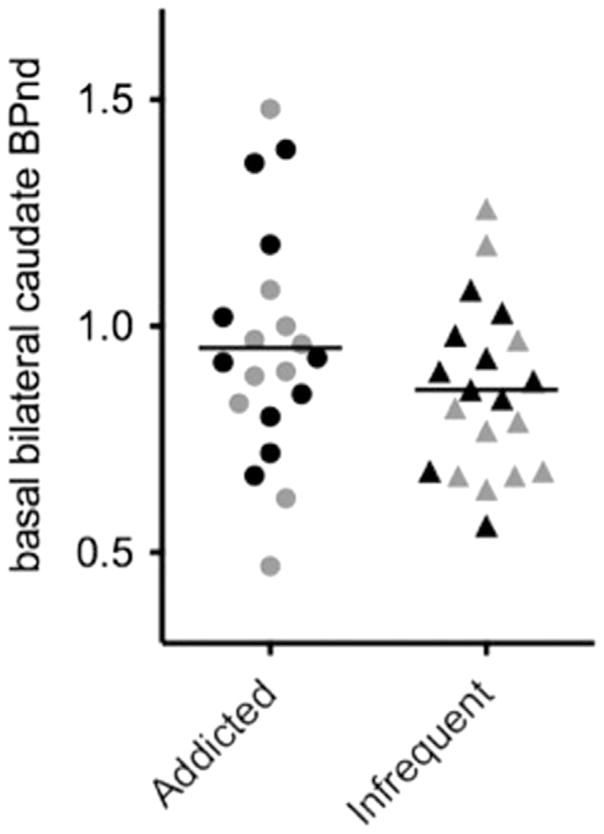
Basal 30-min 123I-IBZM BPnd prior to the active and sham sessions did not significantly differ within the addicted (p=0.90) or infrequent (p=0.78) tanners groups. Therefore, basal scans preceding UVR and non-UVR scans were averaged for each participant. Averaged basal 30-min 123I-IBZM BPnd did not significantly differ between groups (p=0.21) (Fig. 2).
Fig. 2.
Basal bilateral caudate striatal BPnd in addicted and infrequent tanners. There was no significant difference between the groups. Black is pre-active UVR administration; gray is pre-sham UVR administration.
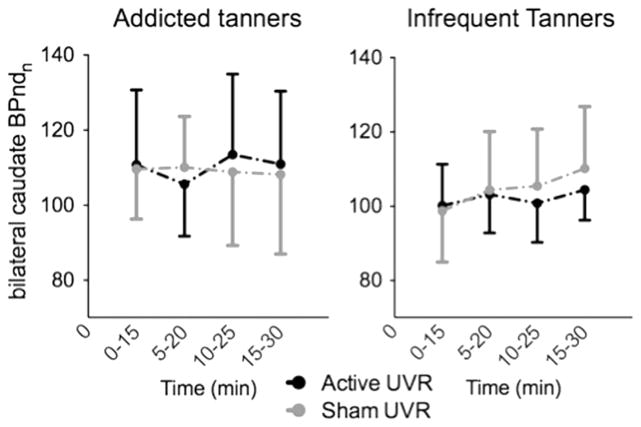
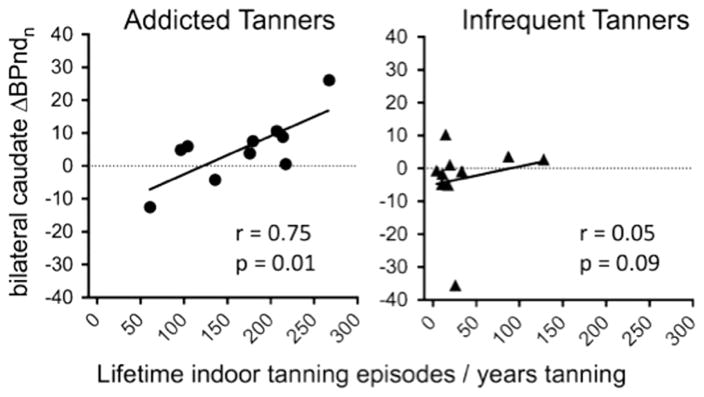
During/following UVR, there was a non-significant increase (p=0.14) in dopamine efflux (decrease in 123I-IBZM nBPnd) in the bilateral caudate in the addicted tanners during the T2 (5–20 min) timeframe relative to the T1 (0–15 min) interval (Fig. 3, left panel). This was followed by a return to T1 levels at T3. The difference between T2 and T3 trended towards significance (p=0.07). These findings suggest a relatively short period of dopamine efflux following UVR administration. Similar changes were not apparent in addicted tanners exposed to sham UVR or infrequent tanners exposed to either UVR or sham UVR (Fig. 3, right panel). No order effect (whether active UVR was administered in the first or second session) was observed for any condition in either group. The change in nBPnd between T1 and T2 (ΔnBPndT1vsT2) demonstrated a significant relationship to tanning severity in the addicted tanners (r=0.75, p=0.01), reflecting that intensity of lifetime tanning was positively correlated with dopamine efflux following UVR (Fig. 4, left panel). A similar relationship was observed in ΔnBPndT2vsT3 (r= −0.62, p=0.05). Significant relationships between tanning severity and ΔnBPnd were not observed in the infrequent tanners (T1vsT2 r=0.18, p=0.61; T2vsT3 r= −.305, p=0.39) (Fig. 4, right panel).
Fig. 3.
Striatal dopamine nBPnd showed a non-significant decrease during/following active UVR, but not sham UVR, in the addicted tanners during the 5–20 min interval –reflecting increased dopamine efflux. No changes were observed during sham UVR in the addicted tanners or during either active or sham UVR in the infrequent tanners. BPnd during each 15-minute block was normalized to baseline BPnd (nBPnd).
Fig. 4.
There was significant relationship between dopamine efflux (T1 – T2) and tanning severity in the addicted tanners only.
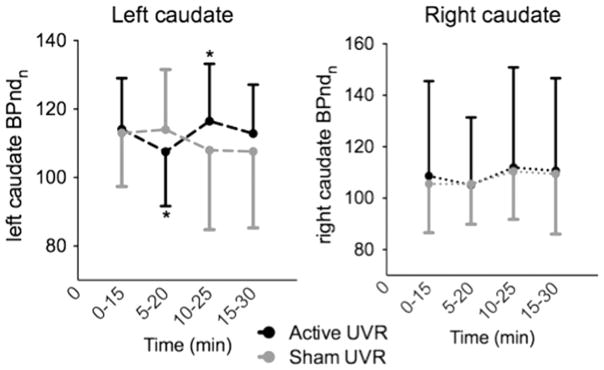
As we had previously observed increased rCBF during UVR administration was localized to the left caudate in addicted tanners (Harrington et al., 2012), post-hoc assessment of nBPnd in the left and right caudate was explored in the addicted tanners. Under active UVR, there was a significant change in 123I-IBZM nBPnd between T1 and T2 (p<0.02) and T2 and T3 (p<0.02) (Fig. 5, left panel). As some addicted tanners received less than 10 min UVR, we assessed whether ΔnBPndT1vsT2 correlated with minutes tanned; it did not (r= −0.03, p=0.94). No significant left caudate changes were seen in response to sham UVR (Fig. 5, left panel) or in the right caudate during either condition (Fig. 5, right panel). A relationship between tanning severity and ΔnBPndT1vsT2 (r =0.66, p=0.035) and ΔnBPndT2vsT3 (r = −0.63, p=0.05) in the left caudate was evident. Despite the lack of a significant change in 123I-IBZM nBPnd in the right caudate, a relationship between tanning severity and ΔnBPndT1vsT2 (r=0.72, p<0.02) and ΔnBPndT2vsT3 (r = −0.56, p=0.08) was demonstrated.
Fig. 5.
UVR induced a statistically significant change (*) in left striatal nBPnd between T1 (0–15 min) and T2 (5–20 min) (p <0.02) and T2 and T3 (10–25 min) (p <0.02), reflecting an increase in dopamine efflux during T2 and a return to basal values at T3. BPnd during each 15-minute block was normalized to baseline BPnd (nBPnd).
As there was a ΔnBPnd UVR response in the left, but not right, caudate, we explored post-hoc whether the left and right basal BPnd differed. Basal left caudate BPnd was significantly higher than the right caudate BPnd (left: 0.94±0.18, right: 0.86±.014; t=4.18; n=20; p=0.0005). Differences between left and right basal BPnd were observed in both the addicted (left: 1.00±0.19; right: 0.90±0.15; t=4.01; p=0.003) and infrequent (left: 0.88±0.16; right=0.83±0.14; t=2.04; p=0.07) tanners. Left and right basal BPnd were highly correlated with one another in both groups (r>0.88, p<0.0007). Neither left or right basal BPnd significantly correlated with ΔnBPnd following UVR onset.
3.4. Craving
There was no within group difference in basal measures of craving prior to active UVR or sham UVR in either the addicted or infrequent group (addicted, p=0.311; infrequent, p=0.5). There was not a statistical difference craving change (preUVR vs. pos-tUVR) following either active or sham UVR in either group (ΔUVR: addicted, p=0.09; infrequent, p=0.50; Δ sham UVR: addicted, p=0.38; infrequent, p=0.66). In the addicted group, 4 participants preferred the UVR session, 2 preferred sham UVR, and 4 had no preference. Four infrequent participants preferred the UVR session; the others had no preference.
However, craving was significantly higher in the addicted tanners prior to sham UVR (p=0.002) and slightly higher prior to active UVR (p=0.062) compared to the infrequent tanners (Table 1). No relationship was seen between craving and dopamine efflux within the left caudate in the addicted group (ΔnBPndT1vsT2 r=0.35, p=0.32; ΔnBPndT2vsT3 r =0.39, p=0.26). Neither the addicted or infrequent tanners showed a significant difference (all ps>0.2) in left caudate ΔnBPndT1vsT2 or ΔnBPndT2vsT3 between those who preferred UVR (n=4) compared to those who had no preference or preferred the sham UVR (n=6).
4. Discussion
Our findings suggest striatal dopamine efflux, primarily in the left caudate, briefly increases in response to UVR administration in tanners with behaviors consistent with an addictive disorder. The intensity of dopaminergic efflux was significantly associated with tanning severity in the addicted but not the infrequent tanners. In contrast to our hypothesis, dopamine efflux did not increase in infrequent tanners and basal striatal dopamine BPnd did not differ between infrequent and addicted tanners. Coupled with the previous preclinical and clinical literature on UVR and behavior, these findings support a biological basis for UVR’s rewarding properties that may underlie the addictive-like properties of tanning.
Although we had hypothesized dopamine efflux would be greater in the infrequent tanners, the increase in UVR-induced dopamine efflux was only observed in the addicted tanners. However, these findings are consistent with studies exploring striatal dopaminergic reactivity in behavioral (not substance-related) addictions. In pathological gamblers, Boileau and colleagues assessed the dopaminergic response to oral amphetamine using a partially selective D3 agonist (Boileau et al., 2013b). Amphetamine-induced dopamine efflux was amplified in the gamblers relative to controls. During a gambling task, patients with Parkinson disease and dopamine agonist-induced pathological gambling also showed a decrease in ventral striatal D2/3 agonist binding potential, indicating greater dopamine efflux, relative to similarly treated patients without pathological gambling (Steeves et al., 2009). Also contrary to our hypothesis, group differences in basal striatal D2/D3 receptors between addicted and infrequent tanners were not observed which, again, mirrored the similarity in D2/3 or D3 substantia nigra and striatal receptor binding reported in pathological gamblers and healthy controls (Boileau et al., 2013a). A more robust striatal dopaminergic response, and absence of long-term evidence of a hypodopaminergic state, may therefore be found in pathological addictive-like disorders associated with a subtler stimulus (e.g., UVR, gambling) relative to highly potent substances of abuse.
The decrease in striatal nBPnd was limited to the left caudate. This mirrors our previous work in which UVR increased left, but not right, caudate rCBF in addicted tanners during UVR relative to sham UVR (Harrington et al., 2012). A recent review of bilaterally in addiction concluded that left fMRI activation peaks were higher in the left hemisphere than the right, although these findings were not specific to the striatum (Gordon, 2015). Left striatal activation, relative to right, has also been associated with sexual desire (Arnow et al., 2002; Demos et al., 2012), monetary reward (Delgado et al., 2000; Lane et al., 1997) and gambling (Steeves et al., 2009). Although Brody et al. (2004) found greater striatal dopamine release during smoking on the right relative to left striatum, most studies have not reported lateralization of dopaminergic efflux during the administration of substances (Barrett et al., 2004; Boileau et al., 2003; Drevets et al., 2001; Fehr et al., 2008; Laruelle et al., 1995; Leyton et al., 2002; Mach et al., 1997; Martinez et al., 2003) or other high-valued rewards (Koepp et al., 1998; Small et al., 2003; Zald et al., 2004). However, some of these studies combined left and right striatal BDnd changes prior to analyses, thus obscuring possible laterality.
While we did not find a group difference between right and left caudate basal D2 BPnd, post-hoc analyses revealed D2 BPnd was higher on the left side in both the infrequent and addicted tanners. This is in contrast to the greater right, relative to left, striatal D2 BPnd reported by others in healthy volunteers [see review in Larisch et al. (1998)] and smokers (Domino et al., 2012). Most other investigators exploring basal striatal D2 BPnd in individuals with substance use disorders relative to healthy controls have not observed left-right laterality (Fehr et al., 2008; Martinez et al., 2007; Volkow et al., 2001; Volkow et al., 1993; Volkow et al., 2002).
Dopamine efflux in our addicted tanning group increased after a 5-min delay and then quickly returned to previous levels (i.e., striatal nBPnd decreased at the 5–20 min interval, reflecting an increase in dopamine, following the onset of UVR and then increased during the 10–25 min interval, reflecting a relative cessation of dopamine efflux). Striatal dopamine release likely occurs through UVB-induced activation of keratinocyte p53, which then cleaves β-endorphin (and adrenocorticotropin, or ACTH) from pro-opiomelanocortin (POMC) (Cui et al., 2007). In addition to binding to epidermal β-endorphin receptors(Kauser et al., 2003), the chronic administration of UVR in rodents increases plasma β-endorphin to physiologically meaningful levels by increasing pain threshold (Fell et al., 2014). Presumably, downstream effects of plasma β-endorphin stimulate ventral tegmental area and ventral striatal opioid receptors, resulting in striatal dopaminergic efflux. While β-endorphin does not appear to cross the blood brain barrier, conditioned place preference (i.e., the return to an environment associated with a previously obtained reward) to intravenously administered β-endorphin has been reported in rodents (Fell et al., 2014). The administration of an opioid antagonist induces opioid-like withdrawal symptoms in both rodents administered chronic UVR (Fell et al., 2014) and humans with addictive-like tanning behaviors (Kaur et al., 2005) and conditioned place aversion is evidenced in response to naloxone-induced withdrawal to UVR (Fell et al., 2014). These findings suggest central nervous system effects of plasma β-endorphins. Although the specific time course from UVR administration to plasma β-endorphin elevation has not been explored, the enzymatic sequence involving the cleavage of pituitary POMC and production of peak plasma ACTH concentrations requires 10–15 min (Adinoff et al., 1991). This is consistent with the increase in striatal dopamine at the 5–20 interval following UVR initiation. Unlike substances of abuse (with half-lives lasting several minutes to hours), UVR would not be expected to result in persistent stimulation following its termination. Thus, the decline in dopamine shortly after UVR cessation is not unexpected.
Strengths of our study include groups matched for age, ethnicity and gender and the use of sham UVR. Potential design confounds include the need to administer UVR for different time intervals to assure addicted tanners received a meaningful stimulus (most frequent tanners administer UVR for 20 min per session) but infrequent tanners were protected from burning. Unexpectedly, craving did not decrease following UVR in the addicted tanners. The cold ambient room temperature may have affected the accurate assessment of changes in craving, as participants reported their high ratings of “desire to tan” following UVR exposure was due to their desire for the heat emanating from the lamp. Nonetheless, addicted tanners reported higher cravings for tanning before and after UVR as compared to the infrequent group. Finally, the signal from 123I-IBZM was not sufficient to provide the spatial resolution necessary to accurately assess nucleus accumbens or putamen dopamine efflux. The 123I-IBZM signal also limited our ability to refine the temporal course of dopamine release.
In summary, these findings support a neurocutaneous system driving the compulsive self-administration of damaging UVR. Due to our relatively small sample size and the modest elevation in dopamine efflux, these observations must be confirmed in pre-clinical models and in a larger cohort of addicted tanners – coupled with concurrent measure of plasma β-endorphin concentrations. Furthermore, it is expected UVR-induced increases in striatal dopamine release will be blocked by opioid antagonists. The confirmation and extension of our findings will guide the way towards effective treatment interventions for individuals compulsively self-administering toxic concentrations of UVR.
Acknowledgments
Funding
This study was funded by Grant R21AR063018 from the National Institutes on Arthritis and Musculoskeletal and Skin Diseases. Screening information was obtained by Research Electronic Data Capture (REDCap), which is supported by UT Southwestern Academic Information Systems, Vanderbilt Research and the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105.
Abbreviations
- 123I-IBZM
123I-Iodobenzamide
- BPnd
Striatal binding potential
Footnotes
Conflict of interest disclosure
None declared.
Contributors
PA, JS, MD, HJ, FF and BA were responsible for the study concept and design. PA, JP, TH, HJ, and BA contributed to the acquisition of data. TH, JS and MD performed the SPECT analysis. PA, JS, JP, TH, HJ, MD and BA assisted with data analysis and interpretation of findings. PA and BA drafted the manuscript. PA, JS, FF, HJ and BA provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- Adinoff B. Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry. 2004;12:305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Risher-Flowers D, De Jong J, Ravitz B, Bone GHA, Nutt DJ, Roehrich L, Martin PR, Linnoila M. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am J Psychiatry. 1991;148:1023–1025. doi: 10.1176/ajp.148.8.1023. [DOI] [PubMed] [Google Scholar]
- Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML, Lue TF, Atlas SW. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002;125:1014–1023. doi: 10.1093/brain/awf108. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A. The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse. 2004;54:65–71. doi: 10.1002/syn.20066. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1979;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bleyer A, Viny A, Barr R. Cancer in 15-to 29-year-olds by primary site. Oncologist. 2006;11:590–601. doi: 10.1634/theoncologist.11-6-590. [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Chugani B, Lobo D, Behzadi A, Rusjan PM, Houle S, Wilson AA, Warsh J, Kish SJ, Zack M. The D2/3 dopamine receptor in pathological gambling: a positron emission tomography study with [11C]-(+)-propyl-hexahydro-naphtho-oxazin and [11C]raclopride. Addiction. 2013a;108:953–963. doi: 10.1111/add.12066. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Chugani B, Lobo DS, Houle S, Wilson AA, Warsh J, Kish SJ, Zack M. In vivo evidence for greater amphetamine-induced dopamine release in pathological gambling: a positron emission tomography study with [C]-(+)-PHNO. Mol Psychiatry. 2013b doi: 10.1038/mp.2013.163. [DOI] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J, Hahn EL, Mandelkern MA. Smoking-induced ventral striatum dopamine release. Am J Psychiatry. 2004;161:1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D’Orazio J, Fung CY, Schanbacher CF, Granter SR, Fisher DE. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci: Off J Soc Neurosci. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino EF, Evans CL, Ni L, Guthrie SK, Koeppe RA, Zubieta JK. Tobacco smoking produces greater striatal dopamine release in G-allele carriers with mu opioid receptor A118G polymorphism. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:236–240. doi: 10.1016/j.pnpbp.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens—Part D: radiation. Lancet Oncol. 2009;10:751–752. doi: 10.1016/s1470-2045(09)70213-x. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, Eberhardt A, Klager M, Smolka MN, Scheurich A, Dielentheis T, Schmidt LG, Rosch F, Bartenstein P, Grunder G, Schreckenberger M. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry. 2008;165:507–514. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- Feldman SR, Liguori A, Kucenic M, Rapp SR, Fleischer AB, Jr, Lang W, Kaur M. Ultraviolet exposure is a reinforcing stimulus in frequent indoor tanners. J Am Acad Dermatol. 2004;51:45–51. doi: 10.1016/j.jaad.2004.01.053. [DOI] [PubMed] [Google Scholar]
- Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin beta-endorphin mediates addiction to UV Light. Cell. 2014;157:1527–1534. doi: 10.1016/j.cell.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MH, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition (SCID-I/P) Biometrics Research Department, New York State Psychiatric Institute; NY: 2002. [Google Scholar]
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- Gordon HW. Laterality of brain activation for risk factors of addiction. Current Drug Abuse Reviews. 2015 doi: 10.2174/1874473709666151217121309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington CR, Beswick TC, Graves M, Jacobe HT, Harris TS, Kourosh S, Devous MD, Sr, Adinoff B. Activation of the mesostriatal reward pathway with exposure to ultraviolet radiation (UVR) vs. sham UVR in frequent tanners: a pilot study. Addict Biol. 2012;17:680–686. doi: 10.1111/j.1369-1600.2010.00312.x. [DOI] [PubMed] [Google Scholar]
- Harrington CR, Beswick TC, Leitenberger J, Minhajuddin A, Jacobe HT, Adinoff B. Addictive-like behaviours to ultraviolet light among frequent indoor tanners. Clin Exp Dermatol. 2011;36:33–38. doi: 10.1111/j.1365-2230.2010.03882.x. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse JJ, Baker MK, Turrisi R, Shields A, Stapleton J, Jain S, Longacre I. Evaluating a measure of tanning abuse and dependence. Arch Dermatol. 2012;148:815–819. doi: 10.1001/archdermatol.2011.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Liguori A, Fleischer AB, Jr, Feldman SR. Side effects of naltrexone observed in frequent tanners: could frequent tanners have ultraviolet-induced high opioid levels? J Am Acad Dermatol. 2005;52:916. doi: 10.1016/j.jaad.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Kaur M, Liguori A, Lang W, Rapp SR, Fleischer AB, Jr, Feldman SR. Induction of withdrawal-like symptoms in a small randomized, controlled trial of opioid blockade in frequent tanners. J Am Acad Dermatol. 2006;54:709–711. doi: 10.1016/j.jaad.2005.11.1059. [DOI] [PubMed] [Google Scholar]
- Kauser S, Schallreuter KU, Thody AJ, Gummer C, Tobin DJ. Regulation of human epidermal melanocyte biology by beta-endorphin. J Investig Dermatol. 2003;120:1073–1080. doi: 10.1046/j.1523-1747.2003.12242.x. [DOI] [PubMed] [Google Scholar]
- Knight JM, Kirincich AN, Farmer ER, Hood AF. Awareness of the risks of tanning lamps does not influence behavior among college students. Arch Dermatol. 2002;138:1311–1315. doi: 10.1001/archderm.138.10.1311. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Brooks DJ, Bench CJ, Grasby PM. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucenic MJ, Patel M, Feldman SR, Liguori A, Fleischer AB. Visual discrimination testing of ultraviolet transmitting and ultraviolet blocking acrylic thermoplastics. Photodermatol Photoimmunol Photomed. 2002;18:228–231. doi: 10.1034/j.1600-0781.2002.02766.x. [DOI] [PubMed] [Google Scholar]
- Kwon HT, Mayer JA, Walker KK, Yu H, Lewis EC, Belch GE. Promotion of frequent tanning sessions by indoor tanning facilities: two studies. J Am Acad Dermatol. 2002;46:700–705. doi: 10.1067/mjd.2002.119560. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Larisch R, Meyer W, Klimke A, Kehren F, Vosberg H, Muller-Gartner HW. Left-right asymmetry of striatal dopamine D2 receptors. Nucl Med Commun. 1998;19:781–787. doi: 10.1097/00006231-199808000-00009. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Rosenblatt W, Zea-Ponce Y, Zoghbi SS, Baldwin RM, Charney DS, Hoffer PB, Kung HF, et al. SPECT imaging of striatal dopamine release after amphetamine challenge. J Nucl Med. 1995;36:1182–1190. [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Mach RH, Nader MA, Ehrenkaufer RL, Line SW, Smith CR, Gage HD, Morton TE. Use of positron emission tomography to study the dynamics of psychostimulant-induced dopamine release. Pharmacol Biochem Behav. 1997;57:477–486. doi: 10.1016/s0091-3057(96)00449-2. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Monfrecola G, Fabbrocini G, Posteraro G, Pini D. What do young people think about the dangers of sunbathing, skin cancer and sunbeds? A questionnaire survey among Italians. Photodermatol Photoimmunol Photomed. 2000;16:15–18. doi: 10.1034/j.1600-0781.2000.160105.x. [DOI] [PubMed] [Google Scholar]
- Mosher CE, Danoff-Burg S. Addiction to indoor tanning: relation to anxiety, depression, and substance use. Arch Dermatol. 2010;146:412–417. doi: 10.1001/archdermatol.2009.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan BV, Feldman SR. Ultraviolet tanning addiction. Dermatol Clin. 2009;27:109–112. doi: 10.1016/j.det.2008.11.007. (v) [DOI] [PubMed] [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacol Bull. 1997;33:17–22. [PubMed] [Google Scholar]
- Poorsattar SP, Hornung RL. UV light abuse and high-risk tanning behavior among undergraduate college students. J Am Acad Dermatol. 2007;56:375–379. doi: 10.1016/j.jaad.2006.08.064. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Speilberger CD. Trait-state anxiety and motor behavior. J Mot Behav. 1971;3:265–279. doi: 10.1080/00222895.1971.10734907. [DOI] [PubMed] [Google Scholar]
- Steeves TD, Miyasaki J, Zurowski M, Lang AE, Pellecchia G, Van Eimeren T, Rusjan P, Houle S, Strafella AP. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132:1376–1385. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding YS, Gatley SJ, Hitzemann R, Wong C, Pappas N. Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res. 2002;116:163–172. doi: 10.1016/s0925-4927(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Warthan MM, Uchida T, Wagner RF., Jr UV light tanning as a type of substance-related disorder. Arch Dermatol. 2005;141:963–966. doi: 10.1001/archderm.141.8.963. [DOI] [PubMed] [Google Scholar]
- Wehner MR, Chren MM, Nameth D, Choudhry A, Gaskins M, Nead KT, Boscardin WJ, Linos E. International prevalence of indoor tanning: a systematic review and meta-analysis. JAMA Dermatol. 2014;150:390–400. doi: 10.1001/jamadermatol.2013.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Seyoum RA, O’Connor SJ, Wang C, Zheng QH, Mock B, Morris ED. Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Res. 2005;29:965–970. doi: 10.1097/01.alc.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]
- Zald DH, Boileau I, El-Dearedy W, Gunn R, McGlone F, Dichter GS, Dagher A. Dopamine transmission in the human striatum during monetary reward tasks. J Neurosci. 2004;24:4105–4112. doi: 10.1523/JNEUROSCI.4643-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller S, Lazovich D, Forster J, Widome R. Do adolescent indoor tanners exhibit dependency? J Am Acad Dermatol. 2006;54:589–596. doi: 10.1016/j.jaad.2005.12.038. [DOI] [PubMed] [Google Scholar]